Abstract
The specificity of the immune response to the 23-valent pneumococcal-polysaccharide (PS) vaccine in healthy adults and to a pneumococcal conjugate vaccine in infants was examined by measuring immunoglobulin G (IgG) antibody titers by enzyme-linked immunosorbent assay (ELISA) and the opsonophagocytosis assay. ELISA measures total antipneumococcal IgG titers including the titers of functional and nonfunctional antibodies, while the opsonophagocytosis assay measures only functional-antibody titers. Twenty-four pairs of pre- and post-pneumococcal vaccination sera from adults were evaluated (ELISA) for levels of IgG antibodies against serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F. Twelve of the pairs were also examined (opsonophagocytosis assay) for their functional activities. The correlation coefficients between assay results for most types ranged from 0.75 to 0.90, but the correlation coefficient was only about 0.6 for serotypes 4 and 19F. The specificities of these antibodies were further examined by the use of competitive ELISA inhibition. A number of heterologous polysaccharides (types 11A, 12F, 15B, 22F, and 33A) were used as inhibitors. Most of the sera tested showed cross-reacting antibodies, in addition to those removed by pneumococcal C PS absorption. Our data suggest the presence of a common epitope that is found on most pneumococcal PS but that is not absorbed by purified C PS. Use of a heterologous pneumococcal PS (22F) to adsorb the antibodies to the common epitope increased the correlation between the IgG ELISA results and the opsonophagocytosis assay results. The correlation coefficient improve from 0.66 to 0.92 for type 4 and from 0.63 to 0.80 for type 19F. These common-epitope antibodies were largely absent in infants at 7 months of age, suggesting the carbohydrate nature of the epitope.
Streptococcus pneumoniae (pneumococcus) is now the leading cause of invasive bacterial disease in children under the age of 5 years. Although there are 90 different pneumococcal serotypes based upon the presence of chemically and immunologically different capsular polysaccharides (PSs), most pneumococcal disease in young children in many countries is caused by less than 12 of these types (7, 8).
The newly U.S. licensed Wyeth Lederle Vaccines seven-valent pneumococcal conjugate vaccine, called Prevnar, containing PS types 4, 6B, 9V, 14, 18C, 19F, and 23F, was shown to be highly effective in reducing the incidence of invasive pneumococcal disease in infants and children under 2 years of age (2, 16). Other pneumococcal conjugate vaccines are in clinical trials (7). These include the 9- and 11-valent formulations, which add types 1 and 5 and types 1, 3, 5, and 7F, respectively. These vaccines will need to be evaluated for safety and efficacy. The ideal study design for evaluation of the efficacies of these vaccines is determination of the incidence of pneumococcal disease in a vaccinated group against the incidence in a nonvaccinated control group, but such trials may be very difficult. Thus, there is a need for in vitro antibody assays that can strongly predict protective efficacy.
Protection against invasive pneumococcal disease is mediated by the presence of opsonic antibodies (12, 22). Both the pneumococcal PS and conjugate vaccines induce type-specific opsonic antibodies (12, 20). Thus, in vitro measurements of opsonophagocytosis may serve as a surrogate for protection. However, opsonophagocytosis assays are labor-intensive and difficult to perform with large numbers of samples. Thus, many laboratories measure antibody binding only by enzyme-linked immunosorbent assay (ELISA). Since clinical protection against pneumococcal infection is mediated by antibodies to the capsular PS, an ELISA that measures immunoglobulin G (IgG) antibody levels may be used in place of opsonophagocytosis assays, provided that a sufficient correlation between the two assays can be shown.
Antibody concentrations and the role of antibody avidity in protection from pneumococcal infections are unclear, but previous studies with Haemophilus influenzae type b conjugate vaccines have shown that high-avidity antibodies perform better on a weight basis in bactericidal assays and are more protective against experimental infection (11).
