Abstract
Carotid atherosclerosis represents a relevant healthcare problem, since unstable plaques are responsible for approximately 15% of neurologic events, namely transient ischemic attack and stroke. Although statins treatment has proven effective in reducing LDL-cholesterol and the onset of acute clinical events, a residual risk may persist suggesting the need for the detection of reliable molecular markers useful for the identification of patients at higher risk regardless of optimal medical therapy. In this regard, several lines of evidence show a relationship among specific biologically active plasma lipids, atherosclerosis, and acute clinical events. We performed a Selected Reaction Monitoring-based High Performance Liquid Chromatography-tandem Mass Spectrometry (SRM-based HPLC-MS/MS) analysis on plasma HDL, LDL, and VLDL fractions purified, by isopycnic salt gradient ultracentrifugation, from twenty-eight patients undergoing carotid endarterectomy, having either a “hard” or a “soft” plaque, with the aim of characterizing the specific lipidomic patterns associated with features of carotid plaque instability. One hundred and thirty lipid species encompassing different lipid (sub)classes were monitored. Supervised multivariate analysis showed that lipids belonging to phosphatidylethanolamine (PE), sphingomyelin (SM), and diacylglycerol (DG) classes mostly contribute to discrimination within each lipoprotein fraction according to the plaque typology. Differential analysis evidenced a significant dysregulation of LDL PE (38:6), SM (32:1), and SM (32:2) between the two groups of patients (adj. p-value threshold = 0.05 and log2FC ≥ |0.58|). Using this approach, some LDL-associated markers of plaque vulnerability have been identified, in line with the current knowledge of the key roles of these phospholipids in lipoprotein metabolism and cardiovascular disease. This proof-of-concept study reports promising results, showing that lipoprotein lipidomics may present a valuable approach for identifying new biomarkers of potential clinical relevance.
Keywords: carotid atherosclerosis, plaque vulnerability, lipoproteins, targeted lipidomics, sphingomyelin, phosphatidylethanolamine
1. Introduction
Atherothrombosis resulting from carotid plaque rupture/erosion is the main contributor to major acute clinical events (https://vizhub.healthdata.org/gbd-compare/ (accessed on 5 October 2022)) including stroke, which represents the second largest cause of mortality and the third largest cause of disability globally, being responsible for 11.59% of total deaths and 5.65% of total disability-adjusted life years (DALYs), respectively.
Although high LDL-cholesterol levels and low HDL-cholesterol levels are well-established risk factors for cardiovascular disease (CVD), several lines of evidence indicate that a residual risk exists in patients who do not fully benefit from statin treatment, suggesting the need to identify novel markers of plaque development and evolution toward instability, useful for selecting the most appropriate patient-centered therapy [1,2]. In the past twenty years, large-scale MS/MS-based technologies have been applied to purified lipoprotein fractions for unravelling their specific protein cargos, in association with CVD and some CVD-associated pathological conditions including kidney disease and type 1 and 2 diabetes mellitus (an updated list of references with the main results can be found in Supplementary Material 1 of [3]). As far as our research group is concerned, we applied shotgun proteomics to the analysis of plasma LDL and HDL, identifying some novel lipoprotein-associated proteins and showing specific signatures for atherosclerotic patients with different types of carotid plaque [3,4], sorted by ultrasonography into hypoechoic (types 1 and 2) or “soft” and hyperechoic (types 3, 4, and 5) or “hard”, according to Gray–Weale classification [5]. Indeed, the improvement of imaging techniques has allowed routine characterization and detection of the features of carotid plaque vulnerability, also providing predictive information in both symptomatic and asymptomatic carotid artery stenosis [6,7,8,9].
Although these studies have proven to be very informative about the numerous functions of each lipoprotein class in relation to CVD, additional information for risk evaluation of acute clinical events onset is currently still missing. Indeed, the multiple biological functions of lipoproteins, particularly of HDL, result from both protein and lipid components, whose alterations are responsible for dysfunctional particles [10].
In the last years, plasma lipidomics have been gaining momentum, as several lines of evidence have shown a relationship among specific plasma lipid species, atherosclerosis [11,12,13,14,15,16,17,18], and the onset of adverse clinical events [19,20,21,22,23,24]. However, only few studies on the association between biologically active lipids, specifically associated with their lipoprotein carriers, and CVD have been reported so far, as also recently reviewed by Ding and Rexrode [25]. This is probably due, at least in part, to the difficulties in applying time-consuming lipoprotein purification procedures requiring specific expertise to large scale studies. Most of the published studies dealt with HDL showing alterations of the phospho- and sphingo-lipidomes in type 1 diabetes [26], type 2 diabetes [27,28], obesity and metabolic syndrome [29,30], dyslipidemia [31,32], and experimental atherosclerosis [33,34]. Some of them reported normalization of the HDL lipidome in metabolic syndrome following either Pitavastatin treatment [35] or weight loss and physical activity [36]. In addition, changes in the LDL lipidome and a consequent reduction of cardiovascular risk were obtained with statin treatment [37] or following phytosterol and omega-3 diet supplementation [38]. The HDL and LDL lipidomes were also analyzed in relation to coronary artery disease (CAD) and acute coronary syndrome (ACS) [39,40]. Furthermore, the HDL phosphosphingolipidome was analyzed in a rare population of subjects with premature CAD having high HDL-cholesterol levels, evidencing distinct signatures with respect to healthy subjects [41]. Very recently, Wang and coworkers developed high-resolution methods to study the relationship between proteome and lipidome of lipoproteins that applied to the analysis of HDL from high-fat, high-cholesterol diet-fed rabbits and ACS patients [42]. Using this approach, they showed that the combined features obtained allowed for better discriminating ACS from healthy individuals than direct proteome and/or lipidome quantification alone.
This study aims to characterize the specific lipidomic profiles of plasma HDL, LDL, and VLDL fractions purified by isopycnic salt gradient ultracentrifugation from atherosclerotic patients undergoing carotid endarterectomy. By applying SRM-based HPLC-MS/MS to the analysis of 130 lipid species, some specific LDL-associated markers of potential clinical relevance were found dysregulated in association with echographic features of carotid plaque vulnerability.
