Abstract
Attachment of Giardia lamblia trophozoites to enterocytes is essential for colonization of the small intestine and is considered a prerequisite for parasite-induced enterocyte dysfunction and clinical disease. In this work, coincubation of Giardia with Int-407 cells, was used as an in vitro model to study the role of cytoskeleton and surface lectins involved in the attachment of the parasite. This interaction was also studied by scanning and transmission electron microscopy. Adherence was dependent on temperature and was maximal at 37°C. It was reduced by 2.5 mM colchicine (57%), mebendazole (10 μg/ml) (59%), 100 mM glucose (26%), 100 mM mannose (22%), 40 mM mannose-6-phosphate (18%), and concanavalin A (100 μg/ml) (21%). No significant modification was observed when Giardia was pretreated with cytochalasins B and D and with EDTA. Giardia attachment was also diminished by preincubating Int-407 cells with cytochalasin B and D (5 μg/ml) (16%) and by glutaraldehyde fixation of intestinal cells and of G. lamblia trophozoites (72 and 100%, respectively). Ultrastructural studies showed that Giardia attaches to the Int-407 monolayer predominantly by its ventral surface. Int-407 cells contact trophozoites with elongated microvilli, and both trophozoite imprints and interactions of Giardia flagella with intestinal cells were also observed. Transmission electron microscopy showed that Giardia lateral crest and ventrolateral flange were important structures in the adherence process. Our results suggest a combination of mechanical and hydrodynamic forces in trophozoite attachment; surface lectins also seem to mediate binding and may be involved in specific recognition of host cells.
Giardia lamblia, a parasitic flagellated protozoan, is the most common causative agent of diarrheal illness worldwide. In spite of significant recent advances in the knowledge on the biochemistry and molecular biology of G. lamblia, little is known about the pathogenesis of symptomatic infections in humans and the factors that determine the variability of the clinical outcome. A combination of parasitic factors and host responses seems to be involved, but damage of the intestinal epithelium by adherent trophozoites of G. lamblia has been proposed as one important mechanism in the pathogenesis of the infection (21). The structural modifications produced by G. lamblia trophozoites on epithelial cells are the result of close attachment of a contractile region of the ventral disk (30).
The mechanism of attachment of trophozoites to intestinal cells has not been established definitively. Evidence supports roles for the ventral disk, which is considered a specific attachment organelle (19), trophozoite contractile elements (12), hydrodynamic and mechanical forces (20), and lectin-mediated binding (8, 26). However, experimental verification has been hindered by the lack of a suitable model. Previous studies of adherence have used a variety of model systems, including synthetic surfaces such as plastic and glass, nonhuman cells such as isolated rat enterocytes and cultured rat enterocyte cell lines, and human cells (8, 15, 21, 22, 27). These models differ in their biological appropriateness for attachment studies and the diversity of findings from them offers no uniformity regarding the importance of microtubules, contractile filaments, or Giardia lectin in the adherence process.
The human Int-407 cell line, used in pathogenic enterobacterium studies, presents a potential alternative model for investigating the interaction of G. lamblia with intestinal cells. Originally used for vaccine production (18), Int-407 was derived from nonmalignant jejunum and ileum of a 2-month-old human embryo, having a complex ultrastructural fimbrial extracellular matrix. More recently, the attachment of the human immunodeficiency virus (1), Salmonella enterica serovar Typhi (31), Escherichia coli (14), and Klebsiella pneumoniae (10) has been investigated with this cell line.
In this work, we characterized the attachment patterns of G. lamblia to Int-407 cells. Our first goal was to determine the experimental conditions required for the maximal adherence in vitro, including time and temperature of incubation, number of cells, and the optimal medium for coincubation. We then examined the implications of cytoskeleton and lectins in this process, and we studied the interactions between Giardia and Int-407 cells by both transmission and scanning electron microscopy.
MATERIALS AND METHODS
Axenic culture of Giardia trophozoites.
Giardia lamblia (WB strain [ATCC 30957] originally from a patient with chronic diarrhea) was obtained from the American Type Culture Collection, Manassas, Va. Trophozoites were maintained in axenic culture, at 37°C, in 10 ml of Diamond's TYI-S-33 modified by Keister (23), in screw-cap cell culture vials. Penicillin G (250 μg/ml), streptomycin sulfate (250 μg/ml), gentamicin sulfate (50 μg/ml), and amphotericin B (0.25 μg/ml) were added during routine culture. Trophozoites attached, of cultures in logarithmic growth phase, were used as inoculum to study G. lamblia adherence to the intestinal cell line. Trophozoites were used for experiments only when they were more than 95% viable as assessed by motility and exclusion of trypan blue.
Epithelial cell line culture.
