Abstract
Prolonged and intensive exercise induces remodeling of all four cardiac chambers, a physiological process which is coined as the “athlete’s heart”. This cardiac adaptation, however, shows overlapping features with non-ischemic cardiomyopathies, such as dilated, arrhythmogenic and hypertrophic cardiomyopathy, also associated with athlete’s sudden cardiac death. Cardiac magnetic resonance (CMR) is a well-suited, highly reproducible imaging modality that can help differentiate athlete’s heart from cardiomyopathy. CMR allows accurate characterization of the morphology and function of cardiac chambers, providing full coverage of the ventricles. Moreover, it permits an in-depth understanding of the myocardial changes through specific techniques such as mapping or late gadolinium enhancement. In this narrative review, we will focus on the certainties and uncertainties of the role of CMR in sports cardiology. The main aspects of physiological adaptation due to regular and intensive sports activity and the application of CMR in highly trained athletes will be summarized.
Keywords: athlete’s heart, cardiovascular magnetic resonance imaging, physiological adaptation, cardiomyopathies
1. Introduction
Cardiac adaptation due to regular and intense exercise is a well-known phenomenon leading to symmetric hypertrophy and volumetric adaptation summarized by the term “athletes’ heart” [1]. The intensive training-induced enlargement of all four cardiac chambers is a well-known attribute of cardiac adaptation. However, it shows overlapping features with important cardiovascular diseases such as dilated (DCM), arrhythmogenic (ACM) and hypertrophic (HCM) cardiomyopathy [2].
Critically, these diseases are among the most important underlying alterations leading to rare (approximately 1 in 50,000) but tragic and highly publicized events of sudden cardiac death (SCD) among young athletes [3,4]. Although up to 80% of athletes are asymptomatic before major adverse cardiovascular events, sophisticated screening tools in a serial fashion can uncover predisposing factors, leading to early diagnosis and target interventions potentially preventing SCD.
Cardiac magnetic resonance (CMR) imaging metrics show high reproducibility and the method is well-suited to differentiate physiological adaptation from pathological alterations [5]. CMR allows accurate characterization of the morphology and function of the cardiac chambers, providing full coverage of the ventricles. Using specific techniques and gadolinium-based contrast material, CMR can also provide tissue level information including myocardial necrosis, fibrosis or edema [6].
To distinguish between physiological and pathological remodeling with high precision, we must establish what normal means within the context of the athlete’s heart. Therefore, we summarize the main aspects of physiological adaptation due to regular and intensive sports activity. Second, we describe the main application of CMR in highly trained athletes. Finally, we provide an overview of critical gaps in the literature.
2. Normal Ranges for Healthy Cardiac Adaptation—What Do I Mean When I Talk about “Normal Values”?
The first description of cardiac dilatation and hypertrophy due to sports activity in cross-country skiers using percussion goes way back to the dawn of the 19th century [7,8]. The introduction of modern imaging techniques, most importantly echocardiography, has revolutionized our understanding of the athlete’s heart. Indeed, it has remained the primary method to investigate physiological cardiac adaptation due to prolonged and intensive exercise. However, CMR is superior at describing subtle alterations and can ensure the clarification of challenging diagnoses. For this reason, CMR is now an important second-line method of investigation for athletes with the suspicion of structural alterations [9].
The cine CMR sequence has been established as the gold-standard method for quantification of ventricular volumes, function and myocardial mass in the general population [6,10,11,12]. However, to date, the only published meta-analysis focusing on the characteristics of the athletes’ heart using CMR is limited to Caucasian, adult, male athletes competing in endurance and mixed sports [13]. Herein, we summarize the main advances in the field and identify critical gaps in the literature regarding the effect of age, sex, ethnicity, sports exposure and post-processing software disparities.
2.1. Age
Cardiac morphology, function and adaptation to exercise changes with age [14,15]. In terms of cardiovascular screening for athletes the recommendation differentiates between young competitive athletes (<35 years) and master athletes (≥35 years) [16]. Adolescent athletes (12–17 years) should be also considered separately, in terms of their adaptation to sports activity due to age-dependent hormonal transition that can potentially unmask genetically determined diseases in this age group [17]. Adolescent athletes have been shown to present only modest increases in left ventricular (LV) cavity size [18], and maximal wall thickness [19] compared to non-athletes. This might suggest that exercise has a different effect on the immature heart, but it may also occur due to lower training intensity and cumulative exercise load at younger ages [18].
Cousergue et al. demonstrated a strong relationship between age-induced changes in LV diastolic function and left atrial (LA) function in healthy young (<35 years) and master (>35 years) athletes using echocardiography [20]. Importantly, Torlasco et al. found key differences in the pattern of adaptation caused by physical activity using CMR. They investigated 237 untrained healthy subjects over a mid-term (6 months) unsupervised physical training, which led to increased ventricular volumes among participants under the age of 35 years, whilst training caused predominantly vascular remodeling among those over 35 years [15]. This is potentially due to the impaired cardiovascular elasticity driven by a reduced number of cardiomyocytes, changes in collagen structure and the reduced responsivity of the heart to sympathetic stimulation as a result of declined cardiac innervation [21,22,23].
2.2. Sex
Despite female athletes taking up to half of the whole athletic population [24], the cardiac adaptation in women is still incompletely understood. Sex differences have been described mainly through studies using echocardiography, generally showing that female athletes have a less pronounced cardiac adaptation compared to man [25]. In contrast, D’Ascenzi has recently shown that highly trained women exhibit a relatively larger increase in cavity dimensions compared to men [26]. In a small athletic cohort, Petersen et al. found no sex-specific differences in training effect on LV and RV volumes, mass indices, and ejection fractions, as well as LV to RV ratios of these volume and mass indices [27].
Csecs et al. on the other hand, examined 327 healthy Caucasian athletes using CMR, including 85 (26%) female athletes to investigate the influence of age, sex, body size, sporting type and training volume on cardiac size and function [28]. They found that both adult and adolescent male athletes showed larger mean ventricular volume and ventricular mass compared with their female counterparts. Importantly, in their multivariate model testing for the potential contributing factors of LV mass index, sex and endurance sports were the strongest. Similarly, Maestrini et al. confirmed that Olympic-level male endurance athletes presented higher volumes and LV mass compared to their female counterparts. Interestingly atria dimensions, systolic function and sphericity index did not differ between sexes in this analysis [29]. Of note, these last two studies did not formally test the relative increase in cardiac measures or the interactions between sex and training load.
Indeed, while the overall size of male athlete’s heart is clearly more enlarged than the female’s, the differences in the relative volumetric and mass changes induced by sports activity are still inconclusive [30].
2.3. Ethnicity
Data regarding ethnic differences using CMR is scarce, despite the well-documented impact of origin on the cardiovascular response to high intensity exercise [31]. In the worst case scenario, this lack of evidence can potentially prevent timely diagnosis and preventive measures among athletes of less well described ethnic origin [32].
Black athletes present predominantly similar exercise-related adaptation as white athletes, however they generally demonstrate an increased LV wall thickness and a more concentric pattern of hypertrophy compared to white athletes [31,33]. Riding et al. have shown competitive male athletes participating in mixed sports exhibit LV hypertrophy more frequently in cases of African-American/Caribbean (9.5%) and West African (5%) origin compared to athletes with West Asian (0.8%), East African (0%), and North African (0%) ancestry even after accounting for size [34]. This trend is rather similar among female athletes too [35].
Critically, echocardiography data from Malhotra et al. [36] shows that mixed-race soccer players have a greater LV wall thickness than white athletes even after indexing for body surface area. In a study of 3000 mixed race, black and white athletes, they found that black athletes have a greater LV wall thickness compared with both white and mixed-race athletes. Moreover, LV wall thickness over 12 mm was present in 7.1% of black athletes, 5.9% of mixed-race athletes, and 1.3% of white athletes. In the future, classification based on genetic ancestry might enable an even more accurate description of cardiovascular adaptation leading to less racial bias in the general and athletic population alike [37].
2.4. Type and Intensity of Sports Exposure
As per the 2020 ESC guidelines, we can differentiate between athletes based on level of sports activity and the type of sports they perform [16]. Comparing elite endurance athletes (sports activity >18 h/week), and regular athletes (sports activity 9–18 h/week) to non-athletes (sports activity <3 h/week) Prakken et al. found that both absolute and body surface area-indexed LV and RV volumes and LV myocardial mass are significantly higher in athletes compared with non-athletes [38]. However, the variation between different levels of exercise is less clearly defined. The relationship between time spent exercising and measures of cardiac remodeling shows an ambiguous relationship, depending to the athletic population in question [28,38].
The differences between sports disciplines are also widely studied, however, it is rather challenging to compare exercise-type related remodeling owing to the differences in the classification of sports in the literature [28,39,40]. In general, endurance sports are associated with ventricular dilatation and eccentric hypertrophy, mixed sports with slightly more modest and balanced dilatation and strength exercise with a more concentric pattern of hypertrophy. However, these are only schematic descriptions and recent data suggest that different sports even within the same discipline might induce certain specific functional alteration or changes in the cardiac deformation [41,42].
In contrast, D’Ascenzi reported that RV volumes and LV mass were independent of sports discipline in their meta-analysis [13]. Of note, the latter study did not differentiate between athletes under and over 35 years and the dataset only contained mixed and endurance athletes.
2.5. Post-Processing Sofware and Contouring Methods
Although it is very well known that different contouring approaches have an important impact on cardiovascular measures [43], no study has formally assessed the potential impact of using different post processing software, or contouring methods in healthy athletes.
3. Hinge Point Fibrosis—Bad Actor or Innocent Bystander?
The right ventricular insertion points (RVIP) to the anterior and posterior ventricular septum, also known as hinge points, are zones of transition where RV and LV muscular fibers cross each other. In these areas, the presence of isolated focal myocardial disarray has been demonstrated thanks to autopsy studies conducted on hearts with HCM [44,45]. Subsequently, isolated myocardial disarray in the RVIP has been seen as a frequent and non-specific finding both in normal (especially athletes) and abnormal conditions (for instance hypertensive cardiomyopathy or aortic stenosis) [46,47].
The reason why RVIP can present myocardial disarray is still a matter of debate. Given their location and configuration, RVIP seem to be particularly sensitive to ventricular pressure overloading. It has been demonstrated in animal models that a raised RV wall stress for increased pressure loading may lead to incremental biventricular fibrosis, predominantly involving the septal hinge points, probably involving increased growth factors signaling, predominately expressed at the level of RVIP [48,49].
Beside histopathology, the presence of an altered myocardial tissue at ventricular interceptions has also been demonstrated by imaging. LGE confined to isolated RVIP (hinge point LGE or “junctional” LGE) is a common finding on CMR imaging. It has raised interest since its first identification in HCM patients, among whom focal fibrosis in the insertion points has been reported in at least 10% of cases [50,51]. However, neither the presence nor the extent of LGE in these areas have been proven to predict adverse events among HCM subjects. Recent studies suggest that RVIP LGE may present different histological characteristics compared to LGE in other areas, with different prognostic implications. Indeed, autopsy analysis have shown that the RVIP region is composed of expanded extracellular space containing interstitial fibrosis, adipose tissue and disarrayed myocytes, while no signs of scarring as a repair process have been identified. Therefore, it was suggested that RVIP LGE presence in HCM could represent gadolinium storage in an expanded extracellular space with disorganized architecture, rather than replacement scarring [51,52].
Hinge point LGE has also been described in other clinical conditions characterized by RV overload [53]. Interestingly, a recent analysis has reported a high prevalence (up to 40%) of RVIP LGE in patients with dilated cardiomyopathy (DCM), with higher LGE extension in the presence of higher wall stress [54].
While LGE imaging is able to evaluate advanced stages of remodeled myocardial tissue, early stages of RVIP disarray may be more subtle and difficult to detect. Newer imaging techniques such as T1-mapping techniques may be more sensitive for the identification of such changes [55,56].
Beyond these considerations, hinge point LGE is also a common finding in healthy subjects with otherwise normal CMR findings and seems to have no prognostic implications [57]. Furthermore, insertion point LGE has been also observed in around 10% of healthy elderly individuals and may form one of the elements of aging hearts [58].
