Abstract
Experimental Borrelia burgdorferi infection of rhesus monkeys is an excellent model of Lyme disease and closely parallels the infection in humans. Little is known about the interaction of host immunity with the spirochete in patients with chronic infection. We hypothesized that rapid development of anti-B. burgdorferi antibody in immunocompetent nonhuman primates (NHPs) is the major determinant of the reduction of the spirochetal load in Lyme borreliosis. This hypothesis was tested by measurement of the spirochetal load by PCR in association with characterization of the anti-B. burgdorferi humoral immune response in immunocompetent NHPs versus that in corticosteroid-treated NHPs. Although anti-B. burgdorferi immunoglobulin G (IgG) antibody was effectively inhibited in dexamethasone (Dex)-treated NHPs, anti-B. burgdorferi IgM antibody levels continued to rise after the first month and reached levels in excess of IgM levels in immunocompetent NHPs. This vigorous production of anti-B. burgdorferi IgM antibodies was also studied in vitro by measurement of antibody produced by B. burgdorferi-stimulated peripheral blood mononuclear cells. Despite these high IgM antispirochetal antibodies in Dex-treated NHPs, spirochetal loads were much higher in these animals. These data indicate that Dex treatment results in interference with isotype switching in this model and provide evidence that anti-B. burgdorferi IgG antibody is much more effective than IgM antibody in decreasing the spirochetal load in infected animals.
Borrelia burgdorferi enters the host via a tick bite, followed by growth through the skin and then dissemination to target organs. The host immune response to B. burgdorferi during Lyme borreliosis results in both protection, by limitation of spirochetal growth, and inflammation, which can be detrimental in that it causes both symptoms and tissue damage (26).
The host immune response to B. burgdorferi has been studied extensively, mostly by in vitro techniques. Cytokines, many of which are produced by Th1 cells, contribute to inflammation (14). Recent work has focused on protective aspects of the immune response important in the development of vaccines (11, 24, 25).
The arm of the immune response believed to be most important in protection and clearance is humoral immunity. This has been investigated in a number of animal models of expermental Lyme borreliosis, including hamsters (13), mice (3, 10, 17), and dogs (27). Vaccine efficacy is thought to be due to the development of protective antibody, and a variety of proteins of B. burgdorferi appear to have protective effects including outer surface protein A (OspA) (8), OspB (9), OspC (17), and decorin binding protein A (5). Clearance of spirochetes naturally occurs with antibody directed against a variety of B. burgdorferi epitopes; the isotype and immunoblot pattern optimal for clearance of active infection by antibody are unknown.
Also, little is known about how the immune response reacts with the spirochete in generating either helpful or harmful immunity. Since the skin is involved early in infection, early immune responses are probably driven by antigen-presenting cells (APCs) and lymphocytes in the skin and draining lymph nodes. This local immune response and the development of high antibody-titers in the serum likely limit spirochetal growth and dissemination.
We hypothesized that interference with humoral immunity to B. burgdorferi would result in higher spirochetal loads in target organs. We also hypothesized that B cells circulating in the peripheral blood can produce anti-B. burgdorferi antibody and that in vitro antibody production by these B cells would mirror the antibody amplitude and isotype in the serum. We tested these hypotheses with the rhesus monkey model of Lyme borreliosis.
MATERIALS AND METHODS
NHPs and spirochetes.
The four adult rhesus macaques (Macaca mulatta; two males and two females) used in this study were housed, cared for, and anesthetized and underwent cisternal punctures as described previously (20). This housing and care were in accordance with the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals (29) in facilities accredited by the American Association for Accreditation of Laboratory Animal Care. Prior to initiation, the study was reviewed and approved by the New Jersey Medical School Animal Care and Use Committee.
Two nonhuman primates (NHPs), designated PAX219 and Z1, respectively, were treated orally with dexamethasone at a dosage considered low to moderate for rhesus macaques (2 mg/kg of body weight/day for 1 week and then 1 mg/kg/day) for 10 weeks after infection; these NHPs are referred to as immunosuppressed (IS1 and IS2, respectively). The other two NHPs, designated E680 and Z23, respectively, did not receive dexamethasone and are referred to as immunocompetent (IC1 and IC2, respectively). Blood was obtained for baseline serum analysis at three times prior to infection. NHPs were necropsied 10 weeks postinfection (p.i.), after euthanasia with ketamine, xylazine, and pentobarbital. Prior to necropsy, the NHPs were perfused with 2 to 4 liters of normal saline.
One million cells of B. burgdorferi of the N40Br strain were used for intradermal inoculations as described previously (20). This strain has been passaged through mouse and NHP brains and has resulted in central nervous system (CNS) invasion in all 21 NHPs tested thus far.
The NHPs were closely monitored for effects of the corticosteroid administration and the spirochetal infection by observation of behavior, weights, and blood testing. The weights obtained were compared to three values obtained before infection and steroid treatment. Complete blood counts, electrolytes, and liver function tests were obtained for the NHPs at 2 to 4 week intervals and compared to three values obtained prior to infection. No significant changes in weight and behavior from preinfection weight or behavior were noted. No changes induced by the dexamethasone administration or infection were noted except for a mild lymphopenia 2 weeks after infection, seen in all infected animals.
Necroscopy and collection of samples.
