Abstract
Background: A high prevalence of dual use of e-cigarettes and conventional cigarettes has been reported across the world. Methods: A systematic search was carried out. We included original articles on any topic relevant to health, excluding mental health, in all languages. The PRISMA guidelines were followed. Both reviewers independently screened and read all publications. We compared dual use with exclusive smoking of conventional cigarettes (ESCC). Results: Fifty-two publications (49 studies) were included. Thirteen papers/10 studies were prospective. There was great heterogeneity across studies. Many methodological weaknesses, such as inaccurate exposure measurement, lack of adjustment for former tobacco consumption, and lack of significance testing were identified. Most prospective studies found dual use to be at least as harmful as ESCC. The longest follow-up was six years. Most of the best available cross-sectional studies found dual use associated with the same and, in several studies, significantly higher risk of self-reported symptoms/disease than in ESCC. The intensity of cigarette smoking seems associated with worse health. Conclusion: Existing studies indicate that dual use is at least as, or probably even more, harmful than ESCC. Due to the predominance of cross-sectional studies and the methodological weaknesses we judged the overall certainty of the evidence as “low certainty”.
Keywords: e-cigarettes, electronic cigarettes, ENDS, vaping, cigarettes, smoking, dual use, health, public health, biomarker
1. Introduction
The number of electronic cigarette/e-cigarette (EC/ECs) users has been increasing rapidly, and in 2021 it was estimated that there were 82 million vapers worldwide [1].
Available evidence on the benefits and risks of EC use are mixed and interpreted differently. Some believe that ECs have the potential to reduce the burden of disease in smokers [2,3] while others worry about the impact on public health and do not recommend, and even ban, their use [4,5]. Debates about the population health impact of alternative nicotine delivery products (i.e., ECs) are ongoing [6]. Public health research can be helpful by providing the needed evidence and facilitating its interpretation in a well-framed decision context.
A very important common reason for EC use in adults is smoking cessation [7,8] and ECs are recommended as cessation tools in some countries [9,10]. Smokers often buy ECs because they want to stop smoking, and though many succeed in switching to ECs for a short period, most relapse to conventional cigarette smoking [11]. The use of ECs and conventional cigarettes at the same time (CC/CCs) is called dual use (DU) [12,13,14,15,16]. A review found that dual users (DUs) perceive ECs as a safer and less addictive alternative to CCs [17].
Several studies across the world have reported a high prevalence of DU [12,13,14,15,18,19,20]. Studies from South Korea found DU in almost all current EC users [21,22,23]. A huge population-based study from the United States of America (USA) reported that more than six out of ten EC users were DUs [24], corresponding to approximately 1–3% of the population [12,22,24,25,26,27]. In contrast, some countries have lower estimates. A large population-based study from the United Kingdom (UK) found that less than 40% of adult EC users were DUs [25].
Dual use might be a short transition period before quitting smoking completely. However, a cohort study with six years of follow-up found that most DUs relapse to exclusive smoking of conventional cigarettes (ESCC) and only few transition exclusively to EC use [28,29]. Several other studies have reported the same similar findings [30,31,32,33,34].
A further concern is that there may not be a significant reduction in DUs’ consumption of CCs [35,36,37]. For example, a study from Poland found that DUs did not smoke fewer conventional cigarettes per day (CPD) than ESCC [38].
While health effects of smoking as well as vaping have been extensively studied, it is also important to understand the health effects of DU, as inhalation of smoke and EC aerosol in combination, in the worst case, could potentially lead to higher pathology than either inhalant alone. To our knowledge, only one review has investigated the health effects of DU (in pregnancy) [39]. The aim of this systematic review was to gather the existing evidence comparing the health effects of DU with the health effects of ESCC.
2. Materials and Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA-2020) guidelines.
Common components of ECs include a battery, heating coil, atomizer that transforms the e-liquid to an aerosol, cartridge that contains the e-liquid, and mouthpiece. Each component has the potential to affect health outcomes independently. The design of ECs has evolved in several ways since their introduction [40,41]. We included all types and all four generations of ECs.
2.1. Eligibility Criteria
Original articles on real-world DU of ECs and CCs on any topic relevant to health in any language.
2.2. Exclusion Criteria
Several cross-sectional studies have investigated the association between mental health problems and DU. These studies were not included, as we found it difficult to distinguish between mental health problems being a result of DU or a predictor of DU. Conference abstracts and dissertations were not included. Animal and human short-term experimental studies with forced switch or forced reduction in number of CPD were not included, as these studies do not reflect real-world use.
2.3. Information Sources
A search was carried out in PubMed, EMBASE, CINAHL, and Cochrane library (Table S1).
2.4. Search Strategy
The first search was conducted on 12 January 2021. The last search was conducted on 27 April 2021. We used the keywords “dual use” AND “e-cigarette” OR “e-cigarettes” OR “electronic cigarette” OR “electrically heated cigarette” OR “electronic nicotine delivery system” OR “electronic nicotine delivery device.” Keywords had to be included in the title, abstract, and/or full text. We used no other filters or limits.
2.5. Selection Process
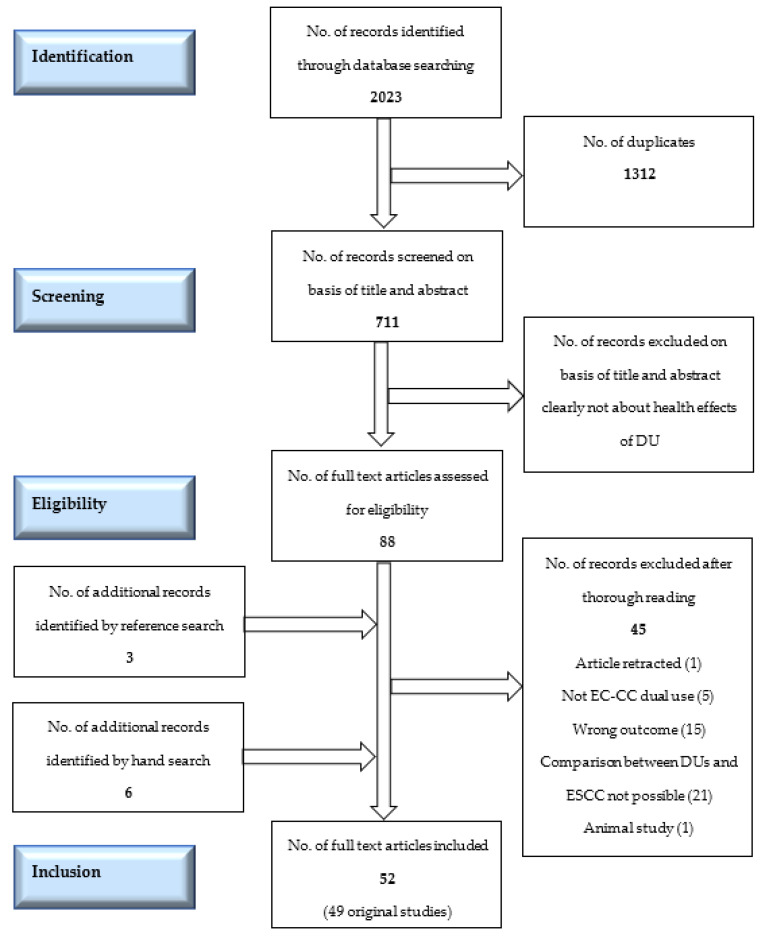
The screening process was blinded (Covidence was used for screening), and agreement of both authors was necessary to include/exclude a title. Eighty-eight papers were assessed for eligibility (Figure 1). All were in English. An overview table of all excluded papers can be found in Table S2. Additionally, references from the screened full text papers were carefully examined for missed papers, and our own reference data base was hand-searched for possible overlooked titles. A total of 52 papers (49 studies) were included in the final analysis.
Figure 1.
The PRISMA flow-chart of the search and inclusion of the papers in the systematic review. DUs= dual users. CC= conventional cigarettes. EC= e-cigarettes. ESCC = exclusive smokers of conventional cigarettes.
2.6. Data Collection Process
During the data collection process, each reviewer independently read the full paper and extracted data from each paper to a predefined table framework. Results from the independent data collection were then compared, discussed, and merged into one detailed table (Table S3). We did not obtain or confirm data from study investigators.
2.7. Risk of Bias and Quality Assessment
As studies used very heterogenic methods and exposure measurement, it was not possible to use a universal assessment method. Prospective studies were assessed by the JBI critical appraisal tool (Appendix S1 in Supplementary Materials). Both reviewers independently assessed the main risks of bias without the use of automated tools. We registered conflict of interest (COI) and the size of the study and looked to see whether data were weighted for non-participation and if the study had taken relevant confounding into account. We further registered if studies had adjusted for former tobacco consumption. When assessing selection bias, we considered sampling, volunteer bias, and attrition bias. If several/many studies had investigated the same outcome, best quality studies were defined as “best available.”
2.8. Effect Measures
Effect measures varied depending on the outcome. Most papers on symptoms or disease risk presented unadjusted and adjusted odds ratios (OR) with a 95% confidence interval (CI). If available, we present adjusted odds ratios (aOR, Appendix S3 in Supplementary Materials). Papers on toxic effects typically presented geometric means and 95% CI and/or ranges and interquartile intervals.
2.9. Data Items
Papers were included if any outcome data comparing DU with ESCC were presented, even if significance levels between ESCC and DU were not shown. We extracted the same predefined information from all papers (Table S3). If data on any variable were missing, we searched in Supplementary Materials and/or in study protocols.
2.10. Synthesis Methods
There was great heterogeneity both in exposure, methods, and outcomes across papers, so merging of results in a meta-analysis was not possible. After completing Table S3, which gave us an overview of all studies, we distributed papers according to two categories: prospective studies (Table 1) and cross-sectional studies (Table 2). Results were synthesized into five overall categories, marked by signs (##; #; ¤; *; **).
Table 1.
