Abstract
Antibody- and complement-mediated phagocytosis is the main defense mechanism against Streptococcus pneumoniae. A standardized, easy to perform phagocytosis assay for pneumococci would be a great asset for the evaluation of the potential efficacy of (experimental) pneumococcal vaccines. Such an assay could replace the laborious phagocytosis assay of viable pneumococci (classical killing assay). Therefore, a newly developed phagocytosis assay based on flow cytometry (flow assay) was compared with the conventional killing assay and enzyme-linked immunosorbent assay (ELISA), using sera obtained from adults pre- and postvaccination with either a bivalent conjugate, a tetravalent conjugate, or the 23-valent polysaccharide vaccine. Highly significant correlations were observed between flow assay phagocytosis titers, killing assay phagocytosis titers, and ELISA antibody titers for serotype 6B and 23F as well. For serotype 19F, strong correlations were only observed between killing assay and ELISA titers. A potential drawback of the flow assay might be the low sensitivity compared with that of the killing assay. The choice of what assay to use, however, will depend on the objectives of the assay. When speed, easy performance, sample throughput, improved worker safety, absence of influence of antibiotics, and absence of false positives are the major criteria, the flow assay is the method of choice. When higher sensitivity is the major requirement, the classical killing assay should be used.
Streptococcus pneumoniae is a major cause of lower respiratory tract infections (18), otitis media (17), sepsis, and meningitis (4, 30, 38) in infants. Pneumococci are increasingly becoming resistant to penicillin (5, 11, 31), and recently vancomycin-tolerant pneumococci have been reported (23). A vaccine to protect humans against pneumococci consisting of the polysaccharides (PS) of the 23 most prevalent serotypes has been available since 1983 (27). These PS are thymus-independent antigens and therefore are poorly immunogenic in infants and do not induce immunological memory. PS conjugated to proteins, however, overcome these problems. These types of antigens are thymus dependent, induce immunological memory, and are immunogenic in infants (33).
Nowadays, several experimental conjugate vaccines are being evaluated in clinical trials (2, 7, 8, 19, 34), and recently the first pneumococcal conjugate vaccine has been licenced in the United States (26). The main defense mechanism against pneumococcal infections is antibody- and complement-mediated phagocytosis. The most commonly used surrogate parameter for protection is PS antibody concentration as measured by enzyme-linked immunosorbent assay (ELISA). This assay, however, does not measure functionality (phagocytic capacity) of the antibodies. Although, in general, strong correlations between PS immunoglobulin G (IgG) antibody titers and phagocytosis titers are measured (11, 14, 31), direct measurement of opsonophagocytosis is a better predictor of in vivo protective capacities of anti-PS antibodies, than anti-PS IgG concentration as measured by ELISA (1, 15, 25, 32). This is especially true when sera of unimmunized subjects are studied (34a). Recently described nonopsonic antibodies that cross-react with different PS in ELISA might explain this lack of correlation between PS antibody levels and protection (21). Therefore, there is a need for an easy-to-perform in vitro phagocytosis assay that can be used as a correlate of efficacy. A number of phagocytosis assays, based on either flow cytometry (6, 10, 20, 24, 28, 36), microscopy (39, 40), radioactivity (37), or cell counting (29), have been developed. To evaluate the phagocytic capacity of large numbers of antisera, these assays should meet a number of criteria. The assays should be easy, fast, and safe to perform and easy to transfer to and standardize in other laboratories. Furthermore, they should be specific and sensitive. Finally, the assays should consume minimal amounts of serum, have a low cost price per sample, and ideally represent the human in vivo defense mechanism. None of the assays described thus far, however, meet all these criteria yet.
We have developed a standardized, easy-to-perform phagocytosis assay based on flow cytometry (flow assay) (14). This flow assay uses human polymorphonuclear granulocytes (PMN) and human IgG-depleted serum as a complement source. Since this assay resembles the defense mechanism in humans it has the potential to correlate with in vivo protection. In a mouse model excellent correlations between flow assay phagocytosis titers and mouse protection have been observed (1; W. T. M. Jansen et al., unpublished results). The killing phagocytosis assay has been standardized by Romero-Steiner et al. (29). This assay is considered to be the “gold standard” and is a predictor for protection from bacteremia in a mouse model (15). It is, however, rather time consuming, requiring much labor and therefore not suited for the evaluation of large numbers of pneumococcal antisera obtained from, e.g., clinical trials. In addition, live human pathogens have to be used on a daily basis.
The aim of this study was to investigate whether the flow assay can be used as an alternative for the killing assay to measure phagocytic capacity of pneumococcal antibodies, measured in pre- and postvaccination sera. Therefore, antisera obtained from vaccinees immunized with the conventional 23-valent PS vaccine or experimental conjugate vaccines were analyzed for anti-PS IgG antibody titers by ELISA and phagocytosis titers as measured by the flow assay and killing assay. Correlations between ELISA and flow and killing assays were determined and advantages and disadvantages of the phagocytosis assays are discussed.
