Abstract
Autophagy is a fundamental catabolic process coordinated by a network of autophagy-related (ATG) proteins. These ATG proteins also perform an important parallel role in “noncanonical” autophagy, a lysosome-associated signaling pathway with key functions in immunity, inflammation, cancer, and neurodegeneration. While the noncanonical autophagy pathway shares the common ATG machinery, it bears key mechanistic and functional distinctions, and is characterized by conjugation of ATG8 to single membranes (CASM). Here, we review the diverse, and still expanding, collection of stimuli and processes now known to harness the noncanonical autophagy pathway, including engulfment processes, drug treatments, TRPML1 and STING signaling, viral infection, and other pathogenic factors. We discuss the multiple associated routes to CASM and assess their shared and distinctive molecular features. By integrating these findings, we propose an updated and unifying mechanism for noncanonical autophagy, centered on ATG16L1 and V-ATPase.
A diverse range of stimuli converge on the V-ATPase–ATG16L1 axis to initiate noncanonical autophagy.
INTRODUCTION
Canonical autophagy, or macroautophagy, is a fundamental catabolic process through which intracellular material is degraded to maintain homeostasis, with vital functions in health and disease (1). The underlying autophagy (ATG) machinery comprises a diverse collection of proteins, from protein and lipid kinases to lipid transfer proteins and ubiquitin-like conjugation systems, which act in concert to target cargoes for lysosomal degradation (2). These cargoes, which include aggregated proteins, damaged organelles, and certain intracellular pathogens, are enwrapped by autophagosomes and directed to the lysosome for digestion, nutrient retrieval, and recycling.
The ATG8 protein family, consisting of ubiquitin-like proteins LC3A/B/C and GABARAP/L1/L2, function as key components of the autophagy machinery (3). During macroautophagy, the ATG8s are conjugated to the lipid, phosphatidylethanolamine (PE), on forming double-membrane autophagosomes (4). This distinctive modification is critical for autophagosome cargo selection, elongation, and closure and represents a defining hallmark event, widely used to detect and assay for autophagy.
Through pioneering work from the Green Lab, and many subsequent studies, we now appreciate that many ATG proteins also “moonlight,” performing critical functions in other parallel processes (5). “Noncanonical” autophagy is an important alternative pathway, defined by the conjugation of ATG8s to single membranes (CASM) (6). CASM targets membranes of the endolysosomal system for ATG8 conjugation, implicating this hallmark event in noncanonical autophagy too and linking ATG proteins to an even broader range of degradative processes with shared links to the lysosome. In this review, we use the terms noncanonical autophagy and CASM somewhat interchangeably, the former being well established in the literature and the latter capturing the specific molecular features.
It is clear that the noncanonical autophagy/CASM pathway displays broad functional significance, with key roles identified in the immune system, vision, cancer and neurodegeneration, and evolutionary conservation (7–13). We are also developing an increasingly detailed understanding of the molecular mechanisms that underlie and define this important autophagy-related signaling pathway.
In this review, we bring together the results of multiple studies, on multiple processes, from multiple laboratories, to propose an updated and unifying mechanism for the induction of noncanonical autophagy/CASM by diverse stimuli.
MOLECULAR FEATURES OF CASM
Canonical and noncanonical autophagy represent closely related, parallel pathways, which not only share overlapping molecular machineries but also bear important differences. In this section, we review the key molecular features that distinguish these alternative branches of ATG-related signaling (Fig. 1).
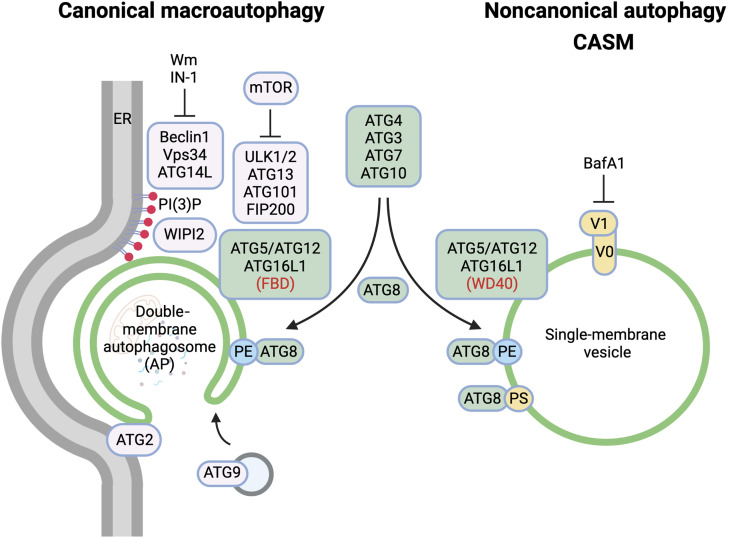
Fig. 1. Molecular features of autophagy-related pathways.
Schematic diagram highlighting the differences in molecular machinery and membrane target between canonical macroautophagy and noncanonical autophagy. BafA1, bafilomycin A1; Wm, wortmannin; IN-1, Vps34 inhibitor 1. Figure generated using Biorender.com
Membranes: Canonical versus noncanonical autophagy
A key distinction between canonical and noncanonical autophagy pathways involves the membranes which they target. During canonical autophagy, the ATG machinery nucleates, expands, and seals de novo, double-membrane autophagosomes using membrane derived from endoplasmic reticulum, mitochondria, or recycling endosomes (14–16). In contrast, noncanonical autophagy targets preformed, single-membrane vesicles or compartments of the endolysosomal system, including phagosomes, macropinosomes, late endosomes, and lysosomes (17). This defining feature of noncanonical autophagy has been clearly demonstrated by many groups using correlative light electron microscopy (CLEM) (18–21).
ATG machinery: Canonical versus noncanonical autophagy
The autophagy machinery comprises a complex collection of ATG proteins, which facilitate autophagosome formation and maturation during macroautophagy. Without the need to initiate de novo autophagosome formation, noncanonical autophagy is independent of much of the upstream autophagy machinery, including ULK1/2, FIP200, ATG13, ATG9, WIPI2, and ATG14L1 (11, 12, 19). However, the core ubiquitin-like conjugation systems that support ATG8 lipidation to membranes, including ATG3, ATG4, ATG5, ATG7, ATG10, ATG12, and ATG16L1, are all similarly essential for CASM and thus shared between macroautophagy and noncanonical autophagy (11, 12, 19).
The differential involvement of these distinct subsets of ATG proteins has been widely used to implicate noncanonical autophagy in specific biological phenotypes through comparative studies of different mouse knockout strains. For instance, a dependence on ATG5/7 and an independence of ULK1/FIP200 are characteristics of noncanonical autophagy used to define its role in inhibiting auto-inflammatory responses to dying cells (11).
ATG16L1: A molecular hub that dissects canonical and noncanonical pathways
ATG16L1, in complex with ATG5 and ATG12, forms part of the core ATG machinery, critical in specifying the membrane site of ATG8 lipidation, during both canonical and noncanonical autophagy (8, 22). During macroautophagy, ATG16L1 is recruited to forming phagophores through its interactions with FIP200 and the PI3P effector WIPI2 (23, 24). Binding to both of these proteins is mediated by the central FIP200 binding domain (FBD) of ATG16L1, and deletion or mutation of this region reduces macroautophagy accordingly. Importantly, however, the FBD region and binding to WIPI2 or FIP200 are not required for CASM (8). Instead, the C-terminal WD40 domain of ATG16L1 is essential to support noncanonical autophagy while conversely being dispensable for macroautophagy (8, 18, 25). This differential dependence on distinct ATG16L1 domains can be used to uncouple the closely related canonical and noncanonical autophagy pathways.
This important discovery provided a previously unknown single system that can cleanly dissect these distinct lipidation pathways. ATG16L1 WD40 deletion mice have thus been generated and used to demonstrate a role for CASM in influenza infection (26) and antigen presentation (8) and in preventing neurodegeneration (27). Further mutational analysis has honed in on critical individual residues within the ATG16L1 WD40 domain, including K490 and F467, which are indispensable for CASM but have no effect on macroautophagy (8). A refined ATG16L1 K490A knock-in single mutant mouse strain has recently been developed, which is specifically deficient for CASM (20). This model targets noncanonical autophagy as precisely as possible and reduces the number of mouse strains required to study this pathway, saving on animal numbers and reducing associated cost implications.
Several proteins have been identified that can bind to the ATG16L1 WD40 domain, including TMEM59 (28), A20 (29), IL-10Rb and IL-2Ry (30), ubiquitin (31), C3 (32), and IFT20 (33), suggesting that it may act as a signaling hub, directing ATG16L1 to different sites within the cell and/or with different partners competing for engagement. Importantly, a critical protein-protein interaction has been identified between the ATG16L1 WD40 domain and vacuolar-type H+-adenosine triphosphatase (V-ATPase) (34), which plays a pivotal role during CASM in a variety of contexts. These finds are discussed further in the “A unifying mechanism for CASM” section.
Alternative lipidation of ATG8s (LC3s and GABARAPs)
A hallmark feature of all autophagy-related pathways is the lipidation of ATG8s through conjugation of the C-terminal glycine to a phospholipid (35). This unusual posttranslational modification can be read out through immunofluorescence, as a relocalization of ATG8 from cytosol to membrane, or by SDS–polyacrylamide gel electrophoresis/Western blotting, where the lipidated ATG8-II form migrates faster than the unlipidated ATG8-I form (36, 37). These analytical techniques represent the most commonly used methods for monitoring both macroautophagy and CASM. However, the shared nature of the conjugation machinery has, historically, made it impossible to distinguish between these two pathways by tracking ATG8 lipidation alone.
Seminal work in yeast, and then mammalian cells, used mass spectrometry and thin-layer chromatography to identify PE as the sole lipid target conjugated to ATG8 during macroautophagy (35, 38, 39). A second lipid, phosphatidylserine (PS), contains the requisite N-terminal amino group for conjugation to ATG8, and this alternative conjugation can be catalyzed in vitro (39). However, only ATG8-PE conjugation could be detected in vivo, and it was long assumed to be the exclusive modification associated with all autophagy processes.
In a recent study, we revisited this assumption and compared the lipid species associated with ATG8 conjugation in macroautophagy and CASM directly (6). Consistent with previous studies, we also found macroautophagy to be exclusively associated with ATG8-PE. Notably, however, we discovered that CASM drives the alternative conjugation of ATG8s to both PE and PS, in response to multiple stimuli, and in all ATG8 isoforms. These findings challenged the existing dogma of PE exclusivity and revealed ATG8-PS as a potential “molecular signature” for CASM.
Many open questions remain in relation to differential ATG8 lipidation. For instance, is ATG8-PS formed in all examples of CASM? ATG8-PS has been detected during phagocytosis, influenza A virus (IAV) infection, and monensin treatment, but other CASM stimuli such as TRPML1 or STING (stimulator of interferon genes) agonists have yet to be tested. Furthermore, the mechanism directing selectivity toward PE or PS conjugation is yet to be fully understood. One simple model suggests that the lipid composition of the target membrane determines the species conjugated, with endolysosomal membranes showing a relative enrichment for PS (40). However, recent lipidomic analyses have detected both PE and PS on isolated autophagosomes, implying that additional means of regulation likely contribute (41). It is conceivable that subcellular conditions, in the vicinity of target membranes, might also influence the process, as has been detected in vitro, where ATG8-PS conjugation is favored by a more alkaline environment and inhibited at neutral pH and by certain phospholipid compositions (42, 43). It also remains possible that additional factors influence lipid selectivity, such as conformational changes in ATG3 localized at different target membranes. It will be of great interest to investigate this area in more detail.
A key molecular difference between ATG8-PE and ATG8-PS relates to subsequent lipid deconjugation by dual activity ATG4 proteases. In both in vitro liposomes and cellular systems, ATG4D, and to some extent ATG4B, deconjugates LC3A-PS less efficiently than LC3A-PE (6). This points to significant, isoform-specific differences between the ATG4s, as well as to altered kinetics for alternatively lipidated ATG8s during CASM versus macroautophagy. During LC3-associated phagocytosis (LAP), the ratio between LC3A-PS and LC3A-PE increases over time, suggesting that ATG8-PS is the longer-lived species. What this means for the function of CASM remains to be fully determined. Persistent ATG8-PS could perhaps drive the prolonged recruitment of ATG8-interacting proteins at endolysosomal membranes and/or the recruitment of an ATG8-PS–specific subset of binding partners. Future work will be needed to delineate the functional consequences of alternative ATG8 lipidation.
The distinctive pharmacology of CASM: Bafilomycin
The V-ATPase is a highly conserved proton pump that acidifies lysosomes, enabling hydrolase activation and degradation (44). Bafilomycin A1 (BafA1) is a V-ATPase inhibitor, which raises lysosomal pH, disrupting lysosomal degradation and blocking autophagic flux (45). In the presence of BafA1, canonical autophagy proceeds, and autophagosomes accumulate, yielding increased levels of lipidated ATG8s from this pathway. In contrast, BafA1 potently inhibits the ATG8 lipidation associated with CASM, in response to a wide range of stimuli (18, 20, 46, 47). BafA1 sensitivity is a defining feature of noncanonical autophagy, which can differentiate this pathway from macroautophagy. This distinctive pharmacology also points toward an important role for V-ATPase in CASM (explored further in the “A unifying mechanism for CASM” section).
The opposing effects of BafA1 on canonical and noncanonical autophagy provide a clear pharmacological means of distinguishing between these closely related pathways. However, experimentally, combining pharmacology with appropriate genetic backgrounds is often the cleanest method of dissecting these pathways. For example, in wild-type cells, BafA1 treatment will increase ATG8 lipidation through canonical autophagy while simultaneously suppressing ATG8 lipidation through CASM. In ATG13 null cells, which are incompetent for macroautophagy, BafA1 inhibition of CASM is more readily detected.
DIVERSE STIMULI DRIVE NONCANONICAL AUTOPHAGY/CASM
The molecular features summarized above have enabled the identification and investigation of noncanonical autophagy/CASM, implicating this pathway in a wide, and still expanding, list of processes, which we review below. For clarity, we have grouped these stimuli into four broad categories: heterophagic engulfments, pharmacological treatments, pathogenic factors, and other candidate CASM-related processes (Fig. 2).
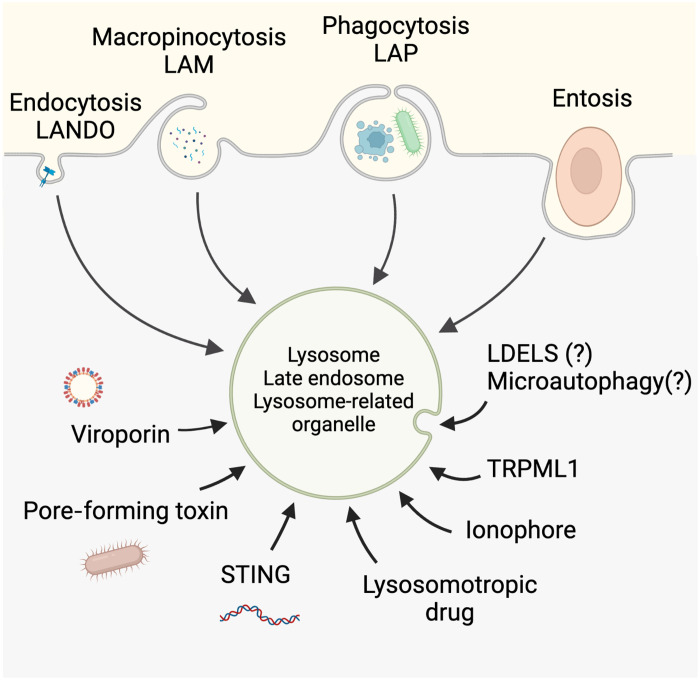
Fig. 2. Diverse routes to noncanonical autophagy and CASM.
