Abstract
Cardiovascular abnormalities, such as left ventricular hypertrophy and valvular disorders, particularly mitral valve prolapse, have been described as highly prevalent among adult patients with autosomal dominant polycystic kidney disease (ADPKD). The present study aimed to assess echocardiographic parameters in a large sample of both normotensive and hypertensive ADPKD patients, regardless of kidney function level, and evaluate their association with clinical and laboratorial parameters. A retrospective study consisted of the analysis of clinical, laboratorial, and transthoracic echocardiograms data retrieved from the medical records of young adult ADPKD outpatients. A total of 294 patients (120 M/174 F, 41.0 ± 13.8 years old, 199 hypertensive and 95 normotensive) with a median estimated glomerular filtration rate (eGFR) of 75.5 mL/min/1.73 m2 were included. The hypertensive group (67.6%) was significantly older and exhibited significantly lower eGFR than the normotensive one. Increased left ventricular mass index (LVMI) was seen in 2.0%, mitral valve prolapse was observed in 3.4%, mitral valve regurgitation in 15.3%, tricuspid valve regurgitation in 16.0%, and aortic valve regurgitation in 4.8% of the whole sample. The present study suggested that the prevalence of mitral valve prolapse was much lower than previously reported, and increased LVMI was not seen in most adult ADPKD patients.
Keywords: ADPKD, cardiovascular, echocardiogram, left ventricular mass index, mitral valve prolapse, polycystic disease, valvar abnormalities
1. Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is one of the most common monogenic kidney diseases, with an estimated prevalence between 1 to 2500 through 1 to 1000 individuals [1,2]. ADPKD is characterized by the formation of cysts along the entire nephron, which increase progressively in number and size, cause architectural destruction of the kidney, triggering a gradual loss of function terminating in chronic kidney disease (CKD) [3]. End-stage chronic kidney disease (ESKD) typically occurs in 50% of patients around the 6th decade of life and corresponds to 5 to 10% of patients under renal replacement therapy [4]. Arterial hypertension (AH), an extrarenal non-cystic manifestation frequently seen in ADPKD is derived from compression of the parenchyma and vasculature by cystic growth, activating renin angiotensin-aldosterone system (RAAS) and renal sympathetic nervous system, causing intrarenal ischemia [5,6]. Reduced exercise-induced vasodilation capacity due to lower systemic nitric oxide levels [7] and non-dipping pattern in ambulatory blood pressure monitoring (ABPM) [8,9], indicating endothelial dysfunction, suggest a future risk of developing hypertension in ADPKD [10]. Early onset AH and the increase in total kidney volume (TKV) are some of the most important surrogate prognostic biomarkers of the progression of the disease [10,11]. Beyond AH and intracranial aneurysms, other cardiovascular abnormalities, such as left ventricular hypertrophy, arrhythmias, valvular disorders, and dilated cardiomyopathy have been described [12,13,14,15], all of them representing potential players in morbidity and mortality in this population [12]. The high incidence of cardiovascular complications may be explained not only by precocious manifestation of hypertension [13] since normotensive ADPKD may also present higher left ventricular mass index (LVMI), dilated cardiomyopathies, and biventricular diastolic dysfunction [16,17]. Moreover, given that polycystins exhibit an important role in heart embryogenesis [18,19] and Pkd1-deficient non-cystic mice already show cardiac dysfunction [20], a primary genetic effect of the dysregulation of polycystin function on cardiovascular abnormalities in ADPKD may exist. Several echocardiographic studies in the last decades have been conducted on different populations, providing some controversial data. The present study aimed to assess cardiac structure and function by echocardiography in a large sample of both normotensive and hypertensive ADPKD patients, regardless of their level of renal function, and evaluate their association with clinical and laboratorial parameters.
2. Materials and Methods
2.1. Study Design and Participants
This is a retrospective study based on the medical records of ADPKD patients followed at the outpatient Polycystic Kidney Disease (PKD) Unit of the Nephrology Division of the Universidade Federal de São Paulo (UNIFESP) from March 2002 through June 2018, whose transthoracic echocardiograms (TTE) were available as part of their routine evaluation. Exclusion criteria were age < 18 years, malignant diseases, and ESKD on dialysis. The diagnosis of ADPKD was confirmed by positive family history and renal cysts according to ultrasonographic diagnostic criteria by Pei et al. [21].
2.2. Ethical Considerations
The study was reviewed and approved by the Research Ethics Advisory Committee of the Universidade Federal de São Paulo (UNIFESP) (CAAE: 01521418.2.0000.5505).
2.3. Data Collection
Demographic, anthropometric, and serum biochemistry data were retrieved from patients’ electronic records. Biochemical parameters considered for the present analysis were serum creatinine, sodium, potassium, uric acid, fasting glucose, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein cholesterol (HDL), and triglycerides. Creatinine was determined by an isotope dilution mass spectrometry (IDMS) traceable method and the estimated Glomerular Filtration Rate (eGFR) was calculated using the CKD-EPI equation. Total Kidney Volume (TKV) was calculated and defined as the mean of both kidneys based on renal ultrasound using a standard formula of a modified ellipsoid for each kidney as follows: Renal volume = 4/3 π (anteroposterior diameter/4 + width/4)2 × length/2. Hypertension at admission was defined based on office or clinic levels of systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg [22] or use of antihypertensive medications. Co-morbidities, such as dyslipidemia, diabetes mellitus, previous acute myocardial infarction (AMI) or coronary artery disease (CAD), stroke, and tobacco use, were also included in the analysis.
Transthoracic echocardiograms (TTE) was performed on a Philips IE33 echocardiography device (Andover, MA, USA) using a S5–1 (1–5 mHz) transducer, capable of obtaining two-dimensional images and analysis of the flow velocity with color and flow mapping techniques and Spectral Doppler. The measurements were obtained as recommended by the American Society of Echocardiography [23] and the Left Ventricle (LV) ejection fraction (EF) was calculated using the modified Simpson’s method. The following echocardiographic parameters were considered for the present analysis: Aortic root diameter (AO) and left atrium (LA) volume, left ventricle (LV) posterior wall (LVPW) thickness, interventricular septum (IVS) thickness, LV ejection fraction (EF) and LV Mass Index (LVMI). The upper limits of normality for LVMI were considered to be 135 g/m2 (males) and 111 g/m2 (females). Valvular disorders included Mitral, Aortic and Tricuspid Valve Regurgitation, Mitral Valve Prolapse (MVP), and Diastolic function. Based on the 2003-Guideline from the American Society of Echocardiography [24], the severity of regurgitation was assessed by doppler echocardiography and grades were classified into mild, moderate, and severe. For the purpose of the present analysis, minimal (trace or trivial) regurgitation was not considered since it can be barely detected and is usually physiologic. For mitral and tricuspid regurgitation, three methods were used to quantify severity: Regurgitant jet area, vena contracta, and flow convergence (PISA), which provides EROA (effective regurgitant orifice area). For aortic regurgitation, the assessment was based on the proximal jet width or cross-sectional area immediately below the aortic valve, within 1 cm of the valve. Mitral valve prolapse was diagnosed as systolic displacement of mitral leaflet into the LA of at least 2 mm from the mitral annular plane [25].