MATERIALS AND METHODS
Vaccines and antisera.
Antisera obtained from adults 1 month after vaccination with a pneumococcal 6A and 23F bivalent CRM197 conjugate vaccine (35) were kindly provided by Wyeth-Lederle Vaccines and Pediatrics, Rochester, N.Y. Antisera from Finnish adults 28 days after vaccination with a tetravalent pneumococcal conjugate vaccine (22), were kindly provided by Tea Nieminen at the National Public Health Institute, Helsinki, Finland. The latter vaccines contained 10 μg of capsular PS of type 6B, 14, 19F, and 23F conjugated to either diphtheria toxoid (Pasteur-Mérieux Connaught, Swiftwater, Pa.) or tetanus toxoid (Pasteur-Mérieux Connaught, Marcy l'Etoile, France). Sera obtained from adults 1 month after vaccination with a 23-valent PS vaccine (Pnu-immune; Wyeth-Lederle Vaccines and Pediatrics) were received from Moon Nahm, Rochester, N.Y.
Flow assay.
The flow cytometry phagocytosis assay was performed as described (14). Pneumococcal strains (serotype 6A, 6B, 19F, and 23F) (American Type Culture Collection [Manassas, Va.] strains), kindly provided by Statens Serum Institut, Copenhagen, Denmark), were grown thrice consecutively to log phase to ensure high-level encapsulation (14) and subsequently were heat inactivated and fluorescein isothiocyanate (FITC) labeled. Human pooled serum was depleted of IgG by protein G affinity chromatography and used as a complement source. Human PMN were isolated from the blood of healthy, Fcγ receptor-typed volunteers (13) by a Ficoll-Histopaque gradient. Only PMN that were heterozygous or homozygous for the His 131 FcγIIa receptor allotype (e.g., receptor allotype with high affinity for IgG2) were used for this study (13). Sera from vaccinees were heated (30 min at 56°C) to inactivate the complement. Samples of 2.5 × 106 pneumococci, a fixed concentration of complement (2% final concentration in the assay), and a diluted serum sample were added per well in a 96-well microtiter plate. After 30 min of opsonization at 37°C on a microtiter plate agitator, 2.5 × 105 PMN per well were added and phagocytosis was performed for another 30 min 37°C on the agitator. After a wash with ice-cold bovine serum albumin-Hanks balanced salt solution, the cells were transferred to fluorescence-activated cell sorter tubes, fixed with paraformaldhyde (2%) in phosphate-buffered saline (PBS), and analyzed in a flow cytometer (FACScan; Becton Dickinson). For an example of the histograms obtained, see reference (14). The percentage of FITC-positive PMN was used as a measure for the phagocytic activity of the serum. Results are expressed as log10 of the phagocytosis titer, in which the phagocytosis titer is the reciprocal serum dilution resulting in 25% FITC-positive PMN. The reproducibility of the assay was controlled by including a positive control serum in each plate.
Killing assay.
The killing assay was performed according to an adapted protocol (3) originally described by Romero-Steiner et al. (29). PMN were isolated from blood of healthy adult donors by dextran sedimentation and Ficoll-Histopaque density gradient centrifugation. Sera were heated (1 h 56°C) to inactivate the complement, and baby rabbit serum was used as an external complement source. Diluted serum samples in Hanks balanced salt solution containing 1% gelatin and bacteria (1,000 CFU per well) were added to 96-well microtiter plates and incubated for 15 min at 37°C in a 5% CO2 atmosphere. Subsequently, complement (12.5% final concentration) and PMN (4 × 105 per well) were added and phagocytosis was performed on a horizontal shaker platform for 45 min at 37°C. From each well a 5-μl aliquot was transferred to plates that were then incubated at 37°C in 5% CO2 overnight, and CFU were counted manually. Opsonophagocytic activity of antibodies was expressed as log10 of the phagocytosis titer, in which the phagocytosis titer is the reciprocal of the serum dilution with 50% viable bacteria compared with the bacterial growth of the controls, which contain no serum. Sera with undetectable phagocytosis titers were reported as having a phagocytosis titer 4, with a titer of 8 being the highest positive result. The reproducibility of the assay was controlled by including a positive control serum in each plate.
EIA.
