Abstract
Sleep is a fundamental, evolutionarily conserved, plastic behavior that is regulated by circadian and homeostatic mechanisms as well as genetic factors and environmental factors, such as light, humidity, and temperature. Among environmental cues, temperature plays an important role in the regulation of sleep. This review presents an overview of thermoreception in animals and the neural circuits that link this process to sleep. Understanding the influence of temperature on sleep can provide insight into basic physiologic processes that are required for survival and guide strategies to manage sleep disorders.
Keywords: temperature, transient receptor potential, sleep, dorsal neuron, preoptic area
1. Introduction
From Drosophila to mammals, sleep is a conservative biological behavior. While sleeping, animals are at risk of predation, but they still spend a lot of time sleeping, suggesting that sleep is important to animals. Recent studies have shown that sleep is essential for replenishing energy after activity, ensuring an optimal physical condition [1], maintaining synaptic stability [2,3,4], regulating immunity [5,6], memory consolidation [7,8], and removing neurotoxic waste [9,10]. Sleep disturbances impair the function of the sympathetic nervous system, leading to metabolic dysregulation [11]. Insufficient sleep during developmental stages can cause a smaller brain and abnormal behavior [12]. In conclusion, sleep is essential for the development, behavior, and survival of organisms. Although the molecular and neural circuits involved in sleep regulation have been extensively studied, the influence of different environmental factors on sleep is not fully understood.
In 1982, Borbely proposed a dual-system model based on previous research results on sleep and used a mathematical model to describe the sleep mechanism [13]. Borbely proposed that sleep is jointly regulated by the rhythmic system (process C) and the sleep-wake homeostatic system (process S). Circadian rhythm determines the periods of wakefulness and sleep over the 24-h daily cycle. Sleep homeostasis preserves physiologic stability; prolonged wakefulness increases the pressure to fall asleep, whereas prolonged sleep time relieves sleep pressure and promotes wakefulness [14]. Studies on the regulatory mechanisms of sleep have identified distinct neural circuits and multiple neurotransmitters that are responsible for maintaining sleep homeostasis and circadian rhythm [15,16,17,18] using model animals such as flies, zebrafish, and mice [8,19]. Based on different model animals, we are increasingly understanding the neural circuits and regulatory molecules that regulate sleep. The regulation of sleep by environmental factors remains to be explored. The brain pathways’ underlying lights impact on sleep have been examined in the current research work [20,21]. It is still unclear how temperature regulation affects sleep.
There are two commonly used methods: one is based on changes in animal sleep-related behaviors and the other is based on changes in brain waves. Five characteristics of sleep in animals include a long period of stillness, higher threshold of response to external stimuli, arousal when stimulated (to distinguish numbness or coma), a distinctive posture, and rebound following sleep deprivation [16,22]. The main criterion for identifying sleep behavior in Drosophila is immobility; flies that are stationary for 5 or more minutes are generally judged to be sleeping [23,24]. The simplicity of this behavior-based definition of sleep makes Drosophila a useful model animal for studying the the complex physiologic mechanisms involved and for identifying novel genes and neurons that regulate this process [25,26,27]. Sleep in mice is analyzed by changes in brain activity [28,29] based on brain waves, and sleep can be categorized as rapid eye movement (REM) sleep and non-REM (NREM). Based on well-established research methods and research models, the researchers explored the effect of temperature on sleep. The present work reviews the current state of knowledge regarding the neural bases of thermoreception and sleep, and the effect of temperature on sleep based on studies in Drosophila and mammals.
2. Effect of Temperature on Sleep
Changes in ambient temperature not only affect ectothermic animals, but also affect the sleep of warm-blooded animals. Many animals build nests or curl up before sleeping to ensure that their bodies stay warm. In mice and humans, sleep can be triggered when the skin warms up [30]. In hot environments, the body cools itself, promoting wakefulness and changing sleep patterns [31]. When exposed to low temperatures, the body produces heat on its own and promotes arousal [32]. Drosophila has a limited ability to control body temperature; therefore, variations in ambient temperature can have a strong impact on behavior. Both prolonged and sudden temperature changes can affect sleep; temperature fluctuations throughout the day are the basis of the circadian rhythm of the rest-activity cycle of Drosophila [33]. Drosophila’s sleep was shown to increase during the day and decrease at night when the ambient temperature shifted from 25 °C to 29 °C [34,35,36]; a suddenly temperature shift from 22 °C to 29 °C decreased sleep during the day and at night [34,37]. Therefore, temperature affects the sleep patterns of Drosophila. By monitoring the sleep of Drosophila species at different latitudes, it was observed that sleep time was related to annual average temperature [38]. Compared with species at low-latitudes, those living at high latitudes had a shorter sleep duration [39]. Additionally, low temperature-dependent splicing increased the transcription level of the daywake (dyw) genes, providing evidence that sleep is regulated by ambient temperature at the molecular level [40,41].
Unlike Drosophila, mammals produce heat by regulating their own metabolism and maintaining their body temperature within a restricted range. Body temperature is regulated by circadian rhythm, and core temperature begins to decline just before sleep and further decreases upon entering NREM sleep. Abnormal circadian rhythms affect body temperature and reduce sleep quality [42]. Humans regulate body temperature in response to elevated temperatures during sleep by increasing the exposed surface area [43,44]. A study of sleep in three geographically segregated human populations found that a lower ambient temperature was associated with different times of sleep onset [45]. Sleep often occurs after sunset when ambient temperature declines; warming of the hands and feet induces NREM sleep [46,47,48], and direct warming of the hypothalamus was shown to promote sleep [49]. Exposure to a warm environment was found to activate hypothalamic neurons in mice, leading to the induction of sleep [50]. According to existing studies, animals’ sleep is regulated by temperature as an environmental cue. Therefore, a foundation for using temperature to treat sleep disorders can be established by comprehending and researching the relationship between temperature and sleep.
This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
3. Thermoreception and Sleep Regulation in Drosophila
3.1. Thermoreception in Drosophila
Drosophila and mammals have thermoreceptors that sense internal and external temperatures, to prevent physiologic injury caused by extreme cold and hot. Drosophila is an ectothermic animal, and changes in environment temperature will directly lead to changes in body temperature. Drosophila has sensitive thermoreceptors that can sense and activate a rapid response to temperature changes [51,52]. Drosophila uses distinct systems at different developmental stages to adapt to temperature variations [53,54], making it an ideal model system for the study of thermoreception.
Animals sense temperature changes through transient receptor potential (TRP) family proteins, which are cation channels present on the cell membrane and in organelles [55,56,57] that conduct Ca2+ influx upon activation [58,59,60]. There are 13 TPR genes in Drosophila [61] (Table 1), that are critical for sensing the external environment and play important roles in vision, olfaction, and thermoreception. In adult Drosophila, environmental temperature is detected by temperature-regulated TRP channels that are expressed in different cell types [62]. dTRPA1 is critical for detecting innocuous temperatures between 20 °C and 29 °C [63]. Anterior cell (AC) neurons [63] in the head and the hot cell neurons in the arista [64] express TRP, sense temperature, and transmit signals to different brain areas that regulate sleep behavior in Drosophila [34,65,66,67]. dTRPA1 is involved in locomotor behavior and synchronization of temperature cycling [68]. Drosophila exhibits bimodal behavior at 25 °C, with characteristic morning and evening activity peaks (M and E peaks) [69]. At physiologic temperatures (29–30 °C), the phase of M and E peaks is advanced and retarded, respectively, leading to sleep induction [70]. Drosophila also uses the taste receptor GR28 to sense temperature changes [71]. The painless and pyrexia gene encode high temperature-sensing receptors; the former is expressed in central and peripheral sensory neurons with an activation threshold of 42 °C [72], whereas the latter mediates heat-activated currents with a threshold of 40 °C [73]. Drosophila lacking pyrexia are heat intolerant, becoming paralyzed upon exposure to 40 °C for 3 min.
Table 1.