2. Results
2.1. Lipoproteins Purification
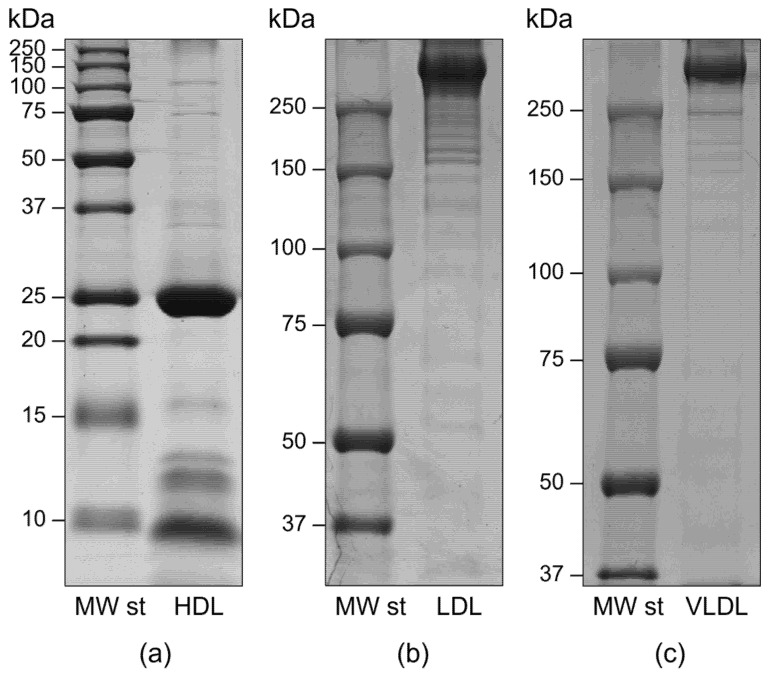
Besides some alternative purification methods such as free solution isotachophoresis and immunoaffinity or size exclusion chromatography, ultracentrifugation in high-salt media represents the most widely used approach [3]. Indeed, the different lipoprotein classes have been historically classified according to their buoyant density. We performed isopycnic salt gradient ultracentrifugation followed by a further step of fraction flotation by high centrifugal fields to obtain highly purified HDL, LDL, and VLDL fractions, as assessed by SDS-PAGE analysis [3,4]. In particular, the electrophoretic profiles of each fraction did not show any gross contamination by plasma proteins, particularly albumin. As expected, apolipoprotein B100 was the most abundant protein in both VLDL and LDL fractions, whereas apolipoprotein AI represented over 80% of HDL apolipoproteins. No HDL contamination by Apo B100-containing lipoproteins was evidenced, as demonstrated by the absence of Apo B100 at the top of the HDL lane (Figure 1).
Figure 1.
Representative mono-dimensional profiles of HDL (a), LDL (b), and VLDL (c) fractions purified by isopycnic salt gradient ultracentrifugation. Apolipoprotein profiles were obtained by SDS-PAGE in either 12% T (for HDL, under reducing conditions) or 6% T (for both LDL and VLDL, under non-reducing conditions) resolving gels.
2.2. Targeted Lipidomics
Targeted lipidomics were performed using a SRM-based HPLC-MS/MS method on HDL, LDL and VLDL lipids fractions extracted with 2:1 chloroform-methanol (v/v) following the Folch procedure [43]. Parameters used for SRM analysis are reported in Supplementary Table S1. Using this approach, one hundred and thirty lipid species belonging to cholesteryl ester (CE), ceramide (Cer), phosphatidylcholine (PC), phosphatidylethanolamine (PE), lysophosphatidylcholine (LPC), lysophosphatidylethanolamine (LPE), sphingomyelin (SM), triacylglycerol (TG), and diacylglycerol (DG) (sub)classes were compared (Supplementary Materials, Table S2). Quality control and data visualization were performed to assess total lipid content in samples before and after normalization, distribution for each lipid (sub)class, and variation coefficients (CV%) for single lipid species, as reported in Supplementary Materials (Figure S1).
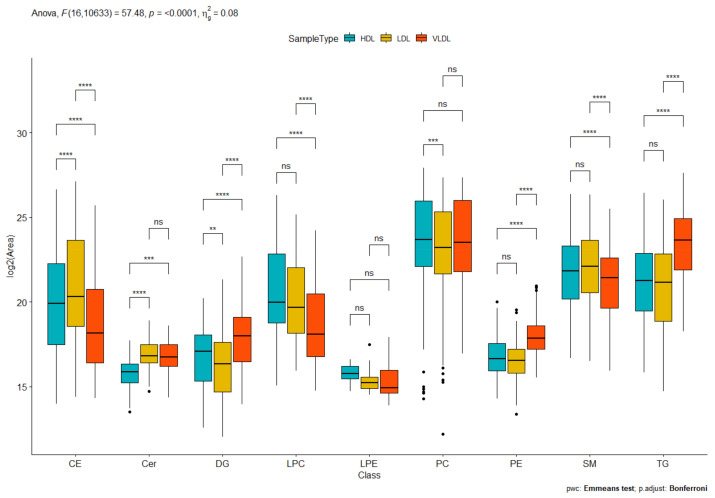
As expected, HDL, LDL, and VLDL fractions differed from each other in terms of distribution of lipid classes, with few exceptions (Figure 2). Indeed, both LDL and HDL showed higher CE levels with respect to VLDL, according to their well-known metabolic roles as CE carriers to the tissues (LDL) or from the tissues to the liver in the reverse cholesterol transport (HDL), whereas VLDL, which is known to be the main plasma carrier of TG from the liver to the tissues, displayed the highest TG contents.
Figure 2.
Box plot reporting the distribution of the nine different lipid classes (cholesteryl ester (CE), ceramide (Cer), phosphatidylcholine (PC), phosphatidylethanolamine (PE), lysophosphatidylcholine (LPC), lysophosphatidylethanolamine (LPE), sphingomyelin (SM), triacylglycerol (TG), and diacylglycerol (DG)) among HDL, LDL, and VLDL. ** p-value < 0.01, *** p-value < 0.001, **** p-value < 0.0001, ns not significant.
We performed a comparative analysis within each lipoprotein fraction sorted according to the plaque typology (“soft” or “hard”), which was determined by ultrasonography. Using this non-invasive routine method, some key characteristics of the lesion such as the fibrous cap thickness, the lipid-core size, the presence of calcifications and/or ulcerations, as well as the degree of stenosis were evaluated, providing useful information on plaque vulnerability. Overall, plaques are defined as “soft” if predominantly hypoechoic (characterized by a large lipid core), or “hard” in the presence of hyperechoic features (mostly fibrotic and/or calcific). Hereafter, for the sake of simplicity, each lipoprotein fraction will be referred to as “hard” or “soft” according to the plaque typology (e.g., HDL “hard”).
Following sorting for the plaque typology, no differences were evidenced between “hard” and “soft” fractions, except for DGs, which were higher in LDL “soft” (Supplementary Materials, Figure S2).
Supervised multivariate analysis showed that lipids belonging to PE, SM, and DG classes mostly contributed to discrimination within each lipoprotein fraction according to the plaque typology (Supplementary Materials, Figure S3).