Before the NHPs were killed they were anesthetized with ketamine (7 mg/kg)-xylazine (3 mg/kg). They were then killed by intravenous administration of an overdose of sodium pentabarbital (65 mg/kg) 10 weeks after inoculation. A complete necroscopy was performed on the NHPs in a laminar-flow hood, beginning with the CNS. During the necropsy the skull was carefully cut laterally and along the midline and the brain was exposed. The brain was separated from the spinal cord by transection of the medulla with a scalpel, and while one investigator was taking sections of the brain for analysis, the other was exposing the spinal cord. After the spinal cord was removed, sections of it were also taken for analysis. Brain and spinal cord samples were quickly excised and kept at 4°C for a few hours. They were subsequently frozen at −70°C. Tissues from uninfected NHPs served as negative controls.
ELISA.
Studies for determination of antibody titers in serum and cerebrospinal fluid (CSF) were performed as described previously (20, 23). In brief, the antigen used in the enzyme-linked immunosorbent assays (ELISAs) and immunoblots were whole-cell sonicates (WCSs) of th strain N40Br. A total of 200 μl of antigen coating solution containing 5 μg of antigen per ml was added to a microtitration plate (Linbro Scientific, Hamden, Conn.) at a concentration of 5 μg/ml, and the plate was incubated overnight at 4°C. The plates were washed three times with phosphate-buffered saline (PBS)–0.05% Tween 20, and 200 μl of the serum was added at a 1/500 dilution. The plates were incubated for 2 h at 37°C and then washed again as described above. A total of 200 μl of horseradish peroxidase-conjugated goat anti-human immunoglobulin of the G (IgG) or M (IgM) isotype (Organon Teknika-Cappel, Malvern, Pa.) was diluted 1:5,000 or 1:10,000 in PBS-Tween 20 and added to each well. Incubation followed for 2 h at 37°C. Goat anti-human antibody reagents were used because they have performed well relative to goat anti-rhesus antibody and were less expensive. The plates were washed, and 200 μl of TMB Microwell Peroxidase Substrate (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) was added to each well, after which 50 μl of 8% sulfuric acid was added to stop the reaction. The plates were read immediately on an ELISA spectrophotometer (Bio-Rad) at 450 nm. On each plate, a standard positive control was run within its linear range of dilutions, and plate-to-plate comparisons were adjusted on the basis of the readings within the linear range of the positive controls on each plate. The raw data are shown in Fig. 1 and 2; preinfection serum and CSF samples from each animal served as negative controls. Background readings, i.e., those for normal serum or CSF samples, were 0.18 to 0.20 by both the IgM and the IgG ELISAs.
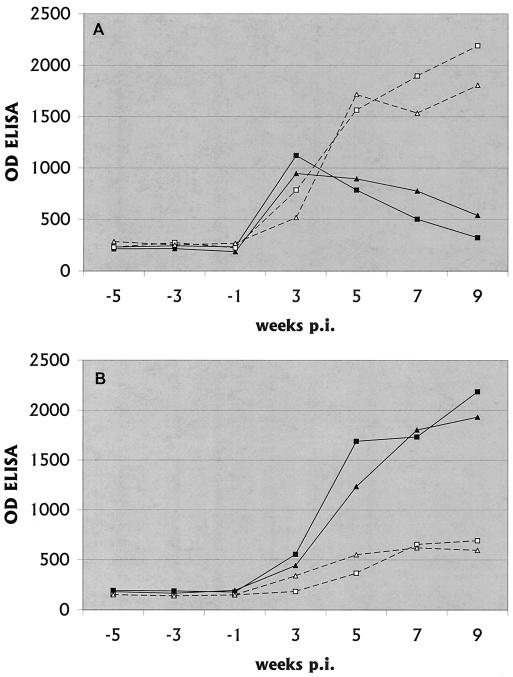
FIG. 1.
Anti-B. burgdorferi IgM (A) and IgG (B) antibody titers in serum. The means of replicate values are shown, with the OD of the ELISA being 1,000 times the recorded value. The duplicate values were within 10% of the mean. The figure was generated from an Excel 1998 spreadsheet by using the Chart Wizard. —, IC1; ▴, IC2; □, IS1; ▵, IS2.
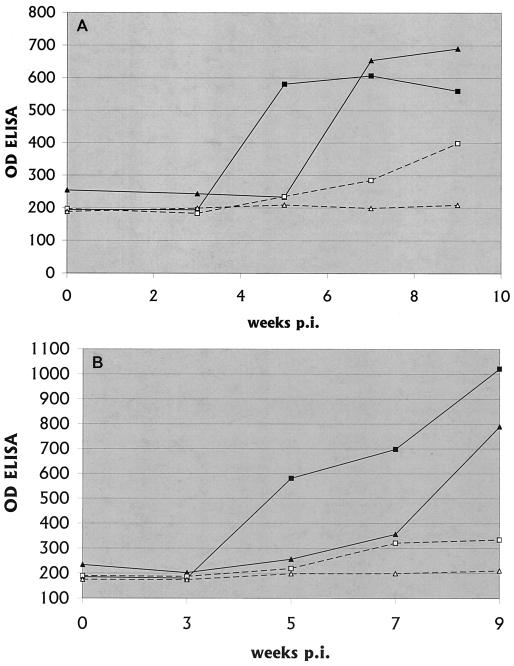
FIG. 2.
Anti-B. burgdorferi IgM (A) and IgG (B) antibody titers in CSF. The means of replicate values are shown, with the OD of the ELISA being 1,000 times the recorded value. The duplicate values were within 10% of the mean. The figure was generated from an Excel 1998 spreadsheet by using the Chart Wizard. —, IC1; ▴, IC2; □, IS1; ▵, IS2.
Immunoblotting.
