Abstract
The gut microbiome is an important factor in human health and disease. While preliminary studies have found some evidence that physical activity is associated with gut microbiome richness, diversity, and composition, this relationship is not fully understood and has not been previously characterized in a large, population-based cohort. In this study, we estimated the association between several measures of physical activity and the gut microbiota in a cohort of 720 Wisconsin residents. Our sample had a mean age of 55 years (range: 18, 94), was 42% male, and 83% of participants self-identified as White. Gut microbial composition was assessed using gene sequencing of the V3-V4 region of 16S rRNA extracted from stool. We found that an increase of one standard deviation in weekly minutes spent in active transportation was associated with an increase in alpha diversity, particularly in Chao1’s richness (7.57, 95% CI: 2.55, 12.59) and Shannon’s diversity (0.04, 95% CI: 0.0008, 0.09). We identified interactions in the association between Inverse Simpson’s diversity and physical activity, wherein active transportation for individuals living in a rural environment was associated with additional increases in diversity (4.69, 95% CI: 1.64, 7.73). We also conducted several permutational ANOVAs (PERMANOVA) and negative binomial regression analyses to estimate the relationship between physical activity and microbiome composition. We found that being physically active and increased physical activity time were associated with increased abundance of bacteria in the family Erysipelotrichaceae. Active transportation was associated with increased abundance of bacteria in the genus Phascolarctobacterium, and decreased abundance of Clostridium. Minutes in active transportation was associated with a decreased abundance of the family Clostridiaceae.
Introduction
The human gut microbiome is home to more than 1,000 unique species of microbes [1] and plays an important role in host physiology, metabolism, nutrition, and immune system development and maintenance [2]. Microbiome dysbioses have been associated with irritable bowel disease [3], Crohn’s disease [4], Type 1 and Type 2 diabetes [5, 6], asthma and allergy [7, 8], and rheumatoid arthritis [9]. Moreover, the gut microbiome has been linked to obesity [10, 11], colorectal cancer [12], cardiovascular disease [13], autism [14, 15], and stress, anxiety, and depression [16]. Because of the vital contributions that gut microbes make to human health, it is important to understand how certain exposures may improve gut health.
Physical activity is a critical component of human health and may alter the health of the gut microbiome. In particular, physical activity has been associated with reduced adiposity, reduced mortality, and improved cardiometabolic health [17]. The relationship between physical activity and the microbiome has been well-established in animal models [18–22]. In humans, the relationship is less well understood. Previous studies that compared athletes with sedentary controls have found that athletes have increased alpha-diversity and enriched functional pathways associated with host health, including amino acid synthesis, carbohydrate metabolism, and short-chain fatty acid synthesis [23, 24]. Acute changes have also been observed in athletes during intense activity [25–27]. While these studies indicate a possible relationship between physical activity and the gut microbiota, they represent extreme physical activity conditions, relative to people with more common physical activity habits [28].
Several studies have been conducted in more general populations, but results have been mixed. Some have found that increased physical activity/fitness is associated with increased alpha-diversity [29], increases in butyrate-producing taxa including Clostridiales, Roseburia, Lachnospiraceae, and Erysipelotrichaceae [29–31], or shifts in the abundance of other taxa [32, 33], whereas others have not observed a relationship [34]. While these studies shed some light on the possible effect of physical activity on the composition, richness, and diversity of the gut microbiome, they are limited by low sample size, with many of them having fewer than 100 participants [30, 33–35]. Here, we address these limitations by considering a large population with 720 participants and estimate the association between both objective and subjective measures of physical activity and the richness, diversity, and composition of the gut microbiome.
Materials and methods
Study population
The data used in this analysis come from the Survey of the Health of Wisconsin (SHOW) and its ancillary microbiome study, Winning the War on Antibiotic Resistance (WARRIOR), whose methods have been described in detail [36–38]. The SHOW and WARRIOR projects were approved by the University of Wisconsin Institutional Review Board (2013–0251). Briefly, SHOW–at its initiation–was an annual cross-sectional survey of a randomly selected, representative sample of Wisconsin residents over the age of 18. Since then, SHOW has expanded to collect longitudinal samples in a subset of adults and comprehensive data on participants’ health and health history, environmental and neighborhood exposures, and several objective physical measures. During 2016 and 2017, as part of the WARRIOR ancillary project, participants also provided stool samples for microbiome analysis and responded to microbiome-specific questionnaires. This analysis uses survey data, microbiome data, and objective measures of physical activity, sedentary time, and sleep data from this study.
Participants were excluded from this analysis if they reported that they were taking antibiotics at the time of stool sample collection; however, those who used antibiotics during the past year were included. Three participants were excluded due to use of antibiotics.
SHOW sampling methods allow for multiple eligible participants per household. The 720 participants who provided high-quality stool samples included in this analysis were from 567 unique households.
DNA extraction, PCR, and sequencing
The DNA extraction methods used in this study have been described in detail previously [37, 39]. Briefly, chemical, heat, and mechanical methods were used to lyse the bacterial cells found in stool samples. The extracted DNA was purified using a phenol-chloroform-isoamyl wash, followed by NucleoSpin Gel and a PCR clean-up kit (Mcherey-Nagel, Germany). The DNA was quantified using PicoGreen in a microplate reader.
The V3-V4 region of the 16S rRNA genes in the extracted DNA was barcoded and amplified as described in Kozich et al. [40]. Following PCR, amplicons were subjected to gel electrophoresis using 1.0% low melt agarose (National Diagnostics, Atlanta, GO). Bands of the approximate amplicon length were extracted and purified using a Zymo gel DNA Recovery Kit (Zymo Research, Irvine, CA, United States). Samples were then quantified using a Qubit® Fluorometer (Invitrogen, San Diego, CA, United States) and pooled to 4nM to construct a sequencing library. Samples were then sequenced on an Illumina MiSeq sequencer (Illumina, Inc., San Diego, CA), using an Illumina MiSeq V2 (2x250bp) Reagent Kit (Illumina, Inc., San Diego, CA) per manufacturer’s instructions.
16s rRNAs sequencing and data processing
Raw sequencing data were processed using mothur (v. 1.43.0) [41] following the Standard Operating Procedure for MiSeq data [40]. Briefly, contigs (overlapping sequences) were aligned using the SILVA database (v. 132) [42], low-quality reads and chimeras detected by UCHIME [43] were removed, and sequences were assigned to operational taxonomic units (OTUs) with a threshold of 97% similarity using the GreenGenes database [44]. Rare OTUs, defined as those whose relative abundance was less than 0.001% of the overall OTU count, were removed. Alpha-diversity metrics were calculated using the phyloseq package in R [45].
Physical activity and objective sleep measures
Participants wore two accelerometers (wGT3xBT—ActiGraph Corporation, Pensacola, FL), one was worn on the hip (to measure physical activity), and the other was worn on the wrist (to measure sleep). Participants were instructed to wear the monitors for seven consecutive days, except during activities that could get the monitor wet, like swimming, showering, or bathing. The hip accelerometer was worn during waking hours only; the wrist accelerometer was worn continuously for 24 hours/day. Data were aggregated into 60 second epochs for scoring and validation.
