Abstract
Coccidioides immitis and Coccidioides posadasii are causative agents of coccidioidomycosis, commonly known as Valley Fever. The increasing Valley Fever cases in the past decades, the expansion of endemic regions, and the rising azole drug-resistant strains have underscored an urgent need for a better understanding of Coccidioides biology and new antifungal strategies. Transporters play essential roles in pathogen survival, growth, infection, and adaptation, and are considered as potential drug targets. However, the composition and roles of transport machinery in Coccidioides remain largely unknown. In this study, genomic data mining revealed an abundant, uncharacterized repertoire of transporters in Coccidioides genomes. The catalog included 1288 and 1235 transporter homologs in C. immitis and C. posadasii, respectively. They were further annotated to class, subclass, family, subfamily and range of substrates based on the Transport Classification (TC) system. They may play diverse roles in nutrient uptake, metabolite secretion, ion homeostasis, drug efflux, or signaling. This study represents an initial effort for a systems-level characterization of the transport machinery in these understudied fungal pathogens.
Keywords: coccidioidomycosis, Coccidioides, transporters, genomics
1. Introduction
Soil-dwelling dimorphic fungi, Coccidioides immitis and Coccidioides posadasii, are causative agents of coccidioidomycosis, commonly known as Valley Fever [1]. While about 60% of infected people show no or minimum symptoms, the remainder develop clinical symptoms, ranging from pneumonia to life threatening, disseminated coccidioidomycosis. There is currently no clinically available vaccine against coccidioidomycosis and treatments are based on standard antifungal therapies.
The development of new antifungal drugs targeting Coccidioides is urgently needed because (1) The rise of incidence of coccidioidomycosis [2]. Incidence was reported to increase by almost 800% from 2000 to 2018 in California, and in 2018 over 15,000 cases were reported to the Centers for Disease Control and Prevention of the United States [3,4]; (2) The increase in the area of endemicity. Historically endemic in the arid and semiarid areas of the southwestern USA, Mexico, Central America, and South America [5], Coccidioides were recently reported to expand to Utah, Oregon, and Washington state [6,7,8,9,10]; (3) The emergence of clinical isolates conferring resistance to antifungals [11,12,13].
The search for new targets against Coccidioides requires a better understanding of Coccidioides biology. Despite recent advances in epidemiology, ecology and population biology [1,14,15], the molecular mechanisms underlying fungal growth, adaptation to the host environment, pathogenesis, and virulence, remain elusive. Difficulties in Coccidioides research partly stem from the fundamental complexity of Coccidioides fungi, which have a unique life cycle, switching between saprobic and parasitic phases. They grow in the soil, cycling between saprobic mycelia and arthroconidia. When the soil is disturbed, the arthroconidia can become airborne and be inhaled by a host. The parasitic phase is initiated when arthroconidia transform into spherules, leading to pulmonary infection.
The completion of the genome sequencing projects for C. immitis and C. posadasii provides us with an unprecedented opportunity to unveil genes and gene products that are essential for their survival and infective potential [10,16,17,18,19]. Instead of an individual-gene based approach, in this study, using genomic and systems biology approaches, we attempted to identify and catalog the transport machinery in C. immitis and C. posadasii. Transporters were chosen as the subject of this study because the complex life cycle of Coccidioides requires a powerful transport system. Transport is a vital function of all living cells. Transporters are an extremely diverse group of proteins that catalyze the transfer of substrates ranging from nutrients, metabolic products, macromolecules, drugs and toxins, to signaling molecules. Depending on the energy sources and translocation mechanisms, transporters can be classified as carriers, channels, pumps, electron-flow carriers, etc. The function of transporters is associated with their structural and topological features, including the presence and number of transmembrane segments. Transporters are believed to be involved in various processes in fungi, including nutrition, signaling, stress response, cellular detoxification, and virulence [20,21]. Most importantly, extensive studies have demonstrated that transporters play an essential role in multidrug resistance in pathogenic fungi [22,23,24,25,26]. In addition, the feasibility of designing inhibitors of transporters important for survival or drug resistance make them potential drug targets [26]. Despite its functional significance, the transport machinery in Coccidioides remains largely unexplored. Our comparative genomic analyses identified 1288 and 1235 putative transporters in C. immitis and C. posadasii, respectively. They may be involved in cellular networks regulating nutrient uptake, metabolite secretion, drug and toxin efflux, maintenance of ion content, macromolecule export, and signaling. This study represents the first step towards a systems-level elucidation of the transport machinery in the complex, yet understudied, Coccidioides.
2. Materials and Methods
2.1. Genomic Data
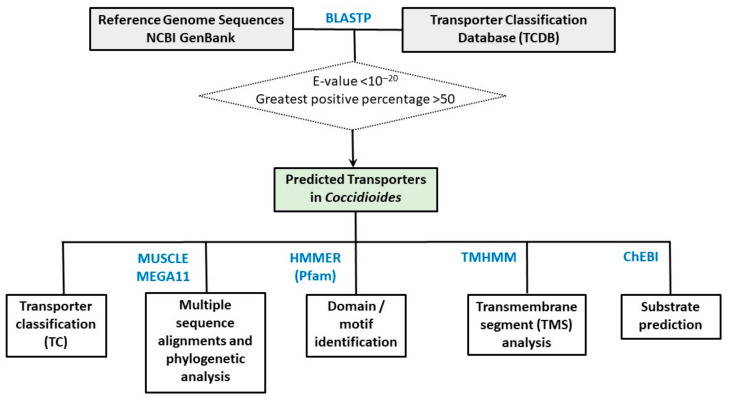
Figure 1 depicts the pipeline of our genomic analysis. The completed reference genome sequences of C. immitis RS strain (BioProject PRJNA12883) and C. posadasii SOWgp strain (BioProject PRJNA9616) were downloaded from the NCBI Genbank database (https://www.ncbi.nlm.nih.gov/data-hub/genome/?taxon=5500, accessed on 20 July 2021) (Table 1) [16,17]. All the amino acid sequences and annotated features were retrieved.
Figure 1.
The pipeline of genomic analysis of Coccidioides transporters. See Materials and Methods for details.
Table 1.
Distribution of transporters in Coccidioides.
| Species | Accession Number | Genome Size (Mbp) | # Proteins | # Transporters | % Transporters |
|---|---|---|---|---|---|
| Coccidioides immitis RS | GCF_000149335.2 | 28.9 | 9910 | 1288 | 13.0 |
| Coccidioides posadasii SOWgp | GCF_000151335.2 | 27.0 | 7227 | 1235 | 17.1 |
The transporter classification database TCDB, https://www.tcdb.org/ (accessed on 20 July 2021), was used as the knowledge database for the identification and classification of transporters in Coccidioides [27,28]. TCDB includes curated transporter sequences, classification, structural, evolutionary, mechanistic, medical and biotechnological information about transport systems from a variety of organisms. A total of 21,373 amino acid sequences of the transporters collected in the TCDB was downloaded.
2.2. Identification, Classification, and Characterization of Putative Transporters in Coccidioides
The BLASTP query of all the proteins in C. immitis RS strain and C. posadasii SOWgp strain against the TCDB database was conducted to identify Coccidioides proteins that were homologs to known or predicted transporters [29]. The cutoff for homologous genes were set as: BLASTP E-value < 10−20 and greatest positive percentage >50. The annotation of Coccidioides transporters was based on the hits in the TCDB with the lowest E-value, and the highest similarity score.
The predicted Coccidioides transporters were further classified into families and subfamilies based on the 5-letter Transport Classification (TC) system [13]. Similar to the Enzyme Commission (EC) system for classification of enzymes, the TC system is formally adopted by the International Union of Biochemistry and Molecular Biology for transporter classification and nomenclature. The 5-letter TC number, in the form of VWXYZ corresponds transporter class, subclass, family, subfamily and the substrate/substrates transported [27].
Conserved domains/motifs in predicted Coccidioides transporter sequences were identified by searching against the Pfam 35.0 database [30], which is a collection of protein families based on hidden Markov models implemented in HMMER [31]. The TMHMM program was used to analyze the transmembrane structures and predict transmembrane segments (TMSs) [32]. The substrates for predicted Coccidioides transporters were predicted based on the Chemical Entities of Biological Interest (ChEBI), an ontology and dictionary focused on small chemical compounds [33].
2.3. Multiple Alignment and Phylogenetic Analysis
Multiple sequence alignments were obtained using the MUSCLE program [34,35]. Phylogenetic trees were inferred by the neighbor-joining methods [36] and the maximum likelihood [37] using MEGA11 [38]. Bootstrap resampling with 1000 pseudo replicates was used to assess statistical support for each branch [39].
3. Results
3.1. C. immitis and C. posadasii Possess a Rich Repertoire of Transporters
To gain insight into the transport machinery of Coccidioides, the protein sequences in the C. immitis RS strain and C. posadasii SOWgp strain were subjected to an exhaustive search against the TCDB database, which has a catalog and a structure-, mechanic-, and phylogeny-based classification of transporters. Stringent threshold of E-value < 10−20 and positive percentage >50 were adopted to ensure the high coverage with low false-positives. A total of 1288 and 1235 transporter homologs were identified in C. immitis and C. posadasii, respectively, which account for 13.0% and 17.1% of their respective proteome (Table 1). The transporter composition in the two organisms was highly similar (Supplementary Table S1): Mutual BLASTP analysis between C. immitis and C. posadasii showed that only 23 transporters were specific to C. immitis, and six transporters were specific to C. posadasii. Our new catalog of transporters included 197 and 103 transporters that were previously annotated by genome-sequencing projects in C. immitis and C. posadasii, respectively [17].
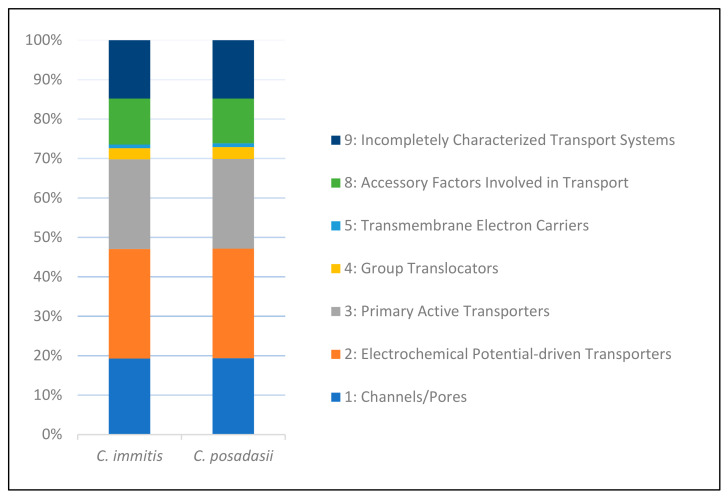
Combining domain specification and TC nomenclature, we further divided the 1288 and 1235 transporter homologs into seven classes, 25 subclasses, and 269 families in C. immitis and C. posadasii (Figure 2 and Table 2). The majority of these predicted transporters were not previously characterized.
