Abstract
Inorganic pollutants in Colombian cocoa (Theobroma cacao L.) agrosystems cause problems in the production, quality, and exportation of this raw material worldwide. There has been an increased interest in bioprospecting studies of different fungal species focused on the biosorption of heavy metals. Furthermore, fungi constitute a valuable, profitable, ecological, and efficient natural soil resource that could be considered in the integrated management of cadmium mitigation. This study reports a new species of Talaromyces isolated from a cocoa soil sample collected in San Vicente de Chucurí, Colombia. T. santanderensis is featured by Lemon Yellow (R. Pl. IV) mycelium on CYA, mono-to-biverticillade conidiophores, and acerose phialides. T. santanderensis is distinguished from related species by its growth rate on CYAS and powdery textures on MEA, YES and OA, high acid production on CREA and smaller conidia. It is differentiated from T. lentulus by its growth rate on CYA medium at 37 °C without exudate production, its cream (R. PI. XVI) margin on MEA, and dense sporulation on YES and CYA. Phylogenetic analysis was performed using a polyphasic approach, including different phylogenetic analyses of combined and individual ITS, CaM, BenA, and RPB2 gene sequences that indicate that it is new to science and is named Talaromyces santanderensis sp. nov. This new species belongs to the Talaromyces section and is closely related to T. lentulus, T. soli, T. tumuli, and T. pratensis (inside the T. pinophilus species complex) in the inferred phylogeny. Mycelia growth of the fungal strains was subjected to a range of 0–400 mg/kg Cd and incorporated into malt extract agar (MEA) in triplicates. Fungal radial growth was recorded every three days over a 13-day incubation period and In vitro cadmium tolerance tests showed a high tolerance index (0.81) when the mycelium was exposed to 300 mg/kg of Cd. Results suggest that T. santanderensis showed tolerance to Cd concentrations that exceed the permissible limits for contaminated soils, and it is promising for its use in bioremediation strategies to eliminate Cd from highly contaminated agricultural soils.
Keywords: fungal systematics, Talaromyces santanderensis, cacao soils, cadmium, mycoremediation
1. Introduction
Heavy metal pollution has become a severe problem worldwide [1]. The cadmium (Cd) released from different sources, whether natural or anthropogenic, can lodge in the soil and therefore bioaccumulate in crops of agricultural interest such as Theobroma cacao L., a native tree from tropical American rainforests, where, until today, it has been found in its wild state, from Peru to Mexico [2]. Cocoa ranks third after sugar and coffee in the world food market, demanded mainly by American and European companies [3]. Nonetheless, the cadmium present in agricultural soils is accumulated by certain species of plants where cocoa is listed [4], representing a problem for the cacao quality, food safety, and the international market. Cadmium can bioaccumulate, is non-biodegradable, and has been determined to be a precursor of several human diseases such as cancer and many other illnesses related to its presence in the human body that causes oxidative stress, inflammation, and tissue damage [5]. Cadmium is found in the earth’s crust at an average concentration of 0.1 mg kg−1 [6]. The average level of cadmium in agricultural soil fluctuates between 0.07 and 1.1 µg g−1, with a natural base level of 0.5 µg g−1 [7].
The production of cocoa beans in Colombia has been evaluated on numerous occasions for its high levels of cadmium. A study by Echeverri & Reyes (2016) [8] showed that the cadmium concentration in chocolate with more than 30% content of Colombian cocoa yielded an average value of 4.0477 mg kg−1 of cadmium. This data puts the health of local producers and consumers at risk and pose a threat to the productive chain of cocoa derivatives because it exceeds the maximum limits established by the recent EU provision (Regulation No. 488/2014 with tolerable values between 0, 1, and 0.80 µg g−1 for derived cocoa products) [9]. This situation limits the growing access to international markets for the Colombian cocoa sector, which in 2018 managed to export a total of 7.056 tons of cocoa to 23 countries and had an FOB income of 16 million USD [10].
The search for different and better strategies to mitigate cadmium concentration in cocoa-producing soils has aroused interest in Central and South American countries. According to a recent study by Bravo et al. (2021) [11], in Colombian cacao-growing farms, Cd concentration in the soil samples ranged from 0,01 mg kg−1 to 27 mg kg−1, especially in the Santander region, where the highest levels of Cd were found. Thus, many genetic, chemical, and agronomic strategies have been applied and studied to counteract the Cd of agricultural soils. Nowadays, mycoremediation has an excellent acceptance in the remediation processes of contaminated soils due to their different advantages, such as the low application cost, small environmental impact, and high effectiveness. Therefore, these advantages reduce the enormous ecological stress caused by heavy metals [12].
Several metal-tolerant filamentous fungi have been isolated from multiple heavy metal contaminated sites and soils [13,14,15,16,17,18]. They have been recognized for their effectiveness in heavy metal remediation processes due to their particular attributes such as rapid growth, ability to thrive in extreme temperature, pH, and tolerance to high concentrations of metals. In the particular case of cadmium stress alleviation in plants, Talaromyces has stood out as a promising endophyte [19,20]. Moreover, for the treatment of substrates and hydrological sources, species such as Talaromyces emersonii and Talaromyces amestolkiae have been used as organic phosphate sources for treating uranium-contaminated water [21,22]. Also, Talaromyces helicus has been widely used to remove cadmium, cobalt, copper, and lead from industrially contaminated sediments [23,24].
Talaromyces spp. is characterized by a cosmopolitan distribution and joint in many substrates (most commonly found on soils) [25]. Furthermore, by their capacity to produce heat-resistant ascospores [26]. This monophyletic genus belongs to the order Eurotiales and contains eight sections (Bacillispori, Helici, Islandici, Purpurei, Subinflati, Talaromyces, Tenues and Trachyspermi) [26]. It was recently classified at the family and genus level, considering its traditional phenotypic characters, such as its texture that can be strictly velutinous to floppy and even synnematous or funiculous. Penicilli, in general, are biverticillate, but certain species can have both biverticillate and monoverticillate with acerose or ampulliform phialides. Conidia are usually described as ellipsoidal to fusiform and globose with a lesser proportion. Its taxonomy has been improved using sequence-based approaches [27]. To date, Talaromyces contains more than 250 described species in the Mycobank database [28] and Talaromyces section (sect.). Talaromyces contains roughly 86 species [29]. Additionally, inside the Talaromyces sect. Talaromyces is located the informal group Talaromyces pinophilus complex, which harbors T. lentulus, T. mae, T. soli, T. malicola, T. adpressus, T. annesophiae, and T. pratensis [29,30]. However, this complex requires improved phylogenetic elucidation to further support this subgroup species’ evolutionary relationships.
In the present study, we performed a collection of an agricultural soil sample from the Santander region in Colombia. After the isolation in multiple media and morphological identification, the Talaromyces sample could not be assigned to any known species. Thus, we performed a polyphasic identification approach by adding phylogenetic analysis of partial ITS, β-tubulin (BenA), calmodulin (CaM), and RNA polymerase II second largest subunit (RPB2) gene sequences, and the use of macro- and micro-morphological data to delimitate the new species in the informal group and genus.
2. Materials and Methods
2.1. Sample Soil Description
A soil composite sample, with high content of Cd (18 mg kg−1), from the Theobroma cacao crop was randomly collected from the village of Monserrate, municipality of San Vicente de Chucurí from the Santander region in Colombia 73.4097601 W decimal longitude, 6.881000 N decimal latitude. The sample for this study was provided by the Colombian Cocoa Growers Federation (Fedecacao) and deposited in the Agro-environmental research laboratory of Biotechnology-LIIBAAN from the University of Santander (UDES). The physical-chemical analysis of the soil sample was carried out by a laboratory certified by the ICA (Colombian Agricultural Institute) according to standard methods (Supplementary Table S1).
2.2. Isolation of Strains and Screening for Cadmium Tolerant Fungi
The fungal strain was initially isolated on Potato Dextrose Agar (PDA, Merck KGaA®, Darmstadt, Germany), and Malt Extract Agar (MEA, Merck KGaA®, Darmstadt, Germany), by serial dilution. Samples were diluted to in sterile water. 0.1 mL of different dilutions were spread on Petri dishes (10 cm diameter) containing 20 mL of medium. The plates were incubated at 25 °C and 35 °C in dark conditions and monitored daily for up to 7 days. Each developed colony was subcultured and isolated on fresh PDA plates supplemented with 25 mg/L of Cadmium adjusted to a pH of 5.5 to 5.7. The plates were incubated under the same conditions.