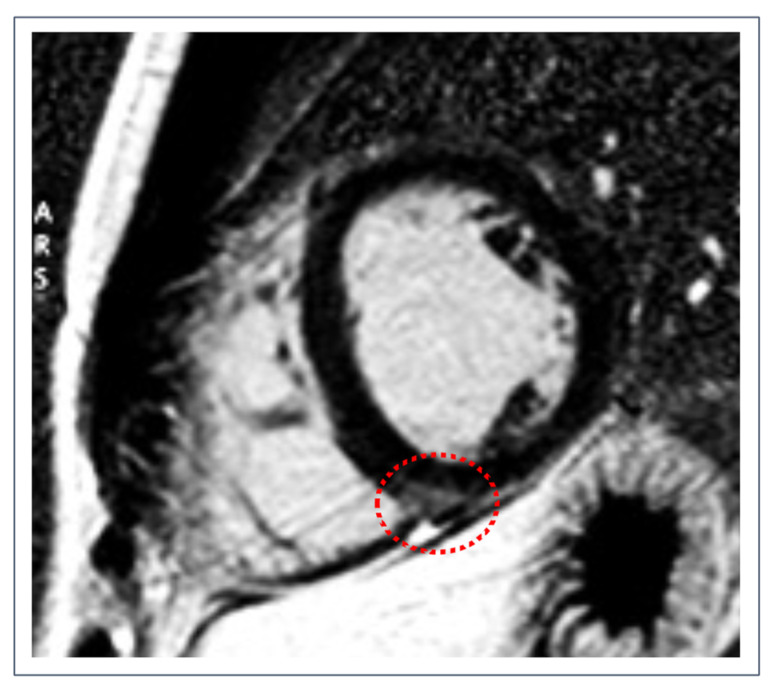
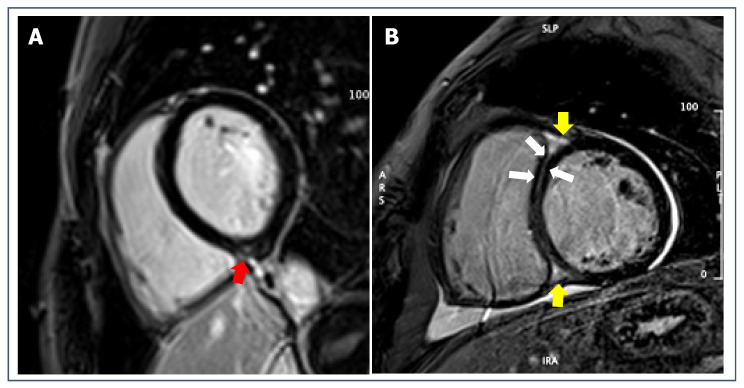
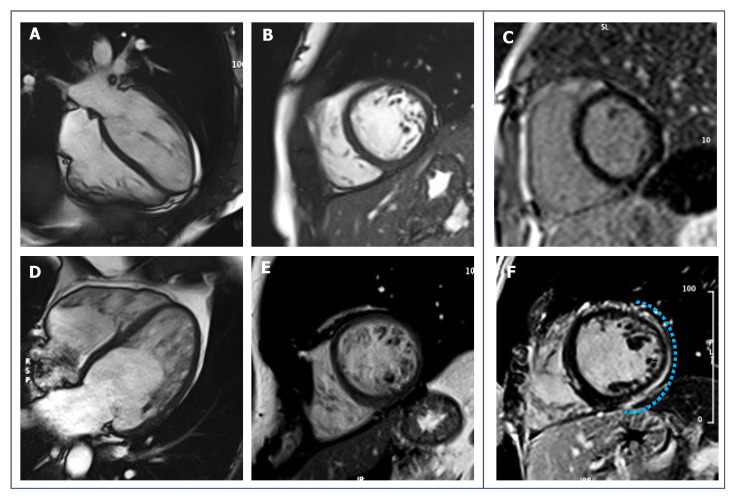
Highly trained endurance athletes (Figure 1) showed a ten-fold increase in the prevalence of focal RVIP LGE as compared to control subjects, along with a globally higher myocardial extracellular volume (ECV) values [59]. The prevalence of RVIP LGE among well-trained athletes may reach up to 30% and has been correlated with the cumulative training load and training intensity [60]. As previously seen for a number of pathological scenarios, this correlation between training load and RVIP alterations may be related to the prolonged pressure and volume overload during intensive exercise, which causes tension on the LV/RV insertion points consequently leading to microinjuries visible as spots of LGE. This hypothesis has been partially confirmed by a recent study evaluating CMR parameters in ultra-marathon runners, which found that athletes with RVIP LGE had higher training volume history and higher right ventricular end-diastolic volume index in comparison to those without LGE. These results may suggest a relationship between hinge-point fibrosis and volume overload [61]. Nonetheless, the total amount of LGE did not significantly differ between athletes and sedentary controls, thus making it challenging to understand the role of endurance training in determining LGE. Finally, the long-term prognostic role of isolated RVIP LGE in the subset of otherwise healthy athletes has yet to be investigated.
Figure 1.
Post-contrast short-axis imaging of a healthy 25-year-old male competitive athlete with isolated RVIP fibrosis (dotted red circle) in the context of an otherwise normal CMR.
4. Mapping Normal Values and Isolated Mapping Alterations—Pathognomic Marker or Misunderstood Measurement Error?
Emerging CMR techniques including native T1 and T2 mapping, have become more widely available in recent years, offering new opportunities for the non-invasive identification of various cardiac pathologies [62]. Native T1 mapping enables the quantitative assessment of tissue characteristics such as myocardial fibrosis [63], amyloid deposition [64] and lipid accumulation [65]. T2 mapping can detect myocardial edema in various settings including acute myocarditis [66]. Therefore, non-contrast mapping techniques might have an impact on the early detection of diffuse pathologies while maintaining a relatively short scan time and without the addition of contrast material.
In spite of the successful application of the technique for various differential diagnostic questions, the sex, ethnicity and training dependence of native T1 and T2 mapping values remain incompletely understood [60]. It is also unclear how recreational sports activity affects these parameters. Importantly, critical differences within scanner types, mapping sequences, and the lack of universal phantom use can hamper the global standardization of mapping values of in the near future [6,43,62].
Native T1 mapping is generally slightly decreased among young, healthy athletes [67,68,69,70], probably due to the increased myocyte mass (causing expansion of intracellular space) in relation to the extracellular space [68,71]. Meanwhile, other authors reported no difference in T1 compared to less active individuals [72,73]. There is less dedicated research regarding the changes of T2 relaxation time with regard to physical activity. Investigating T2 mapping values of healthy elite athletes and volunteers, Szabo et al. reported no differences between the two groups [70].
Notably, limited studies have already demonstrated the additional value of native mapping sequences to better differentiate pathological alterations compared to standard volumetric measures based on cine images. In a small proof-of-concept study, Gastl et al. demonstrated that T2 mapping and deformation imaging may help distinguish left ventricular hypertrophy caused by physiological remodeling due to sports activity and pathological from due to HCM [74]. Similarly, Swoboda demonstrated key differences between HCM patients and athletes even in subjects with gray zone (12–15 mm) hypertrophy using T1 mapping and extracellular volume measurement [71].
Mapping alterations were widely reported in studies investigating the myocardial involvement after SARS-CoV-2 infection in athletes [75,76,77,78,79]. Importantly, these alterations sometimes occurred without any accompanying sign or symptoms of myocardial damage, raising the question of “subclinical” disease versus measurement error. Other studies performed in the general population highlighted the limits of our understanding of these novel and highly sensitive diagnostic tools [80,81]. Experts warned the CMR community to exercise caution when reporting and evaluating isolated mapping alterations, in order to prevent the overdiagnosis of pathologic findings such as myocardial oedema or fibrosis [81,82].
5. Current Evidence of Clinical CMR in the Diagnosis and Management of Athletes
Competitive athletes are a growing population, often (erroneously) considered as “immune” to cardiovascular diseases. Conversely, vigorous physical activity might increase the risk for adverse events up to SCD, especially among subjects with concealed cardiovascular diseases [83].
In the last few years, shared recommendations and pragmatical approaches on athletes’ evaluation have been provided, to distinguish between normality and abnormality [9,84,85]. As mentioned above, CMR use in athletes is rapidly increasing, thanks to the excellent reproducibility, and the large spectrum of morpho-functional information it can provide. In this section, we will look at some of the disease that might be encountered during evaluation of the athlete’s heart.
5.1. Hypertrophic Cardiomyopathy
HCM is one the leading cause of SCD among young athletes [86], and its diagnosis in athletes is not always straightforward. The disease may be suspected when LV end-diastolic wall thickness exceeds 15 mm, since few athletes reach this value of hypertrophy as a physiological adaptation to exercise; the diagnostic suspect is reinforced when other characteristics (ECG, echocardiography, family history or symptoms) are found. However, the differential diagnosis becomes more complex in borderline forms of LV hypertrophy (13–15 mm); indeed, a non-negligible proportion of male healthy athletes (especially those of black ethnicity) reach values of end-diastolic wall thickness between 13 and 16 mm, a range defining the so-called “gray-zone” [39,87].
Notably, the current cut-off values for the diagnosis of HCM are based on echocardiographic, and not CMR, studies. However, when measuring the interventricular septum by echocardiography it is common to wrongly include RV trabeculae, thus overestimating septal thickness. Another ambiguous region is the LV apex, where focal hypertrophy can frequently be unrecognized [88,89,90] (Figure 2). For a more individualized definition of hypertrophy, previous studies have proposed a cut-off of LV thickness indexed to end-diastolic volume [91,92]. However, this may not be applicable to athletes performing strength disciplines, as these sports generally lead to a more concentric LV hypertrophy. Moreover, in athletes with apical phenotype HCM, the cavity size may be larger than expected.
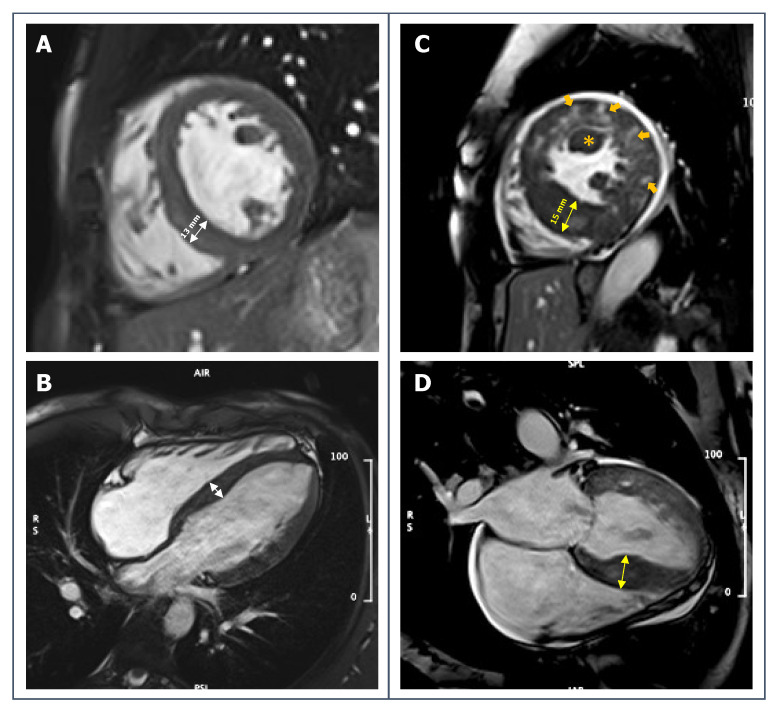
Figure 2.
Left: short-axis (A) and 4-chambers long-axis (B) SSFP-images of a 27-year-old male competitive athlete with borderline LV symmetrical hypertrophy (13 mm, white arrows) in the context of mildly increased biventricular volumes and normal biventricular function and no other symptoms or signs of disease. Right: short-axis (C) and 4-chambers long-axis (D) SSFP-images of a 32-year-old male athlete with increased LV wall thickness especially on interventricular septum (15 mm, yellow arrows), relatively small LV cavity obliterating in systole, papillary muscle hypertrophy (orange asterisk); sequences have been acquired after gadolinium injection and inhomogeneous myocardial signal can be appreciated in these images (orange arrows). The athlete had been referred to CMR for ECG abnormalities.
ECV is typically decreased in athlete’s heart and increased in HCM [71,93]. Moreover, T2 values and native T1 values seem to be significantly prolonged in patients with HCM as compared to healthy athletes [62,74,93].
Contrast-enhanced CMR has been applied to HCM both as an adjunctive diagnostic technique and as a tool for SCD risk stratification [51,94,95]. LGE is present in more than 50% of patients with HCM [96], while it should not be observable in healthy athletes [59,60]. As previously described, the presence of isolated RVIP LGE in HCM does not seem to have prognostic meaning; conversely, the presence of LGE outside hinge-points is associated with increased risk of SCD and overall mortality [51,94,95,97] (Figure 3). Previous studies have also proposed specific cut-off of LGE amount to help in the risk stratification [90]. However, specific studies on the prognostic value of these risk features in competitive athletes are still missing.
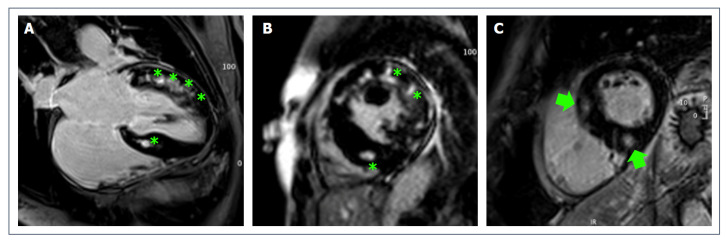
Figure 3.
(A,B): post-contrast imaging (apical 4-chambers view and mid short-axis view) of a 32-year-old male athlete with a diagnosis of HCM with diffuse areas of patchy non-ischemic LGE (green asterisks). (C): short-axis view post-contrast imaging of a 41-year-old female ex-athlete with HCM showing areas of LGE in the basal anteroseptal segment and inferior RVIP (green arrows).
CMR is used for risk stratification according to the 2020 ESC Guidelines on sports cardiology. Importantly, the presence of extensive (≥15% of LV myocardium) LGE may identify individuals at increased risk of ventricular tachyarrhythmias and SCD [95,98,99,100]. In borderline cases, detraining for approximately 3 months may be used to differentiate between the athletes heart and HCM [101]. However, in the absence of high risk features a detraining would not have a consequence on decision-making but might potentially have unnecessarily negative effects on the athlete’s life.
5.2. Dilated Cardiomyopathy
Enlargement of LV cavity is a part of the expression of cardiac adaptation to intense exercise, particularly that with high isotonic/dynamic components. The entity of LV dilatation should therefore be interpreted considering the sports discipline, the body size, the contextual LV hypertrophy, and the harmonic dilatation of the other cardiac chambers [102]. Up to 40% of elite athletes have an increased LV end-diastolic diameter and, in a minority of them, the dilatation reaches values >60 mm with a mildly impaired ejection fraction [39,103]. In these cases, the distinction with mild forms of cardiomyopathy is challenging and of outmost importance, as DCM accounts for up to 8% of SCD in athletes [104,105].
Echocardiographic parameters such as diastolic indexes, strain analysis, LV wall thickness and ejection fraction (at rest and during stress), along with family history, ECG, evaluation of arrhythmias, are the basic diagnostic tools [9]. CMR can offer additional value by providing accurate and reproducible measurements (Figure 4).
