Abstract
Cucurbits powdery mildew (CPM) is one of the main limiting factors of melon cultivation worldwide. Resistance to races 1, 2, and 5 has been reported in the African accession TGR-1551, whose resistance is controlled by a dominant–recessive epistasis. The dominant and recessive quantitative trail loci (QTL) have previously been located in chromosomes 5 and 12, respectively. We used several densely genotyped BC3 families derived from the cross between TGR-1551 and the susceptible cultivar ‘Bola de Oro’ to finely map these resistance regions. The further phenotyping and genotyping of the selected BC5, BC5S1, BC5S2, BC4S1, BC4xPS, and (BC4xPS) S1 offspring allowed for the narrowing of the candidate intervals to a 250 and 381 kb region in chromosomes 5 and 12, respectively. Moreover, the temperature effect over the resistance provided by the dominant gene has been confirmed. High resolution melting markers (HRM) were tightly linked to both resistance regions and will be useful in marker-assisted selection programs. Candidate R genes with variants between parents that caused a potential modifier impact on the protein function were identified within both intervals. These candidate genes provide targets for future functional analyses to better understand the resistance to powdery mildew in melons.
Keywords: powdery mildew, molecular markers, dominant–recessive epistasis, marker-assisted selection, TIR-NBS-LRR
1. Introduction
Melons (Cucumis melo L.) constitute an economically important vegetable crop. In 2019, their world production reached 27,5 million tons [1] (http://faostat3.fao.org accessed on 1 June 2022). Among the most important diseases affecting this crop, cucurbit powdery mildew (CPM), a fungal disease occurring in both field and greenhouse conditions worldwide, reduces its productivity. The occurrence of CPM early in the growing season can lead to a massive reduction in photosynthetic capacity, with negative impacts on plant growth and development, as well as fruit quality [2,3,4]. Two ectoparasites, Golovinomyces orontii (Go) and Podosphaera xanthii (Px), are economically important and distributed worldwide [5], as they are both the two most commonly reported causal agents of CPM [6,7].
Spatiotemporal changes in the geographic distribution of both Go and Px have been observed during the last three decades [3,4,6]. Significant variations in virulence, expressed by numerous pathotypes and/or races and their spatiotemporal fluctuations, have been described for both species [2,3,4,8,9,10,11]. De Miccolis Angelini et al. [12] emphasized that Px has great potential to evolve new, better-adapted genotypes that can overcome the currently used resistance genes and the efficacy of modern fungicides. Variability in Px virulence, recorded as different physiological races, was first observed in the U.S. in 1938, when race 2 appeared shortly after the release of race 1-resistant ‘PMR 45′ produced in Imperial Valley, California [13]. Since then, many physiological races have been identified according to the reactions after infection of a set of differential melon lines, and several cultivars with resistance to some of those races have been described.
To date, several genes and QTL associated with resistance to CPM have been mapped in different melon populations derived from several genetic sources, including Pm-x [14], Pm-x1,5 and Pm-x3 [15], Pm2F [16], QTL (AR5) [17], and Pm-Edisto47-2 [18] on chromosome 2; qPx1-4 [19] on chromosome 4; Pm-w [20], Pm-R [21], Pm-AN [22], qPx1-5 [19], and QTL PmV-1-Pi124112 [23] on chromosome 5; CmPMrs [24] and qPx1-10 [19] on chromosome 10; Pm-y [14], QTL PmXII-1-Pi124112 [23], QTL (AR5) [17], Pm-Edisto47-1 [18], CmPMR1 [24], qPx1-12 [19], qCmPMR-12 [25], and BPm12.1 [26] on chromosome 12; and Pm-1 [27] on LGIX. Most of these resistances are race-specific and controlled by one or two dominant genes. Epistatic effects have been described between CmPMrs and CmPMR1 QTLs [24], as well as between qPx1-5 and qPx1-12 [19]. In addition, several studies reported that Mildew Locus O (MLO) genes act as susceptibility factors in CPM disease and their inactivation of specific MLO genes (knock-out or knock-down) leads to a mediating form of mlo resistance [28,29]. Natarajan et al. [30] carried out a comparative analysis of 14 MLO genes and investigated their SNIPs and InDels variations, finding associations among them.
Among the resistant cultivars described, the Zimbabwean melon TGR-1551 carries resistance to races 1, 2, and 5 of Px. The inheritance of such resistance was studied in a cross of TGR-1551 with the Spanish susceptible cultivar ‘Bola de Oro’ and the results indicated that it was governed by two independent genes, one dominant and one recessive, whose genetic control corresponded to dominant–recessive epistasis [31]. A QTL analysis carried out on the F2 generation derived from the initial cross allowed for the identification of one major QTL (Pm-R) on chromosome 5 for resistance to the three races [32]. A RIL population (F7:F8) obtained from that cross was also evaluated for Px resistance to races 1, 2, and 5, and QTL analyses carried out by Beraldo-Hoischen et al. [33] confirmed the existence of the Pm-R QTL in chromosome 5, associated with the dominant gene. An additional QTL, possibly associated with the recessive gene, was also detected for Px resistance. This QTL is located on chromosome 12 and several microsatellite markers (TJ29, CMBR111, and CMBR150) were described as being associated with resistance [33]. Two QTL associated with resistance to Px had been previously identified on chromosome 12. One of them is the QTL PmXII.1 [23], associated with the Pm-y gene and controlling resistance to races 1, 2, and 5 derived from PI 124112. The direct association of PmXII.1 with the minor QTL reported in TGR-1551 was not possible due to the lack of common molecular markers between both genetic maps [33]. The other QTL was identified by Fukino et al. [17] and associated with one of the two dominant genes for resistance to race 1 described previously in PMR 5 [34], as well as with PmXII.1. This QTL from Fukino et al. [17] was strongly linked to the microsatellite markers associated with the QTL derived from TGR-1551 (TJ29, CMBR111, and CMBR150) [33]. This candidate interval proposed by Fukino et al. [17] has recently been confirmed by Hong et al. [35] and the resistance of MR-1 to race 1 has recently been mapped to the same region [19,24]. More recently, Howlader et al. [36] described three SNP markers associated with specific resistance to race 5 of Px in chromosome 12, in close proximity to the microsatellite markers previously described by Fukino et al. [17] and Beraldo-Hoischen et al. [33]. The recessive gene present in TGR-1551 is unique, since no recessive genes conferring resistance to more than one Px race has been previously reported in melons. This gene could confer a durable resistance to powdery mildew, similar to the resistance conferred by mlo in barley [37]. CPM resistance is an important objective of melon-breeding programs, which implies the identification of molecular markers and possible candidate genes associated with the trait [38].
The objective of this work was to finely map the recessive locus derived from TGR-1551 conferring resistance to Px by analyzing the advanced backcross populations segregating only at the target genomic region associated with the recessive gene previously described. In addition, a fine mapping of the genomic area where the dominant resistant gene is located in chromosome 5 has been carried out. The generations used in this study were constructed from an original BC3F1 (200 families) obtained after three successive backcrosses to the susceptible cultivar ‘Bola de Oro’ (BO).
2. Results
2.1. Selection of BC3, BC3S1, and BC4 Generations
An existing panel of 124 SNP markers evenly distributed throughout the genome (Background markers 1) (Supplementary Table S1) [39,40,41,42,43] were used to genotype 200 plants of the BC3 (TGR-1551 x BO derived) population in the Agena Bioscience Platform. The resistance to CPM derived from TGR-1551 had previously been described as being controlled by a dominant–recessive epistasis, [31] where a QTL related to the dominant gene was located in chromosome 5 [21,32,33] and another QTL linked to the recessive gene mapped in chromosome 12 [33]. A total number of 20 BC3 plants carrying different regions introgressed in these chromosomes were selected (Table 1). For the rest of the genome, a high percentage of the BO genetic background was prioritized. These selected plants were also genotyped in the Agena Bioscience platform with the markers set as CYSDV1, which contained 16 additional SNP markers located in chromosome 5 (markers set CYSDV1) [44] and with the markers sets WMV1 and WMV2 [43], which had 13 and 3 SNP markers in this region, respectively (Supplementary Table S2) (Table 1). The selfing progenies and BC4 offspring (10 to 20 plants) of these 20 plants were genotyped with the set of markers CYSDV1 and CYSDV2 (Supplementary Table S2) [44]. Moreover, those plants were genotyped with the previously developed HRM markers cysdv63 and cysdv65 [44]. These generations were used to finely map the regions associated with resistance.
Table 1.
Genotype for the SNP markers distributed evenly throughout the genome and located in chromosomes 5 and 12 and for the SNPs in the panels CYSDV1, CYSV2, WMV1, and WMV2 for the BC3 plants selected to evaluate their offspring (H: heterozygous; A: homozygous for ‘Bola de Oro’ allele). The genotype for the set CYSDV2 markers has been inferred from the BC3S1 families. The phenotype for the descendants is indicated (SU: susceptible; SE: segregating). Markers for the candidates’ intervals are in bold format highlighted.