Figure 2.
Distribution of transporter types according to the TC system in Coccidioides genomes. Class 1: Channels/Pores; Class 2: Electrochemical Potential-driven Transporters; Class 3: Primary Active Transporters; Class 4: Group Translocators; Class 5: Transmembrane Electron Carriers; Class 8: Accessory Factors Involved in Transport; Class 9: Incompletely Characterized Transport Systems.
Table 2.
Distribution of transporters in each transporter classification (TC) class and subclass in Coccidioides genomes. According to the TC system, the first two letters (VW) correspond to the transporter class and the subclass.
| Class | Subclass | C. immitis | C. posadasii |
|---|---|---|---|
| 1: Channels/Pores | 248 | 239 | |
| 1.A: α-Type Channels | 70 | 67 | |
| 1.B: β-Barrel Porins | 9 | 8 | |
| 1.C: Pore-Forming Toxins (Proteins and Peptides) | 17 | 14 | |
| 1.F: Vesicle Fusion Pores | 17 | 16 | |
| 1.H: Paracellular Channels | 4 | 4 | |
| 1.I: Membrane-bounded Channels | 104 | 103 | |
| 1.N: Cell Fusion Pores | 5 | 5 | |
| 1.P: Non-Envelop Virus Penitration Complex: A complex of host cell proteins that allow non-envelop virus to penetrate the endoplasmic reticular membrane. | 9 | 10 | |
| 1.Q: Fungal Septal Pores | 8 | 8 | |
| 1.R: Membrane Contact Site for Interorganellar Transport | 4 | 3 | |
| 1.W: Phage Portal Protein Subclass | 1 | 1 | |
| 2: Electrochemical Potential-driven Transporters | 358 | 343 | |
| 2.A: Porters (uniporters, symporters, antiporters) | 353 | 338 | |
| 2.D: Transcompartment Lipid Carrier | 5 | 5 | |
| 3: Primary Active Transporters | 293 | 281 | |
| 3.A: P-P-bond-hydrolysis-driven transporters | 244 | 232 | |
| 3.B: Decarboxylation-driven transporters | 3 | 3 | |
| 3.D: Oxidoreduction-driven transporters | 44 | 44 | |
| 3.E: Light absorption-driven transporters | 2 | 2 | |
| 4: Group Translocators | 36 | 37 | |
| 4.C: Acyl CoA ligase-coupled transporters | 23 | 25 | |
| 4.D: Polysaccharide Synthase/Exporters | 8 | 8 | |
| 4.E: Vacuolar Polyphosphate Polymerase-catalyzed Group Translocators | 2 | 2 | |
| 4.F: Choline/EthanolaminePhosphotransferase 1 | 3 | 2 | |
| 5: Transmembrane Electron Carriers | 12 | 12 | |
| 5.B: Transmembrane 1-electron transfer carriers | 12 | 12 | |
| 8: Accessory Factors Involved in Transport | 150 | 140 | |
| 8.A: Auxiliary transport proteins | 150 | 140 | |
| 9: Incompletely Characterized Transport Systems | 191 | 183 | |
| 9.A: Recognized transporters of unknown biochemical mechanism | 74 | 71 | |
| 9.B: Putative transport proteins | 117 | 112 | |
| Total | 1288 | 1235 | |
The most abundant class of transporters is electrochemical potential-driven transporters (Class 2) (Supplementary Table S1). These 358 and 343 secondary carriers account for approximately over 27% of all the transporters in C. immitis and C. posadasii. The majority of the Class 2 transporters belong to porters (TC Subclass 2.A), consisting of uniporters, symporters, and antiporters [40,41]. The porters in fungal species catalyze the uptake of nutrients such as sugars, amino acids, and efflux of toxic compounds and drugs. Among these porters, the major facilitator superfamily (MFS) transporters have been implicated in multidrug resistance in Candida albicans [42,43,44,45], C. glabrata [46,47], C. tropicalis [48], and Aspergillus fumigatus [49]. We identified 140 and 135 MFS transporters in C. immitis and C. posadasii, respectively. See Section 3.2.2 for detailed analysis.
The second most abundant class is primary active transporters (Class 3) (Supplementary Table S1). They represent about 22% of the transporter repertoire in C. immitis and C. posadasii. This class of transporters uses a primary source of energy such as ATP to transport solutes across a membrane against their electrochemical gradient. The members of this class are major players in the uptake and excursion of diverse solutes. Most notably, a superfamily of ATP-binding cassette (ABC) transporters are known to confer efflux-mediated antifungal resistance in pathogenic fungi [21]. Our analysis identified 44 and 38 putative ABC transporters in C. immitis and C. posadasii, respectively. The genomic and phylogenetic analyses of these ABC transporters are seen in Section 3.2.1. In addition, P-type ATPases were found to be present in fungi species such as A. fumigatus, A. nidulans, A. oryzae, C. neoformans, Neurospora crassa, Saccharomyces cerevisiae, and Schizosaccharomyces pombe [50]. These active pumps play important roles in ion homeostasis for fungal cell physiology [51]. C. immitis and C. posadasii genomes contain 20 and 18 P-type ATPases, respectively, including ATPases that translocate calcium, magnesium, copper, phospholipid, and potassium-sodium (Supplementary Table S1).
Class 1 channel/pore proteins are ubiquitous in all living organisms. Over 19% of the transporter homologs in C. immitis and C. posadasii fall within this class. These transporters facilitate diffusion of solutes in an energy-independent mode. The two largest subclasses in Class 1 present in Coccidioides are α-type channels (TC Subclass 1.A) [52], and membrane-bounded channels that form pore complexes (TC Subclass 1.I) [53]. Coccidioides possess various cation channels, including calcium, potassium, and transient receptor potential channels with potential roles in cellular signaling and homeostasis. Homologs of these cation channels are present in S. cerevisiae [54,55], C. albicans [56], Aspergillus spp. [57], and C. neoformans [58]. Although the detailed physiological function of fungal channels is largely unknown, they are considered as potential drug targets [59]. In addition to channels, Coccidioides also possess Nuclear Pore Complex (NPC) transporters (TC family 1.I.1) that bidirectionally transfer macromolecules between the cytoplasm and the nucleus. Besides facilitating nucleocytoplasmic trafficking, the homologs of these porins were shown to be involved in chromatin organization, and gene expression regulation in fungi [53,60,61].
Taking a systems perspective, the TC system also groups various accessory factors that facilitate the transport, but do not directly transport solutes into Class 8. Over 140 predicted homologs fall into Class 8, representing 11% of the total transporter homologs in C. immitis and C. posadasii. The physiological roles of these putative transporters are largely unknown.
3.2. Potential Functionally Important Transporters in C. immitis and C. posadasii
Among the 269 families of predicted transporters, undoubtedly some of the uncharacterized transporters perform important functions in the Coccidioides life cycle, for example, the ABC transporter superfamily (TC 3.A.1) and the major facilitator superfamily (MFS) (TC 2.A.1). Examples of potentially important transporters in Coccidioides and their homologs in other pathogenic fungi are shown in Table 3.
3.2.1. ABC Transporters
The ABC transporter superfamily is one of the largest protein families that are ubiquitous in all three domains of life [62,63,64,65,66,67]. ABC transporters possess two types of characteristic domains: (1) transmembrane domains, allowing substrate recognition and translocation across membranes, and (2) nucleotide-binding domains, allowing ATP binding and hydrolysis to power the transport process [68,69,70]. ABC transporters are believed to play crucial roles in transferring a wide array of substrates such as sugar, ion, lipid, amino acids, peptides, proteins, etc., for nutrition, signaling, and stress response [63]. Notably, ABC transporters have been widely implicated in the development of multidrug resistance (MDR) to antimicrobials or anticancer drugs [71,72,73]. MDR conferred by ABC transporters is increasingly concerning in fungal pathogens [74,75,76,77,78,79,80,81,82,83,84,85,86]. Comprehensive phylogenetic analyses of 27 other fungal species from 18 orders of five fungal phyla defined a complex evolutionary history and classification of fungal ABC transporters [21]. The ABC system in Coccidioides, however, remains to be defined.
Table 3.
Examples of potentially important transporters in Coccidioides and their homologs in other pathogenic fungi.