2.4. Statistical Analysis
Statistical analyses were performed using IBM SPSS version 23.0 (SPSS Inc., Chicago, IL, USA). In all analyses, p < 0.05 was considered significant. Variable distributions were evaluated by the Kolmogorov–Smirnov test. Normally distributed variables were expressed as mean ± standard deviation (SD) and non-normally distributed ones as median (interquartile range). Differences between groups were tested by Mann–Whitney U pairwise or Student t-test according to their distribution. Categorical variables, presented as n (%), were compared using a Chi-square test. A logistic binary regression using left ventricle impaired relaxation (yes or no) as a dependent variable, and a multivariable linear regression using LVMI as a dependent variable, were additionally performed.
3. Results
3.1. Populations Demographic and Clinical Characteristics
From a total of 557 ADPKD patients referred for care at the outpatient PKD unit, TTE data were available for 346. Of these, 52 did not meet inclusion criteria, resulting in 294 subjects for the present analysis. Table 1 summarizes clinical and demographic characteristics of the whole sample as well as according to their classification as hypertensive (n = 199, 67.6%) or normotensive patients (n = 95, 32.4%). Females were predominant in the whole sample (59.2%) as well as Caucasians (61.6%) and mean age was 41.0 ± 13.8 years old. The hypertensive group was significantly older, exhibited significantly higher mean BMI, median TKV, median kidney length and presented with significantly higher rates of dyslipidemia and diabetes than the normotensive group. The percentage of patients with history of AMI, CAD, or stroke did not differ between groups. Within the normotensive group, most patients belonged to stages I, II, or III of CKD, (75.8%, 20.0%, and 4.2%, respectively), contrasting with fewer patients in such classes in the hypertensive group (16.6%, 30.1%, and 33.2%, respectively), with statistically significant differences. CKD stages IV and V were disclosed only among hypertensive patients (16.1% and 4.0%, respectively). The use of tobacco in the whole sample was observed in 13.6%, with no significant differences between hypertensive or normotensive groups. The majority of hypertensive patients used ACEi or ARB (88.9%), diuretics (53.8%), followed by calcium channel blockers or β-blockers, alone or in association (Table 1). None of the normotensive patients used ACEi or ARB.
Table 1.
Demographic and clinical characteristics in ADPKD patients.
| Total (n = 294) |
Hypertensive (n = 199) |
Normotensive (n = 95) |
p Value | |
|---|---|---|---|---|
| Sex (Male) | 120 (40.8) | 79 (39.7) | 41 (43.2) | 0.33 |
| Age (years) | 41.0 ± 13.8 | 46.0 ± 12.1 | 30.0 ± 10.9 | <0.01 |
| BMI (kg/m2) | 25.4 ± 4.6 | 26.5 ± 4.6 | 23.3 ± 3.6 | <0.01 |
| Race | ||||
| Caucasian | 181 (61.6) | 121 (60.8) | 60 (63.1) | 0.87 |
| Non-caucasian | 113 (38.4) | 78 (39.2) | 35 (36.7) | |
| Dyslipidemia | 46 (15.6) | 42 (21.1) | 4 (4.2) | <0.01 |
| Diabetes mellitus | 9 (3.0) | 9 (4.5) | 0 (0) | 0.03 |
| AMI/CAD | 1 (0.3) | 1 (0.5) | 0 (0) | 0.67 |
| Stroke | 5 (1.7) | 5 (2.5) | 0 (0) | 0.14 |
| CKD stages | ||||
| CKD I | 105 (35.7) | 33 (16.6) | 72 (75.8) | <0.001 |
| CKD II | 79 (26.9) | 60 (30.1) | 19 (20.0) | |
| CKD III | 70 (23.8) | 66 (33.2) | 4 (4.2) | |
| CKD IV | 32 (10.9) | 32 (16.1) | 0 (0) | |
| CKD V | 8 (2.7) | 8 (4.0) | 0 (0) | |
| TKV (mL) * | 476.6 (236.2–915.6) | 679.2 (371.1–1124.7) | 237.6 (171.3–375.8) | <0.01 |
| Kidney Length (cm) * | 16.9 (13.4–23.3) | 20.3 (15.6–25.6) | 13.6 (12.0–16.1) | <0.01 |
| Tobacco use | 40 (13.6) | 31 (15.6) | 9 (9.4) | 0.11 |
| Antihypertensive drugs | ||||
| ACEi/ARB | 177 (88.9) | 177 (88.9) | - | - |
| Diuretics | 107 (53.8) | 107 (53.8) | - | - |
| Calcium channel blockers | 54 (27.1) | 54 (27.1) | - | - |
| Beta-blockers | 44 (22.1) | 44 (22.1) | - | - |
Data as n (%), mean ± standard deviation (SD) or median (interquartile interval), BMI (body mass index); AMI (acute myocardial infarction)/CAD (coronary artery disease); CKD (chronic kidney disease), TKV (total kidney volume) ACEi (ACE inhibitors; ARB (Angiotensin II Receptor Blockers) * Missing data: 79.
3.2. Laboratory and Echocardiographic Findings
Table 2 shows the laboratorial and echocardiographic parameters from all patients, also according to the presence or not of hypertension. The hypertensive group presented significantly lower eGFR, higher serum sodium, potassium, uric acid, fasting glucose LDL and triglycerides. With respect to echocardiographic parameters, hypertensive patients exhibited significantly higher mean values of AO and LA diameters, IVS, LVPW thickness, and LVMI than the normotensive group. Only 6 out of 294 patients (2.0%) presented LVMI values above the upper limit of normality. The results of a linear regression using LVMI as a dependent variable are shown in Table 3. In the multivariate model, age and sex were the only independent determinants of LVMI.
Table 2.
Laboratory and echocardiographic parameters in ADPKD patients.