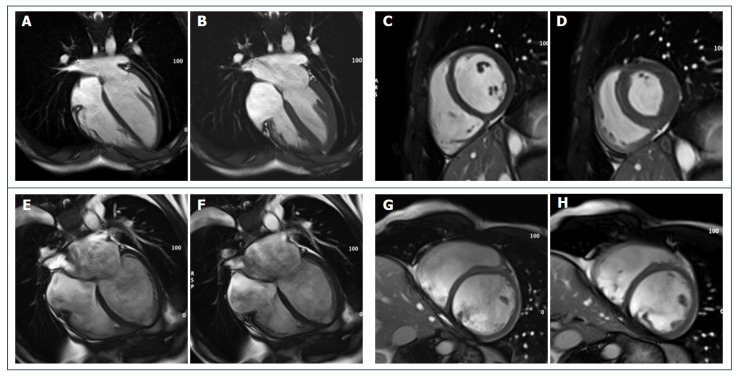
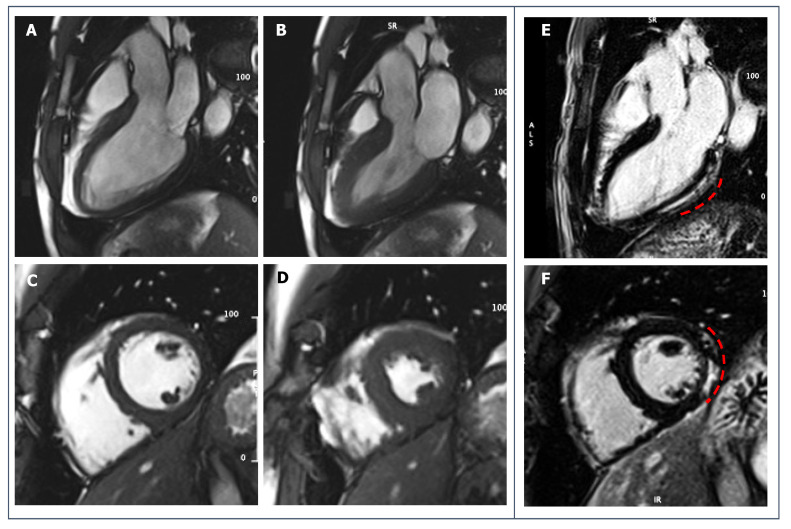
Figure 4.
(A–D): 28-year-old male endurance athlete; SSFP cine sequences (A,C): diastolic frames show mild LV dilatation with harmonic dilatation of the other cardiac chambers and preserved systolic function (B,D): systolic frames. (E,F): SSFP sequences of a 34-year-old male patient with DCM; LV is severely dilated, spherical and moderately dysfunctional (E,G: diastolic frames, F,H: systolic frames).
Recently, the myocardial deformational mechanics by feature-tracking CMR (FT-CMR) have been proposed as an aid in the distinction between adaptation to exercise and pathological dilatation, since DCM has different deformational characteristics as compared to both sedentary and athletes hearts [73]. Moreover mapping technique can be useful in this setting: native T1, ECV and T2 values are all significantly increased in DCM (even in early stages) as compared to normal hearts, and athletes hearts [72].
The main additional value of CMR in this field is the evaluation of fibrosis. The presence of nonischemic LGE (more typically, but not exclusively, as a mid-wall septal stria) is more consistent with DCM, even if the absence of LGE does not exclude the disease [106] (Figure 5). LGE may also be found in the early stages of DCM and is associated with a worsened clinical outcome and the risk of arrhythmic events regardless of the severity of LV dysfunction [107]. Barison et al. have shown that the amount of LGE correlates with major arrhythmic endpoints. They proposed a cutoff of LGE extent at >13% [108]; however, it needs be proven if the same data can be applied to athletes.
Figure 5.
LGE short-axis sequences of a 28-year-old male endurance athlete with athlete’s hearts (A) and of a 34-year-old male patient with DCM (B). In the first case, mild LGE signal is seen in the inferior RVIP (red arrow). In (B), a thin LGE stria can be appreciated in the interventricular (IV) septum (white arrows), and a more pronounced LGE storage is visible in the anterior and inferior RVIP (yellow arrows).
5.3. Arrhythmogenic Cardiomyopathy
Arrhythmogenic cardiomyopathy (ACM) is one of the leading causes of SCD in athletes; moreover, it is well known that intense and repetitive exercise training can accelerate and worsen the course of the disease [109]. The diagnosis of ACM is complex and requires a multiparametric approach [110]; electrical changes, both in the general population and in athletes, the morphological changes may proceed over a long time period [111]. Echocardiography has important limitations in identifying the disease, especially in its early stages. This is true both for the right/biventricular forms (as the complex geometry of RV can be hard to visualize) and for left variant forms, since the disease typically spares the endocardium that contributes most to systolic function and kinetics. Therefore, modern approaches to the diagnosis strongly rely on CMR.
The new diagnostic criteria for ACM [110] requires the presence of at least one morpho-functional (dilatation, kinetics abnormalities and/or dysfunction) or structural (fibrous or fibro-fatty tissue on CMR or endomyocardial biopsy) criteria. In athletes, the cut-off values for RV dilatation proposed in the 2010 criteria [112] may lack specificity because significative RV dilatation can be observed as a physiological adaptation to endurance exercise and increased prolonged RV wall stress [113]. Proper reference values for RV volume in athletes have been reported to differentiate physiologic from pathologic RV dilatation in athletes [13]. Moreover, physiological RV remodeling should be harmonic, different to the predominant dilatation of the outflow tract in ACM patients [114]. RV ejection fraction is frequently reduced in ACM and generally preserved in athletes, although CMR studies have shown that mildly reduced RV function can be observed in up to 5% of elite athletes [115] (Figure 6).
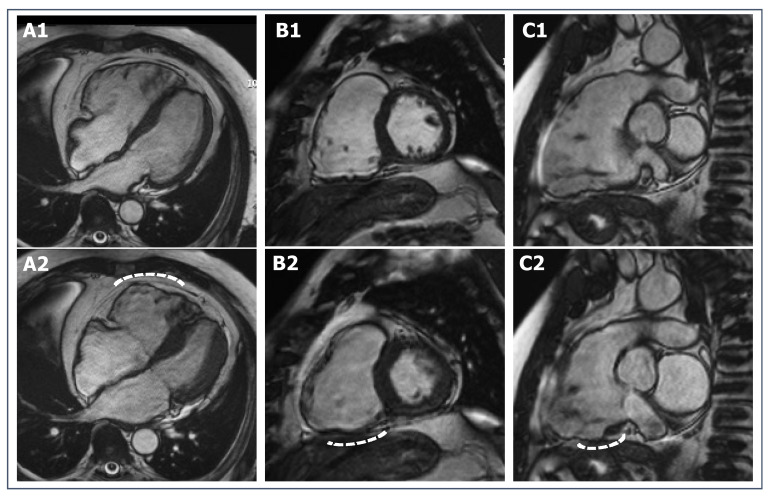
Figure 6.
SSFP cine CMR sequences of a 33-year-old female ex-athlete with arrhythmogenic right ventricular cardiomyopathy. RV dilatation and dysfunction can be appreciated through diastolic and systolic frames ((A1,A2) for 4-chamber view, (B1,B2) for short-axis view, (C1,C2) for RV vertical long-axis view, respectively), as well as wall motion abnormalities (white dotted lines).
Structural evaluation is crucial for the diagnosis of ACM regardless of the exercise level since the disease is characterized by a fibrous (or fibro-fatty) replacement of myocardium. LGE is observed in the majority of cases of ACM. The typical pattern of LGE in left-variant ACM is a subepicardial stria involving more commonly the lateral or infero-lateral wall; concomitant fatty infiltration is often observed in the same regions, even if it is neither specific nor necessary for the diagnosis [116,117] (Figure 7). The assessment of LGE or fatty tissue in RV is challenging because of its thin wall; LGE imaging can be combined with regional wall motion assessment to enhance the sensitivity of CMR [118].
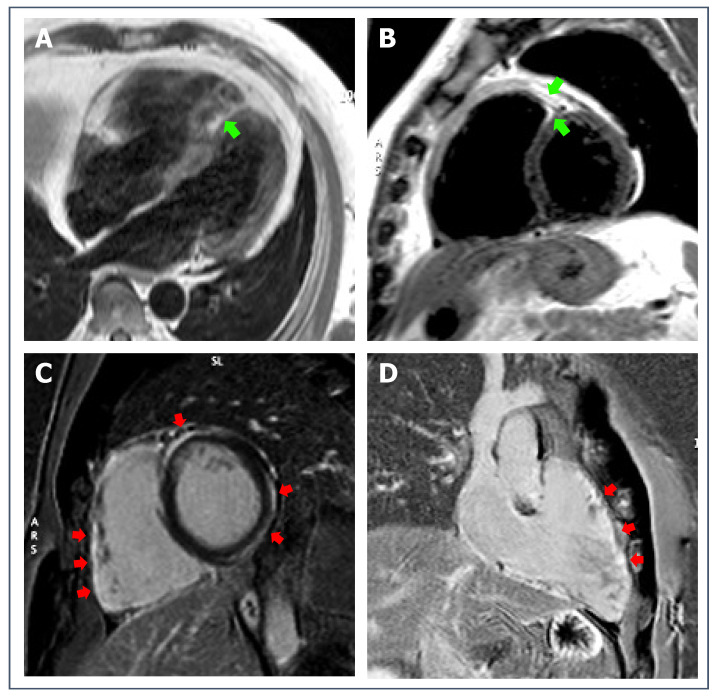
Figure 7.
43-year-old patient with biventricular ACM. T1-weighted sequences (A), 4-chambers view, and (B), short-axis view) show mild signs of fatty infiltration (green arrows). Post-contrast sequences (C), short-axis view, and (D), right heart 2-chambers view) reveal non-ischemic LGE involving both RV and LV walls (red arrows).
Recently, RV strain analysis with FT-CMR has been applied to the field of athletes with suspected ACM, demonstrating good discrimination between athlete’s heart and right-ventricular ACM [119].
5.4. Non-Ischemic Scar
An isolated non-ischemic left ventricular scar (NLVS) is defined as the presence of LGE with a non-ischemic pattern in the LV myocardium in the absence of other distinctive signs of cardiomyopathy. It is an emerging substrate of ventricular arrhythmias and SCD occurring during effort [120,121,122]. NLVS has been originally interpreted as the result of a previous concealed myocarditis, even if it may be the expression of a biventricular or left variant ACM; in the absence of family history, genetic data or other specific features of ACM, the distinction between the two etiologies is challenging. Even the presence of concomitant fatty infiltration is not exclusive of ACM, as it can be part of the natural process of repair after myocardial injury.
CMR offers the possibility of identifying myocardial fibrosis even where other techniques fail. Indeed, since non-ischemic scar by definition spares the endocardium, no wall motion abnormalities or systolic dysfunction are seen, except in cases of extensive scar replacement (Figure 8). ECG abnormalities such as T-wave inversion or low QRS voltages in the limb leads can raise the suspicion of a concealed myocardial fibrosis, but they are not sensitive enough to exclude the disease [123]. When the suspect is raised, CMR is essential for the diagnosis even when second level testing (ECG, echocardiography) is normal.
Figure 8.
39-year-old male football player with uncommon ventricular arrhythmias. No systolic dysfunction or wall motion abnormalities are seen on SSFP cine-sequences (A,B): diastolic and systolic frames of 3-chambers view; (C,D): diastolic and systolic frames of short-axis view. (E,F): post-contrast imaging shows a sub-epicardial stria of LGE involving the basal and middle portion of infero-lateral LV wall (red dotted lines).
In caseswe of an inflammatory etiology of the NLVS, CMR also offers the possibility to display the presence of myocardial edema thanks to T2-weighted sequences and T2 mapping technique indicating an ongoing inflammation [124,125].
The presence of non-ischemic LGE is a marker of possible arrhythmic risk per se. Recently, it has been suggested that LGE amount, but also pattern and localization (for examples, ring-like scar) may carry higher arrhythmic risk [126]. Moreover, the concomitant presence of fatty metaplasia has been associated with negative outcomes [127]. However, the clinical meaning of persistent LGE in an athlete after an acute inflammatory process, in the absence of LV dysfunction or arrhythmias, is still a matter of debate, and it has to be established if there is a specific amount of LGE above which restriction to sports activity should be recommended [128,129].
5.5. Left Ventricular Non-Compaction
Left ventricular non-compaction (LVNC) is characterized by prominent myocardial trabeculations and a thin compacted myocardial layer. The proposed diagnostic criteria rely on the evidence of a high non-compacted to compacted ratio on echocardiography or on CMR [130,131,132,133]. However, fulfilling these morphologic criteria per se has not been associated with adverse LV remodeling or outcome [134]. It is debated whether LVNC should be categorized as an independent disease or not. Critically, there is a genetic and morphological overlap with well described diseases such as DCM. Therefore, categorizing potential dilated LVNC cases as DCM with hypertrabeculation is probably more helpful in clinical practice.
On the other hand, high preload conditions including physiological adaptation to pregnancy or intensive exercise are also associated with increased LV trabeculation. Indeed, almost 20% of asymptomatic athletes can have increased LV trabeculation and up to 8%, in particular those of African or Afro-Caribbean origin, fulfil the criteria for LVNC [135,136,137]. Whether hyper-trabeculation in athletes is a form of incomplete expression of LVNC or just a form of cardiac adaptation to exercise is still uncertain. Although the latter seems more likely, studies on athletes with increased trabeculations have shown that generally this pattern was not associated with LV dysfunction or family history of cardiomyopathy [61]. Key features that should suggest cardiac pathology in the context of LVNC are ongoing cardiac symptoms, decreased systolic function, and a family history of heart failure or SCD [16].
