Abstract
Pachycetus paulsonii, Pachycetus wardii, and Antaecetus aithai are middle Eocene archaeocete whales found in Europe, North America, and Africa, respectively. The three are placed in the new basilosaurid subfamily Pachycetinae. Antaecetus is a new genus known from Egypt and Morocco, and the only pachycetine known from a substantial postcranial skeleton. The skull of A. aithai described here resembles that of Saghacetus osiris in size, but lacks the narrowly constricted rostrum of Saghacetus. Antaecetus is smaller than Pachycetus and its teeth are more gracile. Upper premolars differ in having two rather than three accessory cusps flanking the principal cusp. Pachycetines differ from dorudontines in having elongated posterior thoracic and lumbar vertebrae like those of Basilosaurus, but differ from basilosaurines and from dorudontines in having conspicuously pachyosteosclerotic vertebrae with dense and thickly laminated cortical bone surrounding a cancellous core. Pachycetinae are also distinctive in having transverse processes on lumbar vertebrae nearly as long anteroposteriorly as the corresponding centrum. We infer from their pachyosteosclerotic vertebrae that pachycetines were probably sirenian-like slow swimmers living in shallow coastal seas and feeding on passing fish and mobile invertebrates.
Introduction
The cetacean family Basilosauridae is a cosmopolitan, fully-aquatic group of archaic whales or archaeocetes ranging in age from the late middle Eocene (latest Lutetian or early Bartonian stage-age) through the late Eocene (Priabonian stage-age). Most basilosaurids come from northern hemisphere localities in North Africa, Asia, Europe, and North America [1–4], but basilosaurids are also known from South America and Antarctica in the southern hemisphere [5, 6]. The temporal range of basilosaurids spans an interval from about 41 to 34 million years before present [7].
Fifteen genera and twenty-three species of Basilosauridae appear to be valid (Table 1). Basilosaurus cetoides, Zygorhiza kochii, Dorudon atrox, and Pontogeneus peruvianus are the four genera and species for which good skulls and associated skeletons have been described [1, 8, 9], and much of our understanding of Basilosauridae is based on these specimens. Pontogeneus is the appropriate generic name for P. brachyspondylus [1], and by extension P. peruvianus [10].
Table 1. Temporal and geographic distribution of three subfamilies, 15 genera, and 23 species of Eocene Basilosauridae.
| Genus and species | Species author, year, and page | Holotype | Age | Type locality | North latitude | East longitude |
|---|---|---|---|---|---|---|
| Basilosaurinae | ||||||
| Basilosaurus cetoides | Owen, 1841: 69 [11] | ANSP 12944A | Priabonian | Ouachita River, Louisiana, U.S.A. | 31.92800 | −91.94000 |
| Basilosaurus isis | Beadnell in Andrews, 1904: 214 [12] | CGM 10208 | Priabonian | Birket Qarun, Fayum, Egypt | 29.47200 | 30.35900 |
| Eocetus schweinfurthi | Fraas, 1904: 217 [13] | SMNS 10986 | Bartonian | Gebel Mokattam, Cairo, Egypt | 30.02200 | 31.27300 |
| Eocetus drazindai | Gingerich et al., 1997: 57 [14] | GSP-UM 3193 | Bartonian | Bari Nadi, Punjab, Pakistan | 30.78283 | 70.42783 |
| Basiloterus hussaini | Gingerich et al., 1997: 62 [14] | GSP-UM 3190 | Bartonian | Bari Nadi, Punjab, Pakistan | 30.78783 | 70.44050 |
| Dorudontinae | ||||||
| Dorudon serratus | Gibbes, 1845: 254 [15] | MCZ 8763 | Priabonian | Santee Canal, S. Carolina, U.S.A. | 33.26500 | −79.96000 |
| Zygorhiza kochii | Reichenbach, 1847: 13 [16] | MNB Ma-43248 | Priabonian | Uncertain, Alabama, U.S.A. | 31.68960 | −88.28170 |
| Pontogeneus brachyspondylus | Müller, 1849: 26 [17] | MNB unknown | Priabonian | Uncertain, Alabama, U.S.A. | 31.68960 | −88.28170 |
| Saghacetus osiris | Dames, 1894: 204 [18] | MNB 28388 | Priabonian | Garet el-Esh, Fayum, Egypt | 29.57100 | 30.56500 |
| Dorudon atrox | Andrews, 1906: 255 [19] | CGM 9319 | Priabonian | 12 km WSW Garet Gehannam, Egypt | 29.27300 | 30.03100 |
| Ancalacetus simonsi | Gingerich and Uhen, 1996: 363 [20] | CGM 42290 | Priabonian | Wadi Al Hitan WH-81, Fayum, Egypt | 29.27374 | 30.02344 |
| Chrysocetus healyorum | Uhen and Gingerich, 2001: 3 [21] | SCSM 87.195 | Priabonian | Santee Quarry, Holly Hill, S. Carolina, U.S. | 33.27800 | -80.42300 |
| Stromerius nidensis | Gingerich, 2007: 366 [22] | UM 100140 | Priabonian | Garet el-Esh, Fayum, Egypt | 29.57195 | 30.56637 |
| Masracetus markgrafi | Gingerich, 2007: 375 [22] | SMNS 11414 | Priabonian | Dimeh, Fayum, Egypt | 29.53600 | 30.66900 |
| Pontogeneus peruvianus | Martínez and Muizon, 2011: 518 [5] | MNHN.F.PRU 10 | Priabonian | Paracas Bay, Ica, Peru | −13.88175 | −76.23706 |
| Supayacetus muizoni | Uhen et al., 2011: 960 [23] | MUSM 1465 | Bartonian | AV-17, Ica, Peru | −14.66678 | −75.63515 |
| Ocucajea picklingi | Uhen et al., 2011: 963 [23] | MUSM 1442 | Bartonian | AV-19, Ica, Peru | −14.66830 | −75.63505 |
| Chrysocetus fouadassii | Gingerich and Zouhri, 2015: 278 [24] | FASC Bouj-1 | Bartonian | Sabkha de Gueran, Boujdour, Morocco | 25.12000 | −13.89000 |
| Pachycetinae | ||||||
| Pachycetus paulsonii | Brandt, 1873: 336 [25] | Lost | Bartonian | Chyhyryn, Cherkasy, Ukraine | 49.07300 | 32.66200 |
| Pachycetus wardii | Uhen, 1999: 514 [26] | USNM 310633 | Bartonian | Lanier Quarry, Maple Hill, N. Carolina, U.S. | 34.62500 | −77.67500 |
| Antaecetus aithai | Gingerich and Zouhri, 2015: 280 [24] | FASC Bouj-6 | Bartonian | Sabkha de Gueran, Boujdour, Morocco | 25.07667 | −13.90763 |
| Subfamily incertae sedis | ||||||
| ’Zeuglodon’ wanklyni | Seeley, 1876: 428 [27] | Lost | Bartonian | Barton Cliff, England, U.K. | 50.74280 | −1.65520 |
| ’Pachycetus’ humilis | Van Beneden, 1883: 33 [28] | MMGD NsT-94 | Bartonian | Helmstedt, Niedersachsen, Germany | 52.22900 | 11.01000 |
Basilosaurids are sometimes grouped in a single family without division [3, 5, 9, 24], but there is merit, phenetically at least, in subdividing this based on relative elongation of the posterior thoracic, lumbar, and caudal vertebrae. Basilosaurids with long trunk vertebrae (e.g., Basilosaurus, Eocetus, Basiloterus) are placed in Basilosaurinae, and basilosaurids with short trunk vertebrae (e.g., Dorudon, Zygorhiza, Pontogeneus, Saghacetus, etc.) are placed in Dorudontinae [8, 23, 29–31].
Here, we review the taxonomic history of the enigmatic basilosaurid genus Pachycetus Van Beneden, 1883 [28] and its several nominal species. We recognize that one of these, P. aithai from the late middle Eocene (Bartonian) of Morocco, represents a new genus Antaecetus, which we diagnose with the aid of a new specimen that includes a skull and much of the axial skeleton. Comparison shows that Pachycetus and Antaecetus together represent a new subfamily, Pachycetinae, of divergently specialized archaeocetes that swam and lived much differently from other basilosaurids.
Abbreviations
ANSP: Academy of Natural Sciences, Philadelphia, Pennsylvania, U.S.A.
CGM: Egyptian Geological Museum, Cairo, Egypt
CMM: Calvert Marine Museum, Solomons, Maryland, U.S.A.
CMN: Cossack Museum, Novocherkassk, Rostov, Russia
FSAC: Faculté des Sciences Ain Chock, Université Hassan-II de Casablanca, Morocco
GSP-UM: Geological Survey of Pakistan–University of Michigan collection, Quetta, Pakistan
HMS: Heimatmuseum, Schöningen, Niedersachsen, Germany
KOM: Kirovograd Oblast Museum, Ukraine
KRMHA: Kaliningrad Regional Museum of History and Art, Kaliningrad, Russia
MCZ: Museum of Comparative Zoology, Harvard University, Cambridge, Massachusetts, U.S.A.
MMGD: Museum für Mineralogie und Geologie, Dresden, Sachsen, Germany
MNB: Museum für Naturkunde, Berlin, Germany
MNHN: Muséum Nationale d’Histoire Naturelle, Paris, Île-de-France, France
MUSM: Museo de Historia Natural, Universidad Nacional Mayor de San Marcos, Lima, Peru
NCSM: North Carolina Museum of Natural Sciences, Raleigh, North Carolina, U.S.A.
NHML: Natural History Museum, London, England, U.K.
NMNH-P: National Museum of Natural History, Paleontology, Kyiv, Ukraine
NMR: Natuurhistorisch Museum, Rotterdam, Netherlands
SCSM: South Carolina State Museum, Columbia, South Carolina, U.S.A.
SMNS: Staatliches Museum für Naturkunde, Stuttgart, Baden-Württemberg, Germany
TSNU-GM: Geological Museum, Taras Shevchenko National University, Kyiv, Ukraine
UM: University of Michigan Museum of Paleontology, Ann Arbor, Michigan, U.S.A.
USNM: National Museum of Natural History, Washington, D.C., U.S.A.
Methods
Nomenclatural acts
The electronic edition of this article conforms to the requirements of the amended International Code of Zoological Nomenclature, and hence the new names contained herein are available under that Code from the electronic edition of this article. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix "http://zoobank.org/". The LSID for this publication is: urn:lsid:zoobank.org:pub:23753D98-8394-4D59-A327-93A21BB5EEC5. The electronic edition of this work was published in a journal with an ISSN, has been archived, and is available from the following digital repositories: PubMed Central and LOCKSS.
Permits
No permits were requird for the described study, which complied with all relevant regulations.
History of study
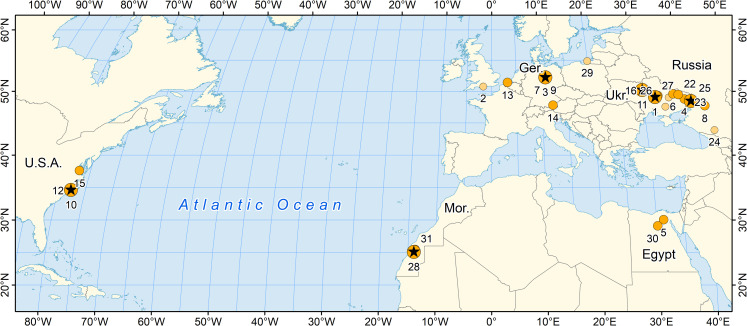
The history of Pachycetus and its constituent and related species is complicated because specimens are relatively rare. Many specimens, including the type specimen of the type species of Pachycetus, are isolated vertebrae or small collections of vertebrae. The specimens have been found on three continents. In addition, Pachycetus itself was omitted from the most thorough reviews following its publication [1, 32]. Localities yielding Pachycetus and its relatives are shown on the map in Fig 1. Pachycetus, constituent and related species, and corresponding locality coordinates are listed as they were published in Table 2.
Fig 1. Distribution of Eocene localities yielding Pachycetus, Antaecetus, or an archaeocete compared to them in Europe, North Africa, and North America.
Localities with stars are those yielding type specimens of named species. Larger colored circles represent specimens identified as Pachycetus, Antaecetus, or a junior synonym. Smaller colored circles are additional records that may represent Pachycetus but remain ambiguous. Literature references and coordinates for each locality are listed in Table 2. Base map from Natural Earth (http://www.naturalearthdata.com/).
Table 2. History of study of Eocene archaeocetes from localities yielding Pachycetus, a synonym, or a closely related contemporary.
| Author | Year | Page | Identification | Country | Locality | North latitude | East longitude | Map symb. | Map label |
|---|---|---|---|---|---|---|---|---|---|
| Brandt | 1873 [25] | 336 | Zeuglodon paulsonii | Ukraine | Chyhyryn | 49.07300 | 32.66200 | T | 1 |
| Paulson (in Brandt) | 1873 [25] | 339 | Zeuglodon rossicus | Ukraine | Chyhyryn | 49.07300 | 32.66200 | — | — |
| Seeley | 1876 [27] | 428 | Zeuglodon wanklyni | England | Barton Cliff | 50.74300 | −1.65500 | O | 2 |
| Van Beneden | 1883 [28] | 32 | Pachycetus robustus | Germany | Helmstedt | 52.22900 | 11.01000 | T | 3 |
| Van Beneden | 1883 [28] | 32 | Pachycetus humilis | Germany | Helmstedt | 52.22900 | 11.01000 | T | — |
| Lutugin | 1894 [33] | 147 | Zeuglodon sp. | Ukraine | Pereshchepnoy | 48.76400 | 38.43900 | P | 4 |
| Andrews | 1907 [34] | 124 | Zeuglodon wanklyni | England | Barton Cliff | 50.74300 | −1.65500 | — | — |
| Stromer | 1908 [35] | 109 | Eocetus schweinfurthi (pt.) | Egypt | Gebel Mokattam | 30.02700 | 31.27300 | P | 5 |
| Fedorovsky | 1912 [36] | 280 | Zeuglodon rossicus | Ukraine | Koropove | 49.58900 | 36.34600 | P | 6 |
| Kuhn | 1935 [37] | 223 | ’Zeuglodon’ cf. Z. isis | Germany | Trendelbusch | 52.18700 | 10.97900 | P | 7 |
| Bogachev | 1959 [38] | 42 | Zeuglodon paulsonii | Russia | Khoroshevskaya | 47.71900 | 42.22600 | P | 8 |
| Halstead and Middleton | 1972 [39] | 186 | Zygorhiza wanklyni | England | Barton Cliff | 50.74300 | −1.65500 | — | — |
| Lienau | 1984 [40] | 73 | Pachycetus robustus | Germany | Treue | 52.17600 | 10.98400 | P | 9 |
| Uhen | 1999 [26] | 514 | Eocetus wardii | U.S.A. | Laniers Pit | 34.62500 | −77.67500 | T | 10 |
| Gritsenko | 2001 [41] | 18 | Platyosphys einori | Ukraine | Pyrohiv, Kyiv | 50.21000 | 30.32000 | T | 11 |
| Uhen | 2001 [42] | 3 | Eocetus wardii | U.S.A. | Rocky Point Quarry | 34.42500 | −77.86830 | P | 12 |
| Post | 2007 [43] | 31 | Archaeoceti indet. | Netherlands | Scheur 10 | 51.41310 | 3.25150 | P | 13 |
| Uhen and Berndt | 2008 [44] | 57 | Eocetus sp. | Germany | Rohrdorf | 47.79694 | 12.17000 | P | 14 |
| Weems et al. | 2011 [45] | 273 | ’Eocetus’ wardii | U.S.A. | Putneys Mill | 37.60400 | −77.09200 | P | 15 |
| Gol’din et al. | 2012 [46] | 105 | ’Eocetus’ sp. | Ukraine | Kurenevka, Kyiv | 50.49500 | 30.43100 | P | 16 |
| Kalmykov | 2012 [47] | 180 | Basilosaurus sp. | Russia | Khoroshevskaya | 47.71900 | 42.22600 | — | — |
| Tesakov et al. | 2012 [48] | 141 | Eocetus sp. | Russia | Khoroshevskaya | 47.71900 | 42.22600 | — | — |
| Zvonok | 2012 [49] | 87 | Basilosauridae indet. | Ukraine | Nagornoye | 49.08300 | 33.13300 | P | 17 |
| Zvonok | 2012 [49] | 88 | Basilosaurus sp. | Ukraine | Subotiv | 49.09200 | 32.54500 | O | 18 |
| Zvonok | 2012 [49] | 88 | Basilosaurus sp. | Ukraine | Pywycha | 49.20200 | 33.12200 | O | 19 |
| Zvonok | 2012 [49] | 88 | Basilosaurus sp. | Ukraine | Nikopol | 47.56900 | 34.39400 | O | 20 |
| Zvonok | 2012 [49] | 88 | Basilosaurus sp. | Ukraine | Pereshchepyne | 49.01500 | 35.36400 | O | 21 |
| Zvonok | 2012 [49] | 88 | Platyosphys paulsoni | Ukraine | Buhaivka | 49.47700 | 37.38500 | P | 22 |
| Zvonok | 2012 [49] | 88 | Archaeoceti | Ukraine | Luhansk | 48.50600 | 39.39300 | O | 23 |
| Zvonok | 2012 [49] | 88 | Basilosauridae indet. | Russia | Pyatigorsk | 43.89900 | 43.11100 | O | 24 |
| Gol’din and Zvonok | 2013 [50] | 255 | Basilotritus uheni | Ukraine | Beloskelevaoye | 48.45000 | 39.64000 | T | 25 |
| Gol’din and Zvonok | 2013 [50] | 259 | Basilotritus sp. | Ukraine | Vlasovka | 49.30000 | 33.26700 | P | 26 |
| Gol’din and Zvonok | 2013 [50] | 260 | Basilotritus sp. | Ukraine | Velykaya Andrusovka | 49.18300 | 32.91700 | P | 27 |
| Gol’din et al. | 2014 [51] | 269 | Basilotritus sp. | Ukraine | Nagornoye | 49.08300 | 33.13300 | — | — |
| Gingerich and Zouhri | 2015 [24] | 280 | Platyosphys aithai | Morocco | Sabkha de Gueran | 25.07667 | −13.90763 | T | 28 |
| Post et al. | 2017 [52] | 50 | Archaeoceti indet. | North Sea | Scheur 10 | — | — | — | — |
| Mychko and Tarasenko | 2020 [53] | 314 | Basilosauridae indet. | Russia | Amber Combine Quarry | 54.86700 | 19.97100 | O | 29 |
| Van Vliet et al. | 2020 [54] | 124 | Pachycetus robustus | Germany | Helmstedt and vicinity | — | — | — | — |
| Davydenko et al. | 2021 [55] | 70 | Basilosauridae incert. sedis | Ukraine | Pyrohiv, Kyiv | 50.21000 | 30.32000 | — | — |
| This study | 2022 | — | Antaecetus sp. | Egypt | Wadi Rayan WR008 | 29.08000 | 30.11900 | P | 30 |
| This study | 2022 | — | Antaecetus aithai | Morocco | El Briej | 25.45100 | −13.72100 | P | 31 |
Locality symbols plotted on the map in Fig 1: O, possible Pachycetus (smaller circle); P, specimen referred to Pachycetus or a junior synonym (larger circle); T, type specimen of a species referred to Pachycetus (star). Label column here gives the number associated with each locality on the map in Fig 1.