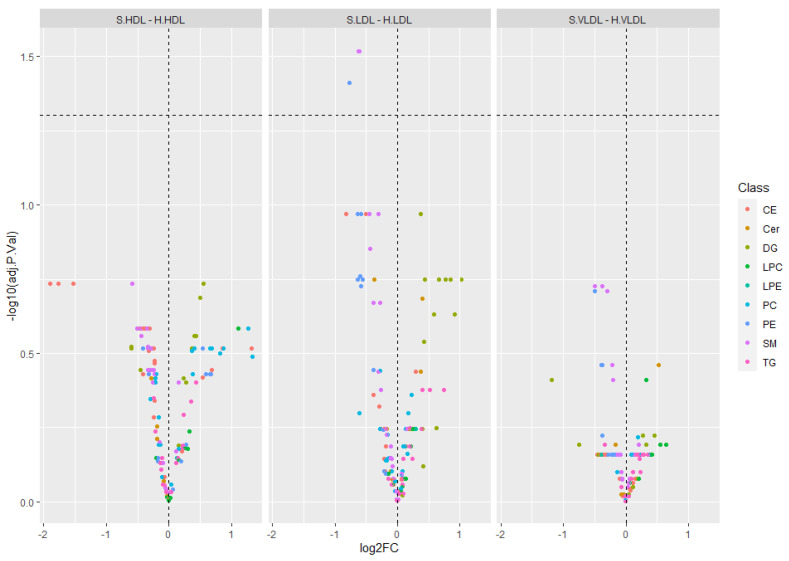
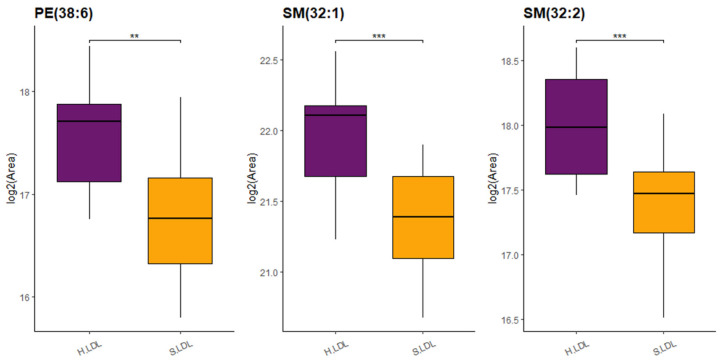
Differential analysis of lipid profiles among “hard” and “soft” fractions evidenced a significant dysregulation of three lipid species, namely PE (38:6), SM (32:1), and SM (32:2), between LDL “soft” vs. LDL “hard” (adj. p-value threshold = 0.05 and log2FC ≥ |0.58|) (Figure 3 and Supplementary Materials, Table S3). Details on relative abundances of the three lipid species significantly dysregulated between LDL “soft” and LDL “hard” are reported in Figure 4.
Figure 3.
Volcano plots showing results of the differential lipid profile analysis (adj. p-value threshold = 0.05 and log2FC ≥ |0.58|) among “hard” and “soft” fractions. Lipids are colored according to the lipid class (CE, Cer, PC, PE, LPC, LPE, SM, TG, DG). PE (38:6), SM (32:1), and SM (32:2) were found significantly dysregulated in LDL “soft” vs. LDL “hard”. SM (32:1) and SM (32:2) are shown as a single purple spot (adjusted p value = 0.03 for both of them; Supplementary Materials, Table S3).
Figure 4.
Boxplot reporting log2(Area) for lipids significantly dysregulated between LDL “soft” (S.LDL) and LDL “hard” (H.LDL): PE (38:6), SM (32:1), and SM (32:2) (** p-value < 0.01, *** p-value < 0.001).
3. Discussion
In the last years, the complexity of the wide array of bioactive lipids carried by lipoproteins is beginning to reveal itself thanks to the emerging MS/MS-based technologies applied to lipidomics [25,44].
Glycerophospholipids and sphingolipids are important regulators of lipoproteins metabolism including the activities of the involved enzymes. Some of them are precursors to several bioactive metabolites including lysophosphatidylcholines and ceramides, which are also involved in cell signaling [45,46,47]. Indeed, dysfunctional sphingolipid metabolism has been implicated in CVD, highlighting the need to delve deeper into lipid biochemistry for a better understanding of the molecular basis of these pathologies [48].
SMs are major structural phospholipids that determine surface pressure in lipoproteins, enhancing rigidity and influencing the activity of enzymes involved in lipid metabolism. In this respect, it is known that SMs strongly inhibit lipoprotein lipase (LPL)-mediated lipolysis [49] and play a key role in determining the lipoprotein CE content by acting as physiological inhibitors of cholesterol esterification by lecithin-cholesterol acyltransferase (LCAT) [50,51], mainly by hindering the binding of the enzyme to the lipoprotein surface [52]. Furthermore, SMs inhibit (whereas Cers activate) the hydrolytic activity of sPLA2, which liberates arachidonic acid from the lipoprotein surface [53].
Interestingly, it has been shown that an increased SMs to PCs ratio makes LDL more susceptible to secretory sphingomyelinase action, leading to the formation of aggregated LDL with high atherogenic potential [54], whereas enrichment of PCs and SMs in HDL strongly influences the rate of reverse cholesterol transport at multiple steps in the process [55,56]. SM levels in HDL have been reported to change in different pathological conditions including CAD [57], in which a reduction has been observed, and hypertension [58], in which an increase has been reported.
Ruut et al., have found that aggregation-prone LDL were enriched in sphingolipids, modifiable and predictive of future cardiovascular deaths [59]. Interestingly, LDL are the main carrier of SMs in circulation (50 mol% of total plasma SMs) [60]. Regarding PEs, Dang et al. have found an important positive association between atherosclerosis extension and progression in an extensive analysis of plasma lipids in ApoE−/− mice [12].
Phospholipids have also proved valuable prognostic markers of cardiovascular disease and total mortality in the Ludwigshafen Risk and Cardiovascular Health study [61].
Recently, we identified a set of plasma lipids composed of seven SMs and three PEs that, even under optimal cholesterol-lowering treatments, allow for discrimination between high-risk CAD patients and controls, suggesting a role for these lipid classes in disease development [17].
In the present study, we applied targeted lipidomics to purified plasma lipoprotein fractions and we report a dysregulation of LDL-associated PE (38:6), SM (32:1), and SM (32:2) between two groups of patients with “hard” or “soft” carotid plaques, homogenous in terms of lipid profile, glycemia, blood pressure, and pharmacological treatments, suggesting their usefulness as potential biomarkers of plaque vulnerability. In any case, these results are in line with the above-mentioned studies showing a contributory role for these phospholipids in lipoprotein metabolism and CVD. Procedures for lipoprotein purification are relatively time-consuming, and, hence, not immediately amenable to application to large casuistries. However, we demonstrated in this proof-of-concept study that targeted lipidomics on purified lipoproteins may have a distinctive advantage over other bulk measurements in the identification of new biomarkers of clinical relevance, which would otherwise be masked by other plasma components. Thus, the evaluation of lipid composition at the lipoprotein level could represent, in perspective, an important tool in precision medicine and diagnostics. Further efforts must be made to validate the obtained results on a large cohort of patients as well as to assess their potential usefulness in risk evaluation of acute clinical events onset.
