Abstract
Gilbert’s syndrome is mainly diagnosed through genetic analysis and is primarily detected through a mutation in the promoter region of the UGT1A1 gene. However, most of the research has been conducted on Caucasian populations. In this study, we studied the Han population in Taiwan to investigate the possibility of other mutations that could cause Gilbert’s syndrome. This study comprised a test group of 45 Taiwanese individuals with Gilbert’s syndrome and 180 healthy Taiwanese individuals as a control group. We extracted DNA from the blood samples and then used Axiom Genome-Wide TWB 2.0 array plates for genotyping. Out of 302,771 single nucleotide polymorphisms (SNPs) from 225 subjects, we detected 57 SNPs with the most significant shift in allele frequency; 27 SNPs among them were located in the UGT1A region. Most of the detected SNPs highly correlated with each other and are located near the first exon of UGT1A1, UGT1A3, UGT1A6, and UGT1A7. We used these SNPs as an input for the machine learning algorithms and developed prediction models. Our study reveals a good association between the 27 SNPs detected and Gilbert’s syndrome. Hence, this study provides a reference for diagnosing Gilbert’s syndrome in the Taiwanese population in the future.
Keywords: Gilbert’s syndrome, genetic factors, single nucleotide polymorphism, machine learning
1. Introduction
Gilbert’s syndrome (GS) is a hereditary disease. The most common physiological symptom is jaundice, caused by the toxic unconjugated bilirubin in the blood (hyperbilirubinemia). However, it does not cause any other abnormal liver functions [1]. Additionally, hepatic processing of drugs metabolized through glucuronidation may be affected in patients with GS, including acetaminophen, nonsteroidal inflammatory drugs, statins, gemfibrozil, human immunodeficiency virus protease inhibitors (indinavir and atazanavir), sorafenib, and irinotecan (CPT-11). It has been found that unconjugated bilirubin has a protective effect against cardiovascular disease, diabetes, and metabolic syndrome [2]. This can be primarily attributed to its antioxidant action and anti-inflammatory properties. However, the risk of GS associated with the neoplastic disease is controversial in several tumors [2]. Bilirubin is a waste product of heme catabolism [3]. The breakdown of hemoglobin releases heme, which is transformed into unconjugated (indirect) bilirubin. The unconjugated bilirubin is generally bound to glucuronic acid, in the liver, by the enzyme uridine diphosphoglucuronate glucuronosyltransferase (UGT) [4]. This enzyme converts the toxic form of bilirubin (unconjugated bilirubin) to its nontoxic form (conjugated bilirubin) and is encoded by the UGT1A1 gene [5]. Conjugated (direct) bilirubin, which is water-soluble, passes from the liver to the gallbladder, where it is mixed with other constituents of bile and finally enters the small intestine [5,6]. Only a small fraction of it is transported into the kidneys and excreted with urine [7]. This contributes to the formation of tea or cola-colored urine and brown-colored stool [8]. However, if the UGT1A1 gene mutates, it causes GS [9,10,11]. Changes in the promoter region of the UGT1A1 gene lead to the dysfunction of the UGT enzyme [12,13]. When UGT enzyme dysfunctions, unconjugated bilirubin is not transformed into conjugated bilirubin. High levels of unconjugated bilirubin in the blood lead to jaundice, characterized by yellow discoloration of the skin and jaundice [14]. Caucasian and African American populations with GS are usually characterized by the homozygous form of the UGT1A1*28 allele (rs34983651), which is a homozygous 2-bp insertion (genotype (TA)7TAA/(TA)7TAA) mutation of the TATA box promoter region of the UGT1A1 gene, resulting in higher concentrations of serum bilirubin [9,12,13,15,16,17]. Recent studies have shown that GS may be associated with UGT1A7 as well [18]. To examine the genotypes of GS in the population of Taiwan, we used the Axiom Genome-Wide TWB2.0 array based on the genotyping data of the Taiwanese subjects [19].