Ukrainian Zeuglodon paulsonii of Brandt (1873)
The first specimens of the archaeocete that is now called Pachycetus were reported by Afanasii Semenovich Rogovich at the Third Russian Congress of Naturalists in Kyiv in 1871. These were then described in a manuscript by Otto Mikhaĭlovich Paulson that was published by Johann Friedrich Brandt. Brandt included the name Zeuglodon paulsonii as a nomen nudem in an abstract [56], and then validated the name in one of many Anhänge or insertions in his 1873 monograph on the fossil and subfossil whales of Europe [25]. Paulson’s illustrations were included as the final plate, plate xxxiv, in Brandt’s 1873 monograph. Rogovich was a botanist and Paulson a zoologist at the Russian Imperial University of St. Vladimir in Kyiv, and Brandt was a zoologist in the Academy of Sciences of St. Petersburg.
According to Paulson, in Brandt [25], three vertebral centra and part of a fourth were available in Kyiv. These came from Eocene strata on the right bank of the Tiasmyn River just south of Chyhyryn (Chigirin or Tschigirin), a small city lying 250 km southeast of Kyiv.
The first archaeocete vertebra to be reported from the Donets River basin of Ukraine was published by Leonid Ivanovich Lutugin in 1894. Lutugin [33] reported “a large vertebra representative of the genus Zeuglodon” found in glauconitic sandstone near a place he called Pereshchepnoy. This is in Luhansk Oblast.
Alexandre S. Fedorowskij was a Kharkiv University professor studying geology, paleontology, and archaeology. In 1912, Fedorowskij [36] described a more complete set of archaeocete vertebrae, which he identified on page 280 as Zeuglodon rossicus = Z. paulsonii. Fedorowskij excavated three vertebrae in 1909 on the right bank of the south-flowing Donets River near Koropove in Kharkiv Oblast, Ukraine, and farm workers there recovered seven more vertebrae. The age of the glauconitic sand yielding these was thought at the time to be early Oligocene. Fedorowskij regarded the 10 vertebrae as seven lumbars, a sacral, and two anterior caudals, publishing three plates of excellent photographic illustrations. The specimens were deposited in the geological collection of Kharkiv University, but are now lost.
Kellogg [1] proposed the new genus Platyosphys for Brandt’s species Zeuglodon paulsonii, based largely on Fedorowskij’s specimen, citing the anteroposteriorly long and relatively flat transverse processes on the vertebrae as characteristic of the genus.
In 2001, after a long hiatus, Volodymyr P. Gritsenko named a new species of Platyosphys, P. einori, based on a Ukrainian specimen, TSNU-GM 2638, from Pyrohiv in Kyiv [41]. Gritsenko gave diagnoses for the family Basilosauridae and the genus Platyosphys, but no diagnosis for the newly named species. He identified the vertebrae of P. einori as caudals, and reported their lengths to range from 220 to 150 mm, with transverse processes nearly as long anteroposteriorly as their corresponding centra. Gritsenko compared the pachyostosis of P. einori to that of Sirenia. Recently Davydenko et al. [55] restudied and reinterpreted TSNU-GM 2638, which they regarded as unidentifiable to genus or species, labeling the specimen “Basilosauridae incertae sedis.” Davydenko et al. identified some of the vertebrae as lumbars. Their descriptions and illustrations indicate that TSNU-GM 2638 is poorly preserved—raising doubt about its perceived distinction from P. paulsonii (see below)
Evgenij Zvonok [49] described large archaeocete teeth and a large archaeocete rib collected in 2010 and 2011 near Nagornoye in eastern Ukraine. These were identified as “Basilosauridae indet.” The largest of the teeth, upper premolar TSNU-GM 15–2, has a crown measuring 49 × 16 mm in length and width, and the rib, TSNU-GM 15–9, is 560 mm long with a maximum diameter near the distal end of 75 mm. Zvonok also provided a map and a list of sites yielding archaeocetes in eastern Ukraine (Subotiv, Pywycha, Nikopol, Pereshchepyne, Buhaivka, and Luhansk) and southwestern Russia (Pyatigorsk).
Pavel Gol’din and co-authors Zvonok and Tatiana Krakhmal’naya described two vertebrae from Kurenevka in Kyiv, Ukraine, which they identified as “Eocetus” sp. [46]. These included a thoracic or lumbar vertebra, NMNH-P OF-1694, and a lumbar vertebra, OF-1695. Both resemble vertebrae previously described as Platyosphys [1, 25, 36].
In the following year Gol’din and Zvonok [50] added three new localities south and east of Kyiv: Beloskelevaoye, Vlasovka, and Velykaya Andrusovka. Specimen NMNH-P OF-2096 from Beloskelevaoye was made the type of the new genus and species Basilotritus uheni. The type comprises a tympanic bulla, a thoracic vertebra, two thoracic centra, and a rib fragment. KOM 44759 P 201– KOM 44762 P 204 from Vlasovka, four vertebral centra, and KOM 44693 P 195 from Velyka Andrusovka, a vertebral centrum, were identified as Basilotritus sp.
The Nagornoye locality yielded specimens, collected in 2004, 2006, 2010, 2011, and 2012, in addition to those described by Zvonok in 2012 [49]. Gol’din et al. [51] studied the new specimens, together with those described earlier, and referred all Nagornoye specimens to “Basilotritus sp.” They reported that all of the Nagornoye specimens came from a 40 cm thick interval of shark-rich glauconitic sand. The most informative new specimens were cervical centra with very small vertebrarterial foramina, and a lumbar centrum. It is an open question whether all of the Nagornoye specimens belong to a single basilosaurid.
As Gingerich and Zouhri wrote previously [24]: Gol’din and Zvonok’s separation of Basilotritus from Platyosphys depended on setting the genus and species Platyosphys paulsonii Brandt, 1873, aside as a nomen dubium, in spite of its stated similarity to Basilotritus uheni, because “the type specimen is considered to be lost” ([50], p. 263). The validity of a genus and species does not depend on the continued availability of a type specimen, but rather on the indication of a tangible specimen and some description of the morphology involved, whether the specimen itself remains available for study or not. An indication and description were clearly provided by Brandt [25, 56], by Fedorowskij [36], and by Kellogg ([1], p. 97).
British Zeuglodon wanklyni of Seeley (1876)
Zeuglodon wanklyni is a species Harry Govier Seeley named in 1876 [27] based on an archaeocete cranium from the Barton Clay at Barton Cliff on the Hampshire coast of southern England. This was evidently a nearly complete cranium when found in 1872, but it was damaged when it was collected. Pieces were salvaged and Seeley made notes on the specimen, which he published four years later. The type is now lost, but the indication and description remain.
The description Seeley [27] gave for the maxillae and maxillary teeth in the type specimen of Zeuglodon wanklyni are informative. Kellogg [1] repeated the descriptions, converting Seeley’s measurements to metric units and referring Z. wanklyni to Zygorhiza. Seeley [27] noted (p. 430) that an isolated anterior tooth (canine?) retained a large pulp cavity, which may mean that the specimen was not fully adult. The sizes Seeley and Kellogg gave for measurable teeth of Z. wanklyni are close to those of deciduous teeth in Zygorhiza kochii published by Kellogg [1], but this does not necessarily mean that they were deciduous.
Andrews [34] described an isolated posterior cervical vertebra of Zeuglodon wanklyni (NHML-M 11090), that resembles C6 of Zygorhiza kochii described by Kellogg [1]. The vertebra was not associated with the skull, but both together suggest that Zeuglodon wanklyni was similar in size to Zygorhiza kochii. One difference is that C6 of Zeuglodon wanklyni has small vertebrarterial foramina (ca. 8 mm in diameter [34]), whereas those of Zygorhiza kochii are large by comparison ([1], p. 134).
Halstead and Middleton [39] described a thoracic vertebra of Zygorhiza wanklyni from Barton, NHML M-12346, which resembles vertebrae here called Pachycetus in having the centrum width substantially greater than the centrum height, in lacking ossified epiphyses, and in having cancellous bone suggesting cartilage where the capitular facets should be. Halstead and Middleton also described another larger thoracic centrum from Barton, NHML M-26552. Finally, NHML M-26553 is an elongated, slightly-flattened, caudal centrum from Barton. The latter two vertebrae were referred to Basilosaurus. None of these vertebrae is complete, and one, two, or all three could possibly represent Pachycetus.
It is not clear that Zeuglodon wanklyni Seeley, 1876, is a species of what is now called Pachycetus, but this is possible because Pachycetus is known from western Europe during deposition of the classic Bartonian strata at Barton Cliff, and one or more vertebrae from Barton appear referable to Pachycetus.
German Pachycetus robustus of Van Beneden (1883)
In 1883 Hanns Bruno Geinitz published the first indication that cetaceans are present in the phosphate beds or Koprolithenlager of Helmstedt, Lower Saxony, in north central Germany. Geinitz [57] described a vertebral centrum from Helmstedt, which he considered to be early Oligocene in age. In a later report Geinitz [58] added a second larger centrum and a large rib. The Helmstedt specimens were then sent to the cetacean authority Pierre-Joseph Van Beneden in Leuven for study.
Van Beneden [28] received four vertebral centra and pieces of two or three ribs from Geinitz. These represented cetaceans of two sizes. Van Beneden attributed the first and largest centrum (now MMGD NsT-90), considered a lumbar, and the largest rib (MMGD NsT-92A) to a new genus and species of mysticete, Pachycetus robustus, similar in size to the living minke whale Balaenoptera acutorostrata. Van Beneden noted that the pedicles of the neural arch were long anteroposteriorly, and the underside of the centrum was distinctive in its flattening and in its furrowed and folded surface. He also noted that the posterior surface of the centrum was substantially larger than the anterior surface. The transverse processes are long anteroposteriorly, but broken near their bases, and it is possible, even likely, that MMGD NsT-90 is a posterior thoracic rather than a lumbar vertebra.
Van Beneden [28] described the rib of P. robustus as a distal half-rib that measures 450 mm in length, 61 × 56 mm in diameter in the middle, swelling to 80 × 46 mm in diameter near the distal end. He compared the rib to that of a sirenian because of its thickness but confirmed it to be cetacean. The genus name Pachycetus was given to acknowledge the thickness of the rib in this larger form, but the vertebral centrum MMGD NsT-90 is the lectotype of Pachycetus robustus ([54], p. 123).
Van Beneden [28] described the second and third centra as thoracics, and considered these to represent one species, which was smaller than Pachycetus robustus. Both centra lack epiphyses. Van Beneden’s fourth centrum is an even smaller anterior thoracic, measuring 40 × 65 × 55 mm in length, width, and height. Van Beneden was unable to recognize facets for rib heads. This fourth centrum (MMGD NsT-94) is the one later illustrated and designated by Kuhn ([37], Fig 4) as the lectotype of Van Beneden’s Pachycetus humilis. Comparison with the pachycetine specimens analyzed here shows that the centrum of P. humilis is not the shape expected for an anterior thoracic of P. robustus (see below). Van Beneden [28] did not publish illustrations of either Pachycetus robustus or P. humilis, and the genus and both species were then seemingly forgotten. Van Beneden mentioned Pachycetus obscurely in his listing of living and fossil whales in museum collections [32], and Kellogg [1] did not mention Pachycetus is his otherwise comprehensive review of Archaeoceti known at the time.
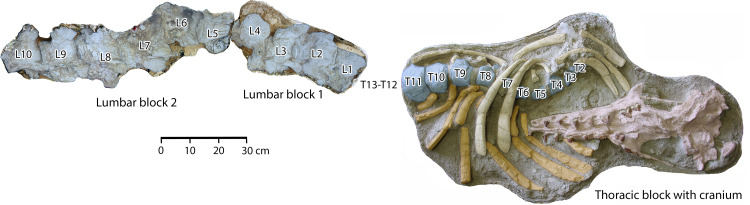
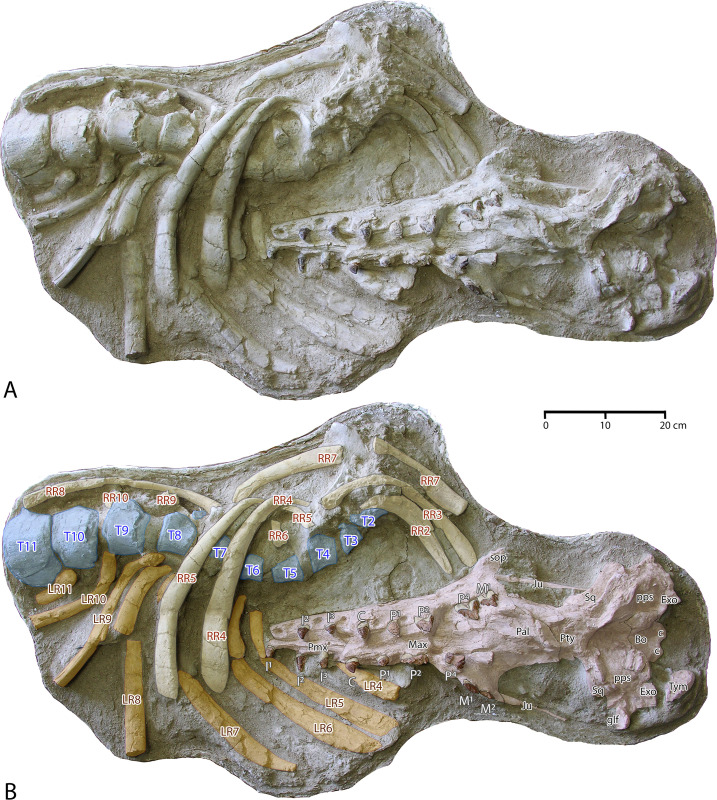
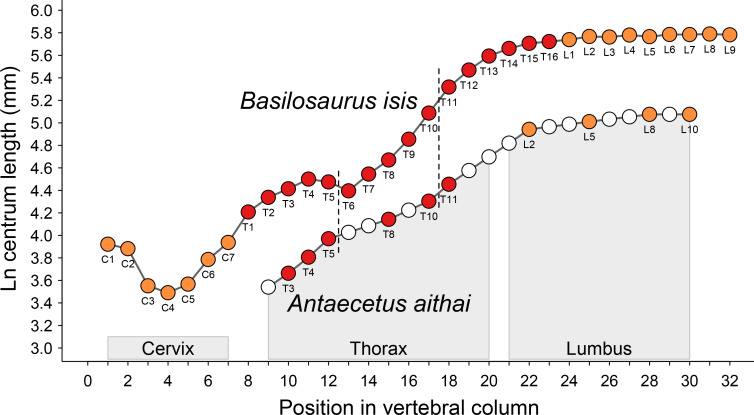
Fig 4. Partial skeleton of Antaecetus aithai, specimen FSAC Bouj-200, as collected and preserved in three blocks of sediment.
Each block was turned before preparation and then cleaned to expose the underside of the block, which is the side shown here. The cranium is shown in red, teeth in brown, vertebrae in blue, left ribs in orange, and right ribs in yellow. Thoracic vertebrae are numbered from T2 through T11 on the assumption that the skeleton had 13 thoracics. Lumbar vertebrae are numbered from L1 through L10. There may have been more than 13 thoracics, and there may have been more than 10 lumbars.
Kuhn [37] redescribed Van Beneden’s specimens of Pachycetus robustus and P. humilis in 1935, illustrated these for the first time, and misinterpreted anterior and posterior in both. Kuhn rejected the name Pachycetus as “uneinheitlich” or “inconsistent” (whatever he meant by this), and identified Van Beneden’s species as “Zeuglodon cf. isis” and “Zeuglodon sp. indet. cf. osiris.” In addition, Kuhn [37] described several new vertebral centra from Trendelbusch, near Helmstedt. The best preserved is a thoracic, lacking epiphyses, that may represent Pachycetus. Treue is another German locality near Helmstedt that has yielded Pachycetus robustus and possibly P. humilis ([40], pp. 72–73).
In 2008 Uhen and Berndt [44] described a vertebral centrum from Rohrdorf in Bavaria that they referred to Eocetus sp. because of its elongated centrum, anteroposterior elongation of the transverse processes, and the distinctively pockmarked surface of the cortical bone. This was subsequently referred to Basilotritus by Gol’din and Zvonok [50] and then to Pachycetus by Van Vliet et al. [54]. The premolar illustrated by Uhen and Berndt [44] is probably also a premolar of Pachycetus.
Finally, in 2020, Van Vliet et al. [54] clarified the systematic position of Pachycetus robustus by adding two lumbar vertebrae, one from Alversdorf (specimen HMS ID20-2/4) and the other from Treue (NMR 9991–13472), both near the type locality of Helmstedt. These are larger and longer than the type specimen of Pachycetus robustus, undoubtedly lumbars, and seemingly confirm synonymy of Pachycetus robustus and Pachycetus paulsonii (see Discussion below).
Egyptian Eocetus (partim) of Stromer (1908)
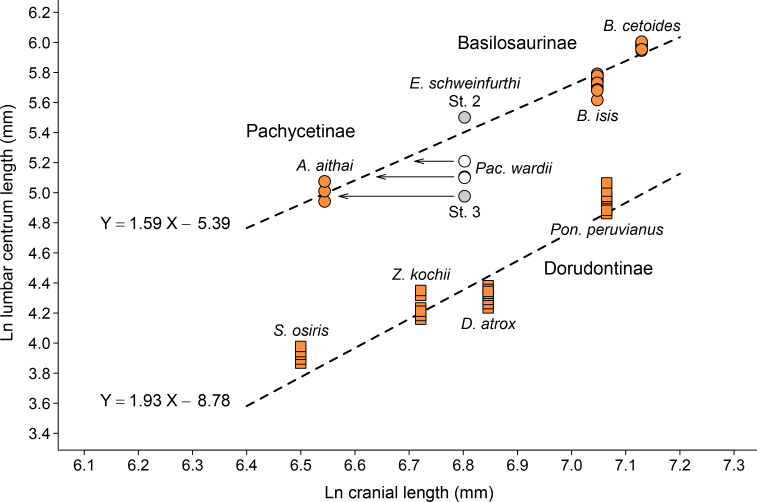
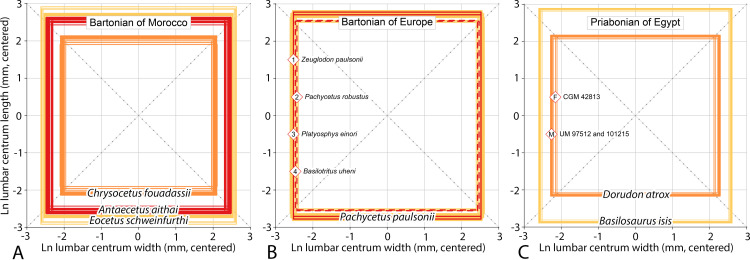
In 1903 Ernst Stromer von Reichenbach described eight archaeocete specimens from Fayum in Egypt, and then when the study went to press added a ninth specimen from high in the Gebel Mokattam stratigraphic section near Cairo ([59], pp. 83–85). The upper Gebel Mokattam interval yielding archaeocetes is now placed in the Giushi Formation and regarded as Bartonian in age [60]. Stromer’s ninth specimen, from Stuttgart, was forwarded for study by Eberhard Fraas. It included two vertebrae, which Stromer called Wirbel 9a and Wirbel 9b. Stromer’s Wirbel 9a, illustrated in his text Fig 1, was described as having a centrum lacking its anterior epiphysis. The centrum, as preserved, measured 245 × 140 × 130 mm in length, width, and height, with a transverse process 155 mm long at its base (making it some 60% of total centrum length). Stromer mentioned that the posterior epiphyseal surface of the centrum was almost flat, nearly circular, and perpendicular to the long axis of the centrum. Wirbel 9b was said to be a piece of vertebral diaphysis that was less complete but seemingly larger than the diaphysis of Wirbel 9a. In a later study Stromer ([35], p. 109) labeled these large lumbar vertebrae ‘Stuttgart 2’ or, when abbreviated, ‘St. 2’ (now SMNS 10934) and identified them as Eocetus schweinfurthi. The genus Eocetus and species E. schweinfurthi were named by Fraas [13, 61] based on ‘St. 1.’ The comparison of vertebral centrum length to skull length in Fig 2 confirms allocation of SMNS 10934 to Eocetus schweinfurthi.