| Total (n = 294) |
Hypertensive (n = 199) |
Normotensive (n = 95) |
p Value | |
|---|---|---|---|---|
| sCreatinine (mg/dL) | 1.08 (0.86–1.57) | 1.29 (0.98–1.92) | 0.89 (0.76–1.00) | <0.01 |
| eGFR (mL/min/1.73 m2) | 75.5 (44.5–101.0) | 56.5 (34.1–81.6) | 105.2 (90.1–115.7) | <0.01 |
| sNa (mEq/L) | 140.0 (138.0–142.8) | 141.0 (138.8–143.0) | 139.0 (137.0–142.0) | <0.01 |
| sK (mEq/L) | 4.4 (4.1–4.7) | 4.4 (4.1–4.8) | 4.25 (4.0–4.6) | 0.02 |
| sUric Acid (mg/dL) | 5.6 (4.6–7.1) | 6.2 (5.0–7.6) | 4.6 (3.9–5.5) | <0.01 |
| sGlucose (mg/dL) | 92.0 (87.0–98.0) | 94.0 (88.0–100.0) | 88.0 (84.5–95.0) | <0.01 |
| HDL (mg/dL) | 49.0 (41.0–57.0) | 48.5 (39.8–55.3) | 50.0 (42.0–60.5) | 0.14 |
| LDL (mg/dL) | 108.0 (88.0–128.0) | 111.0 (93.0–135.0) | 95.5 (75.8–116.0) | <0.01 |
| Triglycerides (mg/dL) | 104.0 (75.0–151.0) | 123.5 (86.8–166.3) | 82.0 (62.0–104.5) | <0.01 |
| Echocardiographic parameters | ||||
| AORTA (mm) | 31.0 (30.0–34.0) | 32.0 (30.0–35.0) | 30.0 (28.0–32.0) | <0.01 |
| LA (mm) | 35.0 (32.0–37.0) | 36.0 (34.0–38.0) | 34.0 (30.0–36.0) | <0.01 |
| IVS (mm) | 9.0 (8.0–10.0) | 9.0 (9.0–10.0) | 8.0 (8.0–9.0) | <0.01 |
| LVPW (mm) | 9.0 (8.0–9.0) | 9.0 (8.0–10.0) | 8.0 (8.0–9.0) | <0.01 |
| LVMI (g/m2) | 78.8 (69.7–90.5) | 81.8 (72.3–91.9) | 75.8 (64.4–84.9) | <0.01 |
| Increased LVMI—n (%) | 6 (2.0) | 6 (2.0) | 0 (0) | |
| EF (%) | 68.0 (65.0–71.0) | 68.0 (66.0–71.0) | 67.5 (64.0–70.0) | 0.23 |
| Impaired LV relaxation—n (%) | 76 (25.8) | 70 (35.2) | 6 (6.3) | <0.001 |
Data expressed in mean (interquartile interval) or number and percentage of patients n (%). Abbreviations: eGFR (estimated Glomerular Filtration Rate) Na (sodium), K (potassium), HDL (high density lipoprotein), LDL (low density lipoprotein), LA (left atrium), IVS (interventricular septum), LVPW (Left Ventricular Posterior Wall) LVMI (Left Ventricular Mass Index), EF (Ejection Fraction), LV (left ventricular).
Table 3.
Possible determinants of LVMI using linear regression.
| LVMI | ||||
|---|---|---|---|---|
| Independent Variables | Univariate | Multivariate * | ||
| β | p | β | p | |
| Age (years) | 0.25 | <0.01 | 0.22 | <0.01 |
| Sex, (M) | 0.15 | 0.02 | 0.19 | <0.01 |
| BMI (kg/m2) | 0.16 | 0.01 | - | - |
| Tobacco use (yes) | 0.15 | 0.02 | - | - |
| Hypertension (yes) | 0.23 | <0.01 | 0.12 | 0.09 |
| Dyslipidemia (yes) | 0.07 | 0.26 | - | - |
| eGFR (mL/min/1.73 m2) | −0.18 | <0.01 | - | - |
| Uric Acid (mg/dL) | 0.11 | 0.14 | - | - |
| LDL (mg/dL) | 0.02 | 0.74 | - | - |
| Triglycerides (mg/dL) | 0.18 | 0.01 | - | - |
| ACEi/ARB (yes) | 0.04 | 0.58 | - | - |
Abbreviations: LVMI (Left Ventricular Mass Index), BMI (Body Mass Index), eGFR (estimated Glomerular Filtration Rate), LDL (low density lipoprotein), ACEi (ACE inhibitors), ARB (Angiotensin II Receptor Blockers). * Run backwards.
A significantly higher mean percentage of patients with impaired LV relaxation was noticed in the hypertensive versus normotensive groups (35.2 vs. 6.3%, p < 0.001), but hypertension was no longer associated with an impaired LV relaxation when performing a logistic binary regression analysis adjusted for age, BMI and eGFR (HR 0.47 [95% CI 0.20–1.23], p = 0.09).
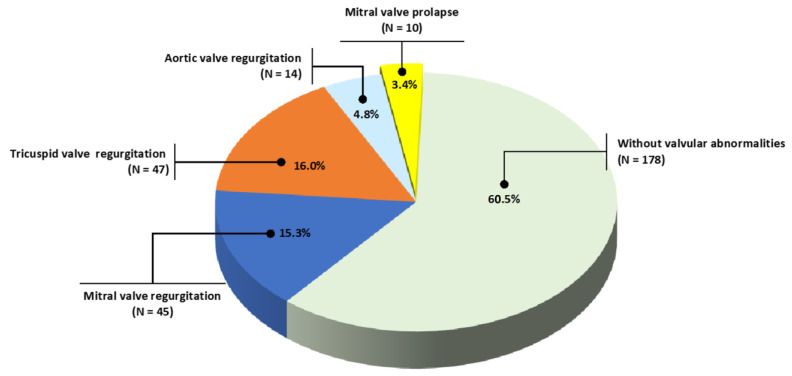
3.3. Valvar Abnormalities
As shown in Figure 1, mitral valve prolapse (MVP) was present in only 3.4% of patients and mitral, aortic, and tricuspid, valve regurgitation in 15.3%, 4.8% and 16.0% of patients. There were no statistical differences between hypertensive or normotensive groups for mitral regurgitation (17.6% vs. 10.5%, respectively, p = 0.08), and tricuspid regurgitation (16.6% vs. 14.7%, respectively, p = 0.41). Aortic regurgitation was significantly higher among hypertensives (6.5 vs. 1.0%, p = 0.03), whereas MVP was higher among normotensives (8.4 vs. 1.0%, p < 0.001).
Figure 1.
Valvar abnormalities.
4. Discussion
Structural and functional cardiovascular alterations, particularly those detected by echocardiography, have been described to be associated with ADPKD [12,13,26,27] and may be important contributors to greater morbidity and mortality in this population [28,29]. However, previous echocardiographic data deriving from most studies conducted between 1980 and 2000 on distinct populations employed different methodologies, yielding heterogeneous results. Herein, we retrospectively assessed echocardiographic parameters and searched for cardiac valvar abnormalities in a large sample of adult patients with ADPKD. Our main findings were a lower prevalence of increased LVMI and valvar lesions than the ones described in most series of adult ADPKD patients, especially concerning mitral valve prolapse (MVP).
Present rates of 3.4% of MVP are similar to the ones described in the general population, around 2.5–3.0% [30,31,32,33,34] but are in contrast with previous rates observed in ADPKD patients ranging from 12% up to 26% in some studies [13,35,36,37,38]. Conversely, some investigators reported rates of 9% for MVP [39], while others had not seen any case of MVP in their sample [40] nor any other cardiac valvar defect at all [41]. In line with the current series, a very recent contemporary cohort of children and young people screened for MVP by Savis et al. [42] showed a prevalence of 0.98% of MVP, without any case of mitral regurgitation. These investigators found variations in normal valvar anatomy in 8.8% of patients, not fulfilling the criteria of true MVP. All this controversy might have accounted for changes in the techniques to evaluate MVP, which were altered in the late 1980s [43,44], or to the redefinition of criteria for echocardiographic parameters dating from the end of the 1990s [33,34]. Therefore, leaflets that appeared to be prolapsed were later found to be normal. The greater part of data investigating valvopathies in ADPKD arising from that period, employed the M-mode echocardiographic method to identify MVP. Subsequently, such technique was shown to fail in displaying the leaflets in relation to their surrounding annular attachments [45], and the results varied widely depending on the orientation of the transducer [33,46]. A study that compared M-mode, two-dimensional, and doppler echocardiography showed that the sensitivity of bidimensional method was significantly higher than M-mode [47]. The association of MVP with age and hypertension in the ADPKD setting is also under debate with some authors finding MVP related to higher blood pressure in children [38], whereas others reported its decrease with age and lack of any association with hypertension [35]. In the current series, MVP is even less prevalent in hypertensive than normotensive ADPKD patients.