Transient receptor potential channels in Drosophila and mammals.
| Channels | Function | Reference |
|---|---|---|
| Drosop h ila | ||
| dTRPA1 | Monitoring temperature fluctuations | [74] |
| A isoform: >26 °C | [75,76,77] | |
| B isoform: >34 °C | [78,79,80,81] | |
| TRPC | Cold avoidance (10 °C) | [74] |
| TRPV | Thermotactic response to cold temperature | [82] |
| TRPP | Thermotactic response to cold temperature | [64] |
| Painless | Avoidance of noxious heat (>40 °C) | [72,83] |
| Pyrexia | Noxious heat resistance (40 °C) | [73,84] |
| Mammals | ||
| TRPV1 | Avoidance of noxious heat (>43 °C) | [85,86] |
| TRPV2 | Avoidance of noxious heat (>52 °C) | [87] |
| TRPV3 | Avoidance of noxious heat (>33 °C) | [88] |
| TRPV4 | Avoidance of noxious heat (27–42 °C) | [89] |
| TRPM8 | Distinguishing between low and medium temperatures (<23–28 °C) | [90] |
| ANKTM1 | Cool temperature avoidance (<18 °C) | [91,92] |
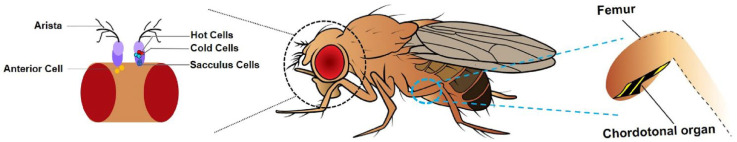
Similar to other animals, adult flies have a temperature-sensing system comprising structures and cells that function in thermoreception; these include arista, which detect warm, hot, and cool temperatures [71,93] as well as anterior cell (AC) neurons in the head [63], sacculus cells in the antennae [64], and chordotonal organs in the body [94,95] (Figure 1).
Figure 1.
Schematic of the anatomy of thermoreception in Drosophila. Left: Hot cells (red) and cold cells (blue) that sense heat and cold are present peripherally in the arista. The Sacculus cells (dark blue) are located in a pouch called the Sacculus within the antennal segments (light and dark purple). Anterior cells (yellow) are found inside the brain, near the antennae. Right: The chordotonal organ (yellow-black stripes) is located at the root of the Drosophila leg.
dTRPA1-expressing ACs are a small group of thermally activated thermal sensors; they are considered as internal thermal sensors because they are in the brain of adult Drosophila, which are known to be autonomously thermosensitive [96]. Additionally, ACs integrate temperature information from peripheral sensors located in the antennae [96]. Drosophila achieves thermorhythmic regulation by aligning physiologic responses to ambient temperature. ACs transmit serotonin signals to small lateral ventral neurons (s-LNvs); these relay the temperature signal to dorsal neuron 2 (DN2), which regulates temperature preference rhythms. The AC-sLNv-DN2 neural circuit sets the preferred temperature before dawn and s-LNvs regulate motor activity and sleep rhythms; predawn environmental temperature may be a wake-up signal that regulates body temperature [97]. ACs have been shown detect changes in environmental temperature and release acetylcholine to activate DN1p and promote wakefulness. DN1p releases the neuropeptide CNMamide to pars intercerebralis (PI) neurons, thereby reducing the amount of sleep at night. Thus, the AC-DN1p-PI neural circuit integrates heat-sensing inputs to promote wakefulness [98].
The antenna is a temperature-sensing organ containing a variety of sensory structures that allow Drosophila to detect temperature changes [99]. Each arista contains brav1- and Gr28b-positive neurons that sense cold and heat [71,100]; temperature signals are transmitted by these cells to different regions of the proximal antennal protocerebrum [64,101], and then to the mushroom body [102]. In the absence of thermosensitive cells in the antenna, the normal response of DN1 neurons is attenuated, leading to disruption of sleep timing during temperature cycling [33]. Temperature signals are transmitted by pyx-expressing neurons in the antenna to ACs and then to the posterior antennal lobe [64,96], which integrates temperature-related information for transmission to higher brain centers.
Cold-sensing neurons in the antenna sense and receive signals from low-temperature stimuli. When the temperature is decreased from 25 °C to ~20 °C, these neurons relay the cold signal to the next neuron, which releases factors that inhibit the activity of sleep-promoting DN1a neurons in the brain [93].
Clock neurons in the brain are synchronized by signals from temperature-responsive peripheral tissues [103]. Loss of nocte alters the structure and function of the chordotonal organ and interferes with the synchronization of behavioral activity to temperature [94]. The nocte mutant flies exhibit abnormal sleep and the expression of PER and TIM in most clock neurons are abnormal during temperature cycling [33]. The nocte protein expressed in the chordotonal organs senses changes in environmental temperature and transmits signals to DNs that regulate activity and sleep by integrating light and temperature signals [104,105].
IR25a is expressed in a subpopulation of chordotonal organ neurons; loss-of-function mutants fail to synchronize with temperature cycling under both constant light and constant dark conditions. Rhythmic oscillations of TIM in DN1 and DN2 are also impaired, suggesting that these neurons receive temperature input from the chordotonal organ and are involved in circadian regulation [95].
The chordotonal organ participates in temperature sensing for circadian clock synchronization. However, direct evidence of temperature sensitivity of this organ in adult Drosophila is lacking, and the putative circuits connecting chordotonal organ neurons and clock neurons in the brain have yet to be described.
3.2. Neural Circuits That Regulate Sleep in Response to Temperature Changes
DN neurons are a heterogeneous group of neurons that can be classified into different subgroups according to gene expression and function [106,107]. DNs have a bidirectional regulatory effect on sleep, promoting both sleep [108] and wakefulness [109] (Figure 2).
Figure 2.
Thermoreceptor circuits in Drosophila. Some thermoreception and circadian neurons in the brain are shown in different colors. The antennae sense cold temperatures, and the second-order thermosensory projection neuron TPN-IIs transmit information from the posterior antennal lobe (PAL) to circadian neurons DN1a to regulate sleep in Drosophila. Arista and AC neurons sense warm temperatures and transmit information to some of the circadian neurons. One of the AC downstream neunon types, a subset of DN1ps (green circles with brown outline), transfers heat signals to PIs (red) to regulate sleep.
Circadian clock activity in different parts of the Drosophila brain is studied by raising or lowering the ambient temperature. DN1p are circadian neurons that are activated and inhibited at low and high environmental temperatures, respectively. In male flies, two distinct sets of TRPA1-expressing thermosensory neurons transmit signals to DN1p at temperatures >29 °C, which influences morning sleep in response to elevated temperatures [37]. The activity of DN1a neurons is inhibited at low temperatures (18 °C). DN1a neurons release the neuropeptide CCHa1 to regulate s-LNv clock neuron activity and also receive PDF signals from s-LNv neurons; this interaction modulates sleep in Drosophila. Thus, DN1a-mediated temperature input may affect other clock neurons [110]. DN1p neurons can also receive high temperature signals from ACs that inhibit neuronal activity and promote wakefulness. Studies have shown that acute heating inhibits CNMa + DN1ps in an AC-independent manner, whereas prolonged heating may activate CNMa + DN1ps through heat-sensing input from AC neurons. That is, CNMa + DN1ps are activated by heat-sensing input from AC neurons to support warmth-induced arousal [98]. The evidence from these studies suggests that DN1 clock neurons serve as a “hub” for temperature input from the periphery and central sleep regulation in Drosophila.
The Drosophila neuropeptide diuretic hormone 31 (DH31) and pigment-dispersing factor receptor (PDFR) regulate nighttime temperature preference. DH31 and PDFR in DN2s regulate the preferred temperature for nighttime sleep onset [111]. DN2 neurons also receive signals from AC cells and sLNvs, with the latter signals peaking before dawn [97].
At the molecular level, temperature regulation involves thermosensitive alternative splicing of clock genes. A high frequency of excision of dmpi8, the temperature-sensitive 3′-terminal intron of the clock gene period (per), inhibits cold-weather sleep as more stable per transcript and protein is produced, leading to earlier peaks of nocturnal activity [112,113]. The dmpi8 splicing efficiency affects the transcription level of dyw [41]. Low temperature-dependent splicing increases dyw mRNA, which inhibits daytime sleep [40].
4. Thermoreception and Sleep Regulation in Mammals
4.1. Thermoreception in Mammals
TRPV1, the most widely studied temperature receptor in mammals, is expressed in the dorsal calcaneal ganglion and trigeminal ganglion (TG). High temperatures activate TRPV1 and cause a painful sensation [55,85]. Trpv1 knockout mice have diminished or absent temperature response [86], underscoring its importance in thermoreception. TRPV2 has 50% sequence similarity to TRPV1 [114,115], is activated at 52 °C, and is primarily expressed in neurons that detect noxious thermal and mechanical stimuli [116]. Loss of TRPV2 did not impair the response of animals to heat [117], implying that TRPV2 is not required for thermoreception in adult mice. TRPV3, which is activated at 33 °C, is expressed in skin keratinocytes, sensory nerve cells, dorsal root ganglion (DRG), TG, and the brain [88,118]. TRPV4 is activated at 27 °C and is expressed in the hypothalamus, sensory neurons and skin keratinocytes [89,119]. TRPM8 was the first receptor to be identified that is activated by cold [120,121,122], and is expressed in the TG and DRG. In addition to TRP channels, ANKTM1, which senses lower temperatures, is only found in the DRG [91].