| Transporter TC 1 | Annotation 2 | Accession Number (C. immitis) |
Accession Number (C. posadasii) |
BLASTP E-Value 3 | Homologous Sequence in Pathogenic Fungi | ||
|---|---|---|---|---|---|---|---|
| Gene/ UniProt ID |
Annotation/Function | Species [References] |
|||||
| 3.A.1.201.10 Multidrug Resistance Exporter (MDR) Family | ABC transporter family protein |
XP_001246263.2 XP_001247009.1 |
XP_003066296.1 XP_003066854.1 |
0 | MDR1 B0Y3B6 |
ABC multidrug transporter | Aspergillus fumigatus [87] |
| 3.A.1.205.6 Pleiotropic Drug Resistance (PDR) Family | ABC transporter | XP_001242727.2 | XP_003069892.1 | 0 | AFR1 Q8X0Z3 |
ABC transporter | Cryptococcus neoformans [88] |
| 3.A.1.205.32 Pleiotropic Drug Resistance (PDR) Family | ABC transporter CDR4 |
XP_001239472.1 XP_001247647.1 |
XP_003065789.1 XP_003067068.1 |
0 | MDR3 F2SG60 |
ABC multidrug transporter | Trichophyton rubrum [89] |
| 3.A.1.208.41 Drug Conjugate Transporter (DCT) Family | ABC multidrug transporter |
XP_001240132.1 XP_004445935.1 |
XP_003066532.1 XP_003069119.1 |
0 | ECDL K0E4D9 |
Antifungal Echonocandin B exporter | Aspergillus fumigatus [90] |
| 3.A.1.212.3 Mitochondrial Peptide Exporter (MPE) Family | ATP-dependent permease MDL1 | XP_001248653.1 | XP_003070931.1 | 0 | MDR2 F2RYR3 |
ABC multidrug transporter |
Trichophyton tonsurans [91] |
| 2.A.1.2.115 Drug:H+ Antiporter-1 (DHA1) Family |
Multidrug resistance protein |
XP_001240201.2 XP_001247717.2 |
XP_003065730.1 XP_003069073.1 |
<2E-59 | QDR2 Q6FSQ7 |
Multidrug transporter | Candida glabrata [47,92] |
| 2.A.1.3.83 Drug:H+ Antiporter-2 (DHA2) Family |
Drug:H+ antiporter-2 |
XP_001241400.2 XP_001241607.2 XP_001247971.2 |
XP_003065523.1 XP_003070493.1 XP_003070583.1 |
<4E-35 | AFLT Q6UEH3 |
Efflux pump aflT | Aspergillus parasiticus [93] |
| 2.A.1.3.73 Drug:H+ Antiporter-2 (DHA2) Family |
Major Facilitator Superfamily protein |
XP_001240495.1 XP_001241711.1 XP_001241807.1 XP_001242730.2 XP_001243393.1 XP_001245646.2 XP_001246829.2 XP_001247440.1 XP_001247620.2 |
XP_003065952.1 XP_003066427.1 XP_003066894.1 XP_003068964.1 XP_003069895.1 XP_003070661.1 XP_003071281.1 |
<4E-21 | MFS1 A4ZGP3 |
Multidrug resistance Mfs1 protein | Zymoseptoria tritici [94] |
| 2.A.39.2 Nucleobase: Cation Symporter-1 (NCS1) Family |
NCS1 nucleoside transporter | XP_001245665.1 | XP_003071266.1 | 1E-54 | FCY21 Q708J7 |
PURINE-CYTOSINE PERMEASE (PCP) | Candida albicans [95] |
| 2.A.40.7 Nucleobase: Cation Symporter-2 (NCS2) Family |
Nucleoside transporter | XP_001244274.1 | XP_003068546.1 | 0 | AzgA Q7Z8R3 |
Purine (hypoxanthine/adenine/guanine) transporter | Aspergillus nidulans [96] |
Note: 1 The 5-letter transporter classification ID, in the form of VWXYZ corresponds transporter class, subclass, family, subfamily and the substrate/substrates transported [27]. 2 Annotation based on transporter classification. 3 The E-value of BLASTP analysis. The lower the E-value, or the closer it is to zero, the more significant the match is.
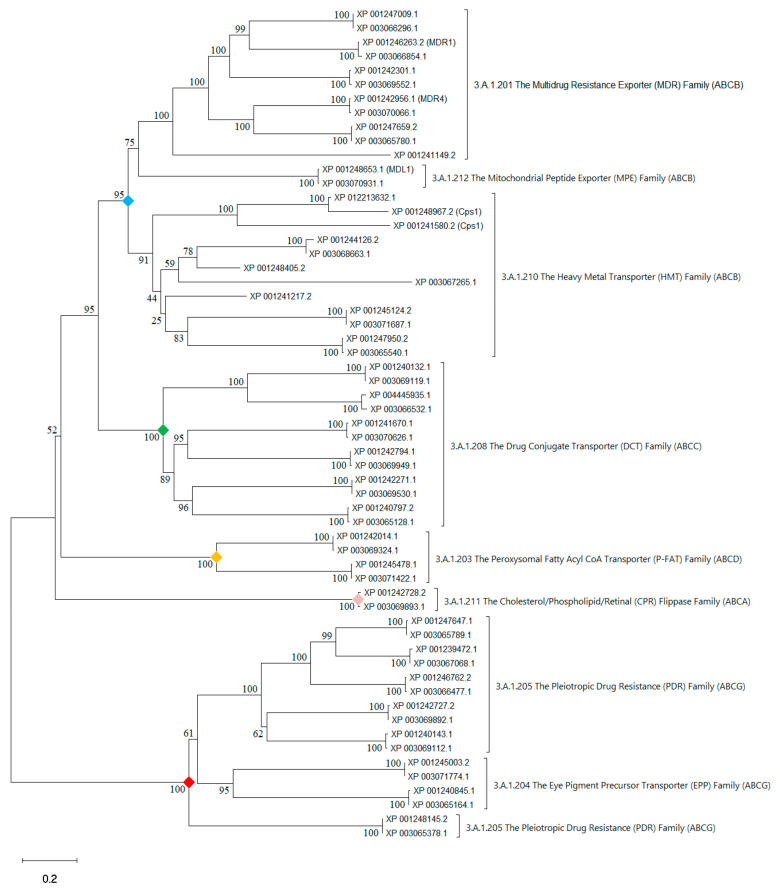
Our genomic analyses identified 44 and 38 putative ABC transporters in C. immitis and C. posadasii, respectively. Phylogenetic analysis revealed that these ABC transporters could be divided into five distinct efflux groups (Figure 3).
Figure 3.
Evolutionary relationships of major ABC transporter families in C. immitis and C. posadasii. The evolutionary history was inferred using the neighbor-joining method [36]. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches [39]. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Poisson correction method [97] and are in the units of the number of amino acid substitutions per site. Evolutionary analyses were conducted in MEGA11 [38]. Accession numbers: XP_0012XXXXX (C. immitis sequences), XP_004445935.1 (C. immitis), and XP_0030XXXXX (C. posadasii sequences). Each colored diamond corresponds to the branching point leading to a specific ABC group.
ABCB, the largest group, includes three families. The Multidrug Resistance Exporter Family (TC 3.A.1.201) has been widely studied in other fungi species such as S. cerevisiae, S. pombe, C. albicans, A. fumigatus, and C. neoformans [21]. Four homologs in C. immitis and C. posadasii are closely related to the MDR1 gene in A. fumigatus, which confers resistance to Cilofungin [87] (Supplementary Table S1). The second member of the ABCB group is the Heavy Metal Transporter (HMT) Family (TC 3.A.1.210). Eight C. immitis and three C. posadasii HMT homologs are closely related to the vacuolar HMT1 gene in S. pombe [98], which is capable of enhancing heavy metal tolerance in a high calcium content. A mitochondrial ATM1 gene in C. posadasii is homologous to mitochondrial iron transporter ATM1 in S. cerevisiae, which is essential for biogenesis of cytosolic iron/sulfur proteins [99,100,101,102]. The third member of the ABCB group is the Mitochondrial Peptide Exporter (MPE) Family (TC 3.A.1.212). ATP-dependent permease MDL1 in C. immitis and its homolog in C. posadasii are evolutionarily related to the MDR2 gene in Trichophyton tonsurans (scalp ringworm fungus), which plays an important role in susceptibility to multiple antifungal drugs [91].
The ABCG group includes two families: (1) The Pleiotropic Drug Resistance (PDR) Family (TC 3.A.1.205). In C. immitis and C. posadasii, a gene is homologous to the Afr1 gene, which confers resistance to azole antifungal drugs including fluconazole in C. neoformans [88]; two genes are homologous to MDR3 of T. tonsurans, which is an important member of the azole efflux pump network [89]. (2) The Eye Pigment Precursor Transporter (EPP) Family (3.A.1.204), which shows sequence similarity to multidrug/pigment exporter, Adp1 in S. cerevisiae [87].
The ABCC group includes the Drug Conjugate Transporter (DCT) Family (TC 3.A.1.208). The Coccidioides sequences in this family are homologous to various drug transporters, for instance, the YOR1 gene in S. cerevisiae conferring resistance to oligomycin, rhodamine B, tetracycline, verapamil, eosin Y and ethidium bromide [103], the YCF1 gene in S. cerevisiae involved in vacuolar metal resistance and drug detoxification [104], the bile acid transporter BAT1 gene in S. cerevisiae, and the EcdL gene in A. fumigatus conferring resistance to antifungal Echnocandin B [90].
The ABCD group includes the Peroxysomal Fatty Acyl CoA Transporter (P-FAT) Family (TC 3.A.1.203). C. immitis and C. posadasii each possesses two paralogs of P-FAT genes, which may be involved in the fatty acid transport across the peroxisomal membrane [105].
Cholesterol/Phospholipid/Retinal (CPR) Flippase Family (TC 3.A.1.211) is the single member of the ABCA group in C. immitis and C. posadasii, which may mediate the efflux of cellular cholesterol and phospholipids [106].
3.2.2. Major Facilitator Superfamily (MFS)
MFS (TC 2.A.1) constitutes a large and diverse superfamily of secondary active transporters. Widespread across all three domains of living organisms, MFS transporters move a broad spectrum of small molecules across membranes to maintain important physiological function of cells [107,108,109]. Similar to the ABC superfamily, MFS has been widely recognized in various pharmacological processes by active excursion of cytotoxic compounds [110]. Mounting evidence suggests that MFS transporters are key mediators of antifungal resistance [20,111,112,113,114,115,116]. While an MFS transporter was shown to have undergone fast evolution in the Coccidioides lineage, indicating potential significance of MFS in Coccidioides adaptation [17], our knowledge of the MFS system in Coccidioides remains minimum.
We identified 140 and 135 MFS transporters in C. immitis and C. posadasii, respectively, representing nearly 11% of the predicted transporters in their genomes. They belonged to 18 families. Of particular interest, abundant members were found in the Drug:H+ Antiporter-1 (DHA1) Family and the DHA2 Family. DHA1 and DHA2 transporters have demonstrated roles in antifungal resistance in fungal species in the genus of Saccharomyces, Candida, Cryptococcus and Aspergillus [20,114].
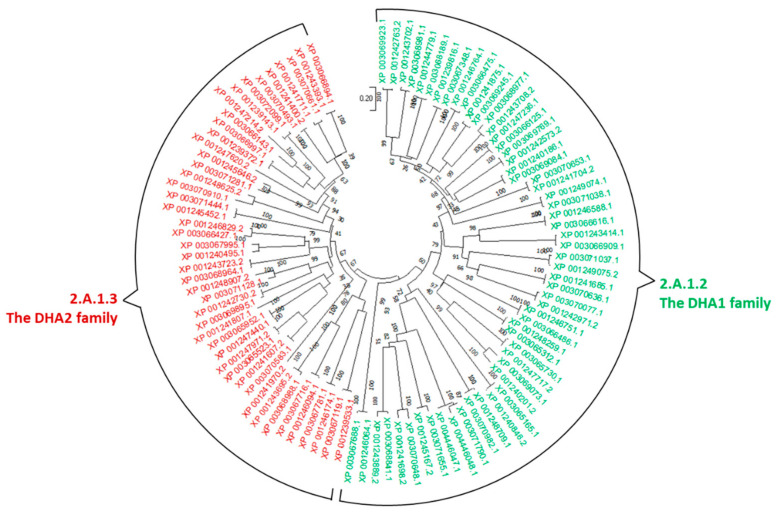
Phylogenetic analysis revealed that DHA1 and DHA2 homologs in Coccidioides are distributed into two clusters, which is consistent with their distinct structural properties (Figure 4).