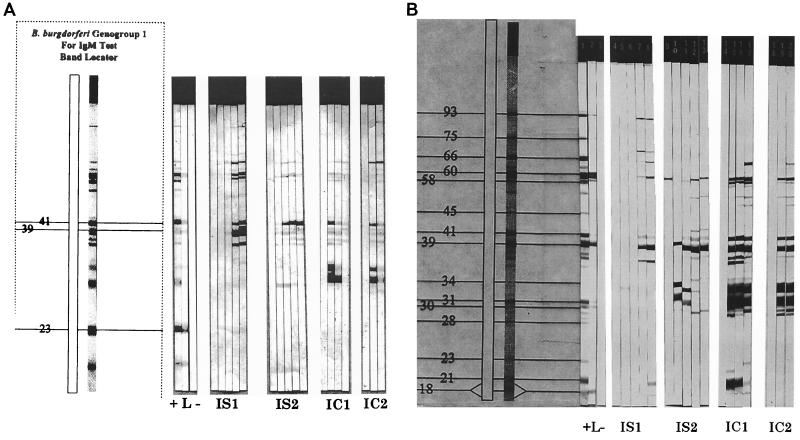
Immunoblotting of NHP sera was performed as described previously (20, 23), except that commercial B. burgdorferi nitrocellulose strips (Microbiology Reference Laboratory, Cypress, Calif.) were used instead of strips made from electrotransferred sodium dodecyl sulfate-polyacrylamide gels. Criteria for immunoblot positivity were the same as those outlined by the Centers for Disease Control and Prevention and the Association of Public Health Laboratory Directors for IgG reactivity for human Lyme disease, a modification of those published by Dressler et al. (7): IgM, reactivity to two or three of proteins of 23, 39, and 41 kDa; IgG, reactivity to five or more of the proteins of 18, 21, 30, 39, 41, 45, 58, 66, and 93 kDa.
In vitro antibody production.
Peripheral blood mononuclear cells (PBMCs) were isolated from fresh venous blood by Ficoll-Hypaque purification as described previously for human blood (22). PBMCs were divided into two groups: one was used as a source for adherent APCs and the other was kept on ice to be used later as the responder population. APCs were purified by allowing them to adhere to 24-well plastic plates, and nonadherent cells were washed away. The medium used in these in vitro studies was AIM-V (Gibco-BRL, Bethesda, Md.). Adherent cells were then incubated with either medium alone or WCSs at 10 μg/ml in medium for 2 h. This pulsing fluid (medium or WCSs) was then washed away, and responder cells were added to the pulsed adherent cells at a concentration of 106/ml. The cells were incubated in a humidified atmosphere with 7% CO2 at 37°C, and the supernatant was harvested at 5 days. No significant anti-B. burgdorferi antibody was produced by PBMCs alone, adherent cells alone, or PBMCs incubated with medium-treated adherent cells. Data are shown in Fig. 1 and 2 for IS1, IS2, and IC1. Adequate blood from IC2 could not be obtained for these studies.
DNA extraction from organs.
Tissue samples were processed by DNA extraction, as follows. The tissue was minced with a scalpel. The minced tissue was brought to a final volume of 0.5 ml with a solution containing 10% sodium dodecyl sulfate and proteinase K. After an overnight incubation at 37°C, the tissue was extracted twice with a 1:1 mixture of phenol and chloroform. A final extraction with chloroform was performed to remove all phenol. One-tenth volume of 3 M sodium acetate (pH 5.4 to 6.0; Sigma Chemical) was then added to the aqeous mixture, followed by the addition of 2 volumes of ice-cold 100% ethanol (Warner-Graham, Cockeysville, Md.). The DNA was precipitated overnight at −20°C and then spun at 14,000 rpm in a Sorval SS-34 rotor for 20 min. The pellet was dried and resuspended in 0.2 ml of TE buffer (10 mM TRIS, 1 mM EDTA [pH 7.6]). Proteinase K was then inactivated by boiling the samples for 5 min. The samples were read on a spectrophotometer at optical densities (ODs) of 260 and 280, and reextraction was performed if the ratio of the OD at 260:OD at 280 was less than 2. The DNA concentration was calculated on the basis of the assumption that 1 OD unit at 260 represents a concentration of 50 μg/ml.
PCR protocol.
The B. burgdorferi gene chosen as the target for amplification was the outer surface protein B (OspB) sequence. OspB-specific primers were ∗OspB122 (5′-CACATCAAAACGCTAAACAAGACCTTCCTG) and ∗OspB462 (5′-TTTATTAGCTTTGAGAGTTTCCTCTGTTATTGA). The OspB-specific PCR protocol was as follows. PCR was carried out by adding 10 μl of extracted DNA in a volume of 50 μl containing 20 mM Tris-HCl (pH 8.4); 50 mM KCl; 0.2 mM (each) dATP, dCTP, and dGTP; 0.19 mM dTTP; 0.01 mM digoxigenin-11-dUTP; 0.5 μM (each) primer (CLONTECH Laboratories, Inc., Palo Alto, Calif.); and 1.25 U of Taq DNA polymerase (Life Technologies, Grand Island, N.Y.). The amplification was achieved with a DNA thermal cycler (Perkin-Elmer Cetus 480). After denaturation at 94°C for 4 min, the amplification was conducted for 35 cycles at 94°C for 45 s for denaturation, 60°C for 45 s for annealing, and 72°C for 2 min for elongation. The final cycle was followed by a single period at 72°C for 7 min. The PCR products were inactivated in the UV reaction chamber and then electrophoresed on a 2% agarose gel and stained with ethidium bromide. The bands were visualized with a UV transilluminator and photographed with a Polaroid camera.
Construction of competitive PCR internal standards.