In response to heat or cold stimuli, somatosensory neurons in the skin send signals to the brain which perceives the change in temperature. In mammals, these neurons are distributed in the DRG [123,124] and are pseudounipolar with a single axon that bifurcates into two branches, one extending to the skin or viscera (detecting changes in environmental and internal core temperature, respectively) [124,125] and the other projecting to the dorsal horn of the spinal cord or spinal trigeminal nucleus in the brainstem.
DRG neurons are the primary afferent neurons of the somatosensory system and convert signals from the external and internal environment into neural activity [126,127,128]. Loss of FGF13 in sensory neurons abolishes the perception of thermal pain without affecting that of mechanical pain [129]. Likewise, ablation of sensory neurons expressing the cold receptor TRPM8 resulted in an inability to perceive cold [130,131,132]. Thus, different temperature receptors in sensory neurons sense temperature changes.
There are four main types of sensory neuron that can be categorized as Aα, Aβ, Aδ, and C fibers, or classified according to the type of stimulus that is detected (mechanical or thermal). Aδ and C fibers include the sensory nerves involved in thermosensation [124,133]. Branches of Aδ and C fibers are widely distributed in the skin and sense different ranges of temperature [134,135,136,137]; this information is encoded as an action potential and transmitted to another axon branch. Aδ axon terminals are distributed in layers I and V of the dorsal horn, while those of C fibers are distributed and form synapses in layers I and II. Temperature information is transmitted via the ascending spinothalamic tract to the thalamus and somatosensory cortex, where temperature perception occurs [125]. Temperature information is also transmitted to the pre-optic area (POA) of the hypothalamus via lateral parabrachial neurons [138,139].
Neurons in the DRG sense temperature changes in most areas of the body, while primary sensory neurons of the TG are mainly distributed in the head and face [140,141,142,143]. The TG is a sensory ganglion that expresses various neuropeptides and signaling molecules and senses temperature changes with a higher temperature threshold and slower action potential conduction velocity than the DRG [142,144].
Warm, hot, and cold stimuli are sensed by different groups of neurons in the TG [121,145,146]. Hypothermia induces the activation of about 15% of non-cold-sensing neurons in the TG [147] that are also involved in temperature perception. The TG contains distinct populations of spinothalamic projection neurons that transmit temperature information to the primary somatosensory cortex [138].
4.2. Association between Temperature and Sleep Regulation
Mammals, unlike Drosophila, are homeotherms, which means that their core body temperature is kept within a specific range. The brain regulates body temperature by boosting heat generation or heat dissipation based on temperature information transmitted from the skin and organs by skin receptors. Mammals’ core and skin temperatures are frequently separated into two categories. Animals’ skin, which is on the outside of them, frequently varies in response to variations in the surrounding temperature, whereas the internal organs and central nervous system have temperatures that are more or less constant [125,148]. Internal organs, the brain, and the spinal cord all keep track of variations in core temperature, and skin receptors send information about environmental temperature to the brain, which regulates body temperature by boosting either heat generation or heat dissipation. The inner core and the skin both communicate temperature data to the POA. Clinical studies have demonstrated that excessively high or low environmental temperature can affect the amount and quality of sleep [149,150,151,152,153].
The hypothalamus receives signals pertaining to temperature changes and participates in thermoregulation; damage to the hypothalamus can also seriously affect sleep. Neurons involved in sleep and temperature regulation are located in the POA [154,155,156] and their activity increases with temperature [157]. The POA is divided into lateral (lPOA), median (MnPOA), and medial (mPOA) nuclei. Activation of GABAergic or galaninergic neurons in the POA promotes sleep [158,159], whereas activation of glutamatergic neurons promotes wakefulness [158,160]. The mPOA is involved in sleep and temperature regulation [161,162]; impaired mPOA function in rats decreased sleep, increased brain temperature, and abolished the ability of the rats to regulate body temperature in a cold environment [161,163]. Elevated orexin levels in the mPOA of lactating rats promoted wakefulness and increased body temperature [164]. Upon exposure to a warm temperature, thermoreceptors in the skin transmit signals to glutamatergic neurons in the LPB nucleus, which activates glutamatergic neurons in the MnPOA and mPOA. MnPOA GABAergic neurons inhibit wakefulness-inducing neurons to promote sleep, while mPOA GABAergic neurons promote hypothermia [50,165]. The lPOA and mPOA regulate sleep in different ways; disruption of the former in rats was shown to reduce sleep while brain temperature was unaffected, although the rats exhibited defects in thermal defense behaviors [161].
During sleep, the temperature of the skin and brain changes. During NREM sleep, the brain and skin temperature decrease; during REM, brain temperature increases. It is suggested that body temperature and sleep stage are closely related. Recording changes in brain temperature while recording neuronal activity found that brain temperature was closely related to changes in sleep at different scales [166,167].
5. Outlook and Discussion
For Drosophila and mammals, temperature provides basic information regarding the environment. A major advance in our understanding of temperature perception was the identification of distinct thermoreceptors in different species and the cloning and characterization of molecular thermosensors. Drosophila is highly sensitive to temperature changes, and it also has a limited number of neurons and multiple genetic tools available, making it amenable to the study of the relationship between temperature and sleep. The current study shows that the transmission of temperature signals in Drosophila has been elucidated. There might, however, yet be undiscovered ways for sensing temperature. The temperature loop can be more clearly described by studying the transmission of temperature signals in different ways [168,169]. It also lays the foundation for exploring the neural circuits of temperature regulation and sleep.
Sleep and body temperature are also closely related in mammals, with both environmental and body temperatures affecting sleep. The POA receives temperature information from the skin and participates in temperature and sleep regulation. Multiple distinct neuron types with different properties and molecular profiles that regulate sleep or body temperature have been identified in the POA. The extent of overlap between temperature-sensing and sleep-regulating POA neuron populations and associated neural circuits are unknown. The current study demonstrates the heterogeneity of POA’s neurons and suggests that various neurons may control various biological processes. Recent single-cell sequencing techniques classify POA neurons more precisely. From there, researchers can manipulate a particular type of POA neuron using light activation or virus injection to better understand how different POA neuron types work in concert to control body temperature and sleep. Most of the current research on sleep in mammals is conducted using mice; as they are nocturnal animals, it is unclear whether the mechanisms of temperature and sleep regulation reflect those in humans. Examining how temperature affects sleep in other species can provide insight into whether the mechanisms that have been described in model organisms are conserved across all animals.
In recent years, researchers have discovered and characterized the networks and neural circuits involved in temperature transmission. However, our understanding of animal thermal sensations still lags behind our understanding of other senses such as sight, smell, and so on. In Drosophila and mammals, many key temperature-sensing neurons and thermoreceptor molecules have not been discovered, and little is known about the roles of known molecules in sleep regulation. Mammalian research advances slowly due to the enormous number of neurons and limited technological capabilities. Given the complexity of mammals’ thermoregulatory systems and temperature sensing networks, it is difficult to fully understand their regulatory processes in the present studies. This makes it challenging to determine how temperature regulation affects sleep. It is anticipated that as technology advances, new neurological pathways controlling sleep through temperature will be discovered, allowing humans to alter the environment’s temperature to enhance sleep quality.
Acknowledgments
We apologize to those whose works have contributed greatly to our knowledge but were not sufficiently reviewed or were not cited owing to space limitations. We thank members of the Han laboratory for proof-reading.