Concentration of IgG antibodies to pneumococcal PS were determined by ELISA for both conjugate vaccine sera (16) and polysaccharide vaccine sera (21). Serotype-specific PS was obtained from the American Type Culture Collection for use as coating antigen. Specimens were preadsorbed with C-PS-enriched absorbent prepared by Wyeth-Lederle Vaccines and Pediatrics. Briefly, the serum samples were diluted 1:100 in PBS–10% fetal calf serum (F-PBS) containing cell wall PS (10 μg/ml). After 30 min at room temperature, threefold dilutions were made in F-PBS and the sera were incubated on the plates for 2 h at 37°C. After washing, antibody binding was detected by alkaline phosphatase-conjugated anti-human IgG (Sigma, St. Louis, Mo.) diluted in F-PBS and incubated for 2 h at 37°C. The color was developed by the substrate p-nitrophenyl phosphate (Sigma) and the absorbance at 405 nm was read on an ELISA reader (Multiscan Labsystems, Helsinki, Finland). Antibody levels in the samples were expressed as log10 of the IgG antibody concentration (in micrograms per milliliter), calculated on the basis of the officially assigned IgG values of the pneumococcal lot 89-SF reference serum (25) (Center for Biological Research and Evaluation, Food and Drug administration, Washington, D.C.).
Statistical analysis.
Killing assay, ELISA, and flow assay were performed in different laboratories. Correlation between flow assay phagocytosis, killing assay phagocytosis, and ELISA antibody titers were analyzed by Pearson bivariate correlation analysis using SPSS 8.0 statistics software. Statistical significance of correlations was assessed by Student's t test. A P value of <0.05 was considered statistically significant.
RESULTS
Comparison between flow assay, killing assay, and ELISA using pneumococcal 23-valent PS vaccine antisera.
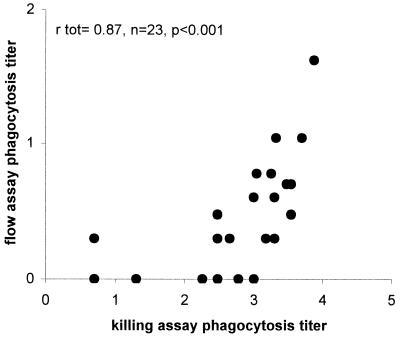
Sera from 23 adults vaccinated with the 23-valent PS vaccine were analyzed for IgG antibody levels in ELISA using PS 6A, 6B, and 19F as coating antigen. The functionality of these serum antibodies was determined in two different phagocytosis assays: the flow assay and the killing phagocytosis assay. Mean antibody titers and phagocytosis titers for the different serotypes are shown in Table 1. In spite of the low phagocytosis titers for serotype 6B in the flow assay, a strong, significant correlation between the flow and killing assays was observed for this serotype (Table 2). However, antisera with opsonophagocytosis titers below 2 (1:100) as measured by the killing assay were not opsonic in the flow assay (Fig. 1), indicating that the flow assay had a lower sensitivity than the killing assay. For serotype 6A, correlations between ELISA, killing assay, and flow assay, titers were all significant but relatively low compared to that serotype or 6B. For serotype 19F, however, the results obtained with the three different assays showed no correlation (Table 2).
TABLE 1.
Mean logarithmic titers obtained by flow assay, killing assay, and ELISA for antisera from adults vaccinated with pneumococcal PS or conjugate vaccine
| Vaccine | Serotype | n | MLTa obtained by:
|
|||||
|---|---|---|---|---|---|---|---|---|
| Flow assay
|
Killing assay
|
ELISA
|
||||||
| Pre | Post | Pre | Post | Pre | Post | |||
| 23-valent PS | 6A | 23 | NDb | 0.21 | ND | 2.41 | ND | 2.30 |
| 6B | 23 | ND | 0.37 | ND | 2.77 | ND | 1.15 | |
| 19F | 23 | ND | 0.83 | ND | 2.64 | ND | 1.13 | |
| Bivalent conjugate | 23F | 46c | 0.25 | 1.32 | 0.23 | 2.53 | 0.52 | 2.12 |
| Tetravalent conjugate | 6B | 34 | 0.25 | 1.07 | 1.28 | 3.50 | 0.27 | 1.31 |
| 19F | 40 | 0.29 | 0.34 | 0.92 | 2.63 | 0.39 | 1.59 | |
| 23F | 40 | 0.21 | 0.62 | 0.88 | 2.85 | 0.33 | 1.54 | |
Mean log10 titers (MLT) were calculated before vaccination (Pre) for conjugate antisera only and after vaccination (Post) for both PS and conjugate vaccine antisera.
ND, not done, because antisera were not available.
MLT for the killing assay was calculated for seven prevaccination and seven postvaccination antisera.
TABLE 2.