Figure 4.
Evolutionary relationships of DHA transporter families in C. immitis and C. posadasii. The sequences in the DHA1 family are marked in green, and the sequences in the DHA2 family are marked in red. The evolutionary history was inferred using the neighbor-joining method [36]. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches [39]. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Poisson correction method [97] and are in the units of the number of amino acid substitutions per site. Evolutionary analyses were conducted in MEGA11 [38]. Accession numbers: XP_0012XXXXX (C. immitis sequences), XP_004445935.1 (C. immitis), and XP_0030XXXXX (C. posadasii sequences).
The DHA1 cluster (TC 2.A.1.2) includes 29 and 28 members in C. immitis and C. posadasii, respectively. These genes display sequence homology to DHA1 genes characterized in other fungi (Supplementary Table S1). For example, both Coccidioides genomes contain paralogs to the HOL1 gene in S. cerevisiae, which is capable of nonselective uptake of histidinol and other cations [117]. Notably, C. immitis and C. posadasii each possesses two paralogs with high sequence similarity to the QDR2 genes in Candida species. QDR2 is known to confer prevalent resistance to a broad spectrum of antifungals including miconazole, clotrimazole, tioconazole, and ketoconazole and quinidine [47,92,118]. One of these two paralogs, XP_001240201.2, appeared to have undergone rapid evolution in C. immitis [17].
The DHA2 cluster (TC 2.A.1.3) includes 24 and 21 members in C. immitis and C. posadasii, respectively. Two paralogs of aflT genes are in the Coccidioides genomes; AflT is an efflux pump in the aflatoxin pathway in filamentous fungus Aspergillus parasiticus [93]. Four paralogs are present in each of the Coccidioides genomes, closely related to the YOR378W gene in S. cerevisiae, which is involved in boron stress tolerance [119]. Abundant copies (nine and seven) of genes are present in C. immitis and C. posadasii, respectively, with high homology to MFS1 genes, which is related to the antifungal resistance in the wheat fungal pathogen Zymoseptoria tritici [94].
3.2.3. Other Novel Transporters
Our catalog of Coccidioides transporters also includes novel transporters with broad implications in fungal physiology. For example, we found members in the Nucleobase: Cation Symporter-1 (NCS1) Family (TC 2.A.39) and in the Nucleobase/Ascorbate Transporter (NAT) or Nucleobase: Cation Symporter-2 (NCS2) Family (TC 2.A.40) [95,120,121], which may be important components of salvage pathways for purine, pyrimidine, and related metabolites [96]. Two paralogs of TRK genes (TC 2.A.38) were identified in Coccidioides. TRK1 and TRK2 were found to participate in potassium uptake and response to internal and external signals in S. cerevisiae and C. albicans [122,123].
3.3. Structural and Biochemical Features of Transporters in C. immitis and C. posadasii
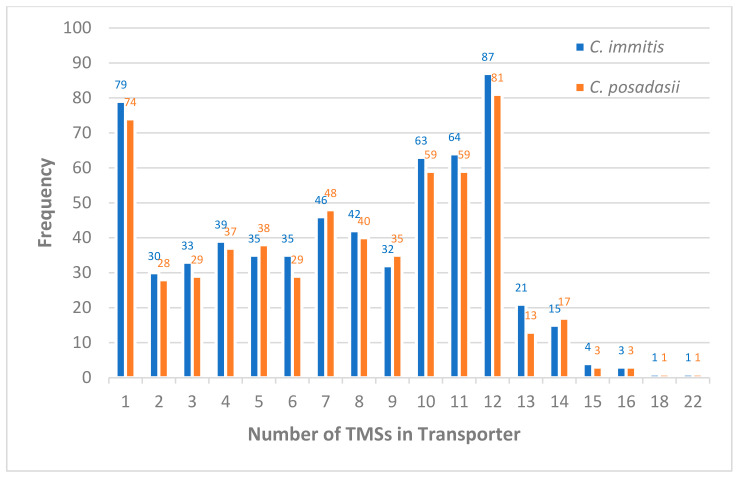
Transmembrane segments (TMSs) are important structural components for transporters to translocate solutes across membranes. The topology of a transporter is specified by the number of the TMSs, and its overall orientation in the membrane [124]. The number of TMSs is a characteristic feature of fungal transporters; for example, different subfamilies of fungal ABC proteins were shown to possess distinct number of TMSs and nucleotide-binding domains, suggesting structure-function correlations [21,125]. To reveal transmembrane topology of predicted transporters in Coccidioides, we performed TMHMM analysis [126]. Similar distributions of TMS topology were shown in two Coccidioides genomes. The number of TMSs range from 0 to 22 (Table 4 and Figure 5). While the structural-functional significance of the TMS topology in Coccidioides is yet to be elucidated, we found that the majority of membrane transporters are channels/pores, electrochemical potential-driven transporters, group translocators, or electron carriers.
Table 4.
Distribution of topological types of transporters in Coccidioides genomes.
| # TMSs | C. immitis | C. posadasii |
|---|---|---|
| 0 | 658 | 640 |
| 1 | 79 | 74 |
| 2 | 30 | 28 |
| 3 | 33 | 29 |
| 4 | 39 | 37 |
| 5 | 35 | 38 |
| 6 | 35 | 29 |
| 7 | 46 | 48 |
| 8 | 42 | 40 |
| 9 | 32 | 35 |
| 10 | 63 | 59 |
| 11 | 64 | 59 |
| 12 | 87 | 81 |
| 13 | 21 | 13 |
| 14 | 15 | 17 |
| 15 | 4 | 3 |
| 16 | 3 | 3 |
| 18 | 1 | 1 |
| 22 | 1 | 1 |
Figure 5.
Distribution of transporter topologies in Coccidioides immitis and Coccidioides posadasii genomes. No TMS helices were identified in 658 predicted transporters in C. immitis and 640 predicted transporters in C. posadasii.
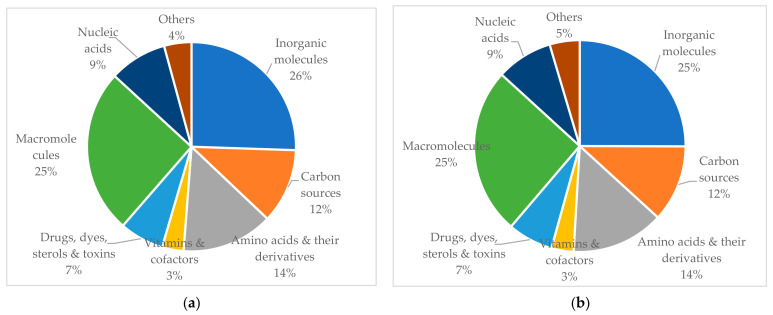
The putative Coccidioides transporters were predicted to be responsible for the translocation of extraordinarily diverse substrates, including inorganic molecules, carbon sources, drugs, toxins, electrons, macromolecules, amino acids and derivatives, nucleic acids, vitamins, and accessory factors (Supplementary Tables S2 and S3). As shown in Figure 6a,b, the two most abundant classes of the substrates in C. immitis and C. posadasii were inorganic molecules and macromolecules. More specifically, about 200 putative transporters were predicted to use cations and proteins as substrates.
Figure 6.
The distribution of substrate types of transporter proteins in Coccidioide genomes. The transporters with no identified substrates were excluded. (a) Distribution of substrate types in C. immitis genome; (b) Distribution of substrate types in C. posadasii genome. The substrates for predicted Coccidioides transporters were predicted based on the Chemical Entities of Biological Interest (ChEBI), an ontology and dictionary focused on small chemical compounds [33].
4. Discussion
Transporters are essential components for the survival of living organisms. The roles of transporters have been demonstrated in a variety of fungi. Our knowledge of the transport machinery in Coccidioides, however, remains limited. The genome annotation laid the groundwork for transporter characterization in C. immitis and C. posadasii [16,17]. To date, only a small number of transporters in Coccidioides have been reported or studied, including MDR1 (a multidrug resistance protein, accession number XP_003069119.1) and PSP1 (a hypothetical lipid transporter, accession number XP_003069236.1) in C. posadasii [127], a transmembrane amino acid transporter CIMG_11858 (accession number XP_012214138.1) and a major facilitator superfamily transporter CIMG_09822 (accession number XP_001240201.2) that showed fast evolution in the Coccidioides lineage [17], an ABC multidrug transporter CIMG_09753 (accession number XP_001240132.1), and a copper transporter CIMG_10037 (accession number XP_001239015.1) in C. immitis [128].
To fill in the critical knowledge gaps in Coccidioides biology, here, for the first time, we present a catalog of 1288 and 1235 putative transporters in C. immitis and C. posadasii, based on exhaustive homology search and comparative genomic analysis. These transporters fall into seven classes, 25 subclasses, and 269 families, with diverse transmembrane topologies and a wide array of substrates. Our hypothesis that Coccidioides fungi possess a rich and powerful transport machinery is justified.
It was estimated by the TCDB that transporters constitute about 10% of all cellular proteins [27]. The high content of transporters found in Coccidioides is likely an attribute of their adaptation to the complex soil ecosystem and the alien mammalian host system. Soil represents one of most challenging natural environments in which microorganisms scavenge nutrients, produce toxins to competing organisms and resist the effects of such cytotoxic substances. A similarly high content of transporters was observed in soil microbials, especially in the genus of Streptomyces [129,130]. However, unlike most Ascomycetes, which are plant pathogens or plant associated, clear evidence suggested that Coccidioides have undergone extensive genomic evolution to adapt to the animal host niche [17]. Such adaptation includes at least two major challenges: first, surviving from a plant-associated to an animal-associated nutritional environment, in a desert or a semi-dessert setting, and second, surviving from host immune detection and defense. The distribution of substrates in the Coccidioides transporters shows that a large number of transporters use proteins, amino acids and derivatives as substrates (Supplementary Tables S2 and S3), indicative of the need for an animal-associated nutritional niche. Moreover, the observed lineage-specific expansion of transporter families including ABC and MFS may be an outcome of positive natural selection in response to the external stress within a human host and to antifungal treatments, thereby contributing to the development of infectious phenotypes in Coccidioides [16,17].
We are bearing in mind that the catalog presented in this study are in silico predictions that await experimental validation. Historically, Coccidioides are understudied, partly due to the required biosafety level 3 (BSL3) laboratory containment and the special expertise needed to manage the aerosol risk posed by the large amounts of spores, and the severity of coccidioidomycosis. Experimental assays standard for other organisms can often be time-consuming or cost-prohibitive. Thanks to the advent of the genomic era, the in silico approach is becoming a cost-effective and efficient approach to identify and prioritize genes for wetlab characterization, especially suitable for non-model organisms such as Coccidioides.