4. Materials and Methods
4.1. Sample Collection
Lipoprotein lipidomics was performed on twenty-eight patients undergoing carotid endarterectomy, enrolled in previously published studies [4,62]. Plaques were classified as soft (n. 16), having hypoechoic features, or hard (n. 12), having hyperechoic features, according to Gray–Weale classification [5]. To reduce confounders, stringent criteria of eligibility for enrolment were used, allowing for the selection of two groups of patients to be as homogenous as possible. Informed consent was obtained before enrolment. Institutional Review Board approval was obtained. The study was conducted in accordance with the ethical principles of the current Declaration of Helsinki. The main clinical parameters of the two groups of patients are summarized in Table 1. Fasting blood samples were harvested before surgery and immediately centrifuged at 2000× g for 10 min at 4 °C to collect plasma fractions, which were stored at −80 °C until analysis.
Table 1.
The main clinical parameters of patients after sorting according to the plaque typology (hyperechoic or “hard” plaques and hypoechoic or “soft” plaques).
| “Hard” (n = 12) | “Soft” (n = 16) | |
|---|---|---|
| Age (Years) * | 66 ± 7.5 | 72.2 ± 7.3 |
| Male Gender | 8 (66.7%) | 13 (81.2%) |
| BMI | 26.5 ± 3.1 | 25.6 ± 3.4 |
| Triglycerides (mg/dL) | 102.1 ± 24 | 125.2 ± 43.8 |
| Total Cholesterol (mg/dL) | 190.3 ± 41.9 | 178.6 ± 47.4 |
| HDL Cholesterol (mg/dL) | 44.8 ± 9.3 | 44.1 ± 17.1 |
| nonHDL Cholesterol (mg/dL) | 145.5 ± 40.2 | 134.5 ± 46.1 |
| LDL Cholesterol (mg/dL) | 125.3 ± 37.5 | 106.8 ± 37.1 |
| TG/HDL-C * | 2.3 ± 0.4 | 3.2 ± 1.6 |
| Cholesterol Lowering Therapy | 9 (75%) | 15 (93.8%) |
| Glycemia (mg/dL) | 105.6 ± 14.3 | 115.6 ± 25.1 |
| HbA1C | 5.84 ± 0.472 | 6.482 ± 0.875 |
| Diabetes | 2 (16.7%) | 5 (41.7%) |
| Glucose Lowering Therapy | 1 (8.3%) | 5 (31.2%) |
| Systolic Blood Pressure (mmHg) | 139.1 ± 11.6 | 135.5 ± 19.4 |
| Diastolic Blood Pressure (mmHg) | 76.2 ± 11.2 | 72.2 ± 8.6 |
| Anti-hypertensive Therapy | 9 (75%) | 12 (75%) |
* p value < 0.05.
4.2. Lipoprotein Isolation and Purity Assessment
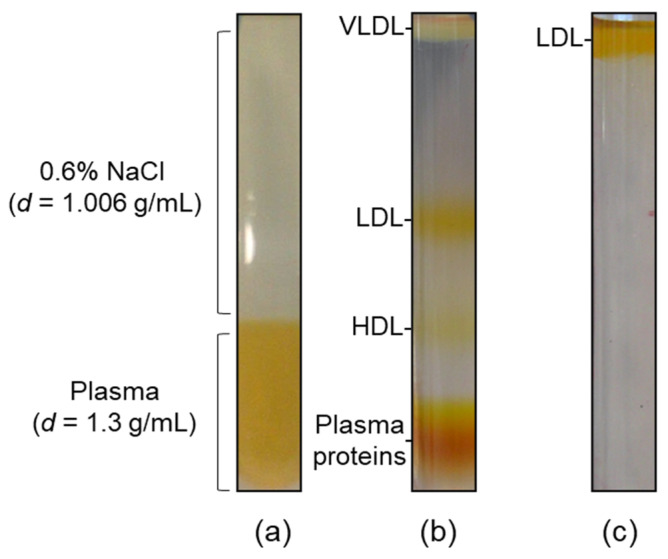
Lipoprotein fractions were purified by isopycnic salt gradient ultracentrifugation (Figure 5) [3,4]. Briefly, 0.9 mL of plasma sample, brought to d = 1.3 g/mL with solid NaBr (472.2 mg NaBr/mL plasma), were gently overlaid with 2.1 mL of a d = 1.006 g/mL solution (0.6% NaCl) (Figure 5a), and centrifuged at 541,000× g for 3 h at 4 °C in a TL-100 series ultracentrifuge equipped with a TLA-100 fixed-angle rotor (Beckman Coulter, Indianapolis, IN, USA) (Figure 5b). Afterwards, VLDL (d = 1.006–1.063 g/mL), LDL (d = 1.063–1.19 g/mL) and HDL (d = 1.19–1.21 g/mL) fractions were collected and further purified by a second centrifugation step, performed at 541,000× g for 2 h in saline solutions at density 1.006, 1.063, and 1.21 g/mL (Figure 5c), respectively, followed by desalting and concentration using Amicon Ultra-0.5 mL centrifugal filter units (10 KDa MWCO, Merck-Millipore, Darmstadt, Germany). The degree of purity was assessed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), as previously described [3].
Figure 5.
Ultracentrifugation procedure for lipoprotein purification. (a) Ultracentrifugation tube containing 0.9 mL of plasma sample added with NaBr, overlaid with 2.1 mL of a 0.6% NaCl solution; (b) self-generated density gradient following isopycnic ultracentrifugation, showing the three lipoprotein fractions under study; (c) LDL on the top of the tube following a washing step at d = 1.063 g/mL.
4.3. HDL, LDL, and VLDL Fractions Processing for HPLC-MS/MS Analysis
HDL, LDL, and VLDL fractions, stored at −20 °C, were thawed at room temperature and immediately subjected to lipid extraction [43]. Each sample was brought to a final volume of 150 μL with 150 mM NaCl, transferred in a 1.5 mL microcentrifuge tube and 600 μL of 0.0625 μM DMS (N,N-dimethylsphingosine (d18:1)), selected as internal standard (ISTD), in MeOH/CHCl3 1/2 (v/v) were added. The biphasic solution thus formed was sonicated for 10 min at room temperature, incubated at 25 °C for 30 min at 1000 rpm in a Thermomixer Compact (Eppendorf, Hamburg, Germany), and then centrifuged at 16,000× g for 10 min at 10 °C in a Microcentrifuge Heraeus Biofuge Fresco (Thermo Scientific, Waltham, MA, USA). From each sample, an aliquot of the lower phase was transferred into a glass HPLC vial, and the HPLC-MS/MS analysis was performed immediately.