Monolayers of Int-407 cells (ATCC CCL6 [derived from human embryonic jejunum and ileum]) were cultured at 37°C in 25-cm2 flasks and grown in RPMI 1640 medium (Gibco BRL) supplemented with 5.0 mM l-glutamine, 20 mM d-glucose, 1.0 mM sodium pyruvate, 10% heat-inactivated (30 min for 56°C) calf bovine serum (Sigma), 10,000 U of penicillin per ml, 10 μg of streptomycin per ml, and 0.5 mg of neomycin per ml in an atmosphere of 5% CO2 and 95% air (29). For adhesion experiments Int-407 cells were trypsinized and then inoculated into wells of a 24-well tissue culture plates (Nunclon multidishes). For ultrastructural adherence study the cells were grown on thermanox tissue culture coverslips which were placed at the bottom of six well tissue culture plates. The cultures were incubated until the monolayers were confluent (3 to 5 days).
Coincubation and attachment assay.
Cultures of Giardia were decanted and remaining attached trophozoites refed with phosphate-buffered saline (PBS) (Dulbecco's formula [pH 7.1]) and chilled on ice until detached. Trophozoites were then centrifuged at 1,000 × g for 10 min, the supernatant was decanted, and the pellet was ressuspended in Int-407 growth medium (see cultures), warmed to 37°C. An aliquot was counted using a hemocytometer (Neubaeur cell counter chamber), and the volume was adjusted to give the desired concentration of trophozoites per milliliter. Medium was aspirated from Int-407 cultures, and the monolayer was gently washed with warmed RPMI 1640 growth medium to remove any cells that had not adhered or debris. Giardia cells were then coincubated with Int-407 cells, and the volume was adjusted to 1 ml per well. Plates were incubated at 37°C in 5% CO2 and 95% air. The time course of attachment was determined over 24 h, and the effect of varying the number of Giardia cells was studied over a range of Giardia/Int-407 ratios from 1:10 to 2:1. An investigation of the impact of temperature on adherence was carried out concurrently at 4 and 37°C. At the end of the incubation periods unattached trophozoites were recovered by gently rinsing the culture plates three times with RPMI culture medium warmed to 37°C. Adherent trophozoites were then recovered by incubation at 4°C for 10 min in cold Ca2+- and Mg2+-free 0.15 M phosphate buffer (Dulbecco's formula [pH 7.1] [Flow Laboratories, United Kingdom]). Adherent and nonadherent trophozoite suspensions were counted in a hemocytometer, and the percentage of Giardia attached to Int-407 was estimated by determining the ratio of attached trophozoites to the total number of Giardia organisms seeded.
To determine the role of components of the Giardia cytoskeleton in attachment, assays were done in the presence of microtubule inhibitors colchicine (2.5 to 5 mM, dissolved in PBS; Sigma) and mebendazole (1.0 to 100 μg/ml; Sigma), and after 30 min of preincubation of trophozoites with the microfilament inhibitors cytochalasin B and D (5 and 10 μg/ml dissolved in dimethyl sulfoxide [DMSO] [1%]; Sigma). The divalent cation dependency of the attachment was determined by incubation of Giardia trophozoites with 10 mM EDTA for 30 min.
To define the possible role of surface lectins in attachment, trophozoites were preincubated in PBS (pH 7.2) containing d-glucose (100 to 200 mM), d-mannose (100 to 200 mM), and mannose-6-phosphate (40 mM). Similarly, the role of mannosyl residues of Int-407 cells was studied by preincubation cell monolayers for 15 min with concanavalin a (ConA) (10 to 100 μg/ml). Further studies were done with preincubation, for 1 h at 37°C, of Int-407 with cythocalasin B and D (2.5 to 5 μg/ml).
In some experiments, Int-407 cell monolayers or trophozoites were fixed with 2.5% glutaraldehyde in 0.15 M NaCl for 15 min and washed with 0.15 M glycine in water and Hanks balanced salt solution to determine whether living cells were needed for binding (27).
Electron microscopy.
Monolayers of Int-407 incubated with trophozoites for 2 and 24 h on thermanox tissue culture coverslips were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.2) for 1 h at 4°C. The specimens were then washed in cacodylate buffer overnight at 4°C. The coverslips were postfixed in 1% osmium tetroxide, in the same buffer as primary fixative, for 2 h at 4°C; dehydrated in acetone; critical point dried using CO2; and sputter coated with gold. The specimens were then examined in a JEOL T330 scanning electron microscope at 15 kV (16).
For transmission electron microscopy, the monolayers of Int-407 incubated with G. lamblia for 2 h at 37°C on thermanox culture coverslips were exposed to osmium vapor to kill cells prior to immersing them in fixative solutions (5). The coverslips were inverted over several drops of 2% osmium contained in a smaller dish for 3 to 5 min. The cells were fixed with glutaraldehyde-osmium (2 and 1%, respectively) in 0.1 M phosphate buffer solution for 1 h at 4°C and rinsed with distilled water two or three times over 10 min. The samples were dehydrated in ethanol and in propylene oxide and embedded in Epon 812 (TAAB) resin. The coverslips were cut and remounted on epoxy blocks (6, 17). Ultrathin sections were stained with lead citrate and uranyl acetate and examined with a JEOL 100S transmission electron microscope.
Controls.
For all experiments, the results were compared with an equal number of control attachment assays. These assays were done at the same time as test wells under identical conditions but without the alteration of the coculture parameter on the compound being tested. Further control experiments were done in medium containing DMSO (1%).
Statistical analysis.