The diverse stimuli that are known to promote CASM on endolysosomal-related vesicles are shown. LANDO, LC3-associated endocytosis; LDELS, LC3-dependent EV loading and secretion. It is unclear whether ATG8 lipidation associated with LDELS or microautophagy constitutes a form of CASM. Figure generated using Biorender.com
Heterophagy and CASM: ATG proteins eating from inside and out
Cells ingest extracellular material through a variety of important engulfment processes, including endocytosis and phagocytosis. These heterophagic pathways not only vary with respect to their target cargoes, their associated fates, and their underlying molecular mechanisms but also share common features, including links to CASM and lysosomes. A level of interplay between heterophagy and autophagy has been suspected for some time, for instance, autophagosomes can fuse with different parts of the endocytic network. However, recent work builds more strongly on this notion, indicating that ATG proteins modulate both the autophagic digestion of intracellular material and the heterophagic processing of extracellular material, as discussed further below.
LC3-associated phagocytosis
Phagocytosis is a receptor-mediated process for the engulfment of large particulate matter (48). Important cargoes include apoptotic or necrotic cells, bacterial and fungal pathogens, and opsonized objects. A range of different receptors, including Toll-like receptors (TLRs), Fc receptors (FcRs), dectins, and complement receptors (CRs), recognize targets and promote signaling cascades that lead to internalization within specialized single-membrane compartments called phagosomes (48). Phagosomes then undergo a series of sequential phosphoinositide changes and protein recruitments that orchestrate their maturation, which is associated with acidification and fusion with lysosomes and, in some cases, generation of reactive oxygen species (ROS). The primary function of phagocytosis is the degradation of internalized cargo, which, in the case of pathogens, is critical for killing and clearance and, in the case of dead cells, removes unwanted cellular material that would otherwise trigger inflammation. Importantly, signals emanating from the phagocytosis of different targets can determine the resulting inflammatory response (49).
In a seminal paper, Sanjuan et al. (13) discovered that phagosomes are targeted by noncanonical autophagy, in a process they termed LAP. Multiple groups have since confirmed this observation and shown that phagosomes housing apoptotic (19, 50) or necrotic cells (50), a variety of bacterial and fungal pathogens (12, 51–54) or antibody-opsonized live cells (55), can be lipidated with various ATG8 proteins and that LAP is evolutionarily conserved (19, 50, 56). Upon infection, LAP appears to represent an important host response to infection, facilitating the efficient killing and clearance of pathogens and directing proinflammatory responses (57, 58). However, the exact downstream molecular mechanisms underlying how LAP orchestrates these functions remain to be fully understood.
LC3-associated macropinocytosis
Macropinocytosis is a macroscale endocytic process that mediates the nonselective, bulk uptake of extracellular fluid and material into the cell. This process is used by Dictyostelium, and some cancers, as a mechanism of nutrient acquisition (59, 60), whereby internalized macromolecules are trafficked to lysosomes for degradation and recycling to meet metabolic demands. Macropinocytosis is also commonly used by macrophages and dendritic cells to sample their surroundings for foreign or unwanted material, which can then be internalized and targeted to specialized degradative compartments for antigen presentation (61). Macropinocytosis can also support the turnover and repair of plasma membrane (62).
A number of studies have demonstrated the targeting of macropinosomes by CASM, in both immune and nonimmune cell types (8, 19, 63), in a process thus termed LC3-associated macropinocytosis (LAM). ATG8/LC3 proteins become transiently conjugated to single-membrane macropinosomes, dependent on the ATG8 conjugation machinery but independent of the upstream ULK1/2 complex (19). The functional outcome of LAM remains to be fully understood. It is interesting to note that not all macropinosomes acquire LC3, with only ~50% being targeted in platelet-derived growth factor (PDGF)–stimulated mouse embryonic fibroblast (MEF) cells (19). Hence, it is intriguing to consider how macropinosomes are targeted, and whether their fates, recycling versus degradation, may perhaps be influenced by their interaction with noncanonical autophagy.
LC3-associated endocytosis
Endocytosis is another fundamental mechanism through which extracellular molecules are engulfed and internalized, with critical cellular roles, such as modulating the degradation and signaling of different receptors [e.g., epidermal growth factor receptor (EGFR)]. Several endocytic pathways internalize cargo through receptor interactions, including both clathrin-dependent endocytosis (CDE) and clathrin-independent endocytosis (CIE) (64). Distinct cargoes thus enter the endosomal system and are subject to multiple fates, including degradation and recycling.
Recently, noncanonical autophagy was shown to target specialized endosomes involved in the uptake of β-amyloid (Aβ), through a process termed LC3-associated endocytosis (LANDO) (27, 65). In this context, CASM does not regulate the degradation of Aβ but rather the recycling of the receptors involved in Aβ internalization (TLR4, CD36, and TREM2), which is important in Alzheimer’s disease and neurodegeneration. Interestingly, CASM may also be involved in the endocytosis and signaling of the receptor tyrosine kinase c-Met (66), suggesting that other endocytic cargoes may harness this LANDO pathway.
Entosis—CASM and cell cannibalism
Entosis is a form of cellular cannibalism in which live and viable epithelial cells are engulfed and internalized by their neighbors, yielding “cell-in-cell” structures commonly observed in cancer (67). A range of tumor-related stimuli, including matrix detachment, aberrant mitosis, glucose starvation, and ultraviolet radiation, induce this homotypic form of cell internalization (67–70), and cell-in-cell frequency correlates with tumor grade. The engulfed cell is then housed within an entotic vacuole, where it can remain viable inside its host for many hours. Ultimately, this vacuole matures and fuses with host cell lysosomes, thereby acidifying and digesting the inner cell in an act of cellular cannibalism. Hence, entosis represents a form of nonapoptotic cell death associated with cell competition within tumors, which can eliminate internalized “loser” cells with aberrant properties (e.g., matrix deadhered, inappropriately dividing) and provide a nutrient source to support “winner” host cell outgrowth (71, 72).
Early studies on entosis revealed, somewhat unexpectedly, that host cell ATG8/LC3 is transiently lipidated to the entotic vacuole during the early stages of its maturation (19, 55). Further analysis revealed that this vacuole is composed of a single membrane, on which the noncanonical autophagy pathway drives CASM. Interestingly, inhibition of ATG8/LC3 recruitment, via ATG5 or ATG7 knockdown, but independent of FIP200, affects inner cell death (19), indicating a role for noncanonical autophagy in this form of “cellular murder” prevalent in cancer.
In summary, a wide range of heterophagic processes are now known to harness noncanonical autophagy/CASM, indicating that conserved ATG machinery facilitates the degradation of material from both inside and outside the cell.
Pharmacological activation of CASM
While most work on noncanonical autophagy has been carried out in the context of heterophagy, with a particular emphasis on LAP, alternative stimuli have since been identified that do not involve macroscale endocytic engulfment. A growing number of studies have now addressed the activation of CASM by pharmacological agents.
Lysosomotropic drugs
Lysosomotropic drugs are hydrophobic, weakly basic compounds that are sequestered, and then accumulated, within the lumen of acidic lysosomes and act to raise their pH and promote their swelling (73, 74). Well known examples, relevant in both research and the clinic, include the antimalarial and anticancer drugs chloroquine (CQ) and hydroxychloroquine (75), the local anesthetics lidocaine and procainamide, and the anti-arrythmia drug betahistine. Many of these drugs have been implicated previously in modulating macroautophagy (74), with CQ commonly used as a lysosome inhibitor to block autophagosome flux. However, comprehensive studies have since demonstrated that all these drugs also robustly induce noncanonical autophagy, in parallel, as can be shown in an ATG13 null background, driving CASM directly on endolysosome membranes (6, 47). The parallel effects of commonly used drugs such as CQ must be considered carefully during experimental design and interpretation.
Ionophores
Similar to lysosomotropic drugs, certain ionophores induce noncanonical autophagy, in parallel to their effects on autophagic flux (47). Nigericin and monensin are ionophores that promote the exchange of potassium and sodium, respectively, for hydrogen ions across membranes, while carbonyl cyanide 3-chlorophenylhydrazone is a proton ionophore. These drugs all induce CASM, by altering the ionic balance of endolysosomes, raising their pH, and driving osmotic effects, as discussed further below. As with lysosomotropic drugs, these compounds regulate canonical and noncanonical autophagy pathways simultaneously, so they must be used and interpreted with care.
Mechanistically, it is important to note that lysosomotropic drugs like CQ, and ionophores like monensin, raise lysosomal pH, similar to BafA1, and yet activate CASM, rather than inhibiting it. This dichotomy provides an important mechanistic clue, implying that the CASM inhibitory effect of BafA1 is related to V-ATPase but independent of its pump activity. We return to this observation in the “A unifying mechanism for CASM” section for further discussion.
Osmotic imbalance
The lysosomotropic and ionophore agents noted above all alter ionic balance, shifting the osmotic properties of intracellular compartments and driving water influx and swelling. Related to this, CASM induced by CQ or monensin is sensitive to aquaporin inhibition (by mercury chloride or phloretin), suggesting that these osmotic effects are mechanistically important (46). Furthermore, cells placed in hypertonic media undergo rapid LC3 lipidation, indicating that osmotic imbalance is sufficient to activate CASM (46). Notably, under these conditions, ATG8/LC3 is only lipidated onto acidic, lysosomal associated membrane protein 1 (LAMP1)-positive vesicles, and this process can be inhibited by BafA1. These data imply that osmotic imbalance is commonly associated with CASM but must act in concert with, and dependent upon, V-ATPase (see the “A unifying mechanism for CASM” section).
TRPML1 agonists
Recent work has identified the lysosomal calcium channel TRPML1 as the first specific protein target that can induce noncanonical autophagy/CASM. Activation of TRPML1, with various small-molecule agonists, is sufficient to drive noncanonical autophagy and ATG8 lipidation directly on lysosome membranes (20). By studying this pathway, we uncovered an important new function for CASM in the regulation of transcription factor EB (TFEB) and lysosomal biogenesis. Activation of CASM by TRPML1 agonists, or other lysosomotropic drugs, activates TFEB in a previously unknown manner, independent of mechanistic target of rapamycin (mTOR) activity and nutrient regulation. This alternative mechanism relies on the ATG8 isoform, GABARAP, binding to the folliculin-interacting protein (FNIP)/folliculin complex. Lipidation of GABARAP during CASM sequesters this complex away from the cytosol, relieving its inhibitory effect on the Rag guanosine triphosphatases (GTPases) and thereby permitting TFEB activation (20).
Drugs driving Golgi ATG8 lipidation
Notably, some other drugs have been identified that induce noncanonical autophagy, including the anti-helminthic drug niclosamide (76) and AMDE-1 (77), which drive ATG8 lipidation on the Golgi compartment.
The studies described above yield important insights into the pharmacology of CASM. This will ultimately be important for the development of drugs to specifically modulate the noncanonical autophagy pathway, both experimentally and clinically. This body of work is also highly instructive with respect to molecular mechanisms. While the drugs described above fall into a wide range of categories, it is notable that all center on the perturbation of ionic, pH, and osmotic balances within intracellular vesicles to induce noncanonical autophagy. Moreover, CASM induced by all these treatments is blocked by BafA1, pointing to a key role for V-ATPase (explored in the “A unifying mechanism for CASM” section).
Given the diverse set of pharmacological agents and treatments that are now known to activate CASM, it is increasingly important to consider this pathway when examining the autophagy-related responses to particular drug interventions. Caution is required when interpreting results, particularly where ATG8 lipidation is used solely as a readout. It seems likely that the role of noncanonical autophagy has been historically underappreciated in the field due to ambiguity in this area and that some functions and phenotypes assigned to the canonical pathway may in fact require CASM as well or instead.
Pathogenic factors, host responses, and CASM
Activation of macroautophagy is a well-established host response to many bacterial and viral pathogens (57). The pathway is often activated as a host defense but can also be usurped by certain pathogens to enable their survival. Recent studies have uncovered a similar interplay between pathogenic factors and noncanonical autophagy, suggesting that this alternative pathway also plays an important role in the host pathogen response.
Viruses and CASM
IAV is a single-stranded RNA virus that enters cells via endocytosis (78). A key factor involved in IAV replication and spread is the M2 protein, an established antiviral target (79). M2 is a viroporin that inserts into membranes and acts as a pH-gated proton channel (80). During viral entry, M2 acidifies the viron interior, which facilitates viron membrane fusion with the endosome, releasing viral RNA into the cytosol. Following this, the M2 protein remains associated with the endosomal membrane, and during viral egress, it alters Golgi pH (81). Recent work has shown that IAV infection, and specifically the action of M2, promotes noncanonical autophagy on, as yet, unidentified intracellular vesicles (6, 8, 82). Furthermore, noncanonical autophagy is important in the host response to IAV (26).
It is tempting to speculate that viroporins from other viruses may have a similar capacity to activate noncanonical autophagy. Coxsackievirus infection induces LC3 lipidation, consistent with many features of CASM (83), and also expresses the viroporin protein 2B. Similarly, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) encodes proteins with viroporin properties, E protein and ORF3a, and has been suggested to modulate autophagy pathways (84). Future work will be required to determine the prevalence of viral-induced noncanonical autophagy/CASM.
STING and CASM
The STING pathway is part of the innate immune response to cytosolic DNA derived from damaged mitochondria (85) or pathogens (86). Engagement of the STING pathway results in activation of interferon regulatory factor 3 (IRF3) and nuclear factor κB (NFκB) and production of type I interferons and inflammatory cytokines. STING activation promotes LC3 lipidation, in a ULK- and Vps34-independent manner (87), which would be consistent with CASM. However, Liu et al. (88) concluded that this nevertheless represents a macroautophagic response, as STING contains an LC3-interacting region (LIR) and is a cargo for autophagosome-mediated degradation. More recently, STING activation has also been shown to also promote noncanonical autophagy (18, 89). STING agonists, cyclic guanosine monophosphate-adenosine monophophate and double-stranded DNA, induce LC3B lipidation to single-membrane vesicles, in the perinuclear region of cells, independent of autophagosome formation. This process depends on V-ATPase and ATG16L1, and has thus been termed VAIL (discussed further in the “A unifying mechanism for CASM” section). The functional role of CASM during STING signaling remains to be determined and will be of great interest to understand further.
