Abstract
Treatment of human spermatozoa with porins or lipopolysaccharide (LPS) increases spontaneous apoptosis in these cells. Porins and LPS were extracted from Salmonella enterica serovar Typhimurium and Pasteurella multocida and were mixed with human spermatozoa for detection of levels of apoptosis.
A relationship between genitourinary tract infections in women and impaired fertility has been postulated for some time. It has already been demonstrated that the endotoxin of Vibrio fetus, which is a common pathogen in rams and bulls 9, is able to immobilize ram and bull spermatozoa. In other studies, a factor from Escherichia coli that immobilizes human spermatozoa was isolated and identified 19. E. coli strains obtained from urinary tract and cervical swab specimen cultures produce a strong depression in the motility and viability of human spermatozoa in vitro. Teague et al. 21 showed that high concentrations of E. coli interfere with the motility of spermatozoa by the attachment of E. coli to the spermatozoa 1. It has also been demonstrated that Ureaplasma urealyticum 12 and Neisseria gonorrhoeae 13 attach themselves to spermatozoa. Gram-negative bacteria release structural components from the outer membrane into the surrounding microenvironment by both cell lysis and secretion during active growth 23. The vaginal tracts of healthy women harbor a wide variety of microbial species. The prevalence of gram-negative bacteria consisting of potentially pathogenic enterobacteria can represent a more or less frequent event.
In previous studies lipopolysaccharide (LPS), porins, and peptidoglycan fragments have been reported to be toxic for human spermatozoa 11 and may lead to reduced fertility or sterility. In recent years several research teams 3, 14, 17 demonstrated the presence of apoptosis in rodent testes. This phenomenon was found essentially in spermatogenic stages VII to XIV and was also found in the postmeiotic stages of dogfish 7. In the ejaculates of infertile men, many spermatozoa present signs of apoptosis 2.
On the basis of these considerations, the purpose of the present study was to verify the probable effects of porins and LPS in increasing the rate of naturally occurring apoptosis of spermatozoa.
Porin was extracted from Salmonella enterica serovar Typhimurium SH 5014 and Pasteurella multocida ATCC 6533 by the method of Nurminen 18. Briefly, 1 g of cell envelopes was suspended in 2% Triton X-100 in 0.01 M Tris-HCl (pH 7.5) containing 10 mM EDTA; after the addition of trypsin (10 mg/1 g of cell envelopes), the pellet was dissolved in sodium dodecyl sulfate (SDS) buffer (4% [wt/vol] in 0.1 M sodium phosphate; pH 7.2) and the solution was applied to an Ultragel ACA 34 column (Pharmacia, Uppsala, Sweden) equilibrated with 0.25% SDS buffer. The fraction containing proteins, identified by determination of the absorption at 280 nm (A280), was extensively dialyzed and checked by SDS-polyacrylamide gel electrophoresis in slabs as described by Laemmli 15. The protein content of the porin preparation was determined by the method of Lowry et al. 16. All possible traces of LPS were revealed on SDS-polyacrylamide gels stained with silver nitrate as described by Tsai and Frasch 22 and by the Limulus amoebocyte lysate assay. In some assays the LPS activity in the porins was neutralized by adding polymyxin B at room temperature for 1 h at a ratio of 1:10.
LPS was isolated by the method of Galanos et al. 10. Briefly, the extraction mixture (90 g of dry phenol plus 11 ml of water-chloroform-petroleum ether at a ratio of 2:5:8, by volume) was added to 1 g of dried bacteria. After being homogenized for 2 min, the suspension was centrifuged and the supernatant was recovered and filtered through filter paper. SDS-gel electrophoresis and subsequent staining were performed as described by Tsai and Frasch 22.
Treatment of human spermatozoa with porins or LPS induces apoptosis in these cells. We used concentrations of LPS or porin which are usually found in vivo during interactions of bacteria and host cells. Considering that the amount of porins (molecular mass, about 36,000 Da) is about 1 × 105 molecules per cell and that the amount of LPS (molecular mass, about 4,500 Da) is about 3.4 × 106 molecules per cell 5, 1 × 108 to 1 × 109 bacterial cells are enough to reach concentrations of about 0.2 to 2.0 μM porin and 0.5 to 5 μM LPS.