While the roles of Coccidioides transporters in physiology, pathogenesis, and stress response are yet to be fully investigated, this study represents an initial attempt to a systems-level understanding of the mechanisms underlying Coccidioides survival and infection. Transporters on the catalog are likely members of the cellular networks associated with nutrient uptake, ion balance, drug excursion, signaling, and regulation. With the availability of high throughput assays, it is possible to integrate various types of omic data to interrogate the expression profiles and associations of transporter genes and their upstream regulators and downstream substrates/effectors in a network perspective [128,131,132,133]. By combining in silico omics-based discovery with wetlab characterization, there is an increased likelihood of identifying new therapeutic targets for these neglected fungal pathogens in the genus of Coccidioides.
In addition to providing a catalog of Coccidioides transporters for characterization, this genomic study also raises an important unanswered question: what are the content and diversity of transporters among other pathogenic fungal species? Published work to date mostly focuses on specific transporters or transporter families. For example, Costa et al. [20] surveyed MFS multidrug transporters in pathogenic fungi such as C. albicans, C. tropicalis, C. parapsilosis, C. guilliermondii, C. lusitaniae, C. glabrata, A. fumigatus, and C. neoformans. Kovalchuk and Driessen [21] performed phylogenetic analysis of fungal ABC transporters. Our future direction will be to predict and classify transporters in other pathogenic fungi and conduct comprehensive comparative genomic analyses of the transporters on their diversity and lineage-specific features.
Acknowledgments
The authors are grateful to Chiung-Yu Hung for helpful discussion. We thank Milton Saier and scientists at the TCDB for making the transporter resources publicly available.
Abbreviations
| ABC | ATP-binding cassette |
| BSL | biosafety level |
| ChEBI | Chemical Entities of Biological Interest |
| CPR | Cholesterol/Phospholipid/Retinal |
| DCT | Drug Conjugate Transporter |
| DHA | Drug:H+ Antiporter |
| EPP | Eye Pigment Precursor Transporter |
| HMT | Heavy Metal Transporter |
| MDR | multidrug resistance |
| MFS | major facilitor superfamily |
| MPE | Mitochondrial Peptide Exporter |
| NCS | Nucleobase: Cation Symporter |
| NPC | Nuclear Pore Complex |
| PDR | Pleiotropic Drug Resistance |
| PFAT | Peroxysomal Fatty Acyl CoA Transporter |
| TC | Transport Classification |
| TCDB | transporter classification database |
| TMS | transmembrane segment |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof8101064/s1, Supplementary Table S1. (A) Putative transporters in C. immitis. (B) Putative transporters in C. posadasii. Supplementary Table S2. Counts of seven classes of transporter proteins according to the substrate types in C. immitis. Transporters without identified substrate(s) were excluded. Supplementary Table S3. Counts of seven classes of transporter proteins according to the substrate types in C. posadasii. Transporters without identified substrate(s) were excluded.
Author Contributions
Conceptualization, J.G., Y.W. and H.C.; Data analysis, H.C., H.Z., D.H.G., Y.W. and J.G.; writing—original draft preparation, Y.W., J.G. and H.C.; writing—review and editing, H.C., H.Z., D.H.G., Y.W. and J.G.; funding acquisition, Y.W. and H.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The accession numbers of all the sequences are included in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by U.S. National Institutes of Health, grant number U19AI166761.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kirkland T.N., Fierer J. Coccidioides immitis and posadasii; A review of their biology, genomics, pathogenesis, and host immunity. Virulence. 2018;9:1426–1435. doi: 10.1080/21505594.2018.1509667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen C., Barker B.M., Hoover S., Nix D.E., Ampel N.M., Frelinger J.A., Orbach M.J., Galgiani J.N. Recent Advances in Our Understanding of the Environmental, Epidemiological, Immunological, and Clinical Dimensions of Coccidioidomycosis. Clin. Microbiol. Rev. 2013;26:505–525. doi: 10.1128/CMR.00005-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention Increase in reported coccidioidomycosis—United States, 1998–2011. MMWR Morb. Mortal. Wkly. Rep. 2013;62:217–221. [PMC free article] [PubMed] [Google Scholar]
- 4.Sondermeyer Cooksey G.L., Nguyen A., Vugia D., Jain S. Regional Analysis of Coccidioidomycosis Incidence-California, 2000–2018. MMWR Morb. Mortal. Wkly. Rep. 2020;69:1817–1821. doi: 10.15585/mmwr.mm6948a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boro R., Iyer P.C., Walczak M.A. Current Landscape of Coccidioidomycosis. J. Fungi. 2022;8:413. doi: 10.3390/jof8040413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention Coccidioidomycosis in workers at an archeologic site—Dinosaur National Monument, Utah, June–July 2001. MMWR Morb. Mortal. Wkly. Rep. 2001;50:1005–1008. [PubMed] [Google Scholar]
- 7.Johnson S.M., Carlson E.L., Fisher F.S., Pappagianis D. Demonstration of Coccidioides immitis and Coccidioides posadasii DNA in soil samples collected from Dinosaur National Monument, Utah. Med. Mycol. 2014;52:610–617. doi: 10.1093/mmy/myu004. [DOI] [PubMed] [Google Scholar]
- 8.Marsden-Haug N., Goldoft M., Ralston C., Limaye A.P., Chua J., Hill H., Jecha L., Thompson G.R., 3rd, Chiller T. Coccidioidomycosis Acquired in Washington State. Clin. Infect. Dis. 2013;56:847–850. doi: 10.1093/cid/cis1028. [DOI] [PubMed] [Google Scholar]
- 9.Litvintseva A.P., Marsden-Haug N., Hurst S., Hill H., Gade L., Driebe E.M., Ralston C., Roe C., Barker B.M., Goldoft M., et al. Valley Fever: Finding New Places for an Old Disease: Coccidioides immitis Found in Washington State Soil Associated With Recent Human Infection. Clin. Infect. Dis. 2015;60:e1–e3. doi: 10.1093/cid/ciu681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teixeira M.d.M., Barker B.M., Stajich J.E. Improved Reference Genome Sequence of Coccidioides immitis Strain WA_211, Isolated in Washington State. Microbiol. Resour. Announc. 2019;8:33. doi: 10.1128/MRA.00149-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kriesel J.D., Sutton D.A., Schulman S., Fothergill A.W., Rinaldi M.G. Persistent pulmonary infection with an azole-resistant Coccidioides species. Med. Mycol. 2008;46:607–610. doi: 10.1080/13693780802140923. [DOI] [PubMed] [Google Scholar]
- 12.Ramani R., Chaturvedi V. Antifungal susceptibility profiles of Coccidioides immitis and Coccidioides posadasii from endemic and non-endemic areas. Mycopathologia. 2007;163:315–319. doi: 10.1007/s11046-007-9018-7. [DOI] [PubMed] [Google Scholar]
- 13.Thompson G.R.T., 3rd, Barker B.M., Wiederhold N.P. Large-Scale Evaluation of In Vitro Amphotericin B, Triazole, and Echinocandin Activity against Coccidioides Species from U.S. Institutions. Antimicrob. Agents Chemother. 2017;61:e02634-16. doi: 10.1128/AAC.02634-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hernandez H., Erives V.H., Martinez L.R. Coccidioidomycosis: Epidemiology, Fungal Pathogenesis, and Therapeutic Development. Curr. Trop. Med. Rep. 2019;6:132–144. doi: 10.1007/s40475-019-00184-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCotter O.Z., Benedict K., Engelthaler D.M., Komatsu K., Lucas K.D., Mohle-Boetani J.C., Oltean H., Vugia D., Chiller T.M., Sondermeyer Cooksey G.L., et al. Update on the Epidemiology of coccidioidomycosis in the United States. Med. Mycol. 2019;57:S30–S40. doi: 10.1093/mmy/myy095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neafsey D.E., Barker B.M., Sharpton T.J., Stajich J.E., Park D.J., Whiston E., Hung C.-Y., McMahan C., White J., Sykes S., et al. Population genomic sequencing of Coccidioides fungi reveals recent hybridization and transposon control. Genome Res. 2010;20:938–946. doi: 10.1101/gr.103911.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharpton T.J., Stajich J.E., Rounsley S.D., Gardner M.J., Wortman J.R., Jordar V.S., Maiti R., Kodira C.D., Neafsey D.E., Zeng Q., et al. Comparative genomic analyses of the human fungal pathogens Coccidioides and their relatives. Genome Res. 2009;19:1722–1731. doi: 10.1101/gr.087551.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teixeira M.M., Alvarado P., Roe C.C., Thompson G.R., 3rd, Patané J.S.L., Sahl J.W., Keim P., Galgiani J.N., Litvintseva A.P., Matute D.R., et al. Population Structure and Genetic Diversity among Isolates of Coccidioides posadasii in Venezuela and Surrounding Regions. mBio. 2019;10:e01976-19. doi: 10.1128/mBio.01976-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Melo Teixeira M., Stajich J.E., Sahl J.W., Thompson G.R., Brem R.B., Dubin C.A., Blackmon A.V., Mead H.L., Keim P., Barker B.M. A chromosomal-level reference genome of the widely utilized Coccidioides posadasii laboratory strain “Silveira”. G3 Genes|Genomes|Genetics. 2022;12:jkac031. doi: 10.1093/g3journal/jkac031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costa C., Dias P.J., Sa-Correia I., Teixeira M.C. MFS multidrug transporters in pathogenic fungi: Do they have real clinical impact? Front. Physiol. 2014;5:197. doi: 10.3389/fphys.2014.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kovalchuk A., Driessen A.J.M. Phylogenetic analysis of fungal ABC transporters. BMC Genom. 2010;11:177. doi: 10.1186/1471-2164-11-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sipos G., Kuchler K. Fungal ATP-Binding Cassette (ABC) Transporters in Drug Resistance & Detoxification. Curr. Drug Targets. 2006;7:471–481. doi: 10.2174/138945006776359403. [DOI] [PubMed] [Google Scholar]