Bacterial toxins and CASM
Many bacterial pathogens hijack the host endocytic pathway and disrupt cell membranes as part of their survival mechanisms. Commonly, this occurs through the expression and secretion of pore-forming toxins (PFTs) (90). One such PFT is vacuolating toxin A (VacA) from Helicobacter pylori. During infection, VacA inserts into the plasma membrane and is internalized into the endocytic system (91), where it forms anion-selective channels. VacA thereby mediates the movement of negatively charged chloride ions into endolysosomes, which is then compensated for by an increase in V-ATPase activity (92). H. pylori infection is known to modulate the canonical autophagy pathway and increase LC3 lipidation (93). However, importantly, it also stimulates noncanonical autophagy and ATG8/LC3 lipidation to enlarged endolysosomal membranes (46). These findings suggest that toxin-induced endolysosomal perturbation could be a common inducer of CASM. Many PFTs have been associated with increased LC3 lipidation, including listeriolysin O (LLO) from Listeria monocytigenes (94), α-hemolysin from Staphylococcus aureus (95), and pneumolysin (PLY) from Streptococcus pneumoniae (53). The action of these PFTs is thought to disrupt and damage endomembranes, which then activates lysophagy. However, it seems possible that they may also act to promote CASM.
The studies described above have uncovered a wide range of pathogenic factors that drive CASM, implicating the pathway as the common host response. Notably, a conserved mechanistic theme emerges, both among these pathogenic factors and with the pharmacological stimuli, linking to ionic, pH, and osmotic imbalance and V-ATPase activity. We return to these mechanistic clues further in the “A unifying mechanism for CASM” section.
Other candidates for CASM?
A number of other cellular processes have been described, which share certain features of CASM, but have not been formally associated with the noncanonical autophagy pathway. These include both degradative and secretory pathways and membrane repair.
Microautophagy
Recent studies have revealed an LC3-dependent, microautophagy pathway in mammalian cells, where endolysosome membrane proteins, such as TRPML1, are internalized into intraluminal vesicles (ILVs) for degradation (96). This is dependent on the core ATG lipidation machinery, but not autophagosome formation, suggesting a possible role for CASM. It remains to be determined whether noncanonical autophagy might also represent a more general mechanism regulating the turnover of membrane proteins.
Secretion: LC3-dependent extracellular vesicle loading
In a pathway termed LC3-dependent extracellular vesicle loading (LDEL), cytosolic proteins, including RNA binding proteins, are loaded into ILVs, via binding to LC3 lipidated on the cytosolic side of multivesicular bodies (MVBs) (97). Fusion of MVBs with the plasma membrane then releases the ILVs as extracellular vesicles, along with their LC3-dependent cargo. This secretion-related example of ATG8/LC3 lipidation shares many common features with CASM, but it remains to be determined whether LDEL depends on exactly the same molecular machinery.
Osteoclasts
During bone degradation and resorption, osteoclasts form a specialized zone of plasma membrane opposed to the bone surface, called the ruffled border, which is partitioned from the rest of the cell membrane via an actin ring. Lysosomes fuse with the ruffled border, secreting their degradative enzymes into the extracellular space. ATG8/LC3 proteins localize and lipidate to the ruffled border and are required for efficient bone degradation (98). This ATG8/LC3 recruitment has been attributed to autophagosome fusion, but this conclusion was based only on the involvement of ATG5, ATG7, and ATG4B, which are also required for CASM. Considering the single-membrane nature of the ruffled border, it seems possible that the ATG8/LC3 recruitment is associated with noncanonical autophagy as well or instead.
As described in this section, a number of different acronyms (LAP, LANDO, and LAM) have been developed to identify a broad range of noncanonical autophagy processes. We propose that CASM can be used as an umbrella term for these diverse but related processes, which captures their key shared features, the conjugation of ATG8 family members to single membranes.
A UNIFYING MECHANISM FOR CASM
The sections above provide an updated overview of the key molecular features, and broad biological stimuli, of noncanonical autophagy/CASM. Taking all of this information into consideration, we have sought to develop a unifying mechanism for CASM that can explain the shared features of diverse processes, from LAP to entosis and viral infection, as well as account for their distinctions. Where do these pathways converge so that many roads lead to CASM (Fig. 3)?
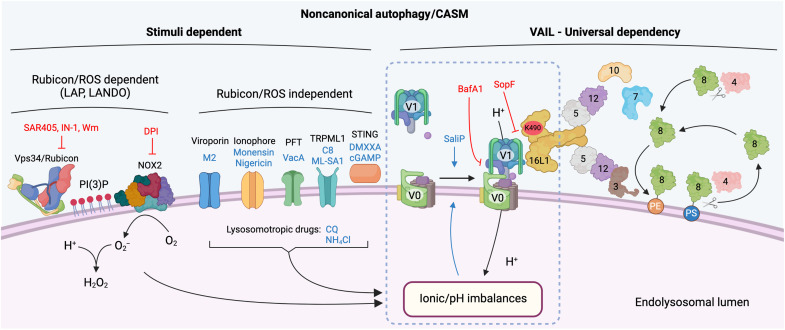
Fig. 3. Regulatory and unifying mechanisms of noncanonical autophagy activation.
An overview of the known mechanisms regulating noncanonical autophagy and CASM. Different stimuli converge to alter the ionic balance and pH of the target endolysosomal vesicle. LAP and LANDO depend on Vps34/Rubicon-mediated activation of NOX2 to induce ROS production, which consumes protons and raises pH. Multiple stimuli, including viroporins, ionophores, PFTs, lysosomotropic drugs, TRPML1 agonists, and STING agonists, all lead to alterations in luminal ion balances and pH independent of Rubicon and ROS. Endolysosomal pH perturbations are sensed through unknown mechanisms and lead to increased association of V0-V1 subunits of the V-ATPase complex. Increased V0-V1 association drives VAIL (V-ATPase–ATG16L1–induced LC3 lipidation) and the recruitment of the ATG16L1 complex through the C-terminal WD40 domain. The autophagy-related ubiquitin-like conjugation systems then target ATG8 proteins for lipidation to PE or PS on the endolysosomal membrane. Pathway inhibitors are in red text, and activators are in blue text. Figure generated using Biorender.com
The V-ATPase–ATG16L1 axis
In considering a possible common mechanism for CASM, V-ATPase and its relationship with ATG16L1 emerged as a central candidate, based on multiple independent lines of evidence, as explored below.
BafA and CASM
As discussed in earlier sections, BafA inhibits CASM in all contexts that have been tested, from heterophagic LAP and entosis (46) to lysosomotropic or ionophore drug treatment (46, 47), activation of TRPML1 (20) and STING (18), as well as in response to pathogenic factors such as VacA (46). Collectively, these studies point to a highly conserved, but incompletely defined, role for V-ATPase in noncanonical autophagy.
Cells, drugs, and vacuoles!
Building on this notion, the wider pharmacology of CASM similarly hints at V-ATPase involvement. Perturbation of ionic, pH, and osmotic balances is intimately linked with CASM, in multiple contexts, and in a BafA1-sensitive manner. Importantly though, many lysosomotropic drugs (e.g., CQ) and ionophores (e.g., monensin), as well as pathogenic factors, raise lysosomal pH in a similar manner to BafA1 but activate CASM rather than inhibit it. This important distinction provides a key mechanistic insight, implying that CASM, and its inhibition by BafA1, is V-ATPase related but must be pump independent.
V0-V1 association and CASM
V-ATPase is a multisubunit proton channel, which can be regulated by a reversible association between its membrane-bound (V0) and cytosolic (V1) sectors (44). Given that CASM is dependent on V-ATPase but likely independent of its pump activity, we hypothesized that it could be linked instead to V0-V1 association.
In recent work (89), we explored this hypothesis directly by comparing BafA1 with saliphenylhalamide (SaliP), a structurally distinct V-ATPase inhibitor. These compounds both block V-ATPase pump activity and raise lysosomal pH (99). However, they bind to distinct sites, and while BafA1 acts to dissociate V0-V1, SaliP instead drives this interaction through covalent adduct formation. Notably, while BafA1 treatment inhibits CASM, in response to diverse stimuli, SaliP treatment instead activates the pathway, driving ATG8/LC3 lipidation (89). These data suggest that forced V0-V1 engagement is sufficient to trigger noncanonical autophagy/CASM. Consistent with this, we find that V0-V1 binding is induced by stimuli that drive CASM (e.g., CQ, monensin, and TRPML1 agonist) but not canonical autophagy (e.g., mTOR inhibitor). Furthermore, inducible recruitment of V1 is observed during CASM induced by both STING activation (18) and LAP (89). Together, these new findings indicate that regulated engagement of V0-V1 is a pivotal molecular event during noncanonical autophagy, which somehow activates CASM.
V-ATPase–ATG16L1 association
A possible solution to this molecular puzzle was provided by an elegant, and entirely separate, study focused on Salmonella infection (34), which identified a direct interaction between V-ATPase and ATG16L1. While this interaction was explored in the context of xenophagy, we were struck by its dependence on the ATG16L1 WD40 domain, which is so strongly associated with CASM. This shared molecular feature hints strongly at a possible mechanistic relationship with noncanonical autophagy.
Through recent work, from our laboratories and others, an analogous, inducible interaction between V-ATPase and ATG16L1 has now been detected during CASM in response to a multitude of stimuli, including IAV infection (82), LAP, treatment with monensin or agonists for TRPML1, and activation of STING during VAIL (18, 89). Notably, this interaction is completely abolished by ATG16L1 K490A mutation, reinforcing its link to the noncanonical autophagy pathway (82, 89). Furthermore, SaliP treatment is sufficient to trigger binding between V-ATPase and ATG16L1 (89), indicating that this protein-protein interaction is tied to the V0-V1 assembly described above.
Together, these data indicate that the V-ATPase–ATG16L1 axis identified during xenophagy plays a similarly pivotal role during CASM, providing a satisfying mechanistic explanation for its unique pharmacology (e.g., BafA1 and SaliP) and conserved features (e.g., pH, ionic, and osmotic changes). Further work will be required to understand this inducible V-ATPase–ATG16L1 interaction in more detail and to establish whether a conformational change occurs upon increased V0-V1 engagement to facilitate binding to ATG16L1.
SopF—Evolutionary clues from pathogen evasion
SopF is a Salmonella effector protein that blocks the interaction between V-ATPase and ATG16L1 by ribosylating Gln124 in the ATP6V0C subunit (34), thus allowing the bacteria to evade host defenses through xenophagy. Several groups have now also shown that SopF inhibits CASM, in response to IAV, monensin (82), LAP (89), and STING (18). Hence, SopF represents a powerful new tool to inhibit noncanonical autophagy/CASM in the cell and provides a strong evolutionary clue regarding the importance of this V-ATPase–ATG16L1 axis. We speculate that CASM represents an important parallel host response that SopF also enables Salmonella to evade (89).
By integrating these diverse sets of data, obtained from numerous independent groups, studying a wide range of autophagy-related processes, we have collated evidence to suggest that V-ATPase plays a universal function during noncanonical autophagy, directly recruiting ATG16L1 to drive CASM. We speculate that induced V0-V1 association permits this interaction, perhaps via conformational change, and that it depends on the K490-containing pocket, on the top face of the ATG16L1 C-terminal WD40 domain. We propose that during CASM, the V-ATPase complex plays an analogous role to WIPI2 during macroautophagy, recruiting ATG16L1 to the appropriate membrane, to specify the site of ATG8 conjugation.
How do the diverse CASM pathways converge at V-ATPase?
A common feature of CASM, which emerges in the “Diverse stimuli drive noncanonical autophagy/CASM” section, is that many, perhaps all, of its stimuli drive the neutralization of endolysosomal pH. We speculate that, to compensate for this, the cell may respond by increasing the association between the V-ATPase cytosolic V1 sector and membrane-bound V0 sector to drive reacidification. As V0-V1 engagement promotes V-ATPase interaction with ATG16L1, we postulate that this molecular event may effectively initiate noncanonical autophagy/CASM (82, 89).
We note that for some CASM stimuli, such as STING agonists, endolysosomal pH change has not been demonstrated to date. Interestingly, there is some evidence that upon activation, STING interacts with the calcium channel TMEM203, which could potentially result in alterations in ionic and pH balance within vesicles (100); however, this possibility remains to be investigated. Hence, some features of the proposed model may require further testing and/or refinement. Nevertheless, this model accounts for many of the shared molecular features and mechanisms of CASM, providing a useful framework to develop our understanding.
Integrating V-ATPase with NOX2, ROS, and Rubicon
While this V-ATPase–ATG16L1 axis provides a compelling, and potentially universal, explanation for CASM, we were left with an outstanding question on how this could be integrated with other known molecular players and mechanisms in noncanonical autophagy. Here, we thought specifically about Rubicon and ROS, which play pivotal, and very well established, roles in LAP (and LANDO) (12, 65).
During phagocytosis, particulate targets, such as dead cells or pathogens, trigger receptor-mediated engulfment into single-membrane phagosomes. These phagosomes then mature through a sequence of protein recruitments and phosphoinositide metabolism (101), which culminates in the transitioning of the phagosome to an acidic, degradative compartment. During maturation, NADPH (reduced form of nicotinamide adenine dinucleotide phosphate) oxidase 2 (NOX2) forms a complex on the phagosome and generates bursts of ROS, a critical step in controlling the function of phagosomes and fate of cargo. Importantly, proteins involved in phagosome maturation and ROS generation are also critical for LAP.
Rubicon (RUN domain and cysteine-rich domain containing, Beclin 1–interacting protein) is part of the class III phosphatidylinositol 3-kinase complex, alongside Vps34, Beclin, and UVRAG, which generates localized phosphatidylinositol-3-phosphate [PI(3)P] on the phagosome membrane (12, 102). Deletion of Rubicon significantly inhibits LAP, without impeding macroautophagy (12); the Vps34 complex containing Rubicon acts as a negative regulator of macroautophagy (103). A key function of phagosomal PI(3)P is then to support the stabilization of NOX2 through binding to the p40phox subunit, which promotes ROS production. Deletion or inhibition of NOX2, or chelation of its ROS products, potently inhibits LAP (12, 104). Thus, targeting of the Rubicon/PI(3)P/ROS axis has been widely used in vitro and in vivo to inhibit and implicate LAP and LANDO in many cellular processes and diseases including infection (12, 51–54), immune responses (11), cancer (7), and neurodegeneration (65).
Importantly, these effects are somewhat context specific, as Rubicon, NOX2, and ROS are not required for other noncanonical autophagy processes. Activation of CASM by ionophores, lysosomotropic drugs, and STING and TRPML1 agonists are all independent of Vps34 activity and PI(3)P formation (20, 46), and Vps34 and ROS are not essential for CASM induced during the formation of endocytic vacuoles in acinar cells (105). So, where do NOX2, ROS, and Rubicon converge with the more universal machinery of CASM?
We have now investigated the possible links between ROS production and the ATG8 conjugation machinery, focusing on the newly defined V-ATPase–ATG16L1 axis (89). Importantly, we noted from the literature, and confirmed experimentally, that phagosomal ROS consume protons and thereby neutralize phagosomal pH (106–108). We reasoned that, in common with other forms of CASM, V-ATPase may therefore be activated in response, through increased V0-V1 engagement, in an attempt to reacidify the compartment. Consistent with this, we found that during LAP, ATG16L1 is recruited to the V-ATPase complex, via its WD40 domain, where it directs ATG8 conjugation to the phagosome membrane, in a manner sensitive to SopF. These findings can thus tie together the well-established requirements for NOX2, ROS, and Rubicon during LAP, with the V-ATPase–ATG16L1 axis common to all forms of CASM.