Each determination was carried out in duplicate and in at least three separate experiments. Results were compared with values for controls that had been run concomitantly and are expressed as mean values ± standard deviations (n ≥ 6) unless otherwise noted. Student's t test was used to assess differences, with a P of <0.05 being considered significant.
RESULTS
Attachment of Giardia to Int-407 cells.
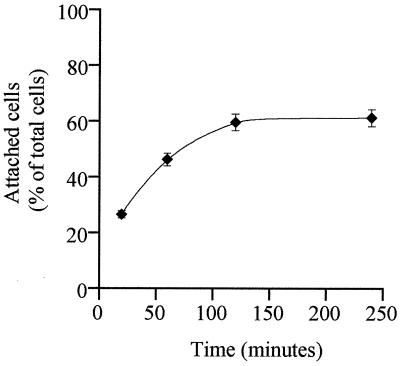
Using an inoculum of 106 trophozoites at 37°C and pH 7.1, Giardia attachment to Int-407 increased with time up to 120 min and then reached a plateau. Adherence was still evident by 24 h. The range of attachment over 1 to 4 h was 46% ± 6% to 60% ± 4% of the total number added. The time course of attachment is shown in Fig. 1. The total number of trophozoites attached after 2 h at pH 7.2 and 37°C increased with the number of Giardia cells seeded, whereas the percentage attached was similar at all Giardia/Int-407 cell ratios (not shown). Attachment was temperature dependent, being maximal at 37°C (59% ± 4%) and virtually abolished at 4°C. The foregoing experiments established the optimal conditions for the attachment assay. Unless otherwise stated, the following results were derived using a parasite/Int-407 cell ratio of 1:1 over 2 h at 37°C, pH 7.2, in 5% CO2 and 95% air.
FIG. 1.
Time course of attachment of G. lamblia trophozoites to Int-407 cell monolayers under standard assay conditions (cell ratio 1:1, 37°C, 5% CO2, and 95% air). Standard errors of the mean (vertical bars) were calculated from data of three experiments (P < 0.01).
Effects of colchicine, mebendazole, cytochalasins, EDTA, and glutaraldehyde fixation.
The involvement of the cytoskeleton and metabolic activity of cells on adherence of trophozoites to the monolayer of intestinal cells is shown in Table 1.
TABLE 1.
Participation of cytoskeleton and metabolic activity of cells in attachment of trophozoites to Int-407 monolayera
| Agents and concn | % Attachment after:
|
||
|---|---|---|---|
| Pretreatment of:
|
Treatment of cocultures | ||
| G. lamblia | Int-407 | ||
| Cytochalasin B (μg/ml) | |||
| 2.5 | 82.8 ± 8 | ||
| 5 | 92 ± 9 | 85 ± 5 | |
| 10 | 96 ± 3 | ||
| Cytochalasin D (μg/ml) | |||
| 2.5 | 89.7 ± 6 | ||
| 5 | 96 ± 4 | 78.1 ± 8 | |
| 10 | 95.2 ± 8 | ||
| EDTA (10 mM) | 89.4 ± 3 | ||
| Glutaraldehyde | 0 | 28 ± 5 | |
| DMSO | 100 ± 3 | 97 ± 5 | |
| Colchicine (mM) | |||
| 2.5 | 42.1 ± 11 | ||
| 23.2 ± 8 | |||
| Mebendazole (μg/ml) | |||
| 1 | 45.7 ± 7 | ||
| 0 | 32.2 ± 4 | ||
| 100 | 14 ± 2 | ||
After treatment of the cells, 106 trophozoites were added to Int-407 monolayers in 24-well tissue culture plates for 2 h at 37°C, and unattached and adherent trophozoites suspensions were counted in a hemocytometer. See the text for different treatments. The results are expressed as a percentage of control values. Standard errors of mean were calculated from data of three experiments. P < 0.05 for all differences of treatment versus the control.
When trophozoites were pretreated with microfilament inhibitors, cytochalasins B and D, and EDTA no significant inhibition was observed. A control, using medium containing 1% DMSO (the solvent for cytochalasin), showed no detrimental effect on attachment. Preincubation of Int-407 monolayers with cytochalasin B and D for 1 h before the coincubation with G. lamblia reduced attachment values to 83.9% ± 6% of control values (mean ± standard error of mean). No marked differences were apparent among the results obtained with different cytochalasins.
Coincubation of Giardia with intestinal cells in the presence of inhibitors of microtubular function, colchicine or mebendazole, resulted in a concentration-dependent reduction in attachment compared with controls. Colchicine (2.5 and 5 mM) reduced attachment to values of 42% ± 10% and 23% ± 8%, respectively. Mebendazole (1, 10, and 100 μg/ml) reduced attachment to values of 45.5% ± 7%, 32% ± 4% and 14% ± 2%, respectively
The participation of metabolic activity of cells on the adherence process was studied by glutaraldehyde fixation of Int-407 cells, which significantly diminished attachment to values to 28% ± 5% of control values, and of trophozoites, which abolished attachment.
Lectin studies.