Prospective studies investigating the potential harm in DUs compared to ESCC.
| First Author, Year of Publication, Country, Conflict of Interest | Method | Participants | Risk of Selection Bias/Weighted Data/Adjusted Analyses/Adjusted for Former Tobacco Consumption | Major Outcomes | Overall Findings Significantly (Higher Risk/Prevalence/Level in ESCC = ## Higher Risk/Prevalence/Level in ESCC, Significance Level Not Tested or Not Significant = # Same Risk/Prevalence/Level in ESCC and DUs = ¤ Higher Risk/Prevalence/Level in DUs, Significance Level Not Tested or Not Significant = * Significantly Higher Risk/Prevalence/Level in DUs = **) |
|
|---|---|---|---|---|---|---|
| Pregnancy and fertility | Cardenas V.M. [42] 2020, USA None |
Pregnancy Risk Assessment Monitoring | 1594 pregnant women | Low/yes/yes/no | Risk of small-for-gestational-age | DUs: higher odds of giving birth to a small-for-gestational-age child than ESCC, but significance level not tested * |
| Clemens M.M. [43] § 2019, USA None |
Pregnancy Risk Assessment Monitoring | 248 pregnant women | Low/yes/yes/no | Carcinogen metabolites (TSNAs) in hair samples + Risk of small-for-gestational-age (SGA) | DUs same level of carcinogen biomarkers as ESCC ¤ DUs had higher risk of small-for-gestational-age than ESCC, but significance not tested * |
|
| Harlow A. [44] 2020, USA None |
Cohort study, online survey | 4586 young women trying to conceive | Low/no/yes/ yes |
Fecundability (menstrual cycle and achieved pregnancy) |
DUs: lower fecundability ratio than ESCC, but not significantly different * | |
| McDonnell BP. [45] 2020, Ireland None |
Pregnancy Risk Assessment Monitoring | 620 pregnant women | Low/no/yes/no | Delivery and neonatal outcomes | DUs: same birthweight, Apgar score and mean gestation at delivery as ESCC ¤ DUs: higher rate of admission to neonatal intensive care unit and higher incidence of birthweight <10th centile than ESCC but significance level not tested * |
|
| Wang X. [46] 2020, USA None |
Pregnancy Risk Assessment Monitoring | 31,973 pregnant women | Low/yes/yes/no | Preterm birth and small-for-gestational-age (SGA) | Similar (elevated) risk of preterm birth and of small-for-gestational-age in DU as ESCC ¤ |
|
| Other | McRobbie H. [47] 2015, UK  Yes Yes |
Smoking cessation study with 4 weeks follow-up | 44 healthy volunteer smokers Use of EC ad libitum |
High/-/no/no | Urinary 3-HPMA, a major metabolite of acrolein and carbon-monoxide (CO) |
DUs had sign. reductions in 3-HPMA and CO after switching from ESCC (significant reduction in cotinine in DU) ## |
| Bhatta D. N. [48] 2020, USA None |
Nationally representative cohort study | 32,320 adults | Low/yes/yes/no | Self-reported respiratory disease (chronic obstructive pulmonary Disease (COPD), chronic bronchitis, emphysema, or asthma) |
DUs: higher odds of reporting of respiratory disease than ESCC but significance level not tested * | |
| Sanou A. Z. [49] 2020, USA None |
Register study using a cohort | 802,621 adult military members |
Low/no/yes/no | Incident cases of acute respiratory infections (in- and outpatient diagnoses) | DUs had higher incident rate of acute respiratory infections than ESCC but significance level not tested * | |
| Flacco M. E. [50] ^^ 2019, Italy  Yes Yes |
Cohort study 48 months | 915 adults | High/no/yes/yes | Changes in self-reported health score and possibly smoking-related disease | DUs: no significant difference in self-reported health score and possible smoking related disease after 4 years than ESCC, but generally worse outcomes in DUs ¤ | |
| Flacco M. E. [29] ^^ 2020, Italy  Yes Yes |
Cohort study 72 months | 912 adults | High/no/yes/yes |
Changes in self-reported health score and possibly smoking-related disease | DUs had higher odds of possibly smoking related disease after 6 years than ESCC but not significant ¤ | |
| Manzoli L. [35] ^^ 2015, Italy None, first 2 years |
Cohort study 12 months | 959 adults with 1-year data | High/no/yes/yes | Self-reported health | DUs: same self-reported health as ESCC ¤ | |
| Manzoli L. [28] ^^ 2017, Italy None, first 2 years |
Cohort study 24 months | 932 adults with 2-year data | High/no/yes/yes | Self-reported health | DUs at baseline: same self-rated health as ESCC and significantly higher probability of serious adverse events than ESCC ¤ ** DUs at 24 months follow-up: significant improvement in self-rated health compared with ESCC ## |
|
| Riehm K. E. [51] 2019, USA  Yes Yes |
Nationally representative cohort | 9588 adolescents | Low/yes/yes/no | Sleep-related complaints | DUs: higher risk of sleep-related complaints than ESCC, but not significant * |
Table 2.
Cross-sectional studies investigating potential harm of DUs compared to ESCC.
| First Author, Year of Publication, Country, Conflict 0f Interest | Method | Participants | Risk of Selection Bias/Weighted Data/Adjusted Analyses/Adjusted for Former Tobacco Consumption | Major Outcomes | Overall Findings Significantly (Higher Risk/Prevalence/Level in ESCC = ## Higher Risk/Prevalence/Level in ESCC, Significance Level Not Tested or Not Significant = # Same Risk/Prevalence/Level in ESCC and DUs = ¤ Higher Risk/Prevalence/Level in DUs, Significance Level Not Tested or Not Significant = * Significantly Higher Risk/Prevalence/Level in DUs = ** |
|
|---|---|---|---|---|---|---|
| Harmful substances | Carroll D.M. [53] 2018, USA None |
Cross-sectional study | 94 volunteer adults of American Indian descent |
High/-/(no)/no | Carcinogen metabolite (NNAL) in urine | DUs same level of carcinogen biomarker as ESCC ¤ |
| Goniewicz M. [15] 2018, USA  Yes Yes |
Cross-sectional analyses of nationally representative cohort study | 5105 adults | Low/yes/yes/no | 50 biomarkers of toxicity (TSNAs, metals, PAHs, and VOCs) in urine |
DUs: significantly higher concentration of most biomarkers of toxicity/carcinogenicity than ESCC ** | |
| Jain R. [54] 2019, USA None |
Cross-sectional analyses of population-based survey | 1139 adults | Low/yes/yes/no | Harmful metals (cadmium, lead, and mercury) in blood | DUs same levels of harmful metals in blood as ESCC ¤ | |
| Keith R. [55] 2020, USA None |
Cross-sectional analysis of cohort study | 371 volunteer adults | Low/no/yes/no | Volatile organic compound (VOC) metabolites in urine | DUs and ESCC had similar levels of most VOC metabolites, except four, which were significantly higher in ESCC than in DU ¤ ## | |
| Piper M [34] 2018, USA None |
Cross-sectional analysis of cohort study | 422 volunteer adults | Low/no/yes/no | Carcinogen metabolite (NNAL) in urine | DUs had significantly lower levels of NNAL than ESCC ## | |
| Prokopowicz A. [52] 2019, Poland  Yes Yes |
Cross-sectional study | 156 young volunteer adults | High/-/yes/no | Harmful metals cadmium and lead in blood | DUs: levels of harmful metals not significantly different than ESCC ¤ | |
| Prokopowicz A. [56] 2020, Poland  Yes Yes |
Cross-sectional study | 88 young volunteer adults | High/-/yes/no | 11 toxic metals in urine | Significance level between ESCC and DUs not tested, but DUs had higher values for 8 out of 11 metals in urine * | |
| Rostron B. L. [57] 2019, USA None |
Cross-sectional analysis of a nationally representative cohort | 2710 adults | Low/yes/yes/no | Carcinogen and toxin exposure, biomarkers (VOCs, PAHs and TSNAs) in urine and blood | DUs: significantly higher levels of some toxic and carcinogenic biomarkers (NNAL, 1-HOP, HPMA and MHB3) compared to ESCC ** ¤ | |
| Shahab L. [58] 2017, UK  Yes Yes |
Cross-sectional study | 181 volunteer adults with long-term use | High/-/yes/yes | Carcinogen and toxin exposure, biomarkers (VOCs and TSNAs) in urine and saliva | DUs and ESCC had similar levels of toxic and carcinogenic substances, but DU had significantly higher level of one carcinogenic substance, benzene than ESCC ¤ ** | |
| Smith D. ^ [59] 2020, Poland, UK and USA  Yes Yes |
Cross-sectional study | 456 volunteer adults with long-term use | High/-/yes/no | Carcinogen and toxin exposure biomarkers (VOCs, TSNAs and minor alkaloids) in urine and saliva | DUs and ESCC had similar levels of toxic and carcinogenic substances, but ESCC had significantly higher level of three TSNAs and acrylonitrile than DUs ¤ ## | |
| Cho J. H. [60] 2016, South Korea None |
Nationally representative survey | 35,904 adolescents | Low/no?/yes/no | Self-reported diagnosed with asthma | DUs higher odds of reporting asthma than ESCC but not significant in adjusted analyses * | |
| Chung S. J. [61] 2019, South Korea None |
Nationally representative survey | 60,040 adolescents | Low/yes/yes/no | Self-reported diagnosed with asthma or/and allergic rhinitis | DUs had higher odds for current allergic rhinitis but lower odds of current asthma than ESCC, but significance level not tested * # | |
| Hedman L. [12] 2018, Sweden  Yes Yes |
2 population-based surveys | 30,272 adults | Low/no/yes/no | Self-reported respiratory symptoms: long-standing cough, sputum production, wheeze | DUs had higher odds of self-reported respiratory symptoms than than ESCC but significance level not tested * |
|
| Lee A. [62] 2019, South Korea None |
Population-based survey | 58,336 adolescents | Low/yes/yes/no | Self-reported asthma, allergic rhinitis and atopic dermatitis | DUs har lower odds of asthma than ESCC, but comparable odds of allergic rhinitis and atopic dermatitis. Significance level not tested # ¤ | |
| Li D. [63] § 2020, USA  Yes Yes |
Nationally representative survey | 28,171 adults | Low/yes/yes/no | Self-reported respiratory symptoms and physical health | DUs same odds of respiratory symptoms as ESCC ¤ DUs: same prevalence of poor physical health as ESCC ¤ |
|
| Osei A. [64] 2020, USA  Yes Yes |
Nationally representative survey | 705,159 adults | Low/yes/yes/no | Self-reported diagnosed with COPD/emphysema/chronic bronchitis | DUs had significantly higher odds of COPD/emphysema/chronic bronchitis than ESCC ** | |