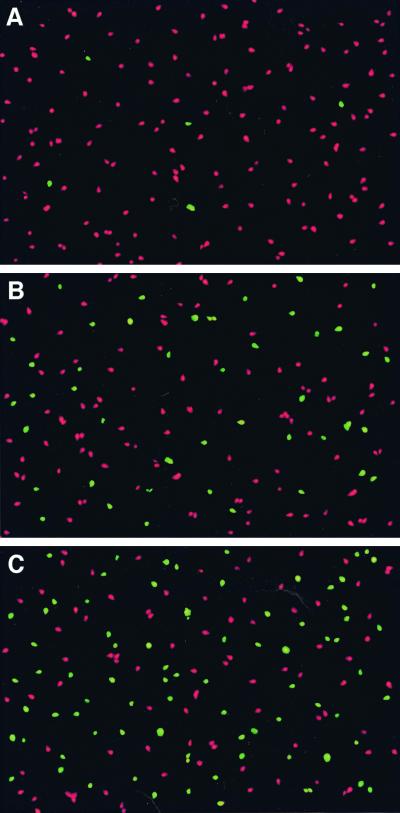
Fresh semen samples were obtained from healthy human volunteers. Aliquots (0.5 ml) of fresh ejaculate were mixed and diluted with 0.5 ml of phosphate-buffered saline (endotoxin-free; 0.5 M; pH 7.2) containing porin or LPS at different concentrations. The assays at 37°C were initiated no later than 1 h after collection of semen. DNA fragmentation in individual apoptotic spermatozoa was detected by the technique of terminal deoxynucleotidyl transferase-mediated UTP nick and labeling (TUNEL) of DNA strand breaks (fluorescein; [In Situ Cell Death Detection Kit; Roche]) according to the instructions of the manufacturer. The negative control consisted of porin- or LPS-treated spermatozoa to which deoxynucleotidiyl transferase was not added (Fig. 1). The effect of porin or LPS is dose dependent and time dependent. At porin and LPS concentrations of 0.2 and 0.5 μM, respectively, it is possible to show an increase in the level of apoptosis, while at higher concentrations (2 and 5 μM), respectively, we observed a greater increase in the level of apoptosis of the spermatozoa compared to that for controls represented by spermatozoa treated by the same protocol but to which porin or LPS was not added. After 1 h the suspensions of spermatozoa treated with 5 μM LPS already showed increases in the level of apoptosis, which reached 40% ± 10%, whereas the 2 μM porin treatment induced a 25% ± 7% increase in the level of apoptosis. The levels of apoptosis of spermatozoa treated with porins or LPS can be summarized as follows: control, 3% ± 2%; 2.0 μM porins, 25% ± 7% (P < 0.001); 2.0 μM porins plus 20 μM polymyxin B, 23% ± 7% (P < 0.001); 5.0 μM LPS, 40% ± 10% (P < 0.001); and 5.0 μM LPS plus 50 μM polymyxin B, 4% ± 2% (valves are the mean ± standard error of three separate experiments, and comparisons between tests were done by Student's t test).
FIG. 1.
Effects of porins and LPS on human spermatozoa after 1 h of incubation. (A) Control; (B) effect of porins (2 μM); (C) effect of LPS (5 μM).
A prolonged treatment of 3 h induces a greater increase in the rate of apoptosis (+20%). A fivefold increase in either the porin or the LPS concentration determines a loss of vitality of the spermatozoa due to the loss of membrane integrity 20. A prolonged treatment of 6 to 12 h shows the diminished vitality of even control spermatozoa. Preincubation of the purified porin preparation for 1 h at 20°C with polymyxin B (ratio, 1:10) 4 to neutralize contaminating LPS conserved the biological activity, while pretreatment of LPS with polymyxin B did not result in biological effects. An aspecific protein such as bovine albumin does not demonstrate any apoptotic activity on spermatozoa under the same experimental conditions. In order to verify whether the apoptotic activity demonstrated by the S serovar Typhimurium porins was specific, we also used porins (37,500 Da) extracted from P. multocida ATCC 6533 as described by Chevalier et al. 8. The purified porins behave like those of serovar Typhimurium; therefore, the apoptotic effect may be a general consequence of the porin activity.
In our tests we used concentrations of surface components from gram-negative bacteria and times of exposure of the spermatozoa to these components that can occur in vivo. The changes in the normal vaginal population, especially the decrease in the numbers of lactobacilli, with a contemporary increase in the number of gram-negative bacteria, cause only modest signs of inflammation. At the same time there may be dramatic changes in the environment as a result of the accumulation of bacterial products which compromise the vitality of the spermatozoa and increase the level of naturally occurring apoptosis.
Our results show that vaginal infections with gram-negative bacteria can lead to infertility as a consequence of the direct action of bacterial components on the viability of spermatozoa.