The present study focused on two important aspects of an immunological assay: specificity and correlation of binding antibodies to functional activity. Purified capsular PSs contain approximately 5% (by weight) contaminating C PS, some of which is covalently bound to the type-specific PS through a peptidoglycan fragment (19). While children and adults have naturally acquired antibodies to the C PS (10), these antibodies are not opsonic and do not protect against pneumococcal infection (14, 21). Therefore, such antibodies should be blocked so that only the levels of antibodies specific to the C PS are measured (13, 20). Other investigators have shown that pneumococcal C PS preparations may also contain non-C PS contaminants that are immunogenic (18, 23). The studies reported here will show the presence of a common epitope in addition to the C PS shared among several pneumococcal PS types and that antibodies to this common epitope are not absorbed by soluble C PS but are removed by using pneumococcal type 22F PS as a second absorbant. Following removal of these additional antibodies, the resulting IgG concentrations correlate more highly with the titers determined by opsonophagocytosis assays.
MATERIALS AND METHODS
Sera and reagents.
Sera obtained before and after immunization of 12 adults with a 23-valent pneumococcal PS vaccine were provided by David Goldblatt, Institute of Child Health, London, United Kingdom. Sera from healthy adults were from our laboratory's serum bank. Postvaccination sera from infants who had received three doses of a tetravalent (types 6B, 14, 19F, and 23F) pneumococcal conjugate vaccine were kindly provided by Merck & Company, West Point, Pa.
The pneumococcal PSs and HL-60 cells were obtained from the American Type Culture Collection (ATCC), Manassas, Va. C PS was obtained from the State Serum Institute, Copenhagen, Denmark. The alkaline phosphatase-labeled anti-IgG antibodies and substrate tablets were obtained from Sigma Chemical Company, St. Louis, Mo. The complement source for the opsonophagocytosis assays was serum from 3- to 4-week-old rabbits (Pel-Freeze, Brown Deer, Wis.). The Centers for Disease Control and Prevention (Atlanta, Ga.) provided the bacterial strains used in the phagocytosis assays. RPMI 1640, penicillin-streptomycin solution, and Hank's balanced salt solution with and without Ca2+ and Mg2+ were obtained from Life Technologies, Grand Island, N.Y. Fetal calf serum was bought from Hyclone Laboratories, Logan, Utah. Todd-Hewitt broth, yeast extract, Bacto Agar, and gelatin were purchased from Difco, Detroit, Mich.
ELISA.
Optimal concentrations of pneumococcal PS and methylated human serum albumin were determined to obtain the highest specific binding to Immulon no. 1 ELISA plates and ranged from 1.0 to 5.0 μg/ml for the PS and 0.5 to 5.0 μg/ml for methylated human serum albumin (4). Coated plates were then left at room temperature for 6 to 16 h. The ELISA was performed as described previously (4).
Inhibition ELISA.
Competitive inhibition ELISA (6) was performed by diluting serum samples in serum conjugate buffer containing 1 μg of C PS per ml plus various concentrations (0.04 to 2.0 μg/ml) of one of five other pneumococcal PS types, type 11A, 12A, 15B, 22F, and 33F PSs. These PSs are present in the 23-valent vaccine but not in the 7-, 9-, or 11-valent conjugate vaccines currently under investigation (7). These PSs have no known cross-reactivity with other pneumococcal PS serogroups (8). PS type 22F was selected because it gave somewhat better absorption compared to the absorptions for the other pneumococcal PS types tested. The optimal concentration was found to be 1.0 μg/ml for C PS absorption and 2.0 μg/ml for PS type 22F absorption. All the serum samples tested were diluted in serum-conjugate buffer containing C PS and PS type 22F. The remainder of the ELISA procedure was as described previously (4).
Opsonophagocytosis assay.
Cells of the HL-60 cell line, a human continuous lymphoblastic cell line were rather than traditional polymorphonuclear leukocytes used as the effector cells. HL-60 cells undergo granulocytic differentiation upon chemical induction for 7 to 10 days with dimethyl formamide. The procedure followed was that previously described by Romero-Steiner et al. (17). Opsonophagocytosis assay titers were expressed as the reciprocal of the highest serum dilution that killed ≥50% of the organisms compared to the number of organisms in the complement control wells. Serum samples were tested beginning at a dilution of 1:8. Samples with a titer lower than 1:8 were considered negative, and the results are reported as a titer of 1:4 for purposes of data analysis.
Statistical analysis.
Correlation coefficients between the ELISA titers (in micrograms per milliliter) and the log2 of the opsonophagocytosis assay titers were calculated with the Instat computer program. A quadrant method of analysis, as described in the Results section, was also used to compare the assays.