Correlation between ELISA, flow assay, and killing assay using antisera from adults vaccinated with pneumococcal PS or conjugate vaccine
| Vaccine | Serotype | nb | Correlation between assaysa
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Flow and killing
|
ELISA and killing
|
Flow and ELISA
|
|||||||||
| r | Pf | Sd | r | P | S | r | P | S | |||
| 23-valent PS | 6A | 23 | 0.58 | 0.01 | 0.16 | 0.17 | 0.01 | 1.34 | 0.60 | 0.01 | 0.48 |
| 6B | 23 | 0.87 | 0.001 | 0.56 | 0.54 | 0.05 | 0.54 | 0.49 | 0.05 | 0.32 | |
| 19F | 23 | 0.06 | NSe | 0.10 | 0.22 | NS | 0.55 | 0.14 | NS | 0.44 | |
| Bivalent conjugate | 23F | 46c | 0.91 | 0.01 | 0.47 | 0.87 | 0.01 | 1.34 | 0.98 | 0.001 | 0.64 |
| Tetravalent conjugate | 6B | 34 | 0.77 | 0.001 | 0.41 | 0.90 | 0.001 | 1.62 | 0.79 | 0.001 | 0.76 |
| 19F | 40 | 0.65 | 0.01 | 0.30 | 0.87 | 0.01 | 1.07 | 0.65 | 0.05 | 0.37 | |
| 23F | 40 | 0.79 | 0.001 | 0.61 | 0.91 | 0.001 | 1.33 | 0.75 | 0.001 | 0.84 | |
The correlation coefficient (r) was calculated by Pearson's bivariate linear correlation analysis.
n is the total number of analyzed antisera per vaccine. For the PS vaccine, only postvaccination antisera were analyzed, whereas for the conjugate vaccines pre- and postvaccination antisera were combined for correlation analysis.
For the killing assay, titers were obtained for 14 antisera only. Therefore, correlations between killing assay and ELISA flow assay and ELISA were calculated for these corresponding 14 antisera.
The slope (S) of the fitted linear curve, Y = Sx + b, was calculated by linear regression analysis.
NS, not significant
P is less than the values given.
FIG. 1.
Correlation between killing assay and flow assay for 23 antisera obtained from adults vaccinated with the 23-valent PS vaccine for serotype 6B pneumococci. The correlation coefficient, rtot, was calculated by Pearson's bivariate correlation analysis.
Comparison between flow-, killing-assay and EIA for conjugate antisera.
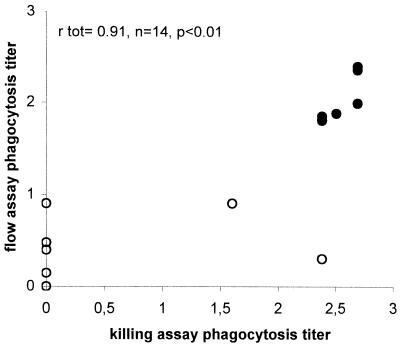
Since newly developed vaccines against S. pneumoniae consist of conjugated saccharides, sera obtained from adults vaccinated with two different experimental conjugate vaccines were analyzed. Sera from 46 vaccinees, before and after vaccination with an experimental 6A-23F bivalent conjugate vaccine, were compared for (functional) antibody levels against serotype 23F as measured by ELISA, flow assay, and killing assay. Mean anti-23F antibody titer and flow and killing assay phagocytosis titers are shown in Table 1. Strong correlations were observed between flow and killing assay titers (Fig. 2), killing assay and ELISA titers (Table 2), and flow assay and ELISA titers (Table 2), the last of these as reported previously in reference (14).
FIG. 2.
Correlation between killing assay and flow assay for 14 antisera obtained from adults vaccinated with a bivalent conjugate vaccine for serotype 23F pneumococci. The correlation coefficient rtot was calculated for total prevaccination (○) and postvaccination (•), antisera by Pearson's bivariate correlation analysis.
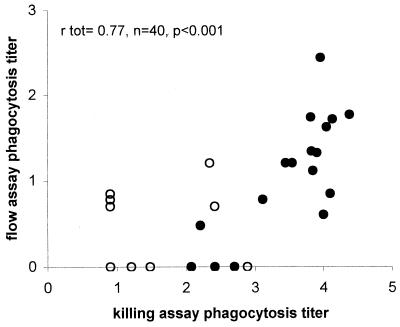
Subsequently ELISA, flow assay, and killing assay titers were determined for 40 sera from adults before and after vaccination with a tetravalent conjugate vaccine. For serotype 6B and 23F, correlations between all three assays were strong and significant (Table 2). For 6B, correlations between the flow assay and killing assay are shown in Fig 3. For serotype 19F, however, significant correlations between the three assays were only observed when all serum samples (pre- and postvaccination) were included.
FIG. 3.
Correlation between killing assay and flow assay for 40 antisera obtained from adults vaccinated with a tetravalent conjugate vaccine for serotype 6B pneumococci. The correlation coefficient rtot was calculated for total prevaccination (○) and postvaccination (•) antisera by Pearson's bivariate correlation analysis.