4.4. HPLC-MS/MS Analysis
An SRM-based HPLC-MS/MS method was used to analyze the lipid extracts. The HPLC system was a Nexera X2 (Shimadzu, Kyoto, Japan) and the mass spectrometer was a QTrap 5500 (SCIEX, Concord, ON, Canada) equipped with a Turbo V ESI source. Vials were put in a refrigerated autosampler at 5 °C and each sample was injected in duplicate (injection volume 0.5 μL). To prevent carry-over, two needle wash solutions were used: MeOH/i-PrOH 50/50 (v/v) and MeOH/CHCl3 1/2 (v/v). Gradient elution at a flow rate of 0.2 mL/min was performed on a Kinetex column packed with a C8 phase (100 × 2.1 mm, 1.7 μm, 100 Å by Phenomenex, Torrance, CA, USA). Mobile phase A was MeOH/H2O/i-PrOH 50/45/5 (v/v) and phase B was MeOH/i-PrOH 50/50 (v/v), both containing 5 mM ammonium formate. The gradient elution program was: 0 min, 65% B; 0.5 min, 65% B; 10 min, 100% B; 15 min, 100% B; 15.1 min, 65% B; 20 min, 65% B. The column temperature was 45 °C. The mass spectrometer was set in positive ion mode and the operating conditions were as follows: curtain gas 30 psi, ion spray voltage 5 kV, probe temperature 200 °C, ion source gas 1 and gas 2 30 psi, declustering potential 100 V, entrance potential 10 V, collision cell exit potential 19 V, collisional gas N2. A study by direct infusion was previously carried out [17] in order to optimize the collision energies for each lipid class and to choose the relative product ions to be used in the SRM analysis (Table S1). A dwell time of 20 msec was used for each transition Q1/Q3. Analyst Software 1.6.3 (SCIEX, Concord, ON, Canada) was used to collect data while lipid peak areas were calculated with MultiQuant 2.1 software (SCIEX, Concord, ON, Canada).
4.5. Lipidomics Data Analysis
Lipidomic experimental data were analyzed using the lipidr package (version 2.8.0) [63] of R software (version 4.1.1). Firstly, lipidomics data were subjected to quality control, then normalized using the Probabilistic Quotient Normalization (PQN) method (lipid areas were compared across samples for each lipid species and then corrected for dilution errors by determining a dilution factor for each sample) [64] and log2 transformed.
Lipid species distributions for each lipid (sub)class were compared between different lipoprotein fractions, irrespective of plaque typology, as well as between the two groups of patients, within the same lipoprotein fraction. Pairwise comparisons between fractions (LDL vs. HDL, VLDL vs. HDL, VLDL vs. LDL) or between plaque typology (“soft” vs. “hard”) were conducted using the estimated marginal means followed by Bonferroni correction.
Considering the whole lipidomics profile, orthogonal partial least-squares discriminant analysis (OPLS-DA), a multivariate supervised method, was performed for each lipoprotein fraction type using the plaque typology as a grouping variable with the aim of revealing patterns in data and discovering lipid levels related to the two different groups of subjects. Therefore, differential analysis was conducted comparing lipid levels in “soft” and “hard” through a moderated t statistic implemented within the limma R package. p-values were corrected using the Benjamini-Hochberg procedure in order to minimize any type I error and thus the occurrence of false positives. Lipids were considered significant and differentially altered with an adjusted p-value < 0.05 and a fold change FC ≤ 1/1.5 or FC ≥ 1.5.
Acknowledgments
The authors wish to thank Rita Spirito and Anna Guarino (Centro Cardiologico Monzino, IRCCS, via Parea, 4-20138 Milano, Italy), and Franco Piredda (Struttura Complessa “Chirurgia Vascolare”, Dip. Cardio-Toraco-Vascolare, AOU of Sassari), who provided clinical support.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms232012449/s1.
Author Contributions
Conceptualization, A.J.L., S.R., G.N. and E.M.; methodology, A.J.L. and S.R.; software, E.M. and N.D.G.; validation, E.M., N.D.G. and G.O.; formal analysis, E.M., N.D.G. and C.C.; investigation, E.M., N.D.G., G.N., C.C., G.O. and A.J.L.; data curation, E.M. and N.D.G.; writing—original draft preparation, A.J.L., G.N., E.M. and N.D.G.; writing—review and editing, A.J.L., G.N., E.M., N.D.G., G.S., S.R. and M.F.; supervision, A.J.L. and S.R.; project administration, A.J.L.; funding acquisition, A.J.L., G.N. and M.F. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Ethical review and approval were waived for this study because samples were obtained from a cohort of atherosclerotic patients and controls enrolled in previously published studies [3,4,62].
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data produced in this study are available in the “Supplementary Materials” or may be requested from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
A.J.L.: G.N., and M.F. thank the University of Sassari (Fondo di Ateneo per la Ricerca 2020) and the Fondazione di Sardegna (BANDO FONDAZIONE DI SARDEGNA 2022 E 2023—PROGETTI DI RICERCA DI BASE DIPARTIMENTALI (D.R.16/2022)) for their financial support. G.N. thanks “Programma Operativo Nazionale (PON) Ricerca e Innovazione 2014–2020—Asse I “Capitale Umano”, Azione I.2—A.I.M. Attraction and International Mobility, Linea 1 Mobilità dei ricercatori, AIM1874325-3, CUP: J54I18000110001”, and “Fondazione di Sardegna 2018–2020 and 2021—Progetti di Ricerca di Base Dipartimentali (D.R. 2397/2021)” for their financial support.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hermus L., Lefrandt J.D., Tio R.A., Breek J.C., Zeebregts C.J. Carotid plaque formation and serum biomarkers. Atherosclerosis. 2010;213:21–29. doi: 10.1016/j.atherosclerosis.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Pirillo A., Bonacina F., Norata G.D., Catapano A.L. The Interplay of Lipids, Lipoproteins, and Immunity in Atherosclerosis. Curr. Atheroscler. Rep. 2018;20:12. doi: 10.1007/s11883-018-0715-0. [DOI] [PubMed] [Google Scholar]
- 3.Finamore F., Nieddu G., Rocchiccioli S., Spirito R., Guarino A., Formato M., Lepedda A.J. Apolipoprotein Signature of HDL and LDL from Atherosclerotic Patients in Relation with Carotid Plaque Typology: A Preliminary Report. Biomedicines. 2021;9:1156. doi: 10.3390/biomedicines9091156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lepedda A.J., Nieddu G., Zinellu E., De Muro P., Piredda F., Guarino A., Spirito R., Carta F., Turrini F., Formato M. Proteomic analysis of plasma-purified VLDL, LDL, and HDL fractions from atherosclerotic patients undergoing carotid endarterectomy: Identification of serum amyloid A as a potential marker. Oxidative Med. Cell Longev. 2013;2013:385214. doi: 10.1155/2013/385214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gray-Weale A.C., Graham J.C., Burnett J.R., Byrne K., Lusby R.J. Carotid artery atheroma: Comparison of preoperative B-mode ultrasound appearance with carotid endarterectomy specimen pathology. J. Cardiovasc. Surg. 1988;29:676–681. [PubMed] [Google Scholar]