- 23.Wolfger H., Mamnun Y.M., Kuchler K. Fungal ABC proteins: Pleiotropic drug resistance, stress response and cellular detoxification. Res. Microbiol. 2001;152:375–389. doi: 10.1016/S0923-2508(01)01209-8. [DOI] [PubMed] [Google Scholar]
- 24.Prasad R., Rawal M.K. Efflux pump proteins in antifungal resistance. Front. Pharmacol. 2014;5:202. doi: 10.3389/fphar.2014.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris A., Wagner M., Du D., Raschka S., Nentwig L.-M., Gohlke H., Smits S.H.J., Luisi B.F., Schmitt L. Structure and efflux mechanism of the yeast pleiotropic drug resistance transporter Pdr5. Nat. Commun. 2021;12:5254. doi: 10.1038/s41467-021-25574-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paul S., Moye-Rowley W.S. Multidrug resistance in fungi: Regulation of transporter-encoding gene expression. Front. Physiol. 2014;5:143. doi: 10.3389/fphys.2014.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saier M.H., Reddy V.S., Moreno-Hagelsieb G., Hendargo K.J., Zhang Y., Iddamsetty V., Lam K.J.K., Tian N., Russum S., Wang J., et al. The Transporter Classification Database (TCDB): 2021 update. Nucleic Acids Res. 2021;49:D461–D467. doi: 10.1093/nar/gkaa1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saier M.H., Jr., Reddy V.S., Tsu B.V., Ahmed M.S., Li C., Moreno-Hagelsieb G. The Transporter Classification Database (TCDB): Recent advances. Nucleic Acids Res. 2016;44:D372–D379. doi: 10.1093/nar/gkv1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 30.Mistry J., Chuguransky S., Williams L., Qureshi M., Salazar G.A., Sonnhammer E.L.L., Tosatto S.C.E., Paladin L., Raj S., Richardson L.J., et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021;49:D412–D419. doi: 10.1093/nar/gkaa913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eddy S.R. Profile hidden Markov models. Bioinformatics. 1998;14:755–763. doi: 10.1093/bioinformatics/14.9.755. [DOI] [PubMed] [Google Scholar]
- 32.Krogh A., Larsson B., von Heijne G., Sonnhammer E.L. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 33.Hastings J., Owen G., Dekker A., Ennis M., Kale N., Muthukrishnan V., Turner S., Swainston N., Mendes P., Steinbeck C. ChEBI in 2016: Improved services and an expanding collection of metabolites. Nucleic Acids Res. 2016;44:D1214–D1219. doi: 10.1093/nar/gkv1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edgar R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madeira F., Pearce M., Tivey A.R.N., Basutkar P., Lee J., Edbali O., Madhusoodanan N., Kolesnikov A., Lopez R. Search and sequence analysis tools services from EMBL-EBI in 2022. Nucleic Acids Res. 2022;50:W276–W279. doi: 10.1093/nar/gkac240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saitou N., Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 37.Felsenstein J. Evolutionary trees from DNA sequences: A maximum likelihood approach. J. Mol. Evol. 1981;17:368–376. doi: 10.1007/BF01734359. [DOI] [PubMed] [Google Scholar]
- 38.Tamura K., Stecher G., Kumar S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021;38:3022–3027. doi: 10.1093/molbev/msab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Felsenstein J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 40.Forrest L.R., Krämer R., Ziegler C. The structural basis of secondary active transport mechanisms. Biochim. Biophys. Acta. 2011;1807:167–188. doi: 10.1016/j.bbabio.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 41.Tanford C. Mechanism of Free Energy Coupling in Active Transport. Annu. Rev. Biochem. 1983;52:379–409. doi: 10.1146/annurev.bi.52.070183.002115. [DOI] [PubMed] [Google Scholar]
- 42.Wirsching S., Michel S., Köhler G., Morschhäuser J. Activation of the Multiple Drug Resistance Gene MDR1 in Fluconazole-Resistant, Clinical Candida albicans Strains Is Caused by Mutations in a trans-Regulatory Factor. J. Bacteriol. 2000;182:400–404. doi: 10.1128/JB.182.2.400-404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wirsching S., Michel S., Morschhauser J. Targeted gene disruption in Candida albicans wild-type strains: The role of the MDR1 gene in fluconazole resistance of clinical Candida albicans isolates. Mol. Microbiol. 2000;36:856–865. doi: 10.1046/j.1365-2958.2000.01899.x. [DOI] [PubMed] [Google Scholar]
- 44.Yamada-Okabe T., Yamada-Okabe H. Characterization of the CaNAG3, CaNAG4, and CaNAG6 genes of the pathogenic fungus Candida albicans: Possible involvement of these genes in the susceptibilities of cytotoxic agents. FEMS Microbiol. Lett. 2002;212:15–21. doi: 10.1111/j.1574-6968.2002.tb11238.x. [DOI] [PubMed] [Google Scholar]
- 45.Li R., Kumar R., Tati S., Puri S., Edgerton M. Candida albicans Flu1-Mediated Efflux of Salivary Histatin 5 Reduces Its Cytosolic Concentration and Fungicidal Activity. Antimicrob. Agents Chemother. 2013;57:1832–1839. doi: 10.1128/AAC.02295-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen K.-H., Miyazaki T., Tsai H.-F., Bennett J.E. The bZip transcription factor Cgap1p is involved in multidrug resistance and required for activation of multidrug transporter gene CgFLR1 in Candida glabrata. Gene. 2007;386:63–72. doi: 10.1016/j.gene.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 47.Costa C., Pires C., Cabrito T.R., Renaudin A., Ohno M., Chibana H., Sá-Correia I., Teixeira M.C. Candida glabrata Drug: H+ Antiporter CgQdr2 Confers Imidazole Drug Resistance, Being Activated by Transcription Factor CgPdr1. Antimicrob. Agents Chemother. 2013;57:3159–3167. doi: 10.1128/AAC.00811-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barchiesi F., Calabrese D., Sanglard D., Falconi Di Francesco L., Caselli F., Giannini D., Giacometti A., Gavaudan S., Scalise G. Experimental Induction of Fluconazole Resistance in Candida tropicalis ATCC 750. Antimicrob. Agents Chemother. 2000;44:1578–1584. doi: 10.1128/AAC.44.6.1578-1584.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bowyer P., Mosquera J., Anderson M., Birch M., Bromley M., Denning D.W. Identification of novel genes conferring altered azole susceptibility in Aspergillus fumigatus. FEMS Microbiol. Lett. 2012;332:10–19. doi: 10.1111/j.1574-6968.2012.02575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thever M.D., Saier M.H., Jr. Bioinformatic Characterization of P-Type ATPases Encoded Within the Fully Sequenced Genomes of 26 Eukaryotes. J. Membr. Biol. 2009;229:115–130. doi: 10.1007/s00232-009-9176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodríguez-Navarro A., Benito B. Sodium or potassium efflux ATPase: A fungal, bryophyte, and protozoal ATPase. Biochim. Biophys. Acta. 2010;1798:1841–1853. doi: 10.1016/j.bbamem.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 52.Grigoryan G., Moore D.T., DeGrado W.F. Transmembrane Communication: General Principles and Lessons from the Structure and Function of the M2 Proton Channel, K+ Channels, and Integrin Receptors. Annu. Rev. Biochem. 2011;80:211–237. doi: 10.1146/annurev-biochem-091008-152423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beck M., Hurt E. The nuclear pore complex: Understanding its function through structural insight. Nat. Rev. Mol. Cell Biol. 2017;18:73–89. doi: 10.1038/nrm.2016.147. [DOI] [PubMed] [Google Scholar]
- 54.Paidhungat M., Garrett S. A homolog of mammalian, voltage-gated calcium channels mediates yeast pheromone-stimulated Ca2+ uptake and exacerbates the cdc1(Ts) growth defect. Mol. Cell. Biol. 1997;17:6339–6347. doi: 10.1128/MCB.17.11.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bertl A., Ramos J., Ludwig J., Lichtenberg-Fraté H., Reid J., Bihler H., Calero F., Martinez P., Ljungdahl P.O. Characterization of potassium transport in wild-type and isogenic yeast strains carrying all combinations of trk1, trk2 and tok1 null mutations. Mol. Microbiol. 2003;47:767–780. doi: 10.1046/j.1365-2958.2003.03335.x. [DOI] [PubMed] [Google Scholar]
- 56.Brand A., Lee K., Veses V., Gow N.A.R. Calcium homeostasis is required for contact-dependent helical and sinusoidal tip growth in Candida albicans hyphae. Mol. Microbiol. 2009;71:1155–1164. doi: 10.1111/j.1365-2958.2008.06592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Benčina M., Bagar T., Lah L., Kraševec N. A comparative genomic analysis of calcium and proton signaling/homeostasis in Aspergillus species. Fungal Genet. Biol. 2009;46((Suppl. 1)):S93–S104. doi: 10.1016/j.fgb.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 58.Liu M., Du P., Heinrich G., Cox G.M., Gelli A. Cch1 Mediates Calcium Entry in Cryptococcus neoformans and Is Essential in Low-Calcium Environments. Eukaryot. Cell. 2006;5:1788–1796. doi: 10.1128/EC.00158-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prole D.L., Taylor C.W. Identification and Analysis of Cation Channel Homologues in Human Pathogenic Fungi. PLoS ONE. 2012;7:e42404. doi: 10.1371/journal.pone.0042404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Theisen U., Straube A., Steinberg G. Dynamic Rearrangement of Nucleoporins during Fungal “Open” Mitosis. Mol. Biol. Cell. 2008;19:1230–1240. doi: 10.1091/mbc.e07-02-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steinberg G., Schuster M., Theisen U., Kilaru S., Forge A., Martin-Urdiroz M. Motor-driven motility of fungal nuclear pores organizes chromosomes and fosters nucleocytoplasmic transport. J. Cell Biol. 2012;198:343–355. doi: 10.1083/jcb.201201087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rees D.C., Johnson E., Lewinson O. ABC transporters: The power to change. Nat. Rev. Mol. Cell Biol. 2009;10:218–227. doi: 10.1038/nrm2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davidson A.L., Dassa E., Orelle C., Chen J. Structure, Function, and Evolution of Bacterial ATP-Binding Cassette Systems. Microbiol. Mol. Biol. Rev. 2008;72:317–364. doi: 10.1128/MMBR.00031-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Davidson A.L., Maloney P.C. ABC transporters: How small machines do a big job. Trends Microbiol. 2007;15:448–455. doi: 10.1016/j.tim.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 65.Dassa E., Bouige P. The ABC of ABCs: A phylogenetic and functional classification of ABC systems in living organisms. Res. Microbiol. 2001;152:211–229. doi: 10.1016/S0923-2508(01)01194-9. [DOI] [PubMed] [Google Scholar]