Preincubation of trophozoites with mannose-6-phosphate (40 mM) reduced attachment to Int-407 to 81% ± 2% of control values. Preincubation of G. lamblia with d-mannose (100 to 200 mM) inhibited trophozoite attachment to 78% ± 3% and 75% ± 4% of control values, respectively. With d-glucose (100 to 200 mM) the attachment was reduced to 73% ± 2% and 71% ± 3% of control values, respectively. Preincubation of Int-407 cell monolayers with ConA (10 to 100 μg/ml) reduced Giardia attachment to 83% ± 2% and 79% ± 2% of control values, respectively (means ± standard errors of mean of three experiments).
Electron microscopy.
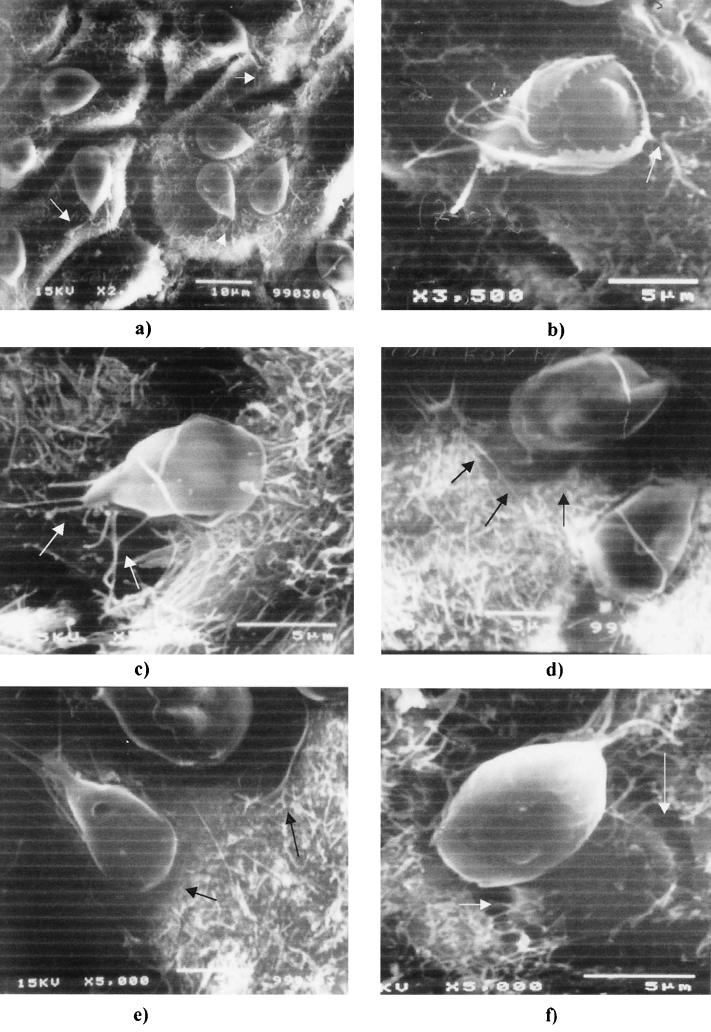
Scanning electron microscopy observations were performed with Giardia incubated with Int-407 cells for different lengths of time. Numerous trophozoites were attached to the surfaces of the Int-407 cells, and most trophozoites were observed with their ventral surfaces applied to the monolayer (Fig. 2a). G. lamblia trophozoites were also seen with their dorsal surfaces opposed to epithelial cells, apparently bound by means other than ventral sucker disk (Fig. 2b). This pattern of adherence is much more evident after 24 h of coincubation. Others interactions between Giardia trophozoites and Int-407 cells were observed: Int-407 cells seemed to contact the trophozoites with fimbrial extension extracellular cell matrix (Fig. 2c), an obvious interaction of posterolateral and caudal flagella with surfaces of Int-407 cells was observed (Fig. 2a to c) (arrows), and circular imprints of G. lamblia trophozoites on Int-407 monolayers were observed (Fig. 2d to f) (arrows).
FIG. 2.
Scanning electron micrographs of G. lamblia attached to Int-407 monolayers. Trophozoites were incubated with Int-407 cells for 2 h (a, c, d, and e) or 24 h (b and f). (a) Most Giardia trophozoites are attached on the Int-407 cell surface, with the ventral disk in contact with the intestinal cell. Note the close interaction of trophozoite flagella with intestinal cells (arrows). (b) Attachment of G. lamblia trophozoites with the dorsal surface. The fimbrial extensions of Int-407 contact the VLF (arrow). (c) The fimbrial extensions (microvilli) of Int-407 cells contact and surround the trophozoite, and the caudal and posterolateral flagella also interact with Int-407 cells (arrows). (d to f) Note the circular imprints of Giardia, after trophozoite detachment, on intestinal cells (arrows).