We note that localized ROS can also inactivate the ATG8-deconjugating activity of ATG4s at the phagosome membrane, through the oxidation of critical cysteine residues in their catalytic sites, leading to sustained ATG8 lipidation (109). Hence, parallel mechanisms may be at play in the context of LAP.
SUMMARY
In this review, we have covered the diversity of processes that harness CASM and discussed both their shared and distinct molecular mechanisms. We posit that these processes all represent examples of the noncanonical autophagy pathway and share a common regulatory mechanism centered on V-ATPase and ATG16L1 at endolysosomal single membranes. However, important process and context differences also exist, with respect to molecular machinery and functional output.
It will be increasingly important within the field to understand precisely which form of noncanonical autophagy/CASM is active in different contexts, as some distinctive mechanisms may be at play. Similarly, because canonical and noncanonical autophagy pathways are regulated by overlapping agonists and inhibitors and share overlapping readouts, there is huge potential for confusion. Rigorous experimental design will be essential to yield unambiguous conclusions.
To assist with providing clarity, we conclude with a summary of molecular features in Fig. 3. We propose that some proteins are associated with specific forms of CASM, for instance, Rubicon for LAP/LANDO or M2 for IAV. In contrast, others are universally required for all forms of noncanonical autophagy, including the core ATG8 conjugation machinery and the V-ATPase–ATG16L1 axis. We suggest that the following features may be considered diagnostic for CASM in future studies:
1) Conjugation of ATG8 to a single membrane (CASM) rather than double-membrane autophagosome.
2)Dependence on the core ATG8 conjugation machinery (ATG3/4/5/7/10/12/16L1), combined with independence from upstream macroautophagy regulators (ULK1/2, FIP200, ATG13/9/14 L1, and WIPI2).
3) Dependence on the ATG16L1 WD40 domain (and K490 residue), combined with independence of its FBD domain.
4) Induction of alternative conjugation of ATG8 to PS rather than exclusively to PE.
5) Dependence on the V-ATPase–ATG16L1 axis, as evidenced by inhibition with BafA1 and/or SopF.
6) We recommend that while dependence on Rubicon and NOX2 can be used to implicate LAP (and LANDO), this should not be used to exclude other forms of noncanonical autophagy and CASM, which couple to the V-ATPase–ATG16L1 axis independently.
Acknowledgments
Funding: This work was supported by grants from the BBSRC, BB/P013384/1 (BBS/E/B/000C0432 and BBS/E/B/000C0434).
Author contributions: J.D. and O.F. conceived and wrote the manuscript.
Competing interests: O.F. is a paid consultant for Casma Therapeutics. All authors declare that they have no other competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper.
REFERENCES AND NOTES
- 1.Choi A. M. K., Ryter S. W., Levine B., Autophagy in human health and disease. N. Engl. J. Med. 368, 651–662 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Feng Y., He D., Yao Z., Klionsky D. J., The machinery of macroautophagy. Cell Res. 24, 24–41 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johansen T., Lamark T., Selective autophagy: ATG8 family proteins, LIR motifs and cargo receptors. J. Mol. Biol. 432, 80–103 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Mizushima N., The ATG conjugation systems in autophagy. Curr. Opin. Cell Biol. 63, 1–10 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Galluzzi L., Green D. R., Autophagy-independent functions of the autophagy machinery. Cell 177, 1682–1699 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durgan J., Lystad A. H., Sloan K., Carlsson S. R., Wilson M. I., Marcassa E., Ulferts R., Webster J., Lopez-Clavijo A. F., Wakelam M. J., Beale R., Simonsen A., Oxley D., Florey O., Non-canonical autophagy drives alternative ATG8 conjugation to phosphatidylserine. Mol. Cell 81, 2031–2040.e8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunha L. D., Yang M., Carter R., Guy C., Harris L., Crawford J. C., Quarato G., Boada-Romero E., Kalkavan H., Johnson M. D. L., Natarajan S., Turnis M. E., Finkelstein D., Opferman J. T., Gawad C., Green D. R., LC3-associated phagocytosis in myeloid cells promotes tumor immune tolerance. Cell 175, 429–441.e16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fletcher K., Ulferts R., Jacquin E., Veith T., Gammoh N., Arasteh J. M., Mayer U., Carding S. R., Wileman T., Beale R., Florey O., The WD40 domain of ATG16L1 is required for its non-canonical role in lipidation of LC3 at single membranes. EMBO J. 37, e97840 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henault J., Martinez J., Riggs J. M., Tian J., Mehta P., Clarke L., Sasai M., Latz E., Brinkmann M. M., Iwasaki A., Coyle A. J., Kolbeck R., Green D. R., Sanjuan M. A., Noncanonical autophagy is required for type I interferon secretion in response to DNA-immune complexes. Immunity 37, 986–997 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim J. Y., Zhao H., Martinez J., Doggett T. A., Kolesnikov A. V., Tang P. H., Ablonczy Z., Chan C. C., Zhou Z., Green D. R., Ferguson T. A., Noncanonical autophagy promotes the visual cycle. Cell 154, 365–376 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez J., Cunha L. D., Park S., Yang M., Lu Q., Orchard R., Li Q.-Z., Yan M., Janke L., Guy C., Linkermann A., Virgin H. W., Green D. R., Noncanonical autophagy inhibits the autoinflammatory, lupus-like response to dying cells. Nature 533, 115–119 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Martinez J., Malireddi R. K. S., Lu Q., Cunha L. D., Pelletier S., Gingras S., Orchard R., Guan J.-L., Tan H., Peng J., Kanneganti T.-D., Virgin H. W., Green D. R., Molecular characterization of LC3-associated phagocytosis reveals distinct roles for Rubicon, NOX2 and autophagy proteins. Nat. Cell Biol. 17, 893–906 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Sanjuan M. A., Dillon C. P., Tait S. W. G., Moshiach S., Dorsey F., Connell S., Komatsu M., Tanaka K., Cleveland J. L., Withoff S., Green D. R., Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature 450, 1253–1257 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Axe E. L., Walker S. A., Manifava M., Chandra P., Roderick H. L., Habermann A., Griffiths G., Ktistakis N. T., Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J. Cell Biol. 182, 685–701 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hailey D. W., Rambold A. S., Satpute-Krishnan P., Mitra K., Sougrat R., Kim P. K., Lippincott-Schwartz J., Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell 141, 656–667 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puri C., Renna M., Bento C. F., Moreau K., Rubinsztein D. C., Diverse autophagosome membrane sources coalesce in recycling endosomes. Cell 154, 1285–1299 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Florey O., Overholtzer M., Autophagy proteins in macroendocytic engulfment. Trends Cell Biol. 22, 374–380 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer T. D., Wang C., Padman B. S., Lazarou M., Youle R. J., STING induces LC3B lipidation onto single-membrane vesicles via the V-ATPase and ATG16L1-WD40 domain. J. Cell Biol. 219, e202009128 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Florey O., Kim S. E., Sandoval C. P., Haynes C. M., Overholtzer M., Autophagy machinery mediates macroendocytic processing and entotic cell death by targeting single membranes. Nat. Cell Biol. 13, 1335–1343 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodwin J. M., Walkup W. G. IV, Hooper K., Li T., Kishi-Itakura C., Ng A., Lehmberg T., Jha A., Kommineni S., Fletcher K., Garcia-Fortanet J., Fan Y., Tang Q., Wei M., Agrawal A., Budhe S. R., Rouduri S. R., Baird D., Saunders J., Kiselar J., Chance M. R., Ballabio A., Appleton B. A., Brumell J. H., Florey O., Murphy L. O., GABARAP sequesters the FLCN-FNIP tumor suppressor complex to couple autophagy with lysosomal biogenesis. Sci. Adv. 7, eabj2485 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell G., Cheng M. I., Chen C., Nguyen B. N., Whiteley A. T., Kianian S., Cox J. S., Green D. R., McDonald K., Portnoy D. A., Listeria monocytogenes triggers noncanonical autophagy upon phagocytosis, but avoids subsequent growth-restricting xenophagy. Proc. Natl. Acad. Sci. U.S.A. 115, E210–E217 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujita N., Itoh T., Omori H., Fukuda M., Noda T., Yoshimori T., The Atg16L complex specifies the site of LC3 lipidation for membrane biogenesis in autophagy. Mol. Biol. Cell 19, 2092–2100 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dooley H. C., Razi M., Polson H. E. J., Girardin S. E., Wilson M. I., Tooze S. A., WIPI2 links LC3 conjugation with PI3P, autophagosome formation, and pathogen clearance by recruiting Atg12-5-16L1. Mol. Cell 55, 238–252 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gammoh N., Florey O., Overholtzer M., Jiang X., Interaction between FIP200 and ATG16L1 distinguishes ULK1 complex-dependent and -independent autophagy. Nat. Struct. Mol. Biol. 20, 144–149 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rai S., Arasteh M., Jefferson M., Pearson T., Wang Y., Zhang W., Bicsak B., Divekar D., Powell P. P., Naumann R., Beraza N., Carding S. R., Florey O., Mayer U., Wileman T., The ATG5-binding and coiled coil domains of ATG16L1 maintain autophagy and tissue homeostasis in mice independently of the WD domain required for LC3-associated phagocytosis. Autophagy 15, 599–612 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y., Sharma P., Jefferson M., Zhang W., Bone B., Kipar A., Bitto D., Coombes J. L., Pearson T., Man A., Zhekova A., Bao Y., Tripp R. A., Carding S. R., Yamauchi Y., Mayer U., Powell P. P., Stewart J. P., Wileman T., Non-canonical autophagy functions of ATG16L1 in epithelial cells limit lethal infection by influenza A virus. EMBO J. 40, e105543 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heckmann B. L., Teubner B. J. W., Boada-Romero E., Tummers B., Guy C., Fitzgerald P., Mayer U., Carding S., Zakharenko S. S., Wileman T., Green D. R., Noncanonical function of an autophagy protein prevents spontaneous Alzheimer’s disease. Sci. Adv. 6, eabb9036 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boada-Romero E., Letek M., Fleischer A., Pallauf K., Ramón-Barros C., Pimentel-Muiños F. X., TMEM59 defines a novel ATG16L1-binding motif that promotes local activation of LC3. EMBO J. 32, 566–582 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slowicka K., Serramito-Gómez I., Boada-Romero E., Martens A., Sze M., Petta I., Vikkula H. K., de Rycke R., Parthoens E., Lippens S., Savvides S. N., Wullaert A., Vereecke L., Pimentel-Muiños F. X., van Loo G., Physical and functional interaction between A20 and ATG16L1-WD40 domain in the control of intestinal homeostasis. Nat. Commun. 10, 1834 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serramito-Gómez I., Boada-Romero E., Villamuera R., Fernández-Cabrera Á., Cedillo J. L., Martín-Regalado Á., Carding S., Mayer U., Powell P. P., Wileman T., García-Higuera I., Pimentel-Muiños F. X., Regulation of cytokine signaling through direct interaction between cytokine receptors and the ATG16L1 WD40 domain. Nat. Commun. 11, 5919 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujita N., Morita E., Itoh T., Tanaka A., Nakaoka M., Osada Y., Umemoto T., Saitoh T., Nakatogawa H., Kobayashi S., Haraguchi T., Guan J. L., Iwai K., Tokunaga F., Saito K., Ishibashi K., Akira S., Fukuda M., Noda T., Yoshimori T., Recruitment of the autophagic machinery to endosomes during infection is mediated by ubiquitin. J. Cell Biol. 203, 115–128 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.King B. C., Kulak K., Krus U., Rosberg R., Golec E., Wozniak K., Gomez M. F., Zhang E., O’Connell D. J., Renström E., Blom A. M., Complement component C3 is highly expressed in human pancreatic islets and prevents β cell death via ATG16L1 interaction and autophagy regulation. Cell Metab. 29, 202–210.e6 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Boukhalfa A., Roccio F., Dupont N., Codogno P., Morel E., The autophagy protein ATG16L1 cooperates with IFT20 and INPP5E to regulate the turnover of phosphoinositides at the primary cilium. Cell Rep. 35, 109045 (2021). [DOI] [PubMed] [Google Scholar]