| Parekh T. [65] 2020, USA None |
Nationally representative survey | 161,965 young adult women | Low/yes/yes/no | Self-reported diagnosed with COPD/emphysema/chronic bronchitis and asthma | DUs had higher odds of asthma and COPD compared than ESCC, but significance level not tested * | |
| Wang J. B. [66] § 2018, USA  Yes Yes |
Internet population -based survey | 39,747 adults | Low/no/yes/no | Self-reported cardiopulmonary symptoms in the last months General health in the last month (SF-12) |
DUs had significantly higher/worse breathing difficulty score than ESCC ** DUs: significantly worse median general health scores than ESCC** DUs: significantly higher prevalence of history of an arrhythmia than ESCC ** |
|
| Wills T. A. [67] 2019, USA None |
Population-based survey | 8087 adults | Low/yes/yes/no | Self-reported diagnosed with asthma, COPD | DUs and ESCC same odds of asthma ¤ DUs higher odds of COPD than ESCC but not significant * |
|
| Wills T.A. [68] 2020, USA None |
Nationally representative youth survey | 14,765 adolescents | Low/yes/yes/no | Self-reported asthma diagnosis | DUs had higher odds of asthma than ESCC but not sign. ¤ Significantly higher in a sensitivity analysis tested in a sample with complete data ** |
|
| Xie Z. [24] 2020, USA None | Nationally representative youth survey | 887,182 adults | Low/yes/yes/no | Self-reported COPD diagnosis told by doctor | DUs had significantly higher risk of self-reported COPD diagnosis told by doctor than ESCC ** | |
|
Cardiovascular and metabolic outcomes
|
Choi D-W [69] 2018, South Korea None |
Nationally representative survey | 8809 adults | Low/yes/yes/yes | Diabetes (HbA1c) | DUs had higher HbA1c levels than ESCC but significance level not tested * |
| Fetterman J. [70] 2020, USA None |
Human clinical study with noninvasive vascular function testing | 467 younger adults | High/-/yes/no | Cardiovascular health (augmentation index) | DUs had similar arterial stiffness as ESCC ¤ | |
| Kim C. [21] 2020, South Korea None |
Population-based survey | 7505 adult men | Low/yes/yes/no | Cardiovascular risk factors (waist circumference, blood pressure, triglycerides, fasting glucose, HDL-cholesterol, diagnosis of metabolic syndrome) |
DUs had significantly higher prevalence odds ratio of cardiovascular risk factors (waist circumference, triglycerides, HDL-cholesterol, blood pressure) and diagnosis of metabolic syndrome than ESCC DUs had similar fasting glucose as ESCC ¤ ** |
|
| Kim T. [22] 2020, South Korea None |
Nationally representative survey | 14,738 adults | Low/yes/yes/no | Cardiovascular risk factors (waist circumference, blood pressure, triglycerides, fasting glucose, HDL-cholesterol, diagnosis of metabolic syndrome) |
DUs had significantly higher odds of abdominal obesity than ESCC ** Other outcomes: no s significant difference but tendency to higher odds in DUs (except blood pressure) # * |
|
| Mainous A. [71] 2020, USA None |
Nationally representative survey | 4659 adults | Low/yes/yes/no | Biomarker of inflammation and predictor of cardiovascular disease (CRP) | DUs had significantly higher odds of elevated CRP than ESCC ** | |
| Miller C. R. [27] 2021, USA  Yes Yes |
Population-based survey | 19,147 adults | Low/yes/yes/no | Self-reported diagnosis of hypertension in the last 12 months | DUs had higher odds for hypertension than ESCC, but significance not reached (0.99 for lower 95%CI) * | |
| Orimoloye O. [72] 2019, USA None |
Population-based survey | 3415 adults | Low/yes/yes/no | Insulin resistance (measured by HOMA-IR and GTT levels) | DUs had same risk of insulin resistance as ESCC ¤ | |
| Osei A. [26] 2019, USA None |
Nationally representative survey | 449,092 adults | Low/yes/yes/no | Self-reported diagnosed with cardiovascular disease (stroke, myocardial infarction or coronary heart disease) |
DUs had significantly higher odds of CVD than ESCC ** DUs had significantly higher odds of premature CVD than ESCC ** |
|
| Parekh T. [73] 2019, USA None |
Nationally representative survey | 161,529 young adults | Low/yes/yes/no | Self-reported stroke | DUs had significantly higher risk of stroke than ESCC ** | |
| Vindhyal M. [74] 2020, USA None |
Nationally representative survey | 16,855 adults | Low/yes/yes/no | Self-reported diagnosed with cardiovascular disease | DUs had higher odds of myocardial infarction and stroke than ESCC, but significantly level not tested * | |
| Other | Akinkugbe A. A. [75] 2019, USA None |
Population-based survey | 13,650 adolescents | Low/yes/yes/no | Self-reported past-year diagnosis with dental problems | DUs: higher odds of dental problems than ESCC, but significance level not tested * |
| Chen D. TH. [25] 2021, United Kingdom None | 4 population-based surveys | 13,077 adults | Low/yes/yes/no | Self-reported experience of COVID-19 symptoms and diagnosis | DUs had higher odds of covid-19 symptoms and higher odds of confirmed/suspected covid-19 diagnosis than ESCC but significance level not tested * | |
| Dinkeloo E. [76] 2019, USA None |
Online survey |
2854 men, soldiers | Low/no/yes/no | Physical activity | DUs: significantly worse fitness than ESCC ** | |
| Gaiha S. M. [77] 2020, USA None |
National online survey |
4351 young adults | Low/yes/yes/no | Self-reported COVID-19 symptoms, testing and diagnosis | DUs higher risk of COVID-19 symptoms and diagnosis than ESCC but significance level not tested * | |
| Kim T. [23] 2021, South Korea None |
Nationally representative population-based survey | 10,692 adults | Low/yes/yes/no | Levels of serum uric acid and hyperuricemia | DUs significantly higher levels of uric acid and prevalence of hyperuricemia than ESCC ** | |
| Leavens E. [78] 2020, USA None |
Interview-survey | 4148 homeless adults | High/no/no/no | Self-reported chronic health conditions | DUs significantly higher rates of asthma ** and cancer compared to ESCC ** | |
| Merianos A. [79] 2021, USA None |
School based nationally representative survey | 11,296 high school students | Low/yes/yes/no | Self-reported duration of sleep | DUs were significantly more likely to report insufficient sleep compared with ESCC ** | |
| Ye D. [80] 2020, USA None |
Human clinical study | 48 adults | High/-/no/no | Systemic inflammation, oxidative stress, angiogenesis and tissue injury/repair in saliva and gingival crevicular fluid (GCF) | DUs: higher levels of most biomarkers of systemic inflammation than ESCC, but no significant difference * |
^ Participants from UK, Shahab 2017, also included in this study. § Also other health outcome than respiratory.  Conflict of interest: pharmaceutical industry.
Conflict of interest: pharmaceutical industry.  Conflict of interest with the tobacco or e-cigarette industry.
Conflict of interest with the tobacco or e-cigarette industry.
3. Results
3.1. Study Design
Table S3 shows the characteristics of the studies, study design, definition of use, and prevalence of use in detail. Most papers (75%) presented results of cross-sectional analyses based on self-reported data from large population-based surveys. Thirteen papers/10 studies reported results from studies with a prospective design (Table 1).
3.2. Definitions of Use (Exposure Measurement)
Exposure measurement was inaccurate in most studies (Appendix S2 in Supplementary Materials) and most papers did not specify the type of EC device or EC-fluid used. The definitions of ESCC, EC users, and DUs were self-reported. Duration and frequency of use of products varied widely. The criteria for current use were “use in the past 30 days” in many studies. Several studies defined smokers and EC users differently, and some had complex definitions. A study including adolescents defined users as “ever users” [62]. Many studies combined daily and non-daily use. Data on the duration of EC use was not presented in most studies. Only two cross-sectional studies distinguished between occasional and daily use [26,64].
3.3. Conflict of Interest
Fourteen (27%) of the papers had a COI: two with an EC manufacturer [52,56], one with the tobacco industry [29], two had received financial support from anonymous contributors [29,50], and the remaining had a COI with pharmaceutical companies.
3.4. Quality Assessment
Overall, 13 of 52 papers (one in four), were rated as having a high risk of selection bias. Only seven papers (five of these were prospective)/three studies had taken former tobacco consumption into account [28,29,35,44,50,58,69]. The overall quality of the prospective studies was generally high, as assessed by the JBI tool (Appendix S1 in Supplementary Materials), except for general inaccurate exposure measurement and lack of adjustment for former tobacco consumption (eight studies). Most of the cross-sectional studies were large, representative of the general population, had weighted data, and had a low risk of bias [26,64]. The primary aim of many studies was to compare EC users with smokers or non-users of tobacco or nicotine products, so they did not test for significance between DU and smokers.
3.5. Findings from the Prospective Studies (Table 1)
Four good-quality pregnancy risk assessment studies investigated DUs’ risk, but significance levels were not tested. Two studies reported that DUs had higher odds of giving birth to a small-for-gestational-age child than ESCC [42,43] and one found higher incidence of birthweight (<10th percentile) and a higher rate of admission to a neonatal intensive care unit in offspring of DUs than in offspring of ESCC [45]. However, the latter study also found the same birthweight, Apgar score, and gestation at delivery in offspring of DUs as in offspring of ESCC [45], and another study found that DUs had the same (elevated) risk of small-for-gestational-age and preterm birth as ESCC [46]. Furthermore, a very large cohort study of young women found a lower but not significantly different fecundability ratio in DUs than in ESCC [44].
