Abstract
Simple Summary
Rynchophorus ferrugineus Olivier, the red palm weevil (RPW), is a damaging insect that often severely infests palm trees. Since insecticides have a detrimental environmental impact and may lead to pesticide resistance, new biological control methods are needed. Bacteria from many different species affect the RPW larval growth, health, and immunity. The work evaluates the crosstalk between the RPW and rhizosphere bacteria. Four bacterial isolates, three belonging to Bacillus cereus and one belonging to B. thuringiensis, had a significantly higher impact on the mortality of the larvae and adults of the RPW than other bacteria that were tested did. The results emphasize the significant potential of developing new microbial resource-based management techniques for this pest.
Abstract
The Red Palm Weevil (Rhynchophorus ferrugineus (Oliv.) (Coleoptera, Dryophthoridae) is a well-known palm tree pest that has caused enormous economic damage all over the globe. Insecticides are still the primary method of controlling this pest at this period. However, field populations of RPW have been shown to be resistant to pesticides. Using Bacillus spp. might be one of the options for controlling R. ferruginous. In this study, 23 species of Bacillus spp. were isolated from the rhizosphere of date palm trees in Al Ahsa Oasis, Saudi Arabia. The isolates were identified using 16S rRNA gene sequencing. R. ferrugineus larvae and adults were tested on sugarcane pieces that were treated with the B. thuringiensis strain PDC-AHSAA1 and B. cereus strains (PDC-AHSAA2, PDC-AHSA3 and PDC-AHSA4). The LC50 values for larvae and adults were quite low when they were compared with those of the other isolated strains. The B. thuringiensis strain PDC-AHSAA1 was more effective against both the larvae and adults. The determined LC50 values for B. thuringiensis ranged from 4.19 × 107–3.78 × 109. After 21 days, the data on larval mortality and body weight were evaluated. The surviving larvae that were treated with the bacterial isolates did not acquire a substantial weight. For the RPW larvae and adults, the mortality and corrected mortality death rates were increased by increasing the concentration of B. thuringiensis. In conclusion, Bacillus-treated diets negatively influenced the growth and development of the RPW. This research reported on the interaction between the RPW and the rhizosphere Bacillus spp. and highlighted the tremendous potential for the development of microbial resource-based control strategies for this pest.
Keywords: Bacillus thuringiensis, Bacillus cereus, Red Palm Weevil, entomopathogenic bacteria, rhizosphere bacteria, date palm
1. Introduction
The Rhynchophorus ferrugineus (Olivier) is the most destructive and devastating pest of many palm plants and it originates from tropical Asia [1]. R. ferrugineus, which is known as the Red Palm Weevil (RPW), affects all palm trees including the date palm (Phoenix dactylifera L.) and the coconut (Cocos nucifera L.) as well as urban palmscapes (e.g., those that are dominated by the Canary Islands date palms) (Phoenix canariensis Chabaud) [2]. The direct losses in date palm production due to their infestation with RPWs reached $5.18 to $25.92 million [3]. It is common for female adults to deposit their eggs in tree wounds, fissures, and cracks. The larvae, which are the most destructive, travel into the palm trunk to create tunnels and enormous holes with damp-fermenting debris, therefore leading to frond deformity and palm death [4,5,6]. Adult RPWs can fly a very long distance, which is a distinct advantage that they have [7,8]. So far, RPWs have been reported in the Middle East, South Asia, and in the Mediterranean area [4,9]. There are costs that are associated with the removal and disposal of dead palms which have lowered property values and caused the degradation of recreational areas and urban wildlife habitats, which have all been results of RPW infestation [10]. None of the natural enemies to control the invasive species that have been transported from other locations were found to reach their full biological potential. Few natural pathogens are known for R. ferrugineus, and its chemical management, even in urban areas, is far from acceptable in the case of the RPW outbreak on Phoenix dactylifera. Prophylactic pesticide applications to protect palms from weevil infestation have resulted in harm and expenses. As a result, controlling the RPW infestation remains a big problem for researchers across the globe.
The RPW is now being controlled by the use of exclusionary quarantines and pheromone traps. On the other hand, insecticide applications remain the most effective method of preventing palm weevil attacks. Insecticides, including carbamates, organophosphates, neonicotinoids, and phenylpyrazoles, for example, have been sprayed on foliage, applied to wounds, used as crown or soil drenches or injected into tree trunks or soil around the base of a tree trunk [10,11,12]. Resistance to these synthetic pesticides such as imidacloprid, chlorpyrifos, and lambda-cyhalothrin has been observed in the field populations of the RPW [13,14,15]. Biocontrol agents offer different benefits over chemical pesticides, such as environmental sustainability and minimal toxicity. Although researchers have utilized Bacillus species (Brevibacillus laterosporus, B. aeruginosa, B. sphaericus, and B. megaterium) against the RPW, none of these pathogens could be considered harmful due to their low pathogenicity [16]. The utilization of natural enemies as means of controlling these pests is an important option. Metarhizium anisopliae, Beauveria bassiana, Steinernema carpocapsae, Serratia marcescens, and Bacillus thuringiensis (Bt) have been demonstrated to be effective against the RPW [17,18,19,20,21,22,23]. It was interesting to explore the potential of date palm rhizosphere bacteria against the RPW.
Several different types of higher organisms inhabit the same ecosystem and may interact with each other, thereby giving the chance for the exchange between microorganisms and such animals and plants. As a result of their close interaction with plants and a wide range of plant-beneficial capabilities, rhizosphere bacteria have been the subject of extensive research. Bacterial pathogens are currently being studied for their possible utility in controlling pests. As an alternative to artificial chemicals, entomopathogenic bacteria exhibit considerable action selectivity and may be a viable option. Different pests may be controlled biologically using products that are based on B. thuringiensis [24]. Coleoptera is often targeted by Bt strains that have been isolated and characterized [1,25]. Evidence has emerged with reference to the possible pathogenicity against pests of various strains of B. thuringiensis [26,27]. After being exposed to B. thuringiensis in the lab, the RPW larvae showed decreased levels of boring activity and eating behavior [10]. The mortality of the RPW larvae that were exposed to B. thuringiensis subsp. kurstaki (Btk) and a polyhedrosis virus was 70% and 61%, respectively [28]. The field tests that have been conducted on these biological agents have so far shown modest success [29,30]. As a result of these findings, new microbes-based pest control techniques should be developed that are based on insect-associated microorganisms.
Currently, the only biological control agents that are available for the suppression of R. ferrugineus are entomopathogenic nematodes and Beauveria bassiana [19], and even with these, an adequate level of control is not always achieved, so the addition of entomopathogenic Bacillus to the RPW management strategies in urban countries would be most encouraged. The potential of Bt and B. cereus as biological control agents against the RPW larvae and adults was investigated which could be extended in the future to utilize these pathogens in management strategies.
2. Materials and Methods
2.1. Collection and Rearing of Rhyncophorus ferrugineus
Larvae and adults were gathered from infested palms, principally Phoenix dactylifera, which were chopped down following phytosanitary procedures for the reduction and prevention of R. ferrugineus with the assistance of the Department of Plant Protection, Palms and Dates Center [12]. Weevils were transported in plastic containers with palm tissues as a food supply in transit. Adults were fed on sugarcane plants (pieces, 4 gm each one) after emerging, and let out to oviposit in polycarbonate tissue culture bottles (350 mL, 75 mm, 109 mm height). In the laboratory, freshly hatched larvae were fed on sugarcane while being cultured in the plastic bottles that are mentioned above (10 larvae per each bottle). Insecticidal activity tests were conducted using larvae that were given untreated and treated sugarcane pieces. Adults were fed on sugarcane after emergence and then, they were moved to the polypropylene tissue culture bottles that are previously described. All of the insects were kept at 27 ± 2 °C at 74 ± 3% relative humidity.