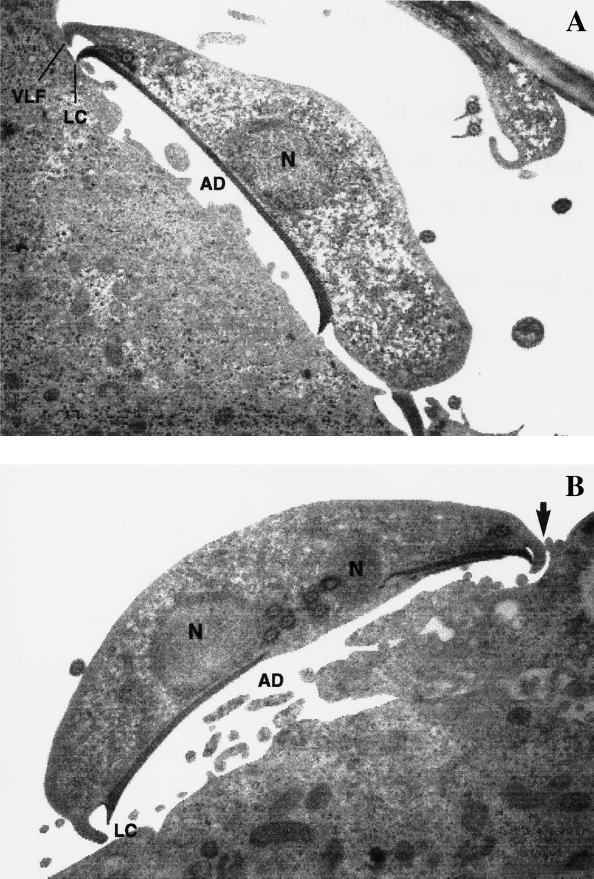
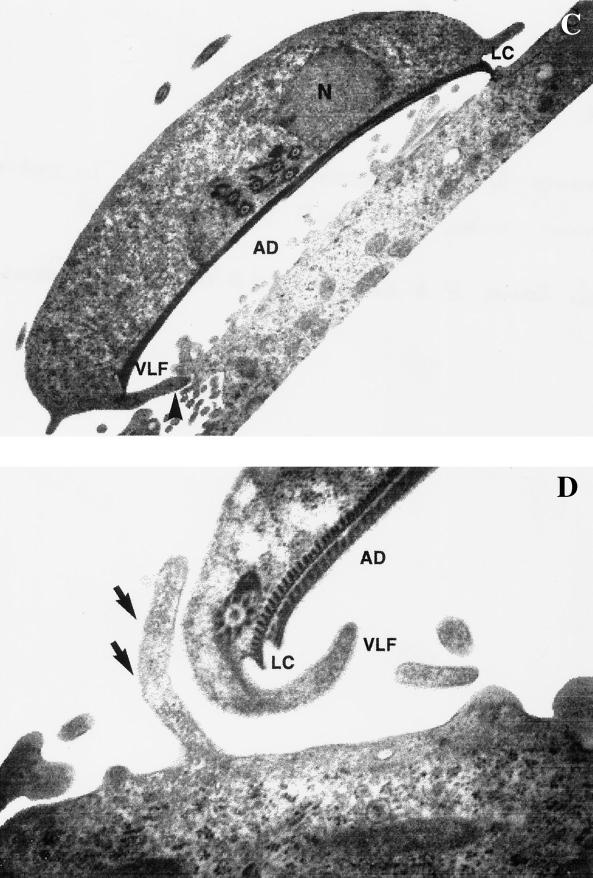
Transmission electron microscopy observations showed that in attached Giardia trophozoites, the lateral crest was in direct contact with Int-407 cells (Fig. 3A) and the ventral disk assumed an arched position. The ventrolateral flange (VLF) also interacts with epithelial cells contacting the substrate laterally or indented between the fimbrial extensions of Int-407 cells (Fig. 3A to C). There were also interactions of the dorsal surface of the Giardia trophozoites with epithelial cell extensions of Int-407 cell membranes, grossly elongated, that surrounded the trophozoites (Fig. 3D).
FIG. 3.
Transmission electron micrographs showing the interactions of G. lamblia trophozoites with Int-407 cells after staining with uranyl and citrate leads. (A) The lateral crest was applied to the substrate and the VLF contacts laterally with the Int-407 cell surface plasma membrane. (B and C) In some cases the VLF indented into the fimbrial extension of intestinal cells (arrow). (D) Some extracelular fimbrial extensions of Int-407 cells surrounding the parasite were grossly elongated (arrow). (A to C) Note the arched ventral disk in attached trophozoites. Magnification: ×11,475 (A), ×13,140 (B), ×12,150 (C), ×33,300 (D). Abbreviations. N, nucleus; AD, adhesive disk; LC, lateral crest.
DISCUSSION
These experiments show that cultured Giardia trophozoites attach firmly to Int-407 cells in vitro and support a role for both cytoskeletal and lectin-mediated mechanisms. This Int-407 cell model seems to be an appropriate model of attachment as it involves a human intestinal cell line and a human isolate of Giardia.
Previous studies of attachment of Giardia trophozoites used glass or plastic as the substrate (12, 15, 34), enterocytes isolated from rodents (21, 26), continuously cultured cell lines (3), and intestinal cell lines (22, 27, 28). Glass or plastic substrates are convenient but are dissimilar to in vivo substrates. The use of freshly isolated enterocytes or villi is tedious and labor-intensive, and these cells may be difficult to manipulate experimentally. Int-407 is a continuously cultured cell line, and like others, it is convenient, easily manipulated, and may exhibit phenomena similar to those occurring in vivo. The Int-407 cell line also seems to be a more physiologic model because these cells are derived from the human small intestine and are a noncarcinoma cell line.
