Abstract
The concentration of fecal mucin and the adhesion of specific probiotics and their combinations in the intestinal mucus of infants during and after rotavirus diarrhea and in healthy children were determined. Mucus was prepared from fecal samples from 20 infants during and after rotavirus diarrhea and from 10 healthy age-matched children. Mucin concentration was determined, and the adhesion of five probiotics—Lactobacillus rhamnosus GG, Lactobacillus casei Shirota, Lactobacillus paracasei F19, Lactobacillus acidophilus LA5, and Bifidobacterium lactis Bb12—and their combinations was tested in vitro. The mean concentrations of fecal mucin during and after rotavirus diarrhea, 15.2 and 14.1 mg/g, were comparable to that in healthy children, 14.9 mg/g. The adherence of probiotics ranged from 1 to 34% in healthy subjects as indicated for the following strains: L. rhamnosus GG, 34%; B. lactis Bb12, 31%; L. acidophilus LA5, 4%; L. paracasei F19, 3%; and L. casei Shirota, 1% (P = 0.0001). The distinctive pattern of probiotic adherence was not influenced by rotavirus diarrhea. The adhesion of Bb12 in the presence of GG increased from 31 to 39% in healthy infants (P = 0.018) and in episodes of diarrhea increased from 26 to 44% (P = 0.001). Rotavirus diarrhea does not decrease the production of fecal mucin or with respect to the adhesion of probiotic bacteria tested in vitro. Combination of specific probiotic strains may enhance adherence in a synergistic manner. Optimal clinical application of these interactions may offer novel therapeutic guidelines for the treatment and prevention of gastrointestinal infections.
An increased propensity to gastrointestinal infections in infants and young children is explained by the immaturity of the gut defense barrier, a protective interface between the internal environment and the constant challenge from potentially pathogenic factors in the external environment (27). Probiotics, live bacterial strains in foods which are beneficial to health, have a positive effect in the prevention and treatment of specific gastrointestinal disorders (26) and in counteracting gut barrier dysfunction associated with inflammation and infection (9, 18). Accordingly, specific probiotic strains have been shown to prevent diarrhea, shorten the duration of diarrheal episodes, and alleviate inflammatory responses (7, 24). The mechanisms behind favourable clinical outcome are still largely unknown. One candidate is enhanced non-specific and antigen-specific immunological response (12, 18).
Adherence to the intestinal epithelium and mucus is associated with stimulation of the immune system (1, 3, 28), and adhesion to the intestinal mucosa is also crucial for transient colonization (5), an important prerequisite for probiotics to control the balance of the intestinal microflora. Intestinal mucus has a dual role; it protects the mucosa from certain microorganisms while providing an initial binding site, nutrient source, and matrix on which bacteria can proliferate. Mucus can inhibit bacterial adhesion to the epithelium (2, 6). It is believed that mucus has receptors mimicking the epithelial cells, to which the bacteria adhere, and the bacteria are consequently prevented from reaching the enterocytes. Mucus is continuously sloughed off into the intestinal lumen and replaced with new mucus secreted by the goblet cells (1, 23, 32). The continuous mucus degradation may explain the transient colonization observed with most probiotic bacteria, but bacteria inhabiting the mucous layer can establish large populations if the multiplication exceeds the turnover and erosion of the mucous layer (2). Intestinal mucus inhibits rotavirus replication (29, 35). Previous studies have shown that the probiotics differ in respect to binding properties and that better adherence seems to correlate with a beneficial health effect (13, 21), but no data are available on adherence properties during diarrhea. Rotaviruses, the major causes of acute diarrhea in infants and young children worldwide (22, 25), interfere with the gut barrier function.
The present study was designed to assess whether fecal mucin levels are affected by rotavirus infection. In addition, the adhesion to mucus of five candidate probiotic strains and their combinations was determined in these clinical situations, since differences in adhesion properties between probiotic strains may affect their efficacy, particularly in adhesion to healthy versus inflamed gut mucosa, and certain combinations may be beneficial.