2.2. Isolation of Rhizosphere Bacteria
Twenty soil samples were collected from date palm rhizosphere in Al Ahsa Oasis, Saudi Arabia. In order to grow bacterial isolates, a soil solution from each sample was well mixed by vortexing, and one loop was placed on nutrient agar (BD Difco™, BD Biosciences, Franklin Lakes, NJ, USA) plates (90 mm Ø) for further investigation. Incubation at 30 °C for 48 h was followed by storage at 4 °C until they were needed. For further isolation and to prevent the presence of background flora, individual colonies were picked up and resealed on new nutrient agar plates. The streaking process was repeated at least five times. Nutrient agar slants were used to cultivate 23 unidentified and purified bacterial strains. The 16S ribosomal DNA universal primers were used to identify the isolates [31]. PCR was performed as explained by Elsharkawy et al. [31]. Samples were properly prepared for dye terminator cycle sequencing using the BigDye Ready Reaction kit (Applied Biosystems, Foster City, CA, USA). The PCR conditions were 3 min at 94 °C, 24 cycles of 30 s at 94 °C for 30 s at 54 °C and 1 min at 72 °C, and then, 10 min at 72 and held at 4 °C. Applied Biosystems Genetic Analyzer which was installed with a 50 cm capillary array was used to conduct DNA sequencing. Sequence scanner software (Applied Biosystems) was utilized to read the sequencing data, and then nucleotide alignment blast was utilized to blast the unidentified sequences online on GenBank’s DNA sequences database. Neighbor-joining (NJ) algorithms were used to create phylogenetic trees from the alignment of the sequences utilizing MEGA 11.0.1 software [32]. Bootstrap analysis with 1000 repetitions of the same software was used to assess the dendrogram’s reliability. There were three separate replicates of this experiment.
2.3. Effect of the Bacterial Isolates on Larvae and Adults of RPW
After 12 h of incubation of the purified isolates at 30 °C, the inoculated Petri plates were counted for the number of colony forming units (CFU). Bacteria that were designated PDC-AHSAA 1, PDC-AHSAA 2, PDC-AHSA 3, and PDC-AHSA 4 demonstrated higher mortality in preliminary trials and were employed in subsequent trials. In diet integration bioassays, bacteria colony-forming units were counted after serial dilutions and adjusted using spectrophotometer. One centimeter of bacterial culture is used to quantify the optical density, which is the amount of light that is absorbed before it is detected by a photodiode. These isolates were tested on adult and younger larvae that were fed using a treated insect diet to determine its biological activity. The diet for the adults and larvae consisted of small, treated sugarcane pieces. The mortality was recorded after three weeks. The bioassay was applied using five different concentrations (1.56 × 107, 3.39 × 108, 8.59 × 109, 4.27 × 1010, and 2.01 × 1011 CFU/mL). LC50 was determined using these rates after initial bracketing results. There was a bacteria-free control treatment. For each experiment, plastic boxes containing 40 RPW second-instar larvae or adults (females or males) for each treatment were starved for 3–4 h before their usage. Electronic balances were used to weigh the sugarcane pieces (before feeding) which were fed to each larva or adult every four days using the same concentration as for the tested bacteria. The sugarcane pieces were immersed for 20 min in either the bacterial suspensions or sterile distilled water before being provided to the larvae [17]. They were then left to air dry for 10 min at room temperature. There were four separate replicates of this experiment.