Hourly data were collected from both the wrist and hip accelerometers and aggregated into six-hour intervals. Of the 720 participants, 625 agreed to participate and contributed at least 1 hour of accelerometry data. The remaining 95 participants did not contribute any accelerometry data. S1 Fig summarizes the intervals with missing data prior to imputation. Because all missing data were imputed, we did not exclude any participants with low wear time. Use of imputation has been shown to be less biased and more precise, compared to methods that discard observations with low wear time [46].
We labeled the period between midnight to 5:59 AM as “night”, 6 AM to 11:59 AM as “morning”, 12 PM to 5:59 PM as “afternoon”, and 6 PM to midnight as “evening”. After aggregation, intervals that did not include at least 300 minutes of wear time out of the possible 360 minutes of measurement were set to missing (and later imputed), while intervals with 300 or more minutes of wear time were scaled up to the full 360 minutes.
Wrist accelerometer measures
Sleep duration was measured via wrist accelerometer. Wrist accelerometer data were aggregated into 60-second epochs for validation, scoring, and analysis. Sleep data were scored both manually and automatically, with in-bed and out-bed times identified manually, based on activity recorded by the accelerometer in conjunction with paper logs filled out by participants. The Cole-Kripke algorithm was used to distinguish sleeping and waking periods [47].
Hip accelerometer measures
Physical activity was measured via hip accelerometer. Freedson cut points were used to distinguish between sedentary, light, moderate, and vigorous physical activity levels [48]. Specifically, Freedson cut points categorize accelerometry data, measured in counts per minute, into metabolic equivalents (METs) categories: light (≤ 2.99 METs), moderate (3.00–5.99 METs), and vigorous (≥6.00 METs). Therefore, any activity that was classified as moderate or vigorous (≥ 3.00 METs) by the Freedson cut points was labeled moderate to vigorous physical activity (MVPA). In these analyses, we used accumulated minutes of activity rather than bouted activity, adjusted to a 7-day week.
Participants were classified as physically active if they met the guideline for completing 150 minutes of moderate or vigorous intensity physical activity per week [49].
Self-reported measures
During the computer assisted personal interview portion of the study, participants were asked, “In a typical week, do you walk or use a bicycle for at least 10 minutes continuously to get to and from places?”. Those who responded yes to this question were classified as participating in active transportation and were asked additional questions to characterize the average time per week that they spent walking or bicycling on a typical day for travel.
Other information
Participants’ primary self-identified race was used to create the categories used in these analyses: Non-Hispanic White, Non-Hispanic Black, Hispanic, and other. Body mass index was calculated based on measures of weight and height that were collected during an in-home interview by trained staff. Weight was measured using a digital calibrated scale (Health-O-Meter 725 KL–Sunbeam Products, Bridgeview, IL), and height was measured in duplicate using a stadiometer (SECA 222 wall-mounted stadiometer–SECA Corp., Hanover, MD).
Participants self-reported their total household income before taxes in the previous 12 months, as well as the household members (including children) who were supported by that income. Households were classified as urban or rural using the 2010 Census urbanized areas and urban cluster classification [50]. Self-reported education was classified as some high school, high school, some college, bachelor’s, and more than bachelors. Never smoking was defined as not smoking more than 100 cigarettes during their entire lives.
Diabetes status was ascertained via a blood sample. Participants were classified as diabetic if their blood HgbA1c level at the time of participation was greater than or equal to 6.5 or if they self-reported a previous physician diagnosis of Type 1 or Type 2 diabetes. Participants were classified as having depression if they reported any depression symptoms or reported taking antidepressant medications in the past 30 days. Depression symptoms were collected during an Audio-Computer Assisted Self-Interview (ACASI) portion of the home visit, using the Patient Health Questionnaire-2 (PHQ-2) [51]. Participants also reported any medications they took in the past 30 days during the computer assisted personal interview (CAPI) portion of the home visit. This list of medications was compared with the RxNorm database to identify antidepressant medications [52]. Proton pump inhibitor and antibiotic use during the past year were gathered using a self-administered questionnaire.
All dietary intake measures (carbohydrates, fat, protein, fiber, and alcohol) were collected using the National Cancer Institute’s Diet History Questionnaire (DHQ) [53]. Participants with dietary variables above the 99th percentile were windsorized to the 99th percentile to account for the positively skewed distribution and known limitations of the dietary instrument. Finally, although not significantly associated with physical activity, we control for sample age in all analyses. Sample age was the time between when the stool sample was produced and when it was put into storage at -80°, as time spent in cold storage during shipping may impact some microbiome measures used in this analysis [54].
Data analysis
Imputation
With the mice package (version 3.14.0) in R, multiple imputation was used to estimate the probable values for all missing data [55]. Predictors were chosen using mice’s quickpred function, whose steps have been previously detailed [56].
Statistical analysis
To estimate the association of physical activity with alpha-diversity, we generated several linear mixed effects models with random intercepts to account for multiple participants from the same household, using R (version 4.1.2). We used physically active status, MVPA minutes per week, use of active transportation, and minutes per week of active transportation as our primary predictors and Chao1, Shannon, and Inverse Simpson as the outcome variables. We performed bivariate regression analyses to test whether a priori possible confounders of the relationship between physical activity and the microbiome were significantly associated with physical activity. Age, race/ethnicity, body mass index, household income, census category, smoking status, diabetes, depression, use of proton pump inhibitors, use of antibiotics, dietary intake of carbohydrates, protein, fat, fiber, and alcohol, sleep duration, sedentary time, and light activity were statistically significantly associated with physical activity (P < 0.05) and were included as covariates in our analyses (S1 Table). We additionally included sex and education as covariates. Tests for significant interaction were performed between each outcome variable of interest and each covariate listed above. Significant interactions are presented in the results where applicable. We tested for non-linearity in each of the primary predictor variables but did not find evidence of a non-linear relationship.
Additionally, we generated several non-metric multi-dimensional scaling plots to visualize differences in microbiota composition by physical activity measures, using Bray-Curtis distance matrices calculated by the vegan package in R [57]. We then ran a PERMANOVA analysis [58] on the distance matrices to examine the association between physical activity and bacterial community composition (n = 1,000 permutations per run). Imputed data was used for these calculations but was unable to be pooled. One imputation was used for each calculation, rather than all imputations pooled together. Each analysis was run on several different imputations to confirm that the results were robust to the choice of imputed data set.
Next, we used negative binomial regression on the raw counts of individual genera and families to estimate the relationship between the abundance of individual taxa and physical activity. To reduce multiple testing and the effect of zero-inflation in our data set, we removed taxa that were comprised of more than 30% zeros. An offset of the log of total reads was used, and all models were adjusted for important covariates. Analyses were adjusted for multiple testing using the Benjamini-Hochberg procedure [59].