Fig 2. Allocation of lumbar vertebrae to the Egyptian basilosaurid Eocetus schweinfurthi.
Two subfamilies, Pachycetinae and Basilosaurinae (circles), have long lumbar centra compared to cranial length; one subfamily, Dorudontinae (squares), has short lumbar centra. Lumbar centrum length in St. 2 (SMNS 10934) matches that expected for the skull length of E. schweinfurthi (SMNS 10986) from the same Bartonian-age strata. The centrum length of St. 3 (10934b) from these strata is shorter than expected. The lumbar centrum of St. 3 has the morphology of a pachycetine, and matches lumbars of Antaecetus aithai in size. The cranium of Pachycetus wardii is not known but it was probably intermediate in length between those of A. aithai and E. schweinfurthi. Cranial and/or lumbar measurements: Antaecetus aithai, this study; Basilosaurus cetoides, Kellogg [1]; Basilosaurus isis, Gingerich et al. (in preparation); Dorudon atrox, Uhen [8]; Eocetus schweinfurthi, Fraas [13] and Stromer [35, 59]; Pachycetus wardii, Uhen [26]; Pontogeneus peruvianus, Martínez-Cáceres et al. [9]; Saghacetus, Gingerich (in preparation); and Zygorhiza kochii, Kellogg [1].
In 1908 Stromer ([35], pp. 109–110) referred a third Stuttgart specimen (‘St. 3’) from Gebel Mokattam to Eocetus schweinfurthi. This included three vertebrae that Stromer interpreted as not fully grown. One of these, lacking epiphyses, had a centrum complete enough to measure. This centrum, smaller and differently shaped than that of ‘St. 2,’ measured “über” 135 × 80 × 65 mm in length, width, and height. Two vertebrae of ‘St. 3’ survive (provisionally numbered SMNS 10934b). Vertebrae of St. 3 (SMNS 10934b) differ from vertebrae of St. 2 (SMNS 10934) in having centra that are smaller and flatter dorsoventrally; having a pachyostotic neural spine, prezygapophyses, and transverse processes; and having anteroposteriorly elongated transverse processes. These are all characteristics of ‘Zeuglodon’ paulsonii described by Paulson in Brandt [25], Pachycetus robustus described by Van Beneden [28], and Platyosphys aithai described by Gingerich and Zouhri [24] (now Antaecetus aithai, see below).
Stromer ([35], p. 110) indicated that the transverse processes of St. 3 arise from the entire length of the diaphysis, making their length minimally about 90% of total centrum length (as distinct from the 60% relative length of transverse processes calculated for St. 2). The contrasting forms of lumbar vertebrae in Eocetus compared to Pachycetus and Antaecetus are illustrated in Fig 2 of Uhen [26]. Eocetus now includes the Bartonian species E. drazindai formerly placed in Priabonian Basilosaurus. Eocetus differs from Basilosaurus in having large lumbar vertebrae with a longer neural arch and longer transverse processes, both relative to centrum length, but Eocetus is closely related and possibly ancestral to Basilosaurus.
Antaecetus aithai is also known from Bartonian-age strata of Fayum in Egypt. This was found in November of 2008 by a University of Michigan field team working at Wadi Rayan locality WR008. The specimen has not been prepared or cataloged in a museum collection.
Russian Zeuglodon paulsonii of Bogachev (1959)
The first Pachycetus specimens from Europe to be found outside Ukraine, Great Britain, and Germany were described by Vladimir Vladimirovich Bogachev from the locality of Khoroshevskaya in the Rostov Oblast of southeastern Russia. Bogachev [38] described four vertebrae identified as Zeuglodon paulsonii, all presumably lumbars, based on notes he made in 1940. These were deposited in the Cossack Museum in Novocherkassk.
Later N. P. Kalmykov [47] and A. S. Tesakov et al. [48] described additional archaeocete remains from Khoroshevskaya found by a local resident. Kalmykov identified a tooth interpreted to be M1 as Basilosaurus sp. The tooth was described as large, but the illustration and measurements are inconsistent, so the size is uncertain. Tesakov et al. described the same specimen as having cervical, thoracic, lumbar, and caudal vertebrae, fragments of limb bones, and a tympanic bulla, but noted that the skull found with the skeleton did not survive. They cited elongation of lumbar centra and transverse processes, vertebral surface texture, and dense layering of cortical bone as characteristic of Eocetus (sensu Uhen [26]), and they identified the Khoroshevskaya specimen as Eocetus, comparable in size or slightly larger than Eocetus wardii.
Another vertebral centrum, KRMHA-KGOM2-13184, was described by Mychko and Tarasenko [53] as a lumbar, but it could possibly be a caudal. Mychko and Tarasenko identified the centrum as Basilosauridae indet.
American Eocetus wardii of Uhen (1999)
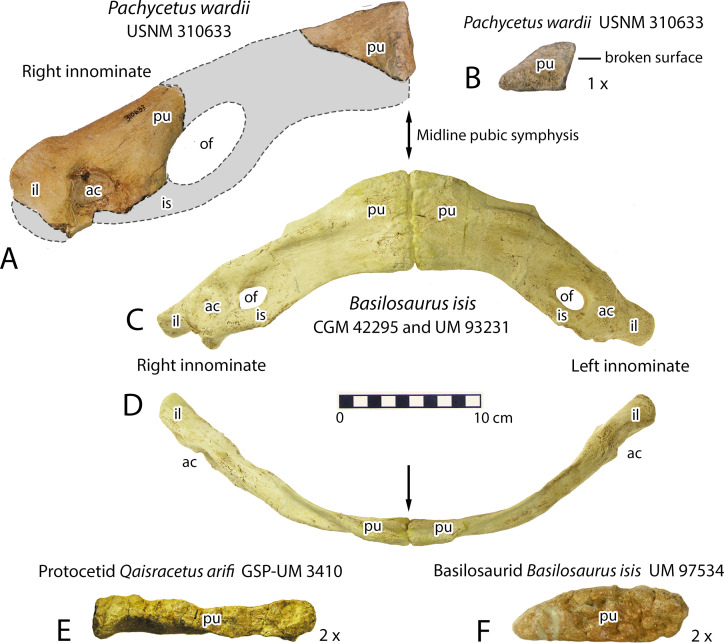
Mark D. Uhen [26] recognized and described a distinctive and important archaeocete specimen from North America in the collection of the United States National Museum of Natural History, which he interpreted as a protocetid and named Eocetus wardii. This was collected by Lauck W. Ward in 1977 from Laniers Pit near Maple Hill, North Carolina, U.S.A. The type specimen, USNM 310633, includes cranial fragments, four thoracic vertebrae, six lumbar vertebrae, a possible caudal vertebra, a complete rib, and a partial innominate. Complete lumbars range in length from 164 to 183 mm. The innominate is especially interesting because of its distinctive difference from other known basilosaurid innominates (see below).
Later Uhen [42] described a second specimen of Eocetus wardii, NCSM 11284, with stylohyals, cervical C7, 12 thoracic vertebrae, and two lumbar vertebrae, sternal elements, ribs, and a scapula. According to Beatty and Geisler [62], this came from the Rocky Point Quarry, near Rocky Point, North Carolina. Centrum lengths for the lumbars from Rocky Point Quarry, both lacking epiphyses, were reported as 134 and 135 mm.
Finally, Weems et al. [45] expanded the geographic range of Eocetus wardii by adding two vertebrae from Putneys Mill in Virginia. One of these vertebrae, an anterior thoracic lacking epiphyses (CMM-V-4334), had a centrum length, as preserved, of 63 mm. The other, a posterior lumbar without epiphyses (CMM-V-4335), had a centrum length of 143 mm.
The species Eocetus wardii named by Uhen [26] has had an interesting taxonomic history. Geisler et al. [63] questioned attribution to Eocetus, and recommended that the species be called ‘Eocetus’ wardii. Gol’din and Zvonok [50] moved E. wardii to Basilotritus when they named Basilotritus uheni. Gingerich and Zouhri [24] referred E. wardii to Platyosphys when they named Platyosphys aithai. Finally, Van Vliet et al. [54] synonymized Basilotritus and Platyosphys with Pachycetus and referred E. wardii to Pachycetus.
North Sea ‘Archaeoceti indet.’ of Post (2007)
In 2007 Klaas Post described three unusual vertebrae recovered by fishermen while trawling across the North Sea bottom off the coast of Belgium and Netherlands [43]. The third of these, NMR 9991–3404, has an elongated lumbar centrum, measuring 190 mm in length without epiphyses. The dorsal surface has a midline crest of bone separating nutrient foramina.
In a later study, Post et al. [52] described a new vertebra, NMR 9991–13472 from ‘Scheur 10’ or channel buoy 10 in the North Sea off the coast of Belgium—which they compared to NMR 9991–3404 and to Eocetus sp. of Uhen and Berndt [44]. All are almost certainly specimens of Pachycetus.
Moroccan Platyosphys aithai of Gingerich and Zouhri (2015)
Platyosphys aithai was named by Gingerich and Zouhri [24] for a series of associated thoracic vertebrae from the Gueran depression in the Sahara southeast of Boujdour, a town on the Atlantic coast of southwest Morocco. Here P. aithai is placed in a new genus Antaecetus. Most thoracic vertebrae of Pachycetus and Antaecetus have centra that become wider from front to back. Anterior thoracics have roughened surfaces for articulation with the heads of ribs—rather than synovial facets—on both the anterior and posterior sides of the centrum. By the middle of the thoracic series there is an anterior capitular depression but none at the posterior end of the centrum. Some middle thoracics have a slender diapophysis projecting from the centrum for articulation with a rib tubercle. The diapophysis is lost on posterior thoracics, and the rib articulations are open capitular depressions on a projecting parapophyseal surface. Known lumbar vertebrae of Antaecetus aithai are rarely complete, but most show the anteroposteriorly long, robust transverse processes extending virtually the entire length of the centrum that are characteristic of Pachycetus and Antaecetus. Here we describe the first skull and articulated partial skeleton of Antaecetus based on a new specimen from Gueran. We also note remains of Antaecetus found at the new locality of El Briej.
Systematic paleontology
The name Archaeoceti was proposed by William Henry Flower for one of three suborders of Cetacea. Flower [64] wrote:
“Among the existing members of the order [Cetacea], there are two very distinct types, the toothed Whales or Odontoceti, and the baleen Whales or Mystacoceti [Mysticeti], which present as many marked distinguishing structural characters as are found between many other divisions of the Mammalia that are reckoned as orders. As the extinct Zeuglodon [Basilosaurus], as far as its characters are known, does not fall into either of these groups, but is in some respects an annectent form, I have placed it provisionally, at least, in a third group by itself, named Archaeoceti.” ([64], pp. 181–182)
Kellogg [1] enshrined Flower’s ‘third group’ in his Review of the Archaeoceti, McKenna and Bell [31] grouped Eocene whales in Archaeoceti, and the name is widely used.
Marx et al. recently wrote in Cetacean Paleobiology ([65], p. 2) that “Taxonomically, cetaceans fall into three major groups: ancient whales (archaeocetes), baleen whales (Mysticeti), and toothed whales (Odontoceti).” The name Archaeoceti, whether Latinized or Anglicized, capitalized or decapitalized, has priority and ample precedent for “annectent” whales appearing in the Eocene. Archaeoceti may be paraphyletic in the sense that a member of the group successfully gave rise to a later group (e.g., Odontoceti, Mysticeti, or both), and the same can be said for Basilosauridae. Here Archaeoceti and Basilosauridae are used to represent taxonomic groups in the form and sense of their original authors, with no implication of group sterility (the group had no descendants) nor holophyly (the group includes all of its descendants).
Mammalia Linnaeus, 1758 [66]
Cetacea Brisson, 1762 [67]
Archaeoceti Flower, 1883 [64]
Basilosauridae Cope, 1868 [68]
Diagnosis
Basilosaurids are middle and late Eocene cetaceans that differ from earlier Pakicetidae, Ambulocetidae, and Protocetidae in lacking upper third molars. They also differ in having well-developed pterygoid sinuses that separate left and right middle and inner ears acoustically, short cervical vertebrae, augmented numbers of thoracic and lumbar vertebrae, forelimbs modified into flippers, and hind limbs reduced in size and no longer articulating with the vertebral column. Basilosaurids were the first whales to become fully aquatic.
Basilosaurids differ from later odontocetes and mysticetes in retaining a dental formula of 3.1.4.2 / 3.1.4.3 with recognizable upper and lower incisor, canine, premolar, and molar teeth. Skulls are not telescoped. There is no evidence of baleen. Basilosaurids retain forelimbs with a moveable elbow, and retain hind limbs with reduced but recognizable innominate, femur, tibia, fibula, ankle, and foot bones. Development of a tail fluke is questionable.
Pachycetinae, new subfamily
urn:lsid:zoobank.org:act:297935C8-D20F-4339-BD95-193709A240FA
Type genus
Pachycetus Van Beneden, 1883 [28].
Included genera
Pachycetus Van Beneden, 1883 [28], and Antaecetus, new genus.
Diagnosis
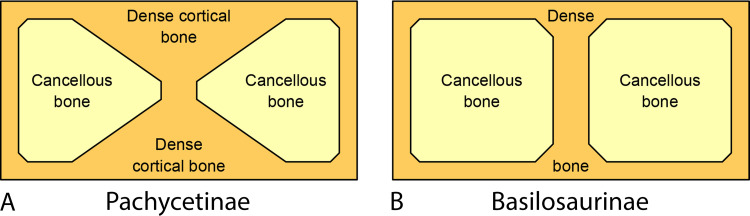
Pachycetinae differ from basilosaurine and dorudontine Basilosauridae in several salient features. Pachycetines have pachyosteosclerotic vertebrae not seen in Basilosaurinae or Dorudontinae, and they have pachyosteosclerotic ribs not seen in Dorudontinae. Cartilagenous and ligamentous connective tissue replaces synovial rib articulations. Thoracic vertebrae increase in size from front to back so rapidly that individual centra have a trapezoidal profile. Lumbar vertebrae are elongated like those of Basilosaurinae but differ in having transverse processes nearly as long anteroposteriorly as the centra from which they arise. Most vertebrae have small vascular openings that give surficial bone a distinctively pitted texture. As interpreted here, the innominate of Pachycetus differs from innominates associated with basilosaurines and dorudontines in having a much larger obturator foramen.
Geological age
Pachycetinae have been reported from strata thought to be middle Eocene, late Eocene, and Oligocene in age, but in recent years a consensus has emerged that most or all pachycetines are Bartonian late middle Eocene. The first appearance of Pachycetinae is probably related in some way to global warming of the late Lutetian thermal maximum (LLTM), to the middle Eocene climatic optimum (MECO), or to the short cooler interval between these events. Sea level rise leading to the global high sea stand characteristic of the Bartonian stage/age started in the cool interval between the LLTM and the MECO [7]. The MECO was a Bartonian climate event in the latter part of magnetochron C18r, but the preceding cool interval started in the latest Lutetian in magnetochron C19n [69]. The boundary between magnetochron C19n and C18r, calibrated at 41.0 million years before present, is a candidate for definition of the Lutetian-Bartonian boundary [7].
Discussion
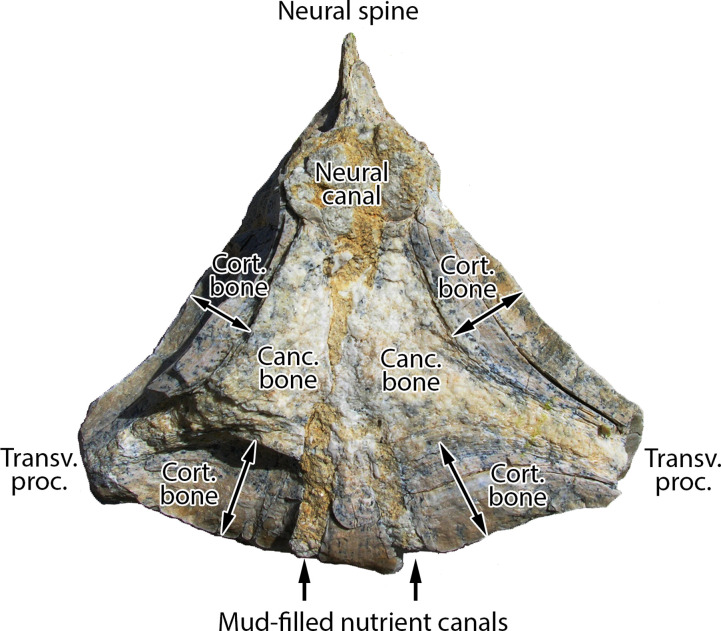
Vertebrae and ribs of Pachycetinae are distinctive in being both pachyostotic, thickened with extra layers of lamellar cortical bone (which inspired the name Pachycetus), and osteosclerotic, having cortical bone that is densely ossified with minimal porosity [70, 71]. The transverse cross section of a pachyosteosclerotic vertebra of Antaecetus is shown in Fig 3. Similar cross sections for vertebrae of Pachycetus are illustrated by Van Vliet et al. in their plate 3: figs. A2 and B1–B3 [54]. Cones of cancellous bone expand from the center of these vertebrae anteriorly and posteriorly toward the vertebral epiphyses. The surrounding cortical bone is perforated by many small vascular canals, and these give the surface of the vertebra a pitted appearance.
Fig 3. Transverse cross section through a caudal vertebra of Antaecetus aithai showing the dense, thickly-laminated, osteosclerotic cortical bone surrounding a central core of cancellous bone.
Cortical bone (arrows) is approximately 2 cm thick on the lateral sides of the centrum and 3 cm thick on the ventral side. Cancellous bone (white) is recrystallized, and the neural canal is now filled with crystalline matrix. Mud-filled nutrient canals are stained yellow. This half-centrum is approximately 14 cm wide, left to right, as shown. Specimen photographed in the field at Gueran.
Ribs are pachyostotic and osteosclerotic, with posterior vertebrosternal and anterior vertebrochondrial ribs displaying the most pachyostsis. Rib articulations with thoracic vertebrae have pitted or roughened surfaces in Antaecetus, indicating that they were cartilaginous and ligamentous rather than synovial. These articular surfaces are not well described for Pachycetus, but where known [26, 50] appear to be pitted or roughened, suggesting that they too were cartilaginous or ligamentous. Lumbar vertebrae of pachycetines are distinctive in having broad anteroposteriorly-elongated transverse processes that approach the length of the centrum.
Pachycetines resemble contemporary basilosaurines and differ from contemporary dorudontines in having elongated trunk vertebrae. However, pachycetines differ from basilosaurines in the pachyosteosclerosis that permeates and envelops their vertebrae. These distinctions have behavioral implications for Pachycetinae (see the Discussion of Locomotion and Behavior below). Phylogenetically, Basilosaurinae and Pachycetinae appear too divergently specialized to have given rise to later whales, leaving generalized Dorudontinae as the group within Basilosauridae most likely to be ancestral to modern Odontoceti and Mysticeti.
The following systematic review summarizes the evidence for recognition of a minimum of three species of Pachycetinae: Pachycetus paulsonii in Europe, Pachycetus wardii in North America, and Antaecetus aithai in Africa.
Genus Pachycetus Van Beneden, 1883
Zeuglodon (in part), Brandt, 1873a [56], p. 112 (nomen nudum). Brandt, 1873b [25], p. 336. Lutugin, 1894 [33], p. 147. Grevé, 1904 [72], p. 67. Fedorowskij, 1912 [36], p. 280. Kuhn, 1935 [37], p. 223. Bogachev, 1959 [38], p. 42.
Pachycetus Van Beneden, 1883 [28], p. 32. Lienau, 1984 [40], p. 73. Van Vliet et al., 2020 [54], p. 124.