2. Results
2.1. Clinical Characteristics
A total of 225 subjects were recruited for this study from August 2013 to April 2021. There were 45 patients with GS and 180 healthy controls. The patients’ GS diagnoses are based on clinical characteristics, with more details provided in Study Subjects. The level of total bilirubin in patients with GS was 1.5–2.8 mg/dL, and the ratio of direct bilirubin to total bilirubin ranged between 0.06–0.19. In comparison, in the healthy controls, the level of total bilirubin was 0.2–0.8 mg/dL, and the ratio of direct bilirubin to total bilirubin ranged between 0.5–1. The demographic and clinical baseline characteristics of the subjects are presented in Table 1. As we know, no significant differences were observed for the other clinical variables.
Table 1.
Baseline characteristics of 225 subjects.
| Healthy Control n = 180 |
Gilbert’s Syndrome n = 45 |
p-Value | |
|---|---|---|---|
| Age, years | 60.45 ± 14.90 | 67.49 ± 13.99 | 0.005 |
| Female, n (%) | 68 (37.8%) | 17 (37.8%) | 1 |
| T_Bilirubin 1 (mg/dL) | 0.63 ± 0.24 | 1.73 ± 0.30 | <0.001 |
| D_Bilirubin 2 (mg/dL) | 0.22 ± 0.08 | 0.22 ± 0.06 | 0.719 |
| D_Bilirubin/T_Bilirubin | 37.82 ± 12.63 | 12.78 ± 3.08 | <0.001 |
| Anemia, n (%) | 11 (6.1%) | 2 (4.4%) | 0.668 |
| WBC 3 (×103/μL) | 6.11 ± 2.64 | 6.17 ± 1.95 | 0.885 |
| RBC 4 (×103/μL) | 4.8 ± 0.60 | 4.86 ± 0.49 | 0.513 |
| Hemoglobin (g/dL) | 14.08 ± 1.42 | 14.3 ± 1.69 | 0.356 |
| Hematocrit (%) | 41.91 ± 3.69 | 42.16 ± 4.07 | 0.689 |
| MCV 5 (fL) | 88.04 ± 7.48 | 87.2 ± 8.14 | 0.504 |
| MCH 6 (pg) | 29.58 ± 2.91 | 29.57 ± 3.34 | 0.993 |
| MCHC 7 (g/dl) | 33.55 ± 1.03 | 33.86 ± 1.22 | 0.092 |
| RDW 8 (%) | 13.39 ± 1.03 | 13.58 ± 1.63 | 0.33 |
| Platelets (×103/μL) | 244.28 ± 62.13 | 244.18 ± 62.05 | 0.992 |
| Transferrin (ng/mL) | 245.36 ± 34.75 | 235.14 ± 35.12 | 0.08 |
1 T_Bilirubin: total bilirubin; 2 D_Bilirubin: direct bilirubin; 3 WBC: white blood cells; 4 RBC: red blood cells; 5 MCV: mean corpuscular volume; 6 MCH: mean corpuscular hemoglobin; 7 MCHC: mean corpuscular hemoglobin concentration; 8 RDW: red blood cell distribution width.
2.2. Genetic Variants Associated with GS
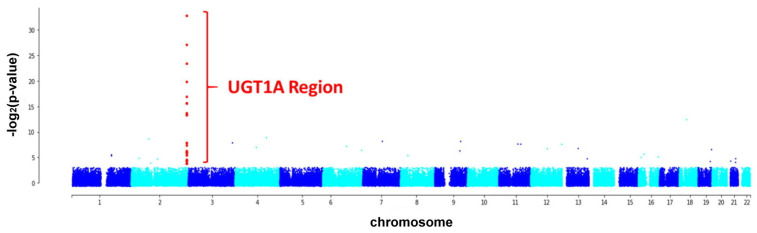
By analyzing the whole-genome SNPs, we found 57 SNPs whose allele frequency variations differed significantly between the test and control groups (p < 0.05, Table S1). Moreover, 27 out of the 57 SNPs were located in the UGT1A gene, and the difference was highly significant (Figure 1).