Results
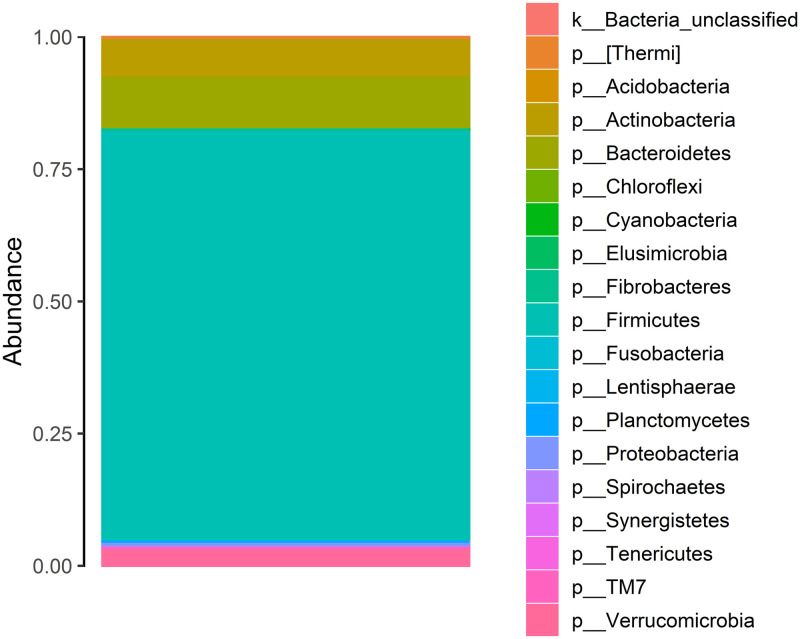
Among the 720 participants, sequencing of the V3-V4 region of the 16S rRNA gene resulted in 23,788,688 reads after filtering of chimeras, low quality reads, and sequences of incorrect length. Samples had an average of 32,632 reads. Filtered reads were assigned to 6,645 unique OTUs. After rarefaction of OTU counts to an even depth of 10,000 reads, there were 4,859 unique taxa. Six participants were removed from analysis due to low read count. After rare taxa were removed, there were 865 unique taxa. Overall, the most abundant phyla were Actinobacteria, Bacteroidetes, Firmicutes, Proteobacteria, and Verrucomicrobia (Fig 1).
Fig 1. A stacked bar plot demonstrating the relative abundance of phyla in our study.
On average, participants were 55 years old (SD: 16). Participants were 58% female, with 83% of participants self-identifying as White, 10% as Black, and as 3.5% Hispanic. Participants had an average body mass index of 30.7 kg/m2 (SD: 7.6) (Table 1).
Table 1. Descriptive sample characteristics, Survey of the Health of Wisconsin, 2016–2017.
| Variable | Mean | SD | N | Percent |
|---|---|---|---|---|
| Age (years) | 54.8 | 16.2 | ||
| Sex | ||||
| Male | 305 | 42.3 | ||
| Female | 415 | 57.6 | ||
| Race/ethnicity | ||||
| White | 596 | 82.8 | ||
| Black | 75 | 10.4 | ||
| Hispanic | 25 | 3.5 | ||
| Other | 24 | 3.5 | ||
| BMI (kg/m2) | 30.7 | 7.6 | ||
| Household income per person (in 1,000 USD) | 30.9 | 22.1 | ||
| Census category | ||||
| Urban | 499 | 69.3 | ||
| Rural | 221 | 30.7 | ||
| Education | ||||
| Some high school | 44 | 6.1 | ||
| High school | 153 | 21.3 | ||
| Some college | 258 | 35.8 | ||
| Bachelor’s | 176 | 24.4 | ||
| More than Bachelor’s | 89 | 12.4 | ||
| Smoking status | ||||
| Current | 96 | 13.4 | ||
| Former | 216 | 29.9 | ||
| Never | 408 | 56.7 | ||
| Diabetes | ||||
| Yes | 113 | 15.7 | ||
| No | 607 | 84.3 | ||
| Depression | ||||
| Yes | 174 | 24.2 | ||
| No | 546 | 75.8 | ||
| PPI use | ||||
| Yes | 105 | 14.6 | ||
| No | 615 | 85.4 | ||
| Antibiotic use | ||||
| Yes | 248 | 34.4 | ||
| No | 472 | 65.6 | ||
| Carbohydrates (g) | 233.8 | 153.6 | ||
| Fat (g) | 78.0 | 48.6 | ||
| Protein (g) | 74.8 | 41.8 | ||
| Fiber (g) | 19.8 | 11.4 | ||
| Alcohol (g) | 10.4 | 25.9 | ||
| Average sleep duration (min/day) | 512.7 | 79.5 | ||
| Average sedentary time (min/day) | 588.8 | 93.0 | ||
| Average light activity (min/day) | 343.8 | 97.9 | ||
| Physically Active | ||||
| Yes | 358 | 49.7 | ||
| No | 362 | 50.5 | ||
| Average MVPA (min/week) | 195.9 | 172.2 | ||
| Active transportation | ||||
| Yes | 148 | 20.5 | ||
| No | 572 | 79.5 | ||
| Active transportation (min/week) | 48.7 | 183.9 |
USD, United States dollar; PPI, proton pump inhibitor; min, minutes.
We investigated several measures of physical activity, and their relationship with alpha-diversity (Table 2). We did not find strong evidence that physically active status, moderate to vigorous physical activity (MVPA) minutes per week, or any active transportation were associated with alpha-diversity. However, a one standard deviation increase in weekly minutes spent in active transportation was associated with an increase of 7.57 (95% CI: 2.55, 12.59) in Chao1’s richness and 0.04 (0.0008, 0.09) in Shannon’s diversity. Due to the compositional nature of our physical activity measures, we also adjusted for light activity rather than sedentary behavior to assess whether changing physical activity by increasing or decreasing sedentary behavior versus light activity had a differential impact on alpha-diversity. There were not substantial differences between models which adjusted for sedentary time versus those that adjusted for light activity (S2 Table).
Table 2. Linear mixed effects model estimates of the relationship between physical activity and alpha-diversity.
| Physical activity measure | Chao1 | Shannon | Inverse Simpson |
|---|---|---|---|
| Estimate (95% CI) | Estimate (95% CI) | Estimate (95% CI) | |
| Physically active (reference = No) | 0.34 (-9.55, 10.23) | 0.007 (-0.08, 0.09) | 0.38 (-0.84, 1.60) |
| MVPA min/week (per pop. SD– 172 min.) | -1.12 (-5.79, 3.54) | -0.02 (-0.06, 0.02) | -0.24 (-0.80, 0.33) |
| Active transportation (reference = No) | 5.86 (-3.52, 15.23) | 0.03 (-0.05, 0.11) | 0.24 (-0.92, 1.39) |
| Active transportation min/week (per pop. SD– 184 min.) | 7.57*** (2.55, 12.59) | 0.04** (0.0008, 0.09) | 0.40 (-0.23, 1.01) |
Linear mixed effects models were adjusted for age, sex, race/ethnicity, body mass index, household income per person, education, census category, smoking status, diabetes, depression, proton pump inhibitor use, antibiotic use, carbohydrate intake, protein intake, fat intake, fiber intake, alcohol intake, average sedentary time per day, average sleep duration, and sample age. Models included random intercepts to account for clustering of participants by household.