2.3. Cadmium Tolerance Test
The cadmium tolerance was evaluated based on the selection of strains whose mycelium initially grew at 25 mg kg−1 of Cd, equal to the control. Tolerant strains were cultivated in higher concentrations of Cd (50, 100, 200, 300, and 400 mg kg−1) in PDA and MEA culture media. Sterile filtered CdCl2 salts (pore size of 0.25 µm) were incorporated, supplemented with 25 mg of streptomycin, and pH was maintained at 5.7. The experiment was performed in triplicate for both the control and the concentrations. Eight-day-old 5 mm diameter mycelium discs were individually inoculated from the pure cultures. Plates were incubated at 25 and 35 ± 1 °C for equal days, during which the radial growth of the mycelium was monitored. The cadmium tolerance potential of the fungal species in the test medium was calculated in relation to the growth control radials (Equation (1)). The tolerance to heavy metals of the fungi was scored as follows: 0.00–0.39 (very low tolerance), 0.40–0.59 (low tolerance), 0.60–0.79 (moderate tolerance), 0.80–0.99 (high tolerance) and ≥1.00 (very high tolerance) [15].
| (1) |
2.4. Cultivation Conditions and Colony Morphology
Colony characters were examined by inoculating the strains on the media proposed by Samson et al. [31]. Isolates were inoculated by placing 1 μL of conidia from semi-solid agar (0.2% agar + 0.05% Tween 80) in 90 mm Petri dishes with Malt Extract Agar (MEA, Merck KGaA®, Darmstadt, Germany), Oatmeal Agar (OA, Merck KGaA®, Darmstadt, Germany), Yeast Extract Sucrose Agar (YES, Merck KGaA®, Darmstadt, Germany), Creatine Sucrose Agar (CREA, Merck KGaA®, Darmstadt, Germany), Czapek Yeast Autolysate Agar (CYA, Merck KGaA®, Darmstadt, Germany), and Czapek Yeast Autolysate Agar + 5% NaCl (CYAS, Merck KGaA®, Darmstadt, Germany). Plates were incubated for seven days at 10 °C, 25 °C, 35 °C and 37 °C in darkness. The Colony features were studied following Visagie et al. 2014 [32] and Yilmaz et al. 2014. [26] Colony diameters were also measured after seven days at 25 °C and 35 °C and photographed (Nikon, camera, model FE-220/X-785), two perpendicular diameters were measured for each colony, and the average was calculated. Phenotypic characteristics, such as obverse and reverse culture appearance, colony texture, mycelium color, sporulation, exudates, and medium changes, were also recorded. The names of colors were referenced by Ridgway [33].
2.5. Microscopic Identification
The microscopic slides were prepared from 7-day-old cultures grown on Malt Extract Agar (MEA, Merck KGaA®, Darmstadt, Germany). The conidia were suspended in 1 mL of 60% lactic acid [26]. Phenotypic characteristics of conidia (shape, ornamentation of the cell wall) and conidiophores (number of branching points between stipe and phialides, and shape/texture of phialides) were recorded. Microscopic examinations were done using a microscope Nikon (model Eclipse Ni-u). Two diameters (length and width) were measured for 100 conidia of each isolate in the Quick Photo Camera software program (Imagen Pro).
2.6. DNA Extraction and Amplification
The isolate was cultured in a Sabouraud dextrose medium for 7 days at 28 °C for DNA extraction. Approximately 100 mg of mycelium was scraped from the surface of the plate using a sterile blade and then transferred to a 1.5 mL sample tube. Total DNA extractions were performed using a phenol-chloroform method with modifications suggested by Chi et al. (2009) [34]. The mycelia were crushed in liquid nitrogen and placed in 1 mL of Lysis Buffer (40 mM Tris-HCl, 20 mM sodium acetate, 10 mM ethylenediaminetetraacetic acid, and 1% sodium dodecyl sulfate, pH 8.0) per 100 mg of powdered mycelium. The polymerase chain reaction (PCR) method was used to amplify marker genes for species; Internal Transcribed spacer (ITS), β-tubulin (BenA), Calmodulin (CaM), and the second subunit of DNA-dependent RNA polymerase II (RPB2).
PCR amplifications were conducted with the following primer pairs: RPB2-5F [35] and bRPB2-7.1R [36] for a fragment of approximately 1000 pb of RPB2 gene. CMD5 and CMD6 primer pair for a fragment of approximately 600 bp of Calmodulin gene. ITS1-F and ITS4-R for a fragment of 500 bp of ITS. T1 and T22 for a fragment of approx 1350 bp of the β-Tubulin gene [37], additional information about used primers can be found in Table 1. The amplification programs were used with the following parameters: for ITS; 5 min at 95 °C, 35 cycles × (1 min 94 °C, 1 min 55 °C, 2 min 72 °C), 5 min at 72 °C. For β-tubulin, 5 min at 95 °C, 35 cycles × (35 s 94 °C, 55 s 55.4 °C, 2 min 72 °C), 5 min at 72 °C. For Calmodulin, it was 5 min at 95 °C, 35 cycles × (1 min 94 °C, 55 s 50 °C, 2 min 72 °C), and 5 min at 72 °C. For RPB2, it was 5 min at 95 °C, 35 cycles × (1 min 94 °C, 55 s 52.5 °C, 2 min 72 °C), and 5 min at 72 °C. All reactions were performed in a C1000 Thermal Cycler (BioRad Technologies). The amplification products were visualized on a 1% agarose gel and quantified in Nanodrop (ThermoFisher) for subsequent shipment to the Sanger sequencing service at the Genecore facility of the Universidad de Los Andes, Colombia. Obtained chromatograms were assembled using Tracy v.0.5.8 [38].
Table 1.
PCR amplification parameters and sequencing primers used for the identification of the fungal isolate.
| Gene | Sequence (5′-3′) | Annealing Temperature (°C) | Size (bp) |
|---|---|---|---|
| ITS | Forward: ITS1-F (TCCGTAGGTGAACCTGCGG) Reverse: ITS4-R (TCCTCCGCTTATTGATATGC) |
55 °C | 500 bp |
| CaM | Forward: CMD5 (CCGAGTACAAGGARGCCTTC) Reverse: CMD6 (CCGATRGAGGTCATRACGTGG) |
50 °C | 600 bp |
| RPB2 | Forward: RPB2-5F (CCRAARTGATCWCKRTCRTC) Reverse: bRPB2-7.1R (CCCATRGCYTGYTTMCCCATDGC) |
52.5 °C | 1000 bb |
| BenA | Forward: T1 (AACATGCGTGAGATTGTAAG) Reverse: T22 (TCTGGATGTTGTTGGGAATCC) |
55.4 °C | 1350 bp |
2.7. Molecular Characterization and Phylogeny
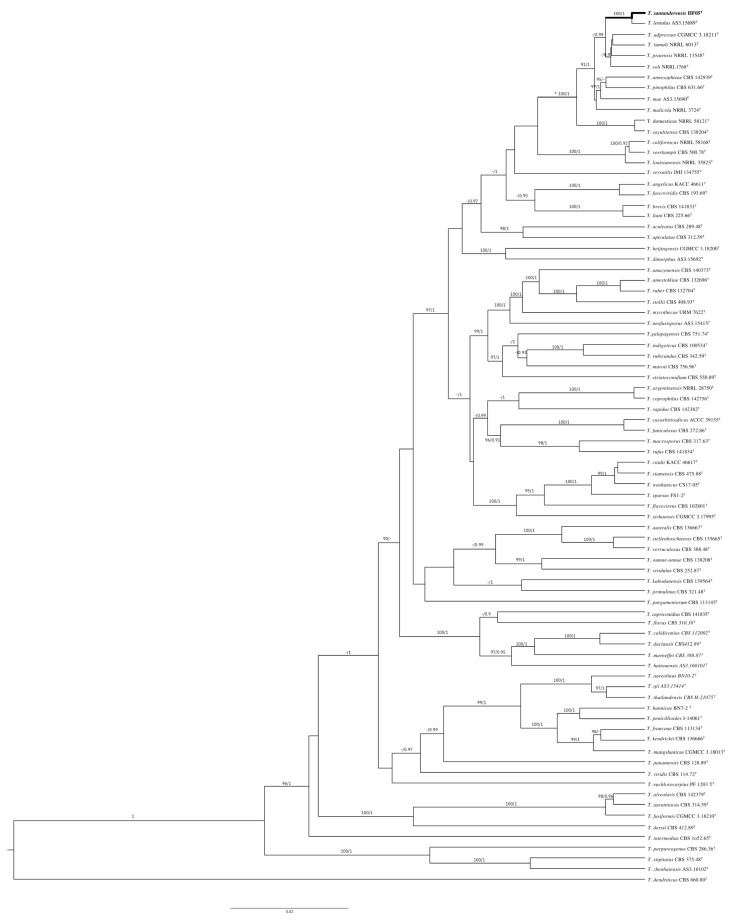
For the phylogenetic reconstruction, a four-gene phylogeny from 80 species described for Talaromyces section Talaromyces was downloaded from NCBI. Strain codes and database accession numbers are indicated in Supplemental Table S1. Phylogenetic analyses were performed with BenA, CaM, RPB2, and ITS sequences individually and concatenated, with T. dendriticus CBS 660.80 of sect. Purpurei as the outgroup.