RESULTS
Twelve pairs of serum samples from adults who were vaccinated with the 23-valent pneumococcal PS vaccine were analyzed by ELISA for titers of IgG antibodies against the seven types contained in the seven-valent conjugate vaccine: types 4, 6B, 9V, 14, 18C, 19F, and 23F. These same serum samples were then examined by the opsonophagocytosis assay with HL-60 cells. Correlation coefficients were calculated between the two assays and gave a range from 0.63 to 0.90, as shown in Table 1.
TABLE 1.
Correlation of ELISA and opsonophagocytosis assay for 12 pairs of pre- and postvaccination serum samples from adultsa
| Pneumococcal PS type |
r |
|---|---|
| 4 | 0.66 |
| 6B | 0.75 |
| 9V | 0.78 |
| 14 | 0.88 |
| 18C | 0.84 |
| 19F | 0.63 |
| 23F | 0.90 |
The sera were assayed by ELISA with only C PS absorption.
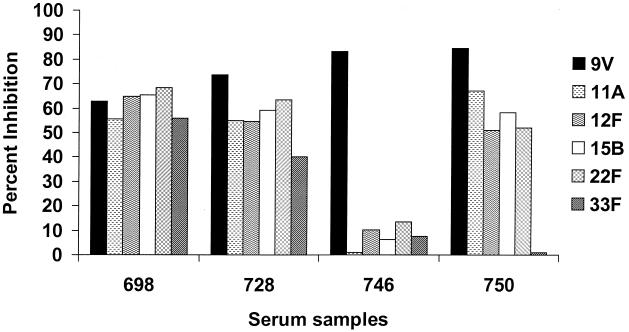
Sera from immunized or nonimmunized adults may have high antibody levels that are not PS specific and that are not absorbed with soluble C PS (18, 23). Sera from four nonimmunized adults were adsorbed with C PS plus the homologous PS type or one of five heterologous PS types and were examined on ELISA plates coated with type 9V PS (Fig. 1). The heterologous PSs were chosen because they are available from ATCC, are not presently included in any pneumococcal conjugate vaccine, and have no shared antigenic cross-reactivity (see Table 6 in reference 7). Adsorption with heterologous pneumococcal PSs reduced antibody binding to the homologous 9V PS. Of the four serum samples, serum sample 746 showed the desired type specificity, being strongly inhibited only by the homologous PS. The other three serum samples were strongly inhibited by all six PSs and thus contained essentially no type 9V-specific antibodies.
FIG. 1.
Competitive ELISA inhibition of pneumococcal PS type 9V antibody with homologous (type 9V) and heterologous (type 11A, 12F, 15B, 22F, and 33F) pneumococcal PSs. The inhibitors were used at 10 μg/ml.
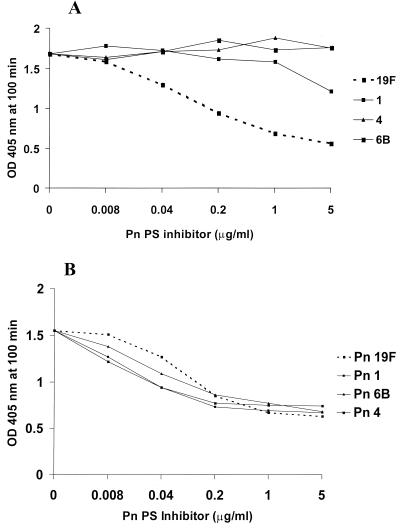
Sera without evident type-specific antibodies are not opsonic. We selected two serum samples with antibody concentrations similar to those for type 19F following C PS absorption; one was strongly opsonic for type 19F and the other was nonopsonic. These two serum samples were then absorbed by the homologous type 19F PS or one of three heterologous pneumococcal PSs and were then examined by ELISA (Fig. 2). Only the type 19F PS removed antibodies to type 19F in the opsonic serum sample, while all four PSs equally absorbed the binding antibody in the nonopsonic serum sample. Thus, antibodies are present to an antigen that is common to these pneumococcal types that are not PS type specific and not opsonic.
FIG. 2.
