Abstract
The assessment of intracellular cytokines at the single-cell level by flow cytometry has recently become a potent tool in many areas of cell biology and in defining the role of cytokines in various human diseases. Three-color flow cytometry for detection of intracellular cytokines combined with simultaneous determination of lymphocytes (CD3+ and CD4+) or monocytes (CD33+ and CD14+) was used for comparison of phytohemagglutinin (PHA)-and phorbol myristate acetate (PMA)-ionomycin-induced production of intracellular cytokines in peripheral blood mononuclear cells (PBMCs) of healthy donors. We found that the number of PBMCs stained for tumor necrosis factor alpha and gamma interferon after 6 h of activation was higher when PMA-ionomycin was used for stimulation, while the frequencies of cells positive for interleukin 4 (IL-4) were similar for both stimulators. However, PMA-ionomycin stimulation caused prominent alterations of cell morphology and membrane expression of CD4 and CD14. In contrast, PHA did not cause downregulation of surface markers and resulted in less pronounced alterations in both forward and side scatter signals during flow cytometry analysis. Moreover, during 48 h of culture PHA stimulated tumor necrosis factor beta and IL-10 production, which was not observed when PMA-ionomycin was used. We conclude that the use of PHA for cell activation may limit in vitro artifacts and allow more precise analysis of intracellular cytokine production in various disease states.
Cytokine production can be detected at the level of a single cell by enzyme-linked immunosorbent assay and SPOT-forming cell (ELISPOT) analysis, limiting dilution analysis, semiquantitative PCR, and in situ hybridization (4, 12). Recently, a number of investigators have used flow cytometry to assess cytokine production at the single-cell level in both humans (6, 15–17, 18) and mice (2, 14). The multiparameter analysis in flow cytometry permits the simultaneous detection of one, two, or more cytokines, which, when combined with determination of the cell surface phenotype, allows Th1 versus Th2 subset detection. Two important developments in the early 1990s have revolutionized this area of cell biology. First, Sander et al. (19) described a microscopic method that detects intracellular cytokines in single cells by using paraformaldehyde fixation, saponin permeabilization, and indirect immunofluorescence staining. Shortly afterward, Jung et al. (8) adapted this method to study the cells stimulated in the presence of monensin, which disrupts intracellular protein transport and causes accumulation of cytokines in the Golgi apparatus. It results in the enhanced cytokine signal that can be detected by flow cytometry. This technique makes it possible to answer, within hours, questions that used to require T-cell cloning over several months. It also has the potential to become the standard assay for examination of cytokine production at the single-cell level ex vivo (22) even when the number of cytokine-producing cells is small.
Unstimulated peripheral blood T lymphocytes spontaneously produce little or no cytokines; thus, in vitro stimulation is required to induce cytokine gene expression. Phorbol myristate acetate (PMA) and ionomycin are commonly used as stimulants. However, the stimulation with these agents leads to rapid downregulation of membrane expression of CD4 (13).
In the present study we describe the three-color flow cytometry method for detection of intracellular cytokines combined with simultaneous analysis of T-lymphocyte (CD3+ CD4+) or monocyte (CD33+ CD14+) determinants, using PMA-ionomycin or phytohemagglutinin (PHA) as stimulators. The data presented demonstrate the advantage of PHA stimulation over PMA-ionomycin stimulation.
MATERIALS AND METHODS
Antibodies.
The following monoclonal antibodies (MAbs) directed against human leukocyte surface markers were used: CD4-fluorescein isothiocyanate (FITC), CD14-FITC as well as anti-human cytokine MAb: anti-tumor necrosis factor alpha (anti-TNF-α)-phycoerythrin (PE) (mouse immunoglobulin G1 [IgG1]), anti-interleukin-4 (anti-IL-4)–PE (rat IgG1), anti-gamma interferon (anti-IFN-γ)-PE (mouse IgG1), anti-tumor necrosis factor beta (anti-TNF-β)-PE (mouse IgG1), anti-interleukin-10 (anti-IL-10)-PE (rat IgG1), and appropriate isotype controls were purchased from Pharmingen/Becton Dickinson (San Diego, Calif.). Anti-CD3-PE/cyanin 5.1 (Cy5) and anti-CD33-PE/Cy5 MAbs and appropriate isotype controls were purchased from Immunotech/Coulter (Marseille, France).