2.4. Effect of B. thuringiensis PDC-Ahsaa 1 on Larval Mortality
For the experiment of mortality, different concentrations of Bt strain PDC-Ahsaa 1 (which showed good results in our preliminary experiments) were used against larvae and adults. Larvae and adults that did not respond to prodding with a needle were deemed to be dead. Following their exposure, the number of dead and living insects was reported every day for 21 days. Treatment of the feed at the same dose for larval bioassay was utilized for the adults. Four sets of ten duplicates for each concentration along with control were performed. For the purposes of this study, we investigated two types of mortality (mortality and corrected mortality) among larvae and adults. The mortality rate of larvae or adults was determined by dividing the total number of larvae that were tested by the number of dead larvae. The corrected mortalities of second-instar larvae were calculated using Abbott’s formula [33]. The effect of different concentrations of the strain PDC-AHSAA 1 on larval weight was measured. There were four separate replicates of this experiment.
2.5. Data Analysis
Confidence intervals (95%), median lethal concentrations (LC50), and median lethal time (LT50) were calculated, as well as the means and standard errors (SE). Tukey’s studentized range test was used to detect the differences between treatment means. At a probability value of 5% (p ≤ 0.05), differences in means were considered significant. Analysis of variance (ANOVA) was utilized to statistically evaluate the data using the SPSS 17.0 for Windows (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Isolation and Identification of Bacterial Isolates
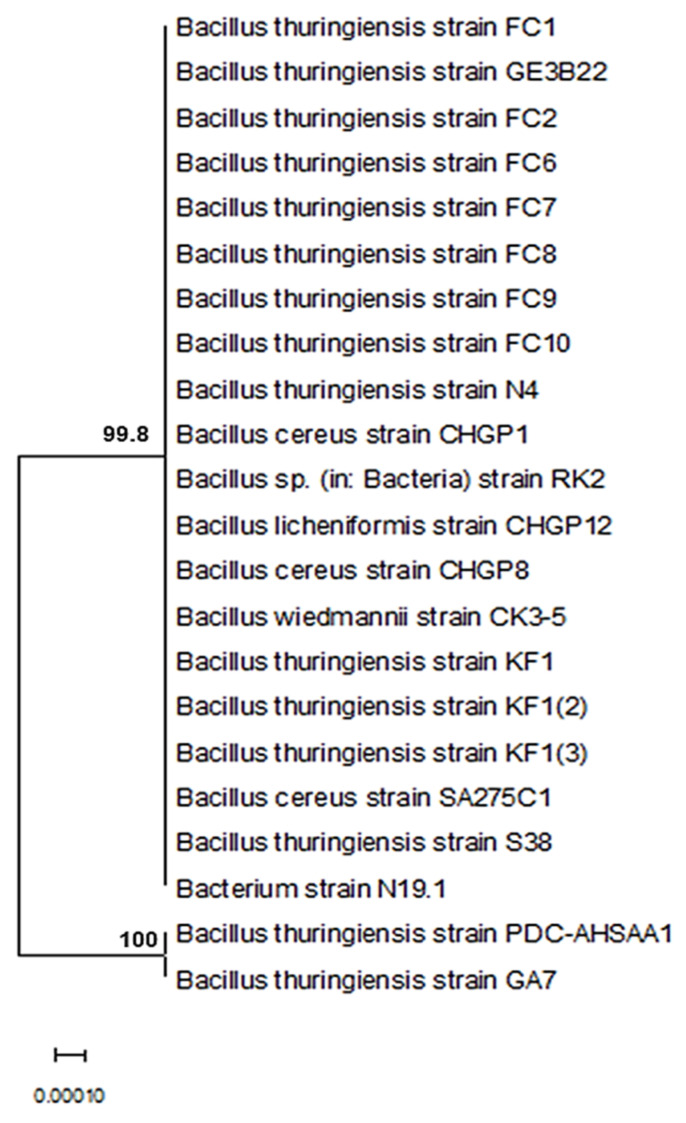
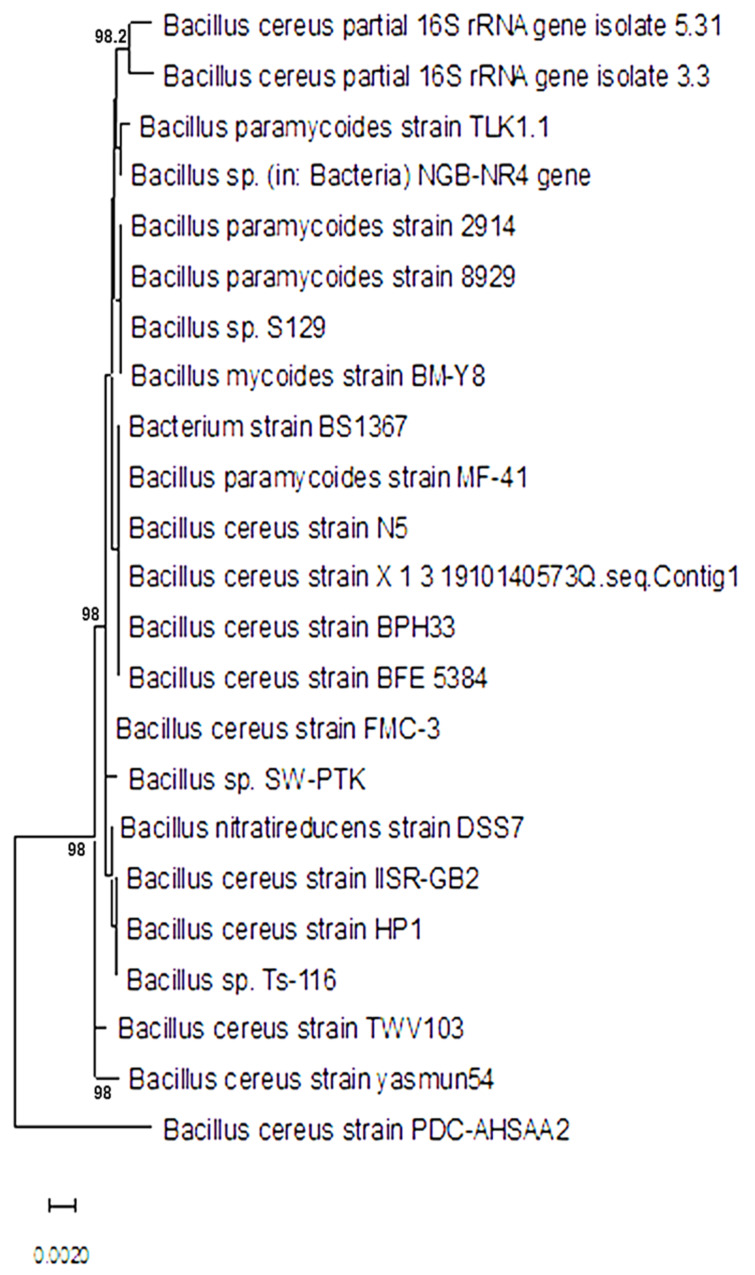
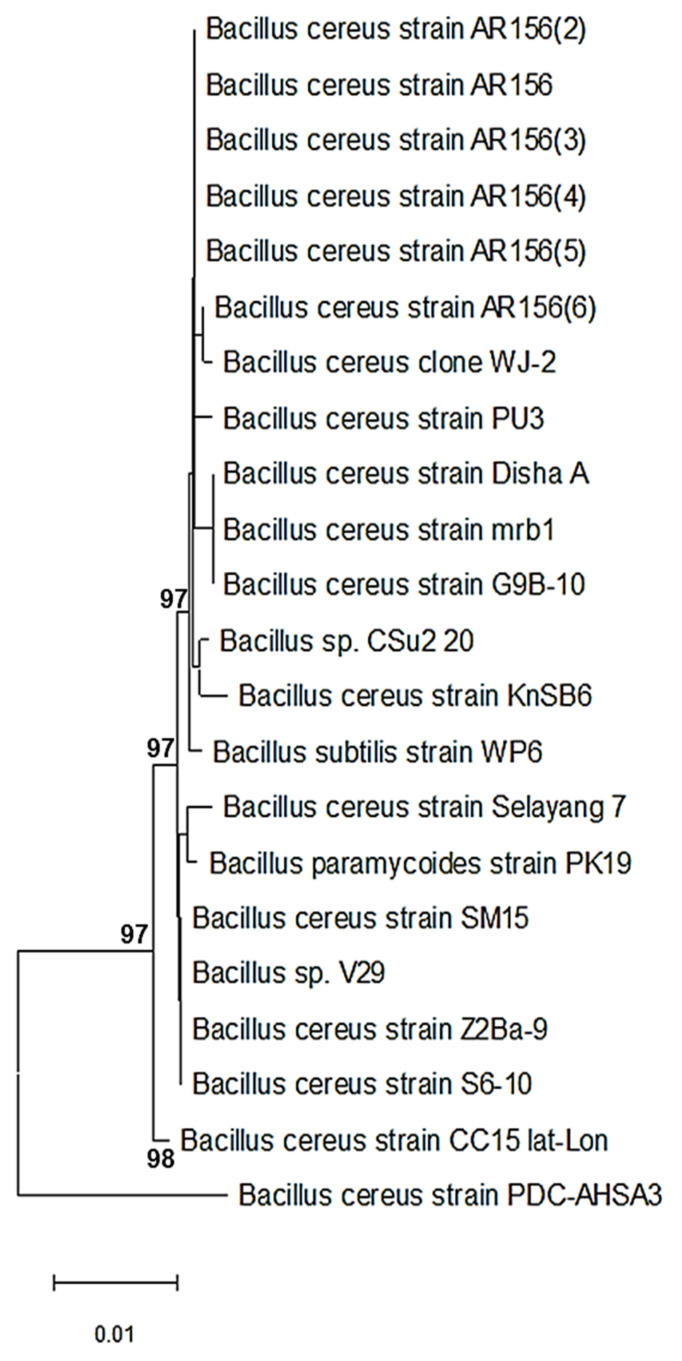
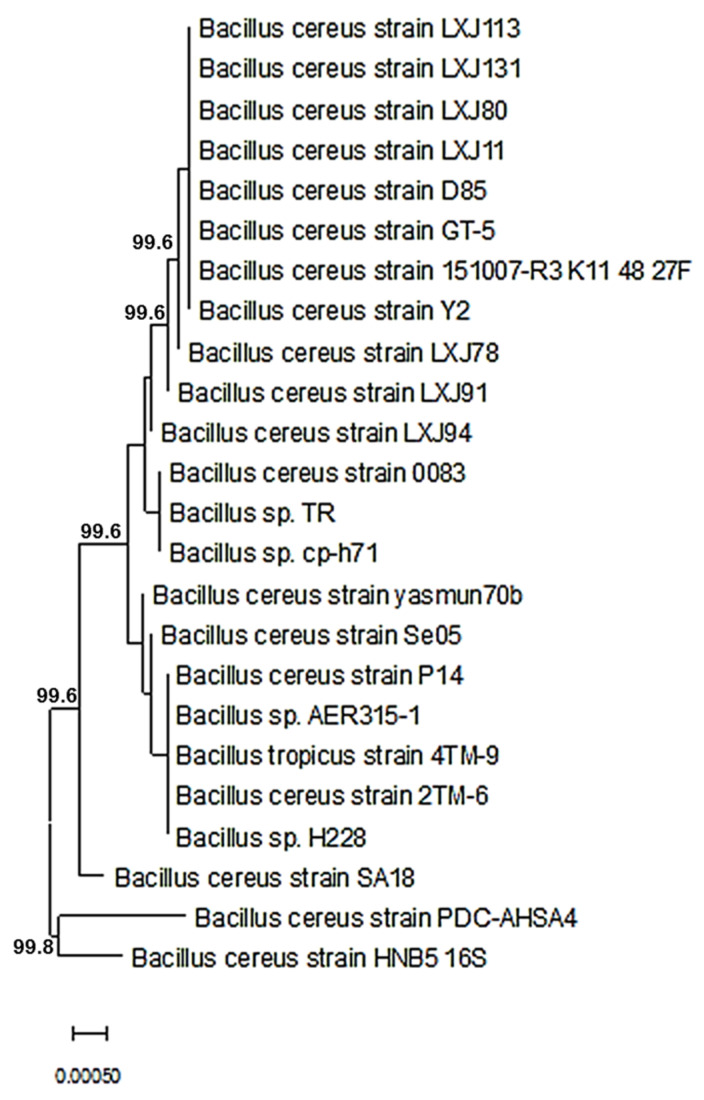
Twenty-three bacterial strains were purified and identified using the sequence of the 16S ribosomal RNA gene. The nucleotide database blasting using a nucleotide query technique demonstrated that the species Bacillus were the most prevalently discovered bacteria. The isolates were identified using amplicons that were amplified by the PCR method. The partial 16S rRNA gene sequences for PDC-AHSAA1, PDC-AHSAA2, PDC-AHSA3, and PDC-AHSA4 were submitted to NCBI with the respective accession numbers ON197897, ON197906, ON358125, and ON358129. The nucleotide alignment revealed that the PDC-AHSAA1 sequences were strongly connected with the B. thuringiensis strain GA7 from Turkey (100% similarity) when they were assessed according to the BLASTn (Figure 1), while the PDC-AHSAA2 sequences were connected with B. cereus from India (98.2% similarity) (Figure 2). The PDC-AHSA3 sequences were connected with the B. cereus strain CC15 lat-Lon from India (97.8% similarity) (Figure 3). Finally, the PDC-AHSA4 sequences were connected with the B. cereus strain HNB5 from China (99.8% similarity) (Figure 4). Among the isolated bacteria from the date palm rhizosphere, the B. thuringiensis strain PDC-AHSAA1, the B. cereus strain PDC-AHSAA2, the B. cereus strain PDC-AHSA3, and the B. cereus strain PDC-AHSA4 were selected for a further investigation. The preliminary bioassay results indicated that the second instar larvae and the adults of the RPW were extremely vulnerable to the isolates PDC-AHSAA1, PDC-AHSAA2, PDC-AHSA3, and PDC-AHSA4, more than any other isolates.
Figure 1.
Phylogenetic dendrogram showing the position of Bacillus thuringiensis strain PDC-AHSAA1 among phylogenetic neighbors.
Figure 2.
Phylogenetic dendrogram showing the position of Bacillus cereus strain PDC-AHSAA2 among phylogenetic neighbors.