DISCUSSION
To examine the potential of the flow assay as an alternative assay for the killing assay, we compared these assays and ELISA using sera from people immunized with different pneumococcal vaccines. In general, comparison yielded highly significant correlations between ELISA, the flow assay, and the killing assay. There were, however, some exceptions. For serotype 19F, correlations between flow assay and killing assay were low, especially when using PS vaccine antisera. This finding is in agreement with the relatively low correlations between ELISA and opsonophagocytosis observed for serotype 19F by Romero-Steiner et al. who used the killing assay (29), suggesting that the extent of correlation between antibody concentration and opsonic capacity of these antibodies might vary between different serotypes.
In general, for PS vaccine antisera correlations between the three assays were relatively low. One of the reasons for the weak correlation between ELISA and phagocytosis assays might be the induction of relatively high amounts of IgM by the PS vaccine in the serum. Recently it has been shown that in addition to IgM and IgG, IgA is also able to mediate phagocytosis of pneumococci (12). Since only IgG antibodies were measured in the ELISA, the presence of IgM and IgA antibodies can obscure the relationship between IgG antibody levels and phagocytosis. Since anti-PS IgG antibody concentrations induced by the PS vaccines in general were rather low compared to those induced by the conjugate vaccines, phagocytosis titers measured were close to the detection limit of the flow assay. This might explain why, in general, correlations between the flow assay and the other assays were weak for serum containing low anti-PS IgG antibody levels (e.g., in prevaccination serum samples and after PS vaccination). As a consequence, the flow assay might be more suited for the evaluation of conjugate vaccine efficacy than for the evaluation of the conventional Pneumovax vaccine.
The killing assay and flow assay differ in assay components and readout system. The possible contribution of each component to the observed differences in phagocytosis titers and sensitivity is discussed below. The flow and killing assays both use PMN as effector cells. These cells bear two Fc receptors for IgG: FcγRIIa and FcγRIIIb. Both receptors display a genetic polymorphism that affects in vitro phagocytosis of pneumococci (13). In the flow assay only FcγRIIa allotypes that were heterozygous for 131 His, i.e., the high-affinity IgG2 receptor allotype, were used. PMN were not allotyped for their FcγR in the killing assay, which might have influenced phagocytosis titers and, as a consequence, affected correlation coefficients between both assays. Furthermore, the assays differ in complement source and complement concentration. The flow assay uses 2% human IgG-depleted serum, whereas the killing assay uses 20% baby rabbit antiserum. Although 20% baby rabbit serum did not significantly alter phagocytosis titers as measured by the flow assay (W. T. M. Jansen et al., unpublished results), it is possible that differences in complement source and concentration are additional factors influencing the correlation between the flow and killing assays. Finally, both assays differ in the strains used for opsonization. In the flow assay, pneumococci are grown thrice to log phase to ensure high-level encapsulation. By this method any opsonophagocytic activity of anti-cell wall PS antibodies is excluded. The very dense encapsulation (electron microscope photographs [data not shown]) ensures high specificity but might to some extent hinder phagocytosis efficiency, thereby reducing the sensitivity of the flow assay.
As stated above, both flow and killing assays use PMN as effector cells. Alternatively, HL60 cell lines can be used as effector cells in a flow cytometry phagocytosis assay (20) and the classical killing phagocytosis assay (29). Although the use of HL60 cell lines circumvents the need for healthy blood donors, this cell line also has some disadvantages. It remains difficult to differentiate this cell line into active phagocytes in most laboratories. Because the differentiation takes up to 8 or 9 days (29), assays using HL60 cell lines are not suited for short-term measurement of opsonophagocytic antibody levels in, for example, patients. Furthermore, the number of cells that remain viable during differentiation is variable (29) and relatively low. Finally, it has been shown that the FcγRIIa on these cells is homozygoous for 131 Arg, which is the low-affinity IgG2 receptor allotype (20). Since in adults the antibody response to PS is mainly of the IgG2 subtype, this is of importance when analyzing phagocytic capacities in sera from adults (13).
Though clinical trials for pneumococcal conjugate vaccine have been recently finished or are still ongoing (9), data about protective opsonic antibody titers are still incomplete. Therefore, the definite choice for a particular in vitro assay as a correlate for pneumococcal vaccine efficacy in humans cannot be made yet. However, both the flow and the killing assays have distinct advantages and disadvantages. The flow assay is easy to set up and perform. It is a fast assay, suitable for the screening of 125 serum samples per day (14). Moreover, since the flow assay does not use viable bacteria it will not be affected by the presence of antibiotics in the serum. All assay components are of human origin, including Fcγ receptor-allotyped PMN. Finally, since this assay only measures serotype-specific phagocytosis of highly encapsulated pneumococci (14), it is unlikely that false positives will occur. A disadvantage of the flow assay is its lower sensitivity. In contrast, the killing assay is highly sensitive but has the disadvantages of being cumbersome to perform and more labor-intensive, requiring live pathogens, and having a higher cost price per sample.