Figure 1.
Manhattan plot visualizing the p-values of the allele frequency changes among the subjects with Gilbert’s syndrome (test group) and healthy individuals (control group). Most of the significant single nucleotide polymorphisms (SNPs) are located in the UGT1A region (chr2:233617645–233773305).
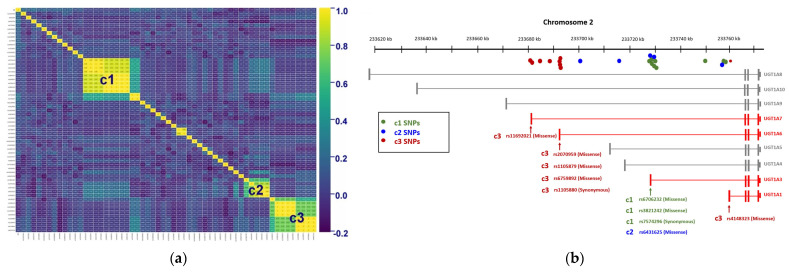
We used the correlation matrix to represent the correlation between these 57 significant SNPs and found that three clusters (c1, c2, and c3) had a high correlation with each other (Figure 2a). The average correlation coefficient of c1 SNPs (n = 9) was 0.791, c2 SNPs (n = 5) was 0.786, and c3 SNPs (n = 9) was 0.804. These three clusters contained SNPs in the same location, the UGT1A region of the genome (Table 2). The UGT1A region includes nine genes: UGT1A1, UGT1A3, UGT1A4, UGT1A5, UGT1A6, UGT1A7, UGT1A9, UGT1A10, and UGT1A8 (in descending order of gene length). Figure 2b shows that the SNPs in the UGT1A region gather around the first exon of the genes UGT1A1, UGT1A3, UGT1A6, and UGT1A7. Hence, the SNPs located near the 5′ end of these genes might be the cause of GS.
Figure 2.
(a) Correlation matrix of 57 significant SNPs (p < 0.05). There are three clusters (c1, c2, and c3) of SNPs that are highly correlated with each other (r > 0.6). All clusters are located in the UGT1A region. (b) The graph shows the distribution of SNPs in the UGT1A region. The three clusters in the correlation matrix are represented by green (c1), blue (c2), and red (c3). Most c1 and c2 SNPs are clustered near the first exon of UGT1A3, and three of them belong to missense variants. Except for rs4148323, which is the missense variant of UGT1A1, the other c3 SNPs are concentrated between the first exon of UGT1A7 and the first exon of UGT1A6, and most of them are missense variants of UGT1A7 or UGT1A6.
Table 2.
The information on SNPs among these tree clusterings which having a high correlation with each other.