Construction of competitive PCR internal standards was carried out as described in the PCR MIMIC Construction Kit (Clontech Laboratories, Inc., The Woodlands, Tex.). Chimeric oligonucleotide amplification primers were synthesized (Genosys Biotechnologies, Inc., The Woodlands, Tex.) with a 20-nucleotide sequence homologous to a neutral DNA template supplied by the manufacturer and a B. burgdorferi-specific sequence homologous to the B. burgdorferi gene of interest, that for OspB. The OspB-specific sequence contains the sequences that serve as the target for amplification of both the wild-type and mimic DNA sequences.
The chimeric primers were ∗A (5′-CACATCAAAACGCTAAACAAGACCTTCCTGCGCAAGTGAAATCTCCTCCGT) and ∗B (5′-TTTATTAGCTTTGAGAGTTTCCTCTGTTATTGACTTGAGTCCATGGGGAGCTTT).
The competitive PCR internal standard, OspB competitor, was synthesized with 0.5 ng of neutral DNA and 1 μl of each chimeric primer (20 μM [each] ∗A and ∗B) in a standard PCR mixture of 50 μl. The OspB competitor was generated with the following PCR program (35 cycles): 94°C for 45 s, 50°C for 45 s, and 72°C for 90 s. After the amplification step, a small aliquot (1 to 3 μl) of the PCR mixture was used to directly ligate the OspB competitor into the TA cloning vector pCR2.1. After construction and amplification, the OspB competitor was approximately 200 bp larger than the OspB amplification product. To confirm that the synthesized OspB competitor incorporated the correct OspB sequences the plasmid containing the OspB competitor was sequenced by using the ABI PRISM Dye Terminator Cycle Sequencing Ready Reaction Kit (Perkin-Elmer). Subsequent to the correct sequencing of the OspB competitor plasmid, Escherichia coli was transformed with the OspB competitor plasmid (Invitrogen Corp., San Diego, Calif.). Growth and preparation of the newly constructed OspB competitor plasmids were performed with the QIAGEN (Chatsworth, Calif.) Plasmid kit.
Screening PCR-ELISA.
All reagents described in this section were products of Boehringer-Mannheim (Indianapolis, Ind.) unless otherwise specified.
(i) Plate coating.
A 96-well Immulon plate (Dynatech, Hamden, Conn.) was coated overnight at 4°C with 5 μg of biotinylated bovine serum albumin per ml in PBS. The plate was then washed with PBS wash buffer (PBS, 0.05% Tween 20). A total of 200 μl (per well) of a 10-μg/ml solution of streptavidin in assay buffer (PBS, 0.5% gelatin, 0.15% Tween 20) was then added, and the plate was incubated at 37°C for 1 h. The plate was washed with PBS wash buffer and was then used for the ELISA.
(ii) Digoxigenin detection.
A hybridization solution was prepared by adding 10 pmol of an internal biotinylated probe for OspB (synthesized and labeled by Lofstrand Labs Limited, Gaithersburg, Md.) per ml to a hybridization buffer (0.15 M NaCl, 0.015 M sodium citrate, 20 mM HEPES, 2 mM EDTA, 0.15% Tween 20). A total of 490 μl of hybridization solution was added to 10 μl of PCR products that had been inactivated in the UV reaction chamber and denatured at 94°C for 5 min. A total of 200 μl of the samples was then added to the well of a coated plate. The plate was incubated at 37°C for 3 h. After the plate was washed three times with washing buffer, 200 μl of peroxidase-labeled anti-digoxigenin antibody, diluted 1:5,000 in assay buffer, was added to each well and the plate was incubated for 30 minutes at 37°C. After the plate was washed, 200 μl of 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) substrate solution was added, the color was allowed to develop fully, and the plate was read on an ELISA plate reader at 405 nm. Samples were considered positive if the OD readings were greater than the mean plus 3.5 standard deviations for four negative samples (two water controls and DNA samples from uninfected brains). Use of these cutoffs resulted in positive signals for less than 1% of samples of DNA from uninfected NHPs.
qt-PCR-ELISA.
The DNA extracts from samples that were positive by the screening analysis described above were further analyzed by quantitative PCR-ELISA (qt-PCR-ELISA). At least two samples, and usually three or four samples, were obtained from each tissue of animal PAX219 (IS1) tested. A range of concentrations of OspB competitor plasmid was added to aliquots containing a total of 500 ng of DNA from the tissue samples, and this mixture of wild-type and competitor DNA was subjected to PCR. In the ELISA, 200 μl of PCR product was analyzed for hybridization to the unique wild-type internal sequence, and an equal volume of PCR product was analyzed for the unique competitor internal sequence.
ODs from the ELISA reader were transferred to an SPSS spreadsheet, and regression analyses were performed. A negatively sloped curve of the OD versus the concentration of competitor was generated from the wild-type DNA hybridization data, and a positively sloped curve was generated from the competitor DNA data. The x-axis intercept of the intersection of these two curves yielded a value for the copy number of the sample. Standard errors of the mean were determined for multiple determinations for the same DNA as well as of the same tissue; standard errors of the mean averaged 11 and 17%, respectively.
RESULTS
Anti-B. burgdorferi antibody response in serum.
In immunocompetent NHPs, there was a brisk rise in serum IgM levels within the first weeks of infection, followed by a decline in IgM levels beginning in the 5th week (Fig. 1). As the IgM levels were declining, IgG levels rose and continued to rise to the end of the study.
In immunosuppressed NHPs, IgM antibody levels continued to rise throughout the experiment so that by week 6 levels were higher than those in immunocompent NHPs and continued to rise. IgG antibodies barely rose in the immunosuppressed NHPs and were low at the time of the necropsy.