Reagents.
Ionomycin and PMA were obtained from Sigma Chemical Co. (St. Louis, Mo.), and PHA was obtained from Murex (Murex Diagnostics, Dartford, United Kingdom). GolgiStop reagent (containing monensin) and reagents for cell fixation and permeabilization (Cytofix/Cytoperm and Perm/Wash, respectively) were purchased from Pharmingen/Becton Dickinson.
Cell culture.
Peripheral blood mononuclear cells (PBMCs) were isolated from EDTA-treated peripheral blood of healthy donors. PBMCs were separated by standard Ficoll-Paque (Pharmacia, Uppsala, Sweden) density gradient centrifugation. Cells were suspended in RPMI 1640 medium supplemented with 10% fetal calf serum (Biochrom, Berlin, Germany), 2 mM glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml (all from Gibco BRL, Karlsruhe, Germany) at a density of 106/ml. To avoid attachment of monocytes to the tubes, PBMCs were cultured in Falcon 2063 nonstick polypropylene round-bottom tubes (Becton Dickinson, San Jose, Calif.). Cells were cultured in the absence or presence of the activators PMA (5 ng/ml) and ionomycin (1 μM) or PHA (2.5 μg/ml) for 6, 12, 24, and 48 h. To inhibit cytokine secretion, monensin (GolgiStop [2 μM]) was added at the begining of short-term cultures (6 and 12 h) or for the last 12 h in case of longer cultures.
Immunostaining of cell surface antigens and for intracellular cytokines.
At the indicated time of culture, the cells were harvested and washed once in ice-cold phosphate-buffered saline (PBS), suspended in a small amount of PBS, and distributed (100 μl per tube) to the Falcon 2054 polystyrene round-bottom tubes (Becton Dickinson) for immunolabeling. As some antibodies which recognize cell surface markers may not bind to fixed or denaturated antigen, immunostaining for the surface determinants was performed with unfixed cells prior to staining for intracellular cytokines. For better discrimination of monocytes and lymphocytes in PBMCs during flow cytometry analysis, fluorochrome-conjugated MAbs against the surface determinants were added to each tube in the following combinations: CD4-FITC or CD14-FITC, CD3-PE/Cy-5 and CD4-FITC, or CD33-PE/Cy-5 and CD14-FITC. The cells were incubated with MAbs or appropriate FITC- or PE/Cy-5-conjugated isotype controls for 30 min on ice, washed twice in ice-cold PBS, fixed, and permeabilized with Cytofix/Cytoperm solution (20 min at 4°C). Then, the cells were washed twice in Perm/Wash solution, and pelleted cells were stained (30 min at 4°C) for intracellular cytokines by using PE-conjugated MAbs against human TNF-α, IL-4, IFNγ, TNF-β, and IL-10. PE-conjugated isotype controls were used in parallel. After the cells were washed twice in PBS with 0.1% bovine serum albumin (BSA; Sigma Chemical Co.), the cells were suspended in PBS-BSA for flow cytometry analysis.
Flow cytometry acquisition and analysis.
Samples were analyzed in a FACS Calibur flow cytometer (Becton Dickinson Immunocytometry Systems, Palo Alto, Calif.) by using Cellquest (version 3.1) software. Typically, list mode data for 50,000 events for PBMCs or 20,000 events for CD3+ or CD33+ cells in a “live-gate” mode were acquired. Single-cell cytokine production was evaluated after forward scatter (FSC) and side scatter (SSC) gating on either lymphocytes or monocytes or was determined for a whole PBMC population. The types of intracellular cytokines were determined in the following cell subpopulations: CD4+ and CD4− lymphocytes, CD14+ and CD14− monocytes (dual-color flow cytometry), or CD4+ and CD4− T cells within CD3+ lymphocytes and CD14+ and CD14− monocytes within CD33+ cells (three-color flow cytometry). Statistical analysis was done by using isotype-matched controls as a reference. Typically, less than 1% positive cells were allowed beyond the statistical marker in the appropriate controls.
Statistical analysis.