| Clustering | SNP | Chromosome | Position (bp) | p-Value | Location |
|---|---|---|---|---|---|
| c1 | rs3755319 | 2 | 233,758,936 | 0.003031869 | UGT1A region: Intronic Variant |
| rs4124874 | 2 | 233,757,013 | 0.005113795 | UGT1A region: Intronic Variant | |
| rs2221198 | 2 | 233,749,977 | 0.00333 | UGT1A region: Intronic Variant | |
| rs7574296 | 2 | 233,729,603 | 0.002707358 | UGT1A3: Synonymous Variant | |
| rs3806597 | 2 | 233,728,923 | 0.026915457 | UGT1A region: Intronic Variant | |
| rs2008595 | 2 | 233,728,546 | 0.026915457 | UGT1A region: Intronic Variant | |
| rs6706232 | 2 | 233,729,207 | 0.0152489 | UGT1A3: Missense Variant | |
| rs3806596 | 2 | 233,729,061 | 0.015291563 | UGT1A region: Intronic Variant | |
| rs3821242 | 2 | 233,729,157 | 0.012727593 | UGT1A3: Missense Variant | |
| c2 | rs17863787 | 2 | 233,702,448 | 0.002450485 | UGT1A region: Intronic Variant |
| rs1976391 | 2 | 233,757,337 | 6.66 × 10−15 | UGT1A region: Intronic Variant | |
| rs1875263 | 2 | 233,716,976 | 0.000759234 | UGT1A region: Intronic Variant | |
| rs6431625 | 2 | 233,729,266 | 1.99 × 10−12 | UGT1A3: Missense Variant | |
| rs1983023 | 2 | 233,728,376 | 8.00 × 10−11 | UGT1A region: Intronic Variant | |
| c3 | rs4148323 | 2 | 233,760,498 | 0.00000151 | UGT1A1: Missense Variant |
| rs7586110 | 2 | 233,681,881 | 0.000000195 | UGT1A region: Intronic Variant | |
| rs10168416 | 2 | 233,688,441 | 0.000000195 | UGT1A region: Intronic Variant | |
| rs11692021 | 2 | 233,682,559 | 5.39 × 10−8 | UGT1A7: Missense Variant | |
| rs2070959 | 2 | 233,693,545 | 0.000000195 | UGT1A6: Missense Variant | |
| rs1105880 | 2 | 233,693,319 | 0.00000193 | UGT1A6: Synonymous Variant | |
| rs1105879 | 2 | 233,693,556 | 0.00000193 | UGT1A6: Missense Variant | |
| rs6759892 | 2 | 233,693,023 | 0.00000193 | UGT1A6: Missense Variant | |
| rs4261716 | 2 | 233,684,471 | 0.00000133 | UGT1A region: Intronic Variant |
2.3. Machine Learning
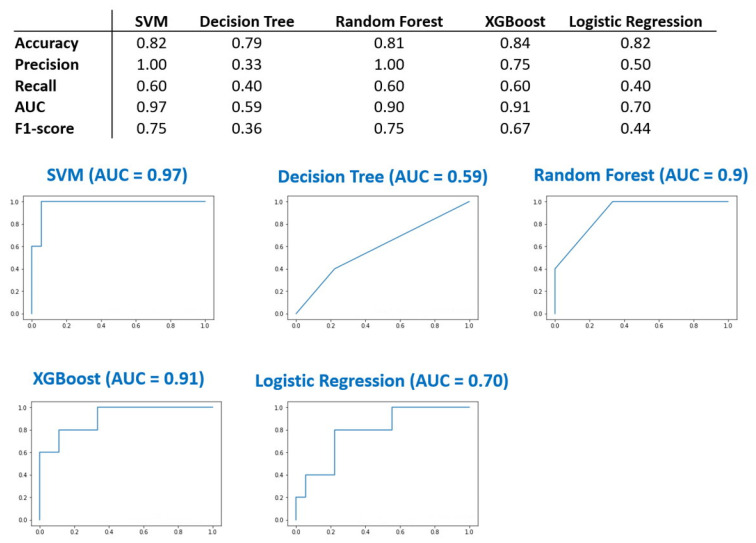
The above results showed that the 27 SNPs located in the UGT1A region exhibit significant differences in the test subjects with GS and are highly correlated with each other. We used these 27 SNPs as input variables for the machine learning models. The prediction models for GS were developed using the algorithms SVM, Random Forest, Logistic Regression, Decision Tree, and XGBoost. SVM and XGBoost perform better in terms of accuracy rate and AUC (Figure 3). SVM and XGBoost displayed an accuracy rate of 0.82 and 0.84, respectively. The AUC of the SVM and XGBoost models were 0.9 and 0.91, respectively. The data demonstrate that these 27 SNPs are a reliable marker in the diagnosis of GS.
Figure 3.
27 SNP signatures were used to build predictive models via five different machine learning methods. Support Vector Machine (SVM), Random Forest, and XGBoost showed better performance of accuracy and area under the receiver operating characteristic (ROC) curve (AUC).