| Number of BC3 Plant | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Markers Set | Marker | Chr a | Genomic Position (bp) | 15 | 19 | 34 | 64 | 78 | 95 | 96 | 105 | 139 | 141 | 166 | 24 | 28 | 37 | 88 | 89 | 146 | 148 | 159 | 198 |
| Background1 | CMPSNP898 | 5 | 256,610 | A | A | A | A | A | A | A | H | A | A | H | A | A | A | H | H | n.d | A | A | A |
| Background1 | CMPSNP387 | 5 | 1,260,621 | A | A | A | H | A | A | A | H | A | A | H | A | A | A | A | H | n.d | A | A | A |
| Background1 | CMPSNP437 | 5 | 1,711,810 | A | A | A | H | A | A | A | A | A | H | H | A | A | A | A | H | n.d | H | A | A |
| Background1 | CMPSNP726 | 5 | 2,373,146 | A | H | A | H | A | A | A | A | A | H | H | A | A | A | A | H | n.d | H | A | A |
| Background1 | SSH9G15 | 5 | 5,781,400 | A | A | A | H | A | A | A | A | A | A | H | H | H | A | A | n.d | n.d | H | A | A |
| CYSDV1 | cysdv10B | 5 | 6,298,039 | A | A | A | H | A | A | A | A | A | A | H | H | H | A | A | A | A | H | H | A |
| CYSDV1 | cysdv11 | 5 | 9,494,514 | A | A | A | H | A | A | A | A | A | A | H | H | H | A | A | A | A | n.d | H | A |
| Background1 | CMPSNP788 | 5 | 12,024,140 | A | A | A | H | A | A | A | A | A | A | H | H | H | A | A | A | A | n.d | H | A |
| WMV1 | b5wmv2 | 5 | 15,026,076 | A | A | A | H | A | A | A | A | A | A | H | H | H | A | A | A | A | H | H | A |
| CYSDV1 | cysdv14 | 5 | 17,216,441 | A | A | A | H | A | A | A | A | A | A | H | H | H | A | A | A | A | n.d | H | A |
| Background1 | 60k41.243 | 5 | 19,612,771 | H | H | A | H | A | H | A | H | A | H | H | A | A | A | A | A | A | n.d | H | A |
| WMV1 | b5wmv3 | 5 | 19,968,717 | n.d | A | A | H | A | A | A | A | A | n.d | H | H | n.d | A | A | A | A | n.d | H | A |
| CYSDV1 | cysdv17 | 5 | 22,544,163 | A | A | A | H | A | A | A | A | A | A | H | H | H | A | A | A | A | n.d | H | A |
| CYSDV1 | cysdv18 | 5 | 24,125,662 | A | H | A | H | A | A | A | A | A | A | H | A | H | A | A | A | A | n.d | H | A |
| CYSDV1 | cysdv19 | 5 | 24,192,280 | A | H | A | H | A | A | A | A | A | A | H | A | H | A | A | A | A | n.d | H | A |
| CYSDV1 | cysdv21 | 5 | 24,427,738 | A | H | A | H | A | A | A | A | A | H | H | A | H | A | A | A | H | n.d | H | A |
| CYSDV1 | cysdv22 | 5 | 24,434,940 | A | H | A | H | A | A | A | A | A | H | H | A | H | A | A | A | H | n.d | H | A |
| CYSDV2 | cysdv40 | 5 | 24,474,795 | A | H | A | H | A | A | A | A | A | H | H | A | H | A | A | A | H | n.d | H | A |
| CYSDV2 | cysdv42 | 5 | 24,608,464 | A | H | A | H | A | A | A | A | A | H | H | A | A | A | A | A | H | n.d | H | A |
| CYSDV2 | cysdv43 | 5 | 24,678,113 | A | H | A | H | A | A | A | A | A | H | H | A | A | A | A | A | H | n.d | H | A |
| CYSDV2 | cysdv44 | 5 | 24,704,705 | A | H | A | H | A | A | A | A | A | H | H | A | A | A | A | A | H | n.d | H | A |
| CYSDV2 | cysdv45 | 5 | 24,761,527 | A | H | A | H | A | A | A | A | A | H | H | A | A | A | A | A | H | n.d | H | A |
| CYSDV2 | cysdv46 | 5 | 24,778,081 | A | H | A | H | A | A | A | A | A | H | H | A | A | A | A | A | H | n.d | H | A |
| CYSDV2 | cysdv48 | 5 | 24,842,569 | A | H | A | H | A | A | A | A | A | H | H | A | A | A | A | A | H | n.d | H | A |
| CYSDV2 | cysdv49 | 5 | 24,842,825 | A | H | A | H | A | A | A | A | A | H | H | A | A | A | A | A | H | n.d | H | A |
| WMV2 | SNP25 | 5 | 24,898,321 | A | H | A | H | A | A | A | A | A | H | H | A | A | A | A | A | H | A | H | A |
| WMV2 | SNP26 | 5 | 25,045,765 | H | H | A | H | A | A | A | A | A | H | H | A | A | A | A | A | H | n.d | H | A |
| CYSDV2 | cysdv50 | 5 | 25,052,002 | H | H | A | H | A | A | A | A | A | H | H | A | A | A | A | A | H | n.d | H | A |
| CYSDV2 | cysdv51 | 5 | 25,132,216 | H | H | A | H | A | H | A | A | A | H | H | A | A | A | A | A | H | n.d | H | A |
| WMV1 | b5wmv4 | 5 | 25,132,292 | H | H | A | H | A | H | A | A | A | H | H | A | A | A | A | A | H | n.d | H | A |
| WMV1 | b5wmv4A | 5 | 25,144,133 | H | H | A | H | A | H | A | A | A | H | H | A | A | A | A | A | H | n.d | H | A |
| CYSDV2 | cysdv53 | 5 | 25,144,085 | H | H | A | H | A | H | A | A | A | H | H | A | A | A | A | A | H | n.d | H | A |
| CYSDV2 | cysdv54 | 5 | 25,210,212 | H | H | A | H | A | H | A | A | A | H | H | A | A | A | A | A | H | n.d | H | A |
| CYSDV2 | cysdv55 | 5 | 25,210,573 | H | H | A | H | A | H | A | A | A | H | H | A | A | A | A | A | H | n.d | H | A |
| CYSDV2 | cysdv56 | 5 | 25,233,221 | H | H | A | H | A | H | A | A | A | H | H | A | A | A | A | A | H | n.d | H | A |
| CYSDV2 | cysdv57 | 5 | 25,341,873 | H | H | A | H | A | H | A | A | A | H | H | A | A | A | A | A | H | n.d | H | A |
| CYSDV2 | cysdv58 | 5 | 25,356,079 | H | H | A | H | A | H | A | A | A | H | H | A | A | A | A | A | H | n.d | H | A |
| CYSDV2 | cysdv59 | 5 | 25,435,244 | H | H | A | H | A | H | A | A | A | H | H | A | A | A | A | A | H | n.d | H | A |
| WMV1 | b5wmv5 | 5 | 25,694,699 | H | H | A | H | A | H | A | A | A | H | H | A | A | A | A | A | H | n.d | H | A |
| CYSDV2 | cysdv60 | 5 | 25,744,140 | H | H | A | H | A | H | A | A | A | H | H | A | A | A | A | A | H | n.d | H | A |
| CYSDV2 | cysdv61 | 5 | 25,756,801 | H | H | A | H | A | H | A | A | A | H | H | A | A | A | A | A | H | n.d | H | A |
| CYSDV2 | cysdv61 | 5 | 25,772,357 | H | H | A | H | A | H | A | A | A | H | H | A | A | A | A | A | H | n.d | H | A |
| CYSDV2 | cysdv62 | 5 | 25,776,015 | H | H | A | H | A | H | A | A | A | H | H | A | A | A | A | A | H | n.d | H | A |
| CYSDV2 | cysdv63 | 5 | 25,782,654 | H | H | A | H | A | H | A | A | A | H | H | A | A | A | A | A | H | n.d | H | A |
| Background1 | CMPSNP464 | 5 | 26,405,006 | H | H | A | H | A | H | A | H | A | A | H | A | A | A | A | A | n.d | A | H | H |
| CYSDV2 | cysdv65 | 5 | 26,408,895 | H | H | A | H | A | H | A | H | A | A | A | A | A | A | A | A | A | n.d | H | H |
| CYSDV2 | cysdv69 | 5 | 26,467,357 | H | H | A | H | A | H | A | H | A | A | A | A | A | A | A | A | A | n.d | H | H |
| CYSDV1 | cysdv24 | 5 | 26,547,204 | H | H | A | A | A | H | A | H | A | A | A | A | A | A | A | A | A | n.d | H | H |
| WMV1 | b5wmv7 | 5 | 26,592,732 | H | H | A | A | A | H | A | H | A | A | A | A | A | A | A | A | A | n.d | H | H |
| WMV1 | b5wmv7B | 5 | 26,592,732 | H | H | A | A | A | H | A | H | A | A | A | A | A | A | A | A | A | n.d | H | H |
| CYSDV1 | cysdv23 | 5 | 26,752,698 | H | H | A | A | A | H | A | H | A | A | A | A | A | A | A | A | A | n.d | H | H |
| CYSDV1 | cysdv25 | 5 | 26,769,546 | H | H | A | A | A | H | A | H | A | A | A | A | A | A | A | A | A | n.d | H | H |
| CYSDV1 | cysdv26 | 5 | 26,940,168 | H | H | A | A | A | H | A | H | A | A | A | A | A | A | A | A | A | n.d | H | H |
| CYSDV1 | cysdv27 | 5 | 26,957,159 | H | H | A | A | A | H | A | H | A | A | A | A | A | A | A | A | A | n.d | H | H |
| WMV1 | b5wmv8 | 5 | 26,963,176 | H | H | A | A | A | H | A | H | A | A | A | A | A | A | A | A | A | n.d | H | H |
| CYSDV1 | cysdv28 | 5 | 27,118,062 | H | H | A | A | A | H | A | H | A | A | A | A | A | A | A | A | A | n.d | H | H |
| WMV1 | b2wmv9 | 5 | 27,273,256 | H | H | A | A | A | H | A | H | A | A | A | A | A | A | A | A | A | n.d | H | H |
| WMV1 | b2wmv10 | 5 | 27,464,146 | H | H | A | A | A | H | A | H | A | A | A | A | A | A | A | A | A | n.d | H | H |
| WMV2 | SNP29 | 5 | 27,538,308 | H | H | A | A | A | H | A | H | A | A | A | A | A | A | A | A | A | n.d | H | H |
| WMV1 | b5wmv11 | 5 | 27,570,154 | H | H | A | A | A | H | A | H | A | A | A | A | A | A | A | A | A | n.d | H | H |
| CYSDV1 | cysdv30B | 5 | 27,570,488 | H | H | A | A | A | H | A | H | A | A | A | A | A | A | A | A | A | n.d | H | H |
| Background1 | AI_13-H12 | 5 | 28,039,739 | H | H | A | A | H | A | A | H | A | A | A | A | A | A | A | A | A | A | H | H |
| Background1 | CMPSNP385 | 12 | 344,819 | A | A | A | A | A | H | A | A | A | A | A | A | A | A | A | H | n.d | A | A | A |
| Background1 | CMPSNP310 | 12 | 5,032,799 | A | A | A | A | A | H | A | A | A | A | A | A | A | A | A | A | n.d | A | A | H |
| Background1 | AI_35-A08 | 12 | 12,750,025 | A | A | A | A | H | A | A | A | A | A | A | A | A | A | A | A | n.d | A | A | H |
| Background1 | ai09g07 | 12 | 16,532,245 | A | A | A | A | H | A | A | A | A | A | A | A | A | A | A | A | n.d | A | A | H |
| Background1 | CMPSNP285 | 12 | 20,421,309 | A | A | H | A | H | H | A | A | H | H | A | A | A | A | H | A | n.d | A | A | H |
| Background1/2 | CMPSNP361 | 12 | 23,000,406 | A | A | H | A | A | H | H | H | H | H | A | A | A | A | H | H | A | H | A | A |
| Background1 | CMPSNP5 | 12 | 24,246,762 | A | A | H | A | A | H | H | A | A | H | A | H | A | A | A | H | n.d | H | A | A |
| Background1 | fr14f22 | 12 | 25,050,570 | H | A | A | A | A | H | H | A | A | H | A | H | A | A | A | H | n.d | A | A | A |
| Background1 | P02.03 | 12 | 25,661,792 | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | n.d | A | A | A |
| SE | SE | SE | SE | SE | SE | SE | SE | SE | SE | SE | SU | SU | SU | SU | SU | SU | SU | SE | SU | ||||
a: chromosome.
2.2. Narrowing of the Candidate Region on Chromosome 5
The 20 BC3S1 families were phenotyped for the resistance against races 1, 2, and 5 of Px (Table 1). The response of the offspring of those plants not carrying a TGR-1551 introgression in chromosome 12 depended on the genotype in chromosome 5. Segregation for the resistance was observed for those families with parents heterozygous for the region between markers cysdv63 (chr5: 25,782,654 bp) and CMPSNP464 (chr5: 26,405,006 bp) (BC3 plants 19, 64, 159, and 166) (Table 1). Contrarily, all the descendants from parents without a TGR-1551 introgression for this region (BC3 plants 28 and 37) were susceptible. Otherwise, BC3 plant 198, whose BC3S1 offspring was susceptible to Px, was homozygous for the BO allele at the locus cysdv63 and heterozygous at the position of the marker CMPSNP464 (Table 1), setting in this marker the lower limit of the candidate resistance region. Moreover, BC3-198 also carried a TGR-1551 introgression at chromosome 12 that seemed to be unrelated to the recessive resistance (Table 1). On the other hand, the family BC3S1-146 was also susceptible but it segregated at the position of the marker cysdv63, whereas it was homozygous for the BO allele at the locus cysdv65 (chr5: 26,408,895 bp), narrowing the candidate interval to a region of ~622 kb between markers cysd63 and CMPSNP464. This same region had been described as related to CYSDV resistance derived from ‘TGR-1551’ [44].