The frequency of cytokine-producing cells was expressed as a percentage of the labeled cells from an individual donor. The Mann-Whitney U test was used to evaluate differences between the groups: P values of <0.05 were considered significant.
RESULTS
Kinetics of intracellular cytokine production by PBMCs following PHA or PMA-ionomycin stimulation.
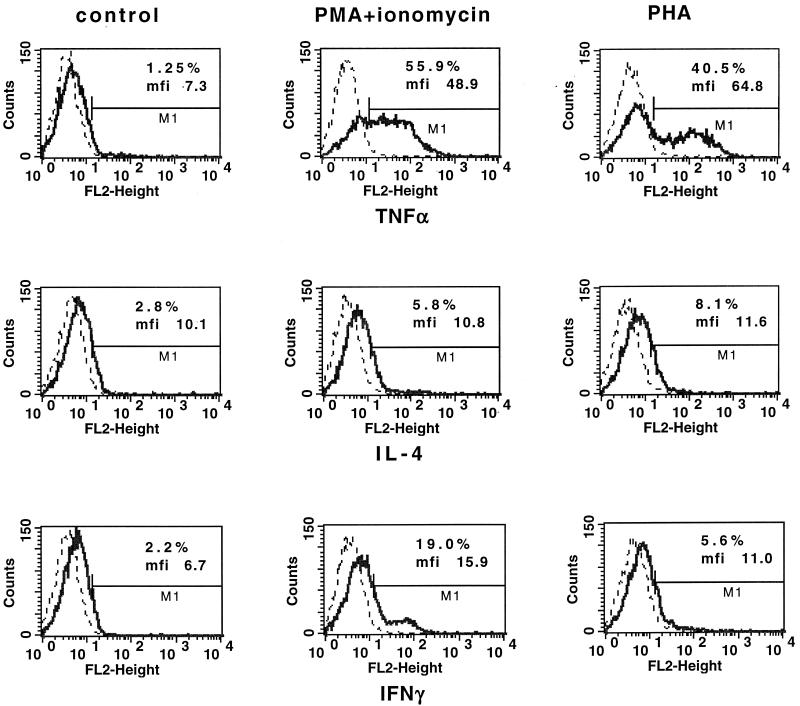
Preliminary experiments were designed to establish optimal conditions for detection of intracellular cytokines. To determine the kinetics of cytokine production, PBMCs from healthy donors were stimulated with PHA or PMA-ionomycin for 6, 12, 24, and 48 h. Then the cells were fixed, permeabilized, stained for TNF-α, TNF-β, IFN-γ, IL-4, or IL-10 by using PE-labeled antibodies, and analyzed by flow cytometry. The frequencies of cytokine-producing cells were determined in a whole PBMC population due to the overlapping clusters of monocytes and lymphocytes after stimulation (see Fig. 2). The presence of intracellular TNF-α, IFN-γ, and IL-4 was detected after 6 h of stimulation with both PMA-ionomycin and PHA (Table 1). However, the number of PBMCs stained for TNF-α and IFN-γ was higher following stimulation with PMA-ionomycin than following stimulation with PHA. The frequency of cells positive for IL-4 was similar for both stimulators. At this time point no IL-10 or TNF-β-producing cells were detected. After 12 h of culture with PMA-ionomycin or PHA, a decrease in the number of TNF-α- and IFN-γ-positive cells and no cells producing IL-10 and TNF-β were observed. Stimulation for 24 h with PHA induced a nonsignificant increase in the frequency of IFN-γ- and IL-10-producing cells, while at 48 h, PHA was superior to PMA-ionomycin in stimulation of IL-10- and TNF-β-producing cells. At this time, PHA but not PMA-ionomycin induced an increase in the frequency of IFN-γ-producing cells, similar to the level observed after stimulation for 24 h. The levels of stimulation of IL-4-producing cells by PMA-ionomycin and PHA were similar at 6 and 12 h, but PHA was superior as a stimulator at 24 and 48 h. However, compared to unstimulated cultures, there was no increased frequency of induction by either stimulants, as culture alone significantly increased the number of IL-4-producing cells, while, in fact, PMA-ionomycin significantly decreased the frequency of such cells.
FIG. 2.