- 66.Albers S.-V., Koning S.M., Konings W.N., Driessen A.J. Insights into ABC Transport in Archaea. J. Bioenerg. Biomembr. 2004;36:5–15. doi: 10.1023/B:JOBB.0000019593.84933.e6. [DOI] [PubMed] [Google Scholar]
- 67.Xiong J., Feng J., Yuan D., Zhou J., Miao W. Tracing the structural evolution of eukaryotic ATP binding cassette transporter superfamily. Sci. Rep. 2015;5:16724. doi: 10.1038/srep16724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saurin W., Hofnung M., Dassa E. Getting In or Out: Early Segregation Between Importers and Exporters in the Evolution of ATP-Binding Cassette (ABC) Transporters. J. Mol. Evol. 1999;48:22–41. doi: 10.1007/PL00006442. [DOI] [PubMed] [Google Scholar]
- 69.Schneider E., Hunke S. ATP-binding-cassette (ABC) transport systems: Functional and structural aspects of the ATP-hydrolyzing subunits/domains. FEMS Microbiol. Rev. 1998;22:1–20. doi: 10.1111/j.1574-6976.1998.tb00358.x. [DOI] [PubMed] [Google Scholar]
- 70.Ford R.C., Beis K. Learning the ABCs one at a time: Structure and mechanism of ABC transporters. Biochem. Soc. Trans. 2019;47:23–36. doi: 10.1042/BST20180147. [DOI] [PubMed] [Google Scholar]
- 71.Juan-Carlos P.-D.M., Perla-Lidia P.-P., Stephanie-Talia M.-M., Mónica-Griselda A.-M., Luz-María T.-E. ABC transporter superfamily. An updated overview, relevance in cancer multidrug resistance and perspectives with personalized medicine. Mol. Biol. Rep. 2021;48:1883–1901. doi: 10.1007/s11033-021-06155-w. [DOI] [PubMed] [Google Scholar]
- 72.Gottesman M.M., Fojo T., Bates S.E. Multidrug resistance in cancer: Role of ATP–dependent transporters. Nat. Rev. Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 73.Piddock L.J.V. Multidrug-resistance efflux pumps-not just for resistance. Nat. Rev. Microbiol. 2006;4:629–636. doi: 10.1038/nrmicro1464. [DOI] [PubMed] [Google Scholar]
- 74.Bissinger P.H., Kuchler K. Molecular cloning and expression of the Saccharomyces cerevisiae STS1 gene product. A yeast ABC transporter conferring mycotoxin resistance. J. Biol. Chem. 1994;269:4180–4186. doi: 10.1016/S0021-9258(17)41760-1. [DOI] [PubMed] [Google Scholar]
- 75.Kolaczkowski M., van der Rest M., Cybularz-Kolaczkowska A., Soumillion J.-P., Konings W.N., Goffeau A. Anticancer Drugs, Ionophoric Peptides, and Steroids as Substrates of the Yeast Multidrug Transporter Pdr5p. J. Biol. Chem. 1996;271:31543–31548. doi: 10.1074/jbc.271.49.31543. [DOI] [PubMed] [Google Scholar]
- 76.Kolaczowski M., Kolaczkowska A., Luczynski J., Witek S., Goffeau A. In Vivo Characterization of the Drug Resistance Profile of the Major ABC Transporters and Other Components of the Yeast Pleiotropic Drug Resistance Network. Microb. Drug Resist. 1998;4:143–158. doi: 10.1089/mdr.1998.4.143. [DOI] [PubMed] [Google Scholar]
- 77.Piper P., Mahé Y., Thompson S., Pandjaitan R., Holyoak C., Egner R., Mühlbauer M., Coote P., Kuchler K. The pdr12 ABC transporter is required for the development of weak organic acid resistance in yeast. EMBO J. 1998;17:4257–4265. doi: 10.1093/emboj/17.15.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Holyoak C.D., Bracey D., Piper P.W., Kuchler K., Coote P.J. The Saccharomyces cerevisiae Weak-Acid-Inducible ABC Transporter Pdr12 Transports Fluorescein and Preservative Anions from the Cytosol by an Energy-Dependent Mechanism. J. Bacteriol. 1999;181:4644–4652. doi: 10.1128/JB.181.15.4644-4652.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Katzmann D.J., Epping E.A., Moye-Rowley W.S. Mutational Disruption of Plasma Membrane Trafficking of Saccharomyces cerevisiae Yor1p, a Homologue of Mammalian Multidrug Resistance Protein. Mol. Cell. Biol. 1999;19:2998–3009. doi: 10.1128/MCB.19.4.2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nishi K., Yoshida M., Nishimura M., Nishikawa M., Nishiyama M., Horinouchi S., Beppu T. A leptomycin B resistance gene of Schizosaccharomyces pombe encodes a protein similar to the mammalian P-glycoproteins. Mol. Microbiol. 1992;6:761–769. doi: 10.1111/j.1365-2958.1992.tb01526.x. [DOI] [PubMed] [Google Scholar]
- 81.Nagao K., Taguchi Y., Arioka M., Kadokura H., Takatsuki A., Yoda K., Yamasaki M. bfr1+, a novel gene of Schizosaccharomyces pombe which confers brefeldin A resistance, is structurally related to the ATP-binding cassette superfamily. J. Bacteriol. 1995;177:1536–1543. doi: 10.1128/jb.177.6.1536-1543.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sanglard D., Odds F.C. Resistance of Candida species to antifungal agents: Molecular mechanisms and clinical consequences. Lancet Infect. Dis. 2002;2:73–85. doi: 10.1016/S1473-3099(02)00181-0. [DOI] [PubMed] [Google Scholar]
- 83.Rogers T.R., Verweij P.E., Castanheira M., Dannaoui E., White P.L., Arendrup M.C., Subcommittee on Antifungal Susceptibility Testing of the EECfAST Molecular mechanisms of acquired antifungal drug resistance in principal fungal pathogens and EUCAST guidance for their laboratory detection and clinical implications. J. Antimicrob. Chemother. 2022;77:2053–2073. doi: 10.1093/jac/dkac161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sanglard D., Coste A.T. Activity of Isavuconazole and Other Azoles against Candida Clinical Isolates and Yeast Model Systems with Known Azole Resistance Mechanisms. Antimicrob. Agents Chemother. 2016;60:229–238. doi: 10.1128/AAC.02157-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Prasad R., De Wergifosse P., Goffeau A., Balzi E. Molecular cloning and characterization of a novel gene of Candida albicans, CDR1, conferring multiple resistance to drugs and antifungals. Curr. Genet. 1995;27:320–329. doi: 10.1007/BF00352101. [DOI] [PubMed] [Google Scholar]
- 86.Sanglard D., Ischer F., Monod M., Bille J. Cloning of Candida albicans genes conferring resistance to azole antifungal agents: Characterization of CDR2, a new multidrug ABC transporter gene. Microbiology. 1997;143:405–416. doi: 10.1099/00221287-143-2-405. [DOI] [PubMed] [Google Scholar]
- 87.Lamping E., Baret P.V., Holmes A.R., Monk B.C., Goffeau A., Cannon R.D. Fungal PDR transporters: Phylogeny, topology, motifs and function. Fungal Genet. Biol. 2010;47:127–142. doi: 10.1016/j.fgb.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Posteraro B., Sanguinetti M., Sanglard D., La Sorda M., Boccia S., Romano L., Morace G., Fadda G. Identification and characterization of a Cryptococcus neoformans ATP binding cassette (ABC) transporter-encoding gene, CnAFR1, involved in the resistance to fluconazole. Mol. Microbiol. 2003;47:357–371. doi: 10.1046/j.1365-2958.2003.03281.x. [DOI] [PubMed] [Google Scholar]
- 89.Monod M., Feuermann M., Salamin K., Fratti M., Makino M., Alshahni M.M., Makimura K., Yamada T. Trichophyton rubrum Azole Resistance Mediated by a New ABC Transporter, TruMDR3. Antimicrob. Agents Chemother. 2019;63:e00863-19. doi: 10.1128/AAC.00863-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bera K., Rani P., Kishor G., Agarwal S., Kumar A., Singh D.V. Structural elucidation of transmembrane domain zero (TMD0) of EcdL: A multidrug resistance-associated protein (MRP) family of ATP-binding cassette transporter protein revealed by atomistic simulation. J. Biomol. Struct. Dyn. 2018;36:2938–2950. doi: 10.1080/07391102.2017.1372311. [DOI] [PubMed] [Google Scholar]
- 91.Martins M.P., Franceschini A.C.C., Jacob T.R., Rossi A., Martinez-Rossi N.M. Compensatory expression of multidrug-resistance genes encoding ABC transporters in dermatophytes. J. Med. Microbiol. 2016;65:605–610. doi: 10.1099/jmm.0.000268. [DOI] [PubMed] [Google Scholar]
- 92.Costa C., Ribeiro J., Miranda I.M., Silva-Dias A., Cavalheiro M., Costa-De-Oliveira S., Rodrigues A.G., Teixeira M.C. Clotrimazole Drug Resistance in Candida glabrata Clinical Isolates Correlates with Increased Expression of the Drug: H(+) Antiporters CgAqr1, CgTpo1_1, CgTpo3, and CgQdr2. Front. Microbiol. 2016;7:526. doi: 10.3389/fmicb.2016.00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yu J., Bhatnagar D., Cleveland T.E. Completed sequence of aflatoxin pathway gene cluster in Aspergillus parasiticus. FEBS Lett. 2004;564:126–130. doi: 10.1016/S0014-5793(04)00327-8. [DOI] [PubMed] [Google Scholar]
- 94.Omrane S., Audéon C., Ignace A., Duplaix C., Aouini L., Kema G., Walker A.-S., Fillinger S. Plasticity of the MFS1 Promoter Leads to Multidrug Resistance in the Wheat Pathogen Zymoseptoria tritici. mSphere. 2017;2:e00393-17. doi: 10.1128/mSphere.00393-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Goudela S., Tsilivi H., Diallinas G. Comparative kinetic analysis of AzgA and Fcy21p, prototypes of the two major fungal hypoxanthine-adenine-guanine transporter families. Mol. Membr. Biol. 2006;23:291–303. doi: 10.1080/09687860600685109. [DOI] [PubMed] [Google Scholar]