Sequences were aligned with MAFFT v 7.453 [39]. For evolution model determination and Maximum Likelihood (ML) analysis, IQTREE [40] was implemented with UltraFast Bootstrap. Bayesian Inference (BI) analysis was performed with BEAST2 with 10.000.000 generations; molecular clock was set as default; consensus tree was calculated with Treeannotator [41]. Substitution models and rates among sites were set as TPM2 + I + G4 for BenA, TIM3e + I + G4 for CaM, TPM2 + I + G4 for RPB2, and TIM2 + F + R3 for ITS for ML and BI analyses. Trees were visualized in Figtree.
3. Results
3.1. Description of New Taxa
Talaromyces santanderensis sp. nov. Guerra-Sierra B. and Arteaga-Figueroa LA. (Figure 1, Figure 2 and Figure 3) MycoBank: MB845323 Etymology: The specific epithet refers to the Santander department in Colombia from where the type of strain was collected. Typification: Colombia, Santander, San Vicente de Chucurí, Finca Monserrate, from rhizosphere soil from Theobroma cacao, 15 February 2019, Guerra-Sierra B., HF05 (holotype strain CBUDES:UDES:3068). GenBank accessions: BenA = OP067657, CaM = OP067656, ITS = OP082331, RPB2 = OP067655. In: Talaromyces sect. Talaromyces. Colony diameter (7 days at 25 °C in mm): CYA 22–24; CYAS 20–22; MEA 34–36; OA 30–32; YES 29–30; PDA 28–35 mm; CREA 5–7. Temperature dependent growth (in mm): CYA 37 °C 26–27; MEA 37 °C 35–38; YES 37 °C 30–32; CYA 10 °C 5–9. Colony characteristics (texture): CYA: floccose at the margin, powdery at center, YES: floccose, MEA and OA: floccose to powdery. Further details about coloring are available in Section 3.3. Micromorphology: Conidiophores mono-to-biverticillate penicilli with acerose-shaped phialides and short blunt necks. Notes: T. santanderensis is distinguished from related species by its growth rate on CYAS and powdery textures on MEA, YES and OA and high acid production on CREA. It is differentiated from T. lentulus by its growth rate on CYA medium at 37 °C without exudate production, its cream (R. PI. XVI) margin on MEA and dense sporulation on YES and CYA.
Figure 1.
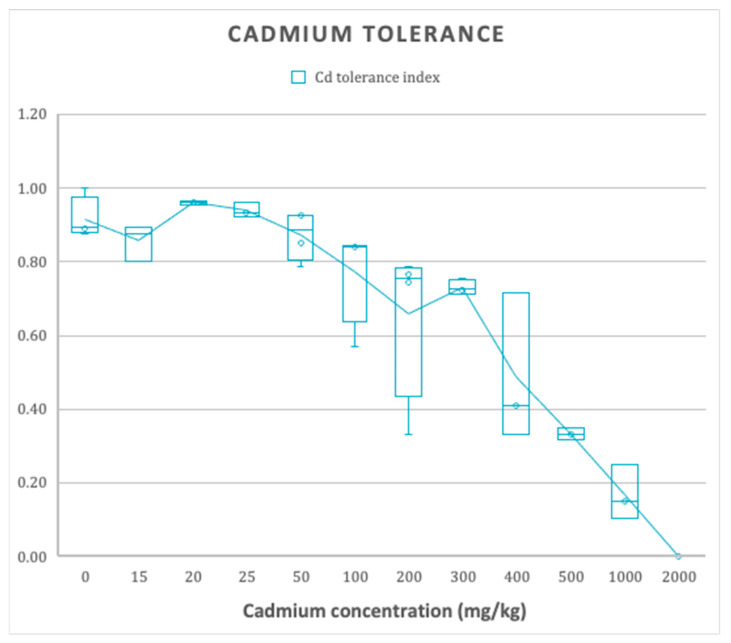
The effect of different concentrations of Cadmium on mycelial radial growth (Cd tolerance index of 3 replicates ± SE).
Figure 2.
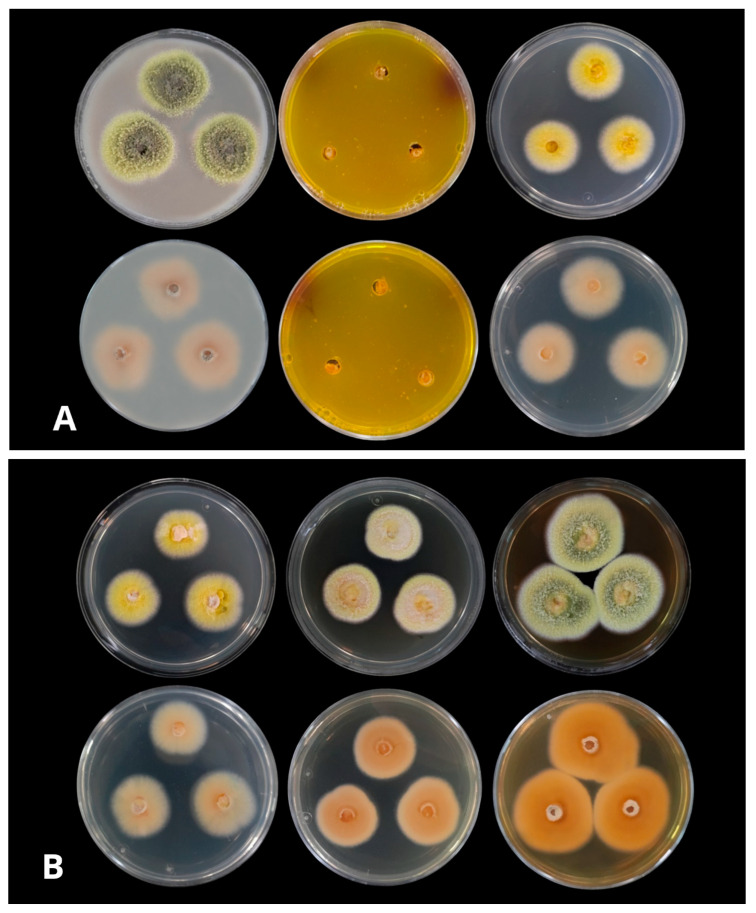
(A): Colonies from left to right (top row) OA, CREA, CYA supplemented with 5% NaCl (CYAS), (bottom row) OA reverse, CREA reverse, and CYA supplemented with 5% NaCl (CYAS) reverse (B): Colonies from left to right (top row) CYA, YES, Malta (bottom row) CYA reverse, YES reverse, and Malta reverse at 25 °C after 1-week incubation.
Figure 3.