The bacteria tested were Lactobacillus rhamnosus GG, Lactobacillus casei strain Shirota, Lactobacillus paracasei F19, Lactobacillus acidophilus LA5, and Bifidobacterium lactis Bb12. All have previously been documented to be safe, to have successful immunological effects, and also to evince antirotavirus activity (10, 11, 17, 23).
MATERIALS AND METHODS
Subjects and study design.
The study was carried out during a rotavirus epidemic season in the Central Hospital of Satakunta, Pori, Finland. The inclusion criterion was rotavirus diarrhea confirmed by enzyme immunoassay (TestPack Rotavirus; Abbot Laboratories). Altogether, 20 admitted patients aged 6 to 42 months were enrolled. Fecal samples were taken during the diarrhea episode and after recovery 1 month later. The control group comprised 10 healthy children matched for age. Their fecal samples were obtained during the same study period. All samples were stored at −20°C until analysis.
Informed consent was obtained from patients' parents, and the protocol was approved by the hospital's Committee on Ethical Practice.
Mucus preparation.
Mucus was prepared from the feces according to the method of Miller and Hoskins (18), as modified by Ouwehand et al. (19). In short, fecal extracts were prepared by suspending the samples in four volumes of ice-cold phosphate-buffered saline containing EDTA, phenylmethylsulfonyl fluoride, sodium azide, and iodoacetamide. The suspensions were centrifuged to remove particulate matter. From the fecal extracts, 100-μl aliquots were frozen for mucin determination. Mucus was isolated from the extracts by dual precipitation with ice-cold ethanol and freeze-dried and resuspended in HEPES-buffered Hanks salt solution (HH) at a concentration of 0.5 mg/ml.
Mucin determination.
Mucin was determined by the alcian blue method of Hall et al. (8). Briefly, fecal extracts were thawed and centrifuged to remove any particulate matter. The samples were appropriately diluted and mixed with alcian blue solution. After incubation, the alcian blue-mucin complex was washed, and the color was released from the complex by sonication in 10% sodium dodecyl sulfate. The absorbance was read at 620 nm and compared to a standard curve of bovine submaxillary mucin (Sigma, St. Louis, Mo.). Mucin levels were determined in duplicate.
Probiotic strains.
Five probiotic strains were tested for their adhesion to the isolated intestinal mucus: L. rhamnosus GG (ATCC 53103; Valio, Helsinki, Finland), L. casei strain Shirota (Yakult, Tokyo, Japan), L. acidophilus LA5 (Chris Hansen, Hørsholm, Denmark), L. paracasei F19 (Arla, Stockholm, Sweden), and B. lactis Bb12 (Chris Hansen). The bacteria were grown overnight at 37°C in MRS (medium for cultivation of lactobacilli; Merck, Darmstadt, Germany) broth containing 10 μl of methyl-1,2-[3H]thymidine (6.7 Ci/mmol; ICN products, Costa Mesa, Calif.) per ml broth to metabolically radiolabel the bacteria. The bacteria were harvested by centrifugation (1,000 × g), washed in HH, and resuspended in HH. The absorbance at 600 nm was adjusted to 0.25 ± 0.01 in order to standardize the bacterial concentration (107 to 108 CFU/ml).
In vitro adhesion assay.
The adhesion of radioactively labeled bacteria was determined according to the method described by Cohen and Laux (4). In brief, mucus preparation (0.5 mg/ml) in HH was centrifuged for 5 min at 2,000 × g to remove undissolved material. Of the clear mucus preparation, 100 μl was immobilized in polystyrene microtiter plate wells (Maxisorp; Nunc, Roskilde, Denmark) by overnight incubation at 4°C. The wells were washed twice with 250 μl of HH. To each well a suspension of 100 μl of radioactively labeled microorganisms was added. After incubation for 1 h at 37°C, the wells were washed twice with 250 μl of HH to remove unattached cells and thereafter incubated for 1 h at 60°C with 250 μl of 1% sodium dodecyl sulfate–NaOH (0.1 mol/liter) to release and lyse bound microorganisms. Radioactivity was determined by liquid scintillation counting. Adhesion was assessed as the percentage of radioactivity recovered from the wells compared to the radioactivity of the bacterial suspension in a 100-μl aliquot added to the wells. The results from the adhesion assay are presented as the means of three independent experiments. Each experiment was performed with three parallels to correct for intra-assay variations.