- 34.Xu Y., Zhou P., Cheng S., Lu Q., Nowak K., Hopp A.-K., Li L., Shi X., Zhou Z., Gao W., Li D., He H., Liu X., Ding J., Hottiger M. O., Shao F., A bacterial effector reveals the V-ATPase-ATG16L1 axis that initiates xenophagy. Cell 178, 552–566.e20 (2019). [DOI] [PubMed] [Google Scholar]
- 35.Ichimura Y., Kirisako T., Takao T., Satomi Y., Shimonishi Y., Ishihara N., Mizushima N., Tanida I., Kominami E., Ohsumi M., Noda T., Ohsumi Y., A ubiquitin-like system mediates protein lipidation. Nature 408, 488–492 (2000). [DOI] [PubMed] [Google Scholar]
- 36.Klionsky D. J., Abdelmohsen K., Abe A., Abedin M. J., Abeliovich H., Acevedo Arozena A., Adachi H., Adams C. M., Adams P. D., Adeli K., Adhihetty P. J., Adler S. G., Agam G., Agarwal R., Aghi M. K., Agnello M., Agostinis P., Aguilar P. V., Aguirre-Ghiso J., Airoldi E. M., Ait-Si-Ali S., Akematsu T., Akporiaye E. T., al-Rubeai M., Albaiceta G. M., Albanese C., Albani D., Albert M. L., Aldudo J., Algül H., Alirezaei M., Alloza I., Almasan A., Almonte-Beceril M., Alnemri E. S., Alonso C., Altan-Bonnet N., Altieri D. C., Alvarez S., Alvarez-Erviti L., Alves S., Amadoro G., Amano A., Amantini C., Ambrosio S., Amelio I., Amer A. O., Amessou M., Amon A., An Z., Anania F. A., Andersen S. U., Andley U. P., Andreadi C. K., Andrieu-Abadie N., Anel A., Ann D. K., Anoopkumar-Dukie S., Antonioli M., Aoki H., Apostolova N., Aquila S., Aquilano K., Araki K., Arama E., Aranda A., Araya J., Arcaro A., Arias E., Arimoto H., Ariosa A. R., Armstrong J. L., Arnould T., Arsov I., Asanuma K., Askanas V., Asselin E., Atarashi R., Atherton S. S., Atkin J. D., Attardi L. D., Auberger P., Auburger G., Aurelian L., Autelli R., Avagliano L., Avantaggiati M. L., Avrahami L., Awale S., Azad N., Bachetti T., Backer J. M., Bae D.-H., Bae J.-S., Bae O.-N., Bae S. H., Baehrecke E. H., Baek S.-H., Baghdiguian S., Bagniewska-Zadworna A., Bai H., Bai J., Bai X.-Y., Bailly Y., Balaji K. N., Balduini W., Ballabio A., Balzan R., Banerjee R., Bánhegyi G., Bao H., Barbeau B., Barrachina M. D., Barreiro E., Bartel B., Bartolomé A., Bassham D. C., Bassi M. T., Bast R. C. Jr., Basu A., Batista M. T., Batoko H., Battino M., Bauckman K., Baumgarner B. L., Bayer K. U., Beale R., Beaulieu J.-F., Beck G. R. Jr., Becker C., Beckham J. D., Bédard P.-A., Bednarski P. J., Begley T. J., Behl C., Behrends C., Behrens G. M. N., Behrns K. E., Bejarano E., Belaid A., Belleudi F., Bénard G., Berchem G., Bergamaschi D., Bergami M., Berkhout B., Berliocchi L., Bernard A., Bernard M., Bernassola F., Bertolotti A., Bess A. S., Besteiro S., Bettuzzi S., Bhalla S., Bhattacharyya S., Bhutia S. K., Biagosch C., Bianchi M. W., Biard-Piechaczyk M., Billes V., Bincoletto C., Bingol B., Bird S. W., Bitoun M., Bjedov I., Blackstone C., Blanc L., Blanco G. A., Blomhoff H. K., Boada-Romero E., Böckler S., Boes M., Boesze-Battaglia K., Boise L. H., Bolino A., Boman A., Bonaldo P., Bordi M., Bosch J., Botana L. M., Botti J., Bou G., Bouché M., Bouchecareilh M., Boucher M.-J., Boulton M. E., Bouret S. G., Boya P., Boyer-Guittaut M., Bozhkov P. V., Brady N., Braga V. M. M., Brancolini C., Braus G. H., Bravo-San Pedro J. M., Brennan L. A., Bresnick E. H., Brest P., Bridges D., Bringer M.-A., Brini M., Brito G. C., Brodin B., Brookes P. S., Brown E. J., Brown K., Broxmeyer H. E., Bruhat A., Brum P. C., Brumell J. H., Brunetti-Pierri N., Bryson-Richardson R. J., Buch S., Buchan A. M., Budak H., Bulavin D. V., Bultman S. J., Bultynck G., Bumbasirevic V., Burelle Y., Burke R. E., Burmeister M., Bütikofer P., Caberlotto L., Cadwell K., Cahova M., Cai D., Cai J., Cai Q., Calatayud S., Camougrand N., Campanella M., Campbell G. R., Campbell M., Campello S., Candau R., Caniggia I., Cantoni L., Cao L., Caplan A. B., Caraglia M., Cardinali C., Cardoso S. M., Carew J. S., Carleton L. A., Carlin C. R., Carloni S., Carlsson S. R., Carmona-Gutierrez D., Carneiro L. A. M., Carnevali O., Carra S., Carrier A., Carroll B., Casas C., Casas J., Cassinelli G., Castets P., Castro-Obregon S., Cavallini G., Ceccherini I., Cecconi F., Cederbaum A. I., Ceña V., Cenci S., Cerella C., Cervia D., Cetrullo S., Chaachouay H., Chae H.-J., Chagin A. S., Chai C.-Y., Chakrabarti G., Chamilos G., Chan E. Y. W., Chan M. T. V., Chandra D., Chandra P., Chang C.-P., Chang R. C. C., Chang T. Y., Chatham J. C., Chatterjee S., Chauhan S., Che Y., Cheetham M. E., Cheluvappa R., Chen C.-J., Chen G., Chen G.-C., Chen G., Chen H., Chen J. W., Chen J.-K., Chen M., Chen M., Chen P., Chen Q., Chen Q., Chen S.-D., Chen S., Chen S. S. L., Chen W., Chen W.-J., Chen W. Q., Chen W., Chen X., Chen Y.-H., Chen Y.-G., Chen Y., Chen Y., Chen Y., Chen Y. J., Chen Y.-Q., Chen Y., Chen Z., Chen Z., Cheng A., Cheng C. H. K., Cheng H., Cheong H., Cherry S., Chesney J., Cheung C. H. A., Chevet E., Chi H. C., Chi S.-G., Chiacchiera F., Chiang H.-L., Chiarelli R., Chiariello M., Chieppa M., Chin L.-S., Chiong M., Chiu G. N. C., Cho D.-H., Cho S.-G., Cho W. C., Cho Y.-Y., Cho Y.-S., Choi A. M. K., Choi E.-J., Choi E.-K., Choi J., Choi M. E., Choi S.-I., Chou T.-F., Chouaib S., Choubey D., Choubey V., Chow K.-C., Chowdhury K., Chu C. T., Chuang T.-H., Chun T., Chung H., Chung T., Chung Y. L., Chwae Y.-J., Cianfanelli V., Ciarcia R., Ciechomska I. A., Ciriolo M. R., Cirone M., Claerhout S., Clague M. J., Clària J., Clarke P. G. H., Clarke R., Clementi E., Cleyrat C., Cnop M., Coccia E. M., Cocco T., Codogno P., Coers J., Cohen E. E. W., Colecchia D., Coletto L., Coll N. S., Colucci-Guyon E., Comincini S., Condello M., Cook K. L., Coombs G. H., Cooper C. D., Cooper J. M., Coppens I., Corasaniti M. T., Corazzari M., Corbalan R., Corcelle-Termeau E., Cordero M. D., Corral-Ramos C., Corti O., Cossarizza A., Costelli P., Costes S., Cotman S. L., Coto-Montes A., Cottet S., Couve E., Covey L. R., Cowart L. A., Cox J. S., Coxon F. P., Coyne C. B., Cragg M. S., Craven R. J., Crepaldi T., Crespo J. L., Criollo A., Crippa V., Cruz M. T., Cuervo A. M., Cuezva J. M., Cui T., Cutillas P. R., Czaja M. J., Czyzyk-Krzeska M. F., Dagda R. K., Dahmen U., Dai C., Dai W., Dai Y., Dalby K. N., Dalla Valle L., Dalmasso G., D’Amelio M., Damme M., Darfeuille-Michaud A., Dargemont C., Darley-Usmar V. M., Dasarathy S., Dasgupta B., Dash S., Dass C. R., Davey H. M., Davids L. M., Dávila D., Davis R. J., Dawson T. M., Dawson V. L., Daza P., de Belleroche J., de Figueiredo P., de Figueiredo R. C. B. Q., de la Fuente J., de Martino L., de Matteis A., de Meyer G. R. Y., de Milito A., de Santi M., de Souza W., de Tata V., de Zio D., Debnath J., Dechant R., Decuypere J.-P., Deegan S., Dehay B., del Bello B., del Re D. P., Delage-Mourroux R., Delbridge L. M. D., Deldicque L., Delorme-Axford E., Deng Y., Dengjel J., Denizot M., Dent P., der C. J., Deretic V., Derrien B., Deutsch E., Devarenne T. P., Devenish R. J., di Bartolomeo S., di Daniele N., di Domenico F., di Nardo A., di Paola S., di Pietro A., di Renzo L., DiAntonio A., Díaz-Araya G., Díaz-Laviada I., Diaz-Meco M. T., Diaz-Nido J., Dickey C. A., Dickson R. C., Diederich M., Digard P., Dikic I., Dinesh-Kumar S. P., Ding C., Ding W. X., Ding Z., Dini L., Distler J. H. W., Diwan A., Djavaheri-Mergny M., Dmytruk K., Dobson R. C. J., Doetsch V., Dokladny K., Dokudovskaya S., Donadelli M., Dong X. C., Dong X., Dong Z., Donohue T. M. Jr., Doran K. S., D’Orazi G., Dorn G. W. II, Dosenko V., Dridi S., Drucker L., du J., du L. L., du L., du Toit A., Dua P., Duan L., Duann P., Dubey V. K., Duchen M. R., Duchosal M. A., Duez H., Dugail I., Dumit V. I., Duncan M. C., Dunlop E. A., Dunn W. A. Jr., Dupont N., Dupuis L., Durán R. V., Durcan T. M., Duvezin-Caubet S., Duvvuri U., Eapen V., Ebrahimi-Fakhari D., Echard A., Eckhart L., Edelstein C. L., Edinger A. L., Eichinger L., Eisenberg T., Eisenberg-Lerner A., Eissa N. T., el-Deiry W. S., el-Khoury V., Elazar Z., Eldar-Finkelman H., Elliott C. J. H., Emanuele E., Emmenegger U., Engedal N., Engelbrecht A.-M., Engelender S., Enserink J. M., Erdmann R., Erenpreisa J., Eri R., Eriksen J. L., Erman A., Escalante R., Eskelinen E.-L., Espert L., Esteban-Martínez L., Evans T. J., Fabri M., Fabrias G., Fabrizi C., Facchiano A., Færgeman N. J., Faggioni A., Fairlie W. D., Fan C., Fan D., Fan J., Fang S., Fanto M., Fanzani A., Farkas T., Faure M., Favier F. B., Fearnhead H., Federici M., Fei E., Felizardo T. C., Feng H., Feng Y., Feng Y., Ferguson T. A., Fernández Á. F., Fernandez-Barrena M. G., Fernandez-Checa J. C., Fernández-López A., Fernandez-Zapico M. E., Feron O., Ferraro E., Ferreira-Halder C. V., Fesus L., Feuer R., Fiesel F. C., Filippi-Chiela E. C., Filomeni G., Fimia G. M., Fingert J. H., Finkbeiner S., Finkel T., Fiorito F., Fisher P. B., Flajolet M., Flamigni F., Florey O., Florio S., Floto R. A., Folini M., Follo C., Fon E. A., Fornai F., Fortunato F., Fraldi A., Franco R., Francois A., François A., Frankel L. B., Fraser I. D. C., Frey N., Freyssenet D. G., Frezza C., Friedman S. L., Frigo D. E., Fu D., Fuentes J. M., Fueyo J., Fujitani Y., Fujiwara Y., Fujiya M., Fukuda M., Fulda S., Fusco C., Gabryel B., Gaestel M., Gailly P., Gajewska M., Galadari S., Galili G., Galindo I., Galindo M. F., Galliciotti G., Galluzzi L., Galluzzi L., Galy V., Gammoh N., Gandy S., Ganesan A. K., Ganesan S., Ganley I. G., Gannagé M., Gao F. B., Gao F., Gao J.-X., García Nannig L., García Véscovi E., Garcia-Macía M., Garcia-Ruiz C., Garg A. D., Garg P. K., Gargini R., Gassen N. C., Gatica D., Gatti E., Gavard J., Gavathiotis E., Ge L., Ge P., Ge S., Gean P.-W., Gelmetti V., Genazzani A. A., Geng J., Genschik P., Gerner L., Gestwicki J. E., Gewirtz D. A., Ghavami S., Ghigo E., Ghosh D., Giammarioli A. M., Giampieri F., Giampietri C., Giatromanolaki A., Gibbings D. J., Gibellini L., Gibson S. B., Ginet V., Giordano A., Giorgini F., Giovannetti E., Girardin S. E., Gispert S., Giuliano S., Gladson C. L., Glavic A., Gleave M., Godefroy N., Gogal R. M. Jr., Gokulan K., Goldman G. H., Goletti D., Goligorsky M. S., Gomes A. V., Gomes L. C., Gomez H., Gomez-Manzano C., Gómez-Sánchez R., Gonçalves D. A. P., Goncu E., Gong Q., Gongora C., Gonzalez C. B., Gonzalez-Alegre P., Gonzalez-Cabo P., González-Polo R. A., Goping I. S., Gorbea C., Gorbunov N. V., Goring D. R., Gorman A. M., Gorski S. M., Goruppi S., Goto-Yamada S., Gotor C., Gottlieb R. A., Gozes I., Gozuacik D., Graba Y., Graef M., Granato G. E., Grant G. D., Grant S., Gravina G. L., Green D. R., Greenhough A., Greenwood M. T., Grimaldi B., Gros F., Grose C., Groulx J.-F., Gruber F., Grumati P., Grune T., Guan J.-L., Guan K.-L., Guerra B., Guillen C., Gulshan K., Gunst J., Guo C., Guo L., Guo M., Guo W., Guo X.-G., Gust A. A., Gustafsson Å. B., Gutierrez E., Gutierrez M. G., Gwak H.-S., Haas A., Haber J. E., Hadano S., Hagedorn M., Hahn D. R., Halayko A. J., Hamacher-Brady A., Hamada K., Hamai A., Hamann A., Hamasaki M., Hamer I., Hamid Q., Hammond E. M., Han F., Han W., Handa J. T., Hanover J. A., Hansen M., Harada M., Harhaji-Trajkovic L., Harper J. W., Harrath A. H., Harris A. L., Harris J., Hasler U., Hasselblatt P., Hasui K., Hawley R. G., Hawley T. S., He C., He C. Y., He F., He G., He R.-R., He X.-H., He Y.-W., He Y.-Y., Heath J. K., Hébert M.-J., Heinzen R. A., Helgason G. V., Hensel M., Henske E. P., Her C., Herman P. K., Hernández A., Hernandez C., Hernández-Tiedra S., Hetz C., Hiesinger P. R., Higaki K., Hilfiker S., Hill B. G., Hill J. A., Hill W. D., Hino K., Hofius D., Hofman P., Höglinger G. U., Höhfeld J., Holz M. K., Hong Y., Hood D. A., Hoozemans J. J. M., Hoppe T., Hsu C., Hsu C.-Y., Hsu L.-C., Hu D., Hu G., Hu H.-M., Hu H., Hu M. C., Hu Y.-C., Hu Z.-W., Hua F., Hua Y., Huang C., Huang H.-L., Huang K.-H., Huang K.-Y., Huang S., Huang S., Huang W. P., Huang Y.-R., Huang Y., Huang Y., Huber T. B., Huebbe P., Huh W.-K., Hulmi J. J., Hur G. M., Hurley J. H., Husak Z., Hussain S. N. A., Hussain S., Hwang J. J., Hwang S., Hwang T. I. S., Ichihara A., Imai Y., Imbriano C., Inomata M., Into T., Iovane V., Iovanna J. L., Iozzo R. V., Ip N. Y., Irazoqui J. E., Iribarren P., Isaka Y., Isakovic A. J., Ischiropoulos H., Isenberg J. S., Ishaq M., Ishida H., Ishii I., Ishmael J. E., Isidoro C., Isobe K.-I., Isono E., Issazadeh-Navikas S., Itahana K., Itakura E., Ivanov A. I., Iyer A. K. V., Izquierdo J. M., Izumi Y., Izzo V., Jäättelä M., Jaber N., Jackson D. J., Jackson W. T., Jacob T. G., Jacques T. S., Jagannath C., Jain A., Jana N. R., Jang B. K., Jani A., Janji B., Jannig P. R., Jansson P. J., Jean S., Jendrach M., Jeon J.-H., Jessen N., Jeung E.-B., Jia K., Jia L., Jiang H., Jiang H., Jiang L., Jiang T., Jiang X., Jiang X., Jiang X., Jiang Y., Jiang Y., Jiménez A., Jin C., Jin H., Jin L., Jin M., Jin S., Jinwal U. K., Jo E.