In conclusion, we partially achieved our goal of establishing our flow assay as an alternative to the killing assay. The preference for either flow or killing assay will depend on which criteria are most important in a particular situation. As long as the sensitivity of the flow assay is high enough to measure minimal protective phagocytosis titer, false negatives can be avoided, whereas false positives will certainly be excluded. In the case of antisera with borderline phagocytic titers, one could fall back on the killing assay for a second opinion.
ACKNOWLEDGMENTS
This study was supported by the World Health Organization Global Program for Vaccines and Immunization, Vaccine Research and Development (V23/181/92), and a travel grant from the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO, 0413-Fin).
We thank Wyeth-Lederle Vaccines and Pediatrics and Pasteur-Mérieux Connaught for their kind gift of pneumococcal vaccination sera.
REFERENCES
- 1.Alonso De Velasco E, Dekker B A, Verheul A F, Feldman R G, Verhoef J, Snippe H. Anti-polysaccharide immunoglobulin isotype levels and opsonic activity of antisera: relationships with protection against Streptococcus pneumoniae infection in mice. J Infect Dis. 1995;172:562–565. doi: 10.1093/infdis/172.2.562. [DOI] [PubMed] [Google Scholar]
- 2.Anderson E L, Kennedy D J, Geldmacher K M, Donnelly J, Mendelman P M. Immunogenicity of heptavalent pneumococcal conjugate vaccine in infants. J Pediatr. 1996;128:649–653. doi: 10.1016/s0022-3476(96)80130-2. [DOI] [PubMed] [Google Scholar]
- 3.Anttila M, Voutilainen M, Jantti V, Eskola J, Kayhty H. Contribution of serotype specific IgG concentration, IgG subclasses, and relative antibody avidity to opsonophagocytic activity against Streptococcus pneumoniae. Clin Exp Immunol. 1999;118:402–407. doi: 10.1046/j.1365-2249.1999.01077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berg S, Trollfors B, Claesson B A, Alestig K, Gothefors L, Hugosson S, Lindquist L, Olcen P, Romanus V, Strangert K. Incidence and prognosis of meningitis due to Haemophilus influenzae, Streptococcus pneumoniae and Neisseria meningitidis in Sweden. Scand J Infect Dis. 1996;28:247–252. doi: 10.3109/00365549609027166. [DOI] [PubMed] [Google Scholar]
- 5.Boswell T C, Frodsham D, Nye K J, Smith E G. Antibiotic resistance and serotypes of Streptococcus pneumoniae at Birmingham Public Health Laboratory, 1989-94. J Infect. 1996;33:17–22. doi: 10.1016/s0163-4453(96)92681-x. [DOI] [PubMed] [Google Scholar]
- 6.Böyum X. Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Investig Suppl. 1986;97:77–89. [PubMed] [Google Scholar]
- 7.Dagan R, Melamed R, Zamir O, Leroy O. Safety and immunogenicity of tetravalent pneumococcal vaccines containing 6B, 14, 19F and 23F polysaccharides conjugated to either tetanus toxoid or diphtheria toxoid in young infants and their boosterability by native polysaccharide antigens. Pediatr Infect Dis J. 1997;16:1053–1059. doi: 10.1097/00006454-199711000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Dagan R, Muallem M, Melamed R, Leroy O, Yagupsky P. Reduction of pneumococcal nasopharyngeal carriage in early infancy after immunization with tetravalent pneumococcal vaccines conjugated to either tetanus toxoid or diphtheria toxoid. Pediatr Infect Dis J. 1997;16:1060–1064. doi: 10.1097/00006454-199711000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Eskola J, Anttila M. Pneumococcal conjugate vaccines. Pediatr Infect Dis J. 1999;18:543–551. doi: 10.1097/00006454-199906000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Gentry M J, Snitily M U, Preheim L C. Phagocytosis of Streptococcus pneumoniae measured in vitro and in vivo in a rat model of carbon tetrachloride-induced liver cirrhosis. J Infect Dis. 1995;171:350–355. doi: 10.1093/infdis/171.2.350. [DOI] [PubMed] [Google Scholar]