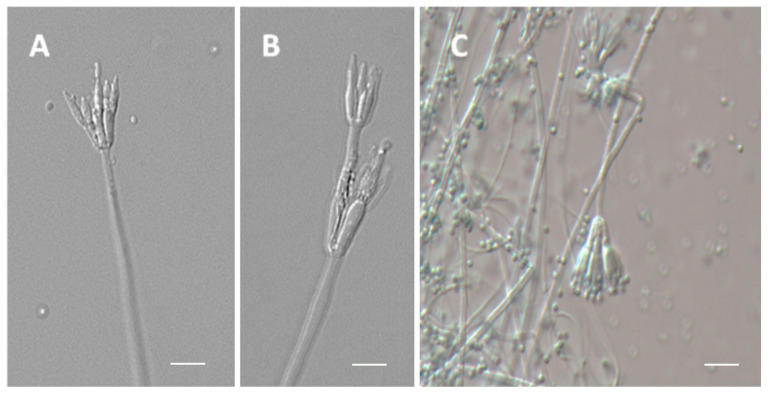
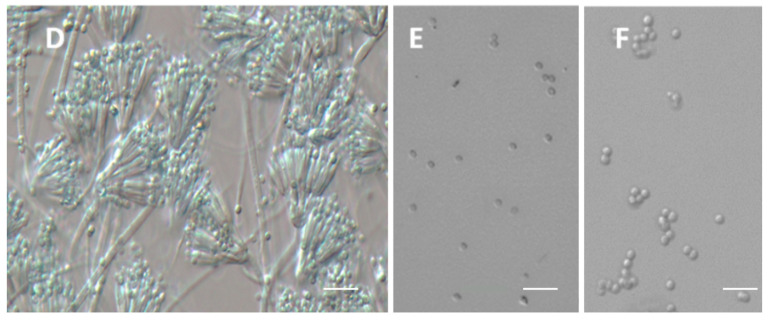
(A–D) Monoverticillate and Biverticillate Conidiophores. (E,F) Conidia. Scale bars = 10 μm.
3.2. Cadmium Tolerance Test
The presence of filamentous fungi in contaminated sites would indicate their adaptation to rough soil conditions and the development of specific mechanisms for such resistance, as shown by the new species of Talaromyces isolated in this study (Figure 1). Talaromyces santanderensis demonstrates a high tolerance rating between 100–400 mg kg−1 cadmium with IC50 = 354.72 mg kg−1, calculated by sigmoid function [42].
3.3. Growth in Different Culture Media
The results of the radial growth diameters and the macro morphological characteristics are presented in Figure 2 and Table 2.
Table 2.
The morphological comparisons of new Talaromyces species and their closely related species.
| Species | CYA mm |
MEA mm |
YES mm |
Conidiophore | Conidia Shape |
Conidia Wall |
Conidia Size-μm |
Source |
|---|---|---|---|---|---|---|---|---|
| T. santanderensis | 22–24 | 34–36 | 26–30 | Mono-to biverticillate |
Slightly globose | Smooth | 1.8–2.2 | Cocoa soil, Santander, Colombia |
| T. pinophilus a | 16–31 | 37–45 | 12–35 | Biverticillate, occasionally monoverticillate | Subglobose to ellipsoidal | Smooth to finely roughened |
2.5–3.5 (−9) ×2.5–3.0 (−5) |
PVC, France |
| T. adpressus b | 32–33 | 42–43 | 42–43 | Biverticillate | Subglobose to ellipsoidal | Smooth | 2.5–4.5 (−4.5) × 2–3.5 |
Indoor environments in Beijing China |
| T. lentulus c | 26–27 | 43–44 | 37–38 | Biverticillate | Globose | Smooth | 2.5–3.0 | Alkaline soil, Yingkou, Shandong, China |
| T. pratensis d | 20–22 | 34–36 | NI | Biverticillate, occasionally monoverticillate | Globose to subglobose, occasionally broadly ellipsoidal | Smooth to finely roughened walls | 2.5–3.0 (−7)× 2.5–3.5 (−4.5) |
Effluent of water treatment plant Cincinnati, W. B. |
| T. soli d | 20–26 | 37–42 | Biverticillate rarely monoverticillate | Subglobose to broadly ellipsoidal | Thick, to finely roughened | 2.5–3.5 (−5.5) × 2.5–3.5 (−4.5) |
Isolated from soil. | |
| T. tumuli d | 19–27 | 36–42 | Biverticillate rarely monoverticillate | Subglobose to broadly ellipsoidal |
Smooth to finely roughened walls | 2.5–3.5 (−7) 2.5–3.5 (−4.5) | Isolated from soil from the big bluestem prairie |
The morphological characteristics of the colonies at 25 °C, 35 °C, and 37 °C were very similar in terms of color and texture in the different culture media tested, observing a change in the growth rate at 10 °C, where a slower growth was observed, and the size of the colonies reached 5–7 mm in diameter, after seven days. Growth rates were better at 25 °C and 35 °C in all culture media except CREA agar, where the mycelium did not develop.
The isolate had a flat-low surface with filamentous colonies and grew moderately on all culture media. The isolate had a low growth rate on CREA medium, where the mycelium did not develop and produced a high acidification (change of medium colour from purple to yellow) at 25 °C, 35 °C, and 37 °C. Colonies had the best growth rates on Malta agar (34–36 mm in diameter) and Oat agar, (30–32 mm in diameter) after seven days at 25 °C. The obverse side on both media was Greenish yellow (R. PI. V), with a wide cream margin (R. PI. XVI) between 6–9 mm. In contrast, the reverse side of the colony was Salmon (R. PI. XIV) on OA and Cinnamon–Rufous (R. PI. XIV) on malt agar. Sporulation was dense and conidia were numerous. Colony texture was floccose to powdery on both media. On YES, they reached a diameter of 29–30 mm after seven days at 25 °C. The colony front was cream-coloured (R. PI. XVI), which became pale orange-yellow with time (R. PI. III), and the back was Rufous-tan (R. PI. XIV). The texture of the colony was floccose. Sporulation was sparse to moderately dense, and conidia were numerous. In CYA supplemented with 5% NaCl (CYAS) and CYA, mycelial growth was slow. Average growth was 22–24 mm in CYA; 26–27 mm in CYA at 37 °C, and 20–22 mm in CYAS, colonies were lemon yellow (R. PI. IV), and the reverse was salmon (R. PI. XIV) (Figure 2A,B). The texture of the colonies was floccose at the margin and compact-powdery at center, sporulation was dense, and conidia were numerous (Figure 2A,B). The isolate showed growth at 25 °C, 35 °C and 37 °C. Soluble pigments were absent in all cultures. Clear exudates were present on Oat and Malt agar.
The growth size (mm) and other morphological aspects of the new Talaromyces species are described and compared with five phylogenetically related Talaromyces species (Table 2).
3.4. Micromorphology Analysis
The isolates showed Conidiophores arising from surface hyphae with smooth-walled stipes (250–330 × 2.0–2.5 μm) (Figure 3A,B). Conidiophores had mono-to-biverticillate penicilli with acerose-shaped phialides and short blunt necks (Figure 3B–D). Conidia are smooth-walled and slightly globose (1.8–2.2 μm in diameter). Loose conidia or chains of conidia were irregularly observed in masses of 6 to 8 conidia (Figure 3E,F). 3–5 Metulae per stipe (9–10 × 2.5–2.8 μm) were observed; phialides (2–4 per metula) were acerose with short collula (7–9 × 1.5–2.0 μm). The stipes were smooth-walled.
The conidia were olive green (R. PI. IV) on OA and MEA medium and pale greenish yellow (R. PI. V) on CYA, CYAS, and YES medium. For ascoma production, OA, MEA and CYA plates were incubated for up to four weeks. No structures of sexual reproduction were found in any of the observed samples for the microscopic analysis.
3.5. Molecular Identification and Sequence Analysis
Sequences were reported to Genbank under the code accesses: OP082331, ITS; OP067657, BenA; OP067656, CaM and OP067655, RPB2. PCR amplification generated amplicons of BenA about 1447 bp, CaM about 449 bp, RPB2 about 1033 bp and ITS about 569 bp. The trimmed alignments of BenA, CaM, RPB2, ITS and the combined BenA-CaM- RPB2-ITS sequences were 520, 639, 1008, 692 and 2859 characters with gaps, respectively.