CI, confidence interval; MVPA, moderate to vigorous physical activity; min, minutes; pop, population; SD, standard deviation.
* p < 0.1.
** p < 0.05.
*** p < 0.01.
Next, we modeled several interactions in the relationship between physical activity and alpha diversity (Table 3). We found that participating in any active transportation in an urban environment was not significantly different from the overall estimate of the impact of active transportation on diversity, while participating in active transportation in a rural environment was associated with an increase of 4.69 (95% CI: 1.64, 7.73). Similarly, we found that an increase of one standard deviation of minutes in active transportation per week was associated with an increase of 2.48 (95% CI: 0.85, 4.12) among those living in a rural environment.
Table 3. Linear mixed effects model estimates of the relationship between physical activity and alpha-diversity, summary of models with interaction.
| Inverse Simpson | ||
|---|---|---|
| Physical Activity Measure | Estimate (95% CI) | Estimate (95% CI) |
| Active transportation (reference = No) | -0.54 (-1.80, 0.73) | |
| Active transportation x Census category (reference = Urban) | 4.69*** (1.64, 7.73) | |
| Active transportation min/week (per pop. SD– 184 min.) | -0.02 (-0.70, 0.65) | |
| Active transportation min/week x Census category (reference = Urban) | 2.48*** (0.85, 4.12) | |
Linear mixed effects models were adjusted for age, sex, race/ethnicity, body mass index, household income per person, education, census category, smoking status, diabetes, depression, proton pump inhibitor use, antibiotics use, carbohydrate intake, protein intake, fat intake, fiber intake, alcohol intake, average sedentary time per day, average sleep duration, and sample age. Models included random intercepts to account for clustering of participants by household.
CI, confidence interval; pop, population; min, minutes. SD, standard deviation.
* p < 0.1.
** p < 0.05.
*** p < 0.01.
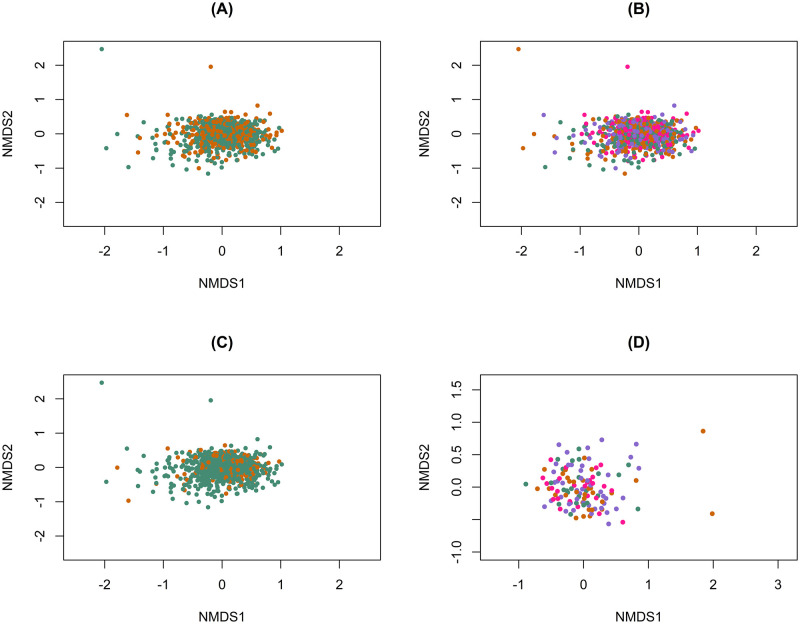
To characterize the association between microbiome composition and physical activity measures, we created non-metric multi-dimensional scaling (NMDS) plots using Bray-Curtis distance matrices colored by measures of physical activity (Fig 2). We did not observe visual clustering by the physical activity variables, but PERMANOVA tests revealed that there were differences in bacterial composition by physically active status (P = 0.001), MVPA minutes per week (P = 0.001), and engagement in active transportation (P = 0.009).
Fig 2. Bray-Curtis dissimilarity distances, colored by measures of physical activity.
(A) Bray-Curtis dissimilarity distances, colored by physically active status. PERMANOVA R2 = 0.004 (P = 0.001). SHOW, 2016–2017. (B) Bray-Curtis dissimilarity distances, colored by quartile of MVPA minutes per week. PERMANOVA R2 = 0.003 (P = 0.001). SHOW, 2016–2017. (C) Bray-Curtis dissimilarity distances colored by engagement in any active transportation. R2 = 0.003 (P = 0.009). SHOW, 2016–2017. (D) Bray-Curtis dissimilarity distances, colored by quartile of minutes in active transportation per week, excluding participants who reported no active transportation (n = 147). PERMANOVA R2 = 0.004 (P = 0.92). SHOW, 2016–2017.
Finally, we conducted several negative binomial regressions on individual genera and family abundance (Table 4). The results presented are statistically significant (P < 0.05) before correction for multiple testing. Results did not remain significant after correction for multiple testing. We found that any active transportation was associated with an increased abundance of genus Phascolarctobacterium and a decreased abundance of Clostridium from family Erysipelotrichaceae (P = 0.047 and 0.03, respectively). Being physically active and increased weekly minutes of MVPA was associated with an increase in the abundance of bacteria from the family Erysipelotrichaceae (P = 0.01). Increased weekly MVPA minutes were also associated with an increase in an unknown family and genus from the order Clostridiales (P = 0.03). Increased minutes in active transportation was associated with a decrease in the abundance of bacteria from the family Clostridiaceae (P = 0.03).
Table 4. Results of negative binomial models, before and after adjustment for multiple testing.
| Genus | Direction | P | P fdr | |
| Active transportation | Phascolarctobacterium | ↑ | 0.047 | 0.6 |
| Clostridium (family: Erysipelotrichaceae) | ↓ | 0.03 | 0.6 | |
| MVPA min/week | Unknown (order: Clostridiales) | ↑ | 0.03 | 0.7 |
| Family | Direction | P | P fdr | |
| Physically active | Erysipelotrichaceae | ↑ | 0.01 | 0.1 |
| MVPA min/week | Erysipelotrichaceae | ↑ | 0.01 | 0.1 |
| Unknown (order: Clostridiales) | ↑ | 0.03 | 0.2 | |
| Minutes in active transportation | Clostridiaceae | ↓ | 0.03 | 0.3 |
Models adjusted for: age, sex, race/ethnicity, body mass index, household income per person, education, census category, smoking status, diabetes, depression, proton pump inhibitor use, antibiotic use, carbohydrate intake, protein intake, fat intake, fiber intake, alcohol intake, average sedentary time per day, average sleep duration, and sample age. fdr, false discovery rate; MVPA, moderate to vigorous physical activity; min, minutes.