Four good-quality papers described results from a cohort study with the longest follow-up that included almost 1400 persons at baseline [29]. There was a low drop-out rate during the six years and the study adjusted for former tobacco consumption and other relevant confounders and used advanced analyses. After one year, DUs had the same self-reported health as ESCC [35]. Two years after baseline, DUs still had the same self-reported health as ESCC and a significantly higher probability of serious adverse events [28]. However, six out of ten DUs stopped using ECs and continued to smoke CCs; those who still were DUs at the 24-month follow-up had significant improvement in self-rated health. After four years, there was no significant difference in self-reported health score and possible smoking-related disease between the DU group and CC users, but the study found generally worse outcomes in DUs [50]. After six years, self-reported health showed a very small change over time in all smoking status groups. Dual users’ risk of a possibly smoking-related disease did not differ significantly from ESCC (aOR of 1.48, 95% CI 0.81–2.70). The results did not differ substantially when the sample was restricted to those who did not switch smoking/vaping group or to those who had their outcomes confirmed through a linkage with hospital discharge abstracts [29].
Two very large, good-quality cohorts investigated pulmonary effects. One study found higher odds of self-reported respiratory disease in DUs [48] and the other found a higher incident rate of acute respiratory infections than in ESCC [49], but significance levels were not tested. A large nationally representative cohort of adolescents found higher, but not statistically significant, risk of sleep-related complaints than ESCC [51].
Thus, all these cohorts, of good quality, found the same or higher risk of negative health outcomes in DUs than in ESCC. This is in contrast with a small smoking cessation study with four weeks follow-up that found reductions in harmful substances in DUs after switching from ESCC [47].
3.6. Cross-Sectional Studies (Table 2)
Ten studies investigated the biomarker levels of harmful and potentially harmful substances (such as tobacco-specific nitrosamines, benzene, metals, or volatile organic compounds) in urine, blood, hair, and saliva of DUs and ESCC. Results of the two largest, best available, nationally representative studies found significantly higher biomarker levels of most of the measured 50 harmful substances [15] and of several toxic and carcinogenic substances [57] in DUs compared to ESCC. Another large, good-quality study found the same biomarker levels of harmful substances [54].
Most of the small studies (six had either a high risk of selection bias and/or lack of adjustment for confounders) found that levels of harmful substances were significantly lower or the same in DUs as in ESCC [52,53,55,56,59]; however, one found that DUs had higher levels for eight out of 11 metals tested [52]. Another study, with high risk of selection bias but adjustment for former tobacco consumption, included volunteers with long-term use of ECs and found that DUs had similar levels of most harmful substances but a significantly higher level of benzene (carcinogenic) than ESCC [58].
Eleven large good-quality surveys (eight of these with low risk of selection bias, weighted data, and adjusted analyses), including between eight and >700,000 persons from the general population, investigated the association between DU and self-reported respiratory symptoms/disease. Most of the surveys found a little higher/or the same (not significant/significance not tested) [12,60,61,63,65,68] odds of asthma or respiratory symptoms in DUs compared with ESCC, or significantly higher odds [66]. One of the surveys, including adolescents, found lower odds of asthma in DUs compared with ESCC, but significance level was not tested [61].
Three good-quality surveys investigated risk of self-reported chronic obstructive pulmonary disorder (COPD) and found significantly higher odds [64] and higher but not significant odds in DUs than in ESCC [67,68].
Ten large, good-quality surveys, including between 3400 and almost 450,00 persons from the general population, investigated the cardiovascular (CVD) and metabolic health effects of DU. The best available of these studies had adjusted for tobacco consumption and found higher HbA1c levels in DUs than in ESCC, but significance levels were not tested [69]. Four of the good-quality surveys investigated cardiovascular risk factors and found that DUs had a significantly higher OR of cardiovascular disease [26], significantly higher prevalence OR of cardiovascular risk factors and diagnosis of metabolic syndrome [21], significantly higher OR of elevated human c-reactive protein (CRP) [71], significantly higher risk of stroke [73], significantly higher prevalence of arrythmia [66], significantly higher OR of elevated CRP [71], and significantly higher OR of abdominal obesity than ESCC [22]. The two remaining surveys found higher OR of myocardial infarction and stroke, but significance level was not tested [65,74], and higher but not significant OR of hypertension [27] in DUs than in ESCC. Furthermore, one survey found that DUs had similar fasting glucose as ESCC [21], and another study found the same levels of insulin resistance [72].
A clinical study performed vascular function testing in almost 500 young persons, and reported that DUs had similar arterial stiffness as ESCC [70]. Eight cross-sectional studies investigated other health outcomes. Large good-quality surveys (only one did not weight data) including adults found that DUs had significantly worse fitness [76] and significantly higher levels of uric acid and prevalence of hyperuricemia [23] compared with ESCC. Further, higher odds of COVID-19 symptoms and higher odds of confirmed/suspected COVID-19 diagnosis were found in DUs [25,77] than in ESCC, but significance levels were not tested. Large surveys including adolescent DUs reported insufficient sleep significantly more often than ESCC [79], and higher odds of dental problems, but significance level was not tested [75]. In a large survey, homeless persons with DU reported significantly higher rates of asthma and cancer compared to ESCC [78]. Finally, in a small human clinical study, DUs had higher levels of most biomarkers of systemic inflammation than ESCC, but the difference was not significant [80].
3.7. Intensity of Smoking or EC use and Impact on Health
Most of the studies did not collect robust data on the level of EC and CC consumption. Those that did, found that DUs smoked the same [15,21,29,35,57,76] or a significantly higher number of CPD as ESCC [66].
A smoking cessation study showed a correlation between the number of CPD and harm in DUs. DUs who had been able to reduce the number of CPD substantially had reduced biomarker levels [47]. Moreover, one of the large, best available surveys showed that the frequency of CC use was positively associated with toxicant concentration [15]. Most of the studies that reported the same or higher tobacco consumption in DUs as in ESCC found significantly worse health outcomes in DUs than in ESCC [15,21,29,57,66,76]. None of the studies where DUs reported smoking a lower number of CPD than ESCC found a significantly worse outcome in DUs [52,59,62]; in fact, one of them found that ESCC had a worse outcome [59].
Only two cross-sectional studies investigated the potential impact of the frequency/dose of EC use by DUs. A survey of good quality found that the risk of premature CVD was significantly higher in DUs with a daily use of ECs than in those with occasional use of ECs [26]. Another survey of good quality found higher OR of COPD with increasing frequency of EC use among people who had never smoked, indicating a stepped harm of EC use [64].
3.8. Synthesis of the Results
Based on a very limited number of prospective studies, DU seems to be at least as harmful as ESCC. The same tendency was found in large, best available cross-sectional studies that found DU associated with the same, and in several studies significantly higher, risk of self-reported symptoms/disease as ESCC and in the largest population-based best available studies that found lower levels of harmful substances ESCC than in DUs. The intensity of smoking seems associated with worse health outcomes in DUs but very few studies investigated this.
Due to the predominance of cross-sectional studies, the inaccurate exposure measurement, and a high risk of reverse causality, we judged the overall certainty of the evidence in this review as “low certainty.”
4. Discussion
4.1. Overall Findings
This is the first systematic review comparing the general health effects of real-world DU with ESCC. We identified 52 papers/49 studies. Only one of four studies had a prospective design. There was great heterogeneity across studies, both in the definition of use, in methodology, and in outcome measurement, so only a narrative review was possible. Many studies did not test for significance. The best available studies found a tendency to at least the same or higher levels of harmful substances, and at least the same or higher risk of harmful effects in DUs compared with ESCC. Due to the predominance of cross-sectional studies, the inaccurate exposure measurement and high risk of reverse causality, the evidence in this review is rated as “low certainty.”
4.2. Comparison with Other Studies and Considerations about Findings
If smokers replaced all/most of the CCs with ECs, there might be a beneficial effect of DU [81]. Short-term experimental studies of forced switch from CCs to ECs have shown reduced levels of harmful substances in DUs compared with ESCC [82], and the degree of reduction to be proportional to the reduced numbers of CPD. However, the “real-world use” studies included in our review found that DUs and ESCC smoked the same amount of tobacco/number of CPD, and one study even found a significantly higher number of CPD in DUs than in ESCC [66]. The lack of significant reduction in number of CPD in DUs agrees with other studies not included in this review [35,36,37,83,84]. The intensity of smoking in DUs was (not surprisingly) found to be associated with worse health outcomes, but the majority of the studies included in the review did not collect robust data on the level of CC consumption.
If DUs do not reduce the number of CPD but use ECs as a supplement, the combination of CCs and ECs might potentially be even more harmful for health than ESCC, as known and unknown [85] harmful substances and transformation products formed by heating of ECs [86] are added on top of the harmful substances in tobacco smoke. The long-term effects of EC use on human health will take a long time to be fully elucidated, but concern has been raised [87,88,89,90,91]. Although several studies have found an increased risk of disease in EC users [48,66,87,92,92,93,94,95] and shown that, e.g., the cardiovascular [96] and pulmonary harm from ECs is biologically plausible [88], at present the risk in the human population is uncertain [81,97].
It was not possible to distinguish the root of a health event/outcome/disease (i.e., caused by tobacco use, or ECs, or a combination), as few studies measured intensity of smoking and even fewer the intensity of EC use. The intensity of EC use was only investigated in two cross-sectional studies, so it is impossible to establish a dose-dependent relationship. Furthermore, the evolving design of the EC devices [98] and the huge heterogeneity of the content of EC-fluid might both have an impact on DU frequency in the population and on the health-related risk. We need further investigation of the impact of dose, frequency, and duration of EC use, as well as the potential biological interactions of ECs and CCs.
We cannot draw conclusions on causality from cross-sectional studies. Moreover, most DUs have been ESCC for decades and might already have had a smoking-related disease before they started vaping. In some studies participants were included if they had ever been diagnosed with, for example, heart disease [26] or stroke [73], but there was no information on whether they had the disease before or after they started using ECs. The prospective study by Bhatta found reverse causality p < 0.001 [48] but only seven papers/four studies adjusted for differences in previous tobacco consumption. The risk of reverse causality is probably the greatest challenge. An Italian good-quality cohort study with six years follow-up, however, had adjusted for duration of tobacco smoking. The study found that the risk of possible smoking-related disease was slightly, but not significantly, worse in persistent DUs [29].