2.4. Limitations
Our study has several limitations. First, only a few subjects were available for analysis. Gilbert’s syndrome is a rare disease and a hereditary genetic disorder. In order to collect more information from the limited number of probes in the SNP array and small sample size, we hope to use whole genome sequencing (WGS) to obtain more SNPs information in the future. Second, validation cohorts of other races or places of residence may be warranted for evaluation in the future. Finally, the current study could not assess clinical events such as drug interactions, cardiovascular disease, diabetes, metabolic syndrome, and cancer outcomes. Therefore, we could not determine a GS-related effect in the above-mentioned clinical events. Moreover, Gilbert syndrome usually is diagnosed until puberty or later. We will design our research in the direction of cohort and longitudinal study so that we may also develop models to predict the opportunity to have Gilbert’s syndrome from childhood.
3. Discussion
We have identified novel biomarkers for the diagnosis of GS through this study. For genetic diagnosis, UGT1A1*28 mutations have been established as a diagnostic marker for GS [15,20]. The genotype (TA)7/(TA)7 insertion in the SNP rs34983651 in the promoter region of UGT1A1 gene (chr2:233760233) promotes the conversion of (TA)6TAA into (TA)7TAA, which impacts UGT1A1 activity and causes the mutations [12].
However, for the Taiwanese Nationwide Cohort examined in this study, we found that UGT1A1*28 or rs34983651 might not be the leading cause of GS. The genome-wide association study shows that although the mutations occur in the UGT1A region, the 27 SNPs identified in this study are positioned around the first exon of the genes UGT1A1, UGT1A3, UGT1A6, and UGT1A7. Studies have reported that mutations in the UGT1A1 region are frequently associated with mutations in UGT1A6, UGT1A3, and UGT1A7 [21,22,23]. This study has identified mutant hotspots in UGTs with greater precision. Out of the 27 SNPs, 8 (rs6706232, rs3821242, rs6431625, rs4148323, rs11692021, rs2070959, rs1105879, and rs6759892) belong to missense variants (Table 2), implying that these 8 SNPs directly affect the activity of these proteins. We used the allele frequencies of these 27 SNPs as the input for the machine learning algorithms to develop predictive models, and the resultant AUC was more than 90%. Since these SNPs have not been reported to be related to GS, we have developed reference data for the diagnosis of GS in the Taiwanese population.
4. Materials and Methods
4.1. Study Subjects
From August 2013 to April 2021, 225 prospective participants were recruited from the Northeastern Taiwan Community Medicine Research Cohort (NTCMRC, ClinicalTrials.gov Identifier: NCT04839796). They were then enrolled and examined in the gastroenterology clinic of the Community Medicine Research Center of Chang Gung Memorial Hospital, Keelung Branch. The participants were divided into 2 groups. The test group comprised 45 subjects with GS and the control group with 180 healthy subjects without GS, and male to female ratio (approx. 1.6:1) was the same in both groups. All subjects underwent clinical examination, blood tests, and assessment of complete individual medical histories. GS was ascertained at total bilirubin >1.4 mg/dL, the ratio of direct bilirubin to total bilirubin was <0.2, and significant jaundice symptoms were also observed, but the levels of glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT) were in the normal range for the liver function tests. In addition, we did not find any abnormalities of the liver, gallbladder, pancreas, spleen, or kidneys or past medical records of chronic hepatitis. Informed consent was provided by all participants. This study conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Institutional Review Board of the Chang Gung Medical Foundation (IRB No: 201800802B0 and 202000077B0A3).
4.2. Clinical Assessment
Personal medical history and questionnaires were collected from 225 participants during recruitment. In addition, all of them were subjected to physical examination and biochemical tests for preliminary clinical evaluation.