Statistics.
A paired t test was applied to compare the mucin concentrations in patients during and after diarrhea, and a t test for independent samples was used to compare patients to healthy controls. Comparison of the adherence of different probiotic strains was by Friedman's nonparametric analysis of variance, and the Wilcoxon signed-rank test was used to compare the adherence of probiotic combinations. Kendall's W, the coefficient of concordance, was used to indicate whether the rank orders of probiotic bacteria were consistent among subjects.
RESULTS
Clinical characteristics.
In most patients diarrhea varied from mild to moderate, and the mean age of the patients was 16.3 (range, 6.0 to 42.0) months and that of the controls was 17.5 (range, 1.6 to 27.6) months. Duration of diarrhea at home was 2.1 (range, 0.5 to 5.0) days, and the number of diarrhoeal stools at home before admission was 8.9 (range, 1 to 25) per patient. Mean weight loss on admission was 320 (range, 0 to 1,000) g, mean percentage of dehydration 2.8 (range, 0 to 5.5)% and the mean consumption of oral rehydration solution during hospitalization was 680 (range, 100 to 1,630) ml. Intravenous rehydration was given to seven patients. The mean total duration of the episode was 6.0 (range, 5.0 to 7.5) days, and the mean duration of hospital stay was 2.9 (range, 1.5 to 6.0) days. The mean weight change in hospital was 115 (range, −250 to 600) g. Three weeks after discharge a mean weight change of 570 (range, −40 to 1,200) g was achieved. In no case was the episode unduly.
Mucin determination.
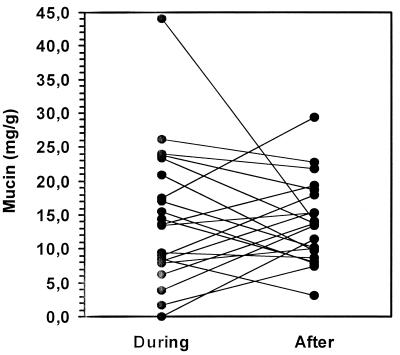
The mean concentration of fecal mucin in healthy controls, 14.9 mg/g (95% confidence interval [CI], 9.9 to 19.9 mg/g), was comparable to that in diarrhea patients, 15.2 mg/g (95% CI, 10.7 to 19.8 mg/g) (P = 0.92). After the episode the mean concentration of mucin was 14.1 mg/g (95% CI, 11.1 to 17.0 mg/g). Figure 1 demonstrates that the mucus concentration was not decreased by diarrhea.
FIG. 1.
Mucin content (milligrams per gram [wet weight]) of feces collected from patients during rotavirus infection and 1 month after recovery.
Adherence of different probiotic bacteria in healthy infants.
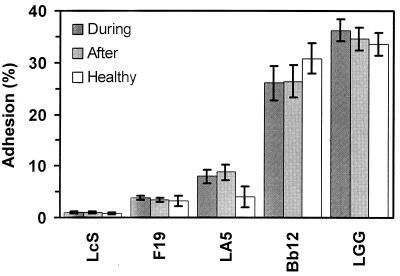
Adherence of the five probiotic bacterial strains to the mucus isolated from healthy infants is presented in Fig. 2. There was a statistically significant difference between the strains (P = 0.0001), the main cause being the typical adherence capacity of different probiotics: the adherence of strain GG was 33.6% (95% CI, 31.4 to 35.8%), strain Bb12 adhered to 30.8% (95% CI, 27.9 to 33.7%), strain LA5 adhered to 4.0% (95% CI, 2.0 to 6.0%), strain F19 adhered to 3.3% (95% CI, 2.2 to 4.3%), and strain Shirota adhered to 0.8% (95% CI, 0.6 to 1.0%). Kendall's W, 0.96 (P = 0.0001), indicated almost complete agreement in the rank orders of probiotic bacteria and their adherence among 10 healthy subjects.