- 6.Saba L., Saam T., Jager H.R., Yuan C., Hatsukami T.S., Saloner D., Wasserman B.A., Bonati L.H., Wintermark M. Imaging biomarkers of vulnerable carotid plaques for stroke risk prediction and their potential clinical implications. Lancet Neurol. 2019;18:559–572. doi: 10.1016/S1474-4422(19)30035-3. [DOI] [PubMed] [Google Scholar]
- 7.Gupta A., Kesavabhotla K., Baradaran H., Kamel H., Pandya A., Giambrone A.E., Wright D., Pain K.J., Mtui E.E., Suri J.S., et al. Plaque echolucency and stroke risk in asymptomatic carotid stenosis: A systematic review and meta-analysis. Stroke. 2015;46:91–97. doi: 10.1161/STROKEAHA.114.006091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brinjikji W., Rabinstein A.A., Lanzino G., Murad M.H., Williamson E.E., DeMarco J.K., Huston J. 3rd. Ultrasound Characteristics of Symptomatic Carotid Plaques: A Systematic Review and Meta-Analysis. Cerebrovasc. Dis. 2015;40:165–174. doi: 10.1159/000437339. [DOI] [PubMed] [Google Scholar]
- 9.Jashari F., Ibrahimi P., Bajraktari G., Gronlund C., Wester P., Henein M.Y. Carotid plaque echogenicity predicts cerebrovascular symptoms: A systematic review and meta-analysis. Eur. J. Neurol. 2016;23:1241–1247. doi: 10.1111/ene.13017. [DOI] [PubMed] [Google Scholar]
- 10.Camont L., Lhomme M., Rached F., Le Goff W., Negre-Salvayre A., Salvayre R., Calzada C., Lagarde M., Chapman M.J., Kontush A. Small, dense high-density lipoprotein-3 particles are enriched in negatively charged phospholipids: Relevance to cellular cholesterol efflux, antioxidative, antithrombotic, anti-inflammatory, and antiapoptotic functionalities. Arterioscler. Thromb. Vasc. Biol. 2013;33:2715–2723. doi: 10.1161/ATVBAHA.113.301468. [DOI] [PubMed] [Google Scholar]
- 11.Ekroos K., Janis M., Tarasov K., Hurme R., Laaksonen R. Lipidomics: A tool for studies of atherosclerosis. Curr. Atheroscler. Rep. 2010;12:273–281. doi: 10.1007/s11883-010-0110-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dang V.T., Huang A., Zhong L.H., Shi Y., Werstuck G.H. Comprehensive Plasma Metabolomic Analyses of Atherosclerotic Progression Reveal Alterations in Glycerophospholipid and Sphingolipid Metabolism in Apolipoprotein E-deficient Mice. Sci. Rep. 2016;6:35037. doi: 10.1038/srep35037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borodzicz S., Czarzasta K., Kuch M., Cudnoch-Jedrzejewska A. Sphingolipids in cardiovascular diseases and metabolic disorders. Lipids Health Dis. 2015;14:55. doi: 10.1186/s12944-015-0053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mantovani A., Bonapace S., Lunardi G., Canali G., Dugo C., Vinco G., Calabria S., Barbieri E., Laaksonen R., Bonnet F., et al. Associations between specific plasma ceramides and severity of coronary-artery stenosis assessed by coronary angiography. Diabetes Metab. 2020;46:150–157. doi: 10.1016/j.diabet.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Hilvo M., Meikle P.J., Pedersen E.R., Tell G.S., Dhar I., Brenner H., Schottker B., Laaperi M., Kauhanen D., Koistinen K.M., et al. Development and validation of a ceramide- and phospholipid-based cardiovascular risk estimation score for coronary artery disease patients. Eur. Heart J. 2020;41:371–380. doi: 10.1093/eurheartj/ehz387. [DOI] [PubMed] [Google Scholar]
- 16.Poss A.M., Maschek J.A., Cox J.E., Hauner B.J., Hopkins P.N., Hunt S.C., Holland W.L., Summers S.A., Playdon M.C. Machine learning reveals serum sphingolipids as cholesterol-independent biomarkers of coronary artery disease. J. Clin. Investig. 2020;130:1363–1376. doi: 10.1172/JCI131838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michelucci E., Giorgi N.D., Finamore F., Smit J.M., Scholte A., Signore G., Rocchiccioli S. Lipid biomarkers in statin users with coronary artery disease annotated by coronary computed tomography angiography. Sci. Rep. 2021;11:12899. doi: 10.1038/s41598-021-92339-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Giorgi N., Michelucci E., Smit J.M., Scholte A., El Mahdiui M., Knuuti J., Buechel R.R., Teresinska A., Pizzi M.N., Roque A., et al. A specific plasma lipid signature associated with high triglycerides and low HDL cholesterol identifies residual CAD risk in patients with chronic coronary syndrome. Atherosclerosis. 2021;339:1–11. doi: 10.1016/j.atherosclerosis.2021.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Stegemann C., Pechlaner R., Willeit P., Langley S.R., Mangino M., Mayr U., Menni C., Moayyeri A., Santer P., Rungger G., et al. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based Bruneck study. Circulation. 2014;129:1821–1831. doi: 10.1161/CIRCULATIONAHA.113.002500. [DOI] [PubMed] [Google Scholar]
- 20.Ellims A.H., Wong G., Weir J.M., Lew P., Meikle P.J., Taylor A.J. Plasma lipidomic analysis predicts non-calcified coronary artery plaque in asymptomatic patients at intermediate risk of coronary artery disease. Eur. Heart J. Cardiovasc. Imaging. 2014;15:908–916. doi: 10.1093/ehjci/jeu033. [DOI] [PubMed] [Google Scholar]
- 21.Cheng J.M., Suoniemi M., Kardys I., Vihervaara T., de Boer S.P., Akkerhuis K.M., Sysi-Aho M., Ekroos K., Garcia-Garcia H.M., Oemrawsingh R.M., et al. Plasma concentrations of molecular lipid species in relation to coronary plaque characteristics and cardiovascular outcome: Results of the ATHEROREMO-IVUS study. Atherosclerosis. 2015;243:560–566. doi: 10.1016/j.atherosclerosis.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 22.Meikle P.J., Wong G., Tsorotes D., Barlow C.K., Weir J.M., Christopher M.J., MacIntosh G.L., Goudey B., Stern L., Kowalczyk A., et al. Plasma lipidomic analysis of stable and unstable coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2011;31:2723–2732. doi: 10.1161/ATVBAHA.111.234096. [DOI] [PubMed] [Google Scholar]