4.3. DNA Extraction from White Blood Cells
Blood samples were collected in citrate-treated tubes and centrifuged at 3000 rpm for 10 min at 4 °C to separate the serum from the cells. Next, the erythrocytes were lysed prior to the phenol/chloroform-based (Sigma Aldrich, St. Louis, MO, USA) DNA extraction process. Lastly, we used 95% isopropanol (J.T. Baker Inc., Philadelphia, PA, USA), followed by 80% ethanol (J.T. Baker Inc., Philadelphia, PA, USA), to obtain the total genomic DNA.
4.4. Whole-Genome Single Nucleotide Polymorphism (SNP) Analysis
We used the Axiom Genome-Wide TWB 2.0 array plates (Thermo Fisher Scientific Inc., Waltham, MA, USA) for the genotyping of genomic DNA samples from the 225 subjects, which is the only means of identifying SNPs in the Taiwanese population, containing 681,796 SNPs. The SNPs whose minor allele frequency was zero and those with more than 10% missing rate were removed. The remaining 302,771 SNPs out of the total 681,796 SNPs were further analyzed.
4.5. Correlation Heatmap and Machine Learning
The genotype was converted to numerals based on the SNP data. The reference for homozygous was considered at 1, for heterozygous at 1.5, and for homozygous alternate at 2. Then, we used the programming language Python 3.8 with package scikit-learn 1.0.2 to analyze the converted data and plotted a correlation heatmap. In addition, we used the scikit-learn 1.0.2 package to develop five different machine-learning models using Support Vector Machine (SVM), Random Forest, Logistic Regression, Decision Tree, and XGBoost and calculated their accuracy, precision, recall, area under the receiver operating characteristic (ROC) curve (AUC) and F1-score to evaluate the performance of the models.
5. Conclusions
Our study reveals that the 27 SNPs play an important role in the Taiwanese population with Gilbert’s syndrome, which differs from the currently known UGT1A1*28 allele (rs34983651) as the basis of diagnosis for Gilbert’s syndrome in other populations. Hence, this study provides a reference for diagnosing Gilbert’s syndrome in the Taiwanese population in the future.
Acknowledgments
We thank the Core Lab of Community Medicine Research Center, Chang Gung Memorial Hospital, Keelung branch, the National Center for Genome Medicine, Academia Sinica, Huey-Kang Sytwu and Y. Henry Sun for scientific communications and assistance.
Abbreviations
| GS | Gilbert’s syndrome |
| UGT | uridine diphosphoglucuronate glucuronosyltransferase |
| GOT | glutamic oxaloacetic transaminase |
| GPT | glutamic pyruvic transaminase |
| SNP | single nucleotide polymorphism |
| SVM | Support Vector Machine |
| ROC | receiver operating characteristic |
| AUC | area under the ROC curve. |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms232012709/s1.
Author Contributions
Conceptualization, P.W.-C.H., Y.-C.S. and C.-L.L.; methodology, P.W.-C.H., Y.-C.S., P.-C.L., X.-Y.L. and Y.-H.K.; validation, P.W.-C.H., P.-C.L., X.-Y.L. and Y.-H.K.; formal analysis, Y.-H.K.; investigation, Y.-C.S. and Y.-H.K.; resources, R.-N.C., C.-T.Y., C.-C.L., Y.-C.S. and C.-L.L.; data curation, Y.-H.K., Y.-C.S. and C.-L.L.; writing—original draft preparation P.W.-C.H. and Y.-C.S.; writing—review and editing, R.-N.C., C.-T.Y., C.-C.L., Y.-C.S. and C.-L.L.; visualization, P.W.-C.H., Y.-C.S., P.-C.L., X.-Y.L. and Y.-H.K.; supervision, C.-C.L., Y.-C.S. and C.-L.L.; project administration, C.-C.L., Y.-C.S. and C.-L.L.; funding acquisition, C.-C.L., Y.-C.S. and C.-L.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Chang Gung Medical Foundation (protocol code 201800289A3/27 August 2021; 201600379B0/7 May 2021; and 201800271B0/11 April 2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
Data available on request due to restrictions, e.g., privacy or ethics. The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the Informed Consent Statement.