The mechanisms of adherence of G. lamblia to intestinal cells is not fully understood, but combining a range of in vitro models, each simulating a part of these interactions, could contribute to the overall understanding of this phenomenon.
Active participation of the mammalian cells during the adhesion process via their cytoskeletal components has been demonstrated with diarrheagenic E. coli strains (24, 33), K. pneumoniae (10), and Vibrio parahaemolyticus (7). These rearrangements include actin polymerization at sites of bacterial adhesion to tissue culture cells and concentration of other cytoskeletal proteins (13). Adherence of G. lamblia to Int-407 cells was inhibited when intestinal cells were pretreated with cytochalasin D, a potent microfilament inhibitor, indicating that adherence of G. lamblia to Int-407 cells occurs with some participation of a microfilament-dependent process. On the other hand, the adherence was significantly inhibited by glutaraldehyde and did not occur at 4°C, indicating that both mammalian cells and G. lamblia must be metabolically active for the attachment process to occur.
The present study supports a primary role for mechanical attachment via the ventral disk with a prominent role for the microtubules. Attachment was inhibited with colchicine and mebendazole, which affect microtubular function. The adherence was not altered when trophozoites were pretreated with EDTA and cytochalasins B and D, which suggests that the Giardia actin-myosin system does not interfere with the attachment process in this in vitro model. Some previous reports' results have differed from these findings. In view of the different models and experimental procedures used to study attachment, it is not surprising that experimental results have not always been concordant. Inge et al. (21), Magne et al. (28), and Kateralis et al. (22) described decreased attachment of Giardia to cells after microtubular inhibition. However, Feely and Erlandsen (11) and MacCabe et al. (27) did not find that colchicine inhibited attachment. Inge et al. (21) did not show that divalent cation depletion or cytochalasin B affected attachment. Gillin and Reiner (15) found little effect of cytochalasins on adherence of trophozoites to glass. Magne et al. (28), who used Caco-2 cells as a model for attachment, reported a biphasic increase of attachment to Caco-2 cells after inhibition of contractile proteins with cytochalasin B.
Earlier studies revealed the presence of two distinct lectins in Giardia, a glucose-mannose-specific lectin and a mannose-6-phosphate binding lectin (8, 32). Lectin-mediated attachment was evident in our in vitro model and was inhibitable by mannose-6-phosphate and ConA, consistent with a mannosyl target for binding, and by glucose and mannose, consistent with a glucose-manose-specific lectin. Evidence suggests that lectin-mediated binding is not the primary mode of attachment of Giardia. Firstly, attachment to synthetic surfaces is avid and is not dependent on receptor-ligand-mediated binding. Secondly, the magnitude of the reduction in attachment is greater after inhibition of cytoskeletal function than with competitive inhibition of lectin-mediated binding. Lastly, although trophozoites are found in various orientations to epithelial cells, most are observed with the ventral surface down.
It is now generally accepted that the force responsible for attachment is a negative pressure beneath the ventral chamber of the disk. The mechanisms by which the sucking force is generated, however, are not fully understood. Of several hypotheses proposed, two deserve consideration: the hydrodynamic model, stressing the involvement of the ventral flagella, and the suction cup model, emphasizing involvement of disk-associated contractile proteins (25). In the first model, the motility and position of the VLF are important components of the system. In the attached parasite, the flange is raised in front of the cell and contacts the substrate laterally. In the suction cup model, the sucking force is generated by the radial contraction of the lateral crest, which may control the diameter of the ventral disk. A change in the size of the ventral disk would produce the buckling or arching of the ventral disk which is a characteristic of attached trophozoites. Chaves and Martinez-Palomo (2) demonstrated that the plasma membrane at the lateral crest of G. lamblia has a low cholesterol content thus providing a greater flexibility and facilitating the contraction of the outer rim of the ventral disk.
Our ultrastructural study showed that the lateral crest and VLF have close contact between parasite and Int-407 cells and seem to be important structures in the adherence process. Based on these observations and the results that demonstrated the involvement of the cytoskeleton, we can conclude that the mechanisms of attachment of G. lamblia to Int-407 cells are essentially a combination of hydrodynamic and mechanical forces.