A complete and multimodal approach is essential for the diagnosis of LVNC, especially in athletes. In suspected cases of LVNC, CMR should be performed to confirm hyper-trabeculation, to better assess LV kinetics and function and to detect areas of LGE that are suggestive for cardiomyopathy [138] (Figure 9). Importantly, the application of published LVNC criteria among athletes is both incorrect and potentially detrimental to patient care.
Figure 9.
(A–C): 19-year-old healthy male runner with hypertrabeculation of LV in the context of an otherwise normal CMR. (D–F): 23-year-old male athlete with a diagnosis of LVNC (later confirmed with genetic testing) with evidence of non-ischemic LGE on post-contrast imaging (blue dotted line).
LGE presence may be seen in LVNC, but the sensitivity is not particularly high [139]. However, its presence may predict adverse outcomes in terms of heart failure and arrhythmias. The few other known predictors of events are LV dilatation and reduced ejection fraction, and a thinned compacted myocardial layer [140,141,142,143].
Recently, mapping studies on LVNC have shown that higher ECV values correlate with worsened outcomes [144]. FT-CMR reports have demonstrated that strain is impaired in patients with LVNC in comparison to patients with normal LV phenotype [145] and that this reduction correlates with the degree of ejection fraction reduction [146]. So far, none of these techniques have been specifically studied in the context of athletes.
5.6. Anomalies of the Coronary Artery Origin
The estimated prevalence of anomalies of the coronary artery origin (ACAO) in the general population is low; nevertheless, it is a well-recognized substrate of SCD in athletes [147]. Higher risk of SCD is carried by ACAO with hemodynamic impact, such as the origin from a wrong aortic sinus with inter-arterial course, or, more uncommonly, left/right coronary artery origin from the pulmonary artery (ALCAPA/ARCAPA) [148,149]. In athletes with a suspicion of ACAO, advanced morphological imaging is recommended [9], by using coronary computed tomography (CCT) or CMR angiography that can depict coronary origins and their proximal course [150,151]. CMR angiography may be a valid alternative to computed tomography in younger athletes with a low risk of coronary artery disease, due to radiation concerns and the possibility of being performed without the use of contrast agents (Figure 10 and Figure 11). CMR angiography can detect the ectopic origin and the course of a coronary artery and quantify the degree of arterial compression thanks to cross-sectional views [95]. Moreover, CMR offers additional information on biventricular function or myocardial viability. However, so far computed tomography remains the first choice in elite athletes or in those with an increased risk of coronary artery disease, due to the possibility of visualizing the atherosclerotic burden [152]. Beyond morphological evaluation, functional assessment to detect exercise-induced ischemia is needed for clinical management and advice on sports participation. Stress echocardiography, nuclear imaging, coronary artery angiography are classically used, depending on the age and risk profile of the athlete. Stress-perfusion CMR has been shown to have good sensitivity and specificity for the non-invasive assessment of myocardial ischemia in the adult [153,154,155] and pediatric [156,157,158] population, with a higher spatial resolution compared to other techniques and a good correlation with invasive functional tools such as fractional flow reserve.
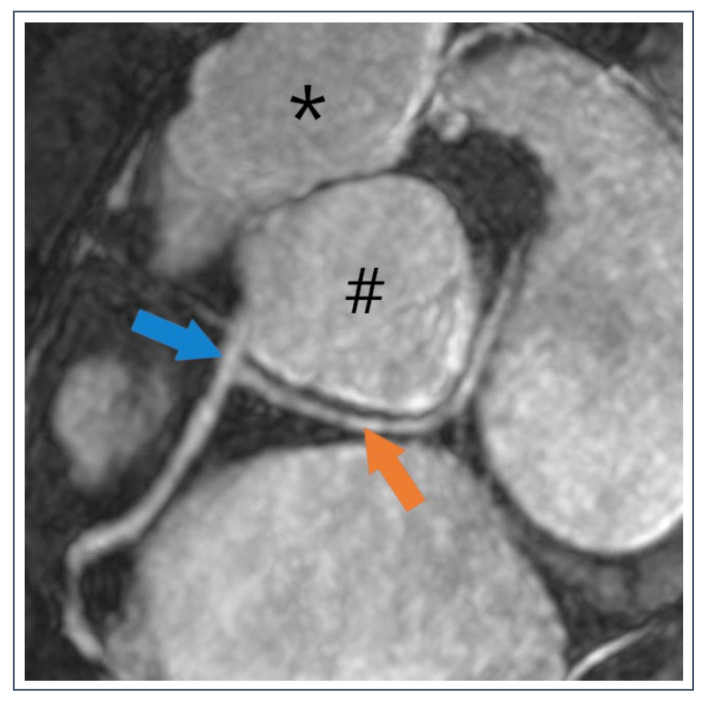
Figure 10.
54-year-old recreational athlete with anomalous circumflex artery (CX) running behind the aorta (#). The CX (orange arrow) originates from the right coronary artery (blue arrow) and has a retroaortic course.
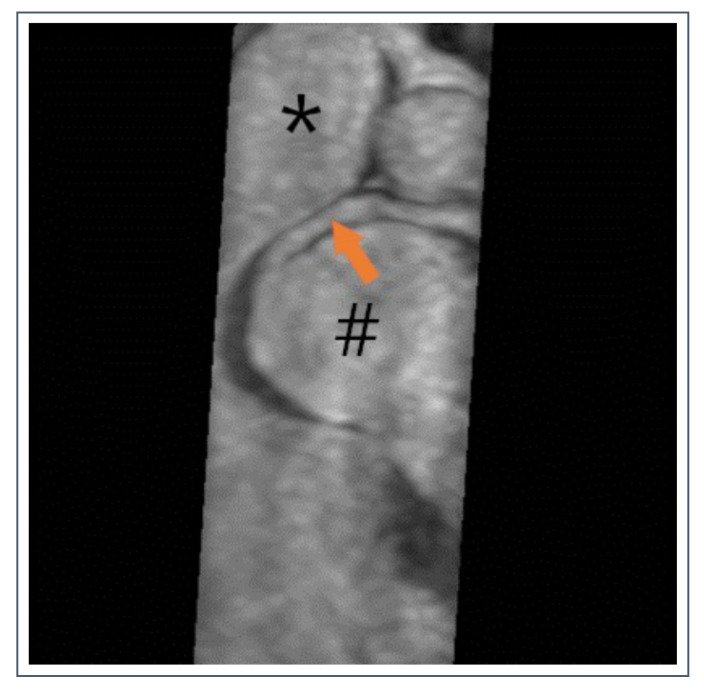
Figure 11.
36-year-old pentathlete with anomalous right coronary artery (RCA). Dominant RCA (orange arrow) originates from the left sinus Valsalva and runs between the aorta (#) and the truncus pulmonalis (*).
The use of the novel CMR conditional ergometer allows an even more physiological approach to detect hemodynamic relevance of anomalous coronary arteries by analyzing regional wall motion during physical exercise. This approach is promising and could be superior to stress echocardiography (especially in patients with poor acoustic windows), however its routine clinical use is not yet fully established. Cardiac motion artefacts at high heart rate as well as breathing artefacts and chest motion artefacts limits image quality, but the improvement of image acquisition techniques (e.g., compressed sensing) will likely extend the possibilities in this field (Figure 12) [159].
Figure 12.
Healthy volunteer is preparing for the stress CMR test.
Finally, evaluation of LGE can offer a demonstration of a previous myocardial ischemia and an aid for risk stratification.
6. CMR Technical Difficulties and Pitfalls
As demonstrated in the previous sections a wide variety of CMR sequences offer a detailed description of the heart making it an imaging modality of increasing popularity. However, CMR is also prone to certain technical difficulties and pitfalls that may entail important limitations. The detailed description of CMR artefacts is beyond the scope of this review, and here we refer to source documents addressing these issues [160]. It should be noted that most clinically used CMR sequences require breath-hold and low premature ventricular beat burden during data collection as they are prone to motion artefacts. Although, novel sequences using artificial intelligence are already employed to produce high-quality images for a wider range of patients. These new tools have the power to further improve and democratize the use of CMR.
Untested or low interobserver variability can cause issues during post-processing as well as different contouring methods. It has been long debated if we should include papillary muscles into the compact myocardium or use a simplified contouring technique excluding the papillary muscles and trabeculae [43]. Apart from significantly reducing segmentation time, emerging machine learning tools might also help standardize these contouring protocols in the future [160]. Caution should be taken during LGE characterization, too [43]. As an example, a septal LAD branch or the aortic outflow tract might be misinterpreted as pathological LGE. Ideally, during the evaluation of LGE, two separate, perpendicular views should confirm the presence of LGE.
7. Gaps in Knowledge and Future Directions
CMR plays a key role in the identification of cardiovascular disease in athletes, and its application is growing fast. In order to create equal opportunities for diagnosis among athletes, we must tackle several issues. Most evidence on athletes come from the male population. Therefore, studies are needed on female athletes’ heart, as it is known that the pattern of remodeling follows relevant sex differences. Moreover, the validation of normal ranges for physiological heart adaptation among all racial groups using CMR is key to promoting unbiased diagnosis making.
Research should focus on establishing robust normal ranges and cut-off values with regards to less well represented groups in the literature. Clear descriptions of standard operating protocols on how metrics were derived should be prioritized and well documented. To enable the clarification of pathological alteration in lower throughput centers, these normal ranges should be openly available to all.
The increasing use of CMR in the pre-participation screening of competitive athletes is partially limited by the higher cost and lower availability in comparison to echocardiography, as well as the impact of possible false-positive results such as the evidence of a focal area of LGE of uncertain meaning.
Myocardial scarring has been included in the novel European Society of Cardiology guidelines [16] as a criteria for exercise recommendation in different pathological conditions for the first time. The adverse effect of the extent of LGE is probably best established among DCM and HCM patients, although there is still a lack of information regarding athletes. LGE extent over 20% of the myocardium is defined as extensive, warranting a ban from high-intensity sports activity, however there is little data in terms of mid-to-long term outcomes of athletes with any type or amount of scarring.
Acknowledgments
We thank the contribution of Attila Toth to this work.