Mitral valve regurgitation was disclosed in 15.3% of the present sample, with no differences between hypertensive and normotensive patients contrasting with the description of up to 31% of ADPKD patients in the literature [36,37]. Despite the difficulty of determining the frequency of this alteration in presumed healthy subjects, Choong et al. [48] described a prevalence of 34% of mitral valve regurgitation in the general population. However, when disregarding minimal grades of mitral valve regurgitation, as we did in our sample, their percentage drops by more than half. Minimal grades of regurgitation in any cardiac valve are recognized to be a normal variant and can occur even when the valvular morphology and leaflet motion are normal [49]. Among patients with ADPKD, Hossack et al. [36] demonstrated mitral valve regurgitation in 31% of cases, but it is noteworthy that these investigators also included minimal mitral valve regurgitation, which represented more than 20% of cases, leading the actual prevalence to decrease to approximately 10%. Lumiaho et al. [35], on the other hand, showed a prevalence of 13% of mitral valve regurgitation in ADPKD, being all the regurgitation grades 2 and 3. When dividing their population according to the presence of hypertension and level of renal function, they observed a significant increase in the frequency of mitral valve regurgitation in hypertensive individuals and individuals with worse renal function, which was not observed in our study (p = 0.08). Interestingly, a very recent small retrospective study with patients who underwent echocardiography and whose genotype was confirmed by genetic testing showed a higher prevalence of mitral valve regurgitation in patients with PKD-1 genotype than in those with PKD-2 or non-PKD-1 (46.9% vs. 8.3% vs. 19.0%), with no other cardiac valve complications [50].
The prevalence of 4.8% of aortic valve regurgitation disclosed in our sample, more common among the hypertensive group, was nevertheless lower than the one reported in the general population of similar age (9%) [48] and also if compared to ADPKD patients, (8% for grades 2 or 3) [35]. In contrast to the present and previous studies, Leier et al. [39] reported a higher prevalence of aortic valve regurgitation than mitral valve regurgitation in a small series of 11 ADPKD patients, evidencing once again the heterogeneity of echocardiographic results. Noteworthy, advanced stages CKD may be accompanied by valve annulus calcification, especially in mitral and aortic valves [51,52,53], but in the present sample, there have been only 4.0% of patients in CKD stage V.
Finally, we observed tricuspid valve regurgitation in 16% of patients (not differing statistically in the presence of hypertension), in line with rates reported by Hossack et al. [36] (15%), but higher than those by Lumiaho et al. [35] (4%). Present percentages were lower than the ones described by healthy individuals of the same age (22%) [48]. The reasons for these differences remain unexplained, but according to Choong et al. [48], valvular regurgitation is not uncommon in structurally normal hearts being mitral and tricuspid the most frequent ones, and as the prevalence increases with aging, a wear-and-tear phenomenon must be balanced against a congenital cause. However, trivial tricuspid regurgitation, which represents more than 10% of their sample, was not included in the present study.
Given that the pathophysiology and epidemiology of cardiac valvar abnormalities, particularly MVP, remain not fully understood even in the general population [54], the underlying mechanisms by which these abnormalities arise in ADPKD, remain even less clear. Additional experimental studies and the advent of newer technologies to carefully measure mitral valve function are still warranted to elucidate if MVP and other valvar disorders are still important extrarenal manifestations in ADPKD.
In the present study, the dimensions of the cardiac chambers and aorta, as well as parameters related to myocardial mass, were within limits established by the American Society of Echocardiography Guidelines, elaborated in conjunction with the European Association of Echocardiography [23]. The majority of measurements in our sample were similar to a report of a Brazilian cohort of asymptomatic individuals, with no history of cardiovascular disease, with the same age and sex distribution, as reported by Ângelo et al. [55]. The present mean values of AO, LA, IVS thickness, LVPW and LVMI were statistically higher for the hypertensive group, although still within the normal range (Table 1). However, when performing a multivariable linear regression analysis, hypertension was not an independent determinant of LVMI (p = 0.09). Of note, when we compared the mean values of LVMI between our normotensive patients with the cohort of healthy individuals reported by Ângelo et al. [55], there were no statistical differences in the groups aged 30–39 years old (79.0 ± 13.5, n = 20 vs. 75.1 ± 14.3, n = 57, p = 0.28) and aged 40–49 years old (72.6 ± 16.5, n = 12 vs. 77.9 ± 13.1, n = 39, p = 0.33), respectively. Moreover, only 2.0% of the present cohort exhibited increased LVMI. Left ventricular hypertrophy (LVH) is highly frequent among pediatric and adult patients with ADPKD [13,16,26,38,41,56,57] associated with hypertension [38,57] or not [8,41,56,58]. According to some studies, increased LVMI in normotensive ADPKD patients correlated with 24h ambulatory systolic BP or with an exaggerated systolic BP response to exercise [8,59]. However, the prevalence of increased LVMI is highly variable in the literature ranging from up to 48% in older series [26] down to 20% or less in more recent reports [60]. Noteworthy, the percentage of increased LVMI measured by magnetic resonance imaging (a gold standard method for assessing LV mass) [14] in the HALT-PKD study [61] involving 558 hypertensive ADPKD patients under pharmacological therapy showed a prevalence of left ventricular hypertrophy (LVH) as low as 0.93%. Potential contributors to the lower LVMI values in this large trial might have been the different imaging technique, an earlier diagnosis of hypertension with more rigorous control of BP, and use of renin–angiotensin–aldosterone system inhibitors by more than 80% [13,14,62], as seems to have been the case in our series as well.