REFERENCES
- 1.Allen W R, Tuttle J P, Eldridge J C. The effect of E. coli on human spermatozoa cyclic adenosine 3′-5′ monophosphate. Fertil Steril. 1979;31:451–452. doi: 10.1016/s0015-0282(16)43946-4. [DOI] [PubMed] [Google Scholar]
- 2.Bacetti B, Collodel G, Piomboni P. Apoptosis in human ejaculated sperm cells (notulae seminologicae) J Submicrosc Cytol Pathol. 1996;28:587–596. [PubMed] [Google Scholar]
- 3.Billig H, Furuta I, Rivier C, Tapanainen J, Parvinen M, Hsueh A W. Apoptosis in testis germ cells: developmental changes in gonadotropin dependence and localization to selective tubule stages. Endocrinology. 1995;136:5–12. doi: 10.1210/endo.136.1.7828558. [DOI] [PubMed] [Google Scholar]
- 4.Blanchard D K, Djeun J K, Friedman J W, Friedman H, Stewart W E. Interferon-gamma induction by lipopolysaccharide: dependence on interleukin-2 and macrophages. J Immunol. 1986;136:321–379. [PubMed] [Google Scholar]
- 5.Brass J M. The cell envelop of gram-negative bacteria: new aspects of its function in transport and chemotaxis. Curr Top Microbiol Immunol. 1986;129:1–92. doi: 10.1007/978-3-642-71399-6_1. [DOI] [PubMed] [Google Scholar]
- 6.Brinkworth M H, Weinbauer G F, Schiatt S, Nieschlag E. Identification of male germ cells undergoing apoptosis in adult rats. J Reprod Fertil. 1995;105:25–33. doi: 10.1530/jrf.0.1050025. [DOI] [PubMed] [Google Scholar]
- 7.Callard G V, Jorgensen J C, Redding J M. Biochemical analysis of programmed cell death during premeiotic stages of spermatogenesis in vivo and in vitro. Dev Genetics. 1995;16:140–147. doi: 10.1002/dvg.1020160207. [DOI] [PubMed] [Google Scholar]
- 8.Chevalier G, Duclohier H, Thomas D, Shechter E, Wroblewski H. Purification or characterization of protein H, the major porin of Pasteurella multocida. J Bacteriol. 1993;175:266–276. doi: 10.1128/jb.175.1.266-276.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dennis S M. Spermicidal activity of bacterial endotoxin. Nature. 1962;195:1227–1228. doi: 10.1038/1951227b0. [DOI] [PubMed] [Google Scholar]
- 10.Galanos C, Luderitz O, Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969;9:245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 11.Galdiero F, Gorga F, Bentivoglio C, Mancuso R, Galdiero E, Tufano M A. The action of LPS porins and peptidoglycan fragments on human spermatozoa. Infection. 1988;16:6–16. doi: 10.1007/BF01644545. [DOI] [PubMed] [Google Scholar]
- 12.Gnarpe H, Friberg J. T-mycoplasma on spermatozoa and infertility. Nature. 1973;245:97–98. doi: 10.1038/245097a0. [DOI] [PubMed] [Google Scholar]
- 13.Gomez C I, Stenbach W A, James A N, Criswell B S, Williams R B. Attachment of Neisseria gonorrhoeae to human sperm. Microscopical study of effect of trypsin and iron. Br J Vener Dis. 1979;55:245–255. doi: 10.1136/sti.55.4.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hikim A P, Wang C, Leung A, Swerdloff R S. Involvement of apoptosis in the induction of germ cell degeneration in adult rats after gonadotropin releasing hormone antagonist treatment. Endocrinology. 1995;136:2770–2775. doi: 10.1210/endo.136.6.7750502. [DOI] [PubMed] [Google Scholar]
- 15.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1985;193:265–275. [PubMed] [Google Scholar]
- 17.Nantel F, Monaco L, Foulkes D, Masquilier D, Lemeur M, Henriksen K, Dirrich A, Parvinen M, Sassone-Corsi P. Spermiogenesis deficiency and germ-cell apoptosis in CREM-mutant mice. Nature. 1996;380:159–162. doi: 10.1038/380159a0. [DOI] [PubMed] [Google Scholar]
- 18.Nurminen M. Isolation of porins trimers. In: Korhonen P K, Dawes E A, Makela P H, editors. Enterobacterial surface antigens methods for molecular characterisation. Amsterdam, The Netherlands: Elsevier Science Publishers (Biomedical Division); 1985. pp. 293–300. [Google Scholar]
- 19.Paulson J D, Polakoski K L. Isolation of a spermatozoa immobilization factor from Escherichia coli. Fertil Steril. 1977;28:182–185. doi: 10.1016/s0015-0282(16)42380-0. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz L M, Osborne B A. Methods in cell biology. 46. Cell death. San Diego, Calif: Academic Press, Inc.; 1995. [Google Scholar]
- 21.Teague N S, Boyarsky S, Gleen J F. Interference of human spermatozoa motility by Escherichia coli. Fertil Steril. 1971;22:281–282. doi: 10.1016/s0015-0282(16)38220-6. [DOI] [PubMed] [Google Scholar]
- 22.Tsai C M, Frasch C E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;179:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H, Nielsen D W, Peterson J W, Klimpel G R. Lipoprotein release by bacteria: potential factor in bacterial pathogenesis. Infect Immun. 1998;66:5196–5201. doi: 10.1128/iai.66.11.5196-5201.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]