Author Contributions
Conceptualization and investigation, L.S., G.B., A.C., A.Z. and H.V.; writing—original draft preparation, L.S. and G.B.; writing—review and editing, A.C., V.J., K.H., F.G., D.B., Z.D. (Zsofia Drobni), Z.D. (Zsofia Dohy), A.Z. and H.V; visualization L.S., G.B., A.C. and H.V.; supervision, M.P.M., D.C., B.M., A.Z. and H.V.; funding acquisition, B.M., A.Z. and H.V. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This project was supported by a grant from the National Research, Development and Innovation Office (NKFIH) of Hungary (K135076 to B.M.) and the „Development of scientific workshops of medical, health sciences and pharmaceutical educations” project. Project identification number: EFOP-3.6.3-VEKOP-16-2017-00009. Project no. TKP2021-NKTA- 46 has been implemented with the support provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021-NKTA funding scheme.This review was supported by the Research Excellence Programme of the Ministry for Innovation and Technology in Hungary within the framework of the Bioimaging Thematic Programme of Semmelweis University and by the Ministry of Innovation LS received funding from the European Association of Cardiovascular Imaging (EACVI Research Grant App000076437). The funders provided support in the form of salaries for authors but did not have any additional role in the review’s content.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Oakley D. General cardiology: The athlete’s heart. Heart. 2001;86:722–726. doi: 10.1136/heart.86.6.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gati S., Sharma S., Pennell D. The Role of Cardiovascular Magnetic Resonance Imaging in the Assessment of Highly Trained Athletes. JACC Cardiovasc. Imaging. 2018;11:247–259. doi: 10.1016/j.jcmg.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 3.D’Silva A., Papadakis M. Sudden cardiac death in athletes. Eur. Cardiol. Rev. 2015;10:48–53. doi: 10.15420/ecr.2015.10.01.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papadakis M., Whyte G., Sharma S. Preparticipation screening for cardiovascular abnormalities in young competitive athletes. BMJ. 2008;337:806–811. doi: 10.1136/bmj.a1596. [DOI] [PubMed] [Google Scholar]
- 5.Ferrari V. The EACVI Textbook of Cardiovascular Magnetic Resonance. Oxford University Press; Oxford, UK: 2018. 35. Athlete’s Heart and Prevention of Sudden Cardiac Death in Athletes; pp. 1–20. [DOI] [Google Scholar]
- 6.Leiner T., Bogaert J., Friedrich M.G., Mohiaddin R., Muthurangu V., Myerson S., Powell A.J., Raman S.V., Pennell D.J. SCMR Position Paper (2020) on clinical indications for cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2020;22:76. doi: 10.1186/s12968-020-00682-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rost R. The athlete’s heart: Historical perspectives—Solved and unsolved problems. Cardiol. Clin. 1997;15:493–512. doi: 10.1016/S0733-8651(05)70355-6. [DOI] [PubMed] [Google Scholar]
- 8.Henschen S. Skilanglauf und Skiwettlauf. Eine medizinische Sportstudie. Mitt Med. Klin. Uppsala. 1899;2:15. [Google Scholar]
- 9.Pelliccia A., Caselli S., Sharma S., Basso C., Bax J.J., Corrado D., D’Andrea A., D’Ascenzi F., Di Paolo F.M., Edvardsen T., et al. European Association of Preventive Cardiology (EAPC) and European Association of Cardiovascular Imaging (EACVI) joint position statement: Recommendations for the indication and interpretation of cardiovascular imaging in the evaluation of the athlete’s he. Eur. Heart J. 2018;39:1949–1969. doi: 10.1093/eurheartj/ehx532. [DOI] [PubMed] [Google Scholar]
- 10.Maceira A.M., Cosin-Sales J., Prasad S.K., Pennell D.J. Characterization of left and right atrial function in healthy volunteers by cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2016;18:64. doi: 10.1186/s12968-016-0284-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petersen S.E., Sanghvi M.M., Aung N., Cooper J.A., Paiva J.M., Zemrak F., Fung K., Lukaschuk E., Lee A.M., Carapella V., et al. The impact of cardiovascular risk factors on cardiac structure and function: Insights from the UK Biobank imaging enhancement study. PLoS ONE. 2017;12:e0185114. doi: 10.1371/journal.pone.0185114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawel-Boehm N., Hetzel S.J., Ambale-Venkatesh B., Captur G., Francois C.J., Jerosch-Herold M., Salerno M., Teague S.D., Valsangiacomo-Buechel E., van der Geest R.J., et al. Reference ranges (“normal values”) for cardiovascular magnetic resonance (CMR) in adults and children: 2020 update. J. Cardiovasc. Magn. Reson. 2020;22:87. doi: 10.1186/s12968-020-00683-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Ascenzi F., Anselmi F., Piu P., Fiorentini C., Carbone S.F., Volterrani L., Focardi M., Bonifazi M., Mondillo S. Cardiac Magnetic Resonance Normal Reference Values of Biventricular Size and Function in Male Athlete’s Heart. JACC Cardiovasc. Imaging. 2019;12:1755–1765. doi: 10.1016/j.jcmg.2018.09.021. [DOI] [PubMed] [Google Scholar]
- 14.Raisi-Estabragh Z., Kenawy A.A.M., Aung N., Cooper J., Munroe P.B., Harvey N.C., E Petersen S., Khanji M.Y. Variation in left ventricular cardiac magnetic resonance normal reference ranges: Systematic review and meta-analysis. Eur. Heart J. Cardiovasc. Imaging. 2020;22:494–504. doi: 10.1093/ehjci/jeaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torlasco C., D’Silva A., Bhuva A.N., Faini A., Augusto J.B., Knott K.D., Benedetti G., Jones S., Van Zalen J., Scully P., et al. Age matters: Differences in exercise-induced cardiovascular remodelling in young and middle aged healthy sedentary individuals. Eur. J. Prev. Cardiol. 2020;28:738–746. doi: 10.1177/2047487320926305. [DOI] [PubMed] [Google Scholar]
- 16.Pelliccia A., Sharma S., Gati S., Bäck M., Börjesson M., Caselli S., Collet J.-P., Corrado D., Drezner J.A., Halle M., et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 2021;42:17–96. doi: 10.1093/eurheartj/ehaa605. [DOI] [PubMed] [Google Scholar]
- 17.Pieles G.E., Stuart A.G. The adolescent athlete’s heart; A miniature adult or grown-up child? Clin. Cardiol. 2020;43:852–862. doi: 10.1002/clc.23417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makan J., Sharma S., Firoozi S., Whyte G., Jackson P.G., McKenna W.J. Physiological upper limits of ventricular cavity size in highly trained adolescent athletes. Heart. 2005;91:495–499. doi: 10.1136/hrt.2004.035121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma S., Maron B.J., Whyte G., Firoozi S., Elliott P., McKenna W.J. Physiologic limits of left ventricular hypertrophy in elite junior athletes: Relevance to differential diagnosis of athlete’s heart and hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2002;40:1431–1436. doi: 10.1016/S0735-1097(02)02270-2. [DOI] [PubMed] [Google Scholar]
- 20.Cousergue C., Saloux E., Reboursi E. Age impacts left atrial functional remodeling in athletes. PLoS ONE. 2022;17:e0271628. doi: 10.1371/journal.pone.0271628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arbab-Zadeh A., Dijk E., Prasad A., Fu Q., Torres P., Zhang R., Thomas J.D., Palmer D., Levine B.D. Effect of aging and physical activity on left ventricular compliance. Circulation. 2004;110:1799–1805. doi: 10.1161/01.CIR.0000142863.71285.74. [DOI] [PubMed] [Google Scholar]
- 22.Gazoti Debessa C.R., Mesiano Maifrino L.B., Rodrigues de Souza R. Age related changes of the collagen network of the human heart. Mech. Ageing Dev. 2001;122:1049–1058. doi: 10.1016/S0047-6374(01)00238-X. [DOI] [PubMed] [Google Scholar]
- 23.Hill J.A., Olson E.N. Cardiac Plasticity. N. Engl. J. Med. 2008;358:1370–1380. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 24.IOC (International Olympic Commitee) Annual Report 2021. IOP; Lausanne, Switzerland: 2021. [Google Scholar]
- 25.Pelliccia A., Maron B.J., Culasso F., Spataro A., Caselli G. Athlete’s Heart in Women: Echocardiographic Characterization of Highly Trained Elite Female Athletes. JAMA. 1996;276:211–215. doi: 10.1001/jama.1996.03540030045030. [DOI] [PubMed] [Google Scholar]
- 26.D’Ascenzi F., Biella F., Lemme E., Maestrini V., di Giacinto B., Pelliccia A. Female Athlete’s Heart: Sex Effects on Electrical and Structural Remodeling. Circ. Cardiovasc. Imaging. 2020;13:E011587. doi: 10.1161/CIRCIMAGING.120.011587. [DOI] [PubMed] [Google Scholar]
- 27.Petersen S.E., Hudsmith L.E., Robson M.D., Doll H.A., Francis J.M., Wiesmann F., Jung B.A., Hennig J., Watkins H., Neubauer S., et al. Sex-specific characterics of cardiac function, geometry, and mass in young adult elite athletes. J. Magn. Reson. Imaging. 2006;24:297–303. doi: 10.1002/jmri.20633. [DOI] [PubMed] [Google Scholar]
- 28.Csecs I., Czimbalmos C., Toth A., Dohy Z., Suhai I.F., Szabo L., Kovacs A., Lakatos B., Sydo N., Kheirkhahan M., et al. The impact of sex, age and training on biventricular cardiac adaptation in healthy adult and adolescent athletes: Cardiac magnetic resonance imaging study. Eur. J. Prev. Cardiol. 2020;27:540–549. doi: 10.1177/2047487319866019. [DOI] [PubMed] [Google Scholar]
- 29.Maestrini V., Birtolo L., Filomena D., Di Giacinto B., Squeo M.R., Mango R., Di Gioia G., Lemme E., Serdoz A., Fallanca A., et al. Gender difference in extreme cardiac remodelling in endurance olympic athletes assessed by non-contrast CMR. Eur. Heart J. Cardiovasc. Imaging. 2021;22:297. doi: 10.1093/ehjci/jeaa356.257. [DOI] [Google Scholar]
- 30.Bryde R., Applewhite A.I., Abu Dabrh A.M., Taylor B.J., Heckman M.G., Filmalter S.E., Pujalte G., Rojas C., Heckman A.J., Brigham T.J., et al. Cardiac structure and function in elite female athletes: A systematic review and meta-analysis. Physiol. Rep. 2021;9:e15141. doi: 10.14814/phy2.15141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozo U., Sharma S. The Impact of Ethnicity on Cardiac Adaptation. Eur. Cardiol. Rev. 2020;15:e61. doi: 10.15420/ecr.2020.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maron B.J., Carney K.P., Lever H.M., Lewis J.F., Barac I., A Casey S., Sherrid M.V. Relationship of race to sudden cardiac death in competitive athletes with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2003;41:974–980. doi: 10.1016/S0735-1097(02)02976-5. [DOI] [PubMed] [Google Scholar]
- 33.Papadakis M., Carre F., Kervio G., Rawlins J., Panoulas V.F., Chandra N., Basavarajaiah S., Carby L., Fonseca T., Sharma S. The prevalence, distribution, and clinical outcomes of electrocardiographic repolarization patterns in male athletes of African/Afro-Caribbean origin. Eur. Heart J. 2011;32:2304–2313. doi: 10.1093/eurheartj/ehr140. [DOI] [PubMed] [Google Scholar]
- 34.Riding N.R., Sharma S., McClean G., Adamuz C., Watt V., Wilson M. Impact of geographical origin upon the electrical and structural manifestations of the black athlete’s heart. Eur. Heart J. 2019;40:50–58. doi: 10.1093/eurheartj/ehy521. [DOI] [PubMed] [Google Scholar]
- 35.Rawlins J., Carré F., Kervio G., Papadakis M., Chandra N., Edwards C., Whyte G.P., Sharma S. Ethnic differences in physiological cardiac adaptation to intense physical exercise in highly trained female athletes. Circulation. 2010;121:1078–1085. doi: 10.1161/CIRCULATIONAHA.109.917211. [DOI] [PubMed] [Google Scholar]
- 36.Malhotra A., Oxborough D., Rao P., Finocchiaro G., Dhutia H., Prasad V., Miller C., Keavney B., Papadakis M., Sharma S. Defining the Normal Spectrum of Electrocardiographic and Left Ventricular Adaptations in Mixed-Race Male Adolescent Soccer Players. Circulation. 2021;143:94–96. doi: 10.1161/CIRCULATIONAHA.120.049740. [DOI] [PubMed] [Google Scholar]
- 37.Borrell L.N., Elhawary J.R., Fuentes-Afflick E., Witonsky J., Bhakta N., Wu A.H.B., Bibbins-Domingo K., Rodríguez-Santana J.R., Lenoir M.A., Gavin J.R., et al. Race and Genetic Ancestry in Medicine—A Time for Reckoning with Racism. Obstet. Gynecol. Surv. 2021;76:395–397. doi: 10.1097/01.ogx.0000767204.20020.0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prakken N.H., Velthuis B.K., Teske A.J., Mosterd A., Cramer M.J. Cardiac MRI reference values for athletes and nonathletes corrected for body surface area, training hours/week and sex. Eur. J. Cardiovasc. Prev. Rehabil. 2010;17:198–203. doi: 10.1097/HJR.0b013e3283347fdb. [DOI] [PubMed] [Google Scholar]
- 39.Pelliccia A., Maron B.J., Spataro A., Proschan M.A., Spirito P. The upper limit of physiologic cardiac hypertrophy in highly trained elite athletes. N. Engl. J. Med. 1991;324:295–301. doi: 10.1056/NEJM199101313240504. [DOI] [PubMed] [Google Scholar]
- 40.Luijkx T., Cramer M.J., Prakken N.H.J., Buckens C.F., Mosterd A., Rienks R., Backx F.J.G., Mali W.P.T.M., Velthuis B.K. Sport category is an important determinant of cardiac adaptation: An MRI study. Br. J. Sports Med. 2012;46:1119–1124. doi: 10.1136/bjsports-2011-090520. [DOI] [PubMed] [Google Scholar]
- 41.Starekova J., Thottakara T., Lund G.K., Welsch G.H., Brunner F.J., Muellerleile K., Adam G., Regier M., Tahir E. Increased myocardial mass and attenuation of myocardial strain in professional male soccer players and competitive male triathletes. Int. J. Cardiovasc. Imaging. 2020;36:2187–2197. doi: 10.1007/s10554-020-01918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martinez V., De La Garza M.S., Grazioli G., Roca E., Brotons D., Sitges M. Cardiac adaptation to endurance exercise training: Differential impact of swimming and running. Eur. J. Sport Sci. 2021;21:844–853. doi: 10.1080/17461391.2020.1789228. [DOI] [PubMed] [Google Scholar]