Specificities of antibodies reactive with pneumococcal type 19F PS following C PS absorption. (A) Results for a serum sample from an adult that had 6.6 μg of type 19F IgG per ml and that was functional by opsonophagocytosis assay. Heterologous pneumococcal PSs of types 1, 4, and 6B were used as inhibitors. The results for the homologous pneumococcal PS, the type 19F PS, is indicated by the broken line. (B) Results for a serum sample from an adult that had 7.7 μg of IgG to type 19F per ml but that was not opsonic for type 19F. OD, optical density; Pn, pneumococcal PS type.
From the data presented in Fig. 1 the pneumococcal type 22F PS was selected for further evaluation as a second absorbent. Assays were performed to determine the appropriate concentration of type 22F PS to be included in the serum dilution buffer. A serum sample with a large proportion of antibodies to the common antigen was used, and the amount of type 22F PS needed to achieve maximal inhibition of antibodies to the common antigen was determined for seven pneumococcal PS types (Fig. 3). Near-optimal inhibition was achieved with about 1 μg of the type 22F PS per ml for six of the types. In contrast, type 22F absorption had no effect on binding of antibody to the type 14 PS. We chose to use 2 μg of the type 22F PS per ml to account for possible lot-to-lot differences in the common cross-reactive component.
FIG. 3.
Determination of optimal type 22F PS concentration for use as an inhibitor. Competitive inhibition with type 22F PS on antibody binding in a serum sample from a healthy adult was done for the seven pneumococcal PS types present in the seven-valent conjugate vaccine. Pn, pneumococcal PS type.
Figure 4 shows the relative effect of C PS absorption compared to that of C PS plus type 22F PS absorption on antibodies measured in a serum sample from a healthy adult against 11 pneumococcal types considered for inclusion in conjugate vaccines. Most of the cross-reactive antibodies were removed by the C PS, with further smaller reductions obtained by the double absorption. The notable exceptions were types 3 and 14, in which type 22F absorption had a minimal effect on the estimated antibody concentrations.
FIG. 4.
Comparative effects of C PS absorption and combined C PS and type 22F PS absorption on a serum sample from an unimmunized adult for 11 pneumococcal types. The C PS and type 22F PS were used at 1 and 2 μg/ml, respectively.
A batch of serotype 22F PS obtained from Merck & Company (Merck & Company supplies the pneumococcal PSs distributed by ATCC) was compared to two other batches of type 22F PS; one was from ATCC and another one was from Wyeth Lederle Vaccines. When present at 2 μg/ml in the serum dilution buffer, all were equally effective at removing the additional antibodies (data not shown).
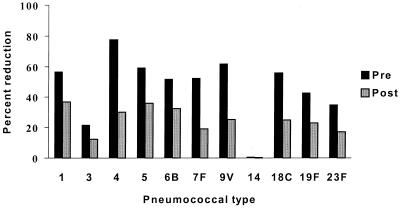
The overall effect of the added type 22F PS absorption on sera from adults pre- and postimmunization with the 23-valent PS vaccine is shown in Fig. 5. The mean percent reduction in antibody binding by type 22F PS for 10 preimmunization serum samples and 18 postimmunization serum samples is shown for 11 pneumococcal types. As expected, the proportion of type-specific antibodies was greater postimmunization. Again, there was no effect of type 22F absorption on antibody binding to type 14 and relatively little effect on antibody binding to type 3. In contrast to adults, infants at 7 months of age have little or no common antigen-reactive antibodies following immunization with a pneumococcal conjugate vaccine (Fig. 6). Data for type 6B are shown, and similar data were obtained for type 23F.
FIG. 5.
Percent reduction of IgG antibody binding in pre- and postvaccination sera following type 22F PS absorption. The mean percent reduction for 10 preimmunization serum samples and 18 postimmunization serum samples are given for 11 pneumococcal types. Percent reduction was calculated as ([IgG] with C PS absorption only − [IgG] with C PS and 22F absorption)/ [IgG] with C PS absorption only × 100.
FIG. 6.
Percent reduction in antibody binding to the type 6B PS by using type 22F PS absorption in postimmunization sera from seven 7-month-old infants. Percent reduction was calculated as described in the legend to Fig. 5.