To narrow the candidate interval in chromosome 5, the heterozygous plants for this region—BC4-159 plants 4 and 11—were selected. The plant BC4(159)4 was crossed with ‘Piel de Sapo’ (PS) to obtain the BC4(159)4xPS offspring. It was also backcrossed to BO to obtain the BC5(159)4 progeny. In the case of the plant BC4(159)11, only the BC5 offspring backcrossed to BO were obtained. These families (approximately six plants each) were genotyped with the HRM markers cysdv63 and cysdv65 and those plants that were heterozygous for both markers were selected and genotyped with background markers 2 set in the Agena Bioscience platform. The plants with the higher genetic background for the BO or PS genome were selected and self-pollinated. Families BC4(159)4xPS-7S1, BC4(159)4xPS-18S1, BC5(159)4-14S1, and BC5(159)11S1 were phenotyped for the resistance to races 1, 2, and 5 of CPM (20 plants per family). They were also genotyped with the HRM markers cysdv63 and cysdv65. The phenotyping/genotyping results of these offspring were compatible with the interval for the dominant gene obtained in the BC3 and BC3S1 analyses. Moreover, three plants were found to be recombinant in the candidate region: (BC4(159)4xPS-7S1 plants 2 and 13 and BC5(159)4-14S1 plant 5). These plants were also genotyped with the HRM markers cysdv626 (chr5: 26,058,307 bp) and cysdv6531 (chr5: 26,309,060 bp) to narrow the interval (Table 2). These three plants were heterozygous at the position of the marker cysdv626 and homozygous for the BO allele for the marker cysdv65. For the marker cysdv6531, BC4(159)4xPS-7S1 plant 2 was heterozygous, while plants BC4(159)4xPS-7S1-13 and BC5(159)4-14S1-5 were homozygous for the BO allele. While the BC4(159)4xPS-7S1 plants 2 and 13 were resistant to CPM, BC5(159)4-14S1 plant 5 was susceptible. These phenotyping/genotyping results enabled us to narrow the candidate interval to a region of 250,753 bp between markers cysdv626 and cysdv6531 (Figure 1). There are 34 predicted genes in the candidate region (Supplementary Table S3). GBS data were used to perform an SNP-calling analysis and SNPeff was used to predict the effects of genetic variants found between TGR-1551 and BO in this candidate region. SNPs were only found in two candidate genes, MELO3C004297 and MELO3C004311, that coded a branched-chain-amino-acid aminotransferase-like protein and a TMV resistant protein N-like, respectively. The detected SNPs had a modifier-predicted impact on the coded protein functions (Supplementary Table S4). One of these candidate genes, MELO3C004311, has also been proposed as the gene conferring resistance in the accession MR-1 [19].
Table 2.
Genotype for SNP markers in the candidate region of chromosome 5 of those BC4xPS-S1 and BC5S1 plants that were recombinant between markers cysdv63 and cysdv65 (A: homozygous for ‘Bola de Oro’ allele; H: heterozygous). The phenotype of the genotyped plants is indicated (SU: susceptible). The physical position of the SNPs in version v.4.0 of the melon genome (available at https://melonomics.net/ (accessed on 1 June 2022)) is indicated.
| Marker Name | Chr a | Genomic Position (bp) v.4.0 | BC4(159)4xPS-7S1-13 | BC4(159)4xPS-7S1-2 | BC5(159)4-14S1-5 |
|---|---|---|---|---|---|
| Cysdv63 | 5 | 25,782,654 | H | H | H |
| Cysdv626 | 5 | 26,058,307 | H | H | H |
| Cysdv6531 | 5 | 26,309,060 | A | H | A |
| Cysdv65 | 5 | 26,408,895 | A | A | A |
| Phenotype | Resistant | Resistant | Susceptible |
a: chromosome
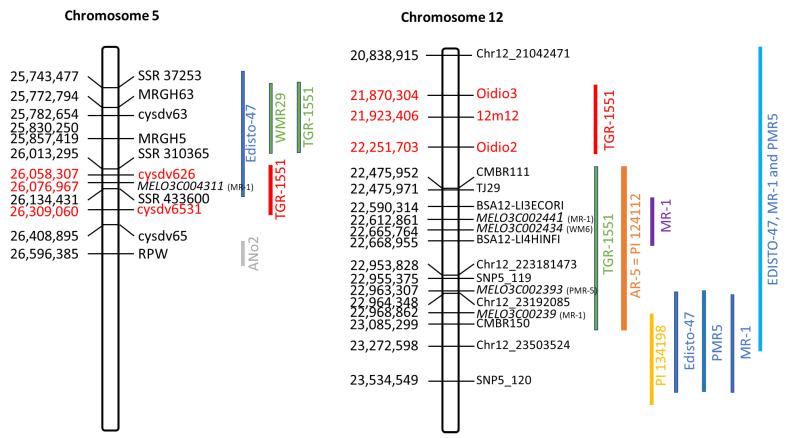
Figure 1.
Genomic regions associated with the resistance to powdery mildew (Podosphaera xanthii) derived from different sources. The results obtained in this work are marked in red and the previous results derived from TGR-1551 are represented in green. The proposed candidate genes for other resistance sources are also indicated. The presented physical positions are refer to the genome version v.4.0 of C. melo (available at https://melonomics.net/ accessed on 1 June 2022).
Several BC5S1 plants, which were homozygous for the TGR-1551 alleles at the candidate region of chromosome 5, were self-pollinated to obtain their BC5S2 offspring. These BC5S2 families were phenotyped for resistance against Px races 1, 2, and 5 and all of them resulted in being resistant, validating the previously obtained results.
2.3. Narrowing of the Candidae Region on Chromosome 12
Eight BC3 plants that only had a TGR-1551 introgression in chromosome 12 (BC3 plants 34, 78, 88, 96, and 139), or with an introgression not related to CPM resistance in chromosome 5 (BC3 plants 24, 89, and 148), were analyzed. The selfing progenies of two of these plants heterozygous for markers CMPSNP285 (chr12: 20,421,309 bp) and CMPSNP361 (chr12: 23,000,406 bp) (BC3 plants 34 and 139) segregated for the resistance in question, while the descendants of plant BC3-88 were susceptible. Four recombinant plants were found according to these two markers (BC3 plants 78, 89, 96, and 148) (Table 1). On the one hand, BC3-78 was heterozygous for the marker CMPSNP285 and homozygous for the BO allele for the marker CMPSNP361 and its offspring segregated for resistance. On the other hand, the BC3 plants 89, 96, and 148 were homozygous for the BO allele for the marker CMPSNP285 and heterozygous for the marker CMPSNP361 with susceptible, segregant, and susceptible progenies, respectively. The high level of recombination in this region demonstrated that the BC3S1-88 offspring were susceptible to CPM even though the BC3-88 plant was heterozygous for both markers in chromosome 12. These results allowed us to initially narrow the candidate interval to a 2.6 Mb region between markers CMPSNP285 and CMPSNP361.
Remarkably, those BC3S1 families whose parents had the TGR-1551 introgressions at both candidate regions of chromosomes 5 and 12 (BC3 plants 95, 105 and 141) had a higher ratio of resistant plants, which agrees with a dominant–recessive epistatic model.
Based on GBS data previously obtained by the research group, 10 high-resolution melting markers (HRM) were designed to narrow the candidate region in chromosome 12 (Supplementary Table S5). These HRM markers were evenly distributed throughout the interval between markers CMPSNP285 and CMPSNP361. Six BC3 plants and their BC3S1 offspring, which had previously been phenotyped for resistance to CPM (BC3S1 families 34, 88, 89, 96, 139, and 148), were genotyped with these 10 HRM markers (Table 3). These results showed that a recombination event had taken place within markers CMPSNP285 and CMPSNP361 in the BC3-88 plant. Only those BC3S1 families whose parents were heterozygous for both markers Oidio2 (chr12: 22,251,703 bp) and Oidio1 (chr12: 22,309,972 bp) (BC3S1 families 34, 96, and 139) segregated for the resistance to CPM. The plant BC3-139 was homozygous for the BO allele at the position of the marker Oidio3 (chr12: 21,870,304 bp) and its selfing progeny was segregant for resistance, setting the upper limit of the candidate region at the position of the locus Oidio3. BC3 plants 88 and 148 were homozygous for the BO allele for the markers Oidio3, Oidio2, and Oidio1 and heterozygous for the marker OidioC (chr12: 22,875,711 bp). Their offspring were susceptible to CPM, narrowing the candidate interval to a ~1 Mb region between markers Oidio3 and OidioC.
Table 3.
SNP markers used to narrow the candidate interval of chromosome 12 (A: homozygous for the ‘Bola de Oro’ allele; H: heterozygous). The molecular markers in the candidate regions are in bold format. The position of the SNPs according to the last version of the genome (v.4.0, available at http//www.melonomics.net accessed on 1 June 2022) is indicated. The phenotype of the genotyped plants is indicated (SU: susceptible).
| BC4(95)10xPS Plants | ||||
|---|---|---|---|---|
| Marker | Chr a | Genomic Position v.4.0 (bp) | 3 | 5 |
| S5_23380459 | 5 | 24,192,281 | A | A |
| cysdv63 | 5 | 25,782,654 | A | A |
| cysdv65 | 5 | 26,408,896 | A | A |
| S5_25653869 | 5 | 26,419,648 | A | A |
| S12_18721450 | 12 | 18,530,394 | A | A |
| Oidio3 | 12 | 21,870,304 | A | A |
| S12_22130778 | 12 | 21,923,406 | A | A |
| Oidio 2 | 12 | 22,251,703 | H | H |
| Oidio1 | 12 | 22,309,972 | H | H |
| CMPSNP361 | 12 | 23,000,406 | H | H |
| Phenotype | SU | SU | ||
a: chromosome.
The genotyping of the BC3S1 families that segregated for CPM resistance showed significative phenotypic differences between those plants that were homozygous for the TGR-1551 allele for the markers Oidio2 and Oidio1 and those that were heterozygous or homozygous for the BO allele at these positions.
Afterwards, BC4-95 plant 10 was selected, as it was heterozygous at the candidate region of chromosome 5 and at the position of the marker SNP361. It was self-pollinated and crossed with the PS cultivar BGCM-126 to obtain the BC4(95)10S1 and BC4(95)10xPS offspring, respectively. Six plants of the BC4(95)10xPS offspring were genotyped with a new set of 160 SNP markers evenly distributed throughout the genome (Background marker 2) (Supplementary Table S1). Moreover, these plants were also genotyped with the HRM markers cysdv63 and cysdv65 (Table 3). Those plants homozygous for the BO allele at the candidate region of chromosome 5 and heterozygous for the marker CMPSNP361 at chromosome 12 were selected (BC4(95)-10xPS plants 3 and 5). These two plants were also genotyped with the HRM markers Oidio3, Oidio2, and Oidio1 (Table 4). Both were homozygous for the BO allele at the position of the markers Oidio3 and S12_22130778 (background marker 2, chr12: 21,923,406 bp) and heterozygous for markers Oidio2 and Oidio1. Their selfing progenies were evaluated for their resistance to races 1, 2, and 5 of CPM. All the BC4(95)-10xPS-S1 plants were susceptible, independently of their genotype for the Oidio2 marker. This allowed us to narrow the candidate interval to a 381,399 bp region between markers Oidio3 and Oidio2 (Figure 1). This region had 50 annotated genes, some of which could be good resistance candidates (Supplementary Table S5).
Table 4.