Eocetus (in part), Stromer, 1908 [35], p. 109. Uhen, 1999 [26], p. 514. Uhen, 2001 [42], p. 3. Uhen and Berndt, 2008 [44], p. 57. Weems et al., 2011 [45], p. 273. Gol’din et al., 2012 [46], p. 104. Tesakov et al. [48], 2012, p. 141.
Platyosphys Kellogg, 1936 [1], p. 97. Gritsenko, 2001 [41], p. 18. Davydenko et al., 2021 [55], p. 70.
Basilosaurus (in part), Halstead and Middleton, 1972 [39], p. 187. Kalmykov, 2012 [47], p. 180.
Archaeoceti indet., Post, 2007 [43], p. 31. Post et al., 2017 [52], p. 50.
Basilosaurinae (in part), Schouten, 2011 [73], p. 19.
Basilotritus Gol’din and Zvonok, 2013 [50], p. 255. Gol’din et al., 2014 [51], p. 269.
Basilosauridae (in part), Mychko and Tarasenko, 2020 [53], p. 314.
Type species
Pachycetus robustus Van Beneden, 1883 [28]. Van Vliet et al. [54] designated P. robustus as the type species of the genus. P. robustus is the type because it is the species on which Pachycetus was based—even though P. robustus is a junior synonym of the first-named species, Zeuglodon paulsonii Brandt, 1873 [25], now included in Pachycetus.
Included species
Zeuglodon paulsonii Brandt, 1873 [25] and Eocetus wardii Uhen, 1999 [26]. ‘Zeuglodon’ wanklyni Seeley, 1876 [27], may belong here as an additional species or a synonym (known material is too fragmentary to tell).
Diagnosis
Differs from Antaecetus gen. nov. in being larger and in having more robust teeth with crenulated enamel. The first upper premolar, P1, differs from that in Antaecetus in being double-rooted or having two fused roots. Posterior upper premolars differ from those of Antaecetus in having four rather than three accessory cusps flanking the central cusp anteriorly and posteriorly. Upper molars differ in lacking any distinct medial swelling in the position of the protocone.
Discussion
In a previous study [24] we stated our reasons for syononymizing Basilotritus Gol’din and Zvonok, 2013, with Platyosphys Kellogg, 1936. Subsequently, Van Vliet et al. [54] recognized that Pachycetus Van Beneden, 1883, is a senior synonym of Platyosphys Kellogg, 1936. Thus Pachycetus is the appropriate generic name for species formerly included in Platyosphys and Basilotritus.
There appear to be two valid species of Pachycetus, one in Europe (P. paulsonii Van Beneden, 1883 [28]) and one in North America (P. wardii Uhen, 1999 [26]). The history of Pachycetus paulsonii is complicated and spans 150 years of study, as the following synonymy for the species shows. Pachycetus wardii was named more recently and is based on more complete specimens, which has simplified its interpretation.
Pachycetus paulsonii (Brandt, 1873b)
Zeuglodon cetoides (in part), Rogovich, 1871 (1873; not seen).
Zeuglodon paulsonii Brandt, 1873a [56], p. 112 (nomen nudum). Brandt, 1873b [25], p. 336. Grevé, 1904 [72], p. 67. Fedorowskij, 1912 [36], p. 280, pl. 1: 1–5, pl. 2: 6–10, pl. 3: 11–18. Bogachev, 1959 [38], p. 42.
Zeuglodon rossicus Paulson in Brandt, 1873b [25], p. 339, pl. 34: 1–6.
Pachycetus robustus Van Beneden 1883 [28], p. 32. Lienau, 1984 [40], p. 73, pl. 9: 13. Van Vliet et al., 2020 [54], p. 124, pl. 2: c1–c4, d1–d2.
Zeuglodon cf. Z. isis, Kuhn, 1935 [37], p. 223, fig. 3a, b.
Basilosaurus sp., Halstead and Middleton, 1972 [39], p. 187, fig. 2. Kalmykov, 2012 [47], p. 180, figs. 2–3.
Platyosphys einori Gritsenko, 2001 [41], p. 18, fig. 2: 1–5, fig. 3: 1–11.
Archaeoceti indet., Post, 2007 [43], p. 31, figs. 3–4. Post et al., 2017 [52], p. 50, figs. 2–3.
Basilosaurinae (in part), Schouten, 2011 [73], p. 19, figs. of NMR 991–3404.
Eocetus sp., Uhen and Berndt, 2008 [44], p. 57, fig. 2. Gol’din et al., 2012 [46], p. 104, figs. 2–4, 5: 2, pl. 1: 1–5, pl. 2: 1–5. Tesakov et al., 2012 [48], p. 141.
Basilosauridae indet., Uhen and Berndt, 2008 [44], p. 59, fig. 4. Zvonok, 2012 [49], p. 87. Mychko and Tarasenko, 2020 [53], p. 314, fig. 1.
Basilotritus uheni Gol’din and Zvonok, 2013 [50], p. 255, figs. 2–6.
Basilotritus sp., Gol’din and Zvonok, 2013 [50], p. 259. Gol’din et al., 2014 [51], p. 269.
Pachycetus sp. indet. A, Van Vliet et al., 2020 [54], p. 126, pl. 1, pl. 2: a1–a3, b1–b3, e1–e2, f1; pl. 3a1–a2, b1–b3, c1–c4, d1–d4.
Type specimen
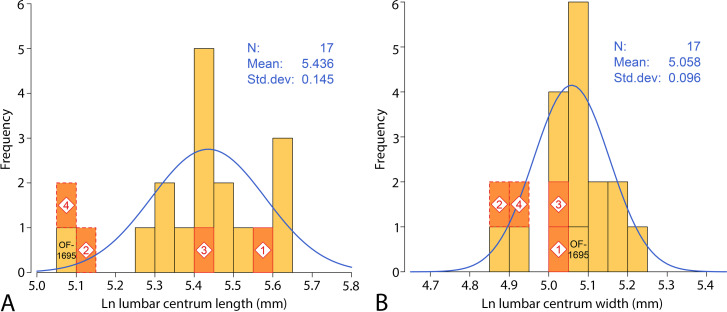
One centrum of a thoracic vertebra and two complete centra of lumbars were found at Chyhyryn in Ukraine. These were described by Paulson in Brandt [25]. Paulson named the species involved Zeuglodon rossicus, but this is a junior objective synonym of Brandt’s name Zeuglodon paulsonii. Kellogg [1] regarded all of Paulson’s vertebrae as co-types, but the holotype is here restricted to the lumbar vertebra retaining both epiphyses and the base of a transverse process (illustrated in Paulson’s fig 2 [25]). According to Gol’din and Zvonok [50], these vertebrae are lost. However, a missing type specimen does not by itself mean a name should be set aside as a nomen dubium, and the type specimen of Zeuglodon paulsonii (now Pachycetus paulsonii) came from a known locality and stratigraphic interval. The type was well illustrated and fully described by Paulson in Brandt [25]. Measurements of the type and other vertebrae of Pachycetus paulsonii are listed in Table 6.
Table 6. Centrum measurements for posterior thoracic and lumbar vertebrae of European Pachycetus paulsonii and its synonyms.
| Author | Genus and species | Locality | Specimen | Pos. | Length | Ant. wid. | Ant. hgt. | Post. wid. | Post. hgt. | Epiphyses |
|---|---|---|---|---|---|---|---|---|---|---|
| Brandt, 1873 [25]: 339 | Zeuglodon paulsonii | Chyhyryn | Lost | L | 260 | 155 | 140 | 155 | 140 | Present |
| Brandt, 1873 [25]: 339 | Zeuglodon paulsonii | Chyhyryn | Lost | L | 228 | 150 | 145 | 150 | 145 | Missing |
| Van Beneden, 1883 [28]: 28 | Pachycetus robustus | Helmstedt | MMGD NsT-90 | T | 160 | 120 | 100 | 120 | 100 | Missing |
| Fedorowskij, 1912 [36]: 260 | Zeuglodon paulsonii | Khoropove | Lost | L | 283 | 161 | 151 | 161 | 151 | Present |
| Fedorowskij, 1912 [36]: 260 | Zeuglodon paulsonii | Khoropove | Lost | L | 282 | 165 | 157 | 165 | 157 | Present |
| Fedorowskij, 1912 [36]: 260 | Zeuglodon paulsonii | Khoropove | Lost | L | 274 | 164 | 157 | 164 | 157 | Present |
| Fedorowskij, 1912 [36]: 260 | Zeuglodon paulsonii | Khoropove | Lost | L | 231 | 157 | 147 | 157 | 147 | Present |
| Kuhn, 1935 [37]: 224 | Pachycetus robustus | Helmstedt | MMGD NsT-90 | T | 150 | 120 | 95 | 110 | 95 | Missing |
| Bogachev, 1959 [38]: 41 | Zeuglodon paulsonii | Khoroshevskaya | CMN | L | 250 | 170 | 150 | 140 | 150 | — |
| Bogachev, 1959 [38]: 41 | Zeuglodon paulsonii | Khoroshevskaya | CMN | L | 240 | 180 | 150 | 160 | 145 | — |
| Bogachev, 1959 [38]: 41 | Zeuglodon paulsonii | Khoroshevskaya | CMN | L | 225 | 175 | 160 | 160 | 160 | — |
| Gritsenko, 2001 [41]: 18* | Platyosphys einori | Pyrohiv, Kyiv | TSNU-GM 2638 | L | 225 | 155 | — | 155 | — | Missing |
| Gritsenko, 2001 [41]: 18* | Platyosphys einori | Pyrohiv, Kyiv | TSNU-GM 2638 | L | 212 | 153 | — | 140 | — | Missing |
| Uhen and Berndt, 2008 [44]: 57 | Eocetus sp. | Rohrdorf | Berndt collection | L | 210 | 161 | — | 161 | — | Missing |
| Gol’din et al., 2012 [46]: 111 | "Eocetus" sp. | Kurenevka | NMNH-P OF-1695 | L | 160 | 147 | 120 | 158 | 134 | Missing |
| Goldin and Zvonok, 2013 [50]: 255 | Basilotritus uheni | Beloskelevatoye | NMNH-P OF-2096 | T | 159 | 98 | 78 | 140 | 80 | — |
| Goldin and Zvonok, 2013 [50]: 260 | Basilotritus sp. | Velykaya Andrusovka | KOM 44693 P 195 | L | 202 | 136 | 126 | 157 | 125 | Missing |
| Gol’din et al., 2014 [51]: 271 | Basilotritus sp. | Nagornoye | NMNH-P Ngr-12 | L | 193 | 186 | 124 | 186 | 124 | Missing |
| Van Vliet et al., 2020 [54], p. 145 | Pachycetus robustus | Helmstedt | MMGD NsT-90 | T | 166 | — | — | 129 | 96 | Missing |
| Van Vliet et al., 2020 [54], p. 146 | Pachycetus sp. | Alversdorf | ID20-2 | L | 239 | 130 | 140 | 131 | 124 | Missing |
| Van Vliet et al., 2020 [54], p. 146 | Pachycetus sp. | Treue | NMR 9991–51759 | L | 225 | 140 | 178 | — | 130 | Missing |
Referred specimens
The principal specimens of Pachycetus paulsonii are listed in Table 6. Additional specimens are listed and illustrated in literature cited in the synonymy above.
Diagnosis
Pachycetus paulsonii differs from Pachycetus wardii in being significantly larger. Lumbar centra with epiphyses average 266 mm in length for P. paulsonii and average 171 mm in length for P. wardii—a difference of 0.44 units on a natural-log scale, which for a linear measurement is equivalent to a difference of about 8 standard deviations.
Provenance
Known specimens are European, from Germany, Russia, Ukraine, United Kingdom, and the North Sea off the coast of Belgium.
Geological age
Most or all well-dated specimens come from the Bartonian stage/age of the late middle Eocene.
Description
The holotype of Pachycetus paulsonii is an anteroposteriorly elongated vertebral centrum retaining both epiphyses. This preserves bases of the anteroposteriorly elongated transverse processes and pedicles for a somewhat less elongated neural arch. Paulson in Brandt [25] gave the length, width, and height of the centrum as 260 × 155 × 140 mm, and the holotype was illustrated in plate 34, fig 2, of Brandt [25]. This plate also shows a somewhat shorter, tapering or trapezoidal thoracic centrum, which is notably narrower at the anterior end and wider at the posterior end. A third centrum illustrated on plate 34 is that of a lumbar showing, again, the characteristically elongated transverse processes. Federowskij [36] described and illustrated several lumbar vertebrae of P. paulsonii and the first-known caudals. Federowskij’s vertebrae are important because of their completeness, preserving neural arches and large flaring metapophyses. Kuhn [37] illustrated the holotype vertebral centrum of Pachycetus robustus Van Beneden, 1883 [28], in his fig 3. He interpreted this as a posterior lumbar and compared it to ‘Zeuglodon’ isis. However, as explained below, it is more likely to be a posterior thoracic of P. paulsonii. Halstead and Middleton [39] illustrated a tapering or trapezoidal centrum that we interpret as a middle thoracic of P. paulsonii.
Vertebrae described by Gritsenko [41] are weathered and so poorly illustrated as to be uninterpretable. This was remedied to some extent by Gol’din and Zvonok [50] and Davydenko et al. [55], who showed that vertebrae Gritsenko interpreted as caudals are really lumbars with anteroposteriorly elongated transverse processes. Uhen and Berndt [44] described a premolar and a lumbar vertebra with an elongated centrum, anteroposteriorly elongated transverse processes, and pockmarked bone. Gol’din et al. [46] described and illustrated two thoracolumbar vertebrae similar to those described by Federowskij a century earlier. Kalmykov [47] illustrated a cervical, a thoracic, and a lumbar vertebra. The first well preserved middle thoracic of P. paulsonii preserving its neural arch was described by Gol’din and Zvonok [50]. The centrum tapers to become wider posteriorly, the neural canal is wide, and the bone of the centrum, neural arch, and neural spine is pachyostotic. Gol’din et al. [51] described cervical vertebrae, which are notable principally for the small size of their vertebrarterial foramina. Van Vliet et al. [54] added a number of cervical, thoracic, lumbar, and caudal vertebrae to Pachycetus paulsonii in what they called Morphotype A. Here again cervicals have small vertebrarterial foramina, thoracic centra are tapering, and lumbars have anteroposteriorly long transverse processes.
Most specimens of Pachycetus paulsonii are vertebrae, but Van Beneden [28] described the distal half of a large pachyostotic rib. Uhen and Berndt [44] illustrated a premolar. Kalmykov [47] described an upper premolar. Zvonok [49] described a relatively flat mesosternal element, rib pieces, and teeth attributable to P. paulsonii. Gol’din and Zvonok [50] described a tympanic with the morphology typical of Basilosauridae, and Gol’din et al. [51] added several teeth and a relatively flat xiphisternum. Van Vliet et al. [54] described and illustrated several teeth and pieces of pachyostotic ribs. Teeth of P. paulsonii are generally similar to those of other Basilosauridae.
Pachycetus wardii (Uhen, 1999)
Holotype
USNM 310633, partial skeleton with the rostral fragment of a skull, vertebrae, ribs, and a partial innominate, found at Lanier’s Pit, Maple Hill, North Carolina.
Referred specimens
CMM V-4334 and 4335, vertebrae. NCSM 11284, partial skeleton with ribs; 11297, vertebrae; 12531, supraoccipital; 13434, vertebral body; 13513, transverse process; 13514, vertebral body; 13676, proximal rib; 13678, partial vertebra; 15663, partial manubrium. USNM 449548, vertebra and ribs.
Diagnosis
Pachycetus wardii differs from Pachycetus paulsonii in being significantly smaller. Lumbar centra with epiphyses average 171 mm in length for P. wardii and average 266 mm in length for P. paulsonii—a difference of 0.44 units on a natural-log scale, which for a linear measurement is equivalent to a difference of about 8 standard deviations.
Provenance
Known specimens are North American and come from the states of North Carolina and Virginia in the eastern United States.
Geological age
The type specimen of Pachycetus wardii is from the Comfort Member of the Castle Hayne Formation [26], which could be late Lutetian or early Bartonian in age, near the beginning of the late middle Eocene [74].
Description
Pachycetus wardii from North America is represented by two partial skeletons that are each more complete than any of their European counterparts. The type, USNM 310633, includes an edentulous rostrum with alveoli for large incisors, canines, and a first premolar. Alveoli for the latter, right P1, show the tooth to have been double-rooted or to have had two fused roots [26].
USNM 310633 includes four thoracic vertebrae, six lumbar vertebrae, and one vertebra tentatively identified as a caudal [26]. P. wardii thoracics have centra increasing in size from anterior to posterior. These are tapered, with the posterior width of each notably greater than the anterior width. Thoracic centrum height is substantially less than centrum width. P. wardii lumbars have centra that change little in size from anterior to posterior. Lumbar centra are more cylindrical in shape than those of thoracics, with anterior and posterior widths being approximately equal. Lumbar centrum height is generally less than centrum width, and the cylindrical shape is thus somewhat flattened dorsoventrally. The caudal is poorly preserved and has never been illustrated. Ribs of Pachycetus wardii are generally osteosclerotic and some are pachyostotic with thickened distal ends [26].
USNM 310633 is distinctive in preserving two pieces of an innominate. One piece has a well-formed acetabulum [26], which is normally where the ilium, ischium, and pubis meet before they co-ossify. There is no suggestion of articulation with a vertebral sacrum, and innominates of Pachycetus wardii were probably anchored in muscles of the ventral body wall—as they were in Basilosaurus and other basilosaurines. A second piece of innominate preserves a portion of the rugose pubic symphysis. We know from comparison of innominates of quadrupedal protocetids with those of fully aquatic dorudontines and basilosaurines that basilosaurids retained a midline pubic symphysis [75] (see Discussion below). However, interpretation of the innominate of Pachycetus is complicated because the intervening piece that connected the acetabulum to the symphysis is missing.
The second of the partial skeletons is NCSM 11284 [42] from the Rocky Point Quarry in Rocky Point, North Carolina [62]. This has a well-preserved series of thoracic and lumbar vertebrae, stylohyals, sternebrae, ribs, and a partial scapula.
Genus Antaecetus, new genus
urn:lsid:zoobank.org:act:5F9DDF63-8AE2-4AA6-9698-38840063555A
Type species
Platyosphys aithai Gingerich and Zouhri, 2015 [24], p. 280.
Included species
Type species only, as Antaecetus aithai.
Diagnosis
Antaecetus has the distinctive pachyosteosclerotic vertebrae of pachycetine basilosaurids but differs from Pachycetus in being smaller and having a notably small cranium. The teeth are more gracile, with smooth rather than crenulated enamel. The first upper premolar, P1, differs from that in Pachycetus in being single-rooted. Posterior upper premolars differ from those of Pachycetus in having three rather than four accessory cusps or denticles flanking the central cusp anteriorly and posteriorly. Upper molars differ in retaining a distinct posteromedial expansion in the position formerly occupied by the protocone.
Etymology
Named for Antaios of Greek mythology (Antaeus in Latin, Anti in Berber), half-giant son of the sea god Poseidon and earth goddess Gaia; combined with cetus (Latin, masc.), whale. According to legend narrated by Plutarch, Antaeus lived in the western desert of North Africa and his tomb was found in what is now Morocco.
Discussion
Antaecetus is known from a skull and much of an associated axial skeleton, both described here. It resembles Pachycetus, but differs in being smaller, and in having a relatively small skull and much smaller and more gracile teeth. Larger, more robust teeth of Pachycetus paulsonii were described by Uhen and Berndt [44], Kalmykov [47], Zvonok [49], Gol’din and Zvonok [50], Gol’din et al. [51], and Van Vliet et al. [54]. The premaxillae of Pachycetus wardii described by Uhen [26] show that it had much larger and more robust incisors than those found in Antaecetus aithai.