3.6. Phylogenetic Analysis
The phylogenetic trees generated by the concatenated BenA-CaM-RPB2-ITS and individual loci show the isolate as a distinct species of sect. Talaromyces. The specific epithet refers to the region of type locality
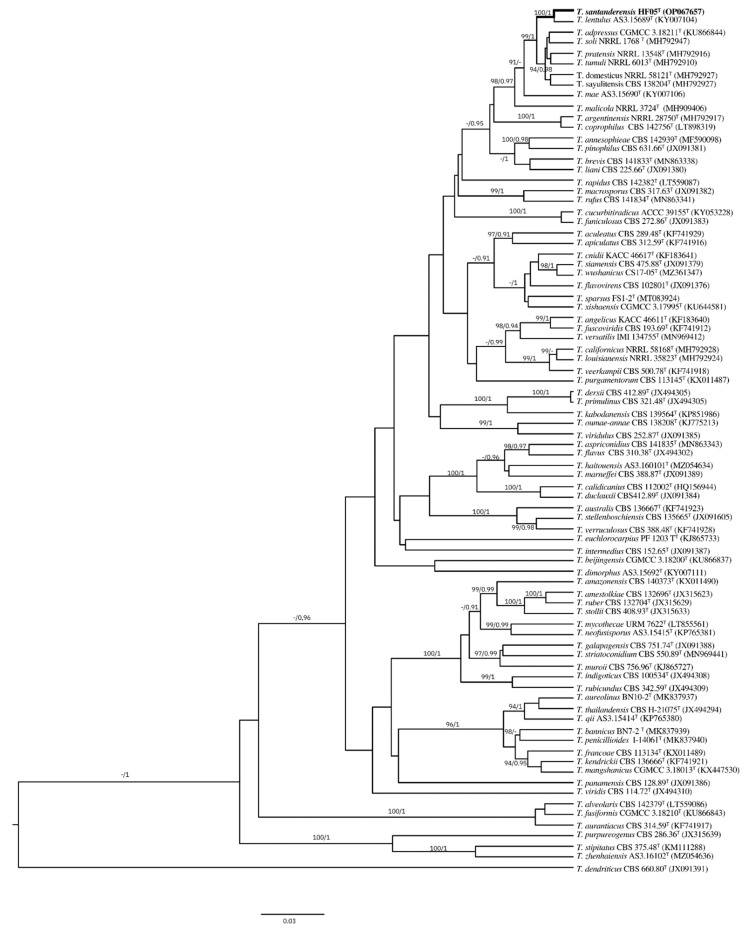
T. santanderensis sp. nov. forms a clade with T. lentulus [46] with 100%/1 bootstrap/pp support in the concatenated BenA-CaM- RPB2-ITS (Figure 4) and individual phylogenies of BenA (Figure 5) and CaM. RPB2 phylogeny supports this clade with 99%/1 bootstrap/pp support. Nevertheless, T. lentulus forms a separate lineage in ITS phylogeny. This clade is located inside the Talaromyces pinophilus complex, which includes T. soli, T. tumuli, T. adpressus, T. pinophilus, T. pratensis, T. domesticus, T. sayulitensis, T. malicola, T. annesophieae, and T. mae [30]. In the concatenated analysis, other clades supported inside the T. pinophilus complex were T. domesticus and T. sayulitensis with 100/1 bootstrap/pp support and T. mae with T. pinophilus and T. annesophieae with 100/1 bootstrap/pp support. In the individual BenA phylogeny, T. domesticus and T. sayulitensis formed a clade with 94/0.98 bootstrap/pp support and T. pinophilus with T. annesophieae with 100/0.98 bootstrap/pp support.
Figure 4.
Topologies reconstructed with ITS, CaM, BenA and RPB2, bootstrap and posterior probability included at the nodes. BI phylogram inferred from the concatenated BenA-CaM-RPB2 sequences. Ultrafast-bootstrap percentages over 90% derived from 10,000 replicates and posterior probabilities over 0.9 of posterior support are indicated at the nodes. T indicates ex-type strains, strains belonging to new species are indicated in boldface. Scale Bar: number of substitutions per nucleotide position.
Figure 5.
Individual phylogeny of BenA. BI phylogram inferred from partial BenA sequences. Ultrafast-bootstrap percentages over 90% derived from 10,000 replicates and posterior probabilities over 0.9 of posterior support are indicated at the nodes. T indicates ex-type strains, strains belonging to new species are indicated in boldface. Scale Bars: number of substitutions per nucleotide position.
4. Discussion
The morphological distinction of the new Talaromyces species proposed in this section (T. santanderensis) can be differentiated from its related species by the distinctive color of the mycelium, the growth rate in different culture media, and the high acid production on CREA, as well as the shape and size of the conidia.
T. santanderensis grows slightly slower than related species on CYA medium (Table 2), displaying a bright Lemon yellow mycelium (R. Pl. IV). Moreover, T. lentulus produces a mycelium close to Pale salmon (R. Pl. XIV), slightly mixed with Naphthalene yellow (R. Pl. XVI). Similarly, T. adpressus, T. pinophilus, T. pratensis, T. soli, and T. tumuli produce white to light yellow mycelium. Meanwhile, reverse coloration displays a higher interspecific variation with: T. santanderensis reverse, salmon (R. PI. XIV); T. lentulus reverse Cinnamon (R. Pl. XXIX), similar to T. adpressus reverse, yellowish brown. T. pratensis, T. soli and T. tumuli reverse is featured by a honey yellow color. Instead, T. pinophilus, features a reverse from yellow ocher with occasional deep red shades to grayish orange to orange. On CYA, T. pinophilus T. pratensis, T. tumuli and T. soli display a funiculose to floccose texture, in contrast, T. lentulus shows a velutinous with overlaid mycelium texture. Distinctively, T. santanderensis features a colony compact-powdery at center and floccose at margin.
Furthermore, T. adpressus, T. lentulus, T. pinophilus, T. soli and T. tumuli produce clear to light yellow exudates. In contrast, T. santanderensis and T. pratensis do not produce exudates on CYA medium. Additionally, T. santanderensis displays dense conidiogenesis and sporulation in this medium, similar to T. tumuli which features moderate to abundant sporulation. Nevertheless, other related species display poor to moderate sporulation.
T. santanderensis (26–27 mm) grows faster than T. lentulus (18–21 mm) on CYA medium at 37 °C. However, this growth rate is comparable with other related species. Additionally, T. santanderensis does not produce exudates in this condition, which T. lentulus does. Furthermore, T. santanderensis grows more in CYAS (20–22 mm) than related species, which display a growth rate between 0–7 mm.
T. santaderensis has a growth rate similar to T. pratensis on MEA, showing a limited growth rate compared to other related species (Table 2). On MEA, T. santanderensis features a greenish yellow (R. PI. V) with a wide cream (R. PI. XVI) margin between 6–9 mm, similarly colored to T. lentulus that displays a near Grayish Olive to Light Grayish Olive (R. Pl. XLVI). Furthermore, T. adpressus, T. pinophilus, T. pratensis, T. tumuli and T. soli display white to light yellow or red mycelia on this medium. On the reverse side, T. santanderensis features a Cinnamon-Rufous (R. P.I. XIV) color, in contrast with T. lentulus that shows a Baryta Yellow (R. Pl. IV) reverse, similar to those of T. adpressus. Nevertheless, T. pinophilus, T. pratensis, T. tumuli, and T. soli feature similar coloration to T. santanderensis. In addition, T. adpressus, T. soli, T. tumuli feature funiculose to floccose texture on MEA, in contrast with T. pinophilus and T. pratensis that show a floccose to funiculose texture. T. santanderensis features on MEA a floccose to powdery texture, in contrast with T. lentulus that displays a velutinous with sparse floccose mycelium overlaid. Clear exudates are produced by T. santanderensis on this medium, similar to those T. adpressus, T. soli and T. tumuli that feature exudates from clear to yellow. T. pinophilus produces exudates from clear to orange-yellow to red on this medium. In contrast, T. lentulus and T. pratensis do not produce exudates on MEA. Moreover, T. santanderensis shows abundant sporulation, similar to T. pratensis and other related species.
On YES, T. santanderensis forms smaller colonies than T. adpressus and T. lentulus (Table 2). T. santanderensis develops Cream mycelium (R. PI. XVI) that turns Pale orange-yellow (R. PI. III) over time, similar to some strains of T. pinophilus, that feature white, yellow and sometimes red mycelium on YES. Similarly, T. adpressus displays white mycelium with occasional red on this medium. Moreover, T. lentulus features a Pale Pinkish Buff (R. Pl. XXIX)* at the colony center and a Citron Yellow (R. Pl. XVI) margin. Furthermore, T. santanderensis displays a reverse Cinnamon-rufous (R. PI. XIV), lighter than T. lentulus reverse Mahogany Red to Burnt Sienna (R. Pl. II). In addition, T. adpressus produces a reverse brown at center and pale brown at edge, similar to T. pinophilus reverse, which ranges from dark brown to golden brownish orange at center and fades into yellow or yellowish orange. T. santaderensis colony texture was floccose, and sporulation was sparse to moderately dense, similar to T. adpressus and T. pinophilus. In contrast, T. lentulus features a colony floccose with short loose funicles at center and velutinous at edge and sparse sporulation.