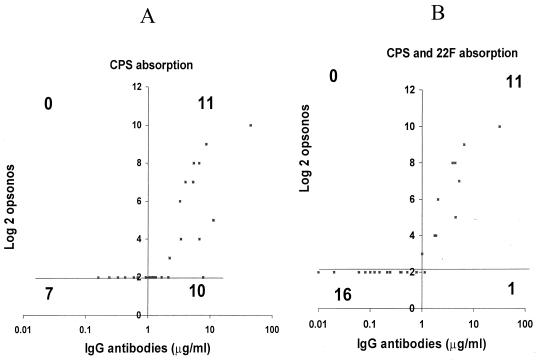
Absorption with C PS plus the type 22F PS removes nonserotype-specific and nonopsonic antibodies, improving the correlation between the IgG antibody concentrations measured by ELISA and the opsonophagocytosis assay titers. This effect is illustrated for types 4 and 19F (Fig. 7 and 8, respectively). Antibody concentrations versus opsonophagocytosis assay titers were examined by a quadrant analysis. For this analysis, the upper left-hand quadrant represents the number of serum samples which are opsonic and which have ≤1.0 μg of IgG per ml as determined by ELISA. The upper right quadrant represents those sera samples that are both opsonic and have IgG concentrations >1.0 μg/ml. The lower left quadrant contains the nonopsonic serum samples with ≤1.0 μg of IgG per ml, and the lower right quadrant contains the number of serum samples that are nonopsonic but that have IgG concentrations of >1.0 μg/ml. Ideally, there should be no datum points in the upper left and lower right quadrants. For type 4, absorption with type 22F reduced the number of nonopsonic serum samples with IgG levels >1 μg/ml from 10 to 1 (Fig. 7). The correlation coefficient increased from 0.66 to 0.92. Similarly for type 19F, after type 22F absorption, the number of nonopsonic serum samples with IgG concentrations >1 μg/ml was reduced from 12 to 5 and the correlation coefficient improved from 0.63 to 0.80 (Fig. 8).
FIG. 7.
Correlation of pneumococcal type 4 ELISA and opsonophagocytosis assay titers. (A) After C PS absorption only. (B) After C PS plus type 22F PS absorption. The quadrant lines were drawn at the 1.0-μg/ml cutoff for ELISA and the log2 (1:4) cutoff for the opsonophagocytosis assay titer. The numbers shown are the number of serum samples in each quadrant. The r values were 0.66 before type 22F PS absorption and 0.92 after type 22F PS absorption.
FIG. 8.
Correlation of pneumococcal type 19F ELISA and opsonophagocytosis assay titers. (A) After C PS absorption. (B) After C PS plus type 22F PS absorption. The quadrant lines were drawn at the 1.0 μg/ml cutoff for the ELISA titer and the log2 (1:4) cutoff for the opsonophagocytosis assay titer. The r value was 0.63 before type 22F PS absorption and 0.80 after type 22F PS absorption.
DISCUSSION
Protection against pneumococcal bacteremia, pneumonia, or otitis media is mediated by antibodies that are opsonic (12). Pneumococcal PSs are immunogenic and induce type-specific protective immunity. Thus, in vitro measurement of opsonophagocytosis by anti-PS antibodies is a surrogate measure of clinical protection. However, due to the difficulties of measuring opsonic antibody titers in large numbers of vaccinees, many laboratories use antibody binding assays, usually ELISA (4, 9).
The pneumococcal cell wall contains a number of carbohydrate structures. All pneumococci express C PS. The C PS is covalently linked to the peptidoglycan and is composed of a tetrasaccharide subunit linked through a phophodiester linkage. It is closely related to the lipoteichoic acid F antigen (19). In encapsulated pneumococci the C PS is covalently linked to the type-specific PS. The nature of the attachment is not known, but it may be analogous to the reported association of the group B carbohydrate and type-specific capsular PS of Streptococcus agalactiae (5). Here, both the group-specific and the type-specific antigens are covalently linked to the peptidoglycan, but to different sugars.