Genotype for SNP markers in the candidate region of chromosome 12 for the BC3 plants selected for not having a TGR-1551 introgression at chromosome 5 (A: homozygous for ‘Bola de Oro’ allele; H: heterozygous). The phenotype of the BC3S1 progenies is indicated (SU: susceptible; SE: segregating). Markers in the candidate interval are in bold format.
| Number of BC3 Plant | ||||||||
|---|---|---|---|---|---|---|---|---|
| Marker | Chr a | Genomic Position (bp) | 34 | 96 | 139 | 88 | 89 | 148 |
| CMPSNP285 | 12 | 20,421,309 | H | A | H | H | A | A |
| Oidio8 | 12 | 20,920,054 | H | A | A | n.d 1 | A | A |
| Oidio5 | 12 | 21,470,961 | H | H | A | n.d | A | A |
| Oidio4 | 12 | 21,866,445 | H | H | A | n.d | A | A |
| Oidio3 | 12 | 21,870,304 | H | H | A | A | A | A |
| Oidio2 | 12 | 22,251,703 | H | H | H | A | A | A |
| Oidio1 | 12 | 22,309,972 | H | H | H | A | A | A |
| OidioC | 12 | 22,875,711 | H | H | H | H | A | H |
| OidoB | 12 | 22,913,643 | H | H | H | H | H | H |
| OidioA | 12 | 22,915,510 | H | H | H | H | H | H |
| CMPSNP361 | 12 | 23,000,406 | H | H | H | H | H | H |
| Phenotype | SE | SE | SE | SU | SU | SU | ||
a: chromosome. 1: missing data.
SNPeff was used to annotate and predict the effects of the SNPs found in two GBS assays between TGR-1551 and BO within this region. SNPs were detected in the sequence of 19 candidate genes in this region. Most of those SNPs had a predicted modifier impact (Supplementary Table S4). Ten of the affected genes coded a serine/threonine-protein kinase. SNPs were also detected in genes coding (3S,6E)-nerolidol synthase 1-like (MELO3C002520), a eukaryotic translation initiation factor-like protein (MELO3C002515), a BTB/POZ domain-containing protein At3g22104 (MELO3C002514), a protein detoxification (MELO3C002526), purine permease 3-like (MELO3C002545), an unknown protein (MELO3C035727), and three receptor-like protein kinases (MELO3C002504, MELO3C002541, and MELO3C002538) (Table 3).
2.4. Changes in Resistance Levels Due to Low Temperatures
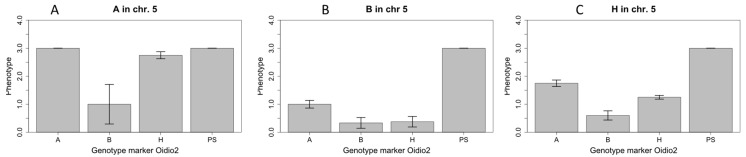
Fifty-seven BC4(95)10S1 plants and five PS plants were phenotyped for the resistance against race 5 of Px. Twelve days after inoculation, the plants of the PS line showed severe infection symptoms. Different levels of fungal sporulation were recorded for the BC4(95)10S1 plants. Thus, the segregation ratio of the BC4(95)10S1 plants observed was 34 resistant:23 susceptible, which is not consistent with a 13:3 ratio corresponding to the independent segregation of two genes, one dominant gene, and one recessive gene. These plants were genotyped with the HRM markers cysdv63, cysdv65, Oidio3, Oidio2, and Oidio1. All the plants were homozygous for the BO allele at the position of the marker Oidio3, while segregation was observed for the rest of them. The combined effect of the QTLs in chromosomes 5 and 12 was analyzed (Figure 2). Those plants that were homozygous for the BO allele at the candidate region in chromosome 5 were only resistant when they were homozygous for the TGR-1551 allele at the locus Oidio2 (Figure 2A). Those plants that were homozygous for the TGR-1551 alleles in the candidate region of chromosome 5 showed mild or no symptoms independently of their genotype at the position of the marker Oidio2 (Figure 2B). There were 25 heterozygous plants at the position of the markers cysdv63 and cysdv65 that were heterozygous or homozygous for the BO allele at the position of the marker Oidio2 in chromosome 12. Among those plants, there were 10 that showed a susceptible phenotype (Figure 2C). The resistance response of the heterozygous plants might have been affected by the lower temperatures recorded during the assay (the medium temperature was lower than 21 °C).
Figure 2.
Interaction between QTL on chromosome 5 (markers cysdv63 and cysdv65) and QTL on chromosome 12 (marker Oidio2) for symptom score at 12 days post-inoculation (bars indicate standard error). Genotype for marker Oidio2: BO: homozygous for ‘Bola de Oro’ allele; H: heterozygous: TGR: homozygous for TGR-1551 allele. Genotype for marker cysdv63: A: homozygous for ‘Bola de Oro’ allele (A); B: homozygous for TGR-1551 allele (B); H: heterozygous; PS: Piel de Sapo control (C).
3. Discussion
In this work, advanced backcross lines derived from an initial cross between TGR-1551 and ‘Bola de Oro’ were used to narrow the candidate regions of the resistance genes to Px and to develop PCR-based markers tightly linked to this resistance. Moreover, thanks to the genotyping achieved via the sequencing of both parents, candidate resistance genes have been provided.
Resistance to Px races 1, 2, and 5 derived from TGR-1551 was initially described as independently controlled by one dominant gene on chromosome 5 and a recessive one [31,32]. The phenotyping of an F2 population derived from the same cross and its genotyping through Bulked Segregant Analysis (BSA) and AFLP markers allowed for the identification of two QTLs, namely, Pm-R1-2 and Pm-R5, the first of them related to resistance to races 1 and 2 and the latter to resistance against race 5 [21]. Two resistance-like genes, MRGH5 and MRGH63, belonging to the NBS-LRR (Nucleotide-binding site and leucine-rich repeats) family, were identified as candidate genes for CPM resistance in the TGR-1551 in the region of chromosome 5. Moreover, the molecular markers developed by Yuste-Lisbona et al. [21] were also linked to the resistance derived from WMR29. The use of advanced backcrossed lines and the genotypic information derived from New Generation-Sequencing technologies (NGS) allowed us to narrow the candidate region related to the resistance to the three races to a 251 kb region between the HRM markers cysdv626 (chr5: 26,058,307 bp) and cysdv6531 (chr5: 26,309,060 bp). This narrowed candidate region contains 34 predicted genes, of which 31 were annotated (Supplementary Table S3), but does not include MRGH63 and MRGH5, which are approximately <275 kb from the candidate interval (Figure 1). Several QTLs linked to Px resistance have been mapped in chromosome 5 (qPx1-5, Pm-AN, Pm-w, Pm-Edisto47-2, and PmV-1-Pi124112) [14,18,19,20,22,23] but only the QTLs qPx1-5 and Pm-AN, linked to resistance to Px race 1 derived from MR-1 and Ano2, respectively, overlapped with the proposed candidate region [19,22] (Figure 1). Moreover, the region in which these resistances are located overlaps with a 760 kb region with the highest concentration of resistance genes in the melon genome [45]. Furthermore, this region has been proven to be highly variable both at the intra- and interspecific levels, explaining the differences in resistance found in different melon genotypes [45].
Most of the annotated genes within the candidate region have resistance-related functions. Among them, there are two genes annotated as “disease resistance proteins” (MELO3C004320 and MELO3C031556), as well as genes related to host pattern recognition receptors (PRR) such as two NBS-LRRs (MELO3C004319 and MELO3C031325) and a “receptor-like cytosolic serine/threonine-protein kinase RBK2” (MELO3C004315). The TMV resistance protein N-like, a TIR-NBS-LRR gene possessing homology with resistance genes [46], was frequently annotated (seven of the annotated genes in the candidate interval). As the dominant resistances of powdery mildew identified so far have been easily broken by newly emerging races [47], it has been postulated that many of the QTLs detected could encode PRRs that would be manipulated by the secreted fungal effectors [48,49]. It has recently been proposed that several dominant resistances against powdery mildew in melon could be caused by missense mutations in different LRR-receptor like kinases and TIR-NBS-LRR genes [19,35]. In this sense, two SNPs with a predicted modifier impact were detected within the sequence of a gene coding TMV resistance protein N-like (MELO3C004311) (Supplementary Table S4), which could play a potential role in an eventual protein product and thereby confer resistance against Px. This TIR-NBS-LRR gene has also been proposed as the candidate gene, derived from MR-1, conferring resistance against Px race 1 [19]. Moreover, six variants with modifier impacts were found within the sequence of the predicted gene MELO3C004297, which was annotated as a “Branched-chain-amino-acid aminotransferase-like protein” (Supplementary Table S4). These kinds of proteins are related to defense responses in several species. A branched chain amino acid aminotransferase, termed TaBCAT1, has been described as a positive regulator of wheat rust (Puccinia triticina) susceptibility, as it had a key role in SA-dependent defense activation [50]. In A. thaliana, the levels of the branched-chain amino acid-related compound ILA correlate with the expression of a glucosyltransferase (UGT76B1) that is induced in response to stress [51]. Therefore, these two genes are the principal candidate resistance genes in the proposed interval.
In addition to the previously cited R genes, two predicted genes were noted to have functions related to cell wall formation (MELO3C002509 and MELO3C002512). The cell wall is one of the primary shields against pathogen infections. It acts as a barrier that pathogens must degrade for infection progression, and it also contains antimicrobial compounds [52,53]. Moreover, cell wall compounds that are released during infection can act as damage-associated molecular patterns (DAMPs), triggering plant immune responses thanks to their perception by PRRs [54,55]. It has been proven that a loss of susceptibility factors increases cell wall thickness, leading to a more effective defense response against powdery mildew [28,56,57,58]. Moreover, the resistance derived from TGR-1551 to Px could be related to post-inoculation modifications of the cell walls, since, when comparing the response of TGR-1551 and BO against infection, a 50% increase in the number of cells with an accumulation of callose in their cell walls is observed in TGR-1551 [59]. There were also two predicted genes related to photosynthetic processes (MELO3C004308 and MELO3C004307). During a powdery mildew infection, the genes involved in photosynthesis and related processes are upregulated in melons [60]. The candidate region also includes the virus aphid transmission resistance gen (Vat) (ten of the predicted genes are annotated as “Vat protein”) [45] carried by TGR-1551 [61]. Moreover, the resistance to CYSDV derived from TGR-1551 has been mapped in the same region [44], which would explain why—while developing a resistant line to CYSDV—the selected plants resulted in being resistant to Px and Aphis gosypii as well, even though no previous selection against the fungus nor the aphid had been made [62].
Regarding the recessive gene, in a previous work, an RIL population derived from a cross between TGR-1551 and BO was evaluated for resistance to the three races of Px and a QTL associated with the recessive gene that was found in chromosome 12 [33]. The SSR markers CMBR111 (chr12: 22,475,952–22,476,042 bp), TJ29 (chr12: 22,475,971–22,476,086 bp), and CMBR150 (chr12: 23,085,299–23,085,068 bp) were found to be linked to this QTL [33]. The same SSR markers were also linked to the resistance QTL(AR5) derived from AR5 [17]. Nevertheless, the phenotyping and genotyping of the advanced backcrossed lines allowed us to narrow the resistance interval to a region of approximately 400 kb between HRM markers Oidio3 (chr12: 21,870,304 bp) and Oidio2 (chr12: 22,251,703 bp) (Table 3). This narrowed region is not located within the previously proposed resistance QTL and is also outside the boundaries proposed for other resistance sources such as Edisto27, MR-1, PMR5, WM6, PI 124112, and PI 134198 [17,19,24,25,26,30,35,36,63] (Figure 1). Nevertheless, the recessive gene present in TGR-1551 is unique, since no recessive genes conferring resistance to more than one Px race have been reported previously in melons, and this resistance could be conferred by a gene different from those derived from other accessions.