- 23.Karjalainen J.P., Mononen N., Hutri-Kahonen N., Lehtimaki M., Hilvo M., Kauhanen D., Juonala M., Viikari J., Kahonen M., Raitakari O., et al. New evidence from plasma ceramides links apoE polymorphism to greater risk of coronary artery disease in Finnish adults. J. Lipid Res. 2019;60:1622–1629. doi: 10.1194/jlr.M092809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meikle P.J., Formosa M.F., Mellett N.A., Jayawardana K.S., Giles C., Bertovic D.A., Jennings G.L., Childs W., Reddy M., Carey A.L., et al. HDL Phospholipids, but Not Cholesterol Distinguish Acute Coronary Syndrome From Stable Coronary Artery Disease. J. Am. Heart Assoc. 2019;8:e011792. doi: 10.1161/JAHA.118.011792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding M., Rexrode K.M. A Review of Lipidomics of Cardiovascular Disease Highlights the Importance of Isolating Lipoproteins. Metabolites. 2020;10:163. doi: 10.3390/metabo10040163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Denimal D., Pais de Barros J.P., Petit J.M., Bouillet B., Verges B., Duvillard L. Significant abnormalities of the HDL phosphosphingolipidome in type 1 diabetes despite normal HDL cholesterol concentration. Atherosclerosis. 2015;241:752–760. doi: 10.1016/j.atherosclerosis.2015.06.040. [DOI] [PubMed] [Google Scholar]
- 27.Kostara C.E., Ferrannini E., Bairaktari E.T., Papathanasiou A., Elisaf M., Tsimihodimos V. Early Signs of Atherogenic Features in the HDL Lipidomes of Normolipidemic Patients Newly Diagnosed with Type 2 Diabetes. Int. J. Mol. Sci. 2020;21:8835. doi: 10.3390/ijms21228835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kostara C.E., Karakitsou K.S., Florentin M., Bairaktari E.T., Tsimihodimos V. Progressive, Qualitative, and Quantitative Alterations in HDL Lipidome from Healthy Subjects to Patients with Prediabetes and Type 2 Diabetes. Metabolites. 2022;12:683. doi: 10.3390/metabo12080683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Denimal D., Nguyen A., Pais de Barros J.P., Bouillet B., Petit J.M., Verges B., Duvillard L. Major changes in the sphingophospholipidome of HDL in non-diabetic patients with metabolic syndrome. Atherosclerosis. 2016;246:106–114. doi: 10.1016/j.atherosclerosis.2015.12.042. [DOI] [PubMed] [Google Scholar]
- 30.Mocciaro G., D’Amore S., Jenkins B., Kay R., Murgia A., Herrera-Marcos L.V., Neun S., Sowton A.P., Hall Z., Palma-Duran S.A., et al. Lipidomic Approaches to Study HDL Metabolism in Patients with Central Obesity Diagnosed with Metabolic Syndrome. Int. J. Mol. Sci. 2022;23:6786. doi: 10.3390/ijms23126786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orsoni A., Therond P., Tan R., Giral P., Robillard P., Kontush A., Meikle P.J., Chapman M.J. Statin action enriches HDL3 in polyunsaturated phospholipids and plasmalogens and reduces LDL-derived phospholipid hydroperoxides in atherogenic mixed dyslipidemia. J. Lipid Res. 2016;57:2073–2087. doi: 10.1194/jlr.P068585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kostara C.E., Tsimihodimos V., Elisaf M.S., Bairaktari E.T. NMR-Based Lipid Profiling of High Density Lipoprotein Particles in Healthy Subjects with Low, Normal, and Elevated HDL-Cholesterol. J. Proteome Res. 2017;16:1605–1616. doi: 10.1021/acs.jproteome.6b00975. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y., Wen S., Jiang M., Zhu Y., Ding L., Shi H., Dong P., Yang J., Yang Y. Atherosclerotic dyslipidemia revealed by plasma lipidomics on ApoE(-/-) mice fed a high-fat diet. Atherosclerosis. 2017;262:78–86. doi: 10.1016/j.atherosclerosis.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 34.Takeda H., Izumi Y., Tamura S., Koike T., Koike Y., Shiomi M., Bamba T. Lipid Profiling of Serum and Lipoprotein Fractions in Response to Pitavastatin Using an Animal Model of Familial Hypercholesterolemia. J. Proteome Res. 2020;19:1100–1108. doi: 10.1021/acs.jproteome.9b00602. [DOI] [PubMed] [Google Scholar]
- 35.Meikle P.J., Wong G., Tan R., Giral P., Robillard P., Orsoni A., Hounslow N., Magliano D.J., Shaw J.E., Curran J.E., et al. Statin action favors normalization of the plasma lipidome in the atherogenic mixed dyslipidemia of MetS: Potential relevance to statin-associated dysglycemia. J. Lipid Res. 2015;56:2381–2392. doi: 10.1194/jlr.P061143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khan A.A., Mundra P.A., Straznicky N.E., Nestel P.J., Wong G., Tan R., Huynh K., Ng T.W., Mellett N.A., Weir J.M., et al. Weight Loss and Exercise Alter the High-Density Lipoprotein Lipidome and Improve High-Density Lipoprotein Functionality in Metabolic Syndrome. Arterioscler. Thromb. Vasc. Biol. 2018;38:438–447. doi: 10.1161/ATVBAHA.117.310212. [DOI] [PubMed] [Google Scholar]
- 37.Chapman M.J., Orsoni A., Tan R., Mellett N.A., Nguyen A., Robillard P., Giral P., Therond P., Meikle P.J. LDL subclass lipidomics in atherogenic dyslipidemia: Effect of statin therapy on bioactive lipids and dense LDL. J. Lipid Res. 2020;61:911–932. doi: 10.1194/jlr.P119000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Padro T., Vilahur G., Sanchez-Hernandez J., Hernandez M., Antonijoan R.M., Perez A., Badimon L. Lipidomic changes of LDL in overweight and moderately hypercholesterolemic subjects taking phytosterol- and omega-3-supplemented milk. J. Lipid Res. 2015;56:1043–1056. doi: 10.1194/jlr.P052217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sutter I., Velagapudi S., Othman A., Riwanto M., Manz J., Rohrer L., Rentsch K., Hornemann T., Landmesser U., von Eckardstein A. Plasmalogens of high-density lipoproteins (HDL) are associated with coronary artery disease and anti-apoptotic activity of HDL. Atherosclerosis. 2015;241:539–546. doi: 10.1016/j.atherosclerosis.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 40.Lee J.H., Yang J.S., Lee S.H., Moon M.H. Analysis of lipoprotein-specific lipids in patients with acute coronary syndrome by asymmetrical flow field-flow fractionation and nanoflow liquid chromatography-tandem mass spectrometry. J. Chromatography. B Anal. Technol. Biomed. Life Sci. 2018;1099:56–63. doi: 10.1016/j.jchromb.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 41.Hancock-Cerutti W., Lhomme M., Dauteuille C., Lecocq S., Chapman M.J., Rader D.J., Kontush A., Cuchel M. Paradoxical coronary artery disease in humans with hyperalphalipoproteinemia is associated with distinct differences in the high-density lipoprotein phosphosphingolipidome. J. Clin. Lipidol. 2017;11:1192–1200.e1193. doi: 10.1016/j.jacl.2017.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang D., Yu B., Li Q., Guo Y., Koike T., Koike Y., Wu Q., Zhang J., Mao L., Tang X., et al. HDL quality features revealed by proteomelipidome connectivity are associated with atherosclerotic disease. J. Mol. Cell Biol. 2022;14:mjac004. doi: 10.1093/jmcb/mjac004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Folch J., Lees M., Sloane Stanley G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226:497–509. doi: 10.1016/S0021-9258(18)64849-5. [DOI] [PubMed] [Google Scholar]