T. santanderensis displays a medium growth rate on OA (30–32 mm). On this medium, T. adpressus features white mycelium, T. pinophilus and T. tumuli show white to yellow mycelia and T. pratensis and T. soli display yellow mycelia. Distinctively, T. santanderensis features a Greenish yellow (R. PI. V), with a wide cream margin (R. PI. XVI) mycelium on OA similar to its coloration on MEA darker at center. On the reverse side, T. santanderensis displays Salmon (R. PI. XIV) coloration, in contrast with T. adpressus pale buff or T. pinophilus uncolored reverse with occasional English red colored strains. Similar to growth in MEA, T. santanderensis displayed a floccose to powdery texture. In comparison, T. pinophilus, T. pratensis, T. soli and T. tumuli feature a funicolose to floccose texture and T. adpressus a floccose texture. On OA, T. santanderensis produces clear exudates and dense sporulation similar to related species. In addition, T. lentulus characters data on OA is not yet available.
T. santanderensis produced a strong acidification in CREA with a limited growth rate, unlike related species. T. pinophilus does not produce strong acidic compounds on CREA medium, only weak acids in some cultures and T. adpressus does not produce acidic compounds on CREA. While T. pratensis, T. soli and T. tumuli produce moderate acidic compounds. Furthermore, related species display a growth greater than 15 mm, instead T. santanderensis, presented a growth between 5–7 mm. Also, the growth of T. lentulus in CREA has not yet been described.
Furthemore, T. santanderensis produces smaller conidia than related species (Table 2) and features mono-to-biverticillade conidiophores. In contrast, related species such as T. pinophilus, T. pratensis, T. soli and T. tumuli produce monoverticillade conidiophores occasionally.
To evaluate the ascoma production on T. santanderensis, OA, MEA, and CYA plates were incubated for up to four weeks. No structures of sexual reproduction were found in any of the observed samples for the microscopic analysis. Nevertheless, other studied species of Talaromyces have shown that they need a longer incubation time for ascospore production (from 6 to 20 weeks) [26]. Sexual reproduction structures are difficult to observe in some species of fungi under laboratory conditions, for which these species have been considered “asexual” or anamorphic, representing a challenge for their study. Some species of ascomycete fungi known as “asexual” can reproduce sexually, induced under laboratory-controlled conditions [47,48]. Therefore, more studies will be required to elucidate if the new species described in this work could develop a teleomorphic state.
It is suggested that T. santanderensis is a new species inside the T. pinophilus complex using a polyphasic approach, including different phylogenetic analyses of combined and individual ITS, CaM, BenA, and RPB2 gene sequences. Nevertheless, relationships between members of the T. pinophilus complex remain disputed. Further barcoding or sampling efforts are needed. T. santanderensis is the fifth species from the Talaromyces sect. Talaromyces described from type material from Colombia, after T. amazonensis, T. francoae, T. purgamentorum, and T. veerkampii [29,49]. Recently, the number of Talaromyces species has increased [49], but further studies are needed to understand the entire biodiversity of the group in Colombia.
Furthermore, a recent study by Bravo et al., 2021 [11] shows the distribution of Cd in soils of cocoa crops in different districts of Colombia, in which Santander had the broadest range of Cd concentrations (0.01–27 mg kg−1) of all the districts analyzed, supporting Cd concentrations found in the studied soil sample (Table S1). T. santanderensis displays a high tolerance index of 0.81 to cadmium in vitro tests (Figure 1) and high IC50 of 354.72 mg kg−1, untrained strains of T. helicus tolerate up to 300 mg kg−1 [24]. It has been reported that this tolerance to metals by filamentous fungi is directly correlated to their isolation soil, the toxicity of the tested metal, its medium concentration, and the competence of the isolate [50]. Additionally, T. santanderensis is featured by high acid production on CREA. Acid synthesis, excretion and binding with cations of cadmium might lead to its immobilization in the crystalline phase of biogenic minerals [51].
This isolate is already adapted to the specific conditions of the soil. This ability to accumulate heavy metals by species of fungi is based on several mechanisms of adaptive genetic and physiological constituents that have been widely described [52,53,54]. Thus, the tolerance of T. santanderensis to cadmium presents a promising opportunity for bioremediation strategies focused on eliminating the stress caused by this heavy metal in the agroecosystems of T. cacao, reducing the bioavailability of the heavy metal and offering a competitive relationship with its niche and microbial community. More studies are needed to understand its role in the rhizospheric community of T. cacao and characterize its potential for bioremediation processes.
Acknowledgments
This study acknowledges the Mycology/Microscopy section from the Agro-environmental Biotechnology Laboratory of the University of Santander and EAFIT University. The authors gratefully acknowledge the supercomputing resources made available by the Apollo Scientific Computing Center of EAFIT University (http://www.eafit.edu.co/apolo, accessed on 28 July 2022) to carry out the research reported in this scientific product and also thank the Federation of Cocoa Growers of Colombia (FEDECACAO) for assistance in obtaining the soil sample.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jof8101042/s1. Table S1: Physical-chemical analysis of the cocoa soil. Table S2: Genbank codes of the strains used for the phylogenetic analysis. Figure S1: BI phylogram inferred from partial CaM sequences. Nodes indicate ultrafast-bootstrap percentages greater than 90% derived from 10,000 replicates and posterior probabilities greater than 0.9 posterior support. T indicates ex-type strains, and strains belonging to new species are indicated in bold. Scale bars: number of substitutions per nucleotide position. Figure S2. BI phylogram inferred from partial RPB2 sequences. Nodes indicate ultrafast-bootstrap percentages greater than 90% derived from 10,000 replicates and posterior probabilities greater than 0.9 posterior support. T indicates ex-type strains, and strains belonging to new species are indicated in bold. Scale bars: number of substitutions per nucleotide position. Figure S3. BI phylogram inferred from partial ITS sequences. Nodes indicate ultrafast-bootstrap percentages greater than 90% derived from 10,000 replicates and posterior probabilities greater than 0.9 posterior support. T indicates ex-type strains, and strains belonging to new species are indicated in bold. Scale bars: number of substitutions per nucleotide position.
Author Contributions
Conceptualization, J.C.A. and B.E.G.S.; methodology, L.A.A.-F., S.S.-P. and B.E.G.S.; formal analysis, J.C.A., B.E.G.S., L.A.A.-F. and S.S.-P.; writing-original draft preparation, B.E.G.S., L.A.A.-F. and J.C.A. writing-review and editing, L.A.A.-F. and S.S.-P.; supervision, J.C.A.; funding acquisition, J.C.A. and B.E.G.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The sequences newly generated in this study can be found in the NCBI database under the codes: OP082331, ITS; OP067657, BenA; OP067656, CaM and OP067655, RPB2.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Legal Considerations
This study was carried out following the collection permit granted by Resolution No. 01749 of 29 December 2017, to the University of Santander (UDES) with NIT number 804.001.890-1, which contributed to the procurement of a composite soil sample of approximately 10 kg, from the Monserrate Farm-municipality of San Vicente de Chucurí. The corresponding credits will be given to the National Federation of Cocoa Growers for the support provided. The strain of T. santanderensis was deposited in the Biological Collection of the Universidad de Santander-Colombia, under the following identification: Biological Record ID: CBUDES: UDES:3068, Record number: CNAT_2LIB_HThCd_02_03, Catalog number: CBUDES-CN-HThCd02. The number of the biological collection registered on the National Single Registry of Biological Collections with Alexander von Humboldt Biological Resources Research Institute- Colombia is RNC: 128. Additionally, the guidelines of the framework contract for access to genetic resources and their derived products No. 127, signed between the Ministry of Environment and Sustainable Development and EAFIT University, were followed. Also, Article 6 of Law 1955 of 2019, the National Development Plan 2018–2022 “Pact for Colombia, Pact for Equity” is issued.