Anti-B. burgdorferi antibody levels in CSF.
As in previous studies, the time of appearance of anti-B. burgdorferi antibody in CSF was delayed relative to the time of appearance in serum. Levels of antibody in CSF in immunosuppressed NHPs were low throughout the study, while these levels in immunocompetent NHPs were positive by 5 weeks p.i. and remained high (Fig. 2).
Immunoblotting of sera. (i) Anti-B. burgdorferi IgM.
In IS1 IgM reactivity did not appear until 5 weeks after infection, at which time bands appeared at 37, 39, 41, 58, 60, and 66 kDa and a new band appeared at 93 kDa (Fig. 3A). This pattern persisted. In IS1, weak reactivity was present at 31, 39, 60, and 66 kDa at 3 weeks and a strong 41-kDa band appeared at 6 weeks. This pattern persisted. In IC1 and IC2 there were strong early responses, with bands at present 31, 34, and 41 kDa at 3 weeks p.i.; these early responses faded. IC1 had no IgM bands by 9 weeks p.i., while IC2 had only a relatively weak 60-kDa band.
FIG. 3.
IgM (A) and IgG (B) immunoblots. Immunoblots for IS1, IS2, IC1, and IC2 are shown at various times after infection. The left section of each panel depicts the proteins present on the blots and bands which count toward positivity by using the criteria of the Centers for Disease Control and Prevention for human immunoblots. Results for controls are represented as high positive (+), low positive (L), and negative (−). Data shown are from weeks 0, 3, 5, 7, and 9 after infection for IS1 and IS2, for weeks 0, 3, 5, and 7 after infection for IC1, and weeks 0, 3, and 5 after infection for IC2. Data for IC1 at week 9 and IC2 at weeks 7 and 9 were inadequate for immunoblot studies.
(ii) Anti-B. burgdorferi IgG.
IS1 did not display IgG bands until 7 weeks p.i., at which time the predominant response was to p39 (Fig. 3B). The IgG response increased slightly from 7 to 9 weeks p.i. In contrast, IS2 displayed bands of 31, 34, and 39 kDa early after infection, despite a relatively low IgG response by ELISA. The sera from neither immunosuppressed NHP demonstrated positivity by the criteria of the Centers for Disease Control and Prevention (7). For IC1, the immunoblot was positive (7) at 3 weeks p.i. (bands of 21, 31, 34, 39, 41, 58, and 60 kDa), with bands of 52 and 55 kDa appearing later. By 9 weeks p.i. there was fading of the 21-kDa band and appearance of the 66-kDa band. For in IC2 the immunoblot was also positive at 3 weeks, but there were not as many bands as for IC1; i.e., for IC2 there were bands of 31, 34, 39, 41, 55, and 60 kDa. By 5 weeks p.i. there were new bands at 52 and 66 kDa.
In vitro antibody production.
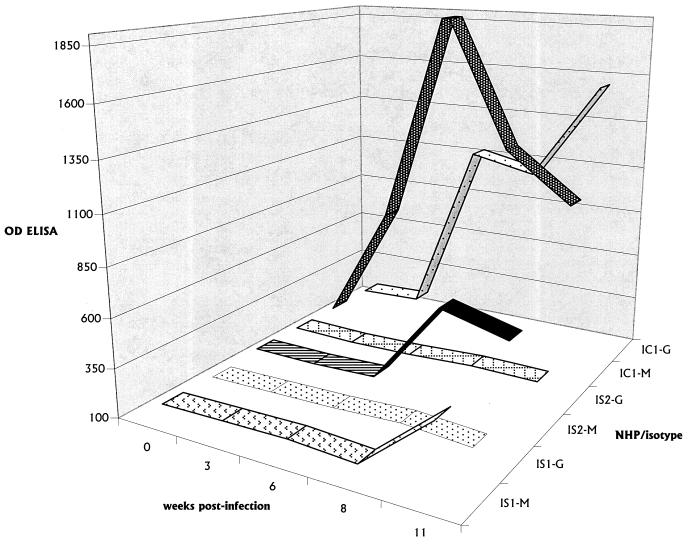
PBMCs produced anti-B. burgdorferi antibody in vitro, stimulated by Borrelia antigens present on B. burgdorferi-pulsed antigen APCs, as shown in Fig. 4. The pattern of antibody production mirrored that present in serum, i.e., low levels of predominantly IgM in IS1 and IS2 and substantial levels of immunoglobulins of both isotypes in IC1 and IC2.
FIG. 4.
In vitro antibody production. In vitro production of IgM in IS1 and IS2 and both IgM and IgG in IC1 is shown as function of the number of weeks p.i. The data shown represent those for samples taken from wells of the cultures on day 6. M, IgM; G, IgG.
Spirochetal dissemination and load. (i) Immunocompetent NHPs (IC1 and IC2).
As in previous studies (19), the spirochetal level was low, but detectable, in immunocompetent NHPs. The level was near the sensitivity of the PCR technique used, i.e., about five copies of B. burgdorferi DNA per 500 ng of DNA extracted from tissue. Screening PCR-ELISAs resulted in positivity for skeletal muscle, nerve, dura mater, brachial plexus, heart, aorta, and bladder tissue samples. Only 7 of 17 (41%) brain stem and spinal cord samples tested were positive. When these samples were tested by the qt-PCR-ELISA technique, a less sensitive technique than the screening PCR-ELISA, all were below the limit of 100 copies (per 500 ng of extracted DNA) necessary for meaningful quantitation by using the qt-PCR-ELISA.
(ii) Immunosuppressed NHPs (IS1 and IS2).