Discussion
Globally, insufficient physical activity is a leading cause of preventable disease, particularly in Western countries such as the United States [60]. In this study, we sought to understand the effects of physical activity on the gut microbiome, including understanding outdoor physical activity, which has recently increased in popularity in the US [61]. This large population-based analysis adds to the growing body of evidence that the gut microbiome may be linked with physical activity. Within this geographically diverse study population, we found that increased weekly minutes in active transportation were associated with increased alpha-diversity of the gut microbiota. Overall, we saw mixed results, consistent with previous study findings. In particular, we did not find strong evidence that being physically active or increased weekly time in MVPA was associated with changes in alpha diversity, while increased minutes in active transportation were associated with increased alpha-diversity. This latter finding is consistent with previous studies that also found a relationship between alpha-diversity and physical activity [23, 29].
We found that the abundance of an unknown family from order Clostridiales was associated with increased weekly MVPA minutes. Clostridiales is a beneficial butyrate-producing taxon which Estaki et al. found to be associated with increased physical fitness [29]. Butyrate has previously been associated with improved gut-barrier integrity [62], and has anti-oxidant, anti-carcinogenic, and anti-inflammatory properties [63]. The abundance of family Erysipelotrichaceae in the gut differed by weekly MVPA minutes. Previous studies have found that Erysipelotrichaceae plays a role in lipid metabolism [64]. Increased abundance of Phascolarctobacterium was associated with active transportation in our study. Previous research has found that higher abundance of Phascolactobacterium was associated with insulin sensitivity [65]. Given the well-established associations between physical activity and metabolic health in general, these findings are consistent and point to a potential pathway by which the gut microbiota may be linked to physical activity and other well established health benefits. Further research using metabolomic and/or functional metagenomics may offer additional insights.
While our findings regarding genera or families associated with physical activity measures were not statistically significant after correction for multiple testing, we present P-values both before and after adjustment while noting that the Benjamini-Hochberg procedure may be an overadjustment because the Benjamini-Hochberg procedure assumes the independence of tests, and gut microbial abundances are correlated [66, 67].
This study is unique in its examination of active transportation, and its findings regarding the interaction between census category and active transportation suggest that spending time outdoors may also be beneficial beyond the benefits of physical activity. Because active transportation necessitates completing physical activity outdoors, while MVPA does not, differences seen in the associations may be due to the added outdoor exposure. A previous study found that time spent outside without physical activity can alter the gut microbiome of children [68], making it plausible that there are additional changes in the gut microbiota specific to exercising outdoors, beyond the activity itself. The interactions we observed between active transportation and rural living support this idea, as the outdoor component of active transportation is likely to be different in those contexts compared to more urban settings. For example, outdoor physical activity in an urban setting may expose individuals to increased levels of traffic-related air pollution, which has been shown to affect the microbiome [69]. However, especially because minutes in active transportation are self-reported, it may be that the relationship between minutes spent in active transportation and the gut microbiome is positively confounded by some additional unmeasured variable. Further investigation into the potential benefits of completing physical activity outdoors is needed to better understand this relationship.
Despite numerous strengths, including large sample size, and objectively measured physical activity, as well as the ability to control for several potential confounders, there are a few limitations to note. First, the study relies on the use of 16S rRNA data, from which we could not identify taxonomy more specific than the genus-level; future studies should leverage species-level taxonomic information and pathway analysis to better understand the mechanisms driving these relationships. Additionally, this study conducted accelerometer measurement for one week. Although 3–5 days of measurement has been shown to be sufficient to estimate typical physical activity and sedentary behavior [70], we did not collect data about habitual physical activity or exercise regimens. Aside from characterizing active transportation, we do not have information on the types of activity participants completed during measurement. Future studies should consider augmenting accelerometry data with details on the types of physical activities performed for a more comprehensive view of physical activity. This study also used actigraphy to estimate sleep durations. Typically, polysomnography would be preferred as the gold standard, but studies have found that actigraphy is an acceptable substitute in healthy adults [71, 72], and the Cole-Kripe algorithm described in this study has been shown to have 83.86% agreement with polysomnography [47]. Furthermore, there may be some residual confounding by several measures. Errors in dietary estimates can be inherently biased and resemble a ‘flattened slope’ wherein those with high intakes tend to under-report and those with low intake tend to over-report [73]. This type of measurement error would induce bias in estimates of the association between dietary variables and the microbiome. Another important potential source of confounding that we were unable to adjust for was environmental exposures. Previous research has found that the gut microbiome can be impacted by exposure to lead [39] and other heavy metals [74], and air pollution [69]. These exposures were not included in our analysis. To the extent that active transportation in an urban environment corresponds with exposure to air pollution, air pollution may be an important confounder of these findings. Finally, while we adjusted for diabetes diagnosis by a physician, we were not able to explicitly adjust for use of anti-diabetic medications. Participants who did not report a diabetes diagnosis, but who were using anti-diabetic medications may have incorrectly been classified as non-diabetics, resulting in the potential for residual confounding.
Conclusions
This study provides some evidence that physical activity is associated with increased abundance of health-promoting gut microbes including Clostridiales. Additionally, we found preliminary evidence that outdoor physical activity has additional benefit to the gut microbiome, compared to physical activity alone. Future research should further evaluate the effects of physical activity on the gut microbiome in comparison with sedentary time spent outdoors and physical activity completed indoors.
Supporting information
Light blue represents intervals with missing data.
(TIF)
Bold cells indicate significance at P < 0.05. MVPA, moderate to vigorous physical activity; CI, confidence interval; Ref, reference; USD, United States dollars; PPI, proton pump inhibitor; NSAID, non-steroidal anti-inflammatory drugs; Avg, average; min, minutes.
(DOCX)
Linear mixed effects models were adjusted for age, sexr, race/ethnicity, body mass index, household income per person, education, census category, smoking status, diabetes, depression, proton pump inhibitor use, antibiotic use carbohydrate intake, fat intake, fiber intake, alcohol intake, average light activity per day, average sleep duration, and sample age. Models included a random intercept to account for clustering of participants by household. CI, confidence interval; MVPA, moderate to vigorous physical activity; pop, population; SD, standard deviation. * p < 0.1; ** p < 0.05; *** p < 0.01.
(DOCX)
Acknowledgments
The authors would like to thank the study participants, staff at the Survey of the Health of Wisconsin, the University of Wisconsin Survey Center, Dr. Safdar’s Infectious Disease Research Lab, and the Suen Lab.
Data Availability
Data cannot be shared publicly because they include potentially identifying information on human subjects. Data can be requested from the Survey of the Health of Wisconsin at their website, show.wisc.edu, or by emailing data@show.wisc.edu.