Figure 3.
Phylogenetic dendrogram showing the position of Bacillus cereus strain PDC-AHSA3 among phylogenetic neighbors.
Figure 4.
Phylogenetic dendrogram showing the position of Bacillus cereus strain PDC-AHSA4 among phylogenetic neighbors.
3.2. Effect of the Isolates on Larvae and Adults of RPW
The data showed that the B. thuringiensis strain PDC-AHSAA1 and the B. cereus strain PDC-AHSAA2 had LC50 values of 4.19 × 107 and 4.96 × 107 for the larvae, respectively. B. thuringiensis PDC-AHSAA1 was shown to be more effective than other isolates were (Table 1). Substantial differences in the 95 % fiducial limit between the LC50 values were regarded as evidence of there being significant differences. The adults were vulnerable to infection in the bioassays with sugarcane pieces that were contaminated by the bacteria. The potential of different bacterial isolates against the females was higher than it was for the males of the RPW. Both of the larvae and adults were affected by B. thuringiensis PDC-AHSAA1 more than they were by the B. cereus strains PDC-AHSAA2, PDC-AHSA3, and PDC-AHSA4 (Table 1).
Table 1.
Insecticidal activity of Bacillus thuringiensis and B. cereus isolates against RPW larvae and adults after three weeks of bioassay.
| RPW | Strain | LC50 | 95% FL | Slope ± SE |
|---|---|---|---|---|
| Larvae | PDC-AHSAA1 | 4.19 × 107 b | 4.02 × 106–2.54 × 108 | 0.91 ± 0.20 |
| PDC-AHSAA2 | 4.96 × 107 b | 4.91 × 106–2.69 × 108 | 1.37 ± 0.34 | |
| PDC-AHSA3 | 2.83 × 108 a | 2.15 × 107–2.79 × 109 | 1.33 ± 0.31 | |
| PDC-AHSA4 | 3.46 × 108 a | 3.13 × 107–3.61 × 109 | 1.12 ± 0.22 | |
| Male | PDC-AHSAA1 | 3.78 × 109 b | 3.66 × 108–3.89 × 1010 | 1.27 ± 0.28 |
| PDC-AHSAA2 | 4.48 × 109 b | 4.15 × 108–2.22 × 1010 | 1.19 ± 0.20 | |
| PDC-AHSA3 | 4.99 × 1010 a | 5.11 × 109–2.58 × 1011 | 1.09 ± 0.19 | |
| PDC-AHSA4 | 5.01 × 1010 a | 5.05 × 109–2.37 × 1011 | 1.67 ± 0.26 | |
| Female | PDC-AHSAA1 | 3.13 × 108 b | 3.12 × 107–1.59 × 109 | 1.57 ± 0.29 |
| PDC-AHSAA2 | 3.98 × 108 b | 3.65 × 107–1.99 × 109 | 1.29 ± 0.33 | |
| PDC-AHSA3 | 4.80 × 109 a | 4.75 × 108–2.65× 1010 | 1.12 ± 0.27 | |
| PDC-AHSA4 | 4.82 × 109 a | 4.95 × 108–2.74 × 1010 | 1.14 ± 0.31 |
When it came to the larvae, the LT50 for the B. thuringiensis PDC-AHSAA1 and B. cereus PDC-AHSAA2 were 10 and 12 days, respectively. The LT50 of PDC-AHSAA1 were 13 and 11 days for males and females of the RPW, respectively (Table 2). The LT50 values of PDC-AHSA4 were 15, 17, and 16 days for the larvae, males, and females of the RPW, respectively (Table 2). The LT50 ranged from 10 to 17 days in the tested bacteria isolates. The results were maintained and extended to 21 days after treatment; however, they were not always statistically different (Table 2).
Table 2.
The impact of Bacillus thuringiensis and B. cereus isolates against RPW larvae and adults (males and females) after 21 days of the bioassay being performed.
| RPW | Strain | LT50 (Days) | 95% FL |
|---|---|---|---|
| Larvae | PDC-AHSAA1 | 10 × 108 c * | 8 × 104–11 × 109 |
| PDC-AHSAA2 | 12 × 108 b | 10 × 104–13 × 109 | |
| PDC-AHSA3 | 15 × 108 a | 14 × 104–16 × 1010 | |
| PDC-AHSA4 | 15 × 108 a | 13 × 104–16 × 1010 | |
| Male | PDC-AHSAA1 | 13 × 108 c | 11 × 104–14 × 109 |
| PDC-AHSAA2 | 15 × 108 b | 12 × 104–15 × 109 | |
| PDC-AHSA3 | 17 × 108 a | 14 × 104–18 × 109 | |
| PDC-AHSA4 | 17 × 108 a | 15 × 104–19 × 108 | |
| Female | PDC-AHSAA1 | 11 × 108 c | 9 × 104–12 × 109 |
| PDC-AHSAA2 | 13 × 108 b | 12 × 104–15 × 109 | |
| PDC-AHSA3 | 16 × 108 a | 13 × 104–17× 1010 | |
| PDC-AHSA4 | 16 × 108 a | 14 × 104–17 × 1010 |
* LT50′s reported in cfu’s/mL.