- 11.Gratten M, Torzillo P, Morey F, Dixon J, Erlich J, Hagger J, Henrichsen J. Distribution of capsular types and antibiotic susceptibility of invasive Streptococcus pneumoniae isolated from aborigines in central Australia. J Clin Microbiol. 1996;34:338–341. doi: 10.1128/jcm.34.2.338-341.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janoff E N, Fasching C, Orenstein J M, Rubins J B, Opstad N L, Dalmasso A P. Killing of Streptococcus pneumoniae by capsular polysaccharide-specific polymeric IgA, complement, and phagocytes. J Clin Investig. 1999;104:1139–1147. doi: 10.1172/JCI6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jansen W T, Breukels M A, Snippe H, Sanders L A, Verheul A F, Rijkers G T. Fcgamma receptor polymorphisms determine the magnitude of in vitro phagocytosis of Streptococcus pneumoniae mediated by pneumococcal conjugate sera. J Infect Dis. 1999;180:888–891. doi: 10.1086/314920. [DOI] [PubMed] [Google Scholar]
- 14.Jansen W T, Gootjes J, Zelle M, Madore D V, Verhoef J, Snippe H, Verheul A F. Use of highly encapsulated Streptococcus pneumoniae strains in a flow-cytometric assay for assessment of the phagocytic capacity of serotype-specific antibodies. Clin Diagn Lab Immunol. 1998;5:703–710. doi: 10.1128/cdli.5.5.703-710.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson S E, Rubin L, Romero-Steiner S, Dykes J K, Pais L B, Rizvi A, Ades E, Carlone G M. Correlation of opsonophagocytosis and passive protection assays using human anticapsular antibodies in an infant mouse model of bacteremia for Streptococcus pneumoniae. J Infect Dis. 1999;180:133–140. doi: 10.1086/314845. [DOI] [PubMed] [Google Scholar]
- 16.Kayhty H, Ahman H, Ronnberg P R, Tillikainen R, Eskola J. Pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine is immunogenic in infants and children. J Infect Dis. 1995;172:1273–1278. doi: 10.1093/infdis/172.5.1273. [DOI] [PubMed] [Google Scholar]
- 17.Klein J O. Otitis media. Clin Infect Dis. 1994;19:823–833. doi: 10.1093/clinids/19.5.823. [DOI] [PubMed] [Google Scholar]
- 18.Korppi M, Koskela M, Jalonen E, Leinonen M. Serologically indicated pneumococcal respiratory infection in children. Scand J Infect Dis. 1992;24:437–443. doi: 10.3109/00365549209052629. [DOI] [PubMed] [Google Scholar]
- 19.Leach A, Ceesay S J, Banya W A, Greenwood B M. Pilot trial of a pentavalent pneumococcal polysaccharide/protein conjugate vaccine in Gambian infants. Pediatr Infect Dis J. 1996;15:333–339. doi: 10.1097/00006454-199604000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Martinez J E, Romero-Steiner S, Pilishvili T, Barnard S, Schinsky M F, Goldblatt D, Carlone G M. A flow cytometric opsonophagocytic assay for measurement of functional antibodies elicited after vaccination with 23-valent pneumococcal polysaccharide vaccine. Clin Diagn Lab Immunol. 1999;6:581–587. doi: 10.1128/cdli.6.4.581-586.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nahm M H, Olander J V, Magyarlaki M. Identification of cross-reactive antibodies with low opsonophagocytic activity for Streptococcus pneumoniae. J Infect Dis. 1997;176:698–703. doi: 10.1086/514093. [DOI] [PubMed] [Google Scholar]
- 22.Nieminen T, Kayhty H, Leroy O, Eskola J. Pneumococcal conjugate vaccination in toddlers: mucosal antibody response measured as circulating antibody-secreting cells and as salivary antibodies. Pediatr Infect Dis J. 1999;18:764–772. doi: 10.1097/00006454-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Novak R, Henriques B, Charpentier E, Normark S, Tuomanen E. Emergence of vancomycin tolerance in Streptococcus pneumoniae. Nature. 1999;399:590–593. doi: 10.1038/21202. [DOI] [PubMed] [Google Scholar]
- 24.Obaro S K, Henderson D C, Monteil M A. Defective antibody-mediated opsonisation of S. pneumoniae in high risk patients detected by flow cytometry. Immunol Lett. 1996;49:83–89. doi: 10.1016/0165-2478(95)02487-5. [DOI] [PubMed] [Google Scholar]
- 25.Quataert S A, Kirch C S, Wiedl L J, Phipps D C, Strohmeyer S, Cimino C O, Skuse J, Madore D V. Assignment of weight-based antibody units to a human antipneumococcal standard reference serum, lot 89-S. Clin Diagn Lab Immunol. 1995;2:590–597. doi: 10.1128/cdli.2.5.590-597.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rennels M B, Edwards K M, Keyserling H L, Reisinger K S, Hogerman D A, Madore D V, Chang I, Paradiso P R, Malinoski F J, Kimura A. Safety and immunogenicity of heptavalent pneumococcal vaccine conjugated to CRM 197 in United States infants. Pediatrics. 1998;101:604–611. doi: 10.1542/peds.101.4.604. [DOI] [PubMed] [Google Scholar]