Funding Statement
This research was supported by a Eunice Kennedy Shriver National Institute of Child Health and Human Development grant to the Center for Demography and Ecology at the University of Wisconsin-Madison (P2C HD047873 and T32 HD07014) and a National Institute on Aging grant to the Center for Demography of Health and Aging at the University of Wisconsin-Madison (P30 AG17266). KCM’s time is also supported by R01 AG061080. Funding for the Survey of the Health of Wisconsin was provided by the Wisconsin Partnership Program PERC Award (233 PRJ 25DJ). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.D’Argenio V, Salvatore F. The role of the gut microbiome in the healthy adult status. Clinica Chimica Acta. 2015. Dec 7;451:97–102. doi: 10.1016/j.cca.2015.01.003 [DOI] [PubMed] [Google Scholar]
- 2.Kinross JM, Darzi AW, Nicholson JK. Gut microbiome-host interactions in health and disease. Genome Medicine. 2011. Mar 4;3(3):14. doi: 10.1186/gm228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamboli CP, Neut C, Desreumaux P, Colombel JF. Dysbiosis in inflammatory bowel disease. Gut. 2004. Jan 1;53(1):1–4. doi: 10.1136/gut.53.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willing B, Halfvarson J, Dicksved J, Rosenquist M, Järnerot G, Engstrand L, et al. Twin Studies Reveal Specific Imbalances in the Mucosaassociated Microbiota of Patients with Ileal Crohn’s Disease. Inflamm Bowel Dis. 2009. May 1;15(5):653–60. doi: 10.1002/ibd.20783 [DOI] [PubMed] [Google Scholar]
- 5.Giongo A, Gano KA, Crabb DB, Mukherjee N, Novelo LL, Casella G, et al. Toward defining the autoimmune microbiome for type 1 diabetes. The ISME Journal. 2011. Jan;5(1):82–91. doi: 10.1038/ismej.2010.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Upadhyaya S, Banerjee G. Type 2 diabetes and gut microbiome: at the intersection of known and unknown. Gut Microbes. 2015. Mar 4;6(2):85–92. doi: 10.1080/19490976.2015.1024918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inoue Y, Shimojo N. Microbiome/microbiota and allergies. Semin Immunopathol. 2015. Jan 1;37(1):57–64. doi: 10.1007/s00281-014-0453-5 [DOI] [PubMed] [Google Scholar]
- 8.Arrieta MC, Finlay B. The intestinal microbiota and allergic asthma. Journal of Infection. 2014. Nov 1;69:S53–5. doi: 10.1016/j.jinf.2014.07.015 [DOI] [PubMed] [Google Scholar]
- 9.Vaahtovuo J, Munukka E, Korkeamäki M, Luukkainen R, Toivanen P. Fecal Microbiota in Early Rheumatoid Arthritis. The Journal of Rheumatology. 2008. Aug 1;35(8):1500–5. [PubMed] [Google Scholar]
- 10.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. PNAS. 2005. Aug 2;102(31):11070–5. doi: 10.1073/pnas.0504978102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013. Aug;500(7464):541–6. doi: 10.1038/nature12506 [DOI] [PubMed] [Google Scholar]
- 12.Sobhani I, Tap J, Roudot-Thoraval F, Roperch JP, Letulle S, Langella P, et al. Microbial Dysbiosis in Colorectal Cancer (CRC) Patients. PLOS ONE. 2011. Jan 27;6(1):e16393. doi: 10.1371/journal.pone.0016393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jie Z, Xia H, Zhong SL, Feng Q, Li S, Liang S, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nature Communications. 2017. Oct 10;8(1):845. doi: 10.1038/s41467-017-00900-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vuong HE, Hsiao EY. Emerging Roles for the Gut Microbiome in Autism Spectrum Disorder. Biological Psychiatry. 2017. Mar 1;81(5):411–23. doi: 10.1016/j.biopsych.2016.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang DW, Park JG, Ilhan ZE, Wallstrom G, LaBaer J, Adams JB, et al. Reduced Incidence of Prevotella and Other Fermenters in Intestinal Microflora of Autistic Children. PLOS ONE. 2013. Jul 3;8(7):e68322. doi: 10.1371/journal.pone.0068322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moloney RD, Desbonnet L, Clarke G, Dinan TG, Cryan JF. The microbiome: stress, health and disease. Mamm Genome. 2014. Feb 1;25(1):49–74. doi: 10.1007/s00335-013-9488-5 [DOI] [PubMed] [Google Scholar]
- 17.Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020. Dec 1;54(24):1451–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang SS, Jeraldo PR, Kurti A, Miller MEB, Cook MD, Whitlock K, et al. Diet and exercise orthogonally alter the gut microbiome and reveal independent associations with anxiety and cognition. Molecular Neurodegeneration. 2014. Sep 13;9(1):36. doi: 10.1186/1750-1326-9-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mika A, Treuren WV, González A, Herrera JJ, Knight R, Fleshner M. Exercise Is More Effective at Altering Gut Microbial Composition and Producing Stable Changes in Lean Mass in Juvenile versus Adult Male F344 Rats. PLOS ONE. 2015. May 27;10(5):e0125889. doi: 10.1371/journal.pone.0125889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Queipo-Ortuño MI, Seoane LM, Murri M, Pardo M, Gomez-Zumaquero JM, Cardona F, et al. Gut Microbiota Composition in Male Rat Models under Different Nutritional Status and Physical Activity and Its Association with Serum Leptin and Ghrelin Levels. PLOS ONE. 2013. May 28;8(5):e65465. doi: 10.1371/journal.pone.0065465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petriz BA, Castro AP, Almeida JA, Gomes CP, Fernandes GR, Kruger RH, et al. Exercise induction of gut microbiota modifications in obese, non-obese and hypertensive rats. BMC Genomics. 2014. Jun 21;15(1):511. doi: 10.1186/1471-2164-15-511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denou E, Marcinko K, Surette MG, Steinberg GR, Schertzer JD. High-intensity exercise training increases the diversity and metabolic capacity of the mouse distal gut microbiota during diet-induced obesity. APSselect. 2016. Apr 26;3(6):E982–93. doi: 10.1152/ajpendo.00537.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clarke SF, Murphy EF, O’Sullivan O, Lucey AJ, Humphreys M, Hogan A, et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014. Dec 1;63(12):1913–20. doi: 10.1136/gutjnl-2013-306541 [DOI] [PubMed] [Google Scholar]
- 24.Barton W, Penney NC, Cronin O, Garcia-Perez I, Molloy MG, Holmes E, et al. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut. 2018. Apr 1;67(4):625–33. doi: 10.1136/gutjnl-2016-313627 [DOI] [PubMed] [Google Scholar]