- 43.Schulz-Menger J., Bluemke D.A., Bremerich J., Flamm S.D., Fogel M.A., Friedrich M.G., Kim R.J., von Knobelsdorff-Brenkenhoff F., Kramer C.M., Pennell D.J., et al. Standardized image interpretation and post-processing in cardiovascular magnetic resonance—2020 update: Society for Cardiovascular Magnetic Resonance (SCMR): Board of Trustees Task Force on Standardized Post-Processing. J. Cardiovasc. Magn. Reson. 2020;22:35. doi: 10.1186/s12968-020-00610-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maron B.J., Anan T.J., Roberts W.C. Quantitative analysis of the distribution of cardiac muscle cell disorganization in the left ventricular wall of patients with hypertonic cardiomyopathy. Circulation. 1981;63:882–894. doi: 10.1161/01.CIR.63.4.882. [DOI] [PubMed] [Google Scholar]
- 45.Becker A.E., Caruso G. Myocardial disarray. A critical review. Br. Heart J. 1982;47:527–538. doi: 10.1136/hrt.47.6.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greenbaum R.A., Ho S.Y., Gibson D.G., Becker A.E., Anderson R.H. Left ventricular fibre architecture in man. Br. Heart J. 1981;45:248–263. doi: 10.1136/hrt.45.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuribayashi T., Roberts W.C. Myocardial disarray at junction of ventricular septum and left and right ventricular free walls in hypertrophic cardiomyopathy. Am. J. Cardiol. 1992;70:1333–1340. doi: 10.1016/0002-9149(92)90771-P. [DOI] [PubMed] [Google Scholar]
- 48.Nielsen E.A., Sun M., Honjo O., Hjortdal V.E., Redington A.N., Friedberg M.K. Dual endothelin receptor blockade abrogates right ventricular remodeling and biventricular fibrosis in isolated elevated right ventricular afterload. PLoS ONE. 2016;11:e0146767. doi: 10.1371/journal.pone.0146767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gold J., Akazawa Y., Sun M., Hunter K.S., Friedberg M.K. Relation between right ventricular wall stress, fibrosis, and function in right ventricular pressure loading. Am. J. Physiol. Heart Circ. Physiol. 2020;318:H366–H377. doi: 10.1152/ajpheart.00343.2019. [DOI] [PubMed] [Google Scholar]
- 50.Choudhury L., Mahrholdt H., Wagner A., Choi K.M., Elliott M.D., Klocke F.J., Bonow R.O., Judd R.M., Kim R.J. Myocardial scarring in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2002;40:2156–2164. doi: 10.1016/S0735-1097(02)02602-5. [DOI] [PubMed] [Google Scholar]
- 51.Chan R.H., Maron B.J., Olivotto I., Assenza G.E., Haas T.S., Lesser J.R., Gruner C., Crean A.M., Rakowski H., Rowin E., et al. Significance of Late Gadolinium Enhancement at Right Ventricular Attachment to Ventricular Septum in Patients with Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2015;116:436–441. doi: 10.1016/j.amjcard.2015.04.060. [DOI] [PubMed] [Google Scholar]
- 52.Shirani J., Pick R., Roberts W.C., Maron B.J. Morphology and significance of the left ventricular collagen network in young patients with hypertrophic cardiomyopathy and sudden cardiac death. J. Am. Coll. Cardiol. 2000;35:36–44. doi: 10.1016/S0735-1097(99)00492-1. [DOI] [PubMed] [Google Scholar]
- 53.Ding W.Y., Cooper R.M., Hasleton J., McKay V., Modi S. Ventricular Hinge Point Fibrosis as a Pathological Marker of Hypertrophic Cardiomyopathy in the Absence of Significant Left Ventricular Hypertrophy? Can. J. Cardiol. 2016;32:1577.e13–1577.e14. doi: 10.1016/j.cjca.2016.02.048. [DOI] [PubMed] [Google Scholar]
- 54.De Lazzari M., Cipriani A., Rizzo S., Famoso G., Giorgi B., Tarantini G., Thiene G., Tona F., Iliceto S., Basso C., et al. Right ventricular junctional late gadolinium enhancement correlates with outcomes in pulmonary hypertension. JACC Cardiovasc. Imaging. 2019;12:936–938. doi: 10.1016/j.jcmg.2018.11.019. [DOI] [PubMed] [Google Scholar]
- 55.Kammerlander A.A., Marzluf B.A., Zotter-Tufaro C., Aschauer S., Duca F., Bachmann A., Knechtelsdorfer K., Wiesinger M., Pfaffenberger S., Greiser A., et al. T1 mapping by CMR imaging: From histological validation to clinical implication. JACC Cardiovasc. Imaging. 2016;9:14–23. doi: 10.1016/j.jcmg.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 56.Moon J.C., Messroghli D.R., Kellman P., Piechnik S.K., Robson M.D., Ugander M., Gatehouse P.D., Arai A., Friedrich M.G., Neubauer S., et al. Myocardial T1 mapping and extracellular volume quantification: A Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J. Cardiovasc. Magn. Reson. 2013;15:92. doi: 10.1186/1532-429X-15-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grigoratos C., Pantano A., Meschisi M., Gaeta R., Ait-Ali L., Barison A., Todiere G., Festa P., Sinagra G., Aquaro G.D. Clinical importance of late gadolinium enhancement at right ventricular insertion points in otherwise normal hearts. Int. J. Cardiovasc. Imaging. 2020;36:913–920. doi: 10.1007/s10554-020-01783-y. [DOI] [PubMed] [Google Scholar]
- 58.North B.J., Sinclair D.A. The intersection between aging and cardiovascular disease. Circ. Res. 2012;110:1097–1108. doi: 10.1161/CIRCRESAHA.111.246876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Domenech-Ximenos B., La Garza M.S.-D., Prat-González S., Sepúlveda-Martínez A., Crispi F., Duran-Fernandez K., Perea R.J., Bijnens B., Sitges M. Prevalence and pattern of cardiovascular magnetic resonance late gadolinium enhancement in highly trained endurance athletes. J. Cardiovasc. Magn. Reson. 2020;22:62. doi: 10.1186/s12968-020-00660-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Małek Ł.A., Bucciarelli-Ducci C. Myocardial fibrosis in athletes—Current perspective. Clin. Cardiol. 2020;43:882–888. doi: 10.1002/clc.23360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Małek Ł.A., Barczuk-Falęcka M., Werys K., Czajkowska A., Mróz A., Witek K., Burrage M.K., Bakalarski W., Nowicki D., Roik D., et al. Cardiovascular magnetic resonance with parametric mapping in long-term ultra-marathon runners. Eur. J. Radiol. 2019;117:89–94. doi: 10.1016/j.ejrad.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 62.Messroghli D.R., Moon J.C., Ferreira V.M., Grosse-Wortmann L., He T., Kellman P., Mascherbauer J.A., Nezafat R., Salerno M., Schelbert E.B., et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2 and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imagin. J. Cardiovasc. Magn. Reson. 2017;19:75. doi: 10.1186/s12968-017-0389-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aherne E., Chow K., Carr J. Cardiac T1 mapping: Techniques and applications. J. Magn. Reson. Imaging. 2020;51:1336–1356. doi: 10.1002/jmri.26866. [DOI] [PubMed] [Google Scholar]
- 64.Lavall D., Vosshage N.H., Geßner R., Stöbe S., Ebel S., Denecke T., Hagendorff A., Laufs U. Native T1 mapping for the diagnosis of cardiac amyloidosis in patients with left ventricular hypertrophy. Clin. Res. Cardiol. 2022:1–9. doi: 10.1007/s00392-022-02005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ponsiglione A., Gambardella M., Green R., Cantoni V., Nappi C., Ascione R., De Giorgi M., Cuocolo R., Pisani A., Petretta M., et al. Cardiovascular magnetic resonance native T1 mapping in Anderson-Fabry disease: A systematic review and meta-analysis. J. Cardiovasc. Magn. Reson. Off. J. Soc. Cardiovasc. Magn. Reson. 2022;24:31. doi: 10.1186/s12968-022-00859-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O’Brien A.T., Gil K.E., Varghese J., Simonetti O.P., Zareba K.M. T2 mapping in myocardial disease: A comprehensive review. J. Cardiovasc. Magn. Reson. Off. J. Soc. Cardiovasc. Magn. Reson. 2022;24:33. doi: 10.1186/s12968-022-00866-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tahir E., Starekova J., Muellerleile K., von Stritzky A., Münch J., Avanesov M., Weinrich J.M., Stehning C., Bohnen S., Radunski A.K., et al. Myocardial Fibrosis in Competitive Triathletes Detected by Contrast-Enhanced CMR Correlates with Exercise-Induced Hypertension and Competition History. JACC Cardiovasc. Imaging. 2018;11:1260–1270. doi: 10.1016/j.jcmg.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 68.McDiarmid A.K., Swoboda P.P., Erhayiem B., Lancaster R.E., Lyall G.K., Broadbent D.A., Dobson L.E., Musa T.A., Ripley D.P., Garg P., et al. Athletic Cardiac Adaptation in Males Is a Consequence of Elevated Myocyte Mass. Circ. Cardiovasc. Imaging. 2016;9:e003579. doi: 10.1161/CIRCIMAGING.115.003579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pujadas S., Doñate M., Li C.H., Merchan S., Cabanillas A., AlOmar X., Pons-Llado G., Serra-Grima R., Carreras F. Myocardial remodelling and tissue characterisation by cardiovascular magnetic resonance (CMR) in endurance athletes. BMJ Open Sport Exerc. Med. 2018;4:10–15. doi: 10.1136/bmjsem-2018-000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Szabo L., Dohy Z., Juhasz V., Balla D., Kiss A.R., Gregor Z., Szucs A., Babity M., Kiss O., Csulak E., et al. How native T1 and T2 mapping is influenced by sex and training load? Cardiac magnetic resonance imaging in young elite athletes and less active individuals. Eur. J. Prev. Cardiol. 2022;29:380–381. doi: 10.1093/eurjpc/zwac056.266. [DOI] [Google Scholar]
- 71.Swoboda P.P., McDiarmid A.K., Erhayiem B., Broadbent D.A., Dobson L.E., Garg P., Ferguson C., Page S.P., Greenwood J.P., Plein S. Assessing Myocardial Extracellular Volume by T1 Mapping to Distinguish Hypertrophic Cardiomyopathy from Athlete’s Heart. J. Am. Coll. Cardiol. 2016;67:2189–2190. doi: 10.1016/j.jacc.2016.02.054. [DOI] [PubMed] [Google Scholar]
- 72.Mordi I., Carrick D., Bezerra H., Tzemos N. T 1 and T 2 mapping for early diagnosis of dilated non-ischaemic cardiomyopathy in middle-aged patients and differentiation from normal physiological adaptation. Eur. Heart J. Cardiovasc. Imaging. 2016;17:797–803. doi: 10.1093/ehjci/jev216. [DOI] [PubMed] [Google Scholar]
- 73.Małek Ł.A., Mazurkiewicz Ł., Marszałek M., Barczuk-Falęcka M., Simon J., Grzybowski J., Miłosz-Wieczorek B., Postuła M., Marczak M. Deformation Parameters of the Heart in Endurance Athletes and in Patients with Dilated Cardiomyopathy—A Cardiac Magnetic Resonance Study. Diagnostics. 2021;11:374. doi: 10.3390/diagnostics11020374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gastl M., Lachmann V., Christidi A., Janzarik N., Veulemans V., Haberkorn S., Holzbach L., Jacoby C., Schnackenburg B., Berrisch-Rahmel S., et al. Cardiac magnetic resonance T2 mapping and feature tracking in athlete’s heart and HCM. Eur. Radiol. 2021;31:2768–2777. doi: 10.1007/s00330-020-07289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rajpal S., Tong M.S., Borchers J., Zareba K.M., Obarski T.P., Simonetti O.P., Daniels C.J. Cardiovascular Magnetic Resonance Findings in Competitive Athletes Recovering from COVID-19 Infection. JAMA Cardiol. 2020;6:116–118. doi: 10.1001/jamacardio.2020.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Starekova J., Bluemke D.A., Bradham W.S., Eckhardt L.L., Grist T.M., Kusmirek J.E., Purtell C.S., Schiebler M.L., Reeder S.B. Evaluation for Myocarditis in Competitive Student Athletes Recovering from Coronavirus Disease 2019 With Cardiac Magnetic Resonance Imaging. JAMA Cardiol. 2021;6:945–950. doi: 10.1001/jamacardio.2020.7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Małek Ł.A., Marczak M., Miłosz-Wieczorek B., Konopka M., Braksator W., Drygas W., Krzywański J. Cardiac involvement in consecutive elite athletes recovered from Covid-19: A magnetic resonance study. J. Magn. Reson. Imaging. 2021;53:1723–1729. doi: 10.1002/jmri.27513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Szabó L., Juhász V., Dohy Z., Fogarasi C., Kovács A., Lakatos B.K., Kiss O., Sydó N., Csulak E., Suhai F.I., et al. Is cardiac involvement prevalent in highly trained athletes after SARS-CoV-2 infection? A cardiac magnetic resonance study using sex-matched and age-matched controls. Br. J. Sports Med. 2021;56:553–560. doi: 10.1136/bjsports-2021-104576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Clark D.E., Parikh A., Dendy J.M., Diamond A.B., George-Durrett K., Fish F.A., Slaughter J.C., Fitch W., Hughes S.G., Soslow J.H. COVID-19 Myocardial Pathology Evaluation in Athletes with Cardiac Magnetic Resonance (COMPETE CMR) Circulation. 2021;143:609–612. doi: 10.1161/CIRCULATIONAHA.120.052573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Petersen S.E., Friedrich M.G., Leiner T., Elias M.D., Ferreira V.M., Fenski M., Flamm S.D., Fogel M., Garg R., Halushka M.K., et al. Cardiovascular Magnetic Resonance for Patients With COVID-19. JACC Cardiovasc. Imaging. 2021;15:685–699. doi: 10.1016/j.jcmg.2021.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Friedrich M.G., Cooper L.T. What we (don’t) know about myocardial injury after COVID-19. Eur. Heart J. 2021;42:1879–1882. doi: 10.1093/eurheartj/ehab145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zorzi A., Cipriani A., Corrado D. COVID-19 viral infection and myocarditis in athletes: The need for caution in interpreting cardiac magnetic resonance findings. Br. J. Sports Med. 2022;56:999–1000. doi: 10.1136/bjsports-2022-105470. [DOI] [PubMed] [Google Scholar]