Funding Statement
This study was supported financially and scientifically by the University of Santander and EAFIT University. The contributions to this research project were aimed at institutional strengthening with external co-financing No. 069-18.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Riaz M., Kamran M., Fang Y., Wang Q., Cao H., Yang G., Deng L., Wang Y., Zhou Y., Anastopoulos I., et al. Arbuscular mycorrhizal fungi-induced mitigation of heavy metal phytotoxicity in metal contaminated soils: A critical review. J. Hazard. Mater. 2021;402:123919. doi: 10.1016/j.jhazmat.2020.123919. [DOI] [PubMed] [Google Scholar]
- 2.Bertoldi D., Barbero A., Camin F., Caligiani A., Larcher R. Multielemental fingerprinting and geographic traceability of Theobroma cacao beans and cocoa products. Food Control. 2016;65:46–53. doi: 10.1016/j.foodcont.2016.01.013. [DOI] [Google Scholar]
- 3.Possú W.B., Navia J.F., Solarte J.G. Socio-economic characterization of the traditional cacao agroforestry system (Theobroma cacao L.) Rev. Cienc. Agríc. 2021;38:17–35. doi: 10.22267/rcia.213802.156. [DOI] [Google Scholar]
- 4.Ramtahal G., Yen I.C., Ahmad N., Bekele I., Bekele F., Maharaj K., Wilson L., Harrynanan L. Prediction of Soil Cadmium Bioavailability to Cacao (Theobroma cacao L.) using Single-Step Extraction Procedures. Commun. Soil Sci. Plant Anal. 2015;46:2585–2594. doi: 10.1080/00103624.2015.1089262. [DOI] [Google Scholar]
- 5.Das S.C., Al-Naemi H.A. Cadmium Toxicity: Oxidative Stress, Inflammation and Tissue Injury. Occup. Dis. Environ. Med. 2019;7:144–163. doi: 10.4236/odem.2019.74012. [DOI] [Google Scholar]
- 6.Ramtahal G., Yen I.C., Hamid A., Bekele I., Bekele F., Maharaj K., Harrynanan L. The Effect of Liming on the Availability of Cadmium in Soils and Its Uptake in Cacao (Theobroma cacao L.) Trinidad & Tobago. Commun. Soil Sci. Plant Anal. 2018;49:2456–2464. doi: 10.1080/00103624.2018.1510955. [DOI] [Google Scholar]
- 7.Kabata-Pendias A. Trace Elements in Soils and Plants. 3rd ed. CRC Press; Boca Raton, FL, USA: 2000. [Google Scholar]
- 8.Echeverry A., Reyes H. Determinación de La Concentración de Cadmio En Un Chocolate Colombiano Con 65% de Cacao y Chocolates Extranjeros Con Diferentes Porcentajes de Cacao. Entre Cienc. Ing. 2016;10:22–32. [Google Scholar]
- 9.European Union Commission Regulation (EU) No 488/2014 of 12 May 2014 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Cadmium in Foodstuffs. [(accessed on 30 July 2022)];J. Eur. Union. 2014 Available online: https://eur–lex.europa.eu/legal–content/EN/TXT/?uri=celex:32014R0488. [Google Scholar]
- 10.Colombian Ministry of Agriculture and Rural Development CADENA DE CACAO. Dirección de Cadenas Agrícolas y Forestales. [(accessed on 28 March 2022)];2019 Available online: https://sioc.minagricultura.gov.co/Cacao/Documentos/2019–06–30%20Cifras%20Sectoriales.pdf.
- 11.Bravo D., Leon-Moreno C., Martínez C., Varón-Ramírez V., Araujo-Carrillo G., Vargas R., Quiroga-Mateus R., Zamora A., Rodríguez E. The First National Survey of Cadmium in Cacao Farm Soil in Colombia. Agronomy. 2021;11:761. doi: 10.3390/agronomy11040761. [DOI] [Google Scholar]
- 12.Bandurska K., Krupa P., Berdowska A., Jatulewicz I., Zawierucha I. Mycoremediation of Soil Contaminated with Cadmium and Lead by Trichoderma sp. Ecol. Chem. Eng. 2021;28:277–286. doi: 10.2478/eces-2021-0020. [DOI] [Google Scholar]
- 13.Văcar C., Covaci E., Chakraborty S., Li B., Weindorf D., Frențiu T., Pârvu M., Podar D. Heavy Metal-Resistant Filamentous Fungi as Potential Mercury Bioremediators. J. Fungi. 2021;7:386. doi: 10.3390/jof7050386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maini Z.A.N., Aribal K.M.J., Narag R.M.A., Melad J.K.L.T., Frejas J.A.D., Arriola L.A.M., Gulpeo P.C.R., Navarrete I.A., Lopez C.M. Lead (II) Tolerance and Uptake Capacities of Fungi Isolated from a Polluted Tributary in the Philippines. Appl. Environ. Biotechnol. 2019;4:18–29. doi: 10.26789/AEB.2019.01.004. [DOI] [Google Scholar]
- 15.Oladipo O.G., Awotoye O.O., Olayinka A., Bezuidenhout C.C., Maboeta M.S. Heavy metal tolerance traits of filamentous fungi isolated from gold and gemstone mining sites. Braz. J. Microbiol. 2018;49:29–37. doi: 10.1016/j.bjm.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohammadian E., Ahari A.B., Arzanlou M., Oustan S., Khazaei S.H. Tolerance to heavy metals in filamentous fungi isolated from contaminated mining soils in the Zanjan Province, Iran. Chemosphere. 2017;185:290–296. doi: 10.1016/j.chemosphere.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 17.Fazli M.M., Soleimani N., Mehrasbi M., Darabian S., Mohammadi J., Ramazani A. Highly cadmium tolerant fungi: Their tolerance and removal potential. J. Environ. Health Sci. Eng. 2015;13:19. doi: 10.1186/s40201-015-0176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ezzouhri L., Castro E., Moya M., Espinola F., Lairini K. Heavy Metal Tolerance of Filamentous Fungi Isolated from Polluted Sites in Tangier, Morocco. Afr. J. Microbiol. Res. 2009;3:35–48. [Google Scholar]
- 19.El-Shafey N.M., Marzouk M.A., Yasser M.M., Shaban S.A., Beemster G.T., AbdElgawad H. Harnessing Endophytic Fungi for Enhancing Growth, Tolerance and Quality of Rose-Scented Geranium (Pelargonium graveolens (L’Hér) Thunb.) Plants under Cadmium Stress: A Biochemical Study. J. Fungi. 2021;7:1039. doi: 10.3390/jof7121039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Shahir A.A., El-Tayeh N.A., Ali O.M., Latef A.A.H.A., Loutfy N. The Effect of Endophytic Talaromyces pinophilus on Growth, Absorption and Accumulation of Heavy Metals of Triticum aestivum Grown on Sandy Soil Amended by Sewage Sludge. Plants. 2021;10:2659. doi: 10.3390/plants10122659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bengtsson L., Johansson B., Hackett T.J., McHale L., McHale A.P. Studies on the biosorption of uranium by Talaromyces emersonii CBS 814.70 biomass. Appl. Microbiol. Biotechnol. 1995;42:807–811. doi: 10.1007/BF00171965. [DOI] [PubMed] [Google Scholar]
- 22.Coelho E., Reis T.A., Cotrim M., Mullan T.K., Renshaw J., Rizzutto M., Corrêa B. Talaromyces amestolkiae uses organic phosphate sources for the treatment of uranium-contaminated water. BioMetals. 2022;35:335–348. doi: 10.1007/s10534-022-00374-9. [DOI] [PubMed] [Google Scholar]
- 23.Massaccesi G., Romero M.C., Cazau M.C., Bucsinszky A.M. Cadmium removal capacities of filamentous soil fungi isolated from industrially polluted sediments, in La Plata (Argentina) World J. Microbiol. Biotechnol. 2002;18:817–820. doi: 10.1023/A:1021282718440. [DOI] [Google Scholar]
- 24.Romero M.C., Reinoso E.H., Urrutia M.I., Kiernan A.M. Biosorption of heavy metals by Talaromyces helicus: A trained fungus for copper and biphenyl detoxification. Electron. J. Biotechnol. 2006;9:3. doi: 10.2225/vol9-issue3-fulltext-11. [DOI] [Google Scholar]
- 25.Sharma P., Singh S.P. Fungi Bio–Prospects in Sustainable Agriculture, Environment and Nano–technology. Elsevier; Amsterdam, The Netherlands: 2021. Role of the Endogenous Fungal Metabolites in the Plant Growth Improvement and Stress Tolerance; pp. 381–401. [DOI] [Google Scholar]