- 25.Zhao X, Zhang Z, Hu B, Huang W, Yuan C, Zou L. Response of Gut Microbiota to Metabolite Changes Induced by Endurance Exercise. Front Microbiol [Internet]. 2018 [cited 2020 Nov 19];9. https://www.frontiersin.org/articles/10.3389/fmicb.2018.00765/full?report=reader [DOI] [PMC free article] [PubMed]
- 26.Keohane DM, Woods T, O’Connor P, Underwood S, Cronin O, Whiston R, et al. Four men in a boat: Ultra-endurance exercise alters the gut microbiome. Journal of Science and Medicine in Sport. 2019. Sep 1;22(9):1059–64. doi: 10.1016/j.jsams.2019.04.004 [DOI] [PubMed] [Google Scholar]
- 27.Grosicki GJ, Durk RP, Bagley JR. Rapid gut microbiome changes in a world-class ultramarathon runner. Physiological Reports. 2019;7(24):e14313. doi: 10.14814/phy2.14313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bassett DR, Wyatt HR, Thompson H, Peters JC, Hill JO. Pedometer-Measured Physical Activity and Health Behaviors in United States Adults. Med Sci Sports Exerc. 2010. Oct;42(10):1819–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Estaki M, Pither J, Baumeister P, Little JP, Gill SK, Ghosh S, et al. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome. 2016. Aug 8;4(1):42. doi: 10.1186/s40168-016-0189-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bressa C, Bailén-Andrino M, Pérez-Santiago J, González-Soltero R, Pérez M, Montalvo-Lominchar MG, et al. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLOS ONE. 2017. Feb 10;12(2):e0171352. doi: 10.1371/journal.pone.0171352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allen JM, Mailing LJ, Niemiro GM, Moore R, Cook MD, White BA, et al. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Medicine & Science in Sports & Exercise. 2018. Apr;50(4):747–57. doi: 10.1249/MSS.0000000000001495 [DOI] [PubMed] [Google Scholar]
- 32.Munukka E, Ahtiainen JP, Puigbó P, Jalkanen S, Pahkala K, Keskitalo A, et al. Six-Week Endurance Exercise Alters Gut Metagenome That Is not Reflected in Systemic Metabolism in Over-weight Women. Front Microbiol [Internet]. 2018 [cited 2020 Nov 19];9. https://www.frontiersin.org/articles/10.3389/fmicb.2018.02323/full [DOI] [PMC free article] [PubMed]
- 33.Whisner CM, Maldonado J, Dente B, Krajmalnik-Brown R, Bruening M. Diet, physical activity and screen time but not body mass index are associated with the gut microbiome of a diverse cohort of college students living in university housing: a cross-sectional study. BMC Microbiol. 2018. Dec 12;18(1):210. doi: 10.1186/s12866-018-1362-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rettedal EA, Cree JME, Adams SE, MacRae C, Skidmore PML, Cameron‐Smith D, et al. Short-term high-intensity interval training exercise does not affect gut bacterial community diversity or composition of lean and overweight men. Experimental Physiology. 2020;105(8):1268–79. doi: 10.1113/EP088744 [DOI] [PubMed] [Google Scholar]
- 35.Shahar RT, Koren O, Matarasso S, Shochat T, Magzal F, Agmon M. Attributes of Physical Activity and Gut Microbiome in Adults: A Systematic Review. Int J Sports Med. 2020. Oct;41(12):801–14. doi: 10.1055/a-1157-9257 [DOI] [PubMed] [Google Scholar]
- 36.Nieto FJ, Peppard PE, Engelman CD, McElroy JA, Galvao LW, Friedman EM, et al. The Survey of the Health of Wisconsin (SHOW), a novel infrastructure for population health research: rationale and methods. BMC Public Health. 2010. Dec 23;10(1):785. doi: 10.1186/1471-2458-10-785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eggers S, Malecki KM, Peppard P, Mares J, Shirley D, Shukla SK, et al. Wisconsin microbiome study, a cross-sectional investigation of dietary fibre, microbiome composition and antibiotic-resistant organisms: rationale and methods. BMJ Open. 2018. Mar 1;8(3):e019450. doi: 10.1136/bmjopen-2017-019450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malecki KMC, Nikodemova M, Schultz AA, LeCaire TJ, Bersch AJ, Cadmus-Bertram L, et al. The Survey of the Health of Wisconsin (SHOW) Program: An Infrastructure for Advancing Population Health. Front Public Health. 2022;10:818777. doi: 10.3389/fpubh.2022.818777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eggers S, Safdar N, Sethi AK, Suen G, Peppard PE, Kates AE, et al. Urinary lead concentration and composition of the adult gut microbiota in a cross-sectional population-based sample. Environment International. 2019. Dec 1;133:105122. doi: 10.1016/j.envint.2019.105122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a Dual-Index Sequencing Strategy and Curation Pipeline for Analyzing Amplicon Sequence Data on the MiSeq Illumina Sequencing Platform. Appl Environ Microbiol. 2013. Sep 1;79(17):5112–20. doi: 10.1128/AEM.01043-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl Environ Microbiol. 2009. Dec 1;75(23):7537–41. doi: 10.1128/AEM.01541-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013. Jan 1;41(D1):D590–6. doi: 10.1093/nar/gks1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011. Aug 15;27(16):2194–200. doi: 10.1093/bioinformatics/btr381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, et al. Greengenes, a Chimera-Checked 16S rRNA Gene Database and Workbench Compatible with ARB. Appl Environ Microbiol. 2006. Jul 1;72(7):5069–72. doi: 10.1128/AEM.03006-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McMurdie PJ, Holmes S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLOS ONE. 2013. Apr 22;8(4):e61217. doi: 10.1371/journal.pone.0061217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Catellier DJ, Hannan PJ, Murray DM, ADDY CL, CONWAY TL, YANG S, et al. Imputation of Missing Data When Measuring Physical Activity by Accelerometry. Med Sci Sports Exerc. 2005. Nov;37(11 Suppl):S555–62. doi: 10.1249/01.mss.0000185651.59486.4e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic Sleep/Wake Identification From Wrist Activity. Sleep. 1992. Sep;15(5):461–9. doi: 10.1093/sleep/15.5.461 [DOI] [PubMed] [Google Scholar]
- 48.Freedson P, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998. May;30(5):777–81. doi: 10.1097/00005768-199805000-00021 [DOI] [PubMed] [Google Scholar]
- 49.U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd edition. U.S. Department of Health and Human Services; 2018.