-K., Johansen T., Johnson D. E., Johnson G. V. W., Johnson J. D., Jonasch E., Jones C., Joosten L. A. B., Jordan J., Joseph A.-M., Joseph B., Joubert A. M., Ju D., Ju J., Juan H.-F., Juenemann K., Juhász G., Jung H. S., Jung J. U., Jung Y.-K., Jungbluth H., Justice M. J., Jutten B., Kaakoush N. O., Kaarniranta K., Kaasik A., Kabuta T., Kaeffer B., Kågedal K., Kahana A., Kajimura S., Kakhlon O., Kalia M., Kalvakolanu D. V., Kamada Y., Kambas K., Kaminskyy V. O., Kampinga H. H., Kandouz M., Kang C., Kang R., Kang T. C., Kanki T., Kanneganti T.-D., Kanno H., Kanthasamy A. G., Kantorow M., Kaparakis-Liaskos M., Kapuy O., Karantza V., Karim M. R., Karmakar P., Kaser A., Kaushik S., Kawula T., Kaynar A. M., Ke P.-Y., Ke Z.-J., Kehrl J. H., Keller K. E., Kemper J. K., Kenworthy A. K., Kepp O., Kern A., Kesari S., Kessel D., Ketteler R., Kettelhut I. C., Khambu B., Khan M. M., Khandelwal V. K. M., Khare S., Kiang J. G., Kiger A. A., Kihara A., Kim A. L., Kim C. H., Kim D. R., Kim D.-H., Kim E. K., Kim H. Y., Kim H.-R., Kim J.-S., Kim J. H., Kim J. C., Kim J. H., Kim K. W., Kim M. D., Kim M.-M., Kim P. K., Kim S. W., Kim S.-Y., Kim Y.-S., Kim Y., Kimchi A., Kimmelman A. C., Kimura T., King J. S., Kirkegaard K., Kirkin V., Kirshenbaum L. A., Kishi S., Kitajima Y., Kitamoto K., Kitaoka Y., Kitazato K., Kley R. A., Klimecki W. T., Klinkenberg M., Klucken J., Knævelsrud H., Knecht E., Knuppertz L., Ko J.-L., Kobayashi S., Koch J. C., Koechlin-Ramonatxo C., Koenig U., Koh Y. H., Köhler K., Kohlwein S. D., Koike M., Komatsu M., Kominami E., Kong D., Kong H. J., Konstantakou E. G., Kopp B. T., Korcsmaros T., Korhonen L., Korolchuk V. I., Koshkina N. V., Kou Y., Koukourakis M. I., Koumenis C., Kovács A. L., Kovács T., Kovacs W. J., Koya D., Kraft C., Krainc D., Kramer H., Kravic-Stevovic T., Krek W., Kretz-Remy C., Krick R., Krishnamurthy M., Kriston-Vizi J., Kroemer G., Kruer M. C., Kruger R., Ktistakis N. T., Kuchitsu K., Kuhn C., Kumar A. P., Kumar A., Kumar A., Kumar D., Kumar D., Kumar R., Kumar S., Kundu M., Kung H.-J., Kuno A., Kuo S.-H., Kuret J., Kurz T., Kwok T., Kwon T. K., Kwon Y. T., Kyrmizi I., la Spada A. R., Lafont F., Lahm T., Lakkaraju A., Lam T., Lamark T., Lancel S., Landowski T. H., Lane D. J. R., Lane J. D., Lanzi C., Lapaquette P., Lapierre L. R., Laporte J., Laukkarinen J., Laurie G. W., Lavandero S., Lavie L., LaVoie M. J., Law B. Y. K., Law H. K. W., Law K. B., Layfield R., Lazo P. A., le Cam L., le Roch K. G., le Stunff H., Leardkamolkarn V., Lecuit M., Lee B.-H., Lee C.-H., Lee E. F., Lee G. M., Lee H.-J., Lee H., Lee J. K., Lee J., Lee J.-H., Lee J. H., Lee M., Lee M. S., Lee P. J., Lee S. W., Lee S.-J., Lee S.-J., Lee S. Y., Lee S. H., Lee S. S., Lee S.-J., Lee S., Lee Y.-R., Lee Y. J., Lee Y. H., Leeuwenburgh C., Lefort S., Legouis R., Lei J., Lei Q.-Y., Leib D. A., Leibowitz G., Lekli I., Lemaire S. D., Lemasters J. J., Lemberg M. K., Lemoine A., Leng S., Lenz G., Lenzi P., Lerman L. O., Lettieri Barbato D., Leu J. I. J., Leung H. Y., Levine B., Lewis P. A., Lezoualc’h F., Li C., Li F., Li F.-J., Li J., Li K., Li L., Li M., Li M., Li Q., Li R., Li S., Li W., Li W., Li X., Li Y., Lian J., Liang C., Liang Q., Liao Y., Liberal J., Liberski P. P., Lie P., Lieberman A. P., Lim H. J., Lim K.-L., Lim K., Lima R. T., Lin C.-S., Lin C.-F., Lin F., Lin F., Lin F.-C., Lin K., Lin K.-H., Lin P.-H., Lin T., Lin W.-W., Lin Y.-S., Lin Y., Linden R., Lindholm D., Lindqvist L. M., Lingor P., Linkermann A., Liotta L. A., Lipinski M. M., Lira V. A., Lisanti M. P., Liton P. B., Liu B., Liu C., Liu C.-F., Liu F., Liu H.-J., Liu J., Liu J.-J., Liu J.-L., Liu K., Liu L., Liu L., Liu Q., Liu R.-Y., Liu S., Liu S., Liu W., Liu X.-D., Liu X., Liu X.-H., Liu X., Liu X., Liu X., Liu Y., Liu Y., Liu Z., Liu Z., Liuzzi J. P., Lizard G., Ljujic M., Lodhi I. J., Logue S. E., Lokeshwar B. L., Long Y. C., Lonial S., Loos B., López-Otín C., López-Vicario C., Lorente M., Lorenzi P. L., Lõrincz P., Los M., Lotze M. T., Lovat P. E., Lu B., Lu B., Lu J., Lu Q., Lu S. M., Lu S., Lu Y., Luciano F., Luckhart S., Lucocq J. M., Ludovico P., Lugea A., Lukacs N. W., Lum J. J., Lund A. H., Luo H., Luo J., Luo S., Luparello C., Lyons T., Ma J., Ma Y., Ma Y., Ma Z., Machado J., Machado-Santelli G. M., Macian F., MacIntosh G. C., MacKeigan J. P., Macleod K. F., MacMicking J. D., MacMillan-Crow L. A., Madeo F., Madesh M., Madrigal-Matute J., Maeda A., Maeda T., Maegawa G., Maellaro E., Maes H., Magariños M., Maiese K., Maiti T. K., Maiuri L., Maiuri M. C., Maki C. G., Malli R., Malorni W., Maloyan A., Mami-Chouaib F., Man N., Mancias J. D., Mandelkow E. M., Mandell M. A., Manfredi A. A., Manié S. N., Manzoni C., Mao K., Mao Z., Mao Z. W., Marambaud P., Marconi A. M., Marelja Z., Marfe G., Margeta M., Margittai E., Mari M., Mariani F. V., Marin C., Marinelli S., Mariño G., Markovic I., Marquez R., Martelli A. M., Martens S., Martin K. R., Martin S. J., Martin S., Martin-Acebes M. A., Martín-Sanz P., Martinand-Mari C., Martinet W., Martinez J., Martinez-Lopez N., Martinez-Outschoorn U., Martínez-Velázquez M., Martinez-Vicente M., Martins W. K., Mashima H., Mastrianni J. A., Matarese G., Matarrese P., Mateo R., Matoba S., Matsumoto N., Matsushita T., Matsuura A., Matsuzawa T., Mattson M. P., Matus S., Maugeri N., Mauvezin C., Mayer A., Maysinger D., Mazzolini G. D., McBrayer M. K., McCall K., McCormick C., McInerney G. M., McIver S. C., McKenna S., McMahon J. J., McNeish I. A., Mechta-Grigoriou F., Medema J. P., Medina D. L., Megyeri K., Mehrpour M., Mehta J. L., Mei Y., Meier U. C., Meijer A. J., Meléndez A., Melino G., Melino S., de Melo E. J. T., Mena M. A., Meneghini M. D., Menendez J. A., Menezes R., Meng L., Meng L.-H., Meng S., Menghini R., Menko A. S., Menna-Barreto R. F. S., Menon M. B., Meraz-Ríos M. A., Merla G., Merlini L., Merlot A. M., Meryk A., Meschini S., Meyer J. N., Mi M.-T., Miao C.-Y., Micale L., Michaeli S., Michiels C., Migliaccio A. R., Mihailidou A. S., Mijaljica D., Mikoshiba K., Milan E., Miller-Fleming L., Mills G. B., Mills I. G., Minakaki G., Minassian B. A., Ming X.-F., Minibayeva F., Minina E. A., Mintern J. D., Minucci S., Miranda-Vizuete A., Mitchell C. H., Miyamoto S., Miyazawa K., Mizushima N., Mnich K., Mograbi B., Mohseni S., Moita L. F., Molinari M., Molinari M., Møller A. B., Mollereau B., Mollinedo F., Mongillo M., Monick M. M., Montagnaro S., Montell C., Moore D. J., Moore M. N., Mora-Rodriguez R., Moreira P. I., Morel E., Morelli M. B., Moreno S., Morgan M. J., Moris A., Moriyasu Y., Morrison J. L., Morrison L. A., Morselli E., Moscat J., Moseley P. L., Mostowy S., Motori E., Mottet D., Mottram J. C., Moussa C. E. H., Mpakou V. E., Mukhtar H., Mulcahy Levy J. M., Muller S., Muñoz-Moreno R., Muñoz-Pinedo C., Münz C., Murphy M. E., Murray J. T., Murthy A., Mysorekar I. U., Nabi I. R., Nabissi M., Nader G. A., Nagahara Y., Nagai Y., Nagata K., Nagelkerke A., Nagy P., Naidu S. R., Nair S., Nakano H., Nakatogawa H., Nanjundan M., Napolitano G., Naqvi N. I., Nardacci R., Narendra D. P., Narita M., Nascimbeni A. C., Natarajan R., Navegantes L. C., Nawrocki S. T., Nazarko T. Y., Nazarko V. Y., Neill T., Neri L. M., Netea M. G., Netea-Maier R. T., Neves B. M., Ney P. A., Nezis I. P., Nguyen H. T. T., Nguyen H. P., Nicot A.-S., Nilsen H., Nilsson P., Nishimura M., Nishino I., Niso-Santano M., Niu H., Nixon R. A., Njar V. C. O., Noda T., Noegel A. A., Nolte E. M., Norberg E., Norga K. K., Noureini S. K., Notomi S., Notterpek L., Nowikovsky K., Nukina N., Nürnberger T., O’Donnell V. B., O’Donovan T., O’Dwyer P. J., Oehme I., Oeste C. L., Ogawa M., Ogretmen B., Ogura Y., Oh Y. J., Ohmuraya M., Ohshima T., Ojha R., Okamoto K., Okazaki T., Oliver F. J., Ollinger K., Olsson S., Orban D. P., Ordonez P., Orhon I., Orosz L., O’Rourke E. J., Orozco H., Ortega A. L., Ortona E., Osellame L. D., Oshima J., Oshima S., Osiewacz H. D., Otomo T., Otsu K., Ou J.-H. J., Outeiro T. F., Ouyang D. Y., Ouyang H., Overholtzer M., Ozbun M. A., Ozdinler P. H., Ozpolat B., Pacelli C., Paganetti P., Page G., Pages G., Pagnini U., Pajak B., Pak S. C., Pakos-Zebrucka K., Pakpour N., Palková Z., Palladino F., Pallauf K., Pallet N., Palmieri M., Paludan S. R., Palumbo C., Palumbo S., Pampliega O., Pan H., Pan W., Panaretakis T., Pandey A., Pantazopoulou A., Papackova Z., Papademetrio D. L., Papassideri I., Papini A., Parajuli N., Pardo J., Parekh V. V., Parenti G., Park J.-I., Park J., Park O. K., Parker R., Parlato R., Parys J. B., Parzych K. R., Pasquet J.-M., Pasquier B., Pasumarthi K. B. S., Patschan D., Patterson C., Pattingre S., Pattison S., Pause A., Pavenstädt H., Pavone F., Pedrozo Z., Peña F. J., Peñalva M. A., Pende M., Peng J., Penna F., Penninger J. M., Pensalfini A., Pepe S., Pereira G. J. S., Pereira P. C., Pérez-de la Cruz V., Pérez-Pérez M. E., Pérez-Rodríguez D., Pérez-Sala D., Perier C., Perl A., Perlmutter D. H., Perrotta I., Pervaiz S., Pesonen M., Pessin J. E., Peters G. J., Petersen M., Petrache I., Petrof B. J., Petrovski G., Phang J. M., Piacentini M., Pierdominici M., Pierre P., Pierrefite-Carle V., Pietrocola F., Pimentel-Muiños F. X., Pinar M., Pineda B., Pinkas-Kramarski R., Pinti M., Pinton P., Piperdi B., Piret J. M., Platanias L. C., Platta H. W., Plowey E. D., Pöggeler S., Poirot M., Polčic P., Poletti A., Poon A. H., Popelka H., Popova B., Poprawa I., Poulose S. M., Poulton J., Powers S. K., Powers T., Pozuelo-Rubio M., Prak K., Prange R., Prescott M., Priault M., Prince S., Proia R. L., Proikas-Cezanne T., Prokisch H., Promponas V. J., Przyklenk K., Puertollano R., Pugazhenthi S., Puglielli L., Pujol A., Puyal J., Pyeon D., Qi X., Qian W.-B., Qin Z.-H., Qiu Y., Qu Z., Quadrilatero J., Quinn F., Raben N., Rabinowich H., Radogna F., Ragusa M. J., Rahmani M., Raina K., Ramanadham S., Ramesh R., Rami A., Randall-Demllo S., Randow F., Rao H., Rao V. A., Rasmussen B. B., Rasse T. M., Ratovitski E. A., Rautou P.-E., Ray S. K., Razani B., Reed B. H., Reggiori F., Rehm M., Reichert A. S., Rein T., Reiner D. J., Reits E., Ren J., Ren X., Renna M., Reusch J. E. B., Revuelta J. L., Reyes L., Rezaie A. R., Richards R. I., Richardson D. R., Richetta C., Riehle M. A., Rihn B. H., Rikihisa Y., Riley B. E., Rimbach G., Rippo M. R., Ritis K., Rizzi F., Rizzo E., Roach P. J., Robbins J., Roberge M., Roca G., Roccheri M. C., Rocha S., Rodrigues C. M. P., Rodríguez C. I., de Cordoba S. R., Rodriguez-Muela N., Roelofs J., Rogov V. V., Rohn T. T., Rohrer B., Romanelli D., Romani L., Romano P. S., Roncero M. I. G., Rosa J. L., Rosello A., Rosen K. V., Rosenstiel P., Rost-Roszkowska M., Roth K. A., Roué G., Rouis M., Rouschop K. M., Ruan D. T., Ruano D., Rubinsztein D. C., Rucker E. B. III, Rudich A., Rudolf E., Rudolf R., Ruegg M. A., Ruiz-Roldan C., Ruparelia A. A., Rusmini P., Russ D. W., Russo G. L., Russo G., Russo R., Rusten T. E., Ryabovol V., Ryan K. M., Ryter S. W., Sabatini D. M., Sacher M., Sachse C., Sack M. N., Sadoshima J., Saftig P., Sagi-Eisenberg R., Sahni S., Saikumar P., Saito T., Saitoh T., Sakakura K., Sakoh-Nakatogawa M., Sakuraba Y., Salazar-Roa M., Salomoni P., Saluja A. K., Salvaterra P. M., Salvioli R., Samali A., Sanchez A. M. J., Sánchez-Alcázar J. A., Sanchez-Prieto R., Sandri M., Sanjuan M. A., Santaguida S., Santambrogio L., Santoni G., dos Santos C. N., Saran S., Sardiello M., Sargent G., Sarkar P., Sarkar S., Sarrias M. R., Sarwal M. M., Sasakawa C., Sasaki M., Sass M., Sato K., Sato M., Satriano J., Savaraj N., Saveljeva S., Schaefer L., Schaible U. E., Scharl M., Schatzl H. M., Schekman R., Scheper W., Schiavi A., Schipper H. M., Schmeisser H., Schmidt J., Schmitz I., Schneider B. E., Schneider E. M., Schneider J. L., Schon E. A., Schönenberger M. J., Schönthal A. H., Schorderet D. F., Schröder B., Schuck S., Schulze R. J., Schwarten M., Schwarz T. L., Sciarretta S., Scotto K., Scovassi A. I., Screaton R. A., Screen M., Seca H., Sedej S., Segatori L., Segev N., Seglen P. O., Seguí-Simarro J. M., Segura-Aguilar J., Seki E., Seiliez I., Sell C., Semenkovich C. F., Semenza G. L., Sen U., Serra A. L., Serrano-Puebla A., Sesaki H., Setoguchi T., Settembre C., Shacka J. J., Shajahan-Haq A. N., Shapiro I. M., Sharma S., She H., Shen C.-K. J., Shen C.-C., Shen H.-M., Shen S., Shen W., Sheng R., Sheng X., Sheng Z.-H., Shepherd T. G., Shi J., Shi Q., Shi Q., Shi Y., Shibutani S., Shibuya K., Shidoji Y., Shieh J.-J., Shih C. M., Shimada Y., Shimizu S., Shin D. W., Shinohara M. L., Shintani M., Shintani T., Shioi T., Shirabe K., Shiri-Sverdlov R., Shirihai O., Shore G. C., Shu C.