Moreover, longer follow-ups are required. In a sample comprised of former smokers, a decade or more would be more appropriate to detect significant risk reductions.
Confounding factors could be another reason for the tendency towards worse health outcomes in DUs but most adjusted for relevant factors, such as exposure to second-hand smoke, and almost all adjusted for sociodemographic factors [61]. Few of the included studies were designed to compare DUs with ESCC and many did not test for significance.
Misclassification might also be a problem regarding smoking/vaping status [87] and the definition of DU also varied a lot across studies. Disease was mostly self-reported, which imposes the risk of both recall and misclassification bias. However, a large retrospective survey using hospital records found a tendency towards a higher risk of respiratory infections in DUs [49].
Conversely, the included studies had also several strengths. The prospective studies were of good quality, except for the exposure measurement, which was mostly inaccurate, and lack of adjustment for former tobacco consumption in most studies. Most of the cross-sectional studies were very large, good-quality population-based studies, reflecting real-world use in a general population. An example of an excellent study (providing the United States Food & Drug Administration (FDA) with detailed information) is the large, nationally representative longitudinal PATH study [99]. Moreover, even though most studies were cross-sectional, many environmental and occupational diseases had been detected first by cross-sectional approaches, long before cohort studies and intervention studies were available. For respiratory disease, Bhatta & Glantz showed a good agreement between longitudinal and cross-sectional results on ECs [48].
Tobacco regulation is a dynamic field. Health authorities rely on a robust science base to develop regulations that improve public health. Well-designed, large prospective studies on health effects of DU, considering not only sociodemographic confounders, but also age at onset of smoking, pack-years of CCs, and, e.g., alcohol consumption, are needed. Furthermore, exposure of CCs and ECs needs to be measured in a more detailed way, so a dose response can be investigated. For ECs, exposure measurement should include type of device, concentration of nicotine used, and duration, frequency (e.g., daily/weekly/monthly use), and intensity of vaping. Measuring vaping intensity is challenging. Puff topography, number of use sessions, and amount of EC liquid consumed are being used in some studies, but all these measures have limitations and development of uniform EC-use intensity measures is needed [100].
Exposure conditions that are relevant to real-world inhalation exposure in humans should be used in human and animal experimental models. The National Academies of Sciences, Engineering, and Medicine (NASEM) report on ECs provides a thorough list of research needs [81].
4.3. Limitations of the Review
Due to the large heterogeneity (methods, exposure, outcomes) of the studies, we were unable to assess study quality in a systematic way nor were we able to perform a meta-analysis. We decided to include all studies, independent of study design and the year of publication. Due to the huge evolution of the EC devices, we might have restricted our search to newer studies, but only four studies were more than five years old (at the time of our search), so the devices used were generally up to date. We could also have restricted our search to prospective studies only but chose to describe if studies were “best available”/had good quality. Moreover, this review did not explore the potential health benefits of DU in smokers who reduce their CPD by, e.g., 50% or more by use of ECs. Many outcomes did not test for significance; therefore, the increased ORs reported in these studies may not indicate a significant increase in adverse outcomes compared to ESCC use. We could have decided not to include studies without a test for significance but chose to include them and show odds ratios in Appendix S3 in Supplementary Materials.
Interpretation of our findings for DUs was challenging because of heterogeneity of the DU group, which can include both habitual heavy smokers who occasionally vape and heavy vapers who only smoke a little. We might have included the experimental forced-switch/CPD reduction studies but they do not reflect real-world use. We might also have excluded the experimental smoking cessation study with very short-term follow-up [47], but smokers could use ECs ad libitum, which reflects real-world use. Furthermore, we might have been more critical regarding the validity of outcomes, such as self-reported symptoms. Finally, an interpretation bias must always be kept in mind.
4.4. Strengths of the Review
Both authors independently read all abstracts and full text of papers and extracted data. Eventual disagreements were discussed. Potential COIs that might influence the findings were described. Quality assessment was performed, even though it could have been more thorough. This review gives a real-world picture of health effects of DU in the general population.
5. Conclusions
A high prevalence of DU has been reported across the world. In some countries, most users of ECs are DUs. This is the first systematic review comparing the health effects of DU with ESCC. Based on a very limited number of prospective studies, DU seems to be at least as, or probably even more harmful than, ESCC. The same tendency was found in cross-sectional studies; several of the best available studies found the same or higher risk of symptoms/disease or level of harmful substances in DUs than in ESCC. The intensity of smoking seems associated with worse health outcomes but very few studies investigated this. Due to the predominance of cross-sectional studies, the inaccurate exposure measurement, and a high risk of reverse causality, we judged the overall certainty of the evidence in this review as “low certainty.” Well-designed prospective studies on health effects of DU are needed.
Acknowledgments
We thank the Danish Heart Foundation for funding of CPs professorship during work on this paper. The Foundation was not involved in the choice of research idea or any step of this study and will first be presented with the results of the study when it is published.
Abbreviations
| ESCC | exclusive smokers of conventional cigarettes |
| DU | dual use of conventional cigarettes and e-cigarettes |
| DUs | dual users of conventional cigarettes and e-cigarettes |
| COPD | chronic obstructive pulmonary disease |
| CRP | human c-reactive protein |
| CVD | cardiovascular disease |
| GCF | gingival crevicular fluid |
| 3-HPMA | Urinary 3-hydroxypropyl mercapturic acid, a major metabolite of acrolein |
| TSNA | Tobacco-specific N-nitrosamines |
| PAH | polycyclic aromatic hydrocarbons |
| HDL | high density lipids |
| GTT | glucose tolerance test |
| HbA1c | glycosylated hemoglobin |
| HPMA | N-acetyl-S-3-hydroxypropylcysteine, a metabolite of acrolein (VOC) |
| MHB3 | a metabolite of 1,3-butadiene (VOC) |
| NNAL | 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol, the principal metabolite of the lung carcinogen NNK (TSNA) |
| PMA | benzene |
| VOC | Volatile organic compounds |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph192013687/s1 Table S1: Systematic search in databases; Table S2: Overview of excluded studies; Table S3: Detailed overview of the included studies; Appendix S1: JBI Checklist for cohort studies; Appendix S2: Definition and prevalence of use. Appendix S3: Characteristics of studies.
Author Contributions
Conceptualization, C.P. and S.K.B.R.; search: S.K.B.R.; methodology, C.P. and S.K.B.R.; validation, C.P. and S.K.B.R.; formal analysis, C.P. and S.K.B.R.; resources, C.P.; data curation, C.P. and S.K.B.R.; writing—original draft preparation, C.P. and S.K.B.R.; writing—review and editing, C.P. and S.K.B.R.; visualization, C.P. and S.K.B.R.; project administration, C.P.; funding acquisition, C.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Links of the databases used in the review: PubMed https://pubmed.ncbi.nlm.nih.gov/ (accessed on 10 October 2022), EMBASE https://www.embase.com/ (accessed on 10 October 2022), CINAHL https://www.ebsco.com/products/research-databases/cinahl-database (accessed on 10 October 2022) (local access), and Cochrane library https://www.cochranelibrary.com/search (accessed on 10 October 2022). All extracted data is available in the original papers; an overview of the extracted data is found in Table S3. Only a narrative review was performed, not a synthesis of data. An overview of excluded studies is available in Table S2.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.GSTHR 82 Million Vapers Worldwide in 2021: The GSTHR Estimate. Briefing Papers: 2022. [(accessed on 10 October 2022)]. Available online: https://gsthr.org/briefing-papers/82-million-vapers-worldwide-in-2021-the-gsthr-estimate/
- 2.McNeill A., Brose L.S., Calder R., Hitchman S., Hajek P., McRobbie H. In: E-Cigarettes: An Evidence Update. England PH, editor. Public Health England; London, UK: 2015. [DOI] [PubMed] [Google Scholar]
- 3.Hartmann-Boyce J., McRobbie H., Butler A.R., Lindson N., Bullen C., Begh R., Theodoulou A., Notley C., Rigotti N.A., Turner T., et al. Electronic cigarettes for smoking cessation. Cochrane Tobacco Addiction Group, editor. Cochrane Database Syst. Rev. 2021;4:CD010216. doi: 10.1002/14651858.CD010216.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tobacco: E-cigarettes. [(accessed on 10 October 2022)]. Available online: https://www.who.int/news-room/questions-and-answers/item/tobacco-e-cigarettes.
- 5.Country Laws Regulating E-cigarettes: A Policy Scan. [(accessed on 10 October 2022)]. Available online: https://www.globaltobaccocontrol.org/en/policy-scan/e-cigarettes/countries?country=263.
- 6.Samet J.M., Barrington-Trimis J. E-Cigarettes and Harm Reduction: An Artificial Controversy Instead of Evidence and a Well-Framed Decision Context. Am. J. Public Health. 2021;111:1572–1574. doi: 10.2105/AJPH.2021.306457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel D., Davis K.C., Cox S., Bradfield B., King B.A., Shafer P., Caraballo R., Bunnell R. Reasons for current E -cigarette use among U.S. adults. Prev. Med. 2016;93:14–20. doi: 10.1016/j.ypmed.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calder R., Gant E., Bauld L., McNeill A., Robson D., Brose L.S. Vaping in Pregnancy: A Systematic Review. Nicotine Tob. Res. 2021;23:1451–1458. doi: 10.1093/ntr/ntab017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Using E-Cigarettes to Stop Smoking. NHS. [(accessed on 10 October 2022)]. Available online: https://www.nhs.uk/live-well/quit-smoking/using-e-cigarettes-to-stop-smoking/
- 10.Harm Reduction and Vaping. Action for Smokefree 2025 (ASH) [(accessed on 10 October 2022)]. Available online: https://www.ash.org.nz/vaping-and-harm-reduction.