Screening PCR-ELISA readily detected B. burgdorferi DNA in almost all subtentorial structures of the CNS tested (11 of 12 [92%]), as well as nerve, muscle, bladder, and cardiac tissues in dexamethasone-treated NHPs. In one of these NHPs, PAX219, DNA specimens were analyzed by qt-PCR-ELISA (Table 1). Only one sample from these animals was below the cutoff able to be quantified, i.e., 100 copies. The remaining tissues displayed a range of spirochetal loads ranging from (hundreds of copies) in CNS tissue, to thousands of copies in nerve tissue, to tens of thousands of copies in skeletal and cardiac muscle tissue.
TABLE 1.
Quantitative analysis of B. burgdorferi DNA
| IS1 (PAX219) sample | Mean no. of copies (103) of B. burgdorferi DNAa |
|---|---|
| Frontal lobe | qt-PCR-ELISA negative |
| Temporal lobe | qt-PCR-ELISA negative |
| Partietal lobe | qt-PCR-ELISA negative |
| Occipital lobe | qt-PCR-ELISA negative |
| Cerebellum | qt-PCR-ELISA negative |
| Midbrain | <0.1b |
| Pons | 0.14 |
| Medulla | 0.21 |
| Cervical spinal cord | 0.26 |
| Thoracic spinal cord | 0.35 |
| Lumbar spinal cord | 0.31 |
| Dura matter | 0.43 |
| Right sciatic nerve | 8.3 |
| Left sciatic nerve | 8.8 |
| Right brachial plexus | 6.5 |
| Left brachial plexus | 7.3 |
| Right median nerve | 6.3 |
| Left median nerve | 5.3 |
| Apex of heart | 33 |
| Base of heart | 28 |
| Right forearm flexor | 16 |
| Left forearm flexor | 21 |
| Right bicep | 16 |
No. of copies of B. burgdorferi genome per 500 ng of tissue from IS1 (PAX219).
PCR-ELISA positive but qt-PCR-ELISA negative.
(iii) Uninfected NHPs.
Tissues from uninfected NHPs served as negative controls. Screening PCR-ELISAs were negative 9 for 26 of these specimens.
DISCUSSION
Lyme borreliosis is a chronic infection in humans which is increasing in prevalence and which results in significant morbidity. The NHP model of Lyme borreliosis consists of chronic inflammation in the CNS, heart, skin, nerve, and muscle, associated with high levels of antibody in serum and CSF, and a strong cellular immune response to spirochetal antigens. This model, which closely parallels the disease in humans (6, 20), provides an opportunity to fill major gaps in our knowledge about Lyme disease in humans.
How spirochetes are cleared in patients with chronic infection is not well understood. A substantial body of work, primarily with experimental models of Lyme borreliosis, has shown that antibody is important in protection (2) but is not completely efficient in clearance of infection, since low-grade infection persists, despite high levels of antibody in the serum (21). Th2-associated cytokines, such as interleukin-4 (IL-4) (4) and IL-6 (18), are important cytokines in the antispirochetal response, probably through their role in the development of the antiborrelia antibody response. In our study, the spirochetal load was much higher in NHPs whose anti-B. burgdorferi antibody response was blunted by dexamethasone than in immunocompetent animals.
The mechanisms of impaired immunity induced by corticosteroid administration are complex and not completely understood. Many cells have intracellular steroid receptors complexed to a heat shock protein, hsp90. Steroids displace hsp90 from the receptor, allowing the receptor to enter the nucleus and bind to the gene regulatory sequences. Corticosteroids have effects on a wide variety of cellular processes and cell types. Thus, the precise steroid effect or combination of effects that to increased spirochetal loads in the corticosteroid-treated NHPs in this study was not determined. However, given previous studies that have demonstrated the importance of anti-B. burgdorferi antibodies in protection from infection, it is likely that the much lower level of specific antibody in the dexamethasone-treated NHPs contributed significantly to the higher spirochetal loads in those animals. Any combination of the lymphoid cells critical to antibody generation (APCs, helper T cells, or B cells) could have been affected.
We assumed at the initiation of the experiment that anti-B. burgdorferi IgM antibody production in the dexamethasone-treated NHPs would be impaired similarly to IgG isotype production. However, the IgM titers in these animals continued to climb through the course of the experiment, surpassed the levels in the immunocompetent animals, and remained high. The IgM antibody produced in the immunosuppressed NHPs, although quantitatively high, did not have the complexity of the IgG response in immunocompetent NHPs. That is, IgM antibody, when tested by immunoblotting, bound predominantly to the 39- and 41-kDa proteins of the spirochete, while the IgG antibody response at the same time p.i. in the immunocompetent NHPs bound to a broader spectrum of spirochetal proteins. This fact, plus the inability of IgM to penetrate significantly into tissue and its relatively poor ability to fix complement, may explain to some extent the relative inability of IgM to limit spirochetal growth.
We tested an assay for in vitro production of antibody. The measurement of in vitro antibody production has proved helpful in learning about production of antibody to other pathogens such as Bordetella pertussis (10a). We hoped that the in vitro antibody produced would reflect the level and isotype of antibody detectable in serum, and this proved to be true. This high degree of correlation of the level of antibody present in the supernatants of PBMCs to that present in the serum of the infected NHPs is important. It allows this system to be used in the future for dissection of conditions necessary for antibody production, such as important cytokines, APCs, peptides of B. burgdorferi, helper T cells, and B-cell populations. For example, IL-6 is a major cytokine produced by mononuclear cells after stimulation with B. burgdorferi (18, 28), plays a role in limiting arthritis (1), and is also a critical cytokine for the generation of specific antibody (15, 16). The in vitro antibody system will allow dissection of the role of IL-6 and other components in the production of specific antibody in the NHP model of Lyme borreliosis.