Beyond the impact of hypertension on echocardiographic alterations in the current study, the hypertensive sub-group was also significantly older, presented higher BMI, TKV and kidney length, more diabetes or features of metabolic syndrome (hyperuricemia, dyslipidemia), and lower kidney function when compared to the normotensive group, suggesting a longer duration of the disease. A recent review of LVH beyond hypertension in ADPKD showed that there are factors other than hypertension that contribute to LVH [63]. Insulin resistance has been associated with LVMI in ADPKD patients independently of age, weight, BP, and albuminuria [64]. The Chronic Renal Insufficiency Cohort (CRIC) Study [65] showed an association between the degree of loss of renal function and abnormalities in cardiac structure, mainly higher left ventricular mass, but polycystic kidney disease had been excluded from the trial. Chen et al. [60] observed an inverse correlation between eGFR and LVMI. In the present series, eGFR was not an independent determinant of LVMI by multivariable linear regression. Park et al. [66] revealed changes in ventricular structure, with a prevalence of 57% of LVH in CKD patients with an eGFR < 45 mL/min/1.73 m² and 32% for those with eGFR > 60 mL/min/1.73 m², but with no evidence of systolic or diastolic dysfunction. Therefore, it is possible that CKD itself, regardless of ADPKD as the underlying disease, is responsible for such findings. Moreover, Martinez-Vea et al. [67] compared echocardiographic parameters of dialysis patients with ADPKD with patients with CKD from other underlying kidney diseases and did not observe any significant difference between the two groups, reinforcing once more that the observed changes might have resulted from decreased renal function and not from ADPKD.
The prevalence of impaired left ventricular relaxation of 25.8% in the present series suggesting diastolic dysfunction, much higher than observed in the general population (11%) [53], was statistically higher among the hypertensive group. However, as aforementioned, the hypertensive group was older and had less preserved kidney function. Nonetheless, according to Otsuka et al. [68], the change in relaxation can be observed even in the early stages of kidney dysfunction. Moreover, other investigators [16,38,56] observed that LV diastolic function was also impaired among normotensive ADPKD patients and was not dependent on age [27]. On the other hand, when using tissue Doppler imaging (TDI) techniques, diastolic dysfunction was not evidenced in young normotensive ADPKD patients [58]. Of note, hypertension was no longer associated with an impaired LV relaxation in our current series when performing a logistic binary regression analysis adjusted for age, BMI and eGFR (p = 0.09).
It seems that more accurate assessment of cardiac diastolic function, such as TDI, spectral Doppler, and speckle strain echocardiography are still needed (especially when ejection fraction is preserved) as more appropriate surrogate markers of diastolic assessment as opposed to a single parameter measurement [69]
Limitations of our study include the characteristic observational and retrospective design, the lack of a control group, no PKD1 or PKD2 genotyping, and determination of kidney volume by ultrasound. However, its strength relies on the larger sample size when compared to the previous reports.
5. Conclusions
In conclusion, the present study disclosed a lower prevalence of mitral valve prolapse and did not show increased LVMI in adult ADPKD patients contrasting with previous reports. Further prospective studies on other populations are warranted to confirm these findings.
Author Contributions
M.B.P., D.R.d.R. and I.P.H. designed the study, M.B.P. and D.R.d.R. conducted data collection. M.B.P., D.R.d.R., F.G.R., E.P. and I.P.H. performed analysis and interpretation of data. M.B.P., D.R.d.R. and F.G.R. wrote the first draft of the manuscript. E.P. and I.P.H. supervised, enriched, and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was reviewed and approved by the Research Ethics Advisory Committee of the Universidade Federal de São Paulo (UNIFESP) (CAAE: 01521418.2.0000.5505).
Informed Consent Statement
Patients informed consent for study participation was waived, since the data evaluated were anonymous and retrospectively assigned.
Data Availability Statement
Data presented in this study will be provided upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, collection, analysis or interpretation of data and in the writing of the manuscript.
Funding Statement
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq, grant 314677/2021-6 (I.P.H.) and Fundação Oswaldo Ramos—Hospital do Rim. D.R.D.R., F.G.R. are supported by a doctoral study scholarship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lanktree M.B., Haghighi A., Guiard E., Iliuta I.-A., Song X., Harris P.C., Paterson A.D., Pei Y. Prevalence Estimates of Polycystic Kidney and Liver Disease by Population Sequencing. J. Am. Soc. Nephrol. 2018;29:2593–2600. doi: 10.1681/ASN.2018050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willey C.J., Blais J.D., Hall A.K., Krasa H.B., Makin A.J., Czerwiec F.S. Prevalence of Autosomal Dominant Polycystic Kidney Disease in the European Union. Nephrol. Dial. Transplant. 2017;32:1356–1363. doi: 10.1093/ndt/gfw240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu A.S.L., Shen C., Landsittel D.P., Grantham J.J., Cook L.T., Torres V.E., Chapman A.B., Bae K.T., Mrug M., Harris P.C., et al. Long-Term Trajectory of Kidney Function in Autosomal-Dominant Polycystic Kidney Disease. Kidney Int. 2019;95:1253–1261. doi: 10.1016/j.kint.2018.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chebib F.T., Torres V.E. Assessing Risk of Rapid Progression in Autosomal Dominant Polycystic Kidney Disease and Special Considerations for Disease-Modifying Therapy. Am. J. Kidney Dis. 2021;78:282–292. doi: 10.1053/j.ajkd.2020.12.020. [DOI] [PubMed] [Google Scholar]
- 5.Schrier R.W. Renal Volume, Renin-Angiotensin-Aldosterone System, Hypertension, and Left Ventricular Hypertrophy in Patients with Autosomal Dominant Polycystic Kidney Disease. J. Am. Soc. Nephrol. 2009;20:1888–1893. doi: 10.1681/ASN.2008080882. [DOI] [PubMed] [Google Scholar]