3.3. Effect of the Bacterial Isolates on Actual Larval Mortality
The mortality of the larvae, males, and females of the RPW that was caused by the treatment diet was high for the B. thuringiensis PDC-AHSAA1. The mortality rates were increased by increasing the concentration of the B. thuringiensis PDC-AHSAA1 in the treated diet. The mortality levels were higher in the treated females when they were compared to those that were reported in the males of the RPW (Table 3). Additionally, there were substantial differences in the overall corrected mortality data after feeding them with five different PDC-AHSAA1 concentrations. The concentration of 2.01 × 1011 CFU/mL had the most harmful impact in the RPW second-instar larvae, males, and females, resulting in mortality rates of 95.93, 90.46, and 93.98 %, respectively (Table 3).
Table 3.
The effects of Bacillus thuringiensis strain PDC-AHSAA1 concentrations on the mortality of RPW larvae and adults after 21 days of bioassay.
| RPW | Strain Concentrations | Mortality (%) | Corrected Mortality (%) |
|---|---|---|---|
| Larvae | 2.01 × 1011 | 95.93 ± 2.34 a | 94.88 ± 2.24 a |
| 4.27 × 1010 | 91.85 ± 2.76 b | 90.47 ± 2.36 b | |
| 8.59 × 109 | 73.22 ± 1.89 c | 72.20 ± 1.78 c | |
| 3.39 × 108 | 71.98 ± 1.69 c | 71.13 ± 1.34 c | |
| 1.56×107 | 58.35± 1.42 d | 57.97± 1.10 d | |
| Control | 2.09 ± 1.13 e | na | |
| Male | 2.01 × 1011 | 90.46 ± 2.84 a | 89.71 ± 2.31 a |
| 4.27 × 1010 | 81.59 ± 2.24 b | 81.02 ± 2.11 b | |
| 8.59 × 109 | 69.57 ± 1.59 c | 68.92 ± 1.36 c | |
| 3.39 × 108 | 63.51 ± 1.36 d | 62.93 ± 1.29 d | |
| 1.56×107 | 50.91 ± 1.09 e | 50.03 ± 1.01 e | |
| Control | 2.35 ± 1.10 f | na | |
| Female | 2.01 × 1011 | 93.98 ± 2.21 a | 93.14 ± 2.13 a |
| 4.27 × 1010 | 88.46 ± 2.30 b | 88.08 ± 1.86 b | |
| 8.59 × 109 | 76.31 ± 1.47 c | 75.92 ± 1.36 c | |
| 3.39 × 108 | 70.29 ± 1.19 d | 69.56 ± 1.15 d | |
| 1.56×107 | 52.99 ± 2.01 e | 51.71 ± 1.91 e | |
| Control | 2.51 ± 1.19 f | na |
na = not applicable.
3.4. Effect of the Bacterial Isolates on Larval Weight
Over the course of the bioassays, the mortality of the RPW larvae that were fed a non-Bt control diet varied from 0% to 3%. Even at the lowest dose that was tested, the treated diet resulted in substantial death rates for the larvae, wherein the mortality rate rose to an extreme 95%. Table 4 shows that there were some impacts on the larval weight and behavior (a decrease in eating and mobility) which were detected. B. thuringiensis is predicted to affect the RPWs that are in older larval stages, and the dose-dependent mortality that we found for the larvae that were exposed was repeatable. It is possible that midgut damage or feeding inhibition occurred among the larvae who survived in the treatments, since the weight of the older instars was lower than that of the control ones.
Table 4.
The effects of Bacillus thuringiensis strain PDC-AHSAA1 concentrations on the weight of RPW larvae after 21 days of bioassay.
| Treatment | Weight (g) ± SE, (n = 30) |
|---|---|
| 2.01 × 1011 | 2.53 ± 0.28 d |
| 4.27 × 1010 | 2.68 ± 0.39 d |
| 8.59 × 109 | 3.35 ± 0.32 c |
| 3.39 × 108 | 3.49 ± 0.24 c |
| 1.56 × 107 | 3.96 ± 0.21 b |
| Control | 4.43 ± 0.23 a |
4. Discussion
Twenty-three purified bacterial strains were isolated from the rhizosphere of date palm trees. The initial bioassays on the R. ferrugineus (RPW) larvae showed that some of the bacterial isolates had bioactivity that could be investigated. Four out of 23 bacterial isolates that were studied, PDC-AHSAA1, PDC-AHSAA2, PDC-AHSA3, and PDC-AHSA4, revealed that they had substantial harmful effects on the larvae and adults. The optical microscopy confirmed that the isolates’ morphological characteristics were rod-shaped, spore-forming, and Gram-positive bacteria. Both PDC-AHSAA1 and PDC-AHSAA2 were extremely effective biocontrol agents, causing death to the larvae and adults. Using a nucleotide query method, the sequences were blasted against a nucleotide database, and Bacillus was identified to be the most prevalent bacterial genera. Three isolates of B. cereus (PDC-AHSAA2, PDC-AHSA3, and PDC-AHSA4) and the B. thuringiensis strain PDC-AHSAA1 were successfully identified. Bacillus thuringiensis (Bt) is often referred to as a soil microorganism simply due to the fact that it has been isolated from this environment for several years [34,35]. Other researchers believe that the Bt spores may only be stored in the soil since they hardly germinate there and need certain nutrients and pH levels for that to occur [36,37]. The native microbiota and soil characteristics, including pH, humidity, mineral and organic matter concentrations, have an immediate impact on the Bt viability, thereby affecting its growth, sporulation, germination, and protein synthesis [37,38]. On the other hand, B. cereus (a taxon that is closely linked to Bt), which has shown the capacity to proliferate in non-sterilized soils, thereby expanding its population up to 20%, seems to be far less affected by these conditions [35]. However, under some circumstances, the Bt and B. cereus spores may germinate and develop successfully, such as in humid, nutrient-rich soils with pH levels that are close to neutral, even when other microbial communities are present [38]. The rhizosphere colonization by B. cereus and Bt has rarely been reported [39,40]. A Bt strain that was isolated from the rhizosphere of Ficus doliaria proved to be very toxic to the Blackfly larvae that were absent in the region [40]. B. cereus has also been reported to germinate and proliferate in the rhizosphere of plants [41]. The phenotypic identification of certain bacteria may be complicated by challenges and overlaps, however the 16S ribosomal DNA-based identification of unknown bacteria is an implemented method [42]. Universal primer mixtures may be used to amplify bacterial DNA [43]. The isolate sequences that were examined using BLASTn, a nucleotide alignment (bl2seq), had a high degree of alignment (100 %). An analysis of the 16S-rRNA sequences of each isolate was carried out using a neighbor-joining approach (NJ), which compared the sequences of the 16S rRNA with others in the database. The Bacillus thuringiensis strain GA7 (from Turkey), the Bacillus cereus strain (from India), the Bacillus cereus strain CC15 lat-Lon (from India), and the Bacillus cereus strain HNB5 (from China) were grouped with the isolates PDC-AHSAA1, PDC-AHSAA2, PDC-AHSA3, and PDC-AHSA4 with high-similarity levels (100, 98.2, 97.5, and 75%, respectively). High-similarity percentages indicated that the PCR products had been totally sequenced. This means that phenotypic characteristics at the species level were supported by the 16S rRNA sequencing-based genotyping.