- 44.Kontush A., Lhomme M., Chapman M.J. Unraveling the complexities of the HDL lipidome. J. Lipid Res. 2013;54:2950–2963. doi: 10.1194/jlr.R036095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meyer zu Heringdorf D., Jakobs K.H. Lysophospholipid receptors: Signalling, pharmacology and regulation by lysophospholipid metabolism. Biochim. Biophys. Acta. 2007;1768:923–940. doi: 10.1016/j.bbamem.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 46.Tani M., Ito M., Igarashi Y. Ceramide/sphingosine/sphingosine 1-phosphate metabolism on the cell surface and in the extracellular space. Cell. signalling. 2007;19:229–237. doi: 10.1016/j.cellsig.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 47.Hannun Y.A., Obeid L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 48.Alewijnse A.E., Peters S.L. Sphingolipid signalling in the cardiovascular system: Good, bad or both? Eur. J. Pharmacol. 2008;585:292–302. doi: 10.1016/j.ejphar.2008.02.089. [DOI] [PubMed] [Google Scholar]
- 49.Saito H., Arimoto I., Tanaka M., Sasaki T., Tanimoto T., Okada S., Handa T. Inhibition of lipoprotein lipase activity by sphingomyelin: Role of membrane surface structure. Biochim. Biophys. Acta. 2000;1486:312–320. doi: 10.1016/S1388-1981(00)00071-8. [DOI] [PubMed] [Google Scholar]
- 50.Subbaiah P.V., Liu M. Role of sphingomyelin in the regulation of cholesterol esterification in the plasma lipoproteins. Inhibition of lecithin-cholesterol acyltransferase reaction. J. Biol. Chem. 1993;268:20156–20163. doi: 10.1016/S0021-9258(20)80707-8. [DOI] [PubMed] [Google Scholar]
- 51.Rye K.A., Hime N.J., Barter P.J. The influence of sphingomyelin on the structure and function of reconstituted high density lipoproteins. J. Biol. Chem. 1996;271:4243–4250. doi: 10.1074/jbc.271.8.4243. [DOI] [PubMed] [Google Scholar]
- 52.Bolin D.J., Jonas A. Sphingomyelin inhibits the lecithin-cholesterol acyltransferase reaction with reconstituted high density lipoproteins by decreasing enzyme binding. J. Biol. Chem. 1996;271:19152–19158. doi: 10.1074/jbc.271.32.19152. [DOI] [PubMed] [Google Scholar]
- 53.Hoofnagle A.N., Vaisar T., Mitra P., Chait A. HDL lipids and insulin resistance. Curr. Diabetes Rep. 2010;10:78–86. doi: 10.1007/s11892-009-0085-7. [DOI] [PubMed] [Google Scholar]
- 54.Schissel S.L., Jiang X., Tweedie-Hardman J., Jeong T., Camejo E.H., Najib J., Rapp J.H., Williams K.J., Tabas I. Secretory sphingomyelinase, a product of the acid sphingomyelinase gene, can hydrolyze atherogenic lipoproteins at neutral pH. Implications for atherosclerotic lesion development. J. Biol. Chem. 1998;273:2738–2746. doi: 10.1074/jbc.273.5.2738. [DOI] [PubMed] [Google Scholar]
- 55.Yancey P.G., de la Llera-Moya M., Swarnakar S., Monzo P., Klein S.M., Connelly M.A., Johnson W.J., Williams D.L., Rothblat G.H. High density lipoprotein phospholipid composition is a major determinant of the bi-directional flux and net movement of cellular free cholesterol mediated by scavenger receptor BI. J. Biol. Chem. 2000;275:36596–36604. doi: 10.1074/jbc.M006924200. [DOI] [PubMed] [Google Scholar]
- 56.Yetukuri L., Soderlund S., Koivuniemi A., Seppanen-Laakso T., Niemela P.S., Hyvonen M., Taskinen M.R., Vattulainen I., Jauhiainen M., Oresic M. Composition and lipid spatial distribution of HDL particles in subjects with low and high HDL-cholesterol. J. Lipid Res. 2010;51:2341–2351. doi: 10.1194/jlr.M006494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Papathanasiou A., Kostara C., Cung M.T., Seferiadis K., Elisaf M., Bairaktari E., Goudevenos I.A. Analysis of the composition of plasma lipoproteins in patients with extensive coronary heart disease using 1H NMR spectroscopy. Hell. J. Cardiol. HJC Hell. Kardiol. Ep. 2008;49:72–78. [PubMed] [Google Scholar]
- 58.Ozerova I.N., Perova N.V., Shchel’tsyna N.V., Mamedov M.N. Parameters of high-density lipoproteins in patients with arterial hypertension in combination with other components of metabolic syndrome. Bull. Exp. Biol. Med. 2007;143:320–322. doi: 10.1007/s10517-007-0100-4. [DOI] [PubMed] [Google Scholar]
- 59.Ruuth M., Nguyen S.D., Vihervaara T., Hilvo M., Laajala T.D., Kondadi P.K., Gistera A., Lahteenmaki H., Kittila T., Huusko J., et al. Susceptibility of low-density lipoprotein particles to aggregate depends on particle lipidome, is modifiable, and associates with future cardiovascular deaths. Eur. Heart J. 2018;39:2562–2573. doi: 10.1093/eurheartj/ehy319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wiesner P., Leidl K., Boettcher A., Schmitz G., Liebisch G. Lipid profiling of FPLC-separated lipoprotein fractions by electrospray ionization tandem mass spectrometry. J. Lipid Res. 2009;50:574–585. doi: 10.1194/jlr.D800028-JLR200. [DOI] [PubMed] [Google Scholar]
- 61.Sigruener A., Kleber M.E., Heimerl S., Liebisch G., Schmitz G., Maerz W. Glycerophospholipid and sphingolipid species and mortality: The Ludwigshafen Risk and Cardiovascular Health (LURIC) study. PLoS ONE. 2014;9:e85724. doi: 10.1371/journal.pone.0085724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lepedda A.J., Lobina O., Rocchiccioli S., Nieddu G., Ucciferri N., De Muro P., Idini M., Nguyen H.Q., Guarino A., Spirito R., et al. Identification of differentially expressed plasma proteins in atherosclerotic patients with type 2 diabetes. J. Diabetes Its Complicat. 2016;30:880–886. doi: 10.1016/j.jdiacomp.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 63.Mohamed A., Molendijk J., Hill M.M. lipidr: A Software Tool for Data Mining and Analysis of Lipidomics Datasets. J. Proteome Res. 2020;19:2890–2897. doi: 10.1021/acs.jproteome.0c00082. [DOI] [PubMed] [Google Scholar]
- 64.Dieterle F., Ross A., Schlotterbeck G., Senn H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics. Anal. Chem. 2006;78:4281–4290. doi: 10.1021/ac051632c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data produced in this study are available in the “Supplementary Materials” or may be requested from the corresponding author.