- 6.Klawitter J., Reed-Gitomer B.Y., McFann K., Pennington A., Klawitter J., Abebe K.Z., Klepacki J., Cadnapaphornchai M.A., Brosnahan G., Chonchol M., et al. Endothelial Dysfunction and Oxidative Stress in Polycystic Kidney Disease. Am. J. Physiol. Renal. Physiol. 2014;307:F1198–F1206. doi: 10.1152/ajprenal.00327.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reinecke N.L., Cunha T.M., Heilberg I.P., Higa E.M.S., Nishiura J.L., Neder J.A., Almeida W.S., Schor N. Exercise Capacity in Polycystic Kidney Disease. Am. J. Kidney Dis. 2014;64:239–246. doi: 10.1053/j.ajkd.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Valero F.A., Martinez-Vea A., Bardají A., Gutierrez C., Garcia C., Richart C., Oliver J.A. Ambulatory Blood Pressure and Left Ventricular Mass in Normotensive Patients with Autosomal Dominant Polycystic Kidney Disease. J. Am. Soc. Nephrol. 1999;10:1020–1026. doi: 10.1681/ASN.V1051020. [DOI] [PubMed] [Google Scholar]
- 9.Vendramini L.C., Dalboni M.A., de Carvalho J.T.G., Jr., Batista M.C., Nishiura J.L., Heilberg I.P. Association of Vitamin D Levels with Kidney Volume in Autosomal Dominant Polycystic Kidney Disease (ADPKD) Front. Med. 2019;6:112. doi: 10.3389/fmed.2019.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu A.S.L., Shen C., Landsittel D.P., Harris P.C., Torres V.E., Mrug M., Bae K.T., Grantham J.J., Rahbari-Oskoui F.F., Flessner M.F., et al. Baseline Total Kidney Volume and the Rate of Kidney Growth Are Associated with Chronic Kidney Disease Progression in Autosomal Dominant Polycystic Kidney Disease. Kidney Int. 2018;93:691–699. doi: 10.1016/j.kint.2017.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rahbari-Oskoui F., Williams O., Chapman A. Mechanisms and Management of Hypertension in Autosomal Dominant Polycystic Kidney Disease. Nephrol. Dial. Transplant. 2014;29:2194–2201. doi: 10.1093/ndt/gft513. [DOI] [PubMed] [Google Scholar]
- 12.Ecder T., Schrier R.W. Cardiovascular Abnormalities in Autosomal-Dominant Polycystic Kidney Disease. Nat. Rev. Nephrol. 2009;5:221–228. doi: 10.1038/nrneph.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuo I.Y., Chapman A.B. Polycystins, ADPKD, and Cardiovascular Disease. Kidney Int. Rep. 2020;5:396–406. doi: 10.1016/j.ekir.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alam A., Perrone R.D. Left Ventricular Hypertrophy in ADPKD: Changing Demographics. Curr. Hypertens. Rev. 2013;9:27–31. doi: 10.2174/1573402111309010005. [DOI] [PubMed] [Google Scholar]
- 15.Chebib F.T., Hogan M.C., El-Zoghby Z.M., Irazabal M.V., Senum S.R., Heyer C.M., Madsen C.D., Cornec-Le Gall E., Behfar A., Harris P.C., et al. Autosomal Dominant Polycystic Kidney Patients May Be Predisposed to Various Cardiomyopathies. Kidney Int. Rep. 2017;2:913–923. doi: 10.1016/j.ekir.2017.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oflaz H., Alisir S., Buyukaydin B., Kocaman O., Turgut F., Namli S., Pamukcu B., Oncul A., Ecder T. Biventricular Diastolic Dysfunction in Patients with Autosomal-Dominant Polycystic Kidney Disease. Kidney Int. 2005;68:2244–2249. doi: 10.1111/j.1523-1755.2005.00682.x. [DOI] [PubMed] [Google Scholar]
- 17.Paavola J., Schliffke S., Rossetti S., Kuo I.Y.-T., Yuan S., Sun Z., Harris P.C., Torres V.E., Ehrlich B.E. Polycystin-2 Mutations Lead to Impaired Calcium Cycling in the Heart and Predispose to Dilated Cardiomyopathy. J. Mol. Cell. Cardiol. 2013;58:199–208. doi: 10.1016/j.yjmcc.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu G., Tian X., Nishimura S., Markowitz G.S., D’Agati V., Park J.H., Yao L., Li L., Geng L., Zhao H., et al. Trans-Heterozygous Pkd1 and Pkd2 Mutations Modify Expression of Polycystic Kidney Disease. Hum. Mol. Genet. 2002;11:1845–1854. doi: 10.1093/hmg/11.16.1845. [DOI] [PubMed] [Google Scholar]
- 19.Boulter C., Mulroy S., Webb S., Fleming S., Brindle K., Sandford R. Cardiovascular, Skeletal, and Renal Defects in Mice with a Targeted Disruption of the Pkd1 Gene. Proc. Natl. Acad. Sci. USA. 2001;98:12174–12179. doi: 10.1073/pnas.211191098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balbo B.E., Amaral A.G., Fonseca J.M., de Castro I., Salemi V.M., Souza L.E., dos Santos F., Irigoyen M.C., Qian F., Chammas R., et al. Cardiac Dysfunction in Pkd1-Deficient Mice with Phenotype Rescue by Galectin-3 Knockout. Kidney Int. 2016;90:580–597. doi: 10.1016/j.kint.2016.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pei Y., Obaji J., Dupuis A., Paterson A.D., Magistroni R., Dicks E., Parfrey P., Cramer B., Coto E., Torra R., et al. Unified Criteria for Ultrasonographic Diagnosis of ADPKD. J. Am. Soc. Nephrol. 2009;20:205–212. doi: 10.1681/ASN.2008050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.James P.A., Oparil S., Carter B.L., Cushman W.C., Dennison-Himmelfarb C., Handler J., Lackland D.T., LeFevre M.L., MacKenzie T.D., Ogedegbe O., et al. 2014 Evidence-Based Guideline for the Management of High Blood Pressure in Adults: Report from the Panel Members Appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 23.Lang R.M., Badano L.P., Mor-Avi V., Afilalo J., Armstrong A., Ernande L., Flachskampf F.A., Foster E., Goldstein S.A., Kuznetsova T., et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Zoghbi W.A., Enriquez-Sarano M., Foster E., Grayburn P.A., Kraft C.D., Levine R.A., Nihoyannopoulos P., Otto C.M., Quinones M.A., Rakowski H., et al. American Society of Echocardiography Report Recommendations for Evaluation of the Severity of Native Valvular Regurgitation with Two-Dimensional and Doppler. J. Am. Soc. Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 25.Zoghbi W.A., Adams D., Bonow R.O., Enriquez-Sarano M., Foster E., Grayburn P.A., Hahn R.T., Han Y., Hung J., Lang R.M., et al. Ase Guidelines and Standards Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017;30:303–371. doi: 10.1016/j.echo.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Chapman A.B., Johnson A.M., Rainguet S., Hossack K., Gabow P., Schrier R.W. Left Ventricular Hypertrophy in Autosomal Dominant Polycystic Kidney Disease. J. Am. Soc. Nephrol. 1997;8:1292–1297. doi: 10.1681/ASN.V881292. [DOI] [PubMed] [Google Scholar]