Several species of Bacillus have been reported to be entomopathogens and/or proteolytic bacteria that cause toxicity [40,41,44,45]. There are six closely related species in the Bacillus cereus group, including B. thuringiensis, B. cereus, B. anthracis, B. mycoides, B. weihenstephanensis, and B. pseudomycoides, which have been found to produce the paralytic toxins sphingomyelinase C and phospholipase C [46,47]. Among the isolated and identified bacteria, B. thuringiensis PDC-AHSAA1 has been demonstrated to have a more than a 95% larval mortality rate. Additionally, the potential of PDC-AHSAA1 on the RPW females was higher than it was for the RPW males. B. thuringiensis has long been regarded as the most important entomopathogenic bacterium in the control of insects. The oriental beetle, the European june beetle, the northern masked chafer, the shining leaf chafer beetle, the common European cockchafer, and the white grub (Coleoptera, Scarabaeidae) have all been effectively treated with the microbial control agents, B. thuringiensis or B. cereus [48,49,50,51]. The differences in the sensitivity across species are thought to be due to toxic protein receptors in the midgut and/or a complex of proteinases in the midgut. The protein is cleaved into a 60-kDa toxin by midgut enzymes which binds to the protein receptors on the brush-border membrane vesicles that are generated from the midgut epithelium. The toxin causes the swelling and the lysis of the gut epithelium by causing holes in the cell membrane [52]. Septicemia and starvation cause the insect’s death. The PDC-AHSAA1 application at the highest concentration that was tested (2.01 × 1011 CFU/mL) resulted in corrected mortality rates of 94.88, 93.14, and 89.71 % for the larvae, females, and males of the RPW, respectively. These results confirm that the larvae were more susceptible to PDC-AHSAA1 than the adults were, and also the males were more resistant than females were. Similarly, the larvae of the RPW were more sensitive to nanochitosan than the adults were, and the males were more resistant to it than the females were [53]. The longest survival period of the female and male that were treated with nanochitosan was 38.5 and 63 days, respectively, which was compared to that being 94.6 and 103 days in the control group, respectively. The larvae’s growth and development were affected by the PDC-AHSAA1-examined concentrations. Although the exact mechanism by which protease inhibitors increase Bt toxin activity is unknown, they speculate that they might inhibit specific gut proteases that would generally inactivate Bt toxins or limit proteolysis of membrane proteins, preventing the degradation of membrane-bound receptors, thus boosting their half-lives and ability to bind Cry toxins. The compound of the proteinases in the midgut of Melolontha melolontha (the European cockchafer) confers that they have a resistance to the Cry8C toxin [54]. The Cry8C toxin is degraded by the proteinases in vulnerable insects to a 67-kDa fragment that cannot be digested further or degraded into fragments of 10 kDa in resistant European cockchafers, thereby rendering it useless. Septicemia in Lymantria dispar (gypsy moth) and other lepidopteran larvae was caused by Enterobacter sp. in the majority of cases, but not in all of the species that were investigated [55,56]. The larvae and adults of the RPW appeared to be infected by the B. cereus and B. thuringiensis bacteria in this investigation. Our results indicate that the strain, B. thuringiensis PDC-AHSAA1, has a significant control impact on RPW larvae and adults.
5. Conclusions
Four isolated bacteria from the date palm rhizosphere were molecularly identified as Bacillus thuringiensis and B. cereus. They exhibited that they had the potential to be very effective against the RPW, and this study was conducted to reduce the consumption of synthetic chemical pesticides that are typically used to control the RPW populations. Due to their rapid death rate, these bacterial isolates may become potential entomopathogenic bacteria for controlling RPWs. Finally, additional research into the potential utility of Bt in managing RPWs under field conditions is needed. Additionally, the method of action and the molecular receptors that are implicated in causing insect death could be investigated in future studies. The study is one of the few to show the impact of entomopathogenic bacteria, B. thuringiensis and B. cereus, on RPW larvae and the first on female and male RPWs.
Acknowledgments
The authors extend their appreciation to Taif University for funding current work by Taif University Researchers Supporting Project number (TURSP-2020/59), Taif University, Taif, Saudi Arabia.
Author Contributions
Conceptualization, M.M.E.; methodology, M.M.E., M.A., Y.M.A. and M.M.H.; software, M.M.E.; validation, M.M.E., R.H.K. and R.S.; formal analysis, M.M.E.; investigation, M.M.E., M.A., Y.M.A., H.E. and M.M.H.; resources, M.M.E.; data curation, M.M.E.; writing—original draft preparation, M.M.E., M.M.H.; writing—review and editing, M.M.H. and H.E.; visualization, M.M.E.; supervision, M.M.E.; project administration, M.M.E.; funding acquisition, M.M.E., M.A., M.M.H., H.E., R.H.K., R.S.B. and R.S. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The funding is by authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Manachini B., Lo Bue P., Peri E., Colazza S. Potential effects of Bacillus thuringiensis against adults and older larvae of Rhynchophorous ferrugineus. IOBC/WPRS Bull. 2009;45:239–242. [Google Scholar]
- 2.Murphy S.T., Briscoe B.R. The red palm weevil as an alien invasive: Biology and the prospects for biological control as a component of IPM. Biocontrol News Inf. 1999;20:35–46. [Google Scholar]
- 3.El-Sabea A.M., Faleiro J., Abo-El-Saad M.M. The threat of red palm weevil Rhynchophorus ferrugineus to date plantations of the Gulf region in the Middle-East: An economic perspective. Outlooks Pest Manag. 2009;20:131–134. doi: 10.1564/20jun11. [DOI] [Google Scholar]
- 4.Gutierrez A., Ruiz V., Molto E., Tapia G., del Mar Tellez M. Development of a bioacoustic sensor for the early detection of red palm weevil (Rhynchophorus ferrugineus Olivier) Crop Prot. 2010;29:671–676. doi: 10.1016/j.cropro.2010.02.001. [DOI] [Google Scholar]
- 5.Knutelski S., Awad M., Łukasz N., Bukowski M., Smiałek J., Suder P., Dubin G., Mak P. Isolation, identification, and bioinformatic analysis of antibacterial proteins and peptides from immunized hemolymph of red palm weevil Rhynchophorus ferrugineus. Biomolecules. 2021;11:83. doi: 10.3390/biom11010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ju R.-T., Wang F., Wan F.-H., Li B. Effect of host plants on development and reproduction of Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) J. Pest Sci. 2011;84:33–39. doi: 10.1007/s10340-010-0323-4. [DOI] [Google Scholar]