The candidate interval contains 50 predicted genes and all of them have been annotated (Supplementary Table S6). Most of the annotated genes have defense-related functions, which might indicate the presence of a resistance cluster. “Kinase binding” is the molecular function most frequently described, and several predicted genes were also annotated as “cysteine-rich receptor-like protein kinase”. There are two predicted genes annotated as “transcription factors” (MELO3C002551 and MELO3C002522) and another as a “eukaryotic translation initiation factor-like protein” (MELO3C002515). Moreover, MELO3C002553 was annotated as a “sugar carrier protein C-like”. Sugar metabolism plays a key role in the plant–pathogen interaction, as bacteria and fungus tend to acquire these metabolites during infection. Modifications in sugar metabolism during infection plays a key role in the susceptibility to all wheat rust and powdery mildew pathogen species in wheat [64]. In grapevine, Hexose Transporter 5 (VvHT5) is strongly upregulated in coordination with Cell Wall Invertase (VcwINV) during powdery and downy mildew infections [65]. The predicted gene MELO3C002550 has been annotated as a “Flowering locus T/terminal flower 1-like protein”. Common genetic and epigenetic regulators for flowering and systemic acquired resistance (SAR) have recently been suggested [66]. There is also one predicted gene implicated in photosynthetic processes (MELO3C002510).
The effect of the detected SNPs was calculated with SNPeff (Supplementary Table S4). Regarding other accessions, different candidate resistance genes have recently been proposed using a similar approach [19,24,25,35]. There were SNPs affecting the coding region of 19 genes located within the candidate region on chromosome 12. Most of those SNPs were located in sequences of genes coding serine/threonine-protein kinases (STKs) and receptor-like serine/threonine-protein kinases (RLKs) (Supplementary Table S4) and frequently had a predicted modifier impact over the protein function. RLKs are key components of the plant immune system, acting in both broad-spectrum, elicitor-initiated defense responses and as dominant resistance genes in race-specific pathogen defense [67]. Moreover, STK domains not only interact with avirulence proteins but also function as signal transduction mediators [68,69]. Mutations in these proteins could lead to a better recognition of PAMPs or DAMPs and, hence, to a faster plant response against Px. A similar phenomenon occurs with serine/threonine-protein kinases, which play a key role in the transduction of the internal and external signals. Some genes coding these kinds of receptors, and located in chromosome 12, have already been proposed as candidate resistance genes derived from MR-1 and PMR5 [19,26,35]. Nevertheless, other candidate genes have been proposed within the same region [24,25]. In addition to the mutations previously described, an SNP was also detected within the coding region of a purine permease. These proteins are involved in cytokinin transport and it has been proven that the suppression of CK transporters can lead to the suppression of the immune response [70]. There was also an SNP that caused the appearance of a new stop codon in the sequence of a gene coding a BTB/POZ domain-containing protein, At3g22104. These proteins are involved in the pathway of protein ubiquitination, and the reduction in ubiquitin levels enhanced the susceptibility to powdery mildew in barley (Hordeum vulgare) [71]. Moreover, other components of the ubiquitination complex have also been related to the plant defense against fungi [72,73]. An SNP with a predicted modifier impact was also found within the 3′UTR region of a gene coding a eukaryotic translation initiation factor (EIF). These proteins have been widely described as susceptibility factors in viral infections, but not similar functions have been documented in plant–fungal interactions. Finally, an SNP that caused the loss of a start codon was also detected within the sequence of a gene coding a (3S,6E)-nerolidol synthase 1-like protein. These proteins are involved in monoterpene and sesquiterpene biosynthesis. The enhanced production of some sesquiterpenes has been related to the inhibition of bacterial growth [74], and many monoterpene-induced transcripts are annotated as either transcription factors or defense genes [75].
A comparative transcriptome profiling of genes and pathways related to resistance against powdery mildew was performed in the contrasting melon genotypes MR-1 and Topmark [76]. The differentially expressed genes (DEGs) detected were classified into seven functional groups: pathogen recognition, signal transduction, transcription factors (TFs), phytoalexin biosynthesis, other primary metabolite functions, Mildew Locus O genes (MLOs), and pathogenesis-related (PR) proteins. The defense-related genes showed an increased expression at the early stage of Px infection in the resistant genotype MR-1, whereas the defense response was suppressed in the susceptible cultivar. Moreover, the expression changes tended to be maintained during the infection [76]. There are also several non-coding RNAs (long non-coding RNAs (lncRNAs) and circular RNAs (circRNAs)) [77,78] that have been described as being related to Px resistance, promoting the expression of genes involved in defense processes. Therefore, the resistance to Px in melons comprises many defense-related mechanisms. The regions presented herein as linked to the resistance derived from TGR-1551 contain several genes involved in the detection of fungal elicitors, signal transduction, and resistance response. A transcriptomic analysis would be needed to identify differentially expressed genes in the candidate regions and further VIGs assays will be performed to functionally validate the proposed candidate genes.
Resistance to powdery mildew has been described as temperature-dependent in different species, including melons and cucumbers [59,79,80,81]. The phenotyping/genotyping results of the BC4(95)10S1 offspring revealed that the resistance conferred by the QTL located in chromosome 5 may decrease under low temperature conditions in heterozygous lines. The plants homozygous for the TGR-1551 allele at locus Oidio2 were not affected by low temperatures, whereas those plants that were heterozygous for the markers cysdv63 and cysdv65 and heterozygous or homozygous for the BO allele at locus Oidio2 showed a decrease in their resistance response (Figure 2C). This condition had not previously been observed in the assays carried out in the same season of different years with higher temperatures. These results were consistent with those observed by Beraldo-Hoischen et al. [59], where TGR-1551 and the NIL21, carrying the QTL Pm-R derived from TGR-1551, were susceptible when inoculated at low temperatures. Under low temperature conditions, a delay in the resistance response was observed and the number of cells with callose accumulation per point of penetration decreased. A similar behavior was observed in the resistant cucumber line PI 1970088-1, where a major QTL was always detected under high and low temperature conditions and four other QTLs were temperature-dependent [82]. Thus, the resistance conferred by the dominant QTL located in chromosome 5 would be temperature-dependent only for the heterozygous plants, while the temperature would not affect the resistance provided by the recessive QTL located in chromosome 12.
4. Materials and Methods
4.1. Plant Material
The resistance source used in this study, TGR-1551, is a Zimbabwean melon cultivar described as resistant to Px races 1, 2, and 5. Generations used in this study come from the breeding program aimed at the introgression of the resistance derived from TGR-1551 to commercial types via backcrosses to the Spanish melon cultivars ‘Bola de Oro’ and ‘Piel de Sapo’. The populations inoculated were constructed from an original BC3 population composed of 200 plants obtained from the F1 TGR-1551 x ‘Bola de Oro’ and three successive backcrosses to the susceptible cultivar ‘Bola de Oro’ (BO). BC3 plants were selected for carrying the candidate regions previously described as linked to Px resistance and with less genetic background from TGR-1551. New backcrosses to BO and self-pollinations were carried out in consecutive years (Supplementary Figure S1). Initially, fifty-two of the BC3 plants were genotyped with markers linked to the candidate resistance region to Px at chromosome 5 (see details below) in 2018. Twenty BC3 plants were selected, backcrossed to BO, and self-pollinated to obtain their corresponding BC4 and BC3S1. In 2019, the BC3S1 and BC4 families generated were genotyped to confirm the presence of the genomic regions associated with resistance to CPM in chromosome 5 and 12. The 20 selected BC3S1 were artificially inoculated with P. xanthii. The corresponding BC4 were then backcrossed to BO and to the CPM-susceptible Spanish cultivar ‘Piel de Sapo’ (PS) to obtain BC5 and BC4 x PS, respectively. In 2020, plants of those populations were genotyped with background and HRM markers to confirm the presence of the genomic regions of interest. Three BC5 and four BC4 x PS plants were selected and self-pollinated to obtain BC5S1 and BC4 x PS-S1 families, respectively. In 2021, the BC5S1 and BC4 x PS-S1 families were genotyped with HRM markers and phenotyped for CPM resistance to narrow the genomic locations of the resistance to Px (Table 1). BC5S1-resistant plants that were homozygous for the TGR-1551 allele at the candidate region of chromosome 5 were self-pollinated and their BC5S2 progenies were phenotyped for CPM resistance.
4.2. Artificial Inoculations
The isolates of Podosphaera xanthii used in this experiment were SF30 (race 1), P-15.0 (race 2 F), and C8 (race 5). They were all collected in melon crops growing under greenhouses in the South of Spain. For each case, and with the help of a brush, a small amount of conidia was taken from the natural mycelial surface present on the melon leaves and placed on cut and previously disinfected cotyledons of cucumber var. ‘Black Beauty’ growing on water agar medium (0.8% agar, 3% benzimidazole, and 3.2% sucrose) in petri dishes under axenic conditions, as described by Yuste-Lisbona et al. [29]. Cotyledons were disinfected (solution of NaClO at 20 gr/L) before use to allow a prolonged preservation of both cotyledon and mildew, avoiding possible contamination of the powdery mildew colonies. Once the pathogen was identified as P. xanthii based on its morphological characters under light microscope examination, monosporic cultures were performed. Natural powdery mildew is composed of a mixture of different genotypes whose competitivity, in particular under in vitro culture conditions, can vary. So, cloning by single conidia isolation seemed to be necessary. An agar-coated eyelash, moistened by medium contact, permitted the straightforward capture of individual conidia under binocular lens, which were then deposited on cotyledons maintained under axenic conditions in individual petri dishes. Colonies appeared after about 15 days under incubation conditions in growth room with controlled temperature (23 °C) and relative humidity (38–65%) at a photoperiod of 16/8 h (light/dark). The races of the different fungal isolates were confirmed by analyzing the reactions of a set of differential melon lines to each isolate, as described by Bardin et al. [8]. Those colonies were then used to carry out the inoculations. The fungus was sub-cultured several times until the inoculation date. The monosporic cultures were maintained under the same conditions of incubation until the inoculation dates.
Three to five plants each from the resistant parental line TGR-1551, the susceptible parental line ‘Bola de Oro’, and their F1 progeny, as well as a variable number of plants of the different generations (Supplementary Figure S1), were inoculated with races 1, 2, and 5 of P. xanthii on the same leaf. Due to availability of inoculum source and considering that response to the three races in TGR-1551-derived plant materials is homogeneous, two of the BC4S1 families of interest were inoculated solely with race 5 in 2020.
All plants were inoculated artificially by depositing a small amount of conidia from each race at two spots (at each side of, and equidistant from, the main leaf vein) on the second true leaf of each plant [83]. Inoculation with the three races was carried out on the same leaf of each plant (Supplementary Figure S2).
The plants were maintained and inoculated in an insect-proof glasshouse during spring, from the middle of March to the middle of June, at temperatures ranging from 18 to 32 °C, the relative humidity ranging between 43–79%, and photoperiod ranging from 12:12 to 16:8 h (light:dark). That period of time is the most adequate to evaluate PM because it is the season when melons are grown under greenhouses in Spain as well as along the Mediterranean basin. Twelve days after inoculation, plants were scored according to the level of sporulation of the fungus, using a scale from 0–3, similar to those previously used by other authors for powdery mildew in melons [21,31,84,85,86] (Supplementary Figure S2). The following classes were established: class 0, no conidia germination; class 1, some conidia germination, no visible sporulation, and no disease progression; class 1.5, low level of sporulation, and the disease does not seem to progress; class 2, moderate level of sporulation and sparse mycelia but the disease progresses; class 2.5, clear sporulation; and class 3, profuse sporulation. Plants in classes 0 and 1 were considered resistant (R), those in class 1.5 were considered moderately resistant (MR), and those in classes from 2 to 3, where the infection progressed, were considered susceptible (S).