The impaired isotype switch in the dexamethasone-treated NHPs was confirmed in the measurement of anti-B. burgdorferi antibody levels in vitro. Although most antibody to B. burgdorferi in an infected NHP is presumably produced in regional lymph nodes, spirochete-specific B cells do circulate in the peripheral blood. These circulating B cells were activated in vitro to produce antibody. The level of production of specific antibody in vitro by these peripheral blood B cells in this study mirrored the levels of production of antibody in the sera of the infected NHPs.
The spirochetal load in immunocompetent NHPs was very low, at or near the sensitivity of the detection of the PCR assay, i.e., 5 to 50 copies per 500 ng of tissue DNA. This level was below the level necessary for meaningful data from the quantitative assay, the qt-PCR-ELISA. The spirochetal load in the dexamethasone-treated NHPs was considerably higher, with the largest concentration being found in skeletal muscle. This tropism of the spirochete to skeletal muscle was unexpected and may have relevance to certain clinical symptoms that occur in infected humans, such as myalgia and fatigue. The dissemination of N40Br, a B. burgdorferi sensu stricto strain, to multiple sites was similar to that observed in our previous studies with NHPs (19). Since in our previous studies we had documented that culture of tissue in this model had a very low yield, tissue culture was not performed in this study to assess spirochetal load. This broad dissemination of sensu stricto strains in nonrodent vertebrates was also seen in a recent study dealing with naturally infected dogs (12); Borrelia garinii isolates, in contrast, appeared to have specific and restricted tropism to the liver. More studies of spirochetal load and tissue tropism in this infection in higher vertebrates are necessary.
In human infections, corticosteroids are sometimes used for the treatment of clinical syndromes such as facial paralysis or arthritis, when these problems are thought to be idiopathic. The data presented above demonstrate the interplay between host immunity and spirochetal infection in Lyme borreliosis and the importance of a competent immune response in limiting the spirochetal load. Since the diagnosis of Lyme borreliosis may sometimes be difficult to make, there is a risk in areas of endemicity of using corticosteroids as therapy in inflammatory syndromes that could represent Lyme disease.
REFERENCES
- 1.Anguita J, Rincon M, Samanta S, Barthold S W, Flavell R A, Fikrig E. Borrelia burgdorferi-infected, interleukin-6-deficient mice have decreased Th2 responses and increased Lyme arthritis. J Infect Dis. 1998;178:1512–1515. doi: 10.1086/314448. [DOI] [PubMed] [Google Scholar]
- 2.Barthold S W. Animal models for Lyme disease. Lab Investig. 1995;72:127–130. [PubMed] [Google Scholar]
- 3.Barthold S W, Feng S, Bockenstedt L K, Fikrig E, Feen K. Protective and arthritis-resolving activity in serum from mice infected with Borrelia burgdorferi. Clin Infect Dis. 1997;25:S9–S17. doi: 10.1086/516166. [DOI] [PubMed] [Google Scholar]
- 4.Brown C R, Reiner S L. Experimental Lyme arthritis in the absence of interleukin-4 or gamma interferon. Infect Immun. 1999;67:3329–3333. doi: 10.1128/iai.67.7.3329-3333.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cassatt D R, Patel N K, Ulbrandt N D, Hanson M S. DbpA, but not OspA, is expressed by Borrelia burgdorferi during spirochetemia and is a target for protective antibodies. Infect Immun. 1998;66:5379–5387. doi: 10.1128/iai.66.11.5379-5387.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coyle P K. Neurological Lyme disease: is there a true animal model? Ann Neurol. 1995;38:560–562. doi: 10.1002/ana.410380403. [DOI] [PubMed] [Google Scholar]
- 7.Dressler F, Whalen J A, Reinhardt B N, Steere A C. Western blotting in the serodiagnosis of Lyme disease. J Infect Dis. 1993;167:392–400. doi: 10.1093/infdis/167.2.392. [DOI] [PubMed] [Google Scholar]
- 8.Fikrig E, Barthold S W, Kantor F S, Flavell R A. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science. 1990;250:553. doi: 10.1126/science.2237407. [DOI] [PubMed] [Google Scholar]
- 9.Fikrig E, Barthold S W, Marcantonio N, Deponte K, Kantor F S, Flavell R A. Roles of OspA, OspB, and flagellin in protective immunity to Lyme borreliosis in laboratory mice. Infect Immun. 1992;60:657–661. doi: 10.1128/iai.60.2.657-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fikrig E, et al. Sera from patients with chronic Lyme disease protect mice from Lyme borreliosis. J Infect Dis. 1994;169:568–574. doi: 10.1093/infdis/169.3.568. [DOI] [PubMed] [Google Scholar]
- 10a.Giacomini E, Urbani F, Ausiello C M, Luzzatti L. Induction of a specific antibody response to Bordetella pertussis antigens in cultures of human peripheral blood mononuclear cells. J Med Microbiol. 1999;48:1081–1086. doi: 10.1099/00222615-48-12-1081. [DOI] [PubMed] [Google Scholar]
- 11.Haupl T, Landgraf S, Netusil P, Biller N, Capiau C, Desmons P, Hauser P, Burmester G R. Activation of monocytes by three OspA vaccine candidates: lipoprotein OspA is a potent stimulator of monokines. FEMS Immunol Med Microbiol. 1997;19:15–23. doi: 10.1111/j.1574-695X.1997.tb01068.x. [DOI] [PubMed] [Google Scholar]