- 7.Hou Y.M., Wu Z.J., Wang C.F. In: The Status and Harm of Invasive Insects in Fujian, China, in Biological Invasions: Problems and Countermeasures. Xie L.H., You M.S., Hou Y.M., editors. Science Press; Beijing, China: 2011. pp. 111–114. [Google Scholar]
- 8.Han Z., Zhou J., Zhong F., Huang Q.L. Research progress on damage and control of Rhynchophorus ferrugineus. Guangdong Agric. Sci. 2013;40:68–71. [Google Scholar]
- 9.Llacer E., Negre M., Jacas J.A. Evaluation of an oil dispersion formulation of imidacloprid as a drench against Rhynchophorus ferrugineus (Coleoptera, Curculionidae) in young palm trees. Pest Manag. Sci. 2012;68:878–882. doi: 10.1002/ps.3245. [DOI] [PubMed] [Google Scholar]
- 10.Milosavljevic I., El-Shafie H.A.F., Faleiro J.R., Hoddle C.D., Lewis M., Hoddle M.S. Palmageddon: The wasting of ornamental palms by invasive palm weevils, Rhynchophorus spp. J. Pest Sci. 2019;92:143–156. doi: 10.1007/s10340-018-1044-3. [DOI] [Google Scholar]
- 11.Faleiro J.R. A review of the issues and management of the red palm weevil Rhynchophorus ferrugineus (Coleoptera: Rhynchophoridae) in coconut and date palm during the last one hundred years. Int. J. Trop. Insect. Sci. 2006;26:135–154. [Google Scholar]
- 12.Pu Y.C., Hou Y.M. Isolation and identification of bacterial strains with insecticidal activities from Rhynchophorus ferrugineus Oliver (Coleoptera: Curculionidae) J. Appl. Entomol. 2016;140:617–626. doi: 10.1111/jen.12293. [DOI] [Google Scholar]
- 13.Hussain A., Rizwan-ul-Haq M., Al-Jabr A.M., Al-Ayied H.Y. Managing invasive populations of red palm weevil: A worldwide perspective. J. Food Agric. Environ. 2013;11:456–463. [Google Scholar]
- 14.Hoddle M.S., Hoddle C.D. How far can the palm weevil, Rhynchophorus vulneratus (Coleoptera: Curculionidae), fly? J. Econ. Entomol. 2016;109:629–636. doi: 10.1093/jee/tov402. [DOI] [PubMed] [Google Scholar]
- 15.Wakil W., Yasin M., Qayyum M.A., Ghazanfar M.U., Al-Sadi A.M., Bedford G.O., Kwon Y.J. Resistance to commonly used insecticides and phosphine fumigant in red palm weevil, Rhynchophorus ferrugineus (Olivier) in Pakistan. PLoS ONE. 2018;13:e0192628. doi: 10.1371/journal.pone.0192628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salama H.S., Foda M.S., El-Bendary M.A., Abdel-Razek A. Infection of red palm weevil, Rhynchophorus ferrugineus, by spore-forming bacilli indigenous to its natural habitat in Egypt. J. Pest Sci. 2004;77:27–31. doi: 10.1007/s10340-003-0023-4. [DOI] [Google Scholar]
- 17.Pu Y.C., Ma T.L., Hou Y.M., Sun M. An entomopathogenic bacterium strain, Bacillus thuringiensis, as a biological control agent against the red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae) Pest Manag. Sci. 2017;73:1494–1502. doi: 10.1002/ps.4485. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J., Qin W.Q., Yan W., Peng Z.Q. Detection of pathogenicity of Metarhizium against Rhynchophorus ferrugineus in laboratory. Chin. J. Trop. Crops. 2012;33:899–905. [Google Scholar]
- 19.Hussain A., Rizwan-ul-Haq M., Al-Ayedh H., Ahmed S., Al-Jabr A.M. Effect of Beauveria bassiana infection on the feeding performance and antioxidant defence of red palm weevil, Rhynchophorus ferrugineus. BioControl. 2015;60:849–859. doi: 10.1007/s10526-015-9682-3. [DOI] [Google Scholar]
- 20.Jalinas J., Güerri-Agulló B., Mankin R.W., López-Follana R., Lopez-Llorca L.V. Acoustic assessment of Beauveria bassiana (Hypocreales: Clavicipitaceae) effects on Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae) larval activity and mortality. J. Econ. Entomol. 2015;108:444–453. doi: 10.1093/jee/tov023. [DOI] [PubMed] [Google Scholar]
- 21.Llácer E., Martínez de Altube M.M., Jacas J.A. Evaluation of the efficacy of Steinernema carpocapsae in a chitosan formulation against the red palm weevil, Rhynchophorus ferrugineus, in Phoenix canariensis. BioControl. 2009;54:559–565. doi: 10.1007/s10526-008-9208-3. [DOI] [PubMed] [Google Scholar]
- 22.Mastore M., Arizza V., Manachini B., Brivio M.F. Modulation of immune responses of Rhynchophorus ferrugineus (Insecta: Coleoptera) induced by the entomopathogenic nematode Steinernema carpocapsae (Nematoda: Rhabditida) Insect Sci. 2015;22:748–760. doi: 10.1111/1744-7917.12141. [DOI] [PubMed] [Google Scholar]
- 23.Banerjee A., Dangar T.K. Pseudomonas aeruginosa, a facultative pathogen of red palm weevil, Rhynchophorus ferrugineus. World J. Microb. Biotechnol. 1995;11:618–620. doi: 10.1007/BF00361002. [DOI] [PubMed] [Google Scholar]
- 24.Sanchis V., Bourguet D. Bacillus thuringiensis: Applications in agriculture and insect resistance management. A review. Agron. Sustain. Dev. 2008;28:11–20. doi: 10.1051/agro:2007054. [DOI] [Google Scholar]
- 25.Wang G., Zhang J., Song F., Gu A., Uwais A., Shao T., Huang D. Recombinant Bacillus thuringiensis strain shows high insecticidal activity against Plutella xylostella and Leptinotarsa decemlineata without affecting nontarget species in the field. J. Appl. Microbiol. 2008;105:1536–1543. doi: 10.1111/j.1365-2672.2008.03866.x. [DOI] [PubMed] [Google Scholar]
- 26.Weathersbee A.A., Lapointe S.L., Shatters R.G. Activity of Bacillus thuringiensis isolates against the root weevil, Diaprepes abbreviatus (Coleoptera: Curculionidae) Fla. Entomol. 2006;89:441–448. doi: 10.1653/0015-4040(2006)89[441:AOBTIA]2.0.CO;2. [DOI] [Google Scholar]
- 27.Martins E.S., Botelho-Praça L., Dumas V.F., Silva-Werneck J.O., Sone E.H., Waga I.C., Berry C., Gomes Monnerat R. Characterization of Bacillus thuringiensis isolates toxic to cotton boll weevil (Anthonomus grandis) Biol. Control. 2007;40:65–68. doi: 10.1016/j.biocontrol.2006.09.009. [DOI] [Google Scholar]
- 28.Alfazairy A.A. Notes on the survival capacity of two naturally occurring entomopathogens of the red palm weevil Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) Egypt J. Biol. Pest Control. 2004;14:423. [Google Scholar]
- 29.Wei G., Bai L., Qu S., Wang S.B. Insect microbiome and their potential application in the insect pest and vector-borne disease control. Acta Microbiol. Sin. 2018;58:1090–1102. [Google Scholar]
- 30.Mazza G., Francardi V., Simoni S., Benvenuti C., Cervo R., Faleiro J.R., Llácer E., Longo S., Nannelli R., Tarasco E., et al. An overview on the natural enemies of Rhynchophorus palm weevils, with focus on R. ferrugineus. Biol. Control. 2014;77:83–92. doi: 10.1016/j.biocontrol.2014.06.010. [DOI] [Google Scholar]