Author Contributions
Y.F. and Y.T. conceptualized the manuscript and wrote the draft. Y.W. prepared the figures. All authors made corrections to the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Jiangsu Natural Science Foundation for Outstanding Young Scientists, BK20180061, granted to Y.T.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vyazovskiy V.V., Delogu A. NREM and REM Sleep: Complementary Roles in Recovery after Wakefulness. Neuroscientist. 2014;20:203–219. doi: 10.1177/1073858413518152. [DOI] [PubMed] [Google Scholar]
- 2.Bellesi M., de Vivo L. Structural synaptic plasticity across sleep and wake. Curr. Opin. Physiol. 2020;15:74–81. doi: 10.1016/j.cophys.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang S., Sigrist S.J. Presynaptic and postsynaptic long-term plasticity in sleep homeostasis. Curr. Opin. Neurobiol. 2020;69:1–10. doi: 10.1016/j.conb.2020.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Tononi G., Cirelli C. Sleep and Synaptic Down-Selection. Eur. J. Neurosci. 2020;51:413–421. doi: 10.1111/ejn.14335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oikonomou G., Prober D.A. Linking immunity and sickness-induced sleep. Science. 2019;363:455–456. doi: 10.1126/science.aaw2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toda H., Williams J.A., Gulledge M., Sehgal A. A sleep-inducing gene, nemuri, links sleep and immune function in Drosophila. Science. 2019;363:509–515. doi: 10.1126/science.aat1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Girardeau G., Lopes-Dos-Santos V. Brain neural patterns and the memory function of sleep. Science. 2021;374:560–564. doi: 10.1126/science.abi8370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donlea J.M. Roles for sleep in memory: Insights from the fly. Curr. Opin. Neurobiol. 2018;54:120–126. doi: 10.1016/j.conb.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Alphen B., Semenza E.R., Yap M., Van Swinderen B., Allada R. A deep sleep stage in Drosophila with a functional role in waste clearance. Sci. Adv. 2021;7:eabc2999. doi: 10.1126/sciadv.abc2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie L., Kang H., Xu Q., Chen M.J., Liao Y., Thiyagarajan M., O’Donnell J., Christensen D.J., Nicholson C., Iliff J.J., et al. Sleep Drives Metabolite Clearance from the Adult Brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bishir M., Bhat A., Essa M.M., Ekpo O., Ihunwo A.O., Veeraraghavan V.P., Mohan S.K., Mahalakshmi A.M., Ray B., Tuladhar S., et al. Sleep Deprivation and Neurological Disorders. BioMed Res. Int. 2020;2020:5764017. doi: 10.1155/2020/5764017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirmiran M., Scholtens J., Van de Poll N., Uylings H., Van der Gugten J., Boer G. Effects of experimental suppression of active (REM) sleep during early development upon adult brain and behavior in the rat. Dev. Brain Res. 1983;7:277–286. doi: 10.1016/0165-3806(83)90184-0. [DOI] [PubMed] [Google Scholar]
- 13.Borbély A.A. A two process model of sleep regulation. Hum. Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 14.Fisher S.P., Foster R.G., Peirson S.N. Circadian Clocks. Springer; Berlin/Heidelberg, Germany: 2013. The Circadian Control of Sleep; pp. 157–183. [DOI] [PubMed] [Google Scholar]
- 15.Joiner W.J. Unraveling the Evolutionary Determinants of Sleep. Curr. Biol. 2016;26:R1073–R1087. doi: 10.1016/j.cub.2016.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keene A.C., Duboue E.R. The origins and evolution of sleep. J. Exp. Biol. 2018;221:jeb159533. doi: 10.1242/jeb.159533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deboer T. Sleep, Neuronal Plasticity and Brain Function. Volume 25. Springer; Berlin/Heidelberg, Germany: 2013. Behavioral and Electrophysiological Correlates of Sleep and Sleep Homeostasis; pp. 1–24. [DOI] [PubMed] [Google Scholar]
- 18.Dement W.C. Sleep Extension: Getting as Much Extra Sleep as Possible. Clin. Sports Med. 2005;24:251–268. doi: 10.1016/j.csm.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 19.Blum I.D., Bell B., Wu M.N. Time for Bed: Genetic Mechanisms Mediating the Circadian Regulation of Sleep. Trends Genet. 2018;34:379–388. doi: 10.1016/j.tig.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santhi N., Ball D.M. Applications in sleep: How light affects sleep. Prog. Brain Res. 2020;253:17–24. doi: 10.1016/bs.pbr.2020.05.029. [DOI] [PubMed] [Google Scholar]
- 21.Mazzotta G.M., Damulewicz M., Cusumano P. Better Sleep at Night: How Light Influences Sleep in Drosophila. Front. Physiol. 2020;11:997. doi: 10.3389/fphys.2020.00997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nath R.D., Bedbrook C.N., Abrams M.J., Basinger T., Bois J.S., Prober D.A., Sternberg P.W., Gradinaru V., Goentoro L. The Jellyfish Cassiopea Exhibits a Sleep-like State. Curr. Biol. 2017;27:2984–2990.e3. doi: 10.1016/j.cub.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaw P.J., Cirelli C., Greenspan R.J., Tononi G. Correlates of Sleep and Waking in Drosophila melanogaster. Science. 2000;287:1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- 24.Hendricks J.C., Finn S.M., Panckeri K.A., Chavkin J., Williams J.A., Sehgal A., Pack A. Rest in Drosophila Is a Sleep-like State. Neuron. 2000;25:129–138. doi: 10.1016/S0896-6273(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 25.Oh Y., Jang D., Sonn J.Y., Choe J. Histamine-HisCl1 receptor axis regulates wake-promoting signals in Drosophila melanogaster. PLoS ONE. 2013;8:e68269. doi: 10.1371/journal.pone.0068269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Damulewicz M., Mazzotta G.M. Corrigendum: One Actor, Multiple Roles: The Performances of Cryptochrome in Drosophila. Front. Physiol. 2020;11:841. doi: 10.3389/fphys.2020.00841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faville R., Kottler B., Goodhill G.J., Shaw P.J., Van Swinderen B. How deeply does your mutant sleep? Probing arousal to better understand sleep defects in Drosophila. Sci. Rep. 2015;5:8454. doi: 10.1038/srep08454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allada R., Siegel J.M. Unearthing the Phylogenetic Roots of Sleep. Curr. Biol. 2008;18:R670–R679. doi: 10.1016/j.cub.2008.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sehgal A., Mignot E. Genetics of Sleep and Sleep Disorders. Cell. 2011;146:194–207. doi: 10.1016/j.cell.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raymann R.J.E.M., Swaab D., Van Someren E.J.W. Skin deep: Enhanced sleep depth by cutaneous temperature manipulation. Brain. 2008;131:500–513. doi: 10.1093/brain/awm315. [DOI] [PubMed] [Google Scholar]
- 31.Keramidas M.E., Botonis P.G. Short-term sleep deprivation and human thermoregulatory function during thermal challenges. Exp. Physiol. 2021;106:1139–1148. doi: 10.1113/EP089467. [DOI] [PubMed] [Google Scholar]
- 32.Okamoto-Mizuno K., Yamashiro Y., Tanaka H., Komada Y., Mizuno K., Tamaki M., Kitado M., Inoue Y., Shirakawa S. Heart rate variability and body temperature during the sleep onset period. Sleep Biol. Rhythm. 2008;6:42–49. doi: 10.1111/j.1479-8425.2008.00335.x. [DOI] [Google Scholar]
- 33.Yadlapalli S., Jiang C., Bahle A., Reddy P., Meyhofer E., Shafer O.T. Circadian clock neurons constantly monitor environmental temperature to set sleep timing. Nature. 2018;555:98–102. doi: 10.1038/nature25740. [DOI] [PubMed] [Google Scholar]