- 12.Hovius K E, Stark L A, Bleumink-Pluym N M, van der Pol I, Verbeek-de Kruif N, Rijpkema S G, Schouls L M, Houwers D J. Presence and distribution of Borrelia burgdorferi sensu lato species in internal organs and skin of naturally infected symptomatic and asymptomatic dogs, as detected by polymerase chain reaction. Vet Q. 1999;21:54–58. doi: 10.1080/01652176.1999.9694992. [DOI] [PubMed] [Google Scholar]
- 13.Johnson R C, Kodner C, Russell M. Passive immunization of hamsters against experimental infection with the Lyme disease spirochete. Infect Immun. 1986;53:713–714. doi: 10.1128/iai.53.3.713-714.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang I, Barthold S W, Persing D H, Bockenstedt L K. T-helper-cell cytokines in the early evolution of murine Lyme arthritis. Infect Immun. 1997;65:3107–3111. doi: 10.1128/iai.65.8.3107-3111.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kopf M, Herren S, Wiles M V, Pepys M B, Kosco-Vilbois M H. Interleukin 6 influences germinal center development and antibody production via a contribution of C3 complement component. J Exp Med. 1998;188:1895–1906. doi: 10.1084/jem.188.10.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.La Flamme A C, Pearce E J. The absence of IL-6 does not affect Th2 cell development in vivo, but does lead to impaired proliferation, IL-2 receptor expression, and B cell responses. J Immunol. 1999;162:5829–5837. [PubMed] [Google Scholar]
- 17.Mbow M L, Gilmore R D, Jr, Titus R G. An OspC-specific monoclonal antibody passively protects mice from tick-transmitted infection by Borrelia burgdorferi B31. Infect Immun. 1999;67:5470–5472. doi: 10.1128/iai.67.10.5470-5472.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pachner A R, Amemiya K, Delaney E, O'Neill T, Hughes C N, Zhang W-F. Interleukin 6 is expressed at high levels in the CNS in Lyme neuroborreliosis. Neurology. 1997;49:147–152. doi: 10.1212/wnl.49.1.147. [DOI] [PubMed] [Google Scholar]
- 19.Pachner A R, Delaney E, O'Neill T. Neuroborreliosis in the non-human primates: Borrelia burgdorferi persists in the central nervous system. Ann Neurol. 1995;38:667–679. doi: 10.1002/ana.410380417. [DOI] [PubMed] [Google Scholar]
- 20.Pachner A R, Delaney E, O'Neill T, Major E. Inoculation of non-human primates with the N40 strain of Borrelia burgdorferi leads to a model of Lyme neuroborreliosis faithful to to the human disease. Neurology. 1995;45:165–172. doi: 10.1212/wnl.45.1.165. [DOI] [PubMed] [Google Scholar]
- 21.Pachner A R, Ricalton N, Delaney E. Comparison of polymerase chain reaction with culture and serology in murine experimental Lyme borreliosis. J Clin Microbiol. 1993;31:208–214. doi: 10.1128/jcm.31.2.208-214.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pachner A R, Steere A C, Sigal L H, Johnson C J. Antigen-specific proliferation of CSF lymphocytes in Lyme disease. Neurology. 1985;35:1642–1644. doi: 10.1212/wnl.35.11.1642. [DOI] [PubMed] [Google Scholar]
- 23.Pachner A R, Zhang W-F, Schaefer H, Schaefer S, O'Neill T. The detection of active infection in Lyme neuroborreliosis in non-human primates: comparison of PCR, culture, and a new bioassay. J Clin Microbiol. 1998;36:3243–3247. doi: 10.1128/jcm.36.11.3243-3247.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Padilla M L, Callister S M, Schell R F, Bryant G L, Jobe D A, Lovrich S D, DuChateau B K, Jensen J R. Characterization of the protective borreliacidal antibody response in humans and hamsters after vaccination with a Borrelia burgdorferi outer surface protein A vaccine. J Infect Dis. 1996;174:739–746. doi: 10.1093/infdis/174.4.739. [DOI] [PubMed] [Google Scholar]
- 25.Philipp M T, Lobet Y, Bohm R P, Jr, Roberts E D, Dennis V A, Gu Y, Lowrie R C, Jr, Desmons P, Duray P H, England J D, Hauser P, Piesman J, Xu K. The outer surface protein A (OspA) vaccine against Lyme disease: efficacy in the rhesus monkey. Vaccine. 1997;15:1872–1887. doi: 10.1016/s0264-410x(97)00133-3. [DOI] [PubMed] [Google Scholar]
- 26.Sigal L H. Lyme disease: a review of aspects of its immunology and immunopathogenesis. Annu Rev Immunol. 1997;15:63–92. doi: 10.1146/annurev.immunol.15.1.63. [DOI] [PubMed] [Google Scholar]
- 27.Straubinger R K, Chang Y F, Jacobson R H, Appel M J. Sera from OspA-vaccinated dogs, but not those from tick-infected dogs, inhibit in vitro growth of Borrelia burgdorferi. J Clin Microbiol. 1995;33:2745–2751. doi: 10.1128/jcm.33.10.2745-2751.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tai K F, Ma Y, Weis J J. Normal human B-lymphocytes and mononuclear cells respond to the mitogenic and cytokine-stimulatory activities of Borrelia burgdorferi and its lipoprotein OspA. Infect Immun. 1994;62:520–528. doi: 10.1128/iai.62.2.520-528.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.U.S. Department of Health and Human Services. Guide for the care and use of laboratory animals. Publication no. 85-23. U.S. Washington, D.C.: Department of Health and Human Services; 1985. [Google Scholar]