The pneumococcal PS antigens used in the ELISA contain about 5% (by weight) of C PS as a contaminant. All individuals including infants have antibodies to the C PS as a result of early exposure to pneumococci and Streptococcus mitis (U. B. S. Sorensen, N. Bergstrom, C. Karlsson, M. Killian, and P. E. Jansson, Second Int. Symp. Pneumococci Pneumococcal Dis. abstr., 2000). The concentrations of antibody to the C PS in unimmunized individuals often exceed those of antibody to the type-specific PSs (Fig. 4). Thus, the current pneumococcal ELISA protocols have an absorption step to remove C PS antibodies (4, 9, 10).
For an ELISA to be used in place of an opsonophagocytosis assay, there should be a strong correlation between the measured antibody concentration and opsonic antibody titers. Thus, the ELISA method should be PS type specific (see below) and correlate well with functional activity (we suggest an r value of ≥0.8 combined with a quadrant analysis). Human antibodies to the pneumococcal C PS are not opsonic (20), and the antibody concentrations measured without removal of these antibodies showed no correlation between the amount of IgG bound and the opsonophagocytosis assay titer.
The specificity of the PS ELISA for a given serotype after removal of C PS antibodies was assessed by inhibition with homologous and heterologous pneumococcal PSs. We consider that there should be <20% inhibition by heterologous PS types, but some sera from healthy nonimmunized adults were found for some types to be nearly equally inhibited by homologous and heterologous pneumococcal PSs. Such sera were often found to be devoid of opsonic antibodies to that type. Yu et al. (23) found that some postvaccination sera positive for pneumococcal PS were much less opsonic for type 6B than would have been predicted from the type 6B antibody concentrations measured by ELISA and that a heterologous PS, type 9V PS, removed most of the nonopsonic antibodies. These observations indicate that absorption with the highly purified soluble C PS alone is not sufficient to remove all common cross-reactive antibodies. Yu et al. (23) stated that the novel epitope was distinct from the C PS, but as outlined below, our working hypothesis is somewhat different.
Another conclusion from the cross-absorption experiments was that the purified pneumococcal type PSs contained another common antigen in addition to the C PS. Our working hypothesis is that this additional common epitope is actually the linkage region between the C PS and the type-specific PS, and this region cannot be present in the highly purified soluble C PS, which is isolated from a rough pneumococcal strain with a C PS capsule (3, 15). In support of this hypothesis was the observation by Yu et al. (23) that a lysate from a nonencapsulated pneumococcal strain was less able to inhibit the cross-reactive antibody than a lysate from an encapsulated strain. It is further supported by the observation by Soininen et al. (18) that the cross-reactive antibodies that remain after C PS absorption were predominantly of the IgG2 subclass, implying that these cross-reactive antibodies were directed against a PS determinant. Furthermore, Bornstein et al. 3 demonstrated antigenic heterogeneity among C PS preparations.
The pneumococcal 11-valent conjugate vaccines under clinical investigation contain types 1, 3, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, and 23F (7). The licensed 23-valent PS vaccine contains 12 additional types, and from these additional types we selected for evaluation as a possible second absorbent types 11A, 12F, 15B, 22F, and 33F, as these types are not included in any present conjugate vaccine, they lack shared type-specific epitopes with other pneumococcal serogroups (8), and they are available from ATCC. Although we chose type 22F, type 22F was not materially different from the other four types tested. Yu et al. (23) have reported that type 9V also contains substantial amounts of a common antigen. However, absorption with the heterologous PS alone could not replace absorption with soluble C PS (C. E. Frasch, unpublished observations).
An important observation was that the antibodies against common epitopes were present in sera from all adults tested but were rarely observed in sera from 7-month-old infants, either those who were vaccinated with the pneumococcal conjugate or those who were nonvaccinated. This suggests that the common antigen is carbohydrate in nature. By contrast, by 7 months of age most all infants had measurable antibodies to the soluble C PS, as C PS absorption reduced antibody binding to the type-specific PS-coated ELISA plates. The ubiquitous presence of C PS on such commensals as Streptococcus mitis may explain the presence of C PS antibodies in the infants.