- 83.Corrado D., Migliore F., Basso C., Thiene G. Exercise and the risk of sudden cardiac death. Herz Kardiovaskuläre Erkrank. 2006;31:553–558. doi: 10.1007/s00059-006-2885-8. [DOI] [PubMed] [Google Scholar]
- 84.Galderisi M., Cardim N., D’Andrea A., Bruder O., Cosyns B., Davin L., Donal E., Edvardsen T., Freitas A., Habib G., et al. The multi-modality cardiac imaging approach to the Athlete’s heart: An expert consensus of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging. 2015;16:353. doi: 10.1093/ehjci/jeu323. [DOI] [PubMed] [Google Scholar]
- 85.Baggish A.L., Battle R.W., Beaver T.A., Border W.L., Douglas P.S., Kramer C.M., Martinez M.W., Mercandetti J.H., Phelan D., Singh T.K., et al. Recommendations on the use of multimodality cardiovascular imaging in young adult competitive athletes: A report from the American Society of Echocardiography in Collaboration with the Society of Cardiovascular Computed Tomography and the Society for Card. J. Am. Soc. Echocardiogr. 2020;33:523–549. doi: 10.1016/j.echo.2020.02.009. [DOI] [PubMed] [Google Scholar]
- 86.Maron B.J., Haas T.S., Doerer J.J., Thompson P.D., Hodges J.S. Comparison of US and Italian experiences with sudden cardiac deaths in young competitive athletes and implications for preparticipation screening strategies. Am. J. Cardiol. 2009;104:276–280. doi: 10.1016/j.amjcard.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 87.Caselli S., Maron M.S., Urbano-Moral J.A., Pandian N.G., Maron B.J., Pelliccia A. Differentiating left ventricular hypertrophy in athletes from that in patients with hypertrophic cardiomyopathy. Am. J. Cardiol. 2014;114:1383–1389. doi: 10.1016/j.amjcard.2014.07.070. [DOI] [PubMed] [Google Scholar]
- 88.Maron M.S., Lesser J.R., Maron B.J. Management implications of massive left ventricular hypertrophy in hypertrophic cardiomyopathy significantly underestimated by echocardiography but identified by cardiovascular magnetic resonance. Am. J. Cardiol. 2010;105:1842–1843. doi: 10.1016/j.amjcard.2010.01.367. [DOI] [PubMed] [Google Scholar]
- 89.Moon J.C.C., Fisher N.G., McKenna W.J., Pennell D.J. Detection of apical hypertrophic cardiomyopathy by cardiovascular magnetic resonance in patients with non-diagnostic echocardiography. Heart. 2004;90:645–649. doi: 10.1136/hrt.2003.014969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rickers C., Wilke N.M., Jerosch-Herold M., Casey S.A., Panse P., Panse N., Weil J., Zenovich A.G., Maron B.J. Utility of cardiac magnetic resonance imaging in the diagnosis of hypertrophic cardiomyopathy. Circulation. 2005;112:855–861. doi: 10.1161/CIRCULATIONAHA.104.507723. [DOI] [PubMed] [Google Scholar]
- 91.Petersen S., Selvanayagam J., Francis J., Myerson S., Wiesmann F., Robson M., Östman-Smith I., Casadei B., Watkins H., Neubauer S. Differentiation of athlete’s heart from pathological forms of cardiac hypertrophy by means of geometric indices derived from cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2005;7:551–558. doi: 10.1081/JCMR-200060631. [DOI] [PubMed] [Google Scholar]
- 92.Czimbalmos C., Csecs I., Toth A., Kiss O., Suhai F.I., Sydo N., Dohy Z., Apor A., Merkely B., Vago H. The demanding grey zone: Sport indices by cardiac magnetic resonance imaging differentiate hypertrophic cardiomyopathy from athlete’s heart. PLoS ONE. 2019;14:e0211624. doi: 10.1371/journal.pone.0211624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Haaf P., Garg P., Messroghli D.R., Broadbent D.A., Greenwood J.P., Plein S. Cardiac T1 mapping and extracellular volume (ECV) in clinical practice: A comprehensive review. J. Cardiovasc. Magn. Reson. 2017;18:89. doi: 10.1186/s12968-016-0308-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bruder O., Wagner A., Jensen C.J., Schneider S., Ong P., Kispert E.-M., Nassenstein K., Schlosser T., Sabin G.V., Sechtem U., et al. Myocardial scar visualized by cardiovascular magnetic resonance imaging predicts major adverse events in patients with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2010;56:875–887. doi: 10.1016/j.jacc.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 95.Mentias A., Raeisi-Giglou P., Smedira N.G., Feng K., Sato K., Wazni O., Kanj M., Flamm S.D., Thamilarasan M., Popovic Z.B., et al. Late gadolinium enhancement in patients with hypertrophic cardiomyopathy and preserved systolic function. J. Am. Coll. Cardiol. 2018;72:857–870. doi: 10.1016/j.jacc.2018.05.060. [DOI] [PubMed] [Google Scholar]
- 96.Maron M.S., Maron B.J. Clinical impact of contemporary cardiovascular magnetic resonance imaging in hypertrophic cardiomyopathy. Circulation. 2015;132:292–298. doi: 10.1161/CIRCULATIONAHA.114.014283. [DOI] [PubMed] [Google Scholar]
- 97.Klopotowski M., Kukula K., Małek Ł.A., Śpiewak M., Polanska-Skrzypczyk M., Jamiołkowski J., Dabrowski M., Baranowski R., Klisiewicz A., Kusmierczyk M., et al. The value of cardiac magnetic resonance and distribution of late gadolinium enhancement for risk stratification of sudden cardiac death in patients with hypertrophic cardiomyopathy. J. Cardiol. 2016;68:49–56. doi: 10.1016/j.jjcc.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 98.Weng Z., Yao J., Chan R.H., He J., Yang X., Zhou Y., He Y. Prognostic value of LGE-CMR in HCM: A meta-analysis. JACC Cardiovasc. Imaging. 2016;9:1392–1402. doi: 10.1016/j.jcmg.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 99.Chan R.H., Maron B.J., Olivotto I., Pencina M.J., Assenza G.E., Haas T., Lesser J.R., Gruner C., Crean A.M., Rakowski H., et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation. 2014;130:484–495. doi: 10.1161/CIRCULATIONAHA.113.007094. [DOI] [PubMed] [Google Scholar]
- 100.Ismail T.F., Jabbour A., Gulati A., Mallorie A., Raza S., Cowling T.E., Das B., Khwaja J., Alpendurada F.D., Wage R., et al. Role of late gadolinium enhancement cardiovascular magnetic resonance in the risk stratification of hypertrophic cardiomyopathy. Heart. 2014;100:1851–1858. doi: 10.1136/heartjnl-2013-305471. [DOI] [PubMed] [Google Scholar]
- 101.Maron B.J. Distinguishing hypertrophic cardiomyopathy from athlete’s heart physiological remodelling: Clinical significance, diagnostic strategies and implications for preparticipation screening. Br. J. Sports Med. 2009;43:649–656. doi: 10.1136/bjsm.2008.054726. [DOI] [PubMed] [Google Scholar]
- 102.Pelliccia A., Culasso F., Di Paolo F.M., Maron B.J. Physiologic left ventricular cavity dilatation in elite athletes. Ann. Intern. Med. 1999;130:23–31. doi: 10.7326/0003-4819-130-1-199901050-00005. [DOI] [PubMed] [Google Scholar]
- 103.Abergel E., Chatellier G., Hagege A., Oblak A., Linhart A., Ducardonnet A., Menard J. Serial left ventricular adaptations in world-class professional cyclists: Implications for disease screening and follow-up. J. Am. Coll. Cardiol. 2004;44:144–149. doi: 10.1016/j.jacc.2004.02.057. [DOI] [PubMed] [Google Scholar]
- 104.Harmon K.G., Drezner J.A., Maleszewski J.J., Lopez-Anderson M., Owens D., Prutkin J.M., Asif I.M., Klossner D., Ackerman M.J. Pathogeneses of sudden cardiac death in national collegiate athletic association athletes. Circ. Arrhythmia Electrophysiol. 2014;7:198–204. doi: 10.1161/CIRCEP.113.001376. [DOI] [PubMed] [Google Scholar]
- 105.Finocchiaro G., Papadakis M., Robertus J.-L., Dhutia H., Steriotis A.K., Tome M., Mellor G., Merghani A., Malhotr A., Behr E., et al. Etiology of sudden death in sports: Insights from a United Kingdom regional registry. J. Am. Coll. Cardiol. 2016;67:2108–2115. doi: 10.1016/j.jacc.2016.02.062. [DOI] [PubMed] [Google Scholar]
- 106.Millar L.M., Fanton Z., Finocchiaro G., Sanchez-Fernandez G., Dhutia H., Malhotra A., Merghani A., Papadakis M., Behr E.R., Bunce N., et al. Differentiation between athlete’s heart and dilated cardiomyopathy in athletic individuals. Heart. 2020;106:1059–1065. doi: 10.1136/heartjnl-2019-316147. [DOI] [PubMed] [Google Scholar]
- 107.Di Marco A., Anguera I., Schmitt M., Klem I., Neilan T.G., White J.A., Sramko M., Masci P.G., Barison A., Mckenna P., et al. Late Gadolinium Enhancement and the Risk for Ventricular Arrhythmias or Sudden Death in Dilated Cardiomyopathy: Systematic Review and Meta-Analysis. JACC Heart Fail. 2017;5:28–38. doi: 10.1016/j.jchf.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 108.Barison A., Aimo A., Mirizzi G., Castiglione V., Ripoli A., Panchetti L., Rossi A., Giannoni A., Startari U., Aquaro G.D., et al. The extent and location of late gadolinium enhancement predict defibrillator shock and cardiac mortality in patients with non-ischaemic dilated cardiomyopathy. Int. J. Cardiol. 2020;307:180–186. doi: 10.1016/j.ijcard.2020.02.028. [DOI] [PubMed] [Google Scholar]
- 109.Corrado D., Basso C., Rizzoli G., Schiavon M., Thiene G. Does Sports Activity Enhance the Risk of Sudden Death in Adolescents and Young Adults? J. Am. Coll. Cardiol. 2003;42:1959–1963. doi: 10.1016/j.jacc.2003.03.002. [DOI] [PubMed] [Google Scholar]
- 110.Corrado D., Perazzolo Marra M., Zorzi A., Beffagna G., Cipriani A., Lazzari M., Migliore F., Pilichou K., Rampazzo A., Rigato I., et al. Diagnosis of arrhythmogenic cardiomyopathy: The Padua criteria. Int. J. Cardiol. 2020;319:106–114. doi: 10.1016/j.ijcard.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 111.Pelliccia A., Di Paolo F.M., Quattrini F.M., Basso C., Culasso F., Popoli G., De Luca R., Spataro A., Biffi A., Thiene G., et al. Outcomes in athletes with marked ECG repolarization abnormalities. N. Engl. J. Med. 2008;358:152–161. doi: 10.1056/NEJMoa060781. [DOI] [PubMed] [Google Scholar]
- 112.Marcus F.I., McKenna W.J., Sherrill D., Basso C., Bauce B., Bluemke D.A., Calkins H., Corrado D., Cox M.G., Daubert J.P., et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/Dysplasia: Proposed modification of the task force criteria. Circulation. 2010;121:1533–1541. doi: 10.1161/CIRCULATIONAHA.108.840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.D’Ascenzi F., Biella F., Lemme E., Maestrini V., Di Giacinto B., Pelliccia A. RV remodeling in Olympic athletes. JACC Cardiovasc. Imaging. 2017;10:385–393. doi: 10.1016/j.jcmg.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 114.Bauce B., Frigo G., Benini G., Michieli P., Basso C., Folino A.F., Rigato I., Mazzotti E., Daliento L., Thiene G., et al. Differences and similarities between arrhythmogenic right ventricular cardiomyopathy and athlete’s heart adaptations. Br. J. Sports Med. 2010;44:148–154. doi: 10.1136/bjsm.2007.042853. [DOI] [PubMed] [Google Scholar]
- 115.Bohm P., Kindermann W., Meyer T., Scharhag J. Right and left ventricular function and mass in male elite master athletes: A controlled contrast-enhanced cardiovascular magnetic resonance study. Circulation. 2016;133:1927–1935. doi: 10.1161/CIRCULATIONAHA.115.020975. [DOI] [PubMed] [Google Scholar]
- 116.Cipriani A., Bauce B., De Lazzari M., Rigato I., Bariani R., Meneghin S., Pilichou K., Motta R., Aliberti C., Thiene G., et al. Arrhythmogenic right ventricular cardiomyopathy: Characterization of left ventricular phenotype and differential diagnosis with dilated cardiomyopathy. J. Am. Heart Assoc. 2020;9:e014628. doi: 10.1161/JAHA.119.014628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cipriani A., Mattesi G., Bariani R., Cecere A., Martini N., De Michieli L., Da Pozzo S., Corradin S., De Conti G., Zorzi A., et al. Cardiac magnetic resonance imaging of arrhythmogenic cardiomyopathy: Evolving diagnostic perspectives. Eur. Radiol. 2022;32:1–13. doi: 10.1007/s00330-022-08958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Aquaro G.D., Barison A., Todiere G., Grigoratos C., Ali L.A., Di Bella G., Emdin M., Festa P. Usefulness of combined functional assessment by cardiac magnetic resonance and tissue characterization versus task force criteria for diagnosis of arrhythmogenic right ventricular cardiomyopathy. Am. J. Cardiol. 2016;118:1730–1736. doi: 10.1016/j.amjcard.2016.08.056. [DOI] [PubMed] [Google Scholar]
- 119.Czimbalmos C., Csecs I., Dohy Z., Toth A., Suhai F.I., Müssigbrodt A., Kiss O., Geller L., Merkely B., Vago H. Cardiac magnetic resonance based deformation imaging: Role of feature tracking in athletes with suspected arrhythmogenic right ventricular cardiomyopathy. Int. J. Cardiovasc. Imaging. 2019;35:529–538. doi: 10.1007/s10554-018-1478-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pilichou K., Mancini M., Rigato I., Lazzarini E., Giorgi B., Carturan E., Bauce B., d’Amati G., Marra M.P., Basso C. Nonischemic left ventricular scar: Sporadic or familial? Screen the genes, scan the mutation carriers. Circulation. 2014;130:e180–e182. doi: 10.1161/CIRCULATIONAHA.114.012515. [DOI] [PubMed] [Google Scholar]
- 121.Whyte G., Sheppard M., George K., Shave R., Wilson M., Prasad S., O’Hanlon R., Sharma S. Post-mortem evidence of idiopathic left ventricular hypertrophy and idiopathic interstitial myocardial fibrosis: Is exercise the cause? Br. J. Sports Med. 2008;42:304–305. doi: 10.1136/bjsm.2007.038158. [DOI] [PubMed] [Google Scholar]