- 11.Hedman L., Galanti M.R., Ryk L., Gilljam H., Adermark L. Electronic cigarette use and smoking cessation in cohort studies and randomized trials: A systematic review and meta-analysis. Tob. Prev. Cessat. 2021;7:62. doi: 10.18332/tpc/142320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hedman L., Backman H., Stridsman C., Bosson J.A., Lundbäck M., Lindberg A., Rönmark E., Ekerljung L. Association of Electronic Cigarette Use with Smoking Habits, Demographic Factors, and Respiratory Symptoms. JAMA Netw. Open. 2018;1:e180789. doi: 10.1001/jamanetworkopen.2018.0789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeon C., Jung K.J., Kimm H., Lee S., Barrington-Trimis J.L., McConnell R., Samet J.M., Jee S.H. E-cigarettes, conventional cigarettes, and dual use in Korean adolescents and university students: Prevalence and risk factors. Drug Alcohol Depend. 2016;168:99–103. doi: 10.1016/j.drugalcdep.2016.08.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sung H.Y., Wang Y., Yao T., Lightwood J., Max W. Polytobacco Use and Nicotine Dependence Symptoms Among US Adults, 2012–2014. Nicotine Tob. Res. 2018;20((Suppl. 1)):S88–S98. doi: 10.1093/ntr/nty050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goniewicz M.L., Smith D.M., Edwards K.C., Blount B.C., Caldwell K.L., Feng J., Wang L., Christensen C., Ambrose B., Borek N., et al. Comparison of Nicotine and Toxicant Exposure in Users of Electronic Cigarettes and Combustible Cigarettes. JAMA Netw. Open. 2018;1:e185937. doi: 10.1001/jamanetworkopen.2018.5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grana R., Benowitz N., Glantz S.A. E-Cigarettes: A Scientific Review. Circulation. 2014;129:1972–1986. doi: 10.1161/CIRCULATIONAHA.114.007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maglia M., Caponnetto P., Di Piazza J., La Torre D., Polosa R. Dual use of electronic cigarettes and classic cigarettes: A systematic review. Addict. Res. Theory. 2018;26:330–338. doi: 10.1080/16066359.2017.1388372. [DOI] [Google Scholar]
- 18.Fears of Growth in Children Vaping Disposables Backed up by New National Survey. Action on Smoking and Health (ash) [(accessed on 10 October 2022)]. Available online: https://ash.org.uk/media-centre/news/press-releases/fears-of-growth-in-children-vaping-disposables-backed-up-by-new-national-survey.
- 19.Christensen T., Welsh E., Faseru B. Profile of e-cigarette use and its relationship with cigarette quit attempts and abstinence in Kansas adults. Prev. Med. 2014;69:90–94. doi: 10.1016/j.ypmed.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Xiao L., Yin X., Di X., Nan Y., Lyu T., Wu Y., Li X. Awareness and prevalence of e-cigarette use among Chinese adults: Policy implications. Tob. Control. 2021;31:498–504. doi: 10.1136/tobaccocontrol-2020-056114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim C.Y., Paek Y.J., Seo H.G., Cheong Y.S., Lee C.M., Park S.M., Park D.W., Lee K. Dual use of electronic and conventional cigarettes is associated with higher cardiovascular risk factors in Korean men. Sci. Rep. 2020;10:5612. doi: 10.1038/s41598-020-62545-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim T., Choi H., Kang J., Kim J. Association between electronic cigarette use and metabolic syndrome in the Korean general population: A nationwide population-based study. PLoS ONE. 2020;15:e0237983. doi: 10.1371/journal.pone.0237983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim T., Kim Y., Kang J. Association of electronic cigarette exposure with serum uric acid level and hyperuricemia: 2016-2017 Korea National Health and Nutritional Examination Survey. PLoS ONE. 2021;16:e0247868. doi: 10.1371/journal.pone.0247868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie Z., Ossip D.J., Rahman I., Li D. Use of Electronic Cigarettes and Self-Reported Chronic Obstructive Pulmonary Disease Diagnosis in Adults. Nicotine Tob. Res. 2020;22:1155–1161. doi: 10.1093/ntr/ntz234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen D.T.H., Kyriakos C.N. Cigarette and E-Cigarettes Dual Users, Exclusive Users and COVID-19: Findings from Four UK Birth Cohort Studies. Int. J. Environ. Res. Public Health. 2021;18:3935. doi: 10.3390/ijerph18083935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osei A.D., Mirbolouk M., Orimoloye O.A., Dzaye O., Uddin S.I., Benjamin E.J., Hall M.E., DeFilippis A.P., Stokes A., Bhatnagar A., et al. Association Between E-Cigarette Use and Cardiovascular Disease Among Never and Current Combustible-Cigarette Smokers. Am. J. Med. 2019;132:949–954.e2. doi: 10.1016/j.amjmed.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 27.Miller C.R., Shi H., Li D., Goniewicz M.L. Cross-Sectional Associations of Smoking and E-cigarette Use with Self-Reported Diagnosed Hypertension: Findings from Wave 3 of the Population Assessment of Tobacco and Health Study. Toxics. 2021;9:52. doi: 10.3390/toxics9030052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manzoli L., Flacco M.E., Ferrante M., La Vecchia C., Siliquini R., Ricciardi W., Marzuillo C., Villari P., Fiore M. Cohort study of electronic cigarette use: Effectiveness and safety at 24 months. Tob. Control. 2017;26:284–292. doi: 10.1136/tobaccocontrol-2015-052822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flacco M.E., Fiore M., Acuti Martellucci C., Ferrante M., Gualano M.R., Liguori G., Bravi F., Pirone G.M., Marzuillo C., Manzoli L. Tobacco vs. electronic cigarettes: Absence of harm reduction after six years of follow-up. Eur. Rev. Med. Pharmacol. Sci. 2020;24:3923–3934. doi: 10.26355/eurrev_202004_20859. [DOI] [PubMed] [Google Scholar]
- 30.Sweet L., Brasky T.M., Cooper S., Doogan N., Hinton A., Klein E.G., Nagaraja H., Quisenberry A., Xi W., Wewers M.E. Quitting Behaviors Among Dual Cigarette and E-Cigarette Users and Cigarette Smokers Enrolled in the Tobacco User Adult Cohort. Nicotine Tob. Res. 2019;21:278–284. doi: 10.1093/ntr/nty222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osibogun O., Bursac Z., Mckee M., Li T., Maziak W. Cessation outcomes in adult dual users of e-cigarettes and cigarettes: The Population Assessment of Tobacco and Health cohort study, USA, 2013–2016. Int. J. Public Health. 2020;65:923–936. doi: 10.1007/s00038-020-01436-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hinton A., Nagaraja H.N., Cooper S., Wewers M.E. Tobacco product transition patterns in rural and urban cohorts: Where do dual users go? Prev. Med. Rep. 2018;12:241–244. doi: 10.1016/j.pmedr.2018.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei L., Muhammad-Kah R.S., Hannel T., Pithawalla Y.B., Gogova M., Chow S., Black R.A. The impact of cigarette and e-cigarette use history on transition patterns: A longitudinal analysis of the population assessment of tobacco and health (PATH) study, 2013–2015. Harm Reduct. J. 2020;17:45. doi: 10.1186/s12954-020-00386-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piper M.E., Baker T.B., Benowitz N.L., Jorenby D.E. Changes in Use Patterns Over 1 Year Among Smokers and Dual Users of Combustible and Electronic Cigarettes. Nicotine Tob. Res. 2020;22:672–680. doi: 10.1093/ntr/ntz065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manzoli L., Flacco M.E., Fiore M., La Vecchia C., Marzuillo C., Gualano M.R., Liguori G., Cicolini G., Capasso L., D’Amario C., et al. Electronic Cigarettes Efficacy and Safety at 12 Months: Cohort Study. PLoS ONE. 2015;10:e0129443. doi: 10.1371/journal.pone.0129443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Etter J.F. A longitudinal study of cotinine in long-term daily users of e-cigarettes. Drug Alcohol Depend. 2016;160:218–221. doi: 10.1016/j.drugalcdep.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Huh J., Leventhal A.M. Intraindividual covariation between e-cigarette and combustible cigarette use in Korean American emerging adults. Psychol. Addict. Behav. 2016;30:246–251. doi: 10.1037/adb0000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goniewicz M.L., Leigh N.J., Gawron M., Nadolska J., Balwicki L., McGuire C., Sobczak A. Dual use of electronic and tobacco cigarettes among adolescents: A cross-sectional study in Poland. Int. J. Public Health. 2016;61:189–197. doi: 10.1007/s00038-015-0756-x. [DOI] [PubMed] [Google Scholar]
- 39.DeVito E.E., Fagle T., Allen A.M., Pang R.D., Petersen N., Smith P.H., Weinberger A.H. Electronic Nicotine Delivery Systems (ENDS) Use and Pregnancy I: ENDS Use Behavior During Pregnancy. Curr. Addict. Rep. 2021;8:347–365. doi: 10.1007/s40429-021-00380-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.E-Cigarette, or Vaping, Product Visual Dictionary. U.S. Department of Health and Human Services. Center for Disease Control and Prevention. [(accessed on 10 October 2022)]; Available online: https://www.cdc.gov/tobacco/basic_information/e-cigarettes/pdfs/ecigarette-or-vaping-products-visual-dictionary-508.pdf.