Antaecetus aithai (Gingerich and Zouhri, 2015)
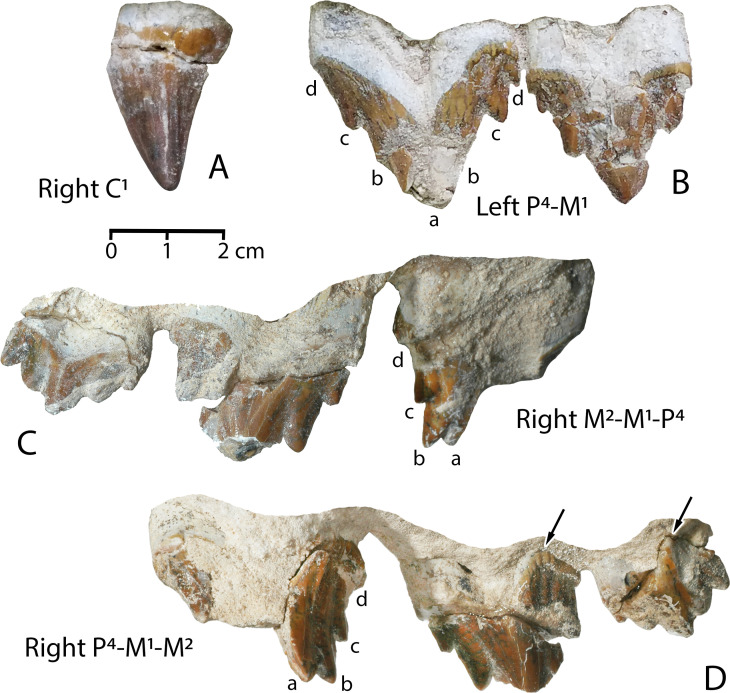
Fig 6. Selected teeth of Antaecetus aithai, specimen FSAC Bouj-200.
A, right upper canine C1 in medial view. B, left upper P4–M1 in lateral view. C, right upper P4–M2 in lateral view. D, right upper P4–M2 in medial view. Apical cusps labeled a are broken on left and right P4; accessory cusps on P4 are labeled, b, c, and d. Arrows point to a posteromedial expansion at the base of the crown on the upper molars; this is broader on M1 and narrower on M2.
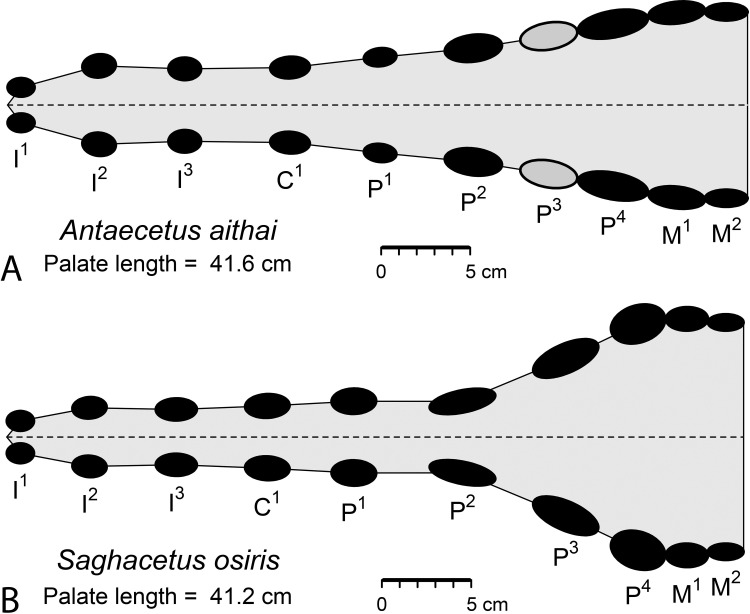
Fig 7. Schematic reconstruction of the palate of Antaecetus aithai compared to that of Saghacetus osiris.
A, palatal map for A. aithai based on FSAC Bouj-200 (Table 3). B, palatal map for S. osiris based on UM 97550. Teeth are shown as ellipses with long axes representing tooth crown lengths and short axes representing tooth crown widths. Spacing is based on diastema length and on the medial-surface-to-midline distance for each tooth. Crown widths for I2 and I3 are assumed to have the same proportion to crown length as that in I1. The missing crown length, width, and height of P3 are assumed to be the same as those for P2. The diastema preceding P3 is arbitrarily assumed to be 10 mm, and the distance from the medial surface of P3 to the midline of the palate is assumed to be the average of crown–midline distances for preceding P2 and following P4. The palate of A. aithai is approximately the same length as that of S. oriris, but palatal and tooth shapes differ.
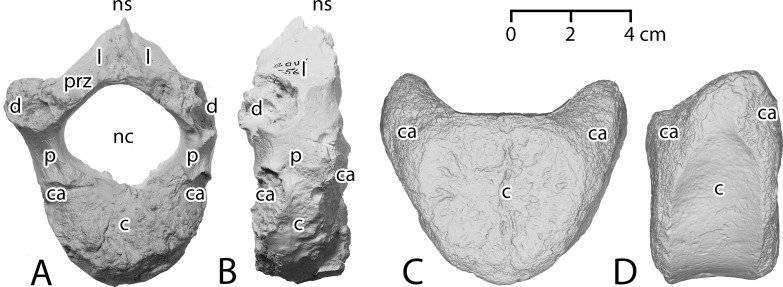
Fig 8. Anterior thoracic vertebrae of Antaecetus aithai and ‘Pachycetus’ humilis.
A, thoracic T1 of A. aithae, FSAC Bouj-56, in anterior view; note the reniform centrum and large neural canal. B, FSAC Bouj-56 in left lateral view. C, centrum of T3 or T4 of ‘P.’ humilis, MMGD NsT-94, in anterior view; note the larger size and more circular centrum. D, MMGD NsT-94 in left lateral view. Illustrations A–B are photographs of a high-fidelity cast, and C–D show a high-resolution 3D digital scan, all reproduced at the same scale. Abbreviations: c, centrum; ca, capitular articulation; d, diapophysis; l, lamina; nc, neural canal; ns, neural spine; p, pedicle; prz, prezygapophysis.
Holotype
FSAC Bouj-6, associated thoracic vertebrae. These were first identified as thoracics T1–T4 [24], but comparisons here indicate they are probably T6, T8, and T11-12.
Referred specimens
FSAC Bouj-7, posterior thoracic vertebra; Bouj-11, three lumbar vertebrae; Bouj-20, partial cranium; Bouj-26, left tympanic bulla; Bouj-200, cranium, thorax, and lumbus described here; measurements of additional specimens are listed in S1 Table.
Provenance
All known specimens of Antaecetus aithai come from the Aridal Formation in the sabkhas of Gueran and El Briej. The new locality of El Briej is located 35 km NNE of Gueran on the map of Gingerich and Zouhri [24].
Horizon and age
Gingerich and Zouhri [24] and Zouhri et al. [76] summarized the geological context of the Aridal Formation at Gueran. The Aridal Formation is found in the sub-basin of Boujdour, which is part of the larger northeast-southwest trending Tarfaya–La’Youn–Ad-Dakhla depositional basin. The larger basin is the onshore part of a passive continental margin on the Atlantic coast of southwestern Morocco. Ratschiller [77, 78] described the stratigraphic sequence of interest as the Gueran Member of his Samlat Formation. Ratschiller divided this member into: (1) a lower 21 m thick chalk with interbedded siltstones and sandstones yielding shark teeth and other fossils; (2) an upper 22 m thick layer of massive white chalk; and (3) a 2 m thick limestone crust or cap rock. Zouhri et al. ([76], fig 2) published another stratigraphic section on the northeastern flank of the Gueran depression that is shorter than the section of Ratschiller, but described in more detail. The fossiliferous interval at Gueran that yields vertebrate remains is a 1-m-thick, white to light-gray, clayey, silty, poorly-sorted sandstone, with component grains ranging from coarse to very fine in size. This fossiliferous bed is 11 m above the base of the section. Gingerich and Zouhri [24] assigned a Bartonian age to the fossiliferous level at Gueran based on the presence of both protocetid and basilosaurid archaeocetes.
Description of FSAC Bouj-200
The principal specimen of Antaecetus aithai described here is the partial skeleton FSAC Bouj-200 (Fig 4), which includes the cranium and thorax in one 110 × 79 cm block of sediment. An articulated sequence of 10 lumbar vertebrae was collected in two additional blocks. Each block was collected in a plaster jacket. The top surface was weathered by exposure in the field, so each block was turned before preparation. The surface visible now in each jacket is the unweathered side that was originally the bottom surface. The cranium is described first, followed by the dentition, thoracic vertebrae, ribs, lumbar vertebrae, and additional elements.
Cranium
The cranium of FSAC Bouj-200 is shown in Fig 5, where the palate and basicranium are exposed. These are distorted slightly, so all measurements are necessarily estimates. The anterior edges of the left and right premaxillae (Pmx) are preserved, as are the posterior surfaces of both occipital condyles (occ). The condylobasal length of the cranium connecting these landmarks is 69.5 cm. The maximum width of the cranium is 31.4 cm, calculated by doubling the distance from the midline of the cranium to its lateral surface just lateral to the right glenoid fossa (glf). The premaxillae are elevated slightly relative to the maxillae (Max), due to compression, and the maxillae are damaged where the upper third premolars, left and right P3, should be (both of these teeth are missing). Bones of the cranium are identified in Fig 5B.
Fig 5. Cranium and thorax of Antaecetus aithai, specimen FSAC Bouj-200.
The cranium is shown in red and the teeth (labeled I1 through M2) are shown in brown. Vertebrae are shown in blue, the left ribs in orange, and the right ribs in yellow. Thoracics and ribs are numbered from T2 through T11 and R2 through R11 on the assumption that the skeleton had 13 thoracics and 13 pairs of ribs. Rib numbers correspond to those of matching vertebrae. Abbreviations: Bo, basioccipital; c, mandibular condyle; Exo, exoccipital; glf, glenoid fossa; Ju, jugal; Max, maxilla; Pal, palatine; Pmx, premaxilla; pps, petrosal and surrounding pterygoid sinus; Pty, pterygoid; sop, supraorbital process of frontal; Sq, squamosal; Tym, tympanic.
Left and right premaxillae (Pmx) each bore three upper incisors (I1-3; I1 is missing on the left side). The suture between the premaxillae and the left and right maxillae (Max) is not visible, but slight displacement and elevation of the premaxillae relative to the maxillae undoubtedly followed the suture. Left and right maxillae each bore seven teeth, of which only the upper canines (C); upper premolars P1-2 and P4; and upper molars M1-2 remain. Upper M2 is missing on the left side. A portion of the left supraorbital process (sop) of the frontal bone is preserved lateral to and above left M1.
The rostral part of the skull is connected to the basicranium by a relatively narrow but robust intertemporal constriction, bordered laterally by large temporal fossae. Palatines (Pal) and pterygoids (Pty) are the principal elements of this constriction that are visible ventrally, and these are not well preserved. Lateral to the temporal fossae, left and right maxillae are connected to the left and right squamosals (Sq) by slender jugals (Ju). The squamosals themselves are not well preserved, and the glenoid fossa (glf) is only present on the right side of the cranium. The only potions of the basicranium that are identifiable are the basioccipital (Bo) with the left and right mandibular condyles (c), and the left and right exoccipitals (Exo). Left and right petrosals and surrounding pterygoid sinuses (pps) are present lateral to the basiocciptal. The petrosals themselves are not well preserved. One tympanic bulla, presumably the right bulla, is preserved floating in matrix just behind the right exoccipital. The tympanic bulla measures approximately 63 × 39 mm in length and width.
Basilosaurid species with good crania include Basilosaurus cetoides and Zygorhiza kochii described by Kellogg [1], Dorudon atrox described by Uhen [8], and Pontogeneus peruvianus described by Martínez-Cáceres et al. [9]. All are similar in having a long and relatively narrow rostrum, a broad supraorbital shield, and a broad braincase, with the facial part of the cranium connected to the braincase by a long and relatively narrow intertemporal region. The cranium of Antaecetus aithai is smaller and more gracile than crania known for other basilosaurids, but it is otherwise typically basilosaurid in form.
Dentition
The upper dental formula of Antaecetus aithai is 3.1.4.2, as is typical for basilosaurids. Upper incisors in the Bouj-200 cranium (I1, I2, and I3) are all single-rooted teeth with simple, laterally-compressed, conical crowns. Incisors at two positions (I1 and I3) have crowns whose projecting height is slightly less than their anteroposterior length. Upper second incisors (I2) have crowns that are significantly higher and more caniniform than the others, with crowns projecting some 1.3 times higher than their anteroposterior length. All have enamel with narrow ridges and shallow grooves running up the surface, and all have a narrow carina or keel of enamel running up the anterior and posterior edges of the crown. The upper canines (C1) are a little larger than the second incisors, but similarly constructed (Fig 6A), with again a single root. The small size of the canine teeth in A. aithai is worthy of note. Canine dimorphism is little studied in archaeocetes [79], but the small canines of Bouj-200 suggest it is female. Upper incisors and canines are separated from adjacent teeth by diastemata of approximately 25 mm (slight distortions of the cranium mean these cannot be measured precisely).
Three of the four upper premolars are present in each maxilla. P1 is a single-rooted tooth. The crown, best preserved on the right side, is smaller but otherwise similar in form to the upper incisors and canines. P2 is a double-rooted tooth. The crown is larger than that of any preceding tooth, but it is not well preserved on either side. Posterior to the crown of P2, there is a substantial gap where there should be, at most, a very small diastema. This is because P3 is missing in Bouj-200. P4 is present and double-rooted. The base of the crown of P4 is well preserved on the left side in Bouj-200, but the surface enamel is somewhat damaged (Fig 6B). The crown is V-shaped in lateral view, with the dentinoenamel junction descending more or less in parallel with the apex of the crown. P4 has a relatively large apical cusp (labeled a in Fig 6) flanked anteriorly and posteriorly by three smaller accessory cusps (labeled b, c, and d) decreasing in size away from the apical cusp. The base of the crown is relatively long and narrow like that of other basilosaurids, but it is more gracile than is typical for basilosaurids. P4 has a weak cingulum on the lateral side of the crown.
The first upper molar, M1, is present in both maxillae of Bouj-200. Left M1 is shown in lateral view in Fig 6B. Right M1 is shown in lateral and medial views in Fig 6C and 6D. M1 is double-rooted. The M1 crown has an apical cusp with two smaller but substantial cusps decreasing in size anterior to the apex and two smaller but substantial cusps decreasing in size posterior to it. The posteromedial margin of the crown is expanded slightly posteromedially (arrow in Fig 6D) but there is no protocone cusp. There is a weak cingulum on the lateral side of the M1 crown, and a stronger cingulum on the medial side of the crown.
The second upper molar, M2, is a simple tooth much smaller than M1. This is shown in lateral and medial views in Fig 6C and 6D. M2 is double-rooted. The M2 crown has an apical cusp and there are again two smaller cusps decreasing in size anterior to the apex and two smaller cusps decreasing in size posterior to it. There is a narrow but distinct medial extension of the medial portion of the crown (arrow in Fig 6D), again with no distinct protocone cusp. The lateral cingulum is weak, and there is seemingly no medial cingulum.
Measurements of the teeth of Bouj-200 are listed in Table 3. This table includes the lengths of diastemata separating successive teeth in the tooth row, and the distance of each tooth from the midline of the cranium. Measurements in Table 3 were used to construct the schematic illustration of the palate shown in Fig 7. The resulting estimate for palate length is 41.6 cm. Thus the length of the palate represents 60% of total skull length. The palate of Antaecetus aithai reconstructed in Fig 7 is similar in size to that of Saghacetus osiris, which is one of the smallest basilosaurids known.
Table 3. Measurements (mm) of teeth and their spacing in the FSAC Bouj-200 cranium of Antaecetus aithai from Boujdour in southwestern Morocco.
| Tooth position | CL (mm) | CW (mm) | CH (mm) | Diast. (mm) | Crown-Mid. (mm) |
| I 1 | 15.2 | 10.5 | 14.2 | — | 4.7 |
| I 2 | 18.9 | — | 25.1 | 26.8 | 15.5 |
| I 3 | 18.4 | — | 17.6 | 29.5 | 14.0 |
| C 1 | 22.3 | 12.2 | 27.2 | 38.8 | 15.0 |
| P 1 | 18.4 | 10.2 | 19.0 | 30.3 | 21.8 |
| P 2 | 32.4 | 15.1 | 28.5 | 27.0 | 24.4 |
| P 3 | — | — | — | — | — |
| P 4 | 39.8 | 15.0 | 32.1 | 0.0 | 38.0 |
| M 1 | 31.8 | 12.3 | 25.1 | 0.0 | 45.8 |
| M 2 | 23.9 | 9.7 | 13.5 | 0.0 | 47.5 |
Abbreviations: CL, mesiodistal crown length; CW, mediolateral crown width; CH, crown height; Diast., diastema separating this from the preceding tooth; Crown-Mid., distance from the medial surface of a tooth crown to the midline of the palate.
Thoracic vertebrae
The ten thoracic vertebrae present in FSAC Bouj-200 are labeled sequentially T2 through T11 in Fig 5B. These numbers were assigned assuming that A. aithai had 13 thoracic vertebrae, but the total number of thoracics is not known. No cervical vertebrae were found with Bouj-200, and it is likely that the most anterior thoracic is missing in Bouj-200 as well. The missing vertebrae may have been destroyed by erosion, or they may remain in a block of sediment that we were unable to locate. In addition, it appears that two posterior thoracic vertebrae were destroyed during excavation when the block of sediment containing the cranium and thorax was separated from the block containing the anterior lumbars. The only parts of the thoracic vertebrae of Bouj-200 that remain are the articulated centra, and these are only visible in ventral view. Rib facets are not visible. The vertebrae were preserved standing upright when they were buried in sediment in the Eocene, and it appears that the neural arches and neural spines were destroyed by weathering when more recent erosion brought the specimen to the surface.
Following the vertebral numbering sequence in Fig 5, the first vertebral centrum that can be measured in Bouj-200 is that of thoracic T3. Centrum lengths of Bouj-200 are listed in Table 4. Centrum widths and heights cannot be measured accurately because they remain embedded in sediment, but it is clear that thoracics of Bouj-200 have tapered centra. The posterior width for a given centrum exceeds the anterior width of the same centrum, and the anterior width for a given centrum is approximately the posterior width of the preceding centrum. Tapered or trapezoidal centra are characteristic of thoracic vertebrae in Pachycetinae.
Table 4. Centrum lengths (mm) of thoracic and lumbar vertebrae in partial skeletons of Antaecetus aithai from Boujdour in southwestern Morocco.
| Vertebral position | FSAC Bouj-6 | FSAC Bouj-7 | FSAC Bouj-11 | FSAC Bouj-56 | FSAC Bouj-200 |
|---|---|---|---|---|---|
| T1 | — | — | — | 33 | — |
| T2 | — | — | — | — | — |
| T3 | — | — | — | — | 39 |
| T4 | 50 | — | — | — | 45 |
| T5 | (54) | — | — | — | 53 |
| T6 | 57 | — | — | — | (56) |
| T7 | (60) | — | — | — | (59) |
| T8 | (63) | — | — | — | 63 |
| T9 | 67 | — | — | — | (68) |
| T10 | 84* | — | — | — | 74 |
| T11 | — | — | — | — | 86 |
| T12 | — | 128 | — | — | (97) |
| T13 | — | — | — | — | (110) |
| L1 | — | — | — | — | (124) |
| L2 | — | — | — | — | 140 |
| L3 | — | — | — | — | (144) |
| L4 | — | — | 151 | — | (147) |
| L5 | — | — | (155) | — | 150 |
| L6 | — | — | 158 | — | (154) |
| L7 | — | — | (163) | — | (157) |
| L8 | — | — | (168) | — | 160 |
| L9 | — | — | (172) | — | (160) |
| L10 | — | — | 175 | — | 160 |
Specimens FSAC Bouj-6, 7, and 11 are illustrated in [24]. Numbers in parentheses are interpolated. There is some ambiguity in measurements of Bouj-200 because it is not clear whether vertebral epiphyses are present or absent. Abbreviations: L, lumbar; T, thoracic. Additional measurements are listed in S1 Table.