4.3. Molecular Markers and Genotyping Methods
Total DNA was extracted from young leaves following the method described by Doyle and Doyle [87] with minor modifications [39]. DNA was quantified using spectrophotometry via a Nanodrop ND-1000 Spectrophotometer v.3.5 (LabTech International, Heathfield, UK) and adjusted to the concentrations suited for the different genotyping analyses.
Previously existing SNPs and new ones developed in this work were used to genotype the different segregating populations. Initially, the 200 BC3 plants were genotyped with an existing panel of 124 SNP markers evenly distributed throughout the genome (Background markers 1) (Supplementary Table S1), implemented in the Agena Bioscience iPLEX®® Gold MassARRAY platform by the Epigenetic and Genotyping unit of the University of Valencia (Unitat Central d’Investigació en Medicina (UCIM), Valencia, Spain). This SNP set had previously been validated in populations derived from ibericus x acidulus melon crosses [39,40,41,42,43]. The selected BC3 plants were genotyped with two additional markers sets, CYSDV1 as well as WMV1 and WMV2, also implemented in the Agena Bioscience Platform, which covered the candidate region on chromosome 5 and had been developed in previous studies [43,44] (Supplementary Table S2). To increase the resolution of the candidate region of chromosome 5, the BC3S1 and BC4 progenies of the 20 selected BC3 plants were also genotyped with the panel sets CYSDV1 and CYSDV2 [44] (Supplementary Table S2). The lectures of a GBS experiment, conducted to perform genetic diversity studies (including TGR-1551 and BO, among many other genotypes), were mapped to the reference melon genome (v.4.0) using bowtie2 v.2.3.4 [88] and an SNP calling was carried out with Freebayes v.1.3.4 [89]. A new set of 160 markers evenly distributed throughout the genome that allowed us to distinguish between the different melon groups was selected (Supplementary Table S1). These 160 markers provided complementary information to that offered by the previously used set of 124 markers. This new set was also implemented in the Agena Bioscience Platform and used to genotype the BC4 x PS, BC4S1 and BC5 progenies. To further narrow the candidate interval in chromosome 12, the marker CMPSNP361 (Supplementary Table S1) and 10 new SNPs, retrieved from the GBS experiment and evenly distributed within the candidate interval, were adapted to a PCR-based protocol for their analysis by high-resolution melting (HRM) (Supplementary Table S5). For a better analysis of the melting curves provided by HRM markers, we avoided selecting SNPs that produce an A/T nucleotide change. These HRM markers were used to genotype selected BC3S1 and BC4 x PS-S1 families. The previously designed HRM markers csydv63 and cysdv65 [44] were used to genotype the BC3S1, BC4, BC4 x PS, BC4 x PS-S1, BC5, and BC5S1 selected families. To narrow the candidate region in chromosome 5, two additional SNPs were adapted for analysis by HRM (csydv626 and cysdv6351) and they were used to genotype the BC4 x PS-S1 and BC5S1 populations. The predicted effect of the SNPs’ variants found between TGR-1551 and BO within the candidate regions was analyzed with SNPeff version 1.3.4 [90].
5. Conclusions
Breeding new cultivars resistant to powdery mildew is the most efficient, durable, and environmentally respectful way to fight this pathogen. The African accession TGR-1551 has been reported as being resistant to CPM races 1, 2, and 5. This work has allowed for the narrowing of the candidate intervals of the dominant and recessive QTLs associated with the resistance to CPM derived from TGR-1551 to a region of approximately 250 kb and 381 kb, respectively. The SNP markers provided herein are a useful resource for the introgression of CPM resistance in commercial melon cultivars and several candidate genes have been proposed. Resistance to CPM is strongly affected by the environmental temperature. Thus, the availability of markers tightly linked to the resistance QTLs is essential for accelerating the introgression of CPM resistance into elite cultivars or landraces.
Acknowledgments
The authors would like to thank E. Jaime, I. Díaz y L. Rodriguez for their technical support in field assays.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms232012553/s1.
Author Contributions
Conceptualization, M.L.G.-G., A.P.-d.-C. and B.P.; methodology, M.L.G.-G. and M.L.-M.; software, M.L.-M.; investigation, M.L.G.-G., B.P., M.L.-M. and A.P.-d.-C.; writing—original draft preparation, review and editing, M.L.-M., A.P.-d.-C., M.L.G.-G. and B.P.; funding acquisition, M.L.G.-G., A.P.-d.-C. and B.P. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Spanish Ministerio de Ciencia e Innovación (MCIN/AEI/10.13039/501100011033), grant number PID2020-116055RB (C21 and C22), and by the Conselleria d’Educació, Investigació, Cultura i Esports de la Generalitat Valenciana, grant number PROMETEO/2021/072 (to promote excellence groups, cofinanced with FEDER funds). M.L. is a recipient of a predoctoral fellowship (PRE2018-083466) of the Spanish Ministerio de Ciencia, Innovación y Universidades co-financed with FSE funds.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.FAO FAOstats, Organización de las Naciones Unidas para la Alimentación y la Agricultura. [(accessed on 1 June 2022)]. Available online: https://www.fao.org/faostat/es/#home.
- 2.Cohen R., Burger Y., Katzir N. Monitoring Physiological Races of Podosphaera Xanthii (Syn. Sphaerothecafuliginea), the Causal Agent of Powdery Mildew in Cucurbits: Factors Affecting Race Identification and the Importance for Research and Commerce. Phytoparasitica. 2004;32:174–183. doi: 10.1007/BF02979784. [DOI] [Google Scholar]
- 3.Lebeda A., Sedlákova B., Krístkova E. Temporal Changes in Pathogenicity Structure of Cucurbit Powdery Mildew Populations. Acta Hortic. 2007;731:381–388. doi: 10.17660/ActaHortic.2007.731.53. [DOI] [Google Scholar]
- 4.Lebeda A., Sedláková B., Křístková E., Vysoudil M. Long-Lasting Changes in the Species Spectrum of Cucurbit Powdery Mildew in the Czech Republic-Influence of Air Temperature Changes or Random Effect? Plant Prot. Sci. 2009;45:S41–S47. doi: 10.17221/2807-PPS. [DOI] [Google Scholar]
- 5.Braun U. Taxonomic Manual of Erysiphales (Powdery Mildews) CBS Biodivers. 2012;11:1–707. [Google Scholar]
- 6.Křístková E., Lebeda A., Sedláková B. Species Spectra, Distribution and Host Range of Cucurbit Powdery Mildews in the Czech Republic, and in Some Other European and Middle Eastern Countries. Phytoparasitica. 2009;34:337–350. doi: 10.1007/s12600-009-0045-4. [DOI] [Google Scholar]
- 7.Lebeda A., Sedláková B., Křístková E., Widrlechner M.P., Kosman E. Understanding Pathogen Population Structure and Virulence Variation for Efficient Resistance Breeding to Control Cucurbit Powdery Mildews. Plant Pathol. 2021;70:1364–1377. doi: 10.1111/ppa.13379. [DOI] [Google Scholar]
- 8.Bardin M., Dogimont C., Nicot P.C., Pitrat M. Genetic Analysis of Resistance of Melon Line PI 124112 to Sphaerotheca Fuliginea and Erysiphe Cichoracearum Studied in Recombinant Inbred Lines; Proceedings of the International Symposium on Cucurbits; Ankara, Turkey. 1 May 1999; pp. 163–168. [Google Scholar]
- 9.Hong Y.J., Hossain M.R., Kim H.T., Park J.I., Nou I.S. Identification of Two New Races of Podosphaera Xanthii Causing Powdery Mildew in Melon in South Korea. Plant Pathol. J. 2018;34:182–190. doi: 10.5423/PPJ.OA.12.2017.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lebeda A., Křístková E., Sedláková B., McCreight J.D., Coffey M.D. New Concept for Determination and Denomination of Pathotypes and Races of Cucurbit Powdery Mildew; Proceedings of the IXth EUCARPIA Meeting on Genetics and Breeding of Cucurbitaceae; Avignon, France. 21–24 May 2008; pp. 125–134. [Google Scholar]
- 11.Lebeda A., Sedláková B. Identification and Survey of Cucurbit Powdery Mildew Races in Czech Populations; Proceedings of the Cucurbitaceae 2006; Asheville, NC, USA. 17–21 September 2006; pp. 444–452. [Google Scholar]
- 12.De Miccolis Angelini R.M., Pollastro S., Rotondo P.R., Laguardia C., Abate D., Rotolo C., Faretra F. Transcriptome Sequence Resource for the Cucurbit Powdery Mildew Pathogen Podosphaera Xanthii. Sci. Data. 2019;6:1–7. doi: 10.1038/s41597-019-0107-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCreight J.D., Coffey M.D., Ando K., Kousik C.S. Cucurbit Powdery Mildew Races on Melon: Current Status in the US; Proceedings of the American Society of Horticulture Science Meeting; Orlando, FL, USA. 31 July–4 August 2018. [Google Scholar]
- 14.Périn C., Hagen L., de Conto V., Katzir N., Danin-Poleg Y., Portnoy V., Baudracco-Arnas S., Chadoeuf J., Dogimont C., Pitrat M. A Reference Map of Cucumis Melo Based on Two Recombinant Inbred Line Populations. Theor. Appl. Genet. 2002;104:1017–1034. doi: 10.1007/s00122-002-0864-x. [DOI] [PubMed] [Google Scholar]
- 15.Fazza A.C., Dallagnol L.J., Fazza A.C., Monteiro C.C., de Lima B.M., Wassano D.T., Aranha Camargo L.E. Mapping of Resistance Genes to Races 1, 3 and 5 of Podosphaera Xanthii in Melon PI 414723. Crop Breed. Appl. Biotechnol. 2013;13:349–355. doi: 10.1590/S1984-70332013000400005. [DOI] [Google Scholar]
- 16.Zhang C., Ren Y., Guo S., Zhang H., Gong G., Du Y., Xu Y. Application of Comparative Genomics in Developing Markers Tightly Linked to the Pm-2F Gene for Powdery Mildew Resistance in Melon (Cucumis Melo L.) Euphytica. 2013;190:157–168. doi: 10.1007/s10681-012-0828-4. [DOI] [Google Scholar]
- 17.Fukino N., Ohara T., Monforte A.J., Sugiyama M., Sakata Y., Kunihisa M., Matsumoto S. Identification of QTLs for Resistance to Powdery Mildew and SSR Markers Diagnostic for Powdery Mildew Resistance Genes in Melon (Cucumis Melo L.) Theor. Appl. Genet. 2008;118:165–175. doi: 10.1007/s00122-008-0885-1. [DOI] [PubMed] [Google Scholar]