- 50.US Census Bureau. 2010 Census Urban and Rural Classification and Urban Area Criteria [Internet]. The United States Census Bureau. 2021 [cited 2020 Nov 20]. https://www.census.gov/programs-surveys/geography/guidance/geo-areas/urban-rural/2010-urban-rural.html
- 51.Kroenke K, Spitzer RL, Williams JBW. The Patient Health Questionnaire-2: Validity of a Two-Item Depression Screener. Medical Care. 2003;41(11):1284–92. doi: 10.1097/01.MLR.0000093487.78664.3C [DOI] [PubMed] [Google Scholar]
- 52.Nelson SJ, Zeng K, Kilbourne J, Powell T, Moore R. Normalized names for clinical drugs: RxNorm at 6 years. J Am Med Inform Assoc. 2011. Jul 1;18(4):441–8. doi: 10.1136/amiajnl-2011-000116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Diet History Questionnaire: Diet*Calc Software [Internet]. 2005 [cited 2020 Nov 20]. https://epi.grants.cancer.gov/dhq/dietcalc/
- 54.Holzhausen EA, Nikodemova M, Deblois CL, Barnet JH, Peppard PE, Suen G, et al. Assessing the impact of storage time on the stability of stool microbiota richness, diversity, and composition. Gut Pathog. 2021. Dec 20;13(1):75. doi: 10.1186/s13099-021-00470-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buuren S van, Groothuis-Oudshoorn K. MICE: Multivariate Imputation by Chained Equations in R. Journal of statistical software [Internet]. 2010. [cited 2020 Nov 20]; Available from: http://localhost/handle/1874/44635 [Google Scholar]
- 56.Buuren S van, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Statistics in Medicine. 1999;18(6):681–94. [DOI] [PubMed] [Google Scholar]
- 57.Oksanen JF, Blanchet G, Friendly M, Kindt R, Legendre P, McGlinn D, et al. Community Ecology Package. 2020. [Google Scholar]
- 58.Anderson MJ. Permutational Multivariate Analysis of Variance (PERMANOVA). In: Wiley StatsRef: Statistics Reference Online [Internet]. American Cancer Society; 2017. [cited 2021 Feb 21]. p. 1–15. http://onlinelibrary.wiley.com/doi/abs/10.1002/9781118445112.stat07841 [Google Scholar]
- 59.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc Series B Stat Metholol. 1995;57(1):289–300. [Google Scholar]
- 60.Guthold R, Stevens GA, Riley LM, Bull FC. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1·9 million participants. The Lancet Global Health. 2018. Oct 1;6(10):e1077–86. doi: 10.1016/S2214-109X(18)30357-7 [DOI] [PubMed] [Google Scholar]
- 61.Kercher VM, Kercher K, Bennion T, Levy P, Alexander C, Amaral PC, et al. 2022 Fitness Trends from Around the Globe. ACSM’s Health & Fitness Journal. 2022. Feb;26(1):21–37. [Google Scholar]
- 62.VanHook AM. Butyrate benefits the intestinal barrier. Science Signaling. 2015. May 26;8(378):ec135–ec135. [Google Scholar]
- 63.Bedford A, Gong J. Implications of butyrate and its derivatives for gut health and animal production. Animal Nutrition. 2018. Jun 1;4(2):151–9. doi: 10.1016/j.aninu.2017.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaakoush NO. Insights into the Role of Erysipelotrichaceae in the Human Host. Frontiers in Cellular and Infection Microbiology [Internet]. 2015 [cited 2022 Apr 5];5. https://www.frontiersin.org/article/10.3389/fcimb.2015.00084 [DOI] [PMC free article] [PubMed]
- 65.Naderpoor N, Mousa A, Gomez-Arango LF, Barrett HL, Dekker Nitert M, de Courten B. Faecal Microbiota Are Related to Insulin Sensitivity and Secretion in Overweight or Obese Adults. Journal of Clinical Medicine. 2019. Apr;8(4):452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schwartzman A, Lin X. The effect of correlation in false discovery rate estimation. Biometrika. 2011. Mar;98(1):199–214. doi: 10.1093/biomet/asq075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stevens JR, Masud AA, Suyundikov A. A comparison of multiple testing adjustment methods with block-correlation positively-dependent tests. PLOS ONE. 2017. Apr 28;12(4):e0176124. doi: 10.1371/journal.pone.0176124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sobko T, Liang S, Cheng WHG, Tun HM. Impact of outdoor nature-related activities on gut microbiota, fecal serotonin, and perceived stress in preschool children: the Play&Grow randomized controlled trial. Scientific Reports. 2020. Dec 15;10(1):21993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alderete TL, Jones RB, Chen Z, Kim JS, Habre R, Lurmann F, et al. Exposure to traffic-related air pollution and the composition of the gut microbiota in overweight and obese adolescents. Environmental Research. 2018. Feb 1;161:472–8. doi: 10.1016/j.envres.2017.11.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Trost SG, McIver KL, Pate RR. Conducting accelerometer-based activity assessments in field-based research. Med Sci Sports Exerc. 2005. Nov;37(11 Suppl):S531–543. doi: 10.1249/01.mss.0000185657.86065.98 [DOI] [PubMed] [Google Scholar]
- 71.Quante M, Kaplan ER, Cailler M, Rueschman M, Wang R, Weng J, et al. Actigraphy-based sleep estimation in adolescents and adults: a comparison with polysomnography using two scoring algorithms. Nat Sci Sleep. 2018. Jan 18;10:13–20. doi: 10.2147/NSS.S151085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Full KM, Kerr J, Grandner MA, Malhotra A, Moran K, Godoble S, et al. Validation of a physical activity accelerometer device worn on the hip and wrist against polysomnography. Sleep Health. 2018. Apr 1;4(2):209–16. doi: 10.1016/j.sleh.2017.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Freedman LS, Schatzkin A, Midthune D, Kipnis V. Dealing With Dietary Measurement Error in Nutritional Cohort Studies. JNCI: Journal of the National Cancer Institute. 2011. Jul 20;103(14):1086–92. doi: 10.1093/jnci/djr189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tu P, Chi L, Bodnar W, Zhang Z, Gao B, Bian X, et al. Gut Microbiome Toxicity: Connecting the Environment and Gut Microbiome-Associated Diseases. Toxics. 2020. Mar;8(1):19. doi: 10.3390/toxics8010019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Light blue represents intervals with missing data.
(TIF)
Bold cells indicate significance at P < 0.05. MVPA, moderate to vigorous physical activity; CI, confidence interval; Ref, reference; USD, United States dollars; PPI, proton pump inhibitor; NSAID, non-steroidal anti-inflammatory drugs; Avg, average; min, minutes.
(DOCX)
Linear mixed effects models were adjusted for age, sexr, race/ethnicity, body mass index, household income per person, education, census category, smoking status, diabetes, depression, proton pump inhibitor use, antibiotic use carbohydrate intake, fat intake, fiber intake, alcohol intake, average light activity per day, average sleep duration, and sample age. Models included a random intercept to account for clustering of participants by household. CI, confidence interval; MVPA, moderate to vigorous physical activity; pop, population; SD, standard deviation. * p < 0.1; ** p < 0.05; *** p < 0.01.
(DOCX)
Data Availability Statement
Data cannot be shared publicly because they include potentially identifying information on human subjects. Data can be requested from the Survey of the Health of Wisconsin at their website, show.wisc.edu, or by emailing data@show.wisc.edu.