FIG. 2.
Adhesion of probiotic bacteria (L. casei Shirota [LcS], L. paracasei F19, L. acidophilus LA5, B. lactis Bb12, and L. rhamnosus GG [LGG]) to mucus isolated from patients with rotavirus infection (During) 1 month after recovery (After), and in age-matched controls (Healthy). Adhesion is expressed as the mean percentage of bacteria that bound to the immobilized mucus relative to the amount of bacteria applied to the mucus. Error bars, 95% CI.
Adherence of different probiotics to mucus during and after diarrhea.
A statistically significant difference in adherence between the various probiotic strains (Friedman test: P < 0.0001) was also observed during the diarrhea episode (Fig. 2): the mean (95% CI) adherence of GG was 36.2% (34.1 to 38.3%), that of Bb12 was 26.1% (22.8 to 29.4%), that of LA5 was 8.1% (6.7 to 9.5%), that of F19 was 3.8% (3.4 to 4.3%), and that of Shirota was 1.0% (0.9 to 1.2%). Kendall's W, 0.98 (P < 0.0001), indicates that the agreement among the 18 diarrhea patients tested was almost complete. The patterns of adherence of specific probiotic strains to mucus isolated from diarrheal patients and healthy controls were indistinguishable. The adhesion of Bb12 during diarrhea was 26.1% (95% CI, 22.8 to 29.4%) and after diarrhea was 26.0% (95% CI, 22.8 to 29.2%), the mean difference being 0.1% (P = 0.84). An exception was the adherence of GG, which was greater during diarrhea, 36.2% (95% CI, 34.1 to 38.3%), than after, 34.6% (95% CI, 32.3 to 36.9%), a mean difference of 1.6% (95% CI, 0.3 to 3.0) (P = 0.02).
Adherence of combinations of probiotic strains.
The adherence of Bb12 in healthy controls increased from 30.8% (95% CI, 27.9 to 33.7%) to 39.4% (95% CI, 34.1 to 44.6%) when in combination with GG (P = 0.018). This effect was even stronger during diarrhea, with adherence when in combination being 44.3% (95% CI, 42.0 to 46.7%) (P = 0.001). Other combinations were no different in their levels of adhesion.
DISCUSSION
The results here indicate that the concentration of fecal mucin does not diminish during an episode of rotavirus diarrhea; the probiotics binding to the intestinal mucus do not thus lose their potential and can be safely used in the treatment of rotavirus diarrhea and in other viral gastrointestinal infections in children. A more rapid exchange of mucus during diarrhea is possible, which may shorten the time for adherence of probiotic bacteria; nevertheless, probiotic bacteria have been shown to enhance the local immune response and to promote recovery from rotavirus diarrhoea (12).
The mucous layer in the intestine is relatively thick (up to 400 μm), and it is in a dynamic state, constantly being synthesized by goblet cells and also degraded. The mucous gel is predominantly water (up to 95%), the mucin content is up to 5%, and other components include lipids, free proteins, and salts. The mucins are the large glycoproteins which are the major organic components of mucus, and the protein content of the mucin is 20%, while carbohydrate comprises 70 to 80% by weight. Intestinal mucin has been shown to inhibit the replication of rotavirus in vitro (3, 29, 35). Calves infected with rotavirus have been shown to have substantially less mucin covering the epithelium of the small intestine and colon than do healthy controls (34), but, as shown here, human infants, at least well-nourished infants, sustain the level of mucin during rotavirus diarrhea.