Free-standing thoracic centra of Antaecetus aithai were illustrated by Gingerich and Zouhri [24], who described FSAC specimen Bouj-6 and mentioned that rib articulations in this species are less an articular facet than a pit or pitted surface. The pits indicate that rib articulations were not synovial like those of basilosaurines and dorudontines, but cartilaginous or ligamentous like those of protosirenid sirenians [80]. Here, after comparison with Bouj-200, we identify the four thoracics included in Bouj-6 as T4, T6, and T9–T10. T10 in Bouj-6 retains a slender diapophysis ([24], fig 8j). Bouj-7 appears to be thoracic T12.
FSAC Bouj-56 (Fig 8A and 8B) is a free-standing first thoracic vertebra, T1, that complements thoracics of Bouj-200 in retaining the neural arch and base of the neural spine. The centrum measures 33 × 56 × 35 mm in length, width, and height. It has reniform anterior and posterior articular surfaces, with a concave dorsal surface that forms the ventral margin of the neural canal. Anterior and posterior surfaces of the centrum expose cancellous bone, and it is not clear whether the epiphyses were ever ossified. The anterolateral surface of the centrum has roughened surfaces for connection to rib capitula at its lateral poles. Capitular depressions are similarly roughened and slightly higher on the posteolateral surface of the centrum. Pedicles are robust, measuring 31 × 13 mm in length and width. These cover virtually the entire anteroposterior length of the centrum. The neural canal is large and almost circular, measuring 40 × 34 mm, with the width being slightly greater than the height. Robust laminae rising from the pedicles converge to enclose the neural canal, forming the dorsal portion of the neural arch. Diapophyses forming the bases for tubercular rib connections project laterally from the left and right pedicle-lamina junctions, but the tubercular rib connections are not preserved. A small prezygapophysis is present on the anterior margin of the right lamina. This is not preserved on the left lamina. The posterior surfaces of the laminae with postzygapophyses are not preserved. The base of the neural spine is small and the spine itself appears to have been gracile and relatively short. T1 of Antaecetus is superficially similar to T1 in Protosiren [80], but differs in the reniform shape of the centrum, in having a relatively wide neural canal, and in having diapophyses positioned higher on the neural arch.
Ribs
Thoracic vertebrae, by definition, have ribs associated with them. In FSAC Bouj-200 it is possible to link each of the preserved ribs to its corresponding thoracic vertebra, and the ribs are numbered accordingly (Fig 5). Ribs 4 through 11 are present on the left side of the specimen (ribs LR4, etc.), and ribs 2 through 10 are present on the right side of the specimen (ribs RR2, etc.). Rib heads are not well preserved. It appears from their preservation in Bouj-200 that the most anterior ribs are robust but not notably pachyostotic. Ribs 4, 5, and 6 have a body that is expanded anteroposteriorly near the distal end, making them not only robust but also pachyostotic. Posterior ribs are robust but again do not appear to be pachyostotic. These differences can be seen in Fig 5. The pachyostotic ribs in Bouj-200 have maximum diameters of 47–48 mm.
Lumbar vertebrae
Ten lumbar vertebrae of FSAC Bouj-200 were recovered in the two blocks of sediment excavated after the skull and thorax were collected. The lumbar blocks are shown in Fig 4 as they were oriented in the field relative to the block containing the skull and thorax. Successive vertebrae in the two blocks are numbered L1 through L10. These are microfractured and slightly deformed in a way that makes preparation difficult. Nevertheless, several features of pachycetine lumbar vertebrae are evident. The lumbars have transverse processes approaching the length of their centra, and many have substantial prezygapophyses or metapophyses extending anteriorly with no corresponding postzygapophyses. The centra of L4 and L5 are oriented in a way that shows paired longitudinal grooves on the ventral surface of the centrum like those illustrated by Fedorowskij [36]. L4 is missing its anterior epiphysis, but enough remains of the diaphysis to show that this was distinctly wider than high. Measurements of lumbar lengths for Bouj-200 are included in Table 4.
The largest lumbar vertebrae of Antaecetus aithai at Gueran and El Briej have centra that measure 190 to 195 mm in length, 140 to 145 mm in width, and 120 to 125 mm in centrum height (S1 Table). These are presumably male. Vertebrae of male A. aithai approach the size of lumbars presumed to be female in contemporary Eocetus schweinfurthi, but vertebrae of male A. aithai are notably smaller than the larger lumbars of male E. schweinfurthi (see Discussion).
Posterior thoracic, lumbar, and anterior caudal vertebrae of Antaecetus and Eocetus sometimes break longitudinally to expose a sagittal section of the centrum, and centra of the two genera are notably different in internal architecture (Fig 9). Antaecetus, and pachycetines in general, have centra with cones of cancellous bone that flare anteriorly and posteriorly from the center of the centrum, where they are surrounded by a thick outer ring of dense cortical bone. The cortical bone thins toward the anterior and posterior ends of the centrum. This architecture was well illustrated for Pachycetus sp. by Van Vliet et al. [54] in their plate 3, figures A1–A2 and B1–B3. In contrast, Eocetus, and basilosaurines in general, have cylinders of cancellous bone filling much of the anterior and posterior half of each centrum ([71], fig 14). The centra of posterior thoracic, lumbar, and anterior caudal vertebrae are consequently much denser in pachycetines than they are in the corresponding vertebrae of basilosaurines.
Fig 9. Internal architecture distinguishing the elongated lumbar vertebrae of Pachycetinae and Basilosaurinae.
A, diagrammatic midsagittal section of the pachycetine Antaecetus aithai showing anterior and posterior cones of cancellous bone surrounded by dense cortical bone. B, diagrammatic midsagittal section of the basilosaurine Eocetus schweinfurthi showing anterior and posterior cylinders of cancellous bone surrounded by dense cortical bone. The proportion of dense cortical bone is much greater in pachycetines than it is in basilosaurines. Drawings are based on midsagittal sections of broken vertebrae observed in the field.
Free-standing lumbar centra of Antaecetus aithai were illustrated by Gingerich and Zouhri [24]. Now, after comparison with FSAC Bouj-200, we identify the three centra of Bouj-11 as lumbars L1, L3, and L7.
Additional elements
There are probably additional skeletal elements of Antaecetus aithai present in the FSAC collection. However, based on field observations, there are at least five species of Basilosauridae and three species of Protocetidae at the localities of Gueran and El Briej, with broadly overlapping ranges of body size. Consequently, few isolated elements can be attributed to established taxa with certainty.
In an earlier study [24], we interpreted the partial cranium FSAC Bouj-20 as belonging to Antaecetus aithai. Now that there is a cranium of A. aithai associated with an axial skeleton, it appears more likely that Bouj-20 is a partial cranium of Eocetus schweinfurthi. It is too large to represent A. aithai.
Discussion
Antaecetus aithai and other Bartonian basilosaurids from Morocco
Most mammals, including whales, have determinate skeletal growth [81, 82], meaning that the skeleton grows ontogenetically until it reachs a definitive size. The most common elements of a mammalian skeleton are vertebrae, which have a range of different pattern-profiles for centrum length plotted against position in the vertebral column. In Basilosauridae, centrum length increases from front to back through the cervical and thoracic vertebrae, reaches a plateau in the lumbar vertebrae, and then decreases in size through the sacral and caudal vertebrae [83, 84]. Determinate growth and the uniformity of lumbar vertebral size means that lumbars are the most useful skeletal elements for recognizing and distinguishing species based on isolated vertebrae.
Fig 10A shows a proportional map of lumbar size and shape in the three common late middle Eocene basilosaurid species from Gueran and El Briej in Morocco: the smaller dorudontine Chrysocetus fouadassii, the medium-sized pachycetine Antaecetus aithai studied here, and the larger basilosaurine Eocetus schweinfurthi. Fig 10A was constructed by mapping lumbar centrum length and width for each vertebra of each species as a rectangle on logarithmic axes. The distance between the top and bottom of a rectangle is the natural log of centrum length for that vertebra. Top and bottom lines are plotted equally distant from the center of the graph. The distance between the left and right sides of each rectangle is the natural log of centrum width for the same vertebra, and again the left and right sides are plotted equally distant from the center of the graph. Logarithms standardize variability. The purpose of this representation is a standardized comparison of size and shape, and a standardized comparison of the variation in size and shape: C. fouadassii has vertebrae smaller than those of A. aithai and E. schweinfurthi, but the variability in size and shape is comparable for all three species.
Fig 10. Proportional maps of centrum size and shape comparing lumbar vertebrae of Moroccan Basilosauridae with European Pachycetus paulsonii and its synonyms.
A, lumbar size and shape in the three common basilosaurids from Gueran and El Briej. All are Bartonian late middle Eocene in age. Dorudontine Chrysocetus fouadassii is the smallest, with lumbars approximately equal in length and width (based on 27 vertebrae of 8 individual specimens). Basilosaurine Eocetus schweinfurthi is the largest, with lumbars longer than they are wide (13 vertebrae of 7 individuals). Pachycetine Antaecetus aithai is intermediate in size, with lumbars longer than they are wide (17 vertebrae of 9 individuals). B, posterior thoracic and lumbar size and shape for 19 centrum lengths and widths listed in Table 6. These are Bartonian late middle Eocene in age. Lumbar type specimens of Zeuglodon paulsonii Brandt, 1873 [25], and Platyosphys einori Gritsenko, 2001 [41], are shown with solid red lines. Slightly smaller posterior thoracic type specimens of Pachycetus robustus Van Beneden, 1883 [28], and Basilotritus uheni Gol’din and Zvonok, 2013 [50], are shown with dashed red lines. All appear to represent a single species, Pachycetus paulsonii. C, lumbar size and shape in male and female Dorudon atrox (CGM 42813 and UM 97512; N = 35) and female Basilosaurus isis (CGM 42195; N = 19) from Wadi Al Hitan in Egypt are shown for comparison. These are Priabonian late Eocene in age. D. atrox is smaller, with shorter and relatively wider lumbars [8]. B. isis is larger, with longer and relatively narrower lumbars (Gingerich et al., in preparation). The variability of the Moroccan taxa is slightly greater than that of Egyptian taxa because more specimens are involved, and measurements of Moroccan taxa were recorded in the field with less measurement precision.
The use of logarithms to compare size in Fig 10A means that the length versus width shapes of the vertebrae are distorted. However, the shapes can still be compared by noting the position of the corners of each rectangle relative to the diagonal lines underlying each plot. Fig 10A shows that Chrysocetus fouadassii has lumbars of approximately equal length and width. Antaecetus aithai and Eocetus schweinfurthi each have lumbars that are notably longer than they are wide. Summary statistics for the three species are listed in Table 5. There are two additional but less common basilosaurids in the Moroccan fauna, not shown here, with lumbar vertebrae wider than they are long. Fig 10A shows how basilosaurid species at Gueran and El Briej can be distinguished based on the size and shape of lumbar vertebrae, even when individual positions within the lumbar series are not known. Any attempted comparison of cervical, thoracic, or caudal vertebrae will only be useful when vertebrae are compared for the same position within the cervical, thoracic, or caudal series.
Table 5. Summary statistics for centrum length, width, and height (mm) in lumbar vertebrae of Chrysocetus fouadassii, Antaecetus aithai, and Eocetus schweinfurthi from Gueran and El Briej in southwestern Morocco.
| Measurement | N | Minimum (mm) | Maximum (mm) | Mean (mm) | Standard deviation (mm) | Coefficient of variation (std. dev./mean) |
|---|---|---|---|---|---|---|
| Chrysocetus fouadassii | ||||||
| Lumbar length | 28 | 45 | 72 | 62.7 | 8.5 | 0.14 |
| Lumbar width | 27 | 50 | 68 | 61.0 | 4.6 | 0.08 |
| Lumbar height | 26 | 43 | 68 | 56.5 | 5.9 | 0.10 |
| Pachycetus aithai | ||||||
| Lumbar length | 28 | 125 | 195 | 165.2 | 20.4 | 0.12 |
| Lumbar width | 17 | 100 | 145 | 127.1 | 14.6 | 0.12 |
| Lumbar height | 16 | 89 | 125 | 106.6 | 13.9 | 0.13 |
| Eocetus schweinfurthi | ||||||
| Lumbar length | 25 | 200 | 345 | 264.0 | 48.0 | 0.18 |
| Lumbar width | 14 | 140 | 190 | 156.3 | 15.8 | 0.10 |
| Lumbar height | 14 | 114 | 170 | 132.6 | 16.4 | 0.12 |
Thoracic and lumbar vertebrae of Pachycetus from Bartonian age strata of Europe are plotted on the proportional map of Fig 10B (see below for Discussion). For comparison, Fig 10C shows a proportional map of lumbar size and shape in skeletons of two late Eocene basilosaurid species from Egypt: Dorudon atrox and Basilosaurus isis. D. atrox has vertebrae smaller than those of B. isis, and their shapes are different, but the size variation and the shape variation of lumbar centra in the two species are comparable. Dorudon atrox has lumbar vertebrae slightly wider than they are long, and the corners of each rectangle fall slightly below the upper diagonals and slightly above the lower diagonals. Basilosaurus isis has vertebrae that are notably longer than they are wide, and the corners of each rectangle fall well above the upper diagonals and well below the lower diagonals. The D. atrox rectangles in Fig 10C include 10 lumbar vertebrae of one specimen (CGM 42813) identified as female, and 25 lumbars of two specimens (UM 97512 and 101215) identified as male. The B. isis rectangles in Fig 10C include 19 lumbar vertebrae of one specimen, CGM 42195, identified as female.
Length profile of Antaecetus aithai vertebrae
The vertebral centrum lengths for the FSAC Bouj-200 partial skeleton of Antaecetus aithai (Table 4) are plotted against their position in the vertebral column in Fig 11. Corresponding centrum lengths for the CGM 42195 skeleton of Basilosaurus isis are used as a model. We assume that Antaecetus aithai had the same number of cervical vertebrae, seven, as in all other archaeocetes, but the number of thoracics and lumbars in the vertebral column of A. aithai is not known. The minimum number of thoracics in other archaeocetes is 13 and the number of lumbars in Bouj-200 is 10, which we take as minima for both the thoracic and lumbar series of A. aithai. Two additional landmarks are available: the first is the inflection point where anterior thoracic lengths increase more slowly (or decrease) and middle thoracic lengths increase more rapidly, and the second is the inflection point where middle thoracic lengths increase more rapidly and posterior thoracic lengths increase more slowly (vertical dashed lines in Fig 11). We take these landmarks to correspond approximately to the points that separate fixed anterior thoracic vertebrae from more-mobile middle and posterior thoracics. Fixed anterior thoracic vertebrae have vertebrosternal ribs that connect each vertebra through its ribs and short costal cartilages directly to the sternum. More-mobile middle thoracic vertebrae have vertebrochondral ribs connecting each vertebra through its ribs and long costal cartilages to the distal ziphisternum. Finally, posterior thoracic vertebrae have floating ribs that are not constrained by costal-cartilage connections to the sternum.
Fig 11. Profile of vertebral centrum length in the FSAC Bouj-200 partial skeleton of Antaecetus aithai compared to the profile for Basilosaurus isis (CGM 42195).
Thoracic vertebrae are shown in red, cervicals and lumbars in orange. Open symbols are interpolated. Dashed lines approximate the inflection points that separate anterior thoracics with fixed vertebrosternal ribs from middle thoracics with more mobile vertebrochondral ribs, and separate middle thoracics from posterior thoracics with floating ribs. Centrum lengths of A. aithai are approximate because these cannot be measured precisely. A. aithai is shown as having 13 thoracic vertebrae, but the number may have been greater.
With these assumptions, we infer that Antaecetus aithai may have had as few as six anterior thoracic vertebrae (as found in the basilosaurine Basilosaurus isis and the dorudontines Dorudon atrox and Pontogeneus peruvianus). A. aithai may have had as few as 7 middle and posterior thoracic vertebrae (many fewer than those in B. isis or D. atrox and P. peruvianus; see, e.g., [8] p. 83, and [9] p. 17). We assume that two posterior thoracics of A. aithai were destroyed when the skeleton was cut in the field to facilitate collection of the skull and thorax, however if three or more thoracics were destroyed during collection, then A. aithai had more than 13 thoracics. Ten centra were recovered when the lumbar vertebrae were collected, but this is a minimum number and there may have been more lumbars.
Synonymy of European Pachycetus
Lumbar vertebrae of Pachycetus from Bartonian age strata of Europe are listed in Table 6, with literature sources and measurements of centrum length, width, and height. These are plotted on the proportional map of Fig 10B for comparison with Moroccan and Egptian forms. Seventeen different lumbar vertebrae are plotted in yellow in the background of Fig 10B. Two of these are type specimens highlighted with solid red lines: the type of Zeuglodon paulsonii Brandt, 1873 [25] (diamond 1) and the type of Platyosphys einori Gritsenko, 2001 [41] (diamond 3). Two posterior thoracic vertebrae are plotted in yellow in the background of Fig 10B. Both are type specimens, highlighted with dashed red lines to distinguish them from type specimens based on lumbars: the type of Pachycetus robustus Van Beneden, 1883 [28] (diamond 2) and the type of Basilotritus uheni Gol’din and Zvonok, 2013 [50] (diamond 4).
Length and width measurements for the 17 lumbar vertebrae in Table 6 are displayed as histograms in Fig 12. The histogram for lumbar length (Fig 12A) spans a range of 0.6 natural log units (5.05 to 5.65 ln units), with a calculated standard deviation of 0.145. This is equivalent to a coefficient of variation of 14.5% for the raw measurements. The histogram for lumbar width (Fig 12B) spans a range of 0.4 natural log units (4.85 to 5.25 ln units), with a calculated standard deviation of 0.096. This is equivalent to a coefficient of variation of 9.6% for the raw measurements. The greater variability of lumbar length is due to variability in the presence of epiphyses, and to the independent variability of diaphysis length (the standard deviation for nine lumbars lacking epiphyses is 0.120).
Fig 12. Variability of lumbar length and width in European Pachycetus paulsonii and its synonyms.
A, histogram of centrum length for 17 lumbar vertebrae listed in Table 6. Type specimens of Zeuglodon paulsonii Brandt, 1873 [25], and Platyosphys einori Gritsenko, 2001 [41], in this sample are labeled with diamonds numbered 1 and 3. Slightly smaller posterior thoracic type specimens of Pachycetus robustus Van Beneden, 1883 [28], and Basilotritus uheni Gol’din and Zvonok, 2013 [50], are labeled with diamonds numbered 2 and 4. OF-1695 is a short-centrum lumbar lacking epiphyses. B, histogram of centrum width for the 17 lumbar vertebrae plotted in panel A. Posterior-thoracic type specimens are shown as well (diamonds 2 and 4). All appear to represent the species Pachycetus paulsonii.
We cannot be certain that all of the lumbar vertebrae listed in Table 6 represent a single species, but close clustering of their measurements in the histograms of Fig 12 makes this plausible. We interpret all to represent Pachycetus paulsonii. The two type specimens that are most different are those representing the two species, Pachycetus robustus and Basilotritus uheni, which are based on posterior thoracic centra. These are relatively short because they are thoracics, but each is as wide as some lumbar vertebrae of P. paulsonii. It will not be surprising if additional archaeocete genera and species are found to be present in Bartonian age strata of Europe, but this has not yet been demonstrated.