- 34.Parisky K.M., Rivera J.L.A., Donelson N.C., Kotecha S., Griffith L.C. Reorganization of Sleep by Temperature in Drosophila Requires Light, the Homeostat, and the Circadian Clock. Curr. Biol. 2016;26:882–892. doi: 10.1016/j.cub.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishimoto H., Lark A.R., Kitamoto T. Factors that Differentially Affect Daytime and Nighttime Sleep in Drosophila melanogaster. Front. Neurol. 2012;3:24. doi: 10.3389/fneur.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Low K.H., Lim C., Ko H.W., Edery I. Natural Variation in the Splice Site Strength of a Clock Gene and Species-Specific Thermal Adaptation. Neuron. 2008;60:1054–1067. doi: 10.1016/j.neuron.2008.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lamaze A., Öztürk-Çolak A., Fischer R., Peschel N., Koh K., Jepson J.E. Regulation of sleep plasticity by a thermo-sensitive circuit in Drosophila. Sci. Rep. 2017;7:40304. doi: 10.1038/srep40304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown E.B., Torres J., Bennick R.A., Rozzo V., Kerbs A., DiAngelo J.R., Keene A.C. Variation in sleep and metabolic function is associated with latitude and average temperature in Drosophila melanogaster. Ecol. Evol. 2018;8:4084–4097. doi: 10.1002/ece3.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Svetec N., Zhao L., Saelao P., Chiu J.C., Begun D.J. Evidence that natural selection maintains genetic variation for sleep in Drosophila melanogaster. BMC Evol. Biol. 2015;15:41. doi: 10.1186/s12862-015-0316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Z., Cao W., Edery I. The SR protein B52/SRp55 regulates splicing of the period thermosensitive intron and mid-day siesta in Drosophila. Sci. Rep. 2018;8:1872. doi: 10.1038/s41598-017-18167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Y., Edery I. Daywake, an Anti-siesta Gene Linked to a Splicing-Based Thermostat from an Adjoining Clock Gene. Curr. Biol. 2019;29:1728–1734.e4. doi: 10.1016/j.cub.2019.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lack L.C., Gradisar M., Van Someren E.J., Wright H.R., Lushington K. The relationship between insomnia and body temperatures. Sleep Med. Rev. 2008;12:307–317. doi: 10.1016/j.smrv.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Raymann R.J.E.M., Swaab D., Van Someren E.J.W. Cutaneous warming promotes sleep onset. Am. J. Physiol. Integr. Comp. Physiol. 2005;288:R1589–R1597. doi: 10.1152/ajpregu.00492.2004. [DOI] [PubMed] [Google Scholar]
- 44.Jansen P., Leineweber M., Thien T. The effect of a change in ambient temperature on blood pressure in normotensives. J. Hum. Hypertens. 2001;15:113–117. doi: 10.1038/sj.jhh.1001134. [DOI] [PubMed] [Google Scholar]
- 45.Yetish G., Kaplan H., Gurven M., Wood B., Pontzer H., Manger P.R., Wilson C., McGregor R., Siegel J.M. Natural Sleep and Its Seasonal Variations in Three Pre-industrial Societies. Curr. Biol. 2015;25:2862–2868. doi: 10.1016/j.cub.2015.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.von Gaudecker J.R. A Feasibility Randomized Controlled Crossover Trial of Home-Based Warm Footbath to Improve Sleep in the Chronic Phase of Traumatic Brain Injury. J. Neurosci. Nurs. 2017;49:386. doi: 10.1097/JNN.0000000000000331. [DOI] [PubMed] [Google Scholar]
- 47.Oshima-Saeki C., Taniho Y., Arita H., Fujimoto E. Lower-limb warming improves sleep quality in elderly people living in nursing homes. Sleep Sci. 2017;10:87–91. doi: 10.5935/1984-0063.20170016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ko Y., Lee J.-Y. Effects of feet warming using bed socks on sleep quality and thermoregulatory responses in a cool environment. J. Physiol. Anthr. 2018;37:13. doi: 10.1186/s40101-018-0172-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McGinty D., Szymusiak R., Thomson D. Preoptic/anterior hypothalamic warming increases EEG delta frequency activity within non-rapid eye movement sleep. Brain Res. 1994;667:273–277. doi: 10.1016/0006-8993(94)91506-7. [DOI] [PubMed] [Google Scholar]
- 50.Harding E.C., Yu X., Miao A., Andrews N., Ma Y., Ye Z., Lignos L., Miracca G., Ba W., Yustos R., et al. A Neuronal Hub Binding Sleep Initiation and Body Cooling in Response to a Warm External Stimulus. Curr. Biol. 2018;28:2263–2273.e4. doi: 10.1016/j.cub.2018.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klein M., Afonso B., Vonner A.J., Hernandez-Nunez L., Berck M., Tabone C.J., Kane E.A., Pieribone V.A., Nitabach M.N., Cardona A., et al. Sensory determinants of behavioral dynamics in Drosophila thermotaxis. Proc. Natl. Acad. Sci. USA. 2014;112:E220–E229. doi: 10.1073/pnas.1416212112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dillon M.E., Wang G., Huey R.B. Global metabolic impacts of recent climate warming. Nature. 2010;467:704–706. doi: 10.1038/nature09407. [DOI] [PubMed] [Google Scholar]
- 53.Sayeed O., Benzer S. Behavioral genetics of thermosensation and hygrosensation in Drosophila. Proc. Natl. Acad. Sci. USA. 1996;93:6079–6084. doi: 10.1073/pnas.93.12.6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Luo L., Gershow M., Rosenzweig M., Kang K., Fang-Yen C., Garrity P.A., Samuel A.D.T. Navigational Decision Making in Drosophila Thermotaxis. J. Neurosci. 2010;30:4261–4272. doi: 10.1523/JNEUROSCI.4090-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Venkatachalam K., Montell C. TRP channels. Annu. Rev. Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng W., Yang F., Takanishi C.L., Zheng J. Thermosensitive TRPV Channel Subunits Coassemble into Heteromeric Channels with Intermediate Conductance and Gating Properties. J. Gen. Physiol. 2007;129:191–207. doi: 10.1085/jgp.200709731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gavva N.R., Treanor J.J., Garami A., Fang L., Surapaneni S., Akrami A., Alvarez F., Bak A., Darling M., Gore A., et al. Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain. 2008;136:202–210. doi: 10.1016/j.pain.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 58.Montell C., Rubin G.M. Molecular characterization of the drosophila trp locus: A putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–1323. doi: 10.1016/0896-6273(89)90069-X. [DOI] [PubMed] [Google Scholar]
- 59.Berry R.E.A. The AASM Manual for the Scoring of Sleep and Associated Events: Rules Terminology and Technical Specifications, Version 2.0. American Academy of Sleep Medicine; Darien, IL, USA: 2012. [Google Scholar]
- 60.Nozadze I., Tsiklauri N., Gurtskaia G., Tsagareli M.G. Role of thermo TRPA1 and TRPV1 channels in heat, cold, and mechanical nociception of rats. Behav. Pharmacol. 2016;27:29–36. doi: 10.1097/FBP.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 61.Fowler M.A., Montell C. Drosophila TRP channels and animal behavior. Life Sci. 2012;92:394–403. doi: 10.1016/j.lfs.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dillon M.E., Wang G., Garrity P.A., Huey R.B. Review: Thermal preference in Drosophila. J. Therm. Biol. 2009;34:109–119. doi: 10.1016/j.jtherbio.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hamada F.N., Rosenzweig M., Kang K., Pulver S.R., Ghezzi A., Jegla T.J., Garrity P.A. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gallio M., Ofstad T.A., Macpherson L.J., Wang J.W., Zuker C.S. The Coding of Temperature in the Drosophila Brain. Cell. 2011;144:614–624. doi: 10.1016/j.cell.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roessingh S., Stanewsky R. The Drosophila TRPA1 Channel and Neuronal Circuits Controlling Rhythmic Behaviours and Sleep in Response to Environmental Temperature. Int. J. Mol. Sci. 2017;18:2028. doi: 10.3390/ijms18102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beckwith E.J., French A.S. Sleep in Drosophila and Its Context. Front. Physiol. 2019;10:1167. doi: 10.3389/fphys.2019.01167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee Y. Contribution of Drosophila TRPA1-Expressing Neurons to Circadian Locomotor Activity Patterns. PLoS ONE. 2013;8:e85189. doi: 10.1371/journal.pone.0085189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee Y., Montell C. Drosophila TRPA1 Functions in Temperature Control of Circadian Rhythm in Pacemaker Neurons. J. Neurosci. 2013;33:6716–6725. doi: 10.1523/JNEUROSCI.4237-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Helfrich-Förster C. Differential Control of Morning and Evening Components in the Activity Rhythm of Drosophila melanogaster—Sex-Specific Differences Suggest a Different Quality of Activity. J. Biol. Rhythm. 2000;15:135–154. doi: 10.1177/074873040001500208. [DOI] [PubMed] [Google Scholar]
- 70.Majercak J., Sidote D., Hardin P.E., Edery I. How a Circadian Clock Adapts to Seasonal Decreases in Temperature and Day Length. Neuron. 1999;24:219–230. doi: 10.1016/S0896-6273(00)80834-X. [DOI] [PubMed] [Google Scholar]