In humans lactobacilli colonize the distal small bowel and the large intestine. Different probiotic bacteria possess various mechanisms, including adhesins and/or coaggregation factors, which aid adhesion and colonization (30). Assessment of bacterial adhesion in vivo is difficult, and in vitro models with cell lines (14) are commonly applied for that purpose. Human ileostomy glycoproteins have been used as a model for the small-intestinal mucus to investigate adhesion of probiotics, and in vitro adhesion to mucous glycoproteins extracted from feces has been shown to correlate with the adhesion to ileostomy glycoproteins (31). Thus, in vitro evaluation of the adhesion to human intestinal mucus provides a suitable model for estimating the ability of probiotics to adhere to intestinal surfaces. It has been suggested that adhesion of lactobacilli to human intestinal mucus has a probiotic function in preventing the adhesion to and colonization of damaged tissue sites by invading pathogens (34). The induction of mucin gene expression is perhaps one of the mechanisms whereby probiotic strains minimize the interplay of other microbes with intestinal mucosal cells and thereby intestinal inflammation (17).
In this study, we characterized the bacterial adhesion to human intestinal mucus isolated from feces during a bout of rotavirus diarrhea and during convalescence. Our results confirm that specific probiotics evince significantly different adherence to the intestinal mucus, and no change in adherence occurred during diarrhea. The adhesion is highly species specific. Our results are comparable to those previously obtained in healthy infants (13, 20, 21). The increased binding of Bb12 in the presence of GG confirms the finding (21). Probiotic strains of high adherence capacity have been demonstrated to enhance the immunoglobulin A response to rotavirus (12). The immune response by GG is similar to the adjuvant action of cholera toxin (15, 16), the adherence properties of which are related to the intensity of the mucosal immune response. GG perhaps influences permeation of antigens through Peyer's patches stimulating an immunoglobulin A-committed B-cell population (12). Thus, the immune response of probiotic strains and their adherence are in a good relation, as is the case with other antigens; the better the adherence is, the stronger the immune response is, but more experimental work is required before this can be confirmed. This study helps us to choose the specific probiotics for clinical use because excellent adherence continues during rotavirus diarrhea, too.
Conclusions. The concentration of human intestinal mucin and the adherence properties of the probiotic strains are altered only negligibly during or after rotavirus diarrhea compared to the status in healthy children, as tested in vitro. The appropriate combination of probiotics seems to increase adhesion and probably also the immune response and may be beneficial in the treatment and prevention of rotavirus diarrhea, possibilities which future clinical trials will investigate.
ACKNOWLEDGMENTS
This study was supported by the Academy of Finland and the Medical Research Funds of Turku University Hospital, Turku, Finland and Satakunta Central Hospital, Pori, Finland.
REFERENCES
- 1.Beachey E H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surfaces. J Infect Dis. 1981;143:325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- 2.Carlstedt-Duke B. The normal microflora and mucin. In: Grubb R, Midtvedt T, Norin E, editors. The regulatory and protective role of the normal microflora. M. New York, N.Y: Stockton Press; 1989. pp. 109–127. [Google Scholar]
- 3.Chen C C, Baylor M, Bass D M. Murine intestinal mucins inhibit rotavirus infection. Gastroenterology. 1993;105:84–92. doi: 10.1016/0016-5085(93)90013-3. [DOI] [PubMed] [Google Scholar]