- 96.Cecchetto G., Amillis S., Diallinas G., Scazzocchio C., Drevet C. The AzgA Purine Transporter of Aspergillus nidulans. Characterization of a protein belonging to a new phylogenetic cluster. J. Biol. Chem. 2004;279:3132–3141. doi: 10.1074/jbc.M308826200. [DOI] [PubMed] [Google Scholar]
- 97.Zuckerkandl E., Pauling L. Evolutionary divergence and convergence in proteins. In: Bryson V., Vogel H.J., editors. Evolving Genes and Proteins. Academic Press; New York, NY, USA: 1965. pp. 97–166. [Google Scholar]
- 98.Ortiz D.F., Kreppel L., Speiser D.M., Scheel G., McDonald G., Ow D.W. Heavy metal tolerance in the fission yeast requires an ATP-binding cassette-type vacuolar membrane transporter. EMBO J. 1992;11:3491–3499. doi: 10.1002/j.1460-2075.1992.tb05431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Srinivasan V., Pierik A.J., Lill R. Crystal Structures of Nucleotide-Free and Glutathione-Bound Mitochondrial ABC Transporter Atm1. Science. 2014;343:1137–1140. doi: 10.1126/science.1246729. [DOI] [PubMed] [Google Scholar]
- 100.Philpott C.C., Leidgens S., Frey A.G. Metabolic remodeling in iron-deficient fungi. Biochim. Biophys. Acta. 2012;1823:1509–1520. doi: 10.1016/j.bbamcr.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kispal G., Csere P., Guiard B., Lill R. The ABC transporter Atm1p is required for mitochondrial iron homeostasis. FEBS Lett. 1997;418:346–350. doi: 10.1016/S0014-5793(97)01414-2. [DOI] [PubMed] [Google Scholar]
- 102.Kispal G., Csere P., Prohl C., Lill R. The mitochondrial proteins Atm1p and Nfs1p are essential for biogenesis of cytosolic Fe/S proteins. EMBO J. 1999;18:3981–3989. doi: 10.1093/emboj/18.14.3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Grigoras I., Lazard M., Plateau P., Blanquet S. Functional characterization of the Saccharomyces cerevisiae ABC-transporter Yor1p overexpressed in plasma membranes. Biochim. Biophys. Acta. 2008;1778:68–78. doi: 10.1016/j.bbamem.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 104.Lazard M., Ha-Duong N.-T., Mounié S., Perrin R., Plateau P., Blanquet S. Selenodiglutathione uptake by the Saccharomyces cerevisiae vacuolar ATP-binding cassette transporter Ycf1p. FEBS J. 2011;278:4112–4121. doi: 10.1111/j.1742-4658.2011.08318.x. [DOI] [PubMed] [Google Scholar]
- 105.Van Roermund C.W.T., Visser W.F., Ijlst L., Waterham H.R., Wanders R.J.A. Differential substrate specificities of human ABCD1 and ABCD2 in peroxisomal fatty acid β-oxidation. Biochim. Biophys. Acta. 2011;1811:148–152. doi: 10.1016/j.bbalip.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 106.Oram J.F., Wolfbauer G., Tang C., Davidson W.S., Albers J.J. An Amphipathic Helical Region of the N-terminal Barrel of Phospholipid Transfer Protein Is Critical for ABCA1-dependent Cholesterol Efflux. J. Biol. Chem. 2008;283:11541–11549. doi: 10.1074/jbc.M800117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Quistgaard E.M., Löw C., Guettou F., Nordlund P. Understanding transport by the major facilitator superfamily (MFS): Structures pave the way. Nat. Rev. Mol. Cell Biol. 2016;17:123–132. doi: 10.1038/nrm.2015.25. [DOI] [PubMed] [Google Scholar]
- 108.Pao S.S., Paulsen I.T., Saier M.H., Jr. Major Facilitator Superfamily. Microbiol. Mol. Biol. Rev. 1998;62:1–34. doi: 10.1128/MMBR.62.1.1-34.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Reddy V.S., Shlykov M.A., Castillo R., Sun E.I., Saier M.H., Jr. The major facilitator superfamily (MFS) revisited. FEBS J. 2012;279:2022–2035. doi: 10.1111/j.1742-4658.2012.08588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Saidijam M., Benedetti G., Ren Q., Xu Z., Hoyle C.J., Palmer S.L., Ward A., Bettaney K.E., Szakonyi G., Meuller J., et al. Microbial Drug Efflux Proteins of the Major Facilitator Superfamily. Curr. Drug Targets. 2006;7:793–811. doi: 10.2174/138945006777709575. [DOI] [PubMed] [Google Scholar]
- 111.Gulshan K., Moye-Rowley W.S. Multidrug Resistance in Fungi. Eukaryot. Cell. 2007;6:1933–1942. doi: 10.1128/EC.00254-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dos Santos S.C., Teixeira M.C., Dias P.J., Sã¡-Correia I. MFS transporters required for multidrug/multixenobiotic (MD/MX) resistance in the model yeast: Understanding their physiological function through post-genomic approaches. Front. Physiol. 2014;5:180. doi: 10.3389/fphys.2014.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cannon R.D., Lamping E., Holmes A.R., Niimi K., Baret P., Keniya M.V., Tanabe K., Niimi M., Goffeau A., Monk B.C. Efflux-Mediated Antifungal Drug Resistance. Clin. Microbiol. Rev. 2009;22:291–321. doi: 10.1128/CMR.00051-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sá-Correia I., dos Santos S.C., Teixeira M.C., Cabrito T.R., Mira N.P. Drug:H+ antiporters in chemical stress response in yeast. Trends Microbiol. 2009;17:22–31. doi: 10.1016/j.tim.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 115.Morschhäuser J. Regulation of multidrug resistance in pathogenic fungi. Fungal Genet. Biol. 2010;47:94–106. doi: 10.1016/j.fgb.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 116.Prasad R., Nair R., Banerjee A. Multidrug transporters of Candida species in clinical azole resistance. Fungal Genet. Biol. 2019;132:103252. doi: 10.1016/j.fgb.2019.103252. [DOI] [PubMed] [Google Scholar]
- 117.Wright M.B., Howell E.A., Gaber R.F. Amino acid substitutions in membrane-spanning domains of Hol1, a member of the major facilitator superfamily of transporters, confer nonselective cation uptake in Saccharomyces cerevisiae. J. Bacteriol. 1996;178:7197–7205. doi: 10.1128/jb.178.24.7197-7205.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Del Sorbo G., Schoonbeek H., De Waard M.A. Fungal Transporters Involved in Efflux of Natural Toxic Compounds and Fungicides. Fungal Genet. Biol. 2000;30:1–15. doi: 10.1006/fgbi.2000.1206. [DOI] [PubMed] [Google Scholar]
- 119.Bozdag G.O., Uluisik I., Gulculer G.S., Karakaya H.C., Koc A. Roles of ATR1 paralogs YMR279c and YOR378w in boron stress tolerance. Biochem. Biophys. Res. Commun. 2011;409:748–751. doi: 10.1016/j.bbrc.2011.05.080. [DOI] [PubMed] [Google Scholar]
- 120.Pantazopoulou A., Diallinas G. Fungal nucleobase transporters. FEMS Microbiol. Rev. 2007;31:657–675. doi: 10.1111/j.1574-6976.2007.00083.x. [DOI] [PubMed] [Google Scholar]
- 121.Krypotou E., Evangelidis T., Bobonis J., Pittis A.A., Gabaldón T., Scazzocchio C., Mikros E., Diallinas G. Origin, diversification and substrate specificity in the family of NCS1/FUR transporters. Mol. Microbiol. 2015;96:927–950. doi: 10.1111/mmi.12982. [DOI] [PubMed] [Google Scholar]
- 122.Zeng G.-F., Pypaert M., Slayman C.L. Epitope Tagging of the Yeast K+ Carrier Trk2p Demonstrates Folding That Is Consistent with a Channel-like Structure. J. Biol. Chem. 2004;279:3003–3013. doi: 10.1074/jbc.M309760200. [DOI] [PubMed] [Google Scholar]
- 123.Masaryk J., Sychrová H. Yeast Trk1 Potassium Transporter Gradually Changes Its Affinity in Response to Both External and Internal Signals. J. Fungi. 2022;8:432. doi: 10.3390/jof8050432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lee H., Kim H. Membrane topology of transmembrane proteins: Determinants and experimental tools. Biochem. Biophys. Res. Commun. 2014;453:268–276. doi: 10.1016/j.bbrc.2014.05.111. [DOI] [PubMed] [Google Scholar]
- 125.Jungwirth H., Kuchler K. Yeast ABC transporters—A tale of sex, stress, drugs and aging. FEBS Lett. 2006;580:1131–1138. doi: 10.1016/j.febslet.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 126.Keogh J.P. Membrane Transporters in Drug Development. Adv. Pharmacol. 2012;63:1131–1138. doi: 10.1016/b978-0-12-398339-8.00001-x. [DOI] [PubMed] [Google Scholar]
- 127.Delgado N., Hung C.-Y., Tarcha E., Gardner M.J., Cole G.T. Profiling gene expression in Coccidioides posadasii. Med Mycol. 2004;42:59–71. doi: 10.1080/1369378031000156890. [DOI] [PubMed] [Google Scholar]
- 128.Carlin A.F., Beyhan S., Peña J.F., Stajich J.E., Viriyakosol S., Fierer J., Kirkland T.N. Transcriptional Analysis of Coccidioides immitis Mycelia and Spherules by RNA Sequencing. J. Fungi. 2021;7:366. doi: 10.3390/jof7050366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhou Z., Sun N., Wu S., Li Y.-Q., Wang Y. Genomic data mining reveals a rich repertoire of transport proteins in Streptomyces. BMC Genom. 2016;17((Suppl. 7)):510. doi: 10.1186/s12864-016-2899-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mendez C., Salas J.A. ABC transporters in antibiotic-producing actinomycetes. FEMS Microbiol. Lett. 1998;158:1–8. doi: 10.1016/S0378-1097(97)00434-5. [DOI] [PubMed] [Google Scholar]
- 131.Duttke S.H., Beyhan S., Singh R., Neal S., Viriyakosol S., Fierer J., Kirkland T.N., Stajich J.E., Benner C., Carlin A.F. Decoding Transcription Regulatory Mechanisms Associated with Coccidioides immitis Phase Transition Using Total RNA. mSystems. 2022;7:e0140421. doi: 10.1128/msystems.01404-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Whiston E., Wise H.Z., Sharpton T.J., Jui G., Cole G.T., Taylor J.W. Comparative Transcriptomics of the Saprobic and Parasitic Growth Phases in Coccidioides spp. PLoS ONE. 2012;7:e41034. doi: 10.1371/journal.pone.0041034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mitchell N.M., Sherrard A.L., Dasari S., Magee D.M., Grys T.E., Lake D.F. Proteogenomic Re-Annotation of Coccidioides posadasii Strain Silveira. Proteomics. 2018;18:1700173. doi: 10.1002/pmic.201700173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The accession numbers of all the sequences are included in the manuscript.