- 18.Ning X., Wang X., Gao X., Zhang Z., Zhang L., Yan W., Li G. Inheritances and Location of Powdery Mildew Resistance Gene in Melon Edisto47. Euphytica. 2014;195:345–353. doi: 10.1007/s10681-013-1000-5. [DOI] [Google Scholar]
- 19.Branham S.E., Kousik C., Mandal M.K., Wechter W.P. Quantitative Trait Loci Mapping of Resistance to Powdery Mildew Race 1 in a Recombinant Inbred Line Population of Melon. Plant Dis. 2021;105:3809–3815. doi: 10.1094/PDIS-12-20-2643-RE. [DOI] [PubMed] [Google Scholar]
- 20.Pitrat M. Linkage Groups in Cucumis Melo L. J. Hered. 1991;82:406–411. doi: 10.1093/oxfordjournals.jhered.a111112. [DOI] [Google Scholar]
- 21.Yuste-Lisbona F.J., Capel C., Gómez-Guillamón M.L., Capel J., López-Sesé A.I., Lozano R. Codominant PCR-Based Markers and Candidate Genes for Powdery Mildew Resistance in Melon (Cucumis melo L.) Theor. Appl. Genet. 2011;122:747–758. doi: 10.1007/s00122-010-1483-6. [DOI] [PubMed] [Google Scholar]
- 22.Wang X., Li G., Gao X., Xiong L., Wang W., Han R. Powdery Mildew Resistance Gene (Pm-AN) Located in a Segregation Distortion Region of Melon LGV. Euphytica. 2011;180:421–428. doi: 10.1007/s10681-011-0406-1. [DOI] [Google Scholar]
- 23.Perchepied L., Bardin M., Dogimont C., Pitrat M. Relationship between Loci Conferring Downy Mildew and Powdery Mildew Resistance in Melon Assessed by Quantitative Trait Loci Mapping. Phytopathology. 2005;95:556–565. doi: 10.1094/PHYTO-95-0556. [DOI] [PubMed] [Google Scholar]
- 24.Cui H., Fan C., Ding Z., Wang X., Tang L., Bi Y., Luan F., Gao P. CmPMRl and CmPMrs Are Responsible for Resistance to Powdery Mildew Caused by Podosphaera Xanthii Race 1 in Melon. Theor. Appl. Genet. 2022;135:1209–1222. doi: 10.1007/s00122-021-04025-4. [DOI] [PubMed] [Google Scholar]
- 25.Cao Y., Diao Q., Chen Y., Jin H., Zhang Y., Zhang H. Development of KASP Markers and Identification of a QTL Underlying Powdery Mildew Resistance in Melon (Cucumis melo L.) by Bulked Segregant Analysis and RNA-Seq. Front. Plant Sci. 2021;11:1819. doi: 10.3389/fpls.2020.593207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li B., Zhao Y., Zhu Q., Zhang Z., Fan C., Amanullah S., Gao P., Luan F. Mapping of Powdery Mildew Resistance Genes in Melon (Cucumis melo L.) by Bulked Segregant Analysis. Sci. Hortic. 2017;220:160–167. doi: 10.1016/j.scienta.2017.04.001. [DOI] [Google Scholar]
- 27.Teixeira A.P.M., Barreto F.A.d.S., Camargo L.E.A. An AFLP Marker Linked to the Pm-1 Gene that Confers Resistance to Podosphaera Xanthii Race 1 in Cucumis melo. Genet. Mol. Biol. 2008;31:547–550. doi: 10.1590/S1415-47572008000300023. [DOI] [Google Scholar]
- 28.Hong C., Weiping K., Junfen L., Jiping L. Analysis of Powdery Mildew Resistance in Wild Melon MLO Mutants. Hortic. Plant J. 2015;1:165–171. doi: 10.16420/J.ISSN.2095-9885.2015-0036. [DOI] [Google Scholar]
- 29.Iovieno P., Andolfo G., Schiavulli A., Catalano D., Ricciardi L., Frusciante L., Ercolano M.R., Pavan S. Structure, Evolution and Functional Inference on the Mildew Locus O (MLO) Gene Family in Three Cultivated Cucurbitaceae spp. BMC Genom. 2015;16:1–13. doi: 10.1186/s12864-015-2325-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Natarajan S., Kim H.T., Thamilarasan S.K., Veerappan K., Park J.I., Nou I.S. Whole Genome Re-Sequencing and Characterization of Powdery Mildew Disease-Associated Allelic Variation in Melon. PLoS ONE. 2016;11:e0157524. doi: 10.1371/journal.pone.0157524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuste-Lisbona F.J., López-Sesé A.I., Gómez-Guillamón M.L. Inheritance of Resistance to Races 1, 2 and 5 of Powdery Mildew in the Melon TGR-1551. Plant Breed. 2010;129:72–75. doi: 10.1111/j.1439-0523.2009.01655.x. [DOI] [Google Scholar]
- 32.Yuste-Lisbona F.J., Capel C., Sarria E., Torreblanca R., Gómez-Guillamón M.L., Capel J., Lozano R., López-Sesé A.I. Genetic Linkage Map of Melon (Cucumis Melo L.) and Localization of a Major QTL for Powdery Mildew Resistance. Mol. Breed. 2011;27:181–192. doi: 10.1007/s11032-010-9421-5. [DOI] [Google Scholar]
- 33.Beraldo-Hoischen P., Gómez-Guillamón M.L., López-Sesé A.I. QTL Associated with One Recessive Gene for Powdery Mildew Resistance in the Melon Genotype TGR-1551; Proceedings of the Xth EUCARPIA Meeting on Genetics and Breeding of Cucurbitaceae; Antalya, Turkey. 15–18 October 2012; pp. 508–513. [Google Scholar]
- 34.Fukino N., Kunihisa M., Matsumoto S. Characterization of Recombinant Inbred Lines Derived from Crosses in Melon (Cucumis melo L.), ‘PMAR No. 5’ × ‘Harukei No. 3’. Breed. Sci. 2004;54:141–145. doi: 10.1270/jsbbs.54.141. [DOI] [Google Scholar]
- 35.Hong J.-E., Hossain M.R., Jung H.-J. Inheritance of Resistance to Race 5 of Powdery Mildew Fungus Podosphaera Xanthii in Melon and Development of Race 5-Specific High Resolution Melting Markers. 2022. [(accessed on 1 June 2022)]. Available online: https://assets.researchsquare.com/files/rs-1433034/v1/dea556c7-b2f4-42cb-a677-789a4e435f56.pdf?c=1659760899.
- 36.Howlader J., Hong Y., Natarajan S., Sumi K.R., Kim H.T., Park J.I., Nou I.S. Development of Powdery Mildew Race 5-Specific SNP Markers in Cucumis Melo L. Using Whole-Genome Resequencing. Hortic. Environ. Biotechnol. 2020;61:347–357. doi: 10.1007/s13580-019-00217-6. [DOI] [Google Scholar]
- 37.Jorgensen J.H. Discovery, Characterization and Exploitation of Mlo Powdery Mildew Resistance in Barley. Euphytica. 1992;63:141–152. doi: 10.1007/BF00023919. [DOI] [Google Scholar]
- 38.Varshney R.K., Ribaut J.-M., Buckler E.S., Tuberosa R., Antoni Rafalski J., Langridge P. Can Genomics Boost Productivity of Orphan Crops? Nat. Publ. Gr. 2012;30:1172–1176. doi: 10.1038/nbt.2440. [DOI] [PubMed] [Google Scholar]
- 39.Esteras C., Formisano G., Roig C., Díaz A., Blanca J., Garcia-Mas J., Gómez-Guillamón M.L., López-Sesé A.I., Lázaro A., Monforte A.J., et al. SNP Genotyping in Melons: Genetic Variation, Population Structure, and Linkage Disequilibrium. Theor. Appl. Genet. 2013;126:1285–1303. doi: 10.1007/s00122-013-2053-5. [DOI] [PubMed] [Google Scholar]
- 40.Leida C., Moser C., Esteras C., Sulpice R., Lunn J., de Langen F., Monforte A.J., Picó B. Variability of Candidate Genes, Genetic Structure and Association with Sugar Accumulation and Climacteric Behavior in a Broad Germplasm Collection of Melon (Cucumis melo L.) BMC Genet. 2015;16:28. doi: 10.1186/s12863-015-0183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perpiñá G., Esteras C., Gibon Y., Monforte A.J., Picó B. A New Genomic Library of Melon Introgression Lines in a Cantaloupe Genetic Background for Dissecting Desirable Agronomical Traits. BMC Plant Biol. 2016;16:154. doi: 10.1186/s12870-016-0842-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sáez C., Esteras C., Martínez C., Ferriol M., Dhillon N.P.S., López C., Picó B. Resistance to Tomato Leaf Curl New Delhi Virus in Melon Is Controlled by a Major QTL Located in Chromosome 11. Plant Cell Rep. 2017;36:1571–1584. doi: 10.1007/s00299-017-2175-3. [DOI] [PubMed] [Google Scholar]
- 43.Pérez-de-Castro A., Esteras C., Alfaro-Fernández A., Daròs J., Monforte A., Picó B., Gómez-Guillamón M. Fine Mapping of Wmv1551, a Resistance Gene to Watermelon Mosaic Virus in Melon. Mol. Breed. 2019;39:93. doi: 10.1007/s11032-019-0998-z. [DOI] [Google Scholar]
- 44.Pérez-De-castro A., López-Martín M., Esteras C., Garcés-Claver A., Palomares-Ríus F.J., Picó M.B., Gómez-Guillamón M.L. Melon Genome Regions Associated with Tgr-1551-Derived Resistance to Cucurbit Yellow Stunting Disorder Virus. Int. J. Mol. Sci. 2020;21:5970. doi: 10.3390/ijms21175970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.González V.M., Aventín N., Centeno E., Puigdomènech P. High Presence/Absence Gene Variability in Defense-Related Gene Clusters of Cucumis melo. BMC Genom. 2013;14:782. doi: 10.1186/1471-2164-14-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marathe R., Anandalakshmi R., Liu Y., Dinesh-Kumar S.P. The Tobacco Mosaic Virus Resistance Gene, N. Mol. Plant Pathol. 2002;3:167–172. doi: 10.1046/j.1364-3703.2002.00110.x. [DOI] [PubMed] [Google Scholar]
- 47.Cui L., Siskos L., Wang C., Schouten H.J., Visser R.G.F., Bai Y. Breeding Melon (Cucumis melo) with Resistance to Powdery Mildew and Downy Mildew. Hortic. Plant J. 2022;8:545–561. doi: 10.1016/j.hpj.2022.07.006. [DOI] [Google Scholar]
- 48.Pedersen C., van Themaat E.V.L., McGuffin L.J., Abbott J.C., Burgis T.A., Barton G., Bindschedler L.V., Lu X., Maekawa T., Weßling R., et al. Structure and Evolution of Barley Powdery Mildew Effector Candidates. BMC Genom. 2012;13:1–21. doi: 10.1186/1471-2164-13-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toruño T.Y., Stergiopoulos I., Coaker G. Plant-Pathogen Effectors: Cellular Probes Interfering with Plant Defenses in Spatial and Temporal Manners. Annu. Rev. Phytopathol. 2016;54:419–441. doi: 10.1146/annurev-phyto-080615-100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Corredor-Moreno P., Minter F., Davey P.E., Wegel E., Kular B., Brett P., Lewis C.M., Morgan Y.M.L., Pérez L.A.M., Korolev A.V., et al. The Branched-Chain Amino Acid Aminotransferase TaBCAT1 Modulates Amino Acid Metabolism and Positively Regulates Wheat Rust Susceptibility. Plant Cell. 2021;33:1728. doi: 10.1093/plcell/koab049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Von Saint Paul V., Zhang W., Kanawati B., Geist B., Faus-Keßler T., Schmitt-Kopplin P., Schäffner A.R. The Arabidopsis Glucosyltransferase UGT76B1 Conjugates Isoleucic Acid and Modulates Plant Defense and Senescence. Plant Cell. 2011;23:4124–4145. doi: 10.1105/tpc.111.088443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bacete L., Mélida H., Miedes E., Molina A. Plant Cell Wall-Mediated Immunity: Cell Wall Changes Trigger Disease Resistance Responses. Plant J. 2018;93:614–636. doi: 10.1111/tpj.13807. [DOI] [PubMed] [Google Scholar]