- 31.Elsharkawy M.M., Elsawy M.M., Ismail I.A. Mechanism of resistance to Cucumber mosaic virus elicited by inoculation with Bacillus subtilis subsp. subtilis. Pest Manag. Sci. 2022;78:86–94. doi: 10.1002/ps.6610. [DOI] [PubMed] [Google Scholar]
- 32.Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abbott W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1995;18:265–267. doi: 10.1093/jee/18.2.265a. [DOI] [Google Scholar]
- 34.Martin P.A.W., Travers R.S. Worldwide abundance and distribution of Bacillus thuringiensis isolates. Appl. Environ. Microbiol. 1989;55:2437–2442. doi: 10.1128/aem.55.10.2437-2442.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akiba Y. Microbial ecology of Bacillus thuringiensis VI. Germination of Bacillus thuringiensis spore in the soil. Appl. Entomol. Zool. 1986;21:76–80. doi: 10.1303/aez.21.76. [DOI] [Google Scholar]
- 36.Polanczyk R.A., Zanúncio J.C., Alves S.B. Relationship between chemical properties of the soil and the occurrence of Bacillus thuringiensis. Ciênc. Rural. 2009;39:1–5. doi: 10.1590/S0103-84782009000100001. [DOI] [Google Scholar]
- 37.Akiba Y., Sekijima Y., Aizawa K., Fujiyoshi N. Microbial ecological studies on Bacillus thuringiensis. II. Dynamics of Bacillus thuringiensis in sterilized soil. Jpn. J. Appl. Entomol. Zool. 1977;21:41–46. doi: 10.1303/jjaez.21.41. [DOI] [Google Scholar]
- 38.West A.W., Burges H.D., Dixon T.J. Survival of Bacillus thuringiensis and Bacillus cereus spore inocula in soil: Effects of ph, moisture, nutrient availability and indigenous microorganisms. Soil Biol. Biochem. 1985;17:657–665. doi: 10.1016/0038-0717(85)90043-4. [DOI] [Google Scholar]
- 39.Bullied W.J., Buss T.J., Vessey J.K. Bacillus cereus UW85 inoculation effects on growth, nodulation, and N accumulation in grain legumes: Field studies. Can. J. Plant Sci. 2002;82:291–298. doi: 10.4141/P01-048. [DOI] [Google Scholar]
- 40.Rabinovitch L., Fátima C., Cavados G., Chaves J.Q., Silva K.R.A., Seldin L. A new strain of Bacillus thuringiensis serovar israelensis very active against Blackfly larvae. Mem. Inst. Oswaldo Cruz. 1999;94:683–685. doi: 10.1590/S0074-02761999000500024. [DOI] [PubMed] [Google Scholar]
- 41.Halverson L.J., Clayton M.K., Handelsman J. Population biology of Bacillus cereus UW85 in the rhizosphere of field-grown soybeans. Soil Biol. Biochem. 1993;25:485–493. doi: 10.1016/0038-0717(93)90074-L. [DOI] [Google Scholar]
- 42.Drancourt M., Bollet C., Carlioz A., Martelin R., Gayral J.P., Raoult D. 16S ribosomal DNA sequence analysis of a large collection of environmental and clinical unidentifiable bacterial isolates. J. Clin. Microbiol. 2000;38:3623–3630. doi: 10.1128/JCM.38.10.3623-3630.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barghouthi S.A. A universal method for the identification of bacteria based on general PCR primers. Indian J. Microbiol. 2011;51:430–444. doi: 10.1007/s12088-011-0122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sezen K., Demir I., Demirbag Z. Investigations on bacteria as a potential biological control agent of summer chafer, Amphimallon solstitiale L. (Coleoptera: Scarabaeidae) J. Microbiol. 2005;43:463–468. [PubMed] [Google Scholar]
- 45.Visotto L.E., Oliveira M.G.A., Ribon A.O.B., MaresGuia T.R., Guedes R.N.C. Characterization and identification of proteolytic bacteria from the gut of the velvet bean caterpillar (Lepidoptera: Noctuidae) Environ. Entomol. 2009;38:1078–1085. doi: 10.1603/022.038.0415. [DOI] [PubMed] [Google Scholar]
- 46.Lysenko O. Pathogenicity of Bacillus cereus for insects. I. Production of phospholipase C. Folia Microbiol. 1972;17:221–227. doi: 10.1007/BF02875817. [DOI] [PubMed] [Google Scholar]
- 47.Nishiwaki H., Ito K., Otsuki K., Yamamoto H., Komai K. Purification and functional characterization of insecticidal sphingomyelinase C produced by Bacillus cereus. Eur. J. Biochem. 2004;271:601–606. doi: 10.1111/j.1432-1033.2003.03962.x. [DOI] [PubMed] [Google Scholar]
- 48.Guttmann D.M., Ellar D.J. Phenotypic and genotypic comparisons of 23 strains from the Bacillus cereus complex for a selection of known and putative B. thuringiensis virulence factors. FEMS Microbiol. Lett. 2000;188:7–13. doi: 10.1111/j.1574-6968.2000.tb09160.x. [DOI] [PubMed] [Google Scholar]
- 49.Selvakumar G., Mohan M., Sushil S.N., Kundu S., Bhatt J.C., Gupta H.S. Characterization and phylogenetic analysis of an entomopathogenic Bacillus cereus strain WGPSB-2 (MTCC7182) isolated from white grub Anomala dimidiata (Coleoptera: Scarabaeidae) Biocontrol Sci. Technol. 2007;17:525–534. doi: 10.1080/09583150701311663. [DOI] [Google Scholar]
- 50.Sushil S.N., Mohan M., Selvakumar G., Bhatt J.C., Gupta H.S. Isolation and toxicity evaluation of bacterial entomopathogens against phytophagous white grubs (Coleoptera: Scarabaeidae) in Indian Himalayan hills. Int. J. Pest Manag. 2008;54:301–307. doi: 10.1080/09670870802187274. [DOI] [Google Scholar]
- 51.Sezen K., Demir I., Demirbağ Z. Identification and pathogenicity of entomopathogenic bacteria from common cockchafer, Melolontha melolontha L. (Col., Scarabaeidae) N. Z. J. Crop Horticul. Sci. 2007;35:79–85. doi: 10.1080/01140670709510171. [DOI] [Google Scholar]
- 52.Bravo A., Gomez I., Conde J., Munoz-Garay C., Sanchez J., Miranda R., Zhuang M., Gill S.S., Soberon M. Oligomerization triggers binding of a Bacillus thuringiensis Cry1Ab pore-forming toxin to aminopeptidase N receptor leading to insertion into membrane microdomains. Biochim. Biophys. Acta. 2004;1667:38–46. doi: 10.1016/j.bbamem.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 53.Habood E.A.M., Sayed R.M., Rizk S.A., El-Sharkawy A.Z.E., Badr N.F. Nanochitosan to control the red palm weevil Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) J. Radiat. Res. Appl. Sci. 2022;15:7–17. doi: 10.1016/j.jrras.2022.03.013. [DOI] [Google Scholar]
- 54.Wagner W., Mohrlen F., Schnetter W. Characterization of the proteolytic enzymes in the midgut of the European cockchafer, Melolontha melolontha (Coleoptera: Scarabaeidae) Insect Biochem. Mol. Biol. 2002;32:803–814. doi: 10.1016/S0965-1748(01)00167-9. [DOI] [PubMed] [Google Scholar]
- 55.Broderick N.A., Raffa K.F., Handelsman J. Midgut bacteria required for Bacillus thuringiensis insecticidal activity. Proc. Natl. Acad. Sci. USA. 2006;103:15196–15199. doi: 10.1073/pnas.0604865103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Broderick N.A., Robinson C.J., McMahon M.D., Holt J., Handelsman J., Raffa K.F. Contributions of gut bacteria to Bacillus thuringiensis-induced mortality vary across a range of Lepidoptera. [(accessed on 21 April 2022)];BMC Biol. 2009 7:11. doi: 10.1186/1741-7007-7-11. Available online: http://www.biomedcentral.com/1741-7007/7/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available upon request from the corresponding author.