Systematic position of Pachycetus humilis
Van Beneden [28] named the genus Pachycetus and its type species Pachycetus robustus based on what he called a lumbar centrum, MMGD NsT-90, from the phosphate beds at Helmstedt in Germany. MMGD NsT-90 is probably a posterior thoracic of Pachycetus paulsonii [54]. Van Beneden [28] named a smaller archaeocete Pachycetus humilis from the same phosphate beds. The type specimen of P. humilis, MMGD NsT-94, is illustrated in Fig 8C and 8D, where it is compared to FSAC Bouj-56 described here. The NsT-94 centrum, lacking epiphyses, is larger, measuring 40 × 65 × 55 mm in length, width, and height [28]. Pachycetine thoracics increase markedly in size from front to back (Fig 11), and based on size alone the NsT-94 centrum might be considered an anterior thoracic vertebra conspecific with P. robustus. However, the centrum height and the more circular centrum outline of NsT-94 contrast with the broader, shallower vertebral centra of pachycetine thoracics [24, 26, 42, 50]. Thus we agree with Van Vliet et al. [54] that NsT-94 and ‘Pachycetus’ humilis are likely to represent a second Helmstedt archaeocete distinct from Pachycetus.
Interpretation of the innominate of Pachycetus wardii
Relatively complete innominates are known for the basilosaurines Basilosaurus cetoides [85] and Basilosaurus isis [75], for the dorudontines Chrysocetus healyorum [21] and Dorudon atrox (unpublished), and for two transitional early mysticetes [86, 87]. A partial innominate is known for the dorudontine Pontogeneus peruvianus [9]. Basilosaurine and dorudontine innominates are similar in having a relatively small acetabulum (frequently injured and remodeled in life); a small, oval obturator foramen; and a prominent midline symphysis (Fig 13).
Fig 13. Innominate of Pachycetus wardii compared to those of Basilosaurus isis.
A, right innominate of P. wardii, USNM 310633, in ventral view (anterior at the top). Two pieces are preserved, but the shape of bone connecting these, in gray, is conjectural, as are the length and width of the innominate as a whole. B, pubic symphysis of Pachycetus wardii, USNM 316033, in medial view. C–D, pelvic model for B. isis based on right and left innominates, CGM 42295 and UM 93231, in ventral view (anterior at the top) and in anterior view. E, pubic symphysis of the protocetid Qaisracetus arifi, GSP-UM 3410 [88] in medial view. F, pubic symphysis of the basilosaurid Basilosaurus isis, UM 97534, in medial view. Articular surfaces for all three symphyses are similarly rugose. GSP-UM 3410 and UM 97534 are shown at twice the scale of the other images. Abbreviations: ac, acetabulum; il, ilium; is, ischium; of, obturator foramen; pu, pubis.
Uhen [26] described a partial innominate of Pachycetus wardii (Fig 13A) which is part of the type specimen USNM 310633. He placed the species initially in Eocetus, at a time when Eocetus was regarded as a protocetid, recognizing that the innominate of P. wardii was different from those of known protocetids. The only landmark that can be identified with certainty on the partial innominate described by Uhen is the acetabulum. Here we add a second piece of innominate, a portion of the pubic symphysis (Fig 13A), which was found among bone remnants of USNM 310633. The two pieces, acetabular and symphyseal, may be parts of the same innominate, but they do not contact. Consequently, the outline of the P. wardii innominate in Fig 13 is still uncertain.
The ramus and symphysis of the Pachycetus wardii innominate are compared to complete left and right innominates of Basilosaurus isis in Fig 13. Landmarks identifiable in both species are the acetabulum for articulation with the head of the femur, and the midline pubic symphysis (arrows) for articulation with the opposite innominate. If the obturator foramen is correctly identified in P. wardii, then it was much larger than that of Basilosaurus, Chrysocetus, or Dorudon. Our interpretation of the innominate of P. wardii differs from that of Uhen [26] in three ways: (1) we regard Uhen’s ischium as a portion of the ilium; (2) we regard his ilium as the pubic ramus; and (3) we regard his edge of the obturator foramen as a short segment of the outer edge of the innominate. The innominate of P. wardii is not only different from those of protocetids in lacking a sacral articulation, but it is also different from innominates known for basilosaurines and dorudontines.
Lucas [85], Gidley [89], Kellogg [1], Gol’din [90], Martínez et al. [9], Lambert et al. [86], and Buono et al. [87], interpreted the innominates of Basilosaurus and related forms differently from Gingerich et al. [75], who we follow here. The innominate of a basilosaurid differs from the pelvic bone of a modern cetacean in having a well-defined obturator foramen and a recognizable acetabular fossa. The pelvic bone of modern cetaceans lacks these landmarks; is reduced in size; and has the ilium, ischium, and pubis fused or reduced by attrition to a single element.
According to Martínez et al. ([9], p. 130) and other authors, the pelvic bone of modern cetaceans has the orientation typical of quadrupedal mammals (including protocetid archaeocetes), with (1) the ilium anterior to the ischium; and (2) the ischium dorsal to the pubis. On the first point, Martínez et al. cited Struthers [91]—but Struthers did not use the term ilium in reference to cetaceans. Struthers reasoned (page 148), echoing Flower ([92], p. 293), that the modern cetacean pelvic bone is “represented by the ischium alone.” On the second point, Martínez et al. cited Struthers [91] and Andrews [93], but Struthers did not distinguish the pubis from the ischium, and Andrews [93] did not mention either an ischium or a pubis.
The innominates of basilosaurids differ from those of quadrupedal mammals and from those of protocetids in that they no longer articulated with sacral vertebrae, but this does not mean left and right innominates no longer articulated with each other. Surficial bone of the basilosaurid innominate is smooth, except for one rugose surface that resembles the pubic symphysis in a protocetid (compare Fig 13B, 13E and 13F). The basilosaurid symphyseal surface is a little shorter anteroposteriorly than the symphyseal surface in a protocetid of similar size, but this shortening is expected in an element that is itself reduced in size. When left and right innominates are known for the same basilosaurid individual, the left and right symphyseal surfaces match closely in size.
Finally, the natural curvature of basilosaurid innominates (seen in Fig 13D) is similar to curvature in the pubic rami of a protocetid: both follow the curvature of a transverse section of the animal’s ventral body wall where the innominates are located near the base of the tail. This curvature is a functional necessity because left and right acetabula for articulation of the hind limbs, and the midline pubic anchorage for genitalia must all remain near the body surface. The observed curvature would be inexplicable if the innominates were longitudinal elements separated from each other and oriented anteroposteriorly in the lateral wall of the body.
Locomotion and behavior of pachycetine archaeocetes
Salient features of the FSAC Bouj-200 skeleton and referred specimens of Antaecetus aithai relevant to its locomotion and behavior are:
the skull is small relative to the size of the vertebrae, and the teeth are relatively small and gracile;
vertebrae increase rapidly in size through the thorax, and remain relatively long through the lumbar series;
transverse processes on lumbar vertebrae are nearly as long anteroposteriorly as the vertebral centra;
most vertebrae and some ribs are conspicuously pachyostotic and osteosclerotic; and
rib articulations are cartilaginous and ligamentous, facilitating enlargement and reduction of thorax volume.
Pachycetus paulsonii and P. wardii are similar to A. anthai in the elongation, pachyostosis, and osteosclerosis of posterior thoracic, lumbar, and anterior caudal vertebrae, but the two Pachycetus species appear to have retained larger skulls and more robust teeth.
Skeletal elongation in pachycetines is similar to that in Basilosaurus (Fig 11), and the pelvic girdle and hind limb appear to have been similarly modified (Fig 13). We infer from their elongated torso and reduced hind limbs that Antaecetus and Pachycetus swam, like Basilosaurus [84, 94], by undulation of the body as a whole. However, in contrast to Basilosaurus, anteroposterior elongation of the transverse processes in Antaecetus and Pachycetus means that there was little space between adjacent transverse processes for muscle contraction—minimizing the potential for lateral bending and lateral undulation of the vertebral column. With this constraint, swimming in Antaecetus and Pachycetus was limited to dorsoventral undulation.
Antaecetus and Pachycetus differ from Basilosaurus in having much greater development of bone density: vertebrae as well as ribs are characterized by pachyostosis and osteosclerosis. Ballasting like this may be associated with increased lung volume, and the two together are arguably advantageous in an animal that: (1) feeds in relatively shallow water, at or near the sea bottom; (2) hovers or swims slowly; and (3) relies on free air in the lungs as an oxygen store [95]. Cartilaginous and ligamentous rib articulations may have enabled enlargement of the thorax to increase air intake at the sea surface, and then facilitated collapse of the thorax as air was exhaled to minimize buoyancy on the sea bottom. The locomotor cost of such extensive pachyostosis and osteosclerosis in a swimmer is reduction of the ability to accelerate and maneuver.
From this we infer that pachycetines were probably slow swimmers feeding in the shallow neritic zone of coastal seas. However, Antaecetus, with its relatively small and gracile teeth, cannot have fed on plants as sirenians do, nor hard-shelled invertebrates as sea otters do. Small gracile teeth make it unlikely that Antaecetus fed on the sea bottom because ingested sediment would make such delicate teeth wear rapidly. As a slow swimmer, Antaecetus is not likely to have been a pursuit predator but rather an ambush predator, lying in wait for passing fish or mobile invertebrates of some kind. Mammals require oxygen, so Antecetus necessarily rose to the surface from time to time to breathe as modern cetaceans do.
Conclusions
Pachycetus paulsonii, Pachycetus wardii, and Antaecetus aithai are middle Eocene archaeocetes found in Europe, North America, and Africa, respectively. The three are placed in the new basilosaurid subfamily Pachycetinae. Within Basilosauridae, basilosaurine and pachycetinae genera differ from dorudontines in having posterior thoracic, lumbar, and anterior caudal vertebrae with elongated centra. Pachycetines differ from basilosaurines in having conspicuously pachyostotic vertebrae with thick, dense, laminated cortical bone surrounding a cancellous core. Pachycetinae are also distinctive in having transverse processes on lumbar vertebrae nearly as long anteroposteriorly as the corresponding centrum.
Antaecetus is a new genus and the only pachycetine known from a cranium and substantial axial skeleton. It is smaller than Pachycetus, and differs in having smaller teeth and possibly a smaller skull relative to its body size. The skull of A. aithai described here resembles that of Saghacetus osiris in size, but lacks the narrowly constricted rostrum of S. osiris. Teeth of Antaecetus are more gracile than those of Pachycetus and upper premolars differ in having two rather than three accessory cusps flanking the principal cusp. The vertebral length profile of Antaecetus parallels that of Basilosaurus in having an inflection between anterior and middle thoracics and another between middle and posterior thoracics. The innominate known for Pachycetus is different from that of basilosaurines and dorudontines, but similarly reduced by comparison with earlier protocetids. It is doubtful that Pachycetus or any basilosaurid used its reduced hind limbs in locomotion.
We infer that pachycetines were probably sirenian-like, slow, torso- and tail-powered swimmers feeding in the shallow neritic zone of coastal seas, lying in wait to ambush fish and mobile invertebrates, and rising to the surface when necessary to breathe.
Supporting information
Specimens come from two localities in southwestern Morocco, Gueran and El Breij. FSAC numbers are given where these have been assigned. Positions represented are Th, thoracic; L, lumbar; or Ca, caudal. Measurements of the centrum are length (L), anterior width (AW), anterior height (AH), posterior width (PW), and posterior height (PH), Measurements of the neural canal are width (NCW), and height (NCH). Measurements of the neural arch are centrum length anterior to the neural arch (NA1), anteroposterior length of the base of the neural arch (NA2), centrum length posterior to the neural arch (NA3), and length of NA2 as a proportion of NA1+NA2+NA3 (NA2P). Measurements of the transverse process are centrum length anterior to the transverse process (TP1), anteroposterior length of the base of the transverse process (TP2), centrum length posterior to the transverse process (TP3), and length of TP2 as a proportion of TP1+TP2+tP3 (TP2P). Numbers in square brackets are interpolated.
(XLSX)
Acknowledgments
Said Ait Ha found and collected the skeleton of Antaecetus aithai, FSAC Bouj-200, described here. We thank Markus Wilmsen, Museum für Mineralogie und Geologie, Senckenberg Naturhistorische Sammlungen Dresden, and Thomas Reuter, Landesamt für Archäologie Sachsen, Dresden, for providing research-quality digital images of the Pachycetus robustus and P. humilis type specimens (©Landesamt für Archäologie Sachsen); 3D models were produced by Adam Rountrey, University of Michigan Museum of Paleontology (archived as UM 118359 and 118560, respectively). Mark Uhen, George Mason University, provided casts of the Eocetus wardii type specimen (archived as UM 118567). We also thank Pavel Gol’din of the I. I. Schmalhausen Institute of Zoology, National Academy of Sciences of Ukraine, for providing casts of four basilosaurid teeth from Nagornoye (archived as UM 118562–118565).
Mark Uhen of George Mason University and Reinhard Ziegler of the Staatliches Museum für Naturkunde, Stuttgart, clarified our understanding of specimens of Eocetus and what we here identify as Antaecetus in the Stuttgart collection. Hans-Jürgen Berndt allowed us to study specimens of Pachycetus from Rohrdorf in his collection. Nicholas Pyenson provided access to the type specimen of Pachycetus wardii in the U. S. National Museum, and B. Holly Smith helped with its study. William Sanders prepared, molded, and cast some of the specimens studied here at the University of Michigan. Iyad Zalmout helped in distinguishing the anterior thoracic of Antaecetus from vertebrae of Protosiren. Finally, we thank Olivier Lambert and an anonymous reviewer for helpful reviews that improved the manuscript substantially.
Field research in Morocco was supported by grants 9202–12 and 9765–15 from the National Geographic Society, by Hassan II University of Casablanca, Morocco, and by the Museum of Paleontology at the University of Michigan. The manuscript was drafted when Samir Zouhri was a 2021 Visiting Scholar at the University of Michigan supported by the Moroccan-American Fulbright Commission.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
S.Z. 9202-12 National Geographic Society https://www.nationalgeographic.org/funding-opportunities/ The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. S.Z. 9765-15 National Geographic Society https://www.nationalgeographic.org/funding-opportunities/ The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kellogg R. A review of the Archaeoceti. Carnegie Institution of Washington Publications. 1936;482:1–366. [Google Scholar]
- 2.Gingerich PD. Cetacea. In: Werdelin L, Sanders WJ, editors. Cenozoic Mammals of Africa. Berkeley: University of California Press; 2010. p. 873–899. [Google Scholar]
- 3.Uhen MD. A review of North American Basilosauridae. Alabama Museum of Natural History Bulletin. 2013;31(2):1–45. [Google Scholar]
- 4.Uhen MD. Basilosaurids and kekenodontids. In: Würsig B, Thewissen JGM, Kovacs KM, editors. Encyclopedia of Marine Mammals, 3rd ed. London: Academic Press; 2018. p. 78–80. doi: 10.1016/B978-0-12-804327-1.00061-3 [DOI] [Google Scholar]
- 5.Martínez-Cáceres M, Muizon C de. A new basilosaurid (Cetacea, Pelagiceti) from the late Eocene to early Oligocene Otuma Formation of Peru. Comptes Rendus Palevol. 2011;10:517–526. doi: 10.1016/j.crpv.2011.03.006 [DOI] [Google Scholar]
- 6.Buono MR, Fernández MS, Reguero MA, Marenssi SA, Santillana SN, Mörs T. Eocene basilosaurid whales from the La Meseta Formation, Marambio (Seymour) Island, Antarctica. Ameghiniana. 2016;53:296–315. doi: 10.5710/AMGH.02.02.2016.2922 [DOI] [Google Scholar]
- 7.Speijer RP, Pälike H, Hollis CJ, Hooker JJ, Ogg JG. The Paleogene Period (GTS 2020). In: Gradstein FM, Ogg JG, Schmitz MD, Ogg GM, editors. Geologic Time Scale 2020. Amsterdam: Elsevier; 2020. p. 1087–1140. doi: 10.1016/B978-0-12-824360-2.00028-0 [DOI] [Google Scholar]
- 8.Uhen MD. Form, function, and anatomy of Dorudon atrox (Mammalia, Cetacea): an archaeocete from the middle to late Eocene of Egypt. University of Michigan Papers on Paleontology. 2004;34:1–222. [Google Scholar]
- 9.Martínez-Cáceres M, Lambert O, Muizon C de. The anatomy and phylogenetic affinities of Cynthiacetus peruvianus, a large Dorudon-like basilosaurid (Cetacea, Mammalia) from the late Eocene of Peru. Geodiversitas. 2017;39(1):7–163. doi: 10.5252/g2017n1a1 [DOI] [Google Scholar]
- 10.Gingerich PD. New partial skeleton and relative brain size in the late Eocene archaeocete Zygorhiza kochii (Mammalia, Cetacea) from the Pachuta Marl of Alabama, with a note on contemporaneous Pontogeneus brachyspondylus. Contributions from the Museum of Paleontology, University of Michigan. 2015;32:161–188. [Google Scholar]
- 11.Owen R. Observations on the Basilosaurus of Dr. Harlan (Zeuglodon cetoides Owen). Transactions of the Geological Society of London, Series 2. 1841;6(1):69–79. [Google Scholar]
- 12.Andrews CW. Further notes on the mammals of the Eocene of Egypt, Part III. Geological Magazine (Decade V), London. 1904;1:211–215. doi: 10.1017/S0016756800119624 [DOI] [Google Scholar]
- 13.Fraas E. Neue Zeuglodonten aus dem unteren Mitteleocän vom Mokattam bei Cairo. Geologische und Paläontologische Abhandlungen, Neue Folge, Jena. 1904;6:197–220. [Google Scholar]