- 71.Ni L., Bronk P., Chang E.C., Lowell A.M., Flam J.O., Panzano V.C., Theobald D., Griffith L., Garrity P.A. A gustatory receptor paralogue controls rapid warmth avoidance in Drosophila. Nature. 2013;500:580–584. doi: 10.1038/nature12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sokabe T., Tsujiuchi S., Kadowaki T., Tominaga M. Drosophila Painless Is a Ca2+-Requiring Channel Activated by Noxious Heat. J. Neurosci. 2008;28:9929–9938. doi: 10.1523/JNEUROSCI.2757-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee Y., Lee Y., Lee J., Bang S., Hyun S., Kang J., Hong S.T., Bae E., Kaang B.K., Kim J. Pyrexia is a new thermal transient receptor potential channel endowing tolerance to high temperatures in Drosophila melanogaster. Nat. Genet. 2005;37:305–310. doi: 10.1038/ng1513. [DOI] [PubMed] [Google Scholar]
- 74.Rosenzweig M., Kang K., Garrity P.A. Distinct TRP channels are required for warm and cool avoidance in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 2008;105:14668–14673. doi: 10.1073/pnas.0805041105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kwon Y., Shim H.-S., Wang X., Montell C. Control of thermotactic behavior via coupling of a TRP channel to a phospholipase C signaling cascade. Nat. Neurosci. 2008;11:871–873. doi: 10.1038/nn.2170. [DOI] [PubMed] [Google Scholar]
- 76.Shen W.L., Kwon Y., Adegbola A.A., Luo J., Chess A., Montell C. Function of Rhodopsin in Temperature Discrimination in Drosophila. Science. 2011;331:1333–1336. doi: 10.1126/science.1198904. [DOI] [PubMed] [Google Scholar]
- 77.Rosenzweig M., Brennan K.M., Tayler T.D., Phelps P.O., Patapoutian A., Garrity P.A. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes Dev. 2005;19:419–424. doi: 10.1101/gad.1278205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Viswanath V., Story G.M., Peier A.M., Petrus M.J., Lee V.M., Hwang S.W., Patapoutian A., Jegla T. Opposite thermosensor in fruitfly and mouse. Nature. 2003;423:822–823. doi: 10.1038/423822a. [DOI] [PubMed] [Google Scholar]
- 79.Zhong L., Bellemer A., Yan H., Honjo K., Robertson J., Hwang R.Y., Pitt G.S., Tracey W.D. Thermosensory and Nonthermosensory Isoforms of Drosophila melanogaster TRPA1 Reveal Heat-Sensor Domains of a ThermoTRP Channel. Cell Rep. 2011;1:43–55. doi: 10.1016/j.celrep.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kang K., Panzano V.C., Chang E.C., Ni L., Dainis A.M., Jenkins A.M., Regna K., Muskavitch M.A.T., Garrity P.A. Modulation of TRPA1 thermal sensitivity enables sensory discrimination in Drosophila. Nature. 2011;481:76–80. doi: 10.1038/nature10715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Neely G.G., Keene A.C., Duchek P., Chang E.C., Wang Q.-P., Aksoy Y.A., Rosenzweig M., Costigan M., Woolf C.J., Garrity P., et al. TrpA1 Regulates Thermal Nociception in Drosophila. PLoS ONE. 2011;6:e24343. doi: 10.1371/journal.pone.0024343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kwon Y., Shen W.L., Shim H.-S., Montell C. Fine Thermotactic Discrimination between the Optimal and Slightly Cooler Temperatures via a TRPV Channel in Chordotonal Neurons. J. Neurosci. 2010;30:10465–10471. doi: 10.1523/JNEUROSCI.1631-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tracey W.D., Jr., Wilson R.I., Laurent G., Benzer S. painless, a Drosophila Gene Essential for Nociception. Cell. 2003;113:261–273. doi: 10.1016/S0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- 84.Wolfgang W., Simoni A., Gentile C., Stanewsky R. The Pyrexia transient receptor potential channel mediates circadian clock synchronization to low temperature cycles in Drosophila melanogaster. Proc. R. Soc. B Boil. Sci. 2013;280:20130959. doi: 10.1098/rspb.2013.0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Caterina M.J., Schumacher M.A., Tominaga M., Rosen T.A., Levine J.D., Julius D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 86.Caterina M.J., Leffler A., Malmberg A.B., Martin W.J., Trafton J., Petersen-Zeitz K.R., Koltzenburg M., Basbaum A.I., Julius D. Impaired Nociception and Pain Sensation in Mice Lacking the Capsaicin Receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 87.Bender F., Schnitzler M.M.Y., Li Y., Ji A., Weihe E., Gudermann T., Schäfer M. The Temperature-Sensitive Ion Channel TRPV2 is Endogenously Expressed and Functional in the Primary Sensory Cell Line F-11. Cell. Physiol. Biochem. 2005;15:183–194. doi: 10.1159/000083651. [DOI] [PubMed] [Google Scholar]
- 88.Smith G.D., Gunthorpe M.J., Kelsell R.E., Hayes P.D., Reilly P., Facer P., Wright J.E., Jerman J.C., Walhin J.-P., Ooi L., et al. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature. 2002;418:186–190. doi: 10.1038/nature00894. [DOI] [PubMed] [Google Scholar]
- 89.Güler A.D., Lee H., Iida T., Shimizu I., Tominaga M., Caterina M. Heat-Evoked Activation of the Ion Channel, TRPV4. J. Neurosci. 2002;22:6408–6414. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dhaka A., Murray A.N., Mathur J., Earley T.J., Petrus M.J., Patapoutian A. TRPM8 Is Required for Cold Sensation in Mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 91.Story G.M., Peier A.M., Reeve A.J., Eid S.R., Mosbacher J., Hricik T.R., Earley T.J., Hergarden A.C., Andersson D.A., Hwang S.W., et al. ANKTM1, a TRP-like Channel Expressed in Nociceptive Neurons, Is Activated by Cold Temperatures. Cell. 2003;112:819–829. doi: 10.1016/S0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 92.Belmonte C., Viana F. Molecular and Cellular Limits to Somatosensory Specificity. Mol. Pain. 2008;4:14. doi: 10.1186/1744-8069-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alpert M.H., Frank D.D., Kaspi E., Flourakis M., Zaharieva E.E., Allada R., Para A., Gallio M. A Circuit Encoding Absolute Cold Temperature in Drosophila. Curr. Biol. 2020;30:2275–2288.e5. doi: 10.1016/j.cub.2020.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sehadova H., Glaser F.T., Gentile C., Simoni A., Giesecke A., Albert J.T., Stanewsky R. Temperature entrainment of Drosophila’s circadian clock involves the gene nocte and signaling from peripheral sensory tissues to the brain. Neuron. 2009;64:251–266. doi: 10.1016/j.neuron.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 95.Chen C., Buhl E., Xu M., Croset V., Rees J., Lilley K.S., Benton R., Hodge J., Stanewsky R. Drosophila Ionotropic Receptor 25a mediates circadian clock resetting by temperature. Nature. 2015;527:516–520. doi: 10.1038/nature16148. [DOI] [PubMed] [Google Scholar]
- 96.Tang X., Platt M.D., Lagnese C.M., Leslie J.R., Hamada F.N. Temperature Integration at the AC Thermosensory Neurons in Drosophila. J. Neurosci. 2013;33:894–901. doi: 10.1523/JNEUROSCI.1894-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tang X., Roessingh S., Hayley S.E., Chu M.L., Tanaka N.K., Wolfgang W., Song S., Stanewsky R., Hamada F.N. The role of PDF neurons in setting the preferred temperature before dawn in Drosophila. eLife. 2017;6:e23206. doi: 10.7554/eLife.23206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jin X., Tian Y., Zhang Z.C., Gu P., Liu C., Han J. A subset of DN1p neurons integrates thermosensory inputs to promote wakefulness via CNMa signaling. Curr. Biol. 2021;31:2075–2087.e6. doi: 10.1016/j.cub.2021.02.048. [DOI] [PubMed] [Google Scholar]