- 41.Rankin G.D., Wingfors H., Uski O., Hedman L., Ekstrand-Hammarström B., Bosson J., Lundbäck M. The toxic potential of a fourth-generation E-cigarette on human lung cell lines and tissue explants. J. Appl. Toxicol. 2019;39:1143–1154. doi: 10.1002/jat.3799. [DOI] [PubMed] [Google Scholar]
- 42.Cardenas V.M., Cen R., Clemens M.M., Moody H.L., Ekanem U.S., Policherla A., Fischbach L.A., Eswaran H., Magann E.F., Delongchamp R.R., et al. Use of Electronic Nicotine Delivery Systems (ENDS) by pregnant women I: Risk of small-for-gestational-age birth. [(accessed on 10 October 2022)];Tob. Induc. Dis. 2019 17:44. doi: 10.18332/tid/106089. Available online: http://www.journalssystem.com/tid/Use-of-Electronic-Nicotine-Delivery-Systems-by-Pregnant-Women-I-Risk-of-Small-for,106089,0,2.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clemens M.M., Cardenas V.M., Fischbach L.A., Cen R., Siegel E.R., Eswaran H., Ekanem U.S., Policherla A., Moody H.L., Magann E.F., et al. Use of electronic nicotine delivery systems by pregnantwomen II: Hair biomarkers for exposures to nicotine andtobacco-specific nitrosamines. [(accessed on 10 October 2022)];Tob. Induc. Dis. 2019 17:50. doi: 10.18332/tid/105387. Available online: http://www.journalssystem.com/tid/Use-of-Electronic-Nicotine-Delivery-Systems-by-Pregnant-Women-II-Hair-Biomarkers,105387,0,2.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harlow A.F., Hatch E.E., Wesselink A.K., Rothman K.J., Wise L.A. Electronic Cigarettes and Fecundability: Results From a Prospective Preconception Cohort Study. Am. J. Epidemiol. 2021;190:353–361. doi: 10.1093/aje/kwaa067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McDonnell B., Dicker P., Regan C. Electronic cigarettes and obstetric outcomes: A prospective observational study. BJOG Int. J. Obstet. Gy. 2020;127:750–756. doi: 10.1111/1471-0528.16110. [DOI] [PubMed] [Google Scholar]
- 46.Wang X., Lee N.L., Burstyn I. Smoking and use of electronic cigarettes (vaping) in relation to preterm birth and small-for-gestational-age in a 2016 U.S. national sample. Prev. Med. 2020;134:106041. doi: 10.1016/j.ypmed.2020.106041. [DOI] [PubMed] [Google Scholar]
- 47.McRobbie H., Phillips A., Goniewicz M.L., Smith K.M., Knight-West O., Przulj D., Hajek P. Effects of Switching to Electronic Cigarettes with and without Concurrent Smoking on Exposure to Nicotine, Carbon Monoxide, and Acrolein. Cancer Prev. Res. 2015;8:873–878. doi: 10.1158/1940-6207.CAPR-15-0058. [DOI] [PubMed] [Google Scholar]
- 48.Bhatta D.N., Glantz S.A. Association of E-Cigarette Use with Respiratory Disease Among Adults: A Longitudinal Analysis. Am. J. Prev. Med. 2020;58:182–190. doi: 10.1016/j.amepre.2019.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanou A.Z., Ziadeh C., Stahlman S., Clausen S.S. Acute Respiratory Infections Among Active Component Service Members Who Use Combustible Tobacco Products and/or E-cigarette/Vaping Products, U.S. Armed Forces, 2018–2019. MSMR. 2020;27:2–7. [PubMed] [Google Scholar]
- 50.Flacco M.E., Ferrante M., Flore M., Marzuillo C., La Vecchia C., Gualano M.R., Liguori G., Fragassi G., Carradori T., Bravi F., et al. Cohort study of electronic cigarette use: Safety and effectiveness after 4 years of follow-up. Eur. Rev. Med. Pharmacol. Sci. 2019;23:402–412. doi: 10.26355/eurrev_201901_16789. [DOI] [PubMed] [Google Scholar]
- 51.Riehm K.E., Rojo-Wissar D.M., Feder K.A., Mojtabai R., Spira A.P., Thrul J., Crum R.M. E-cigarette use and sleep-related complaints among youth. J. Adolesc. 2019;76:48–54. doi: 10.1016/j.adolescence.2019.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prokopowicz A., Sobczak A., Szuła-Chraplewska M., Ochota P., Kośmider L. Exposure to Cadmium and Lead in Cigarette Smokers Who Switched to Electronic Cigarettes. Nicotine Tob. Res. 2019;21:1198–1205. doi: 10.1093/ntr/nty161. [DOI] [PubMed] [Google Scholar]
- 53.Carroll D.M., Wagener T.L., Peck J.D., Brame L.S., Thompson D.M., Stephens L.D., Campbell J.E., Beebe L.A. Biomarkers of Exposure in ENDS Users, Smokers, and Dual Users of American Indian Descent. Tob. Regul. Sci. 2018;4:3–15. doi: 10.18001/TRS.4.2.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jain R.B. Concentrations of cadmium, lead, and mercury in blood among US cigarettes, cigars, electronic cigarettes, and dual cigarette-e-cigarette users. Environ. Pollut. 2019;251:970–974. doi: 10.1016/j.envpol.2019.05.041. [DOI] [PubMed] [Google Scholar]
- 55.Keith R.J., Fetterman J.L., Orimoloye O.A., Dardari Z., Lorkiewicz P.K., Hamburg N.M., DeFilippis A.P., Blaha M.J., Bhatnagar A. Characterization of Volatile Organic Compound Metabolites in Cigarette Smokers, Electronic Nicotine Device Users, Dual Users, and Nonusers of Tobacco. Nicotine Tob. Res. 2020;22:264–272. doi: 10.1093/ntr/ntz021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prokopowicz A., Sobczak A., Szdzuj J., Grygoyć K., Kośmider L. Metal Concentration Assessment in the Urine of Cigarette Smokers Who Switched to Electronic Cigarettes: A Pilot Study. Int. J. Environ. Res. Public Health. 2020;17:1877. doi: 10.3390/ijerph17061877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rostron B.L., Corey C.G., Chang J.T., van Bemmel D.M., Miller M.E., Chang C.M. Associations of Cigarettes Smoked Per Day with Biomarkers of Exposure Among U.S. Adult Cigarette Smokers in the Population Assessment of Tobacco and Health (PATH) Study Wave 1 (2013–2014) Cancer Epidemiol. Biomark. Prev. 2019;28:1443–1453. doi: 10.1158/1055-9965.EPI-19-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shahab L., Goniewicz M.L., Blount B.C., Brown J., McNeill A., Alwis K.U., Feng J., Wang L., West R. Nicotine, Carcinogen, and Toxin Exposure in Long-Term E-Cigarette and Nicotine Replacement Therapy Users: A Cross-sectional Study. Ann. Intern. Med. 2017;166:390. doi: 10.7326/M16-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith D.M., Shahab L., Blount B.C., Gawron M., Kosminder L., Sobczak A., Xia B., Sosnoff C.S., Goniewicz M.L. Differences in Exposure to Nicotine, Tobacco-Specific Nitrosamines, and Volatile Organic Compounds among Electronic Cigarette Users, Tobacco Smokers, and Dual Users from Three Countries. Toxics. 2020;8:88. doi: 10.3390/toxics8040088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cho J.H., Paik S.Y. Association between Electronic Cigarette Use and Asthma among High School Students in South Korea. Fehrenbach H, editor. PLoS ONE. 2016;11:e0151022. doi: 10.1371/journal.pone.0151022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chung S.J., Kim B.K., Oh J.H., Shim J.S., Chang Y.S., Cho S.H., Yang M.S. Novel tobacco products including electronic cigarette and heated tobacco products increase risk of allergic rhinitis and asthma in adolescents: Analysis of Korean youth survey. Allergy. 2020;75:1640–1648. doi: 10.1111/all.14212. [DOI] [PubMed] [Google Scholar]
- 62.Lee A., Lee S.Y., Lee K.S. The Use of Heated Tobacco Products is Associated with Asthma, Allergic Rhinitis, and Atopic Dermatitis in Korean Adolescents. Sci. Rep. 2019;9:17699. doi: 10.1038/s41598-019-54102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li D., Sundar I.K., McIntosh S., Ossip D.J., Goniewicz M.L., O’Connor R.J., Rahman I. Association of smoking and electronic cigarette use with wheezing and related respiratory symptoms in adults: Cross-sectional results from the Population Assessment of Tobacco and Health (PATH) study, wave 2. Tob. Control. 2019;29:140–147. doi: 10.1136/tobaccocontrol-2018-054694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Osei A.D., Mirbolouk M., Orimoloye O.A., Dzaye O., Uddin S.I., Benjamin E.J., Hall M.E., DeFilippis A.P., Bhatnagar A., Biswal S.S., et al. Association Between E-Cigarette Use and Chronic Obstructive Pulmonary Disease by Smoking Status: Behavioral Risk Factor Surveillance System 2016 and 2017. Am. J. Prev. Med. 2020;58:336–342. doi: 10.1016/j.amepre.2019.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parekh T., Owens C., Fay K., Phillips J., Kitsantas P. Use of e-Cigarettes and Development of Respiratory Conditions in Women of Childbearing Age. South Med. J. 2020;113:448–494. doi: 10.14423/SMJ.0000000000001158. [DOI] [PubMed] [Google Scholar]
- 66.Wang J.B., Olgin J.E., Nah G., Vittinghoff E., Cataldo J.K., Pletcher M.J., Marcus G.M. Cigarette and e-cigarette dual use and risk of cardiopulmonary symptoms in the Health eHeart Study. PLoS ONE. 2018;13:e0198681. doi: 10.1371/journal.pone.0198681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wills T.A., Pagano I., Williams R.J., Tam E.K. E-cigarette use and respiratory disorder in an adult sample. Drug Alcohol Depend. 2019;194:363–370. doi: 10.1016/j.drugalcdep.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wills T.A., Choi K., Pagano I. E-Cigarette Use Associated with Asthma Independent of Cigarette Smoking and Marijuana in a 2017 National Sample of Adolescents. J. Adolesc. Health. 2020;67:524–530. doi: 10.1016/j.jadohealth.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Choi D.W., Jeon J., Lee S., Han K.T., Park E.C., Jang S.I. Association between Smoking Behavior Patterns and Glycated Hemoglobin Levels in a General Population. Int. J. Environ. Res. Public Health. 2018;15:2260. doi: 10.3390/ijerph15102260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fetterman J.L., Keith R.J., Palmisano J.N., McGlasson K.L., Weisbrod R.M., Majid S., Bastin R., Stathos M.M., Stokes A.C., Robertson R.M., et al. Alterations in Vascular Function Associated with the Use of Combustible and Electronic Cigarettes. JAHA. 2020;9:e014570. doi: 10.1161/JAHA.119.014570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mainous A.G., Yadav S., Hong Y.R., Huo J. E-Cigarette and Conventional Tobacco Cigarette Use, Dual Use, and C-Reactive Protein. J. Am. Coll. Cardiol. 2020;75:2271–2273. doi: 10.1016/j.jacc.2020.02.061. [DOI] [PubMed] [Google Scholar]