The disease-causing mechanisms in giardiasis are poorly understood. The pathogenesis of the diarrhea and malabsorption associated with this infection is multifactorial. There is, however, a dominant pathophysiologic feature: the impairment of digestive and absorptive functions of enterocytes due to alteration of their microvillus surface area. In the upper small intestine of the infected host the villi become shortened and thickened and may be the cause of diarrhea. The ephitelial cells in the affected area are vacuolized and compressed, and some of them are severely damaged (9). Giardia trophozoites attach to the epithelium and have been shown by electromicroscopy to disrupt and distort microvilli at the site where the ventral disk interfaces with the microvillus membrane. It is possible therefore that physical factors involved in this cell-cell interaction might account for microvillus damage at the site of adherence (9). Our electron microscopy study suggested that adherent trophozoites of G. lambia induce direct cell damage, since some Int-407 cells appeared disrupted with trophozoite imprints at the site of attachment. Furthermore, the trophozoites appeared to indent into the Int-407 cell membrane, and the microvilli surrounding the attached parasite were grossly elongated. These morphological alterations induced by G. lamblia trophozoites may be involved in pathological processes of diarrhea by affecting intestinal cell function. In human giardiasis, morphological abnormalities have been associated with deficiences of disaccharidases and some other brush border digestive enzymes (lactase, trehalase, sucrase, maltase, and alkaline phosphatase) in the microvillus membrane (9). Further experiments, namely, determination of dissacharidase activity, plasma membrane potential, or cell viability, are needed to clarify the alterations induced by G. lamblia that result from a close and stable interaction with Int-407 cells.
The importance of adherence for the survival of G. lamblia in vivo has been emphasized by Crouch et al. (4), who concluded that parasite survival is dependent on the cells' attachment abilities, without which they would be swept away by peristaltic waves, and that further, the adherence process may be a potential target for attack by chemotherapeutic mechanisms. The cellular model used in the present study may be applied to the evaluation of antigiardial agents that act by either prevention of attachment or induction of detachment of organisms.
There was an obviously active interaction between Int-407 and G. lamblia; both cells need to be metabolically active (alive) for the adherence process to occur. In this study we demonstrated that Int-407 is a useful cellular model for Giardia adherence studies and could be applied both to study the action of antigiardial drugs and to study the mechanisms involved in clinical disease.
REFERENCES
- 1.Bourinbaiar A R, Nagorny R. Inhibitory effect of natural interferon alpha on immunodeficiency virus type 1 transmission from epithelial cells to lymphocytes in vitro. Eur J Pharmacol. 1993;230:15–22. doi: 10.1016/0014-2999(93)90404-6. [DOI] [PubMed] [Google Scholar]
- 2.Chaves B, Martinez-Palomo A. Giardia lamblia: freeze-fracture ultrastructure of the ventral disc plasma membrane. J Eukaryot Microbiol. 1995;42:136–141. doi: 10.1111/j.1550-7408.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- 3.Chavez B F, Knaippe L, Gonzalez-Mariscal, Martinez-Palomo A. Giardia lamblia: electrophysiology and ultrastructure of cytopathology in culture epithelial cells. Exp Parasitol. 1986;61:379–389. doi: 10.1016/0014-4894(86)90194-3. [DOI] [PubMed] [Google Scholar]
- 4.Crouch A A, Seow W K, Whitman L M, Smith S E, Thong Y H. Inhibition of adherence of Giardia intestinalis by human neutrophils and monocytes. Trans R Soc Trop Med Hyg. 1991;85:375–379. doi: 10.1016/0035-9203(91)90297-c. [DOI] [PubMed] [Google Scholar]
- 5.Dykstra M J. A manual of applied techniques for biological electron microscopy. New York, N.Y: Plenum Press; 1993. pp. 60–64. [Google Scholar]
- 6.Dykstra M J. A manual of applied techniques for biological electron microscopy. New York, N.Y: Plenum Press; 1993. pp. 72–73. [Google Scholar]
- 7.Fabri A, Falzano L, Frank C, Donelli G, Matarrese P, Raimondi F, Fasano A, Fiorentini C. Vibrio parahaemolyticus thermostable direct hemolysin modulates cytoskeletal organization and calcium homeostasis in intestinal cultured cells. Infect Immun. 1999;67:1139–1148. doi: 10.1128/iai.67.3.1139-1148.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farthing M J, Pereira M E A, Keusch G T. Description and characterization of a surface lectin from Giardia lamblia. Infect Immun. 1986;51:661–667. doi: 10.1128/iai.51.2.661-667.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farthing M J G. The molecular pathogenesis of giardiasis. J Pediatr Gastroenterol Nutr. 1997;24:79–88. doi: 10.1097/00005176-199701000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Favre-Bonte S, Darfeuille-Michaux A, Forestier C. Aggregative adherence of Klebsiella pneumoniae to human intestine-407 cells. Infect Immun. 1995;63:1318–1328. doi: 10.1128/iai.63.4.1318-1328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feely D E, Erlandsen S L. Effect of cytochalasin B low Ca2+ concentration, iodoacetic acid and quinacrine-HCl on the attachment of Giardia trophozoites in vitro. J Parasitol. 1982;68:869–873. [PubMed] [Google Scholar]