- 99.Budelli G., Ni L., Berciu C., van Giesen L., Knecht Z.A., Chang E.C., Kaminski B., Silbering A.F., Samuel A., Klein M., et al. Ionotropic Receptors Specify the Morphogenesis of Phasic Sensors Controlling Rapid Thermal Preference in Drosophila. Neuron. 2019;101:738–747.e3. doi: 10.1016/j.neuron.2018.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Benton R. Chemical sensing in Drosophila. Curr. Opin. Neurobiol. 2008;18:357–363. doi: 10.1016/j.conb.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 101.Li K., Gong Z. Feeling Hot and Cold: Thermal Sensation in Drosophila. Neurosci. Bull. 2016;33:317–322. doi: 10.1007/s12264-016-0087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Frank D.D., Jouandet G.C., Kearney P., Macpherson L., Gallio M. Temperature representation in the Drosophila brain. Nature. 2015;519:358–361. doi: 10.1038/nature14284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.George R., Stanewsky R. Peripheral Sensory Organs Contribute to Temperature Synchronization of the Circadian Clock in Drosophila melanogaster. Front. Physiol. 2021;12:622545. doi: 10.3389/fphys.2021.622545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen C., Xu M., Anantaprakorn Y., Rosing M., Stanewsky R. nocte Is Required for Integrating Light and Temperature Inputs in Circadian Clock Neurons of Drosophila. Curr. Biol. 2018;28:1595–1605.e3. doi: 10.1016/j.cub.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 105.Edery I. A Stretch from the Periphery Helps Brain Clocks Feel the Daily Heat. Neuron. 2009;64:157–160. doi: 10.1016/j.neuron.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 106.Guo F., Holla M., Díaz M.M., Rosbash M. A Circadian Output Circuit Controls Sleep-Wake Arousal in Drosophila. Neuron. 2018;100:624–635.e4. doi: 10.1016/j.neuron.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 107.Zhang Y., Liu Y., Bilodeau-Wentworth D., Hardin P.E., Emery P. Light and Temperature Control the Contribution of Specific DN1 Neurons to Drosophila Circadian Behavior. Curr. Biol. 2010;20:600–605. doi: 10.1016/j.cub.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guo F., Yu J., Jung H.J., Abruzzi K.C., Luo W., Griffith L., Rosbash M. Circadian neuron feedback controls the Drosophila sleep–activity profile. Nature. 2016;536:292–297. doi: 10.1038/nature19097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kunst M., Hughes M.E., Raccuglia D., Felix M., Li M., Barnett G., Duah J., Nitabach M.N. Calcitonin Gene-Related Peptide Neurons Mediate Sleep-Specific Circadian Output in Drosophila. Curr. Biol. 2014;24:2652–2664. doi: 10.1016/j.cub.2014.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fujiwara Y., Hermann-Luibl C., Katsura M., Sekiguchi M., Ida T., Helfrich-Förster C., Yoshii T. The CCHamide1 Neuropeptide Expressed in the Anterior Dorsal Neuron 1 Conveys a Circadian Signal to the Ventral Lateral Neurons in Drosophila melanogaster. Front. Physiol. 2018;9:1276. doi: 10.3389/fphys.2018.01276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Goda T., Tang X., Umezaki Y., Chu M.L., Kunst M., Nitabach M.N., Hamada F.N. Drosophila DH31 Neuropeptide and PDF Receptor Regulate Night-Onset Temperature Preference. J. Neurosci. 2016;36:11739–11754. doi: 10.1523/JNEUROSCI.0964-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen W.-F., Low K.H., Lim C., Edery I. Thermosensitive Splicing of a Clock Gene and Seasonal Adaptation. Cold Spring Harb. Symp. Quant. Biol. 2007;72:599–606. doi: 10.1101/sqb.2007.72.021. [DOI] [PubMed] [Google Scholar]
- 113.Majercak J., Chen W.F., Edery I. Splicing of the period gene 3′-terminal intron is regulated by light, circadian clock factors, and phospholipase C. Mol. Cell Biol. 2004;24:3359–3372. doi: 10.1128/MCB.24.8.3359-3372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Caterina M.J., Rosen T.A., Tominaga M., Brake A.J., Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- 115.Montell C., Birnbaumer L., Flockerzi V. The TRP Channels, a Remarkably Functional Family. Cell. 2002;108:595–598. doi: 10.1016/S0092-8674(02)00670-0. [DOI] [PubMed] [Google Scholar]
- 116.Kojima I., Nagasawa M. Trpv2. Handb. Exp. Pharmacol. 2014;222:247–272. doi: 10.1007/978-3-642-54215-2_10. [DOI] [PubMed] [Google Scholar]
- 117.Park U., Vastani N., Guan Y., Raja S.N., Koltzenburg M., Caterina M.J. TRP Vanilloid 2 Knock-Out Mice Are Susceptible to Perinatal Lethality But Display Normal Thermal and Mechanical Nociception. J. Neurosci. 2011;31:11425–11436. doi: 10.1523/JNEUROSCI.1384-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xu H., Ramsey I.S., Kotecha S.A., Moran M.M., Chong J.A., Lawson D., Ge P., Lilly J., Silos-Santiago I., Xie Y., et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418:181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- 119.Peier A.M., Reeve A.J., Andersson D.A., Moqrich A., Earley T.J., Hergarden A.C., Story G.M., Colley S., Hogenesch J.B., McIntyre P., et al. A Heat-Sensitive TRP Channel Expressed in Keratinocytes. Science. 2002;296:2046–2049. doi: 10.1126/science.1073140. [DOI] [PubMed] [Google Scholar]
- 120.McKemy D.D., Neuhausser W.M., Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 121.Yarmolinsky D.A., Peng Y., Pogorzala L.A., Rutlin M., Hoon M.A., Zuker C.S. Coding and Plasticity in the Mammalian Thermosensory System. Neuron. 2016;92:1079–1092. doi: 10.1016/j.neuron.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gavva N.R., Davis C., Lehto S.G., Rao S., Wang W., Zhu D.X. Transient Receptor Potential Melastatin 8 (TRPM8) Channels are Involved in Body Temperature Regulation. Mol. Pain. 2012;8:36. doi: 10.1186/1744-8069-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xiao R., Xu X.S. Temperature Sensation: From Molecular Thermosensors to Neural Circuits and Coding Principles. Annu. Rev. Physiol. 2021;83:205–230. doi: 10.1146/annurev-physiol-031220-095215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vriens J., Nilius B., Voets T. Peripheral thermosensation in mammals. Nat. Rev. Neurosci. 2014;15:573–589. doi: 10.1038/nrn3784. [DOI] [PubMed] [Google Scholar]
- 125.Romanovsky A.A., Almeida M.C., Garami A., Steiner A., Norman M.H., Morrison S.F., Nakamura K., Burmeister J.J., Nucci T.B. The Transient Receptor Potential Vanilloid-1 Channel in Thermoregulation: A Thermosensor It Is Not. Pharmacol. Rev. 2009;61:228–261. doi: 10.1124/pr.109.001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Basbaum A.I., Bautista D.M., Scherrer G., Julius D. Cellular and Molecular Mechanisms of Pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Craig A.D. How do you feel? Interoception: The sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 128.Wang F., Bélanger E., Côté S.L., Desrosiers P., Prescott S.A., Côté D.C., De Koninck Y. Sensory Afferents Use Different Coding Strategies for Heat and Cold. Cell Rep. 2018;23:2001–2013. doi: 10.1016/j.celrep.2018.04.065. [DOI] [PubMed] [Google Scholar]
- 129.Yang L., Dong F., Yang Q., Yang P.-F., Wu R., Wu Q.-F., Wu D., Li C.-L., Zhong Y.-Q., Lu Y.-J., et al. FGF13 Selectively Regulates Heat Nociception by Interacting with Nav1.7. Neuron. 2017;93:806–821.e9. doi: 10.1016/j.neuron.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 130.Knowlton W.M., Palkar R., Lippoldt E.K., McCoy D.D., Baluch F., Chen J., McKemy D.D. A Sensory-Labeled Line for Cold: TRPM8-Expressing Sensory Neurons Define the Cellular Basis for Cold, Cold Pain, and Cooling-Mediated Analgesia. J. Neurosci. 2013;33:2837–2848. doi: 10.1523/JNEUROSCI.1943-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Milenkovic N., Zhao W.-J., Walcher J., Albert T., Siemens J., Lewin G.R., Poulet J.F.A. A somatosensory circuit for cooling perception in mice. Nat. Neurosci. 2014;17:1560–1566. doi: 10.1038/nn.3828. [DOI] [PubMed] [Google Scholar]
- 132.Pogorzala L.A., Mishra S.K., Hoon M.A. The cellular code for mammalian thermosensation. J. Neurosci. 2013;33:5533–5541. doi: 10.1523/JNEUROSCI.5788-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dubin A.E., Patapoutian A. Nociceptors: The sensors of the pain pathway. J. Clin. Investig. 2010;120:3760–3772. doi: 10.1172/JCI42843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Campero M., Baumann T.K., Bostock H., Ochoa J.L. Human cutaneous C fibres activated by cooling, heating and menthol. J. Physiol. 2009;587:5633–5652. doi: 10.1113/jphysiol.2009.176040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hensel H., Iggo A. Analysis of cutaneous warm and cold fibres in primates. Pflug. Arch. 1971;329:1–8. doi: 10.1007/BF00586896. [DOI] [PubMed] [Google Scholar]
- 136.Iriuchijima J., Zotterman Y. The Specificity of Afferent Cutaneous C Fibres in Mammals. Acta Physiol. Scand. 1960;49:267–278. doi: 10.1111/j.1748-1716.1960.tb01952.x. [DOI] [PubMed] [Google Scholar]
- 137.Duclaux R., Kenshalo D.R. Response characteristics of cutaneous warm receptors in the monkey. J. Neurophysiol. 1980;43:1–15. doi: 10.1152/jn.1980.43.1.1. [DOI] [PubMed] [Google Scholar]