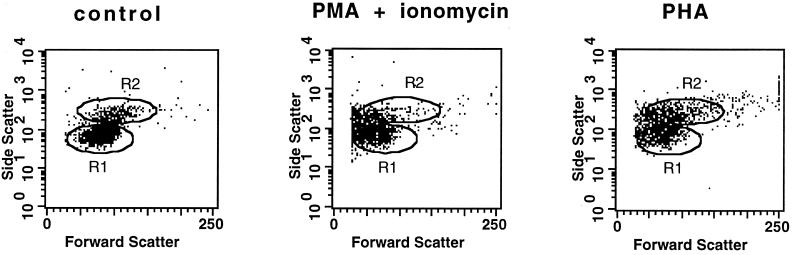
Alterations of cell size (FSC) and cell granularity (SSC) of PBMCs after activation with PMA-ionomycin or PHA. PBMCs were cultured for 6 h in the medium (left) or were stimulated with PMA-ionomycin (middle) or PHA (right) in the presence of monensin. Dot plots (FSC versus SSC) show the changes in cellular morphology after cell activation. Regions R1 and R2, defined in a control, correspond to lymphocytes and monocytes, respectively.
TABLE 1.
Kinetics of TNF-α, IFN-γ, IL-4, TNF-β, and IL-10 production in PBMCs following PMA-ionomycin or PHA stimulation
| Time and treatment | Frequency of cytokine-producing cells (%)a
|
||||
|---|---|---|---|---|---|
| TNF-α | IFN-γ | IL-4 | TNF-β | IL-10 | |
| 6 h | |||||
| Medium | 1.2 (0.8–2.4) | 1.4 (0.6–3.0) | 1.5 (0.9–3.0) | 2.7 (1.6–3.4) | 2.2 (1.0–3.4) |
| PHA | 23.4 (16.3–45.9)b | 6.2 (4.4–10.3)b | 6.9 (2.8–8.1)b | 3.4 (2.7–6.1) | 3.9 (1.1–5.1) |
| PMA-ionomycin | 46.0 (28.6–63.2)b | 10.5 (7.6–19.0)b | 5.8 (2.9–9.8)b | 4.6 (2.4–7.2) | 4.3 (3.6–6.8) |
| 12 h | |||||
| Medium | 2.1 (1.1–4.2) | 2.3 (1.0–4.3) | 3.0 (1.5–5.2) | 1.5 (0.1–2.9) | 1.6 (0.8–3.0) |
| PHA | 17.4 (11.7–22.8)b | 5.4 (4.0–9.7) | 5.3 (3.8–9.1) | 1.8 (0.9–4.4) | 2.2 (0.6–3.8) |
| PMA-ionomycin | 29.3 (19.4–39.6)b | 9.2 (6.2–15.8)b | 4.7 (2.9–8.7) | 2.5 (1.5–5.8) | 2.4 (1.5–4.8) |
| 24 h | |||||
| Medium | 15.3 (7.2–26.7) | 6.7 (1.5–11.5) | 11.7 (6.6–20.8) | 2.8 (1.0–4.7) | 3.2 (1.0–5.2) |
| PHA | 22.0 (16.8–36.2) | 8.2 (4.0–17.8) | 12.3 (6.9–26.0) | 3.1 (1.4–6.4) | 5.3 (2.2–7.4) |
| PMA-ionomycin | 26.0 (9.1–42.6) | 5.2 (3.1–12.5) | 4.1 (2.1–8.8) | 0.5 (0.1–2.3) | 2.6 (0.9–4.7) |
| 48 h | |||||
| Medium | 11.1 (4.8–29.3) | 3.9 (1.2–8.0) | 19.8 (9.0–34,8) | 4.0 (1.1–7.5) | 2.0 (0.3–3.2) |
| PHA | 10.2 (3.7–16.3) | 7.7 (4.1–13.4) | 22.0 (11.0–43.3) | 9.5 (6.2–16.9)b | 8.3 (1.6–12.8)b |
| PMA-ionomycin | 6.5 (0.6–12.2) | 6.4 (1.6–10.7) | 10.7 (5.5–23.8) | 5.5 (1.9–8.9) | 3.9 (0.9–7.6) |
Values are medians and ranges for five different donors.
P < 0.05.