- 14.Gingerich PD, Arif M, Bhatti MA, Anwar M, Sanders WJ. Basilosaurus drazindai and Basiloterus hussaini, new Archaeoceti (Mammalia, Cetacea) from the middle Eocene Drazinda Formation, with a revised interpretation of ages of whale-bearing strata in the Kirthar Group of the Sulaiman Range, Punjab (Pakistan). Contributions from the Museum of Paleontology, University of Michigan. 1997;30(2):55–81. [Google Scholar]
- 15.Gibbes RW. Description of the teeth of a new fossil animal found in the green-sand of South Carolina. Proceedings of the Academy of Natural Sciences, Philadelphia 1845;2:254–256. [Google Scholar]
- 16.Reichenbach HGL. Systematisches. In: Carus CG, editor. Resultate geologischer, anatomischer und zoologischer Untersuchungen über das unter dem Namen Hydrarchos. Dresden: Arnold; 1847. p. 13–15. [Google Scholar]
- 17.Müller JP. Über die fossilen Reste der Zeuglodonten von Nordamerica, mit Rücksicht auf die europaischen Reste aus dieser Familie. Berlin: G. Reimer; 1849. [Google Scholar]
- 18.Dames WB. Über Zeuglodonten aus Aegypten und die Beziehungen der Archaeoceten zu den übrigen Cetaceen. Geologische und Paläontologische Abhandlungen, Jena. 1894;5:189–222. [Google Scholar]
- 19.Andrews CW. A descriptive catalogue of the Tertiary Vertebrata of the Fayum, Egypt. London: British Museum (Natural History); 1906. [Google Scholar]
- 20.Gingerich PD, Uhen MD. Ancalecetus simonsi, a new dorudontine archaeocete (Mammalia, Cetacea) from the early late Eocene of Wadi Hitan, Egypt. Contributions from the Museum of Paleontology, University of Michigan. 1996;29(13):359–401. [Google Scholar]
- 21.Uhen MD, Gingerich PD. New genus of dorudontine archaeocete (Cetacea) from the middle-to-late Eocene of South Carolina. Marine Mammal Science. 2001;17:1–34. doi: 10.1111/j.1748-7692.2001.tb00979.x [DOI] [Google Scholar]
- 22.Gingerich PD. Stromerius nidensis, new archaeocete (Mammalia, Cetacea) from the upper Eocene Qasr el-Sagha Formation, Fayum, Egypt. Contributions from the Museum of Paleontology, University of Michigan. 2007;31:363–378. [Google Scholar]
- 23.Uhen MD, Pyenson ND, Devries TJ, Urbina M, Renne PR. New middle Eocene whales from the Pisco Basin of Peru. Journal of Paleontology. 2011;85:955–969. doi: 10.1666/10-162.1 [DOI] [Google Scholar]
- 24.Gingerich PD, Zouhri S. New fauna of archaeocete whales (Mammalia, Cetacea) from the Bartonian middle Eocene of southern Morocco. Journal of African Earth Sciences. 2015;111:273–286. doi: 10.1016/j.jafrearsci.2015.08.006 [DOI] [Google Scholar]
- 25.Brandt JF. Untersuchungen über die fossilen und subfossilen Cetaceen Europa’s. Mémoires de l’Académie Impériale des Sciences de Saint-Pétersbourg, VII Série. 1873;20:1–372. [Google Scholar]
- 26.Uhen MD. New species of protocetid archaeocete whale, Eocetus wardii (Mammalia: Cetacea) from the middle Eocene of North Carolina. Journal of Paleontology. 1999;73:512–528. [Google Scholar]
- 27.Seeley HG. Notice of the occurrence of remains of a British fossil Zeuglodon (Z. wanklyni Seeley) in the Barton Clay of the Hampshire coast. Quarterly Journal of the Geological Society of London. 1876;32:428–432. [Google Scholar]
- 28.Van Beneden P-J. Sur quelques ossements de cétacés fossiles, recueillis dans des couches phosphatées entre l’Elbe et le Weser. Bulletin de l’Academie Royale des Sciences, des Lettres, et des Beaux-Arts de Belgique, Bruxelles. 1883;Série 3, Tome 6(7):27–33. [Google Scholar]
- 29.Miller GS. The telescoping of the cetacean skull. Smithsonian Miscellaneous Collections. 1923;76:1–70. [Google Scholar]
- 30.Slijper EJ. Cetaceen Die, Vergleichend-Anatomisch und Systematisch. Capita Zoologica. 1936;6–7:1–590. [Google Scholar]
- 31.McKenna MC, Bell SK. Classification of Mammals Above the Species Level. New York: Columbia University Press; 1997. [Google Scholar]
- 32.Van Beneden P-J. Description des ossements fossiles des environms d’Anvers. Cinquième partie. Annales du Musée Royal d’Histoire Naturelle de Belgique, Série Paléontooogique. 1886;13:1–139. [Google Scholar]
- 33.Lutugin LI. Geological research carried out in 1893 in the northern part of the Donetz coal basin (in Russian). Izviestiia Geologicheskago Komiteta. 1894;13(2):129–148. [Google Scholar]
- 34.Andrews CW. Note on the cervical vertebra of a Zeuglodon from the Barton Clay of Barton Cliff (Hampshire). Quarterly Journal of the Geological Society of London. 1907;63:124–127. [Google Scholar]
- 35.Stromer von Reichenbach E. Die Archaeoceti des ägyptischen Eozäns. Beiträge zur Paläontologie und Geologie Österreich-Ungarns und des Orients, Vienna. 1908;21:106–178. [Google Scholar]
- 36.Fedorowskij AS. Discovery of a fossil cetacean in the Zmievsky District of Kharkov Province (in Russan). Trudy Obshchestva Ispytateleĭ Prirody pri Imperatorskom Kharʹkovskom Universitet. 1912;45:253–287. [Google Scholar]
- 37.Kuhn O. Archäoceten aus dem norddeutschen Alttertiär. Zentralblatt für Mineralogie, Geologie und Paläontologie, Stuttgart, Abteilung B. 1935;1935:219–226. [Google Scholar]
- 38.Bogachev VV. Remains of a cetacean from the Oligocene of Tsimlyanskaya Station (in Russian). Trudy Instytuta Mineral’nykh Resursiv Akademii Nauk USSR, Kiev. 1959;1:40–42. [Google Scholar]
- 39.Halstead LB, Middleton JA. Notes on fossil whales from the upper Eocene of Barton, Hampshire. Proceedings of the Geologists Association, London. 1972;83:185–190. [Google Scholar]
- 40.Lienau H-W. Die marinen Deckschichten (Mitteleozän-Unteroligozän) der Helmstedter Braunkohlen (Niedersachsen, BRD). Zeitschrift Documenta Naturae. 1984;22:1–120. [Google Scholar]
- 41.Gritsenko VP. New species Platyosphys einori (Archaeoceti) from Oliogocene deposits of Kiev (in Ukrainian). Visnyk Heolohila Kyivskyi Natsionalyi Universytet Imeni Tarasa Shevchenka, Geologiya. 2001;20:17–20. [Google Scholar]
- 42.Uhen MD. New material of Eocetus wardii (Mammalia, Cetacea), from the middle Eocene of North Carolina. Southeastern Geology. 2001;40:135–148. [Google Scholar]
- 43.Post K. Raadsels uit de Noordzee [Riddles from the North Sea]. Cranium. 2007;24(2):31–38. [Google Scholar]
- 44.Uhen MD, Berndt H-J. First record of the archaeocete whale family Protocetidae from Europe. Fossil Record: Mitteilungen aus dem Museum für Naturkunde in Berlin. 2008;11:57–60. doi: 10.1002/mmng.200800001 [DOI] [Google Scholar]
- 45.Weems RE, Edwards LE, Osborne JE, Alford AA. An occurrence of the protocetid whale ‘Eocetus’ wardii in the middle Eocene Piney Point Formation of Virginia. Journal of Paleontology. 2011;85(2):271–278. doi: 10.1666/10-083.1 [DOI] [Google Scholar]
- 46.Gol’din PE, Zvonok EA, Krakhmal’naya T. New materials of ’Eocetus’ sp. (Mammalia, Cetacea) from the Eocene of Ukraine (in Ukranian). Geolog Ukrainy. 2012;39:104–113. [Google Scholar]
- 47.Kalmykov NP. New finding of the ancient whale Basilosaurus (Cetacea, Archaeoceti: Basilosauridae) in the Lower Don area. Doklady Earth Sciences. 2012;442:178–180. [Google Scholar]
- 48.Tesakov AS, Aleksandrova GH, Tarasenko KK, Timonina GI, Titov VV. Palynological characteristics of Paleogene sediments and the find of an ancient whale on the southwestern coast of Tsimlyan Reservoir (Rostov region) (in Russian). Bulletin of the Regional Interdepartmental Stratigraphic Commissions for the Center and South of the Russian Platform. 2012;5:138–143. [Google Scholar]
- 49.Zvonok EA. Problematic remains of Eocene cetaceans from Nagornoe field (Kirovograd region, Ukraine) and the importance of Archaeoceti for stratigraphic research (in Russian). Ukrainian Geologist. 2012;37:87–93. [Google Scholar]
- 50.Gol’din PE, Zvonok EA. Basilotritus uheni, a new cetacean (Cetacea, Basilosauridae) from the late middle Eocene of eastern Europe. Journal of Paleontology. 2013;87:254–268. doi: 10.1666/12-080R.1 [DOI] [Google Scholar]
- 51.Gol’din PE, Zvonok EA, Rekovets L, Kovalchuk A, Krakhmalnaya T. Basilotritus (Cetacea: Pelagiceti) from the Eocene of Nagornoye (Ukraine): new data on anatomy, ontogeny and feeding of early basilosaurids. Comptes Rendus Palevol. 2014;13:267–276. doi: 10.1016/j.crpv.2013.11.002 [DOI] [Google Scholar]
- 52.Post K, Hoekman A, De Wilde B. Oerwalvissen op de bodem van de Noordzee [Primeval whales on the bottom of the North Sea]. Cranium. 2017;34(1):48–51. [Google Scholar]
- 53.Mychko EV, Tarasenko KK. The first finding of Basilosauridae (Mammalia: Cetacea) in the upper Eocene of the Baltic States (Russia, Kaliningrad Region). Paleontological Journal. 2020;54(3):311–318. doi: 10.1134/S0031030120030119 [DOI] [Google Scholar]
- 54.Van Vliet HJ, Bosselaers M, Vahldiek B-W, Paymans T, Verheijen I. Eocene cetaceans from the Helmstedt region, Germany, with some remarks on Platyosphys, Basilotritus and Pachycetus. Cainozoic Research. 2020;20(1):121–148. [Google Scholar]
- 55.Davydenko S, Shevchenko T, Ryabokon T, Tretiakov R, Gol’din P. A giant Eocene whale from Ukraine uncovers early Cetacean adaptations to the fully aquatic life. Evolutionary Biology. 2021;48(1):67–80. doi: 10.1007/s11692-020-09524-8 [DOI] [Google Scholar]
- 56.Brandt JF. Über bisher in Russland gefundene Reste von Zeuglodonten. Bulletin de l’Academie Impériale des Sciences de Saint-Pétersbourg. 1873;18:574. [Google Scholar]
- 57.Geinitz HB. Die sogenannten Koprolithenlager von Helmstedt, Büddenstedt und Schleweke bei Harzburg. Abhandlungen der naturwissenschaftlichen Gesellschaft Isis in Dresden. 1883;1:3–14. [Google Scholar]
- 58.Geinitz HB. Ueber neue Funde in den Phosphatlagern von Helmstedt, Büddenstedt und Schleweke. Abhandlungen der naturwissenschaftlichen Gesellschaft Isis in Dresden. 1883;1:37–46. [Google Scholar]
- 59.Stromer von Reichenbach E. Zeuglodon-Reste aus dem oberen Mitteleocän des Fajum. Beiträge zur Paläontologie und Geologie Österreich-Ungarns und des Orients, Vienna. 1903;15:65–100. [Google Scholar]
- 60.Gingerich PD. Marine mammals (Cetacea and Sirenia) from the Eocene of Gebel Mokattam and Fayum, Egypt: stratigraphy, age, and paleoenvironments. University of Michigan Papers on Paleontology. 1992;30:1–84. [Google Scholar]
- 61.Fraas E. Neue Zeuglodonten aus dem unteren Mitteleocän vom Mokattam bei Cairo. Geologisches Zentralblatt, Leipzig. 1904;5(8):374. [Google Scholar]
- 62.Beatty BL, Geisler J. A stratigraphically precise record of Protosiren (Protosirenidae, Sirenia) from North America. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen. 2010;258:185–194. doi: 10.1127/0077-7749/2010/0095 [DOI] [Google Scholar]
- 63.Geisler JH, Sanders AE, Luo Z. A new protocetid whale (Cetacea: Archaeoceti) from the late middle Eocene of South Carolina. American Museum Novitates. 2005;3480:1–65. [Google Scholar]
- 64.Flower WH. On the arrangement of the orders and families of existing Mammalia. Proceedings of the Zoological Society of London. 1883;1883:178–186. [Google Scholar]
- 65.Marx FG, Lambert O, Uhen MD. Cetacean Paleobiology. Chichester UK: Wiley Blackwell; 2016. [Google Scholar]
- 66.Linnaeus C. Systema Naturae, tenth edition (Systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus I. Editio decima, reformata). Holmiae (Stockholm): Laurentii Salvii; 1758.
- 67.Brisson M-J. Regnum Animale in Classes IX. Distributum, sive Synopsis Methodica. Leiden: Lugduni Batavorum, Theodorum Haak; 1762. [Google Scholar]
- 68.Cope ED. An addition to the vertebrate fauna of the Miocene period, with a synopsis of the extinct Cetacea of the United States. Proceedings of the Academy of Natural Sciences, Philadelphia. 1868;19:138–156. [Google Scholar]
- 69.Bohaty SM, Zachos JC, Florindo F, Delaney ML. Coupled greenhouse warming and deep-sea acidification in the middle Eocene. Paleoceanography. 2009;24(2):1–16. doi: 10.1029/2008PA001676 [DOI] [Google Scholar]
- 70.Vd Buffrénil, Ricqlès AJd, Ray CE, Domning DP. Bone histology of the ribs of the archaeocetes (Mammalia: Cetacea). Journal of Vertebrate Paleontology. 1990;10:455–466. [Google Scholar]
- 71.Houssaye A, Tafforeau P, de Muizon C, Gingerich PD. Transition of Eocene whales from land to sea: evidence from bone microstructure. PLoS One. 2015;10(e0118409):1–28. doi: 10.1371/journal.pone.0118409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grevé KH. Fossile und recent Wale des russischen Reichsgebietes. Korrespondenzblatt des Naturforschenden Vereins zu Riga. 1904;47:67–76. [Google Scholar]
- 73.Schouten S. De wervels van Basilosauridae: een overzicht van en een vergelijking met raadselachtige vondsten uit de Noordzee. Cranium. 2011;28(2):17–25. [Google Scholar]
- 74.Hazel JE, Bybell LM, Edwards LE, Jones GD, Ward LW. Age of the Comfort Member of the Castle Hayne Formation, North Carolina. Geological Society of America Bulletin. 1984;95:1040–1044. doi: [DOI] [Google Scholar]
- 75.Gingerich PD, Smith BH, Simons EL. Hind limbs of Eocene Basilosaurus isis: evidence of feet in whales. Science. 1990;249(4965):154–7. doi: 10.1126/science.249.4965.154 [DOI] [PubMed] [Google Scholar]
- 76.Zouhri S, Gingerich PD, Adnet S, Bourdon E, Jouve S, Khalloufi B, et al. Middle Eocene vertebrates from the Sabkha of Gueran, southern Morocco. Comptes Rendus Geoscience. 2018;350(6):310–318. doi: 10.1016/j.crte.2018.06.006 [DOI] [Google Scholar]
- 77.Ratschiller LK. Sahara, correlazioni geologico-lithostratigrafiche fra Sahara Centrale ed Occidentale (con note geologiche generali e brevi cenni sulle possibilità petrolifere dell’Africa Nord-Occidentale). Memorie del Museo Tridentino di Scienze Naturali, Trento. 1967;16 (2):53–190. [Google Scholar]
- 78.Ratschiller LK. Lithostratigraphy of the northern Spanish Sahara. Memorie del Museo Tridentino di Scienze Naturali, Trento. 1970;18:1–80. [Google Scholar]
- 79.Gingerich PD, Haq M-u, Koenigswald Wv, Sanders WJ, Smith BH, Zalmout IS. New protocetid whale from the middle Eocene of Pakistan: birth on land, precocial development, and sexual dimorphism. PLoS One. 2009;4 (e4366):1–20. doi: 10.1371/journal.pone.0004366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zalmout IS, Gingerich PD. Late Eocene sea cows (Mammalia, Sirenia) from Wadi Al Hitan in the Western Desert of Fayum, Egypt. University of Michigan Papers on Paleontology. 2012;37:1–158. http://hdl.handle.net/2027.42/94568. [Google Scholar]
- 81.Mumby HS, Chapman SN, Crawley JAH, Mar KU, Htut W, Thura Soe A, et al. Distinguishing between determinate and indeterminate growth in a long-lived mammal. BMC Evolutionary Biology. 2015;15(1):214. doi: 10.1186/s12862-015-0487-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Manabe A, Yamakawa T, Ohnishi S, Akamine T, Narimatsu Y, Tanaka H, et al. A novel growth function incorporating the effects of reproductive energy allocation. PLoS One. 2018;13(6):e0199346:1–18. doi: 10.1371/journal.pone.0199346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gingerich PD. Paleobiological perspectives on Mesonychia, Archaeoceti, and the origin of whales. In: Thewissen JGM, editor. Emergence of Whales: Evolutionary Patterns in the Origin of Cetacea. New York: Plenum Press; 1998. p. 423–449. [Google Scholar]
- 84.Buchholtz EA. Vertebral osteology and swimming style in living and fossil whales (Order: Cetacea). Journal of Zoology. 2001;253(2):175–190. doi: 10.1017/S0952836901000164 [DOI] [Google Scholar]
- 85.Lucas FA. The pelvic girdle of Zeuglodon, Basilosaurus cetoides (Owen), with notes on the other portions of the skeleton. Proceedings of the US National Museum. 1900;23:327–331. [Google Scholar]
- 86.Lambert O, Martínez-Cáceres M, Bianucci G, Di Celma C, Salas-Gismondi R, Steurbaut E, et al. Earliest mysticete from the late Eocene of Peru sheds new light on the origin of baleen whales. Current Biology. 2017;27(10):1535-41.e2:1–7. doi: 10.1016/j.cub.2017.04.026 [DOI] [PubMed] [Google Scholar]
- 87.Buono MR, Fordyce RE, Marx FG, Fernandez MS, Reguero MA. Eocene Antarctica: a window into the earliest history of modern whales. Advances in Polar Science. 2019;30(3):293–302. doi: 10.13679/j.advps.2019.0005 [DOI] [Google Scholar]
- 88.Gingerich PD, M-u, Khan IH, Zalmout IS. Eocene stratigraphy and archaeocete whales (Mammalia, Cetacea) of Drug Lahar in the eastern Sulaiman Range, Balochistan (Pakistan). Contributions from the Museum of Paleontology, University of Michigan. 2001;30:269–319. [Google Scholar]
- 89.Gidley JW. A recently mounted Zeuglodon skeleton in the United States National Museum. Proceedings of the US National Museum. 1913;44:649–654. [Google Scholar]
- 90.Gol’din PE. Naming an innominate: pelvis and hindlimbs of Miocene whales give an insight into evolution and homology of cetacean pelvic girdle. Evolutionary Biology. 2014:473–479. [Google Scholar]
- 91.Struthers J. On the bones, articulations, and muscles of the rudimentary hind-limb of the Greenland right-whale (Balaena mysticetus). Journal of Anatomy and Physiology. 1881;15(2)141-176, (3)301-321. [PMC free article] [PubMed] [Google Scholar]
- 92.Flower WH. An Introduction to the Osteology of the Mammalia. 2nd ed. London: Macmillan; 1876. [Google Scholar]
- 93.Andrews RC. A remarkable case of external hind limbs in a humpback whale. American Museum Novitates. 1921;9:1–6. [Google Scholar]
- 94.Gingerich PD, Antar MSM, Zalmout IS. Aegicetus gehennae, a new late Eocene protocetid (Cetacea, Archaeoceti) from Wadi Al Hitan, Egypt, and the transition to tail-powered swimming in whales. PLoS One. 2019;14(12):e0225391:1–55. doi: 10.1371/journal.pone.0225391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Taylor MA. Functional significance of bone ballast in the evolution of buoyancy control strategies by aquatic tetrapods. Historical Biology. 2000;14:15–31. doi: 10.1080/10292380009380550 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Specimens come from two localities in southwestern Morocco, Gueran and El Breij. FSAC numbers are given where these have been assigned. Positions represented are Th, thoracic; L, lumbar; or Ca, caudal. Measurements of the centrum are length (L), anterior width (AW), anterior height (AH), posterior width (PW), and posterior height (PH), Measurements of the neural canal are width (NCW), and height (NCH). Measurements of the neural arch are centrum length anterior to the neural arch (NA1), anteroposterior length of the base of the neural arch (NA2), centrum length posterior to the neural arch (NA3), and length of NA2 as a proportion of NA1+NA2+NA3 (NA2P). Measurements of the transverse process are centrum length anterior to the transverse process (TP1), anteroposterior length of the base of the transverse process (TP2), centrum length posterior to the transverse process (TP3), and length of TP2 as a proportion of TP1+TP2+tP3 (TP2P). Numbers in square brackets are interpolated.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.