- 53.Miedes E., Vanholme R., Boerjan W., Molina A. The Role of the Secondary Cell Wall in Plant Resistance to Pathogens. Front. Plant Sci. 2014;5:1–13. doi: 10.3389/fpls.2014.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Voxeur A., Habrylo O., Guénin S., Miart F., Soulié M.C., Rihouey C., Pau-Roblot C., Domon J.M., Gutierrez L., Pelloux J., et al. Oligogalacturonide Production upon Arabidopsis Thaliana-Botrytis Cinerea Interaction. Proc. Natl. Acad. Sci. USA. 2019;116:19743–19752. doi: 10.1073/pnas.1900317116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Azevedo Souza C., Li S., Lin A.Z., Boutrot F., Grossmann G., Zipfel C., Somerville S.C. Cellulose-Derived Oligomers Act as Damage-Associated Molecular Patterns and Trigger Defense-like Responses. Plant Physiol. 2017;173:2383–2398. doi: 10.1104/pp.16.01680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nie J., Wang Y., He H., Guo C., Zhu W., Pan J., Li D., Lian H., Pan J., Cai R. Loss-of-Function Mutations in CsMLO1 Confer Durable Powdery Mildew Resistance in Cucumber (Cucumis sativus L.) Front. Plant Sci. 2015;6:1155. doi: 10.3389/fpls.2015.01155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vogel J.P., Raab T.K., Schiff C., Somerville S.C. PMR6, a Pectate Lyase-like Gene Required for Powdery Mildew Susceptibility in Arabidopsis. Plant Cell. 2002;14:2095–2106. doi: 10.1105/tpc.003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vogel J.P., Raab T.K., Somerville C.R., Somerville S.C. Mutations in PMR5 Result in Powdery Mildew Resistance and Altered Cell Wall Composition. Plant J. 2004;40:968–978. doi: 10.1111/j.1365-313X.2004.02264.x. [DOI] [PubMed] [Google Scholar]
- 59.Beraldo-Hoischen P., Hoefle C., López-Sesé A.I. Fungal Development and Callose Deposition in Compatible and Incompatible Interactions in Melon Infected with Powdery Mildew. Pathogens. 2021;10:873. doi: 10.3390/pathogens10070873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Polonio Á., Pineda M., Bautista R., Martínez-Cruz J., Pérez-Bueno M.L., Barón M., Pérez-García A. RNA-Seq Analysis and Fluorescence Imaging of Melon Powdery Mildew Disease Reveal an Orchestrated Reprogramming of Host Physiology. Sci. Rep. 2019;9:1–16. doi: 10.1038/s41598-019-44443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sarria-Villada E., Garzo E., Lopez-Sese A., Fereres A., Gomez-Guillamon M.L. Hypersensitive Response to Aphis Gossypii Glover in Melon Genotypes Carrying the Vat Gene. J. Exp. Bot. 2009;60:3269–3277. doi: 10.1093/jxb/erp163. [DOI] [PubMed] [Google Scholar]
- 62.Palomares-Rius F., Garcés-Claver A., Picó B., Esteras C., Yuste-lisbona F., Gómez-Guillamón M. ‘Carmen’, a Yellow Canary Melon Breeding Line Resistant to Podosphaera xanthii, Aphis gossypii, and Cucurbit Yellow Stunting Disorder Virus. Hort Sci. 2018;53:1072–1075. doi: 10.21273/HORTSCI13013-18. [DOI] [Google Scholar]
- 63.Liu L., Chen Y., Zhenghong S., Zang H., Zhu W. A Sequence-Amplified Characterized Region Marker for a Single, Dominant Gene in Melon PI 134198 that Confers Resistance to a Unique Race of Podosphaera xanthii in China. Am. Soc. Hortic. Sci. 2010;45:1407–1410. doi: 10.21273/HORTSCI.45.9.1407. [DOI] [Google Scholar]
- 64.Moore J.W., Herrera-Foessel S., Lan C., Schnippenkoetter W., Ayliffe M., Huerta-Espino J., Lillemo M., Viccars L., Milne R., Periyannan S., et al. A Recently Evolved Hexose Transporter Variant Confers Resistance to Multiple Pathogens in Wheat. Nat. Genet. 2015;47:1494–1498. doi: 10.1038/ng.3439. [DOI] [PubMed] [Google Scholar]
- 65.Hayes M.A., Feechan A., Dry I.B. Involvement of Abscisic Acid in the Coordinated Regulation of a Stress-Inducible Hexose Transporter (VvHT5) and a Cell Wall Invertase in Grapevine in Response to Biotrophic Fungal Infection. Plant Physiol. 2010;153:211–221. doi: 10.1104/pp.110.154765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Banday Z.Z., Nandi A.K. Interconnection between Flowering Time Control and Activation of Systemic Acquired Resistance. Front. Plant Sci. 2015;6:1–11. doi: 10.3389/fpls.2015.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ramonell K.M., Goff K.E. The Role and Regulation of Receptor-Like Kinases in Plant Defense. Gene Regul. Syst. Biol. 2007;1:167–175. [PMC free article] [PubMed] [Google Scholar]
- 68.Martin G.B., Brommonschenkel S.H., Chunwongse J., Frary A., Ganal M.W., Spivey R., Wu T., Earle E.D., Tanksley S.D. Map-Based Cloning of a Protein Kinase Gene Conferring Disease Resistance in Tomato. Science. 1993;262:1432–1436. doi: 10.1126/science.7902614. [DOI] [PubMed] [Google Scholar]
- 69.Song W.Y., Wang G.L., Chen L.L., Kim H.S., Pi L.Y., Holsten T., Gardner J., Wang B., Zhai W.X., Zhu L.H., et al. A Receptor Kinase-Like Protein Encoded by the Rice Disease Resistance Gene, Xa21. Science. 1995;270:1804. doi: 10.1126/science.270.5243.1804. [DOI] [PubMed] [Google Scholar]
- 70.Wang S., Wang S., Sun Q., Yang L., Zhu Y., Yuan Y., Hua J. A Role of Cytokinin Transporter in Arabidopsis Immunity. Mol. Plant Microbe Interact. 2017;30:325–333. doi: 10.1094/MPMI-01-17-0011-R. [DOI] [PubMed] [Google Scholar]
- 71.Dong W., Nowara D., Schweizer P. Protein Polyubiquitination Plays a Role in Basal Host Resistance of Barley. Plant Cell. 2006;18:3321–3331. doi: 10.1105/tpc.106.046326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Deng F., Guo T., Lefebvre M., Scaglione S., Antico C.J., Jing T., Yang X., Shan W., Ramonell K.M. Expression and Regulation of ATL9, an E3 Ubiquitin Ligase Involved in Plant Defense. PLoS ONE. 2017;12:e0188458. doi: 10.1371/JOURNAL.PONE.0188458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li W., Zhong S., Li G., Li Q., Mao B., Deng Y., Zhang H., Zeng L., Song F., He Z. Rice RING Protein OsBBI1 with E3 Ligase Activity Confers Broad-Spectrum Resistance against Magnaporthe Oryzae by Modifying the Cell Wall Defence. Cell Res. 2011;21:835–848. doi: 10.1038/cr.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang M., Sanchez-Moreiras A.M., Abel C., Sohrabi R., Lee S., Gershenzon J., Tholl D. The Major Volatile Organic Compound Emitted from Arabidopsis Thaliana Flowers, the Sesquiterpene (E)-β-Caryophyllene, Is a Defense against a Bacterial Pathogen. New Phytol. 2012;193:997–1008. doi: 10.1111/j.1469-8137.2011.04001.x. [DOI] [PubMed] [Google Scholar]
- 75.Godard K.A., White R., Bohlmann J. Monoterpene-Induced Molecular Responses in Arabidopsis Thaliana. Phytochemistry. 2008;69:1838–1849. doi: 10.1016/j.phytochem.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 76.Zhu Q., Gao P., Wan Y., Cui H., Fan C., Liu S., Luan F. Comparative Transcriptome Profiling of Genes and Pathways Related to Resistance against Powdery Mildew in Two Contrasting Melon Genotypes. Sci. Hortic. 2018;227:169–180. doi: 10.1016/j.scienta.2017.09.033. [DOI] [Google Scholar]
- 77.Sun J., Dong Y., Wang C., Xiao S., Jiao Z., Gao C. Identification and Characterization of Melon Circular RNAs Involved in Powdery Mildew Responses through Comparative Transcriptome Analysis. PeerJ. 2021;9:e11216. doi: 10.7717/peerj.11216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou X., Cui J., Cui H., Jiang N., Hou X., Liu S., Gao P., Luan Y., Meng J., Luan F. Identification of LncRNAs and Their Regulatory Relationships with Target Genes and Corresponding MiRNAs in Melon Response to Powdery Mildew Fungi. Gene. 2020;735:144403. doi: 10.1016/j.gene.2020.144403. [DOI] [PubMed] [Google Scholar]
- 79.Fukino N., Yoshioka Y., Sugiyama M., Sakata Y., Matsumoto S. Identification and Validation of Powdery Mildew (Podosphaera Xanthii)-Resistant Loci in Recombinant Inbred Lines of Cucumber (Cucumis sativus L.) Mol. Breed. 2013;32:267–277. doi: 10.1007/s11032-013-9867-3. [DOI] [Google Scholar]
- 80.Hosoya K., Kuzuya M., Murakami T., Kato K., Narisawa K., Ezura H. Impact of Resistant Melon Cultivars on Sphaerotheca Fuliginea. Plant Breed. 2000;119:286–288. doi: 10.1046/j.1439-0523.2000.00489.x. [DOI] [Google Scholar]
- 81.Tores J.A., Gomez-Guillamon M.L., Canovas I. Temperature-Conditioned Response to Sphaerotheca Fuliginea Race 1 in the Spanish Melon Cultivar ANC57. Cucurbit Genet. Coop. Rep. 1996;19:59–60. [Google Scholar]
- 82.Sakata Y., Kubo N., Morishita M., Kitadani E., Sugiyama M., Hirai M. QTL Analysis of Powdery Mildew Resistance in Cucumber (Cucumis sativus L.) Theor. Appl. Genet. 2006;112:243–250. doi: 10.1007/s00122-005-0121-1. [DOI] [PubMed] [Google Scholar]
- 83.Ferriere H., Molot P.M. Effect of Leaf Position on the Susceptibility of Melon Plants to Artificial Infection with Powdery Mildew, Sphaerotheca Fuliginea. J. Phytopathol. 1988;121:250–254. doi: 10.1111/j.1439-0434.1988.tb04451.x. [DOI] [Google Scholar]
- 84.Bohn G.W., Whitaker T.W. Genetics of resistance to powdery mildew race 2 in muskmelon. Phytopathology. 1964;54:587–591. [Google Scholar]
- 85.Boiteux L.S., Reifschneider F.J.B., Pessoa H.B.S.V. Phenotypic Expression of Quantitative and Qualitative Components of Partial Resistance to Powdery Mildew (Sphaerotheca Fuliginea Race 1) in Melon (Cucumis melo) Germplasm. Plant Breed. 1995;114:185–187. doi: 10.1111/j.1439-0523.1995.tb00789.x. [DOI] [Google Scholar]
- 86.McCreight J.D. Genes for Resistance to Powdery Mildew Races 1 and 2 U.S. in Melon PI 313970. HortScience. 2003;38:591–594. doi: 10.21273/HORTSCI.38.4.591. [DOI] [Google Scholar]
- 87.Doyle J., Doyle J. Isolation of Plant DNa from Fresh Tissue. Focus. 1990;12:13–15. [Google Scholar]
- 88.Langmead B., Salzberg S.L. Fast Gapped-Read Alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Garrison E., Marth G. Haplotype-Based Variant Detection from Short-Read Sequencing. [(accessed on 12 October 2022)];arXiv. 2012 Available online: https://arxiv.org/abs/1207.3907.1207.3907 [Google Scholar]
- 90.Cingolani P., Platss A., Wang L., Coon M., Nguyen T., Wang L., Land S.J., Lu X., Ruden D.M. A Program for Annotating and Predicting the Effects of Single Nucleotide Polymorphisms, SnpEff. Fly. 2012;6:80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.