In order to be used in place of functional assays, the results of an ELISA method should, at a minimum, correlate well (r ≥ 0.8) with those of the opsonophagocytosis assay. We also recommend that the data be examined visually (for example, by quadrant analysis) to evaluate the number of individual serum samples with discordant results. The correlation between antibody binding by ELISA and opsonphagocytosis assay for 24 serum samples from adults is shown in Table 1. Serotypes 4 and 19F showed the weakest correlation when the sera were absorbed only with C PS. Removal of additional nonopsonic antibodies by type 22F absorption increased the correlation coefficients between antibody binding and opsonophagocytic assay titers for types 4 and 19F from 0.66 and 0.63 before type 22F absorption to 0.92 and 0.80 after type 22F absorption. Thus, combined C PS and type 22F PS absorption increases the type specificity and improves the correlation with functional activity.
Although the thrust of our work has been to improve the specificity in measurement of pneumococcal vaccine-induced antibody titers in healthy individuals, retrieval of optimal pneumococcal antibody specificity in sera from patients, including immunocompromised individuals, is also important, because these individuals may have high levels of common antigen-reactive antibodies, which do not appear to contribute to protection.
In conclusion, we have found that absorption with a heterologous pneumococcal PS blocks binding of nonopsonic antibodies and by so doing have made the PS ELISA more predictive of functional activity. On the basis of the results presented in this report, we recommend that sera be absorbed with both C PS and type 22F PS, recognizing that the type 22F PS was not materially superior to other heterologous PS types evaluated. Additional studies are needed to examine the age-related acquisition of the common antigen antibodies. We are investigating whether the linkage region of the C PS to the type-specific PS carries the common epitope.
REFERENCES
- 1.Anttila M, Eskola J, Åhman H, Käyhty H. Differences in the avidity of antibodies evoked by four different pneumococcal conjugate vaccines in early childhood. Vaccine. 1999;17:1970–1977. doi: 10.1016/s0264-410x(98)00458-7. [DOI] [PubMed] [Google Scholar]
- 2.Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen J R, Elvin L, Ensor K M, Hackell J, Siber G, Malinoski F, Madore D, Chang I, Kohberger R, Watson W, Austrian R, Edwards K Northern California Kaiser Permanente Vaccine Study Center Group. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr Infect Dis J. 2000;19:187–195. doi: 10.1097/00006454-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Bornstein D L, Schiffman G, Bernheimer H P, Austrian R. Capsulation of pneumococcus with soluble C-like (Cs) polysaccharide. J. Biological and genetic properties of Cs pneumococcal strains. J Exp Med. 1968;128:1385–1400. doi: 10.1084/jem.128.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Concepcion N, Frasch C E. Evaluation of previously assigned antibody concentrations in pneumococcal polysaccharide reference serum 89SF by the method of cross-standardization. Clin Diagn Lab Immunol. 1998;5:199–204. doi: 10.1128/cdli.5.2.199-204.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng L Y, Kasper D L, Krick T P, Wessels M R. Characterization of the linkage between the type III capsular polysaccharide and the bacterial cell wall of group B Streptococcus. J Biol Chem. 2000;275:7497–7504. doi: 10.1074/jbc.275.11.7497. [DOI] [PubMed] [Google Scholar]
- 6.Frasch C E, Concepcion N F. Specificity of human antibodies reactive with pneumococcal C polysaccharide. Infect Immun. 2000;68:2333–2337. doi: 10.1128/iai.68.4.2333-2337.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hausdorff W P, Bryant J, Paradiso P R, Siber G R. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. Clin Infect Dis. 2000;30:100–121. doi: 10.1086/313608. [DOI] [PubMed] [Google Scholar]