Taken together, the frequency of TNF-α-producing PBMCs was highest after 6 h of culture for both stimuli used; however, PMA-ionomycin induced approximately twice more TNF-α-positive cells than PHA did. The maximum frequency of IFN-γ-positive cells was observed after 6 h of stimulation with PMA-ionomycin. In the case of PHA stimulation, a statistically significant increased frequency of IFN-γ-positive cells was detected after 6 h. A significant level of IL-4-producing cells was detected after 6 h of stimulation with PHA and PMA-ionomycin. Optimal intracellular detection of IL-10 and TNF-β was observed after 48 h of cell culture with PHA stimulation. In that case, PMA-ionomycin stimulation was ineffective.
PHA-stimulated PBMCs produce a wider range of cytokines than the range produced following activation with PMA-ionomycin.
It is known that treatment with PMA-ionomycin yields robust cell activation and potent cytokine production (16, 18). Our data support this observation; however, in our hands during all time points, in comparison to PHA PMA-ionomycin was not effective for stimulation of TNF-β- and IL-10-producing cells (Table 1). As PHA was also at least as effective as PMA-ionomycin for IL-4 stimulation after culture for 6 h (Fig. 1), these data show that PMA-ionomycin induces rather Th1-type cytokine profiles, whereas PHA stimulates both Th1 and Th2 cytokines.
FIG. 1.
Flow cytometry analysis of intracellular cytokine expression in control PBMC (left set of panels) and following either PMA-ionomycin activation (middle set of panels) or PHA activation (right set of panels). PBMCs were cultured in medium alone or were stimulated with PMA-ionomycin or PHA in the presence of monensin for 6 h and stained with PE-labeled anti-cytokine MAbs. Histogram overlays show the FL2 (orange fluorescence) intensity corresponding to a given cytokine (solid line) compared to the intensity for the isotype-specific control (dotted line). The numbers indicate the percentages of positive cells and the mean fluorescence intensity (mfi). The results from one representative experiment of five experiments performed are shown.
Alterations of cell morphology and membrane CD4 and CD14 expression following PMA-ionomycin stimulation.
To establish which population of PBMCs is the main source of a given cytokine, simultaneous analysis of intracellular cytokine expression and cell phenotype was used. To distinguish more precisely lymphocytes and monocytes (apart from FSC and SSC gating) staining of cells with anti-CD4 or anti-CD14 MAbs was applied. As it has been previously reported that the surface CD4 molecule is rapidly and completely downregulated in response to PMA (7, 13), CD4 MAbs could not be used to delineate the CD4+ cells after PMA-ionomycin stimulation. This phenomenon was also observed in our study (see Fig. 3A). Moreover, downregulation of CD14 expression and the changes in the FSC and SSC signals of cells treated with PMA-ionomycin were also noticed (Fig. 2 and 3B), making the clusters of lymphocytes and monocytes very difficult to distinguish. In addition, a significant proportion of dead cells was already detected after 6 h of culture in the presence of PMA-ionomycin (data not shown). These alterations were much less pronounced or not observed when PHA was used as a stimulant (Fig. 2 and 3).
FIG. 3.
Surface CD4 and CD14 and intracellular TNF-α expression in PBMCs. PBMCs were cultured for 6 h in medium (left panels) or were stimulated with PMA-ionomycin (middle panels) or PHA (right panels) in the presence of monensin. Dot plots of CD4-FITC (FL1 [green fluorescence]) (A) or CD14-FITC (B) versus TNF-α–PE (FL2 [orange fluorescence]) expression in the whole PBMC population are shown. Numbers show percentages of positive cells. Data from one representative experiment of three experiments performed are shown.
Methodology for precise analysis of the type of cells producing cytokines.
Culture of PBMCs in the presence of a mitogenic stimulus leads to lymphocyte proliferation. During flow cytometry analysis, these blast cells give rise to FSC and SSC signals similar to those of monocytes. Thus, clusters of blasts and monocytes are overlapping. As the CD4 marker is not useful for distinguishing between lymphocytes and monocytes, we combined the detection of intracellular cytokine expression with simultaneous determination of CD4 and CD3 or CD14 and CD33 for lymphocytes and monocytes, respectively. In this set of experiments PBMCs cultured for different periods of time with or without PMA-ionomycin or PHA were incubated with anti-CD4-FITC and anti-CD3-PE/Cy-5 or anti-CD14-FITC and anti-CD33-PE/Cy-5 and then, after fixation and permeabilization, were stained with PE-labeled MAbs directed against various cytokines. The CD3+ or CD33+ cells were aquired in a live-gate mode. By this approach the precise characterization of CD4+ and CD4− T lymphocytes and CD14+ and CD14− monocytes producing cytokines was possible (Fig. 4 and 5).