- 122.Di Gioia C.R.T., Giordano C., Cerbelli B., Pisano A., Perli E., de Dominicis E., Poscolieri B., Palmieri V., Ciallella C., Zeppilli P., et al. Nonischemic left ventricular scar and cardiac sudden death in the young. Hum. Pathol. 2016;58:78–89. doi: 10.1016/j.humpath.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 123.De Lazzari M., Zorzi A., Cipriani A., Susana A., Mastella G., Rizzo A., Rigato I., Bauce B., Giorgi B., Lacognata C., et al. Relationship between electrocardiographic findings and cardiac magnetic resonance phenotypes in arrhythmogenic cardiomyopathy. J. Am. Heart Assoc. 2018;7:e009855. doi: 10.1161/JAHA.118.009855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Caforio A.L.P., Pankuweit S., Arbustini E., Basso C., Gimeno-Blanes J., Felix S.B., Fu M., Heliö T., Heymans S., Jahns R., et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013;34:2636–2648. doi: 10.1093/eurheartj/eht210. [DOI] [PubMed] [Google Scholar]
- 125.Ferreira V.M., Schulz-Menger J., Holmvang G., Kramer C.M., Carbone I., Sechtem U., Kindermann I., Gutberlet M., Cooper L.T., Liu P., et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: Expert recommendations. J. Am. Coll. Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 126.Muser D., Nucifora G., Muser D., Nucifora G., Pieroni M., Castro S.A., Arroyo R.C., Maeda S., Benhayon D.A., Liuba I., et al. Prognostic value of nonischemic ringlike left ventricular scar in patients with apparently idiopathic nonsustained ventricular arrhythmias. Circulation. 2021;143:1359–1373. doi: 10.1161/CIRCULATIONAHA.120.047640. [DOI] [PubMed] [Google Scholar]
- 127.Di Bella G., Gentile G., Irsuti F., Giuseppe R., Clemenza F., Mamone G., Donato R., De Luca A., Bogaert J., Aquaro G.D. Prognostic Role of Left Ventricular Intramyocardial Fatty Metaplasia in Patients With Previous Myocarditis (MYOFAT Study) Am. J. Cardiol. 2021;143:135–144. doi: 10.1016/j.amjcard.2020.12.029. [DOI] [PubMed] [Google Scholar]
- 128.Pelliccia A., Solberg E.E., Papadakis M., Adami P.E., Biffi A., Caselli S., La Gerche A., Niebauer J., Pressler A., Schmied C.M., et al. Recommendations for participation in competitive and leisure time sport in athletes with cardiomyopathies, myocarditis, and pericarditis: Position statement of the Sport Cardiology Section of the European Association of Preventive Cardiology (EAPC) Eur. Heart J. 2019;40:19–33. doi: 10.1093/eurheartj/ehy730. [DOI] [PubMed] [Google Scholar]
- 129.Maron B.J., Zipes D.P., Kovacs R.J. Eligibility and Disqualification Recommendations for Competitive Athletes with Cardiovascular Abnormalities: Preamble, Principles, and General Considerations: A Scientific Statement from the American Heart Association and American College of Cardiology. Circulation. 2015;132:e256–e261. doi: 10.1161/CIR.0000000000000236. [DOI] [PubMed] [Google Scholar]
- 130.Jenni R., Oechslin E., Schneider J., Jost C.A., Kaufmann P.A. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: A step towards classification as a distinct cardiomyopathy. Heart. 2001;86:666–671. doi: 10.1136/heart.86.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chin T.K., Perloff J.K., Williams R.G., Jue K., Mohrmann R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation. 1990;82:507–513. doi: 10.1161/01.CIR.82.2.507. [DOI] [PubMed] [Google Scholar]
- 132.Petersen S.E., Selvanayagam J.B., Wiesmann F., Robson M.D., Francis J.M., Anderson R.H., Watkins H., Neubauer S. Left ventricular non-compaction: Insights from cardiovascular magnetic resonance imaging. J. Am. Coll. Cardiol. 2005;46:101–105. doi: 10.1016/j.jacc.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 133.Jacquier A., Thuny F., Jop B., Giorgi R., Cohen F., Gaubert J.-Y., Vidal V., Bartoli J.M., Habib G., Moulin G. Measurement of trabeculated left ventricular mass using cardiac magnetic resonance imaging in the diagnosis of left ventricular non-compaction. Eur. Heart J. 2010;31:1098–1104. doi: 10.1093/eurheartj/ehp595. [DOI] [PubMed] [Google Scholar]
- 134.Ivanov A., Dabiesingh D.S., Bhumireddy G.P., Mohamed A., Asfour A., Briggs W.M., Ho J., Khan S.A., Grossman A., Klem I., et al. Prevalence and prognostic significance of left ventricular noncompaction in patients referred for cardiac magnetic resonance imaging. Circ. Cardiovasc. Imaging. 2017;10:e006174. doi: 10.1161/CIRCIMAGING.117.006174. [DOI] [PubMed] [Google Scholar]
- 135.Gati S., Chandra N., Bennett R.L.E., Reed M., Kervio G., Panoulas V.F., Ghani S., Sheikh N., Zaidi A., Wilson M., et al. Increased left ventricular trabeculation in highly trained athletes: Do we need more stringent criteria for the diagnosis of left ventricular non-compaction in athletes? Heart. 2013;99:401–408. doi: 10.1136/heartjnl-2012-303418. [DOI] [PubMed] [Google Scholar]
- 136.Luijkx T., Cramer M.J., Zaidi A., Rienks R., Senden P.J., Sharma S., van Hellemondt F.J., Buckens C.F., Mali W.P., Velthuis B.K. Ethnic differences in ventricular hypertrabeculation on cardiac MRI in elite football players. Neth. Heart J. 2012;20:389–395. doi: 10.1007/s12471-012-0305-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Caselli S., Ferreira D., Kanawati E., Di Paolo F., Pisicchio C., Jost C.A., Spataro A., Jenni R., Pelliccia A. Prominent left ventricular trabeculations in competitive athletes: A proposal for risk stratification and management. Int. J. Cardiol. 2016;223:590–595. doi: 10.1016/j.ijcard.2016.08.272. [DOI] [PubMed] [Google Scholar]
- 138.Gati S., Papadakis M., van Niekerk N., Reed M., Yeghen T., Sharma S. Increased left ventricular trabeculation in individuals with sickle cell anaemia: Physiology or pathology? Int. J. Cardiol. 2013;168:1658–1660. doi: 10.1016/j.ijcard.2013.03.039. [DOI] [PubMed] [Google Scholar]
- 139.Nucifora G., Aquaro G.D., Pingitore A., Masci P.G., Lombardi M. Myocardial fibrosis in isolated left ventricular non-compaction and its relation to disease severity. Eur. J. Heart Fail. 2011;13:170–176. doi: 10.1093/eurjhf/hfq222. [DOI] [PubMed] [Google Scholar]
- 140.Vaidya V.R., Lyle M., Miranda W.R., Farwati M., Isath A., Patlolla S.H., Hodge D.O., Asirvatham S.J., Kapa S., Deshmukh A.J., et al. Long-term survival of patients with left ventricular noncompaction. J. Am. Heart Assoc. 2021;10:e015563. doi: 10.1161/JAHA.119.015563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Casas G., Limeres J., Oristrell G., Gutierrez-Garcia L., Andreini D., Borregan M., Larrañaga-Moreira J.M., Lopez-Sainz A., Codina-Solà M., Teixido-Tura G., et al. Clinical risk prediction in patients with left ventricular myocardial noncompaction. J. Am. Coll. Cardiol. 2021;78:643–662. doi: 10.1016/j.jacc.2021.06.016. [DOI] [PubMed] [Google Scholar]
- 142.Ramchand J., Podugu P., Obuchowski N., Harb S.C., Chetrit M., Milinovich A., Griffin B., Burrell L.M., Tang W.H.W., Kwon D.H., et al. Novel Approach to Risk Stratification in Left Ventricular Non-Compaction Using A Combined Cardiac Imaging and Plasma Biomarker Approach. J. Am. Heart Assoc. 2021;10:e019209. doi: 10.1161/JAHA.120.019209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Grigoratos C., Barison A., Ivanov A., Andreini D., Amzulescu M.S., Mazurkiewicz L., De Luca A., Grzybowski J., Masci P.G., Marczak M., et al. Meta-analysis of the prognostic role of late gadolinium enhancement and global systolic impairment in left ventricular noncompaction. JACC Cardiovasc. Imaging. 2019;12:2141–2151. doi: 10.1016/j.jcmg.2018.12.029. [DOI] [PubMed] [Google Scholar]
- 144.Araujo-Filho J.A.B., Assuncao A.N., De Melo M.D.T., Bière L., Lima C.R., Dantas R.N., Nomura C.H., Salemi V.M.C., Jerosch-Herold M., Parga J. Myocardial T1 mapping and extracellular volume quantification in patients with left ventricular non-compaction cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging. 2018;19:888–895. doi: 10.1093/ehjci/jey022. [DOI] [PubMed] [Google Scholar]
- 145.Dreisbach J.G., Mathur S., Houbois C.P., Oechslin E., Ross H., Hanneman K., Wintersperger B.J. Cardiovascular magnetic resonance based diagnosis of left ventricular non-compaction cardiomyopathy: Impact of cine bSSFP strain analysis. J. Cardiovasc. Magn. Reson. 2020;22:9. doi: 10.1186/s12968-020-0599-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Szűcs A., Kiss A.R., Gregor Z., Horváth M., Tóth A., Dohy Z., Szabó L.E., Suhai F.I., Merkely B., Vágó H. Changes in strain parameters at different deterioration levels of left ventricular function: A cardiac magnetic resonance feature-tracking study of patients with left ventricular noncompaction. Int. J. Cardiol. 2021;331:124–130. doi: 10.1016/j.ijcard.2021.01.072. [DOI] [PubMed] [Google Scholar]
- 147.Angelini P. Normal and anomalous coronary arteries: Definitions and classification. Am. Heart J. 1989;117:418–434. doi: 10.1016/0002-8703(89)90789-8. [DOI] [PubMed] [Google Scholar]
- 148.Basso C., Maron B.J., Corrado D., Thiene G. Clinical profile of congenital coronary artery anomalies with origin from the wrong aortic sinus leading to sudden death in young competitive athletes. J. Am. Coll. Cardiol. 2000;35:1493–1501. doi: 10.1016/S0735-1097(00)00566-0. [DOI] [PubMed] [Google Scholar]
- 149.Ripley D.P., Saha A., Teis A., Uddin A., Bijsterveld P., Kidambi A., McDiarmid A.K., Sivananthan M., Plein S., Pennell D.J., et al. The distribution and prognosis of anomalous coronary arteries identified by cardiovascular magnetic resonance: 15 year experience from two tertiary centres. J. Cardiovasc. Magn. Reson. 2014;16:34. doi: 10.1186/1532-429X-16-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bunce N.H., Lorenz C.H., Keegan J., Lesser J., Reyes E.M., Firmin D., Pennell D.J. Coronary artery anomalies: Assessment with free-breathing three-dimensional coronary MR angiography. Radiology. 2003;227:201–208. doi: 10.1148/radiol.2271020316. [DOI] [PubMed] [Google Scholar]
- 151.Prakken N.H., Cramer M.J., Olimulder M.A., Agostoni P., Mali W.P., Velthuis B.K. Screening for proximal coronary artery anomalies with 3-dimensional MR coronary angiography. Int. J. Cardiovasc. Imaging. 2010;26:701–710. doi: 10.1007/s10554-010-9617-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Angelini P., Cheong B.Y., De Rosen V.V.L., Lopez A., Uribe C., Masso A.H., Ali S.W., Davis B.R., Muthupillai R., Willerson J.T. High-risk cardiovascular conditions in sports-related sudden death: Prevalence in 5169 school children screened via cardiac magnetic resonance. Texas Heart Inst. J. 2018;45:205–213. doi: 10.14503/THIJ-18-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Greenwood J.P., Maredia N., Younger J., Brown J.M., Nixon J., Everett C.C., Bijsterveld P., Ridgway J.P., Radjenovic A., Dickinson C.J., et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): A prospective trial. Lancet. 2012;379:453–460. doi: 10.1016/S0140-6736(11)61335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schwitter J., Wacker C.M., Wilke N., Al-Saadi N., Sauer E., Huettle K., Schönberg S.O., Luchner A., Strohm O., Ahlstrom H., et al. MR-IMPACT II: Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary artery disease Trial: Perfusion-cardiac magnetic resonance vs. single-photon emission computed tomography for the detection of coronary artery disease: A comparative. Eur. Heart J. 2013;34:775–781. doi: 10.1093/eurheartj/ehs022. [DOI] [PubMed] [Google Scholar]
- 155.Greenwood J.P., Ripley D.P., Berry C., McCann G.P., Plein S., Bucciarelli-Ducci C., Dall’Armellina E., Prasad A., Bijsterveld P., Foley J.R., et al. Effect of care guided by cardiovascular magnetic resonance, myocardial perfusion scintigraphy, or NICE guidelines on subsequent unnecessary angiography rates: The CE-MARC 2 randomized clinical trial. JAMA. 2016;316:1051–1060. doi: 10.1001/jama.2016.12680. [DOI] [PubMed] [Google Scholar]
- 156.Scannell C.M., Hasaneen H., Greil G., Hussain T., Razavi R., Lee J., Pushparajah K., Duong P., Chiribiri A. Automated quantitative stress perfusion cardiac magnetic resonance in pediatric patients. Front. Pediatr. 2021;9:699497. doi: 10.3389/fped.2021.699497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Agrawal H., Wilkinson J.C., Noel C.V., Qureshi A.M., Masand P.M., Mery C.M., Sexson-Tejtel S.K., Molossi S. Impaired myocardial perfusion on stress CMR correlates with invasive FFR in children with coronary anomalies. J. Invasive Cardiol. 2021;33:E45–E51. doi: 10.25270/jic/20.00237. [DOI] [PubMed] [Google Scholar]
- 158.Clemente A., Del Borrello M., Greco P., Mannella P., Di Gregorio F., Romano S., Morra A. Anomalous origin of the coronary arteries in children: Diagnostic role of three-dimensional coronary MR angiography. Clin. Imaging. 2010;34:337–343. doi: 10.1016/j.clinimag.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 159.Craven T.P., Tsao C.W., La Gerche A., Simonetti O.P., Greenwood J.P. Exercise cardiovascular magnetic resonance: Development, current utility and future applications. J. Cardiovasc. Magn. Reson. 2020;22:65. doi: 10.1186/s12968-020-00652-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ferreira P.F., Gatehouse P.D., Mohiaddin R.H., Firmin D.N. Cardiovascular magnetic resonance artefacts. J. Cardiovasc. Magn. Reson. 2013;15:41. doi: 10.1186/1532-429X-15-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.