- 8.Henrichsen J. Six newly recognized types of Streptococcus pneumoniae. J Clin Microbiol. 1995;33:2759–2762. doi: 10.1128/jcm.33.10.2759-2762.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Käyhty H, Åhman H, Rönnberg P R, Tillikainen R, Eskola J. Pneumococcal polysaccharide meningococcal outer membrane protein complex conjugate vaccine is immunogenic in infants and children. J Infect Dis. 1995;172:1273–1278. doi: 10.1093/infdis/172.5.1273. [DOI] [PubMed] [Google Scholar]
- 10.Koskela M. Serum antibodies to pneumococcal C polysaccharide in children: response to acute pneumococcal otitis media or to vaccination. Pediatr Infect Dis J. 1997;6:519–526. doi: 10.1097/00006454-198706000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Lucas A H, Granoff D M. Functional differences in idiotypically defined IgG1 anti-polysaccharide antibodies elicited by vaccination with Haemophilus influenzae type B polysaccharide-protein conjugates. J Immunol. 1995;154:4195–4202. [PubMed] [Google Scholar]
- 12.Musher D, Chapman A J, Goree A, Jonsson S, Briles D E, Baughn R E. Natural and vaccine-related immunity to Strepococcus pneumoniae. J Infect Dis. 1986;154:245–256. doi: 10.1093/infdis/154.2.245. [DOI] [PubMed] [Google Scholar]
- 13.Musher D M, Groover J E, Rowland J M, Watson D A, Struewing J B, Baughn R E, Mufson M A. Antibody to capsular polysaccharides of Streptococcus pneumoniae: prevalence, persistence, and response to revaccination. Clin Infect Dis. 1993;17:66–73. doi: 10.1093/clinids/17.1.66. [DOI] [PubMed] [Google Scholar]
- 14.Musher D M, Watson D A, Baughn R E. Does naturally acquired IgG antibody to cell wall polysaccharide protect human subjects against pneumococcal infection? J Infect Dis. 1990;161:736–740. doi: 10.1093/infdis/161.4.736. [DOI] [PubMed] [Google Scholar]
- 15.Pedersen F K, Henrichsen J, Sorensen U S, Nielsen L L. Anti-C-carbohydrate antibodies after pneumococcal vaccination. Acta Pathol Microbiol Immunol Scand Sect C. 1982;90:353–255. doi: 10.1111/j.1699-0463.1982.tb01462.x. [DOI] [PubMed] [Google Scholar]
- 16.Rennels M B, Edwards K M, Keyserling H L, Reisinger K S, Hogerman D A, Madore D V, Chang I, Paradiso P R, Malinoski F J, Kimura A. Safety and immunogenicity of heptavalent pneumococcal vaccine conjugated to CRM197 in United States infants. Pediatrics. 1998;101:604–611. doi: 10.1542/peds.101.4.604. [DOI] [PubMed] [Google Scholar]
- 17.Romero-Steiner S, LiButti D E, Pais L, Dykes J, Anderson P, Whitin J, Keyserling H, Carlone G. Standardization of an opsonophagocytic assay for the measurement of functional activity against Streptococcus pneumoniae using differentaited HL-60 cells. Clin Diagn Lab Immunol. 1997;4:415–422. doi: 10.1128/cdli.4.4.415-422.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soininen A, Dobbelstein G, Oomen L, Kayhty H. Are the enzyme immunoassays for antibodies to pneumococcal capsular polysaccharides type specific? Clin Diagn Lab Immunol. 2000;7:468–476. doi: 10.1128/cdli.7.3.468-476.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sorensen U B S, Henrichsen J, Chen H-C, Szu S C. Covalent linkage between the capsular polysaccharide of Streptococcus pneumoniae revealed by immunochemical methods. Microb Pathog. 1990;8:325–334. doi: 10.1016/0882-4010(90)90091-4. [DOI] [PubMed] [Google Scholar]
- 20.Vioarsson G, Jónsdóttir I, Jónsson S, Valdimarsson H. Opsonization and antibodies to capsular and cell wall polysaccharides of Streptococcus pneumoniae. J Infect Dis. 1994;170:592–599. doi: 10.1093/infdis/170.3.592. [DOI] [PubMed] [Google Scholar]
- 21.Watson D A, Musher D M. Assessment of responses to pneumococcal infection and vaccination using enzyme-linked immunoassay. Serodiagn Immunother Dis. 1993;5:131–133. [Google Scholar]
- 22.Winkelstein J A. The role of complement in the host's defense against Streptococcus pneumoniae. Rev Infect Dis. 1981;3:289–298. doi: 10.1093/clinids/3.2.289. [DOI] [PubMed] [Google Scholar]
- 23.Yu X H, Sun Y, Frasch C, Concepcion N, Nahm M H. Pneumococcal capsular polysaccharide preparations may contain non-C-polysaccharide contaminants that are immunogenic. Clin Diagn Lab Immunol. 1999;6:519–524. doi: 10.1128/cdli.6.4.519-524.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]