- 4.Cohen P S, Laux D C. Bacterial adhesion to and penetration of intestinal mucus in vitro. Methods Enzymol. 1995;253:309–314. doi: 10.1016/s0076-6879(95)53026-6. [DOI] [PubMed] [Google Scholar]
- 5.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;6:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forstner G, Sherman P, Forstner J. Mucus: function and structure. In: Boedeker E C, editor. Attachment of organisms to the gut mucosa. Boca Raton, Fla: CRC Press; 1984. pp. 13–21. [Google Scholar]
- 7.Guarino A, Canani R B, Spagnuolo M I, Albano F, di Benedetto L. Oral bacterial therapy reduces the duration of symptoms and viral excretion in children with mild diarrhoea. J Pediatr Gastroenterol Nutr. 1998;25:516–519. doi: 10.1097/00005176-199711000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Hall R L, Miller R J, Peatfield A C, Richardson P S, Williams I, Lampert I. A colorimetric assay for mucous glycoproteins using alcian blue. Biochem Soc Trans. 1980;8:72. doi: 10.1042/bst0080072. [DOI] [PubMed] [Google Scholar]
- 9.Isolauri E, Juntunen M, Rautanen T, Sillanaukee P, Koivula T. A human Lactobacillus strain (Lactobacillus GG) promotes recovery from acute diarrhoea in children. Pediatrics. 1991;88:90–97. [PubMed] [Google Scholar]
- 10.Isolauri E, Kaila M, Mykkänen H, Ling W H, Salminen S. Oral bacteriotherapy for viral gastroenteritis. Dig Dis Sci. 1994;39:2595–2600. doi: 10.1007/BF02087695. [DOI] [PubMed] [Google Scholar]
- 11.Kaila M, Isolauri E, Saxelin M, Arvilommi H, Vesikari T. Viable versus inactivated Lactobacillus strain GG in acute rotavirus diarrhoea. Arch Dis Child. 1995;72:51–53. doi: 10.1136/adc.72.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaila M, Isolauri E, Soppi E, Virtanen E, Laine E, Arvilommi H. Enhancement of the circulating antibody secreting cell response in human diarrhoea by a human Lactobacillus strain. Pediatr Res. 1992;32:141–144. doi: 10.1203/00006450-199208000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Kirjavainen P V, Ouwehand A C, Isolauri E, Salminen S J. The ability of probiotic bacteria to bind to human intestinal mucus. FEMS Microbiol Lett. 1998;67:185–189. doi: 10.1111/j.1574-6968.1998.tb13226.x. [DOI] [PubMed] [Google Scholar]
- 14.Lehto E M, Salminen S. Adhesion of two Lactobacillus strains, one Lactococcus and one Propionibabacterium strain to cultured human intestinal Caco-2 cell Line. Biosci Microflora. 1997;16:13–17. [Google Scholar]
- 15.Lycke N, Holmgren J. Strong adjuvant properties of cholera toxin on gut mucosal immune responses to orally presented antigens. Immunology. 1986;59:301–308. [PMC free article] [PubMed] [Google Scholar]
- 16.Lycke N, Karlsson U, Sjölander A, Magnusson K-E. The adjuvant action of cholera toxin is associated with increased intestinal permeability for luminal antigens. Scand J Immunol. 1991;33:691–698. doi: 10.1111/j.1365-3083.1991.tb02542.x. [DOI] [PubMed] [Google Scholar]
- 17.Mack D R, Wei S, Hoollingsworth M A. Modulation in the expression of intestinal MUC3 mucin by probiotic microbes. J Pediatr Gastroenterol Nutr. 2000;31(Suppl. 2):S277. [Google Scholar]
- 18.Majamaa H, Isolauri E, Saxelin M, Vesikari T. Lactic acid bacteria in the treatment of acute rotavirus gastroenteritis. J Pediatr Gastroenterol Nutr. 1995;20:333–338. doi: 10.1097/00005176-199504000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Miller R S, Hoskins L C. Mucin degradation in human colon ecosystems. Fecal population densities of mucin-degrading bacteria estimated by a “most probable number” method. Gastroenterology. 1981;81:759–765. [PubMed] [Google Scholar]
- 20.Ouwehand A C, Kirjavainen P V, Grönlund M-M, Isolauri E, Salminen S. Adhesion of probiotic micro-organisms to intestinal mucus. Int Dairy J. 1999;9:623–630. [Google Scholar]