- 12.Feely D E, Schollmeyer J V, Erlandsen S L. Giardia: distribution of contractile proteins in attachment organelle. Exp Parasitol. 1982;53:145–154. doi: 10.1016/0014-4894(82)90100-x. [DOI] [PubMed] [Google Scholar]
- 13.Finlay B B, Rosenshine I, Donnenberg M S, Kaper J. Cytoskeletal composition of attaching and effacing lesions associated with enteropathogenic Escherichia coli adherence to HeLa cells. Infect Immun. 1982;60:2541–2543. doi: 10.1128/iai.60.6.2541-2543.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fratamico P M, Bahaduri S, Buchanan R L. Studies on Escherichia coli serotype O157:H7 strains containing a 60-Mda plasmid and on 60-Mda plasmid-cultured derivates. J Med Microbiol. 1993;87:245–250. doi: 10.1099/00222615-39-5-371. [DOI] [PubMed] [Google Scholar]
- 15.Gillin F D, Reiner D S. Attachment of the flagellate Giardia lamblia: role of reducing agents, serum, temperature, and ionic composition. Mol Cell Biol. 1982;2:369–77. doi: 10.1128/mcb.2.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonçalves C A, Figueiredo M H, Bairos V A. Three-dimensional organisation of the elastic fibres in the rat lung. Anat Rec. 1995;243:63–70. doi: 10.1002/ar.1092430108. [DOI] [PubMed] [Google Scholar]
- 17.Hemphill A, Croft S L. Electron microscopy in parasitology. In: Rogan M T, editor. Analytical parasitology. Berlin, Germany: Springer; 1997. pp. 227–268. [Google Scholar]
- 18.Henle G, Deinhardt F. The establishment of strains of human cells in tissue culture. J Immunol. 1957;79:54–59. [PubMed] [Google Scholar]
- 19.Holberton D V. Fine structure of the ventral disc apparatus and the mechanism of attachment in the flagellated Giardia muris. J Cell Sci. 1973;13:11–41. doi: 10.1242/jcs.13.1.11. [DOI] [PubMed] [Google Scholar]
- 20.Holberton D V. Attachment of Giardia—a hydrodynamic model based on flagellar activity. J Exp Biol. 1974;60:207–221. doi: 10.1242/jeb.60.1.207. [DOI] [PubMed] [Google Scholar]
- 21.Inge P M G, Edson C M, Farthing M J G. Attachment of Giardia lamblia to rat intestinal epithelial cells. Gut. 1988;29:795–801. doi: 10.1136/gut.29.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katelaris P H, Naeem A, Farthing M J G. Attachment of Giardia lamblia trophozoites to a cultured human intestinal cell line. Gut. 1995;37:512–518. doi: 10.1136/gut.37.4.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keister B D. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans R Soc Trop Med Hyg. 1983;77:487–488. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- 24.Knutton S, Baldwin T, Williams P H, McNeish A S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989;60:2083–2091. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kulda J, Nohyhová E. Giardia in humans and animals. In: Kreier J P, editor. Parasitic protozoa. New York, N.Y: Academic Press; 1995. pp. 241–244. [Google Scholar]
- 26.Lev B, Ward H, Keusch G T, Pereira M E A. Lectin activation in Giardia lamblia by host protease: a novel host parasite interaction. Science. 1986;232:71–73. doi: 10.1126/science.3513312. [DOI] [PubMed] [Google Scholar]
- 27.MacCabe R E, Yu G S M, Conteas C, Morrill P R, MacMorrow B. In vitro model of attachment of Giardia intestinalis trophozoites to IEC-6 cells, an intestinal cell line. Antimicrob Agents Chemother. 1991;35:29–35. doi: 10.1128/aac.35.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magne D, Lavennec L, Chochillon C, Gorenflot A, Meillet D, Kapel N, Raichvarg D, Savec J, Gobert J G. Role of cytoskeleton and surface lectins in Giardia duodenalis attachment to Caco 2 cells. Parasitol Res. 1991;77:659–662. doi: 10.1007/BF00928679. [DOI] [PubMed] [Google Scholar]
- 29.Sarem F, Sarem-Damerdji L O, Nicolas J P. Comparison of the adherence of three Lactobacillus strains to Caco-2 and Int-407 human intestinal cell lines. Lett Appl Microbiol. 1996;22:439–442. doi: 10.1111/j.1472-765x.1996.tb01198.x. [DOI] [PubMed] [Google Scholar]
- 30.Smith P D. Pathophysiology and immunology of giardiasis. Annu Rev Med. 1985;36:295–307. doi: 10.1146/annurev.me.36.020185.001455. [DOI] [PubMed] [Google Scholar]
- 31.Tartera C, Metcalf E S. Osmolarity and growth phase overlap on regulation of Salmonella typhi adherence to and invasion of human intestinal cells. Infect Immun. 1993;61:3084–3089. doi: 10.1128/iai.61.7.3084-3089.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ward H D, Lev B I, Kane A V, Keusch G T, Pereira M E A. Identification and characterization of taglin, a mannose-6-phosphate binding, trypsin activated lectin from Giardia lamblia. Biochemistry. 1987;26:8869–8875. doi: 10.1021/bi00400a027. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto T, Kaneko M, Changchawalit S, Serichantalergs O, Ijuin S, Echeverria P. Actin accumulation associated with clustered and localized adherence in Escherichia coli isolated from patients with diarrhea. Infect Immun. 1994;62:2917–2929. doi: 10.1128/iai.62.7.2917-2929.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zenian A, Gillin F D. Interactions of Giardia lamblia with human intestinal mucus: enhancement of trophozoite attachment to glass. J Protozool. 1985;32:664–668. doi: 10.1111/j.1550-7408.1985.tb03098.x. [DOI] [PubMed] [Google Scholar]