- 27.de Almeida E.A.F., de Oliveira E.I., Lopes J.A., Almeida A.G., Lopes M.G., Prata M.M. Diastolic Function in Several Stages of Chronic Kidney Disease in Patients with Autosomal Dominant Polycystic Kidney Disease: A Tissue Doppler Imaging Study. Kidney Blood Press. Res. 2007;30:234–239. doi: 10.1159/000104092. [DOI] [PubMed] [Google Scholar]
- 28.Perrone R.D., Ruthazer R., Terrin N.C. Survival after End-Stage Renal Disease in Autosomal Dominant Polycystic Kidney Disease: Contribution of Extrarenal Complications to Mortality. Am. J. Kidney Dis. 2001;38:777–784. doi: 10.1053/ajkd.2001.27720. [DOI] [PubMed] [Google Scholar]
- 29.Fick G.M., Johnson A.M., Hammond W.S., Gabow P.A. Causes of Death in Autosomal Dominant Polycystic Kidney Disease. J. Am. Soc. Nephrol. 1995;5:2048–2056. doi: 10.1681/ASN.V5122048. [DOI] [PubMed] [Google Scholar]
- 30.Hickey A.J., Wolfers J., Wilcken D.E. Mitral-Valve Prolapse: Prevalence in an Australian Population. Med. J. Aust. 1981;1:31–33. doi: 10.5694/j.1326-5377.1981.tb135284.x. [DOI] [PubMed] [Google Scholar]
- 31.Darsee J.R., Mikolich J.R., Nicoloff N.B., Lesser L.E. Prevalence of Mitral Valve Prolapse in Presumably Healthy Young Men. Circulation. 1979;59:619–622. doi: 10.1161/01.CIR.59.4.619. [DOI] [PubMed] [Google Scholar]
- 32.Procacci P.M., Savran S.V., Schreiter S.L., Bryson A.L. Prevalence of Clinical Mitral-Valve Prolapse in 1169 Young Women. N. Engl. J. Med. 1976;294:1086–1088. doi: 10.1056/NEJM197605132942004. [DOI] [PubMed] [Google Scholar]
- 33.Freed L.A., Levy D., Levine R.A., Larson M.G., Evans J.C., Fuller D.L., Lehman B., Benjamin E.J. Prevalence and Clinical Outcome of Mitral-Valve Prolapse. N. Engl. J. Med. 1999;341:1–7. doi: 10.1056/NEJM199907013410101. [DOI] [PubMed] [Google Scholar]
- 34.Devereux R.B., Brown W.T., Kramer-Fox R., Sachs I. Inheritance of Mitral Valve Prolapse: Effect of Age and Sex on Gene Expression. Ann. Intern. Med. 1982;97:826–832. doi: 10.7326/0003-4819-97-6-826. [DOI] [PubMed] [Google Scholar]
- 35.Lumiaho A., Ikäheimo R., Miettinen R., Niemitukia L., Laitinen T., Rantala A., Lampainen E., Laakso M., Hartikainen J. Mitral Valve Prolapse and Mitral Regurgitation Are Common in Patients with Polycystic Kidney Disease Type 1. Am. J. Kidney Dis. 2001;38:1208–1216. doi: 10.1053/ajkd.2001.29216. [DOI] [PubMed] [Google Scholar]
- 36.Hossack K.F., Leddy C.L., Johnson A.M., Schrier R.W., Gabow P.A. Echocardiographic Findings in Autosomal Dominant Polycystic Kidney Disease. N. Engl. J. Med. 1988;319:907–912. doi: 10.1056/NEJM198810063191404. [DOI] [PubMed] [Google Scholar]
- 37.Timio M., Monarca C., Pede S., Gentili S., Verdura C., Lolli S. The Spectrum of Cardiovascular Abnormalities in Autosomal Dominant Polycystic Kidney Disease: A 10-Year Follow-up in a Five-Generation Kindred. Clin. Nephrol. 1992;37:245–251. [PubMed] [Google Scholar]
- 38.Ivy D.D., Shaffer E.M., Johnson A.M., Kimberling W.J., Dobin A., Gabow P.A. Cardiovascular Abnormalities in Children with Autosomal Dominant Polycystic Kidney Disease. J. Am. Soc. Nephrol. 1995;5:2032–2036. doi: 10.1681/ASN.V5122032. [DOI] [PubMed] [Google Scholar]
- 39.Leier C.V., Baker P.B., Kilman J.W., Wooley C.F. Cardiovascular Abnormalities Associated with Adult Polycystic Kidney Disease. Ann. Intern. Med. 1984;100:683–688. doi: 10.7326/0003-4819-100-5-683. [DOI] [PubMed] [Google Scholar]
- 40.Harrap S.B., Davies D.L., Macnicol A.M., Dominiczak A.F., Fraser R., Wright A.F., Watson M.L., Briggs J.D. Renal, Cardiovascular and Hormonal Characteristics of Young Adults with Autosomal Dominant Polycystic Kidney Disease. Kidney Int. 1991;40:501–508. doi: 10.1038/ki.1991.238. [DOI] [PubMed] [Google Scholar]
- 41.Saggar-Malik A.K., Missouris C.G., Gill J.S., Singer D.R.J., Markandu N.D., Macgregor G.A. Left Ventricular Mass in Normotensive Subjects with Autosomal Dominant Polycystic Kidney Disease. BMJ. 1994;309:1617. doi: 10.1136/bmj.309.6969.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savis A., Simpson J.M., Kabir S., Peacock K., Beardsley H., Sinha M.D. Prevalence of Cardiac Valvar Abnormalities in Children and Young People with Autosomal Dominant Polycystic Kidney Disease. Pediatr. Nephrol. 2022. pp. 1–5. online ahead of print . [DOI] [PubMed]
- 43.Levine R.A., Handschumacher M.D., Sanfilippo A.J., Hagege A.A., Harrigan P., Marshall J.E., Weyman A.E. Three-Dimensional Echocardiographic Reconstruction of the Mitral Valve, with Implications for the Diagnosis of Mitral Valve Prolapse. Circulation. 1989;80:589–598. doi: 10.1161/01.CIR.80.3.589. [DOI] [PubMed] [Google Scholar]
- 44.Levine R.A., Stathogiannis E., Newell J.B., Harrigan P., Weyman A.E. Reconsideration of Echocardiographic Standards for Mitral Valve Prolapse: Lack of Association between Leaflet Displacement Isolated to the Apical Four Chamber View and Independent Echocardiographic Evidence of Abnormality. J. Am. Coll. Cardiol. 1988;11:1010–1019. doi: 10.1016/S0735-1097(98)90059-6. [DOI] [PubMed] [Google Scholar]
- 45.Sahn D.J., Wood J., Allen H.D., Peoples W., Goldberg S.J. Echocardiographic Spectrum of Mitral Valve Motion in Children with and without Mitral Valve Prolapse: The Nature of False Positive Diagnosis. Am. J. Cardiol. 1977;39:422–431. doi: 10.1016/S0002-9149(77)80100-8. [DOI] [PubMed] [Google Scholar]
- 46.Markiewicz W., London E., Popp R.L. Effect of Transducer Placement on Echocardiographic Mitral Valve Motion. Am. Heart J. 1978;96:555–556. doi: 10.1016/0002-8703(78)90172-2. [DOI] [PubMed] [Google Scholar]
- 47.Abbasi A.S., Decristofaro D., Anabtawi J., Irwin L. Mitral Valve Prolapse: Comparative Value of M-Mode, Two-Dimensional and Doppler Echocardiography. J. Am. Coll. Cardiol. 1983;2:1219–1223. doi: 10.1016/S0735-1097(83)80354-4. [DOI] [PubMed] [Google Scholar]
- 48.Choong C.Y., Abascal V.M., Weyman J., Levine R.A., Gentile F., Thomas J.D., Weyman A.E. Prevalence of Valvular Regurgitation by Doppler Echocardiography in Patients with Structurally Normal Hearts by Two-Dimensional Echocardiography. Am. Heart J. 1989;117:636–642. doi: 10.1016/0002-8703(89)90739-4. [DOI] [PubMed] [Google Scholar]
- 49.Lancellotti P., Tribouilloy C., Hagendorff A., Popescu B.A., Edvardsen T., Pierard L.A., Badano L., Zamorano J.L., Bruder O., Cosyns B., et al. Recommendations for the Echocardiographic Assessment of Native Valvular Regurgitation: An Executive Summary from the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging. 2013;14:611–644. doi: 10.1093/ehjci/jet105. [DOI] [PubMed] [Google Scholar]