-W., Shukla D., Sibirny A. A., Sica V., Sigurdson C. J., Sigurdsson E. M., Sijwali P. S., Sikorska B., Silveira W. A., Silvente-Poirot S., Silverman G. A., Simak J., Simmet T., Simon A. K., Simon H.-U., Simone C., Simons M., Simonsen A., Singh R., Singh S. V., Singh S. K., Sinha D., Sinha S., Sinicrope F. A., Sirko A., Sirohi K., Sishi B. J. N., Sittler A., Siu P. M., Sivridis E., Skwarska A., Slack R., Slaninová I., Slavov N., Smaili S. S., Smalley K. S. M., Smith D. R., Soenen S. J., Soleimanpour S. A., Solhaug A., Somasundaram K., Son J. H., Sonawane A., Song C., Song F., Song H. K., Song J.-X., Song W., Soo K. Y., Sood A. K., Soong T. W., Soontornniyomkij V., Sorice M., Sotgia F., Soto-Pantoja D. R., Sotthibundhu A., Sousa M. J., Spaink H. P., Span P. N., Spang A., Sparks J. D., Speck P. G., Spector S. A., Spies C. D., Springer W., Clair D. S., Stacchiotti A., Staels B., Stang M. T., Starczynowski D. T., Starokadomskyy P., Steegborn C., Steele J. W., Stefanis L., Steffan J., Stellrecht C. M., Stenmark H., Stepkowski T. M., Stern S. T., Stevens C., Stockwell B. R., Stoka V., Storchova Z., Stork B., Stratoulias V., Stravopodis D. J., Strnad P., Strohecker A. M., Ström A.-L., Stromhaug P., Stulik J., Su Y.-X., Su Z., Subauste C. S., Subramaniam S., Sue C. M., Suh S. W., Sui X., Sukseree S., Sulzer D., Sun F.-L., Sun J., Sun J., Sun S.-Y., Sun Y., Sun Y., Sun Y., Sundaramoorthy V., Sung J., Suzuki H., Suzuki K., Suzuki N., Suzuki T., Suzuki Y. J., Swanson M. S., Swanton C., Swärd K., Swarup G., Sweeney S. T., Sylvester P. W., Szatmari Z., Szegezdi E., Szlosarek P. W., Taegtmeyer H., Tafani M., Taillebourg E., Tait S. W. G., Takacs-Vellai K., Takahashi Y., Takáts S., Takemura G., Takigawa N., Talbot N. J., Tamagno E., Tamburini J., Tan C.-P., Tan L., Tan M. L., Tan M., Tan Y.-J., Tanaka K., Tanaka M., Tang D., Tang D., Tang G., Tanida I., Tanji K., Tannous B. A., Tapia J. A., Tasset-Cuevas I., Tatar M., Tavassoly I., Tavernarakis N., Taylor A., Taylor G. S., Taylor G. A., Taylor J. P., Taylor M. J., Tchetina E. V., Tee A. R., Teixeira-Clerc F., Telang S., Tencomnao T., Teng B.-B., Teng R.-J., Terro F., Tettamanti G., Theiss A. L., Theron A. E., Thomas K. J., Thomé M. P., Thomes P. G., Thorburn A., Thorner J., Thum T., Thumm M., Thurston T. L. M., Tian L., Till A., Ting J. P. Y., Titorenko V. I., Toker L., Toldo S., Tooze S. A., Topisirovic I., Torgersen M. L., Torosantucci L., Torriglia A., Torrisi M. R., Tournier C., Towns R., Trajkovic V., Travassos L. H., Triola G., Tripathi D. N., Trisciuoglio D., Troncoso R., Trougakos I. P., Truttmann A. C., Tsai K.-J., Tschan M. P., Tseng Y.-H., Tsukuba T., Tsung A., Tsvetkov A. S., Tu S., Tuan H.-Y., Tucci M., Tumbarello D. A., Turk B., Turk V., Turner R. F. B., Tveita A. A., Tyagi S. C., Ubukata M., Uchiyama Y., Udelnow A., Ueno T., Umekawa M., Umemiya-Shirafuji R., Underwood B. R., Ungermann C., Ureshino R. P., Ushioda R., Uversky V. N., Uzcátegui N. L., Vaccari T., Vaccaro M. I., Váchová L., Vakifahmetoglu-Norberg H., Valdor R., Valente E. M., Vallette F., Valverde A. M., van den Berghe G., van den Bosch L., van den Brink G. R., van der Goot F. G., van der Klei I. J., van der Laan L. J. W., van Doorn W. G., van Egmond M., van Golen K. L., van Kaer L., van Lookeren Campagne M., Vandenabeele P., Vandenberghe W., Vanhorebeek I., Varela-Nieto I., Vasconcelos M. H., Vasko R., Vavvas D. G., Vega-Naredo I., Velasco G., Velentzas A. D., Velentzas P. D., Vellai T., Vellenga E., Vendelbo M. H., Venkatachalam K., Ventura N., Ventura S., Veras P. S. T., Verdier M., Vertessy B. G., Viale A., Vidal M., Vieira H. L. A., Vierstra R. D., Vigneswaran N., Vij N., Vila M., Villar M., Villar V. H., Villarroya J., Vindis C., Viola G., Viscomi M. T., Vitale G., Vogl D. T., Voitsekhovskaja O. V., von Haefen C., von Schwarzenberg K., Voth D. E., Vouret-Craviari V., Vuori K., Vyas J. M., Waeber C., Walker C. L., Walker M. J., Walter J., Wan L., Wan X., Wang B., Wang C., Wang C.-Y., Wang C., Wang C., Wang C., Wang D., Wang F., Wang F., Wang G., Wang H.-J., Wang H., Wang H.-G., Wang H., Wang H.-D., Wang J., Wang J., Wang M., Wang M.-Q., Wang P.-Y., Wang P., Wang R. C., Wang S., Wang T.-F., Wang X., Wang X.-J., Wang X.-W., Wang X., Wang X., Wang Y., Wang Y., Wang Y., Wang Y.-J., Wang Y., Wang Y., Wang Y. T., Wang Y., Wang Z.-N., Wappner P., Ward C., Ward D. M. V., Warnes G., Watada H., Watanabe Y., Watase K., Weaver T. E., Weekes C. D., Wei J., Weide T., Weihl C. C., Weindl G., Weis S. N., Wen L., Wen X., Wen Y., Westermann B., Weyand C. M., White A. R., White E., Whitton J. L., Whitworth A. J., Wiels J., Wild F., Wildenberg M. E., Wileman T., Wilkinson D. S., Wilkinson S., Willbold D., Williams C., Williams K., Williamson P. R., Winklhofer K. F., Witkin S. S., Wohlgemuth S. E., Wollert T., Wolvetang E. J., Wong E., Wong G. W., Wong R. W., Wong V. K. W., Woodcock E. A., Wright K. L., Wu C., Wu D., Wu G. S., Wu J., Wu J., Wu M., Wu M., Wu S., Wu W. K. K., Wu Y., Wu Z., Xavier C. P. R., Xavier R. J., Xia G.-X., Xia T., Xia W., Xia Y., Xiao H., Xiao J., Xiao S., Xiao W., Xie C.-M., Xie Z., Xie Z., Xilouri M., Xiong Y., Xu C., Xu C., Xu F., Xu H., Xu H., Xu J., Xu J., Xu J., Xu L., Xu X., Xu Y., Xu Y., Xu Z.-X., Xu Z., Xue Y., Yamada T., Yamamoto A., Yamanaka K., Yamashina S., Yamashiro S., Yan B., Yan B., Yan X., Yan Z., Yanagi Y., Yang D.-S., Yang J.-M., Yang L., Yang M., Yang P.-M., Yang P., Yang Q., Yang W., Yang W. Y., Yang X., Yang Y., Yang Y., Yang Z., Yang Z., Yao M.-C., Yao P. J., Yao X., Yao Z., Yao Z., Yasui L. S., Ye M., Yedvobnick B., Yeganeh B., Yeh E. S., Yeyati P. L., Yi F., Yi L., Yin X.-M., Yip C. K., Yoo Y.-M., Yoo Y. H., Yoon S.-Y., Yoshida K.-I., Yoshimori T., Young K. H., Yu H., Yu J. J., Yu J.-T., Yu J., Yu L., Yu W. H., Yu X.-F., Yu Z., Yuan J., Yuan Z.-M., Yue B. Y. J. T., Yue J., Yue Z., Zacks D. N., Zacksenhaus E., Zaffaroni N., Zaglia T., Zakeri Z., Zecchini V., Zeng J., Zeng M., Zeng Q., Zervos A. S., Zhang D. D., Zhang F., Zhang G., Zhang G.-C., Zhang H., Zhang H., Zhang H., Zhang H., Zhang J., Zhang J., Zhang J., Zhang J., Zhang J.-P., Zhang L., Zhang L., Zhang L., Zhang L., Zhang M.-Y., Zhang X., Zhang X. D., Zhang Y., Zhang Y., Zhang Y., Zhang Y., Zhang Y., Zhao M., Zhao W.-L., Zhao X., Zhao Y. G., Zhao Y., Zhao Y., Zhao Y.-X., Zhao Z., Zhao Z. J., Zheng D., Zheng X.-L., Zheng X., Zhivotovsky B., Zhong Q., Zhou G.-Z., Zhou G., Zhou H., Zhou S.-F., Zhou X.-J., Zhu H., Zhu H., Zhu W.-G., Zhu W., Zhu X.-F., Zhu Y., Zhuang S.-M., Zhuang X., Ziparo E., Zois C. E., Zoladek T., Zong W.-X., Zorzano A., Zughaier S. M., Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12, 1–222 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mizushima N., Yoshimori T., How to interpret LC3 immunoblotting. Autophagy 3, 542–545 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Kirisako T., Ichimura Y., Okada H., Kabeya Y., Mizushima N., Yoshimori T., Ohsumi M., Takao T., Noda T., Ohsumi Y., The reversible modification regulates the membrane-binding state of Apg8/Aut7 essential for autophagy and the cytoplasm to vacuole targeting pathway. J. Cell Biol. 151, 263–276 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sou Y. S., Tanida I., Komatsu M., Ueno T., Kominami E., Phosphatidylserine in addition to phosphatidylethanolamine is an in vitro target of the mammalian Atg8 modifiers, LC3, GABARAP, and GATE-16. J. Biol. Chem. 281, 3017–3024 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Fairn G. D., Schieber N. L., Ariotti N., Murphy S., Kuerschner L., Webb R. I., Grinstein S., Parton R. G., High-resolution mapping reveals topologically distinct cellular pools of phosphatidylserine. J. Cell Biol. 194, 257–275 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schütter M., Giavalisco P., Brodesser S., Graef M., Local fatty acid channeling into phospholipid synthesis drives phagophore expansion during autophagy. Cell 180, 135–149.e14 (2020). [DOI] [PubMed] [Google Scholar]
- 42.Nakatogawa H., Oh-oka K., Ohsumi Y., Lipidation of Atg8: How is substrate specificity determined without a canonical E3 enzyme? Autophagy 4, 911–913 (2008). [DOI] [PubMed] [Google Scholar]
- 43.Oh-oka K., Nakatogawa H., Ohsumi Y., Physiological pH and acidic phospholipids contribute to substrate specificity in lipidation of Atg8. J. Biol. Chem. 283, 21847–21852 (2008). [DOI] [PubMed] [Google Scholar]
- 44.Collins M. P., Forgac M., Regulation and function of V-ATPases in physiology and disease. Biochim. Biophys. Acta Biomembr. 1862, 183341 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamamoto A., Tagawa Y., Yoshimori T., Moriyama Y., Masaki R., Tashiro Y., Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct. Funct. 23, 33–42 (1998). [DOI] [PubMed] [Google Scholar]
- 46.Florey O., Gammoh N., Kim S. E., Jiang X., Overholtzer M., V-ATPase and osmotic imbalances activate endolysosomal LC3 lipidation. Autophagy 11, 88–99 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jacquin E., Leclerc-Mercier S., Judon C., Blanchard E., Fraitag S., Florey O., Pharmacological modulators of autophagy activate a parallel noncanonical pathway driving unconventional LC3 lipidation. Autophagy 13, 854–867 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Freeman S. A., Grinstein S., Phagocytosis: Receptors, signal integration, and the cytoskeleton. Immunol. Rev. 262, 193–215 (2014). [DOI] [PubMed] [Google Scholar]
- 49.Yin C., Heit B., Cellular responses to the efferocytosis of apoptotic cells. Front. Immunol. 12, 631714 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martinez J., Almendinger J., Oberst A., Ness R., Dillon C. P., Fitzgerald P., Hengartner M. O., Green D. R., Microtubule-associated protein 1 light chain 3 alpha (LC3)-associated phagocytosis is required for the efficient clearance of dead cells. Proc. Natl. Acad. Sci. U.S.A. 108, 17396–17401 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Gluschko A., Herb M., Wiegmann K., Krut O., Neiss W. F., Utermöhlen O., Krönke M., Schramm M., The β2 integrin Mac-1 induces protective LC3-associated phagocytosis of Listeria monocytogenes. Cell Host Microbe 23, 324–337.e325 (2018). [DOI] [PubMed] [Google Scholar]
- 52.Hubber A., Kubori T., Coban C., Matsuzawa T., Ogawa M., Kawabata T., Yoshimori T., Nagai H., Bacterial secretion system skews the fate of Legionella-containing vacuoles towards LC3-associated phagocytosis. Sci. Rep. 7, 44795 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Inomata M., Xu S., Chandra P., Meydani S. N., Takemura G., Philips J. A., Leong J. M., Macrophage LC3-associated phagocytosis is an immune defense against Streptococcus pneumoniae that diminishes with host aging. Proc. Natl. Acad. Sci. U.S.A. 117, 33561–33569 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Köster S., Upadhyay S., Chandra P., Papavinasasundaram K., Yang G., Hassan A., Grigsby S. J., Mittal E., Park H. S., Jones V., Hsu F.-F., Jackson M., Sassetti C. M., Philips J. A., Mycobacterium tuberculosis is protected from NADPH oxidase and LC3-associated phagocytosis by the LCP protein CpsA. Proc. Natl. Acad. Sci. U.S.A. 114, E8711–E8720 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim S. E., Zhang J., Jiang E., Overholtzer M., Amino acids and mechanistic target of rapamycin regulate the fate of live engulfed cells. FASEB J. 35, e21909 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fazeli G., Trinkwalder M., Irmisch L., Wehman A. M., C. elegans midbodies are released, phagocytosed and undergo LC3-dependent degradation independent of macroautophagy. J. Cell Sci. 129, 3721–3731 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levine B., Mizushima N., Virgin H. W., Autophagy in immunity and inflammation. Nature 469, 323–335 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Upadhyay S., Philips J. A., LC3-associated phagocytosis: Host defense and microbial response. Curr. Opin. Immunol. 60, 81–90 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Commisso C., Davidson S. M., Soydaner-Azeloglu R. G., Parker S. J., Kamphorst J. J., Hackett S., Grabocka E., Nofal M., Drebin J. A., Thompson C. B., Rabinowitz J. D., Metallo C. M., Vander Heiden M. G., Bar-Sagi D., Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 497, 633–637 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams T. D., Kay R. R., The physiological regulation of macropinocytosis during Dictyostelium growth and development. J. Cell Sci. 131, jcs213736 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Doodnauth S. A., Grinstein S., Maxson M. E., Constitutive and stimulated macropinocytosis in macrophages: Roles in immunity and in the pathogenesis of atherosclerosis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 374, 20180147 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sønder S. L., Häger S. C., Heitmann A. S. B., Frankel L. B., Dias C., Simonsen A. C., Nylandsted J., Restructuring of the plasma membrane upon damage by LC3-associated macropinocytosis. Sci. Adv. 7, eabg1969 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Florey O., Overholtzer M., Macropinocytosis and autophagy crosstalk in nutrient scavenging. Philos. Trans. R. Soc. Lond. B Biol. Sci. 374, 20180154 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kumari S., Mg S., Mayor S., Endocytosis unplugged: Multiple ways to enter the cell. Cell Res. 20, 256–275 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heckmann B. L., Teubner B. J. W., Tummers B., Boada-Romero E., Harris L., Yang M., Guy C. S., Zakharenko S. S., Green D. R., LC3-associated endocytosis facilitates β-amyloid clearance and mitigates neurodegeneration in murine Alzheimer’s disease. Cell 178, 536–551.e14 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barrow-McGee R., Kishi N., Joffre C., Ménard L., Hervieu A., Bakhouche B. A., Noval A. J., Mai A., Guzmán C., Robbez-Masson L., Iturrioz X., Hulit J., Brennan C. H., Hart I. R., Parker P. J., Ivaska J., Kermorgant S., Beta 1-integrin-c-Met cooperation reveals an inside-in survival signalling on autophagy-related endomembranes. Nat. Commun. 7, 11942 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Overholtzer M., Mailleux A. A., Mouneimne G., Normand G., Schnitt S. J., King R. W., Cibas E. S., Brugge J. S., A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell 131, 966–979 (2007). [DOI] [PubMed] [Google Scholar]