- 21.Ouwehand A C, Tölkkö S, Salminen S. The mucus binding of Bifidobacterium lactis Bb12 is increased in the presence of Lactobacillus GG and L. delbrueckii ssp bulgaricus. Lett Appl Microbiol. 2000;30:10–13. doi: 10.1046/j.1472-765x.2000.00590.x. [DOI] [PubMed] [Google Scholar]
- 22.Parashar U D, Holman R C, Clarke M J, Breese J S, Glass R J. Hospitalizations associated with rotavirus diarrhoea in the United States, 1993 through 1995: surveillance based on the new ICD-9-CM rotavirus-specific diagnostic code. J Infect Dis. 1998;177:13–17. doi: 10.1086/513808. [DOI] [PubMed] [Google Scholar]
- 23.Peach S L, Tabaqchali S. Some studies of the bacterial flora associated with the mucosa of the human gastrointestinal tract. Nahrung. 1984;28:627–634. doi: 10.1002/food.19840280619. [DOI] [PubMed] [Google Scholar]
- 24.Saavedra J M, Bauman N A, Oung I, Perman J A, Yolken R H. Feeding of Bifidobacterium bifidum and Streptococcus thermophilus to infants in hospital for prevention of diarrhoea and shedding of rotavirus. Lancet. 1994;344:1046–1049. doi: 10.1016/s0140-6736(94)91708-6. [DOI] [PubMed] [Google Scholar]
- 25.Saidi S M, Iijima Y, Sang W K, Mwangudza A K, Oundo J O, Taga K, Aihara M, Nagayama K, Yamamoto H, Waiyaki P G, Honda T. Epidemiological study on infectious diarrheal diseases in children in a coastal rural area of Kenya. Microbiol Immunol. 1997;41:773–778. doi: 10.1111/j.1348-0421.1997.tb01925.x. [DOI] [PubMed] [Google Scholar]
- 26.Salminen S, Bouley C, Boutron-Rualt M-C, Cummings J H, Franck A, Gibson G R, Isolauri E, Moreau M C, Roberfroid M, Rowland I. Functional food science and gastrointestinal physiology and function; the role of prebiotics and probiotics. Br J Nutr. 1998;80(Suppl. 1):147–171. doi: 10.1079/bjn19980108. [DOI] [PubMed] [Google Scholar]
- 27.Sanderson I R, Walker W A. Uptake and transport of macromolecules by the intestine: possible role in clinical disorders (an update) Gastroenterology. 1993;104:622–639. doi: 10.1016/0016-5085(93)90436-g. [DOI] [PubMed] [Google Scholar]
- 28.Schiffrin E J, Brassart D, Servin A L, Rochat F, Donnet-Hughes A. Immune modulation of blood leukocytes in humans by lactic acid bacteria: criteria for strain selection. Am J Clin Nutr. 1997;66:515–520. doi: 10.1093/ajcn/66.2.515S. [DOI] [PubMed] [Google Scholar]
- 29.Superti F, Marziano M L, Tinari A, Donelli G. Effects of polyions on the infectivity of SA-11 rotavirus in LCC-MK2 cells. Comp Immunol Microbiol Infect Dis. 1993;16:55–62. doi: 10.1016/0147-9571(93)90061-9. [DOI] [PubMed] [Google Scholar]
- 30.Tannock G W. Normal microflora. An introduction to microbes inhabiting the human body. London, United Kingdom: Chapman and Hall; 1995. [Google Scholar]
- 31.Tuomola E M, Ouwehand A C, Salminen S J. Human ileostomy glycoproteins as a model for small intestinal mucus to investigate adhesion of probiotics. Lett Appl Microbiol. 1999;28:159–163. doi: 10.1046/j.1365-2672.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- 32.Van der Waaij L A, Limburg P C, Mesander G, van der Waaij D. In vivo IgA coating of anaerobic bacteria in human faeces. Gut. 1996;38:348–354. doi: 10.1136/gut.38.3.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varshney K C, Bridger J C, Parsons K R, Cook R, Teucher J, Hall G A. The lesions of rotavirus infection in 1- and 10-day-old gnotobiotic calves. Vet Pathol. 1995;32:619–627. doi: 10.1177/030098589503200602. [DOI] [PubMed] [Google Scholar]
- 34.Vaughan E E, Mollet B. Probiotics in the new millenium. Nahrung. 1999;43:148–153. doi: 10.1002/(SICI)1521-3803(19990601)43:3<148::AID-FOOD148>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 35.Yolken R H, Ojeh C, Khatri I A, Sajjan U, Forstner J F. Intestinal mucins inhibit rotavirus replication in an oligosaccharide-dependent manner. J Infect Dis. 1994;169:1002–1006. doi: 10.1093/infdis/169.5.1002. [DOI] [PubMed] [Google Scholar]