- 138.Nakamura K. Central circuitries for body temperature regulation and fever. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2011;301:R1207–R1228. doi: 10.1152/ajpregu.00109.2011. [DOI] [PubMed] [Google Scholar]
- 139.Clapham J.C. Central control of thermogenesis. Neuropharmacology. 2012;63:111–123. doi: 10.1016/j.neuropharm.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 140.Beitel R.E., Dubner R. Fatigue and adaptation in unmyelinated (C) polymodal nociceptors to mechanical and thermal stimuli applied to the monkey’s face. Brain Res. 1976;112:402–406. doi: 10.1016/0006-8993(76)90295-X. [DOI] [PubMed] [Google Scholar]
- 141.Beitel R.E., Dubner R. Response of unmyelinated (C) polymodal nociceptors to thermal stimuli applied to monkey’s face. J. Neurophysiol. 1976;39:1160–1175. doi: 10.1152/jn.1976.39.6.1160. [DOI] [PubMed] [Google Scholar]
- 142.Cuellar J.M., Manering N.A., Klukinov M., Nemenov M.I., Yeomans D.C. Thermal Nociceptive Properties of Trigeminal Afferent Neurons in Rats. Mol. Pain. 2010;6:39. doi: 10.1186/1744-8069-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Le Pichon C.E., Chesler A.T. The functional and anatomical dissection of somatosensory subpopulations using mouse genetics. Front. Neuroanat. 2014;8:21. doi: 10.3389/fnana.2014.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Viatchenko-Karpinski V., Gu J.G. Effects of cooling temperatures and low pH on membrane properties and voltage-dependent currents of rat nociceptive-like trigeminal ganglion neurons. Mol. Pain. 2018;14:1744806918814350. doi: 10.1177/1744806918814350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Madrid R., De la Pena E., Donovan-Rodríguez T., Belmonte C., Viana F. Variable Threshold of Trigeminal Cold-Thermosensitive Neurons Is Determined by a Balance between TRPM8 and Kv1 Potassium Channels. J. Neurosci. 2009;29:3120–3131. doi: 10.1523/JNEUROSCI.4778-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vriens J., Owsianik G., Hofmann T., Philipp S.E., Stab J., Chen X., Benoit M., Xue F., Janssens A., Kerselaers S., et al. TRPM3 Is a Nociceptor Channel Involved in the Detection of Noxious Heat. Neuron. 2011;70:482–494. doi: 10.1016/j.neuron.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 147.Kanda H., Gu J.G. Effects of cold temperatures on the excitability of rat trigeminal ganglion neurons that are not for cold sensing. J. Neurochem. 2015;141:532–543. doi: 10.1111/jnc.13511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kanosue K., Crawshaw L.I., Nagashima K., Yoda T. Concepts to utilize in describing thermoregulation and neurophysiological evidence for how the system works. Eur. J. Appl. Physiol. 2009;109:5–11. doi: 10.1007/s00421-009-1256-6. [DOI] [PubMed] [Google Scholar]
- 149.Chimed-Ochir O., Ando S., Murakami S., Kubo T., Ishimaru T., Fujino Y., Ikaga T. Perception of feeling cold in the bedroom and sleep quality. Nagoya J. Med. Sci. 2021;83:705–714. doi: 10.18999/nagjms.83.4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Szymusiak R. Body temperature and sleep. Handb. Clin. Neurol. 2018;156:341–351. doi: 10.1016/b978-0-444-63912-7.00020-5. [DOI] [PubMed] [Google Scholar]
- 151.Te Lindert B.H.W., van Someren E.J.W. Skin temperature, sleep, and vigilance. Handb. Clin. Neurol. 2018;156:353–365. doi: 10.1016/B978-0-444-63912-7.00021-7. [DOI] [PubMed] [Google Scholar]
- 152.Xu X., Lian Z., Shen J., Lan L., Sun Y. Environmental factors affecting sleep quality in summer: A field study in Shanghai, China. J. Therm. Biol. 2021;99:102977. doi: 10.1016/j.jtherbio.2021.102977. [DOI] [PubMed] [Google Scholar]
- 153.Harding E., Franks N.P., Wisden W. The Temperature Dependence of Sleep. Front. Neurosci. 2019;13:336. doi: 10.3389/fnins.2019.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nakamura K., Morrison S.F. A thermosensory pathway mediating heat-defense responses. Proc. Natl. Acad. Sci. USA. 2010;107:8848–8853. doi: 10.1073/pnas.0913358107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Siemens J., Kamm G.B. Cellular populations and thermosensing mechanisms of the hypothalamic thermoregulatory center. Pflügers Arch.-Eur. J. Physiol. 2018;470:809–822. doi: 10.1007/s00424-017-2101-0. [DOI] [PubMed] [Google Scholar]
- 156.Tan C.L., Knight Z.A. Regulation of Body Temperature by the Nervous System. Neuron. 2018;98:31–48. doi: 10.1016/j.neuron.2018.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gong H., Szymusiak R., King J., Steininger T., McGinty D. Sleep-related c-Fos protein expression in the preoptic hypothalamus: Effects of ambient warming. Am. J. Physiol. Integr. Comp. Physiol. 2000;279:R2079–R2088. doi: 10.1152/ajpregu.2000.279.6.R2079. [DOI] [PubMed] [Google Scholar]
- 158.Vanini G., Bassana M., Mast M., Mondino A., Cerda I., Phyle M., Chen V., Colmenero A.V., Hambrecht-Wiedbusch V.S., Mashour G.A. Activation of Preoptic GABAergic or Glutamatergic Neurons Modulates Sleep-Wake Architecture, but Not Anesthetic State Transitions. Curr. Biol. 2020;30:779–787.e4. doi: 10.1016/j.cub.2019.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kroeger D., Absi G., Gagliardi C., Bandaru S.S., Madara J.C., Ferrari L.L., Arrigoni E., Münzberg H., Scammell T.E., Saper C.B., et al. Galanin neurons in the ventrolateral preoptic area promote sleep and heat loss in mice. Nat. Commun. 2018;9:4129. doi: 10.1038/s41467-018-06590-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mondino A.A., Hambrecht-Wiedbusch V., Li D., York A.K. Glutamatergic neurons in the preoptic hypothalamus promote wakefulness, destabilize NREM sleep, suppress REM sleep, and regulate cortical dynamics. J. Neurosci. 2021;41:3462–3478. doi: 10.1523/JNEUROSCI.2718-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Srividya R., Mallick H., Kumar V. Differences in the effects of medial and lateral preoptic lesions on thermoregulation and sleep in rats. Neuroscience. 2006;139:853–864. doi: 10.1016/j.neuroscience.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 162.Lu J., Greco M.A., Shiromani P., Saper C.B. Effect of Lesions of the Ventrolateral Preoptic Nucleus on NREM and REM Sleep. J. Neurosci. 2000;20:3830–3842. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ray B., Mallick H.N., Kumar V.M. Changes in sleep–wakefulness in the medial preoptic area lesioned rats: Role of thermal preference. Behav. Brain Res. 2005;158:43–52. doi: 10.1016/j.bbr.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 164.Rivas M., Serantes D., Peña F., González J., Ferreira A., Torterolo P., Benedetto L. Role of Hypocretin in the Medial Preoptic Area in the Regulation of Sleep, Maternal Behavior and Body Temperature of Lactating Rats. Neuroscience. 2021;475:148–162. doi: 10.1016/j.neuroscience.2021.08.034. [DOI] [PubMed] [Google Scholar]
- 165.Zhao Z.-D., Yang W.Z., Gao C., Fu X., Zhang W., Zhou Q., Chen W., Ni X., Lin J.-K., Yang J., et al. A hypothalamic circuit that controls body temperature. Proc. Natl. Acad. Sci. USA. 2017;114:2042–2047. doi: 10.1073/pnas.1616255114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Csernai M., Borbély S., Kocsis K., Burka D., Fekete Z., Balogh V., Káli S., Emri Z., Barthó P. Dynamics of sleep oscillations is coupled to brain temperature on multiple scales. J. Physiol. 2019;597:4069–4086. doi: 10.1113/JP277664. [DOI] [PubMed] [Google Scholar]
- 167.Sheroziya M., Timofeev I. Moderate Cortical Cooling Eliminates Thalamocortical Silent States during Slow Oscillation. J. Neurosci. 2015;35:13006–13019. doi: 10.1523/JNEUROSCI.1359-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Marin E., Büld L., Theiss M., Sarkissian T., Roberts R.J., Turnbull R., Tamimi I.F., Pleijzier M., Laursen W.J., Drummond N., et al. Connectomics Analysis Reveals First-, Second-, and Third-Order Thermosensory and Hygrosensory Neurons in the Adult Drosophila Brain. Curr. Biol. 2020;30:3167–3182. doi: 10.1016/j.cub.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Barbagallo B., Garrity P.A. Temperature sensation in Drosophila. Curr. Opin. Neurobiol. 2015;34:8–13. doi: 10.1016/j.conb.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.