- 68.Chen R., Ram A., Albeck J. G., Overholtzer M., Entosis is induced by ultraviolet radiation. iScience 24, 102902 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Durgan J., Tseng Y.-Y., Hamann J. C., Domart M.-C., Collinson L., Hall A., Overholtzer M., Florey O., Mitosis can drive cell cannibalism through entosis. eLife 6, e27134 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hamann J. C., Surcel A., Chen R., Teragawa C., Albeck J. G., Robinson D. N., Overholtzer M., Entosis Is induced by glucose starvation. Cell Rep. 20, 201–210 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krishna S., Palm W., Lee Y., Yang W., Bandyopadhyay U., Xu H., Florey O., Thompson C. B., Overholtzer M., PIKfyve regulates vacuole maturation and nutrient recovery following engulfment. Dev. Cell 38, 536–547 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun Q., Luo T., Ren Y., Florey O., Shirasawa S., Sasazuki T., Robinson D. N., Overholtzer M., Competition between human cells by entosis. Cell Res. 24, 1299–1310 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Duve C., de Barsy T., Poole B., Trouet A., Tulkens P., van Hoof F., Commentary. Lysosomotropic agents. Biochem. Pharmacol. 23, 2495–2531 (1974). [DOI] [PubMed] [Google Scholar]
- 74.Marceau F., Bawolak M.-T., Lodge R., Bouthillier J., Gagné-Henley A., Gaudreault R. C., Morissette G., Cation trapping by cellular acidic compartments: Beyond the concept of lysosomotropic drugs. Toxicol. Appl. Pharmacol. 259, 1–12 (2012). [DOI] [PubMed] [Google Scholar]
- 75.Solomon V. R., Lee H., Chloroquine and its analogs: A new promise of an old drug for effective and safe cancer therapies. Eur. J. Pharmacol. 625, 220–233 (2009). [DOI] [PubMed] [Google Scholar]
- 76.Liu Y., Luo X., Shan H., Fu Y., Gu Q., Zheng X., Dai Q., Xia F., Zheng Z., Liu P., Yin X.-M., Hong L., Li M., Niclosamide triggers non-canonical LC3 lipidation. Cell 8, 248 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gao Y., Liu Y., Hong L., Yang Z., Cai X., Chen X., Fu Y., Lin Y., Wen W., Li S., Liu X., Huang H., Vogt A., Liu P., Yin X.-M., Li M., Golgi-associated LC3 lipidation requires V-ATPase in noncanonical autophagy. Cell Death Dis. 7, e2330 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sieczkarski S. B., Whittaker G. R., Influenza virus can enter and infect cells in the absence of clathrin-mediated endocytosis. J. Virol. 76, 10455–10464 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liang R., Li H., Swanson J. M., Voth G. A., Multiscale simulation reveals a multifaceted mechanism of proton permeation through the influenza A M2 proton channel. Proc. Natl. Acad. Sci. U.S.A. 111, 9396–9401 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shimbo K., Brassard D. L., Lamb R. A., Pinto L. H., Ion selectivity and activation of the M2 ion channel of influenza virus. Biophys. J. 70, 1335–1346 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sakaguchi T., Leser G. P., Lamb R. A., The ion channel activity of the influenza virus M2 protein affects transport through the Golgi apparatus. J. Cell Biol. 133, 733–747 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ulferts R., Marcassa E., Timimi L., Lee L. C., Daley A., Montaner B., Turner S. D., Florey O., Baillie J. K., Beale R., Subtractive CRISPR screen identifies the ATG16L1/vacuolar ATPase axis as required for non-canonical LC3 lipidation. Cell Rep. 37, 109899 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mohamud Y., Shi J., Tang H., Xiang P., Xue Y. C., Liu H., Ng C. S., Luo H., Coxsackievirus infection induces a non-canonical autophagy independent of the ULK and PI3K complexes. Sci. Rep. 10, 19068 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Koepke L., Hirschenberger M., Hayn M., Kirchhoff F., Sparrer K. M., Manipulation of autophagy by SARS-CoV-2 proteins. Autophagy 17, 2659–2661 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rongvaux A., Jackson R., Harman C. C. D., Li T., West A. P., de Zoete M. R., Wu Y., Yordy B., Lakhani S. A., Kuan C.-Y., Taniguchi T., Shadel G. S., Chen Z. J., Iwasaki A., Flavell R. A., Apoptotic caspases prevent the induction of type I interferons by mitochondrial DNA. Cell 159, 1563–1577 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Barber G. N., STING: Infection, inflammation and cancer. Nat. Rev. Immunol. 15, 760–770 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gui X., Yang H., Li T., Tan X., Shi P., Li M., du F., Chen Z. J., Autophagy induction via STING trafficking is a primordial function of the cGAS pathway. Nature 567, 262–266 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu D., Wu H., Wang C., Li Y., Tian H., Siraj S., Sehgal S. A., Wang X., Wang J., Shang Y., Jiang Z., Liu L., Chen Q., STING directly activates autophagy to tune the innate immune response. Cell Death Differ. 26, 1735–1749 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hooper K. M., Jacquin E., Li T., Goodwin J. M., Brumell J. H., Durgan J., Florey O., V-ATPase is a universal regulator of LC3 associated phagocytosis and non-canonical autophagy. J. Cell Biol. 221, e202105112 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Los F. C., Randis T. M., Aroian R. V., Ratner A. J., Role of pore-forming toxins in bacterial infectious diseases. Microbiol. Mol. Biol. Rev. 77, 173–207 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Garner J. A., Cover T. L., Binding and internalization of the Helicobacter pylori vacuolating cytotoxin by epithelial cells. Infect. Immun. 64, 4197–4203 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Genisset C., Puhar A., Calore F., de Bernard M., Dell’Antone P., Montecucco C., The concerted action of the Helicobacter pylori cytotoxin VacA and of the v-ATPase proton pump induces swelling of isolated endosomes. Cell. Microbiol. 9, 1481–1490 (2007). [DOI] [PubMed] [Google Scholar]
- 93.Terebiznik M. R., Raju D., Vázquez C. L., Torbricki K., Kulkarni R., Blanke S. R., Yoshimori T., Colombo M. I., Jones N. L., Effect of Helicobacter pylori’s vacuolating cytotoxin on the autophagy pathway in gastric epithelial cells. Autophagy 5, 370–379 (2009). [DOI] [PubMed] [Google Scholar]
- 94.Meyer-Morse N., Robbins J. R., Rae C. S., Mochegova S. N., Swanson M. S., Zhao Z., Virgin H. W., Portnoy D., Listeriolysin O is necessary and sufficient to induce autophagy during Listeria monocytogenes infection. PLOS ONE 5, e8610 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.López de Armentia M. M., Gauron M. C., Colombo M. I., Staphylococcus aureus alpha-toxin induces the formation of dynamic tubules labeled with LC3 within host cells in a Rab7 and Rab1b-dependent manner. Front. Cell. Infect. Microbiol. 7, 431 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee C., Lamech L., Johns E., Overholtzer M., Selective lysosome membrane turnover is induced by nutrient starvation. Dev. Cell 55, 289–297.e4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Leidal A. M., Huang H. H., Marsh T., Solvik T., Zhang D., Ye J., Kai F.-B., Goldsmith J., Liu J. Y., Huang Y.-H., Monkkonen T., Vlahakis A., Huang E. J., Goodarzi H., Yu L., Wiita A. P., Debnath J., The LC3-conjugation machinery specifies the loading of RNA-binding proteins into extracellular vesicles. Nat. Cell Biol. 22, 187–199 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.DeSelm C. J., Miller B. C., Zou W., Beatty W. L., van Meel E., Takahata Y., Klumperman J., Tooze S. A., Teitelbaum S. L., Virgin H. W., Autophagy proteins regulate the secretory component of osteoclastic bone resorption. Dev. Cell 21, 966–974 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xie X. S., Padron D., Liao X., Wang J., Roth M. G., de Brabander J. K., Salicylihalamide A inhibits the V0 sector of the V-ATPase through a mechanism distinct from bafilomycin A1. J. Biol. Chem. 279, 19755–19763 (2004). [DOI] [PubMed] [Google Scholar]
- 100.Li Y., James S. J., Wyllie D. H., Wynne C., Czibula A., Bukhari A., Pye K., Bte Mustafah S. M., Fajka-Boja R., Szabo E., Angyal A., Hegedus Z., Kovacs L., Hill A. V. S., Jefferies C. A., Wilson H. L., Yongliang Z., Kiss-Toth E., TMEM203 is a binding partner and regulator of STING-mediated inflammatory signaling in macrophages. Proc. Natl. Acad. Sci. U.S.A. 116, 16479–16488 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Levin R., Grinstein S., Schlam D., Phosphoinositides in phagocytosis and macropinocytosis. Biochim. Biophys. Acta 1851, 805–823 (2015). [DOI] [PubMed] [Google Scholar]
- 102.Yang C. S., Lee J.-S., Rodgers M., Min C.-K., Lee J.-Y., Kim H. J., Lee K.-H., Kim C.-J., Oh B., Zandi E., Yue Z., Kramnik I., Liang C., Jung J. U., Autophagy protein Rubicon mediates phagocytic NADPH oxidase activation in response to microbial infection or TLR stimulation. Cell Host Microbe 11, 264–276 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Matsunaga K., Saitoh T., Tabata K., Omori H., Satoh T., Kurotori N., Maejima I., Shirahama-Noda K., Ichimura T., Isobe T., Akira S., Noda T., Yoshimori T., Two Beclin 1-binding proteins, Atg14L and Rubicon, reciprocally regulate autophagy at different stages. Nat. Cell Biol. 11, 385–396 (2009). [DOI] [PubMed] [Google Scholar]
- 104.Huang J., Canadien V., Lam G. Y., Steinberg B. E., Dinauer M. C., Magalhaes M. A. O., Glogauer M., Grinstein S., Brumell J. H., Activation of antibacterial autophagy by NADPH oxidases. Proc. Natl. Acad. Sci. U.S.A. 106, 6226–6231 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.De Faveri F., Chvanov M., Voronina S., Moore D., Pollock L., Haynes L., Awais M., Beckett A. J., Mayer U., Sutton R., Criddle D. N., Prior I. A., Wileman T., Tepikin A. V., LAP-like non-canonical autophagy and evolution of endocytic vacuoles in pancreatic acinar cells. Autophagy 16, 1314–1331 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Westman J., Grinstein S., Determinants of phagosomal pH during host-pathogen interactions. Front. Cell Dev. Biol. 8, 624958 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mantegazza A. R., Savina A., Vermeulen M., Pérez L., Geffner J., Hermine O., Rosenzweig S. D., Faure F., Amigorena S., NADPH oxidase controls phagosomal pH and antigen cross-presentation in human dendritic cells. Blood 112, 4712–4722 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Savina A., Jancic C., Hugues S., Guermonprez P., Vargas P., Moura I. C., Lennon-Duménil A.-M., Seabra M. C., Raposo G., Amigorena S., NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell 126, 205–218 (2006). [DOI] [PubMed] [Google Scholar]
- 109.Ligeon L. A., Pena-Francesch M., Vanoaica L. D., Núñez N. G., Talwar D., Dick T. P., Münz C., Oxidation inhibits autophagy protein deconjugation from phagosomes to sustain MHC class II restricted antigen presentation. Nat. Commun. 12, 1508 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]