- 26.Yilmaz N., Visagie C., Houbraken J., Frisvad J., Samson R. Polyphasic taxonomy of the genus Talaromyces. Stud. Mycol. 2014;78:175–341. doi: 10.1016/j.simyco.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun B.-D., Chen A.J., Houbraken J., Frisvad J., Wu W.-P., Wei H.-L., Zhou Y.-G., Jiang X.-Z., Samson R.A. New section and species in Talaromyces. MycoKeys. 2020;68:75–113. doi: 10.3897/mycokeys.68.52092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crous P.W., Gams W., Stalpers J.A., Robert V., Stegehuis G. MycoBank: An Online Initiative to Launch Mycology into the 21st Century. Stud. Mycol. 2004;50:19–22. [Google Scholar]
- 29.Wang X.-C., Zhuang W.-Y. New Species of Talaromyces (Trichocomaceae, Eurotiales) from Southwestern China. J. Fungi. 2022;8:647. doi: 10.3390/jof8070647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peterson S.W., Jurjević Ž. The Talaromyces pinophilus species complex. Fungal Biol. 2019;123:745–762. doi: 10.1016/j.funbio.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Samson R., Yilmaz N., Houbraken J., Spierenburg H., Seifert K., Peterson S., Varga J., Frisvad J. Phylogeny and nomenclature of the genus Talaromyces and taxa accommodated in Penicillium subgenus Biverticillium. Stud. Mycol. 2011;70:159–183. doi: 10.3114/sim.2011.70.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Visagie C., Hirooka Y., Tanney J., Whitfield E., Mwange K., Meijer M., Amend A., Seifert K., Samson R. Isolated from house dust samples collected around the world. Stud. Mycol. 2014;78:63–139. doi: 10.1016/j.simyco.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ridgway R. Color Standards and Color Nomenclature. Washington, DC, USA: 1912. [(accessed on 28 March 2022)]. Available online: https://library.si.edu/digital–library/book/colorstandardsc00ridg. [Google Scholar]
- 34.Chi M.-H., Park S.-Y., Lee Y.-H. A Quick and Safe Method for Fungal DNA Extraction. Plant Pathol. J. 2009;25:108–111. doi: 10.5423/PPJ.2009.25.1.108. [DOI] [Google Scholar]
- 35.Liu Y.J., Whelen S., Hall B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 1999;16:1799–1808. doi: 10.1093/oxfordjournals.molbev.a026092. [DOI] [PubMed] [Google Scholar]
- 36.Matheny P.B. Improving phylogenetic inference of mushrooms with RPB1 and RPB2 nucleotide sequences (Inocybe; Agaricales) Mol. Phylogenet. Evol. 2005;35:1–20. doi: 10.1016/j.ympev.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 37.O’Donnell K., Cigelnik E. Two Divergent Intragenomic rDNA ITS2 Types within a Monophyletic Lineage of the Fungus Fusarium Are Nonorthologous. Mol. Phylogenet. Evol. 1997;7:103–116. doi: 10.1006/mpev.1996.0376. [DOI] [PubMed] [Google Scholar]
- 38.Rausch T., Fritz M.H.-Y., Untergasser A., Benes V. Tracy: Basecalling, alignment, assembly and deconvolution of sanger chromatogram trace files. BMC Genom. 2020;21:230. doi: 10.1186/s12864-020-6635-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katoh K., Asimenos G., Toh H. Multiple Alignment of DNA Sequences with MAFFT. In: Posada D., editor. Bioinformatics for DNA Sequence Analysis. Humana Press; Totowa, NJ, USA: 2009. pp. 39–64. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen L.-T., Schmidt H.A., Von Haeseler A., Minh B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2014;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bouckaert R., Heled J., Kühnert D., Vaughan T., Wu C.-H., Xie D., Suchard M.A., Rambaut A., Drummond A.J. BEAST 2: A Software Platform for Bayesian Evolutionary Analysis. PLoS Comput. Biol. 2014;10:e1003537. doi: 10.1371/journal.pcbi.1003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meddings J.B., Scott R.B., Fick G.H. Analysis and comparison of sigmoidal curves: Application to dose-response data. Am. Am. J. Physiol.—Gastrointest. Liver Physiol. 1989;257:G982–G989. doi: 10.1152/ajpgi.1989.257.6.G982. [DOI] [PubMed] [Google Scholar]
- 43.Houbraken J., de Vries R.P., Samson R.A. Modern Taxonomy of Biotechnologically Important Aspergillus and Penicillium Species. Adv. Appl. Microbiol. 2014;86:199–249. doi: 10.1016/b978-0-12-800262-9.00004-4. [DOI] [PubMed] [Google Scholar]
- 44.Fujii T., Hoshino T., Inoue H., Yano S. Taxonomic Revision of the Cellulose–Degrading Fungus Acremonium Cellulolyticus Nomen Nudum to Talaromyces Based on Phylogenetic Analysis. FEMS Microbiol. Lett. 2014;351:32–41. doi: 10.1111/1574-6968.12352. [DOI] [PubMed] [Google Scholar]
- 45.Chen A., Sun B., Houbraken J., Frisvad J., Yilmaz N., Zhou Y., Samson R. New Talaromyces species from indoor environments in China. Stud. Mycol. 2016;84:119–144. doi: 10.1016/j.simyco.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang X.-Z., Yu Z.-D., Ruan Y.-M., Wang L. Three New Species of Talaromyces Sect. Talaromyces Discovered from Soil in China. Sci. Rep. 2018;8:4932. doi: 10.1038/s41598-018-23370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Böhm J., Hoff B., O’Gorman C.M., Wolfers S., Klix V., Binger D., Zadra I., Kürnsteiner H., Pöggeler S., Dyer P.S., et al. Sexual reproduction and mating-type–mediated strain development in the penicillin-producing fungus Penicillium chrysogenum. Proc. Natl. Acad. Sci. USA. 2013;110:1476–1481. doi: 10.1073/pnas.1217943110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ropars J., Dupont J., Fontanillas E., de la Vega R.C.R., Malagnac F., Coton M., Giraud T., López-Villavicencio M. Sex in Cheese: Evidence for Sexuality in the Fungus Penicillium Roqueforti. PLoS ONE. 2012;7:e49665. doi: 10.1371/journal.pone.0049665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Z.-K., Wang X.-C., Zhuang W.-Y., Cheng X.-H., Zhao P. New Species of Talaromyces (Fungi) Isolated from Soil in Southwestern China. Biology. 2021;10:745. doi: 10.3390/biology10080745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruta L., Paraschivescu C., Matache M., Avramescu S., Farcasanu I.C. Removing heavy metals from synthetic effluents using “kamikaze” Saccharomyces cerevisiae cells. Appl. Microbiol. Biotechnol. 2009;85:763–771. doi: 10.1007/s00253-009-2266-3. [DOI] [PubMed] [Google Scholar]
- 51.Urik M. Ph.D. Thesis. Faculty of Chemistry, Brno University of Technology; Brno, Czech Republic: 2018. Interactions of Toxic Metals and Metaloids with Fungi. [Google Scholar]
- 52.Kumar V., Dwivedi S.K. Mycoremediation of heavy metals: Processes, mechanisms, and affecting factors. Environ. Sci. Pollut. Res. 2021;28:10375–10412. doi: 10.1007/s11356-020-11491-8. [DOI] [PubMed] [Google Scholar]
- 53.Khan I., Aftab M., Shakir S., Ali M., Qayyum S., Rehman M.U., Haleem K.S., Touseef I. Mycoremediation of heavy metal (Cd and Cr)–polluted soil through indigenous metallotolerant fungal isolates. Environ. Monit. Assess. 2019;191:585. doi: 10.1007/s10661-019-7769-5. [DOI] [PubMed] [Google Scholar]
- 54.Purohit J., Chattopadhyay A., Biswas M.K., Singh N.K. Fungal Biology. Springer International Publishing; Berlin/Heidelberg, Germany: 2018. Mycoremediation of Agricultural Soil: Bioprospection for Sustainable Development; pp. 91–120. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequences newly generated in this study can be found in the NCBI database under the codes: OP082331, ITS; OP067657, BenA; OP067656, CaM and OP067655, RPB2.