Conflicts of Interest
The authors declare no conflict of interest.
The Animal Study Protocol
Not applicable.
Clinical Trial Registration
Northeastern Taiwan Community Medicine Research Cohort (NTCMRC, ClinicalTrials.gov Identifier: NCT04839796).
Funding Statement
This research was funded by Chang Gung Memorial Hospital Research Project Grants number CLRPG2L0052, CRRPG2H0041-4, CMRPG2K0141-2, CMRPG2J0011, CMRPG2L0311, and CRRPG2F0011-2; and Ministry of Science and Technology (MOST) grant number Most109-2311-B182A-001.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arias I.M. Gilbert’s syndrome. Br. Med. J. 1968;2:702. doi: 10.1136/bmj.2.5606.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erlinger S., Arias I.M., Dhumeaux D. Inherited disorders of bilirubin transport and conjugation: New insights into molecular mechanisms and consequences. Gastroenterology. 2014;146:1625–1638. doi: 10.1053/j.gastro.2014.03.047. [DOI] [PubMed] [Google Scholar]
- 3.Vitek L., Schwertner H.A. The heme catabolic pathway and its protective effects on oxidative stress-mediated diseases. Adv. Clin. Chem. 2007;43:1–57. doi: 10.1016/s0065-2423(06)43001-8. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh S.S., Sappal B.S., Kalpana G.V., Lee S.W., Chowdhury J.R., Chowdhury N.R. Homodimerization of human bilirubin-uridine-diphosphoglucuronate glucuronosyltransferase-1 (UGT1A1) and its functional implications. J. Biol. Chem. 2001;276:42108–42115. doi: 10.1074/jbc.M106742200. [DOI] [PubMed] [Google Scholar]
- 5.Fevery J. Bilirubin in clinical practice: A review. Liver Int. 2008;28:592–605. doi: 10.1111/j.1478-3231.2008.01716.x. [DOI] [PubMed] [Google Scholar]
- 6.Tiribelli C., Ostrow J.D. Intestinal flora and bilirubin. J. Hepatol. 2005;42:170–172. doi: 10.1016/j.jhep.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Gollan J.L., Dallinger K.J., Billing B.H. Excretion of conjugated bilirubin in the isolated perfused rat kidney. Clin. Sci. Mol. Med. 1978;54:381–389. doi: 10.1042/cs0540381. [DOI] [PubMed] [Google Scholar]
- 8.Hoilat G.J., John S. BTI-StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2022. Bilirubinuria. [Google Scholar]
- 9.Rodrigues C., Vieira E., Santos R., de Carvalho J., Santos-Silva A., Costa E., Bronze-da-Rocha E. Impact of UGT1A1 gene variants on total bilirubin levels in Gilbert syndrome patients and in healthy subjects. Blood Cells Mol. Dis. 2012;48:166–172. doi: 10.1016/j.bcmd.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Shi X., Aronson S., Khan A.S., Bosma P.J. A novel UGT1A1 gene mutation causing severe unconjugated hyperbilirubinemia: A case report. BMC Pediatr. 2019;19:173. doi: 10.1186/s12887-019-1555-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agrawal S.K., Kumar P., Rathi R., Sharma N., Das R., Prasad R., Narang A. UGT1A1 gene polymorphisms in North Indian neonates presenting with unconjugated hyperbilirubinemia. Pediatr. Res. 2009;65:675–680. doi: 10.1203/PDR.0b013e31819ed5de. [DOI] [PubMed] [Google Scholar]
- 12.Ostanek B., Furlan D., Mavec T., Lukac-Bajalo J. UGT1A1(TA)n promoter polymorphism--a new case of a (TA)8 allele in Caucasians. Blood Cells Mol. Dis. 2007;38:78–82. doi: 10.1016/j.bcmd.2006.10.160. [DOI] [PubMed] [Google Scholar]