- 50.Miyamoto R., Sekine A., Fujimaru T., Suwabe T., Mizuno H., Hasegawa E., Yamanouchi M., Chiga M., Mori T., Sohara E., et al. Echocardiographic Findings and Genotypes in Autosomal Dominant Polycystic Kidney Disease. Kidney Dis. 2021;8:246–252. doi: 10.1159/000520300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.London G.M., Pannier B., Marchais S.J., Guerin A.P. Calcification of the Aortic Valve in the Dialyzed Patient. J. Am. Soc. Nephrol. 2000;11:778–783. doi: 10.1681/ASN.V114778. [DOI] [PubMed] [Google Scholar]
- 52.Ureña-Torres P., D’Marco L., Raggi P., García–Moll X., Brandenburg V., Mazzaferro S., Lieber A., Guirado L., Bover J. Valvular Heart Disease and Calcification in CKD: More Common than Appreciated. Nephrol. Dial. Transplant. 2019;35:2046–2053. doi: 10.1093/ndt/gfz133. [DOI] [PubMed] [Google Scholar]
- 53.Fischer M., Baessler A., Hense H.W., Hengstenberg C., Muscholl M., Holmer S., Döring A., Broeckel U., Riegger G., Schunkert H. Prevalence of Left Ventricular Diastolic Dysfunction in the Community: Results from a Doppler Echocardiographic-Based Survey of a Population Sample. Eur. Heart J. 2003;24:320–328. doi: 10.1016/S0195-668X(02)00428-1. [DOI] [PubMed] [Google Scholar]
- 54.Delling F.N., Vasan R.S. Epidemiology and Pathophysiology of Mitral Valve Prolapse: New Insights into Disease Progression, Genetics, and Molecular Basis. Circulation. 2014;129:2158–2170. doi: 10.1161/CIRCULATIONAHA.113.006702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ângelo L.C.S., Vieira M.L.C., Rodrigues S.L., Morelato R.L., Pereira A.C., Mill J.G., Krieger J.E. Echocardiographic Reference Values in a Sample of Asymptomatic Adult Brazilian Population. Arq. Bras. Cardiol. 2007;89:184–190. doi: 10.1590/S0066-782X2007001500007. [DOI] [PubMed] [Google Scholar]
- 56.Bardaji A., Martinez Vea A., Gutierrez C., Ridao C., Richart C., Oliver J.A. Left Ventricular Mass and Diastolic Function in Normotensive Young Adults with Autosomal Dominant Polycystic Kidney Disease. Am. J. Kidney Dis. 1998;32:970–975. doi: 10.1016/S0272-6386(98)70071-X. [DOI] [PubMed] [Google Scholar]
- 57.Cadnapaphornchai M.A., McFann K., Strain J.D., Masoumi A., Schrier R.W. Increased Left Ventricular Mass in Children with Autosomal Dominant Polycystic Kidney Disease and Borderline Hypertension. Kidney Int. 2008;74:1192–1196. doi: 10.1038/ki.2008.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Almeida E.A.F., de Oliveira E.I., Lopes J.A., Almeida A.G., Prata M.M. Tissue Doppler Imaging in the Evaluation of Left Ventricular Function in Young Adults with Autosomal Dominant Polycystic Kidney Disease. Am. J. Kidney Dis. 2006;47:587–592. doi: 10.1053/j.ajkd.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 59.Martinez-Vea A., Bardají A., Gutierrez C., García C., Peralta C., Marcas L., Oliver J.A. Exercise Blood Pressure, Cardiac Structure, and Diastolic Function in Young Normotensive Patients with Polycystic Kidney Disease: A Prehypertensive State. Am. J. Kidney Dis. 2004;44:216–223. doi: 10.1053/j.ajkd.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 60.Chen H., Watnick T., Hong S.N., Daly B., Li Y., Seliger S.L. Left Ventricular Hypertrophy in a Contemporary Cohort of Autosomal Dominant Polycystic Kidney Disease Patients. BMC Nephrol. 2019;20:386. doi: 10.1186/s12882-019-1555-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schrier R.W., Abebe K.Z., Perrone R.D., Torres V.E., Braun W.E., Steinman T.I., Winklhofer F.T., Brosnahan G., Czarnecki P.G., Hogan M.C., et al. Blood Pressure in Early Autosomal Dominant Polycystic Kidney Disease. N. Engl. J. Med. 2014;371:2255–2266. doi: 10.1056/NEJMoa1402685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perrone R.D., Abebe K.Z., Schrier R.W., Chapman A.B., Torres V.E., Bost J., Kaya D., Miskulin D.C., Steinman T.I., Braun W., et al. Cardiac Magnetic Resonance Assessment of Left Ventricular Mass in Autosomal Dominant Polycystic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2011;6:2508–2515. doi: 10.2215/CJN.04610511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oto O.A., Edelstein C.L. The Pathophysiology of Left Ventricular Hypertrophy, beyond Hypertension, in Autosomal Dominant Polycystic Kidney Disease. Nephron. 2022. pp. 1–9. online ahead of print . [DOI] [PubMed]
- 64.Lumiaho A., Pihlajamäki J., Hartikainen J., Ikäheimo R., Miettinen R., Niemitukia L., Lampainen E., Laakso M. Insulin Resistance Is Related to Left Ventricular Hypertrophy in Patients with Polycystic Kidney Disease Type 1. Am. J. Kidney Dis. 2003;41:1219–1224. doi: 10.1016/S0272-6386(03)00354-8. [DOI] [PubMed] [Google Scholar]
- 65.Lash J.P., Go A.S., Appel L.J., He J., Ojo A., Rahman M., Townsend R.R., Xie D., Cifelli D., Cohan J., et al. Chronic Renal Insufficiency Cohort (CRIC) Study: Baseline Characteristics and Associations with Kidney Function. Clin. J. Am. Soc. Nephrol. 2009;4:1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park M., Hsu C.-Y., Li Y., Mishra R.K., Keane M., Rosas S.E., Dries D., Xie D., Chen J., He J., et al. Associations between Kidney Function and Subclinical Cardiac Abnormalities in CKD. J. Am. Soc. Nephrol. 2012;23:1725–1734. doi: 10.1681/ASN.2012020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martinez-Vea A., Bardaji A., Gutierrez C., Garcia C., Peralta C., Aguilera J., Sanchez P., Vidiella J., Angelet P., Compte T., et al. Echocardiographic Evaluation in Patients with Autosomal Dominant Polycystic Kidney Disease and End-Stage Renal Disease. Am. J. Kidney Dis. 1999;34:264–272. doi: 10.1016/S0272-6386(99)70354-9. [DOI] [PubMed] [Google Scholar]
- 68.Otsuka T., Suzuki M., Yoshikawa H., Sugi K. Left Ventricular Diastolic Dysfunction in the Early Stage of Chronic Kidney Disease. J. Cardiol. 2009;54:199–204. doi: 10.1016/j.jjcc.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 69.Dokainish H. Left Ventricular Diastolic Function and Dysfunction: Central Role of Echocardiography. Glob. Cardiol. Sci. Pract. 2015;2015:3. doi: 10.5339/gcsp.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data presented in this study will be provided upon reasonable request.