- 72.Orimoloye O.A., Uddin S.I., Chen L.C., Osei A.D., Mirbolouk M., Malovichko M.V., Sithu I.D., Dzaye O., Conklin D.J., Srivastava S., et al. Electronic cigarettes and insulin resistance in animals and humans: Results of a controlled animal study and the National Health and Nutrition Examination Survey (NHANES 2013–2016) PLoS ONE. 2019;14:e0226744. doi: 10.1371/journal.pone.0226744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Parekh T., Pemmasani S., Desai R. Risk of Stroke with E-Cigarette and Combustible Cigarette Use in Young Adults. Am. J. Prev. Med. 2020;58:446–452. doi: 10.1016/j.amepre.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 74.Vindhyal M.R., Okut H., Ablah E., Ndunda P.M., Kallail K.J., Choi W.S. Cardiovascular Outcomes Associated with Adult Electronic Cigarette Use. [(accessed on 10 October 2022)];Cureus. 2020 12:e9618. doi: 10.7759/cureus.9618. Available online: https://www.cureus.com/articles/38144-cardiovascular-outcomes-associated-with-adult-electronic-cigarette-use. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Akinkugbe A.A. Cigarettes, E-cigarettes, and Adolescents’ Oral Health: Findings from the Population Assessment of Tobacco and Health (PATH) Study. JDR Clin. Transl. Res. 2019;4:276–283. doi: 10.1177/2380084418806870. [DOI] [PubMed] [Google Scholar]
- 76.Dinkeloo E., Grier T.L., Brooks R.D., Jones B.H. Vaping, Smoking, and the Physical Fitness of Active Young Men. Am. J. Prev. Med. 2020;58:e31–e37. doi: 10.1016/j.amepre.2019.08.015. [DOI] [PubMed] [Google Scholar]
- 77.Gaiha S.M., Cheng J., Halpern-Felsher B. Association Between Youth Smoking, Electronic Cigarette Use, and COVID-19. J. Adolesc. Health. 2020;67:519–523. doi: 10.1016/j.jadohealth.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leavens E.L., Ford B.R., Ojo-Fati O., Winkelman T.N., Vickery K.D., Japuntich S.J., Busch A.M. Electronic cigarette use patterns and chronic health conditions among people experiencing homelessness in MN: A statewide survey. BMC Public Health. 2020;20:1889. doi: 10.1186/s12889-020-09919-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Merianos A.L., Jandarov R.A., Choi K., Fiser K.A., Mahabee-Gittens E.M. Combustible and electronic cigarette use and insufficient sleep among U.S. high school students. Prev. Med. 2021;147:106505. doi: 10.1016/j.ypmed.2021.106505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.YYe D., Gajendra S., Lawyer G., Jadeja N., Pishey D., Pathagunti S., Lyons J., Veazie P., Watson G., McIntosh S., et al. Inflammatory biomarkers and growth factors in saliva and gingival crevicular fluid of e-cigarette users, cigarette smokers, and dual smokers: A pilot study. J. Periodontol. 2020;91:1274–1283. doi: 10.1002/JPER.19-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems. Board on Population Health and Public Health Practice, Health and Medicine Division. National Academies of Sciences, Engineering, and Medicine . In: Public Health Consequences of E-Cigarettes. Stratton K., Kwan L.Y., Eaton D.L., editors. National Academies Press; Washington, DC, USA: 2018. [(accessed on 10 October 2022)]. Available online: https://www.nap.edu/catalog/24952. [PubMed] [Google Scholar]
- 82.Czoli C.D., Fong G.T., Goniewicz M.L., Hammond D. Biomarkers of Exposure Among “Dual Users” of Tobacco Cigarettes and Electronic Cigarettes in Canada. Nicotine Tob. Res. 2019;21:1259–1266. doi: 10.1093/ntr/nty174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim J., Lee S. Daily Cigarette Consumption and Urine Cotinine Level between Dual Users of Electronic and Conventional Cigarettes, and Cigarette-Only Users. J. Psychoact. Drugs. 2020;52:20–26. doi: 10.1080/02791072.2019.1706791. [DOI] [PubMed] [Google Scholar]
- 84.Beard E., Brown J., Michie S., West R. Is prevalence of e-cigarette and nicotine replacement therapy use among smokers associated with average cigarette consumption in England? A time-series analysis. BMJ Open. 2018;8:e016046. doi: 10.1136/bmjopen-2017-016046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tehrani M.W., Newmeyer M.N., Rule A.M., Prasse C. Characterizing the Chemical Landscape in Commercial E-Cigarette Liquids and Aerosols by Liquid Chromatography–High-Resolution Mass Spectrometry. Chem. Res. Toxicol. 2021;34:2216–2226. doi: 10.1021/acs.chemrestox.1c00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pisinger C., Døssing M. A systematic review of health effects of electronic cigarettes. Prev. Med. 2014;69:248–260. doi: 10.1016/j.ypmed.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 87.Wills T.A., Soneji S.S., Choi K., Jaspers I., Tam E.K. E-cigarette use and respiratory disorders: An integrative review of converging evidence from epidemiological and laboratory studies. Eur. Respir. J. 2021;57:1901815. doi: 10.1183/13993003.01815-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Davis L.C., Sapey E., Thickett D.R., Scott A. Predicting the pulmonary effects of long-term e-cigarette use: Are the clouds clearing? Eur. Respir. Rev. 2022;31:210121. doi: 10.1183/16000617.0121-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Espinoza-Derout J., Shao X.M., Lao C.J., Hasan K.M., Rivera J.C., Jordan M.C., Echeverria V., Roos K.P., Sinha-Hikim A.P., Friedman T.C. Electronic Cigarette Use and the Risk of Cardiovascular Diseases. Front. Cardiovasc. Med. 2022;9:879726. doi: 10.3389/fcvm.2022.879726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Skotsimara G., Antonopoulos A.S., Oikonomou E., Siasos G., Ioakeimidis N., Tsalamandris S., Charalambous G., Galiatsatos N., Vlachopoulos C., Tousoulis D. Cardiovascular effects of electronic cigarettes: A systematic review and meta-analysis. Eur. J. Prev. Cardiolog. 2019;26:1219–1228. doi: 10.1177/2047487319832975. [DOI] [PubMed] [Google Scholar]
- 91.Armendáriz-Castillo I., Guerrero S., Vera-Guapi A., Cevallos-Vilatuña T., García-Cárdenas J.M., Guevara-Ramírez P., López-Cortés A., Pérez-Villa A., Yumiceba V., Zambrano A.K., et al. Genotoxic and Carcinogenic Potential of Compounds Associated with Electronic Cigarettes: A Systematic Review. BioMed. Res. Int. 2019;2019:1386710. doi: 10.1155/2019/1386710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alzahrani T., Pena I., Temesgen N., Glantz S.A. Association between electronic cigarette use and myocardial infarction. Am. J. Prev. Med. 2018;55:455–461. doi: 10.1016/j.amepre.2018.05.004. Erratum in Am. J. Prev. Med. 2019, 57, 579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sharma A., Gupta I., Venkatesh U., Singh A.K., Golamari R., Arya P. E-cigarettes and myocardial infarction: A systematic review and meta-analysis. Int. J. Cardiol. 2022;28:S0167527322013158. doi: 10.1016/j.ijcard.2022.09.007. [DOI] [PubMed] [Google Scholar]
- 94.Patel U., Patel N., Khurana M., Parulekar A., Patel A., Ortiz J.F., Patel R., Urhoghide E., Mistry A., Bhriguvanshi A., et al. Effect Comparison of E-Cigarette and Traditional Smoking and Association with Stroke—A Cross-Sectional Study of NHANES. Neurol. Int. 2022;14:441–452. doi: 10.3390/neurolint14020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang I., Sandeep S., Rodriguez J. The oral health impact of electronic cigarette use: A systematic review. Crit. Rev. Toxicol. 2020;50:97–127. doi: 10.1080/10408444.2020.1713726. [DOI] [PubMed] [Google Scholar]
- 96.Kennedy C.D., van Schalkwyk M.C.I., McKee M., Pisinger C. The cardiovascular effects of electronic cigarettes: A systematic review of experimental studies. Prev. Med. 2019;127:105770. doi: 10.1016/j.ypmed.2019.105770. [DOI] [PubMed] [Google Scholar]
- 97.Banks E., Yazidjoglou A., Brown S., Nguyen M., Martin M., Beckwith K., Daluwatta A., Campbell S., Joshy G. Electronic Cigarettes and Health Outcoomes: Systematic Review of Global Evidence. National Centre for Epidemiology and Population Health: Australian National University. [(accessed on 10 October 2022)]. Available online: https://openresearch-repository.anu.edu.au/bitstream/1885/262914/1/Electronic%20cigarettes%20health%20outcomes%20review_2022_WCAG.pdf.
- 98.Williams M., Talbot P. Design Features in Multiple Generations of Electronic Cigarette Atomizers. Int. J. Environ. Res. Public Health. 2019;16:2904. doi: 10.3390/ijerph16162904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.PATH Study Findings Give Insight into Flavored Tobacco, Health Effects of E-Cigarettes, and Adult Use of Cigars and Hookah. U.S. Food & Drug Administration. [(accessed on 10 October 2022)]; Available online: https://www.fda.gov/tobacco-products/research/path-study-findings-give-insight-flavored-tobacco-health-effects-e-cigarettes-and-adult-use-cigars.
- 100.Soule E., Bansal-Travers M., Grana R., McIntosh S., Price S., Unger J.B., Walton K. Electronic cigarette use intensity measurement challenges and regulatory implications. Tob. Control. 2021 doi: 10.1136/tobaccocontrol-2021-056483. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Links of the databases used in the review: PubMed https://pubmed.ncbi.nlm.nih.gov/ (accessed on 10 October 2022), EMBASE https://www.embase.com/ (accessed on 10 October 2022), CINAHL https://www.ebsco.com/products/research-databases/cinahl-database (accessed on 10 October 2022) (local access), and Cochrane library https://www.cochranelibrary.com/search (accessed on 10 October 2022). All extracted data is available in the original papers; an overview of the extracted data is found in Table S3. Only a narrative review was performed, not a synthesis of data. An overview of excluded studies is available in Table S2.