- 13.Raijmakers M.T., Jansen P.L., Steegers E.A., Peters W.H. Association of human liver bilirubin UDP-glucuronyltransferase activity with a polymorphism in the promoter region of the UGT1A1 gene. J. Hepatol. 2000;33:348–351. doi: 10.1016/S0168-8278(00)80268-8. [DOI] [PubMed] [Google Scholar]
- 14.Fargo M.V., Grogan S.P., Saguil A. Evaluation of Jaundice in Adults. Am. Fam. Physician. 2017;95:164–168. [PubMed] [Google Scholar]
- 15.Gil J., Sasiadek M.M. Gilbert syndrome: The UGT1A1*28 promoter polymorphism as a biomarker of multifactorial diseases and drug metabolism. Biomark. Med. 2012;6:223–230. doi: 10.2217/bmm.12.4. [DOI] [PubMed] [Google Scholar]
- 16.Bosma P.J., Chowdhury J.R., Bakker C., Gantla S., de Boer A., Oostra B.A., Lindhout D., Tytgat G.N., Jansen P.L., Oude Elferink R.P., et al. The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert’s syndrome. N. Engl. J. Med. 1995;333:1171–1175. doi: 10.1056/NEJM199511023331802. [DOI] [PubMed] [Google Scholar]
- 17.Bosma P., Chowdhury J.R., Jansen P.H. Genetic inheritance of Gilbert’s syndrome. Lancet. 1995;346:314–315. doi: 10.1016/S0140-6736(95)92203-2. [DOI] [PubMed] [Google Scholar]
- 18.Teng H.C., Huang M.J., Tang K.S., Yang S.S., Tseng C.S., Huang C.S. Combined UGT1A1 and UGT1A7 variant alleles are associated with increased risk of Gilbert’s syndrome in Taiwanese adults. Clin. Genet. 2007;72:321–328. doi: 10.1111/j.1399-0004.2007.00873.x. [DOI] [PubMed] [Google Scholar]
- 19.Wei C.Y., Yang J.H., Yeh E.C., Tsai M.F., Kao H.J., Lo C.Z., Chang L.P., Lin W.J., Hsieh F.J., Belsare S., et al. Genetic profiles of 103,106 individuals in the Taiwan Biobank provide insights into the health and history of Han Chinese. NPJ Genom. Med. 2021;6:10. doi: 10.1038/s41525-021-00178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wagner K.H., Shiels R.G., Lang C.A., Seyed Khoei N., Bulmer A.C. Diagnostic criteria and contributors to Gilbert’s syndrome. Crit. Rev. Clin. Lab. Sci. 2018;55:129–139. doi: 10.1080/10408363.2018.1428526. [DOI] [PubMed] [Google Scholar]
- 21.Urawa N., Kobayashi Y., Araki J., Sugimoto R., Iwasa M., Kaito M., Adachi Y. Linkage disequilibrium of UGT1A1*6 and UGT1A1*28 in relation to UGT1A6 and UGT1A7 polymorphisms. Oncol. Rep. 2006;16:801–806. doi: 10.3892/or.16.4.801. [DOI] [PubMed] [Google Scholar]
- 22.Ramirez J., Mirkov S., House L.K., Ratain M.J. Glucuronidation of OTS167 in Humans Is Catalyzed by UDP-Glucuronosyltransferases UGT1A1, UGT1A3, UGT1A8, and UGT1A10. Drug Metab. Dispos. 2015;43:928–935. doi: 10.1124/dmd.115.063271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peters W.H., te Morsche R.H., Roelofs H.M. Combined polymorphisms in UDP-glucuronosyltransferases 1A1 and 1A6: Implications for patients with Gilbert’s syndrome. J. Hepatol. 2003;38:3–8. doi: 10.1016/S0168-8278(02)00306-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available on request due to restrictions, e.g., privacy or ethics. The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the Informed Consent Statement.