- 27.Robbins J B, Austrian R, Lee C J, Rastogi S C, Schiffman G, Henrichsen J, Makela P H, Broome C V, Facklam R R, Tiesjema R H. Considerations for formulating the second-generation pneumococcal capsular polysaccharide vaccine with emphasis on the cross-reactive types within groups. J Infect Dis. 1983;148:1136–1159. doi: 10.1093/infdis/148.6.1136. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez M E, van der Pol W L, Sanders L A, van de Winkel J G. Crucial role of FcgammaRIIa (CD32) in assessment of functional anti-Streptococcus pneumoniae antibody activity in human sera. J Infect Dis. 1999;179:423–433. doi: 10.1086/314603. [DOI] [PubMed] [Google Scholar]
- 29.Romero-Steiner S, Libutti D, Pais L B, Dykes J, Anderson P, Whitin J C, Keyserling H L, Carlone G M. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin Diagn Lab Immunol. 1997;4:415–422. doi: 10.1128/cdli.4.4.415-422.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sankilampi U, Herva E, Haikala R, Liimatainen O, Renkonen O V, Leinonen M. Epidemiology of invasive Streptococcus pneumoniae infections in adults in Finland. Epidemiol Infect. 1997;118:7–15. doi: 10.1017/s0950268896007169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schreiber J R, Jacobs M R. Antibiotic-resistant pneumococci. Pediatr Clin N Am. 1995;42:519–537. doi: 10.1016/s0031-3955(16)38977-5. [DOI] [PubMed] [Google Scholar]
- 32.Shatz D V, Schinsky M F, Pais L B, Romero Steiner S, Kirton O C, Carlone G M. Immune responses of splenectomized trauma patients to the 23-valent pneumococcal polysaccharide vaccine at 1 versus 7 versus 14 days after splenectomy. J Trauma. 1998;44:760–765. doi: 10.1097/00005373-199805000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Shelly M A, Jacoby H, Riley G J, Graves B T, Pichichero M, Treanor J J. Comparison of pneumococcal polysaccharide and CRM197-conjugated pneumococcal oligosaccharide vaccines in young and elderly adults. Infect Immun. 1997;65:242–247. doi: 10.1128/iai.65.1.242-247.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sigurdardottir S T, Vidarsson G, Gudnason T, Kjartansson S, Kristinsson K G, Jonsson S, Valdimarsson H, Schiffman G, Schneerson R, Jonsdottir I. Immune responses of infants vaccinated with serotype 6B pneumococcal polysaccharide conjugated with tetanus toxoid. Pediatr Infect Dis J. 1997;16:667–674. doi: 10.1097/00006454-199707000-00009. [DOI] [PubMed] [Google Scholar]
- 34a.Soininen A, van den Dobbelsteen G, Oomen L, Käyhty H. Are the enzyme immunoassays for antibodies to pneumococcal capsular polysaccharides serotype specific? Clin Diagn Lab Immunol. 2000;7:468–476. doi: 10.1128/cdli.7.3.468-476.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steinhoff M C, Edwards K, Keyserling H, Thoms M L, Johnson C, Madore D, Hogerman D. A randomized comparison of three bivalent Streptococcus pneumoniae glycoprotein conjugate vaccines in young children: effect of polysaccharide size and linkage characteristics. Pediatr Infect Dis J. 1994;13:368–372. doi: 10.1097/00006454-199405000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Tino M J, Wright J R. Surfactant protein A stimulates phagocytosis of specific pulmonary pathogens by alveolar macrophages. Am J Physiol. 1996;270:L677–L686. doi: 10.1152/ajplung.1996.270.4.L677. [DOI] [PubMed] [Google Scholar]
- 37.Vitharsson G, Jonsdottir I, Jonsson S, Valdimarsson H. Opsonization and antibodies to capsular and cell wall polysaccharides of Streptococcus pneumoniae. J Infect Dis. 1994;170:592–599. doi: 10.1093/infdis/170.3.592. [DOI] [PubMed] [Google Scholar]
- 38.Voss L, Lennon D, Okesene Gafa K, Ameratunga S, Martin D. Invasive pneumococcal disease in a pediatric population, Auckland, New Zealand. Pediatr Infect Dis J. 1994;13:873–878. doi: 10.1097/00006454-199410000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Weaver T, Hall C L, Kachel D L, Ward R P, Williams M D, Perry D G, Wisniowski P, Martin W J. Assessment of in vivo attachment/phagocytosis by alveolar macrophages. J Immunol Methods. 1996;193:149–156. doi: 10.1016/0022-1759(96)00031-2. [DOI] [PubMed] [Google Scholar]
- 40.Wehle K, Kupper T, Marzahn S, Pfitzer P. Identification of phagocytosed Pneumocystis carinii in human pulmonary alveolar macrophages. Cytopathology. 1993;4:225–229. doi: 10.1111/j.1365-2303.1993.tb00092.x. [DOI] [PubMed] [Google Scholar]