FIG. 4.
Intracellular cytokine expression in gated CD3+ T cells by three-color flow cytometry. PBMCs cultured for 6 h in medium (left set of panels) or stimulated with PMA-ionomycin (middle set of panels) or PHA (right set of panels) in the presence of monensin were labeled for the surface expression of CD3 (PE/Cy5) and CD4 (FITC) and for the intracellular presence of different cytokines (PE). Dot plots of CD4-FITC (FL1 [green fluorescence]) versus relevant cytokine-PE (FL2 [orange fluorescence]) after T-cell gating according to CD3-PE/Cy5 (FL3 [red fluorescence]) expression are shown. Numbers show the percentages of CD4− and CD4+ T cells producing cytokines set according to the isotype-matched control. Data from one representative experiment of five experiments performed are shown.
FIG. 5.
Intracellular TNF-α and IL-4 expression in gated CD33+ monocytes by three-color flow cytometry. PBMCs cultured for 6 h in medium (left set of panels) or stimulated with PMA-ionomycin (middle set of panels) or PHA (right set of panels) in the presence of monensin were labeled for surface expression of CD33 (PE/Cy5) and CD14 (FITC) and for intracellular TNF-α or IL-4 by using PE-conjugated MAbs. CD33+ gated monocytes were analyzed on FL1 (FITC) versus FL2 (PE) dot plots to discriminate cytokine expression in CD14− and CD14+ monocytes. Data from one representative experiment of five experiments performed are shown.
Intracellular production of TNF-α, IFN-γ, and IL-4 by lymphocytes and monocytes defined by use of two-color immunophenotyping.
The methodology described above was used for detection of some cytokines in the cytoplasms of lymphocytes and monocytes. The typical pattern of cytokines detected in the cytoplasms of gated CD3+ lymphocytes and CD33+ monocytes after 6 h of stimulation with PMA-ionomycin or PHA is shown in Fig. 4 and 5. Summarized data from an analysis of T cells and monocytes from five different donors are presented in Table 2 and Table 3. These results show that within the CD3+ population, TNF-α and IL-4 are produced mainly by CD3+ and CD4+ cells stimulated with both activators. In contrast, IFN-γ was detected at a similar frequency in the CD3+ and CD4+ T cells and the CD3+ and CD4− T cells when PMA-ionomycin was used as an activator and mainly in the CD3+ and CD4+ T cells following stimulation with PHA. In the case of CD33+ monocytes, TNF-α was produced predominantly by CD33+ and CD14+ monocytes. These data are even more pronounced when only CD14+ or CD14− cells are considered for analysis (Table 3). No IL-4 production was detected within CD33+ cells. Within both CD3+ and CD33+ cell populations, the decrease in the surface expression of CD4 and CD14 was observed after PMA-ionomycin treatment (41.0% ± 9.9% versus 66.2% ± 2.5% for CD4+ cells in control cultures and 53.4% ± 6.9% versus 87.7% ± 1.5% for CD14+ cells in control cultures). This was much less pronounced or even not observed when PHA was used as the activator (60.3% ± 5.3% versus 66.2% ± 2.5% for CD4+ cells in control cultures and 80.1% ± 3.4% versus 87.7% ± 1.5% for CD14+ cells in control cultures).
TABLE 2.
Cytokine production by CD3+ cells and subpopulations of CD4+ and CD4− T cells after 6 h of stimulation with PMA-ionomycin or PHA
| Treatment | Frequency of cytokine-producing cells (%)a
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| TNF-α
|
IFN-γ
|
IL-4
|
|||||||
| CD3+ | CD4+ | CD4− | CD3+ | CD4+ | CD4− | CD3+ | CD4+ | CD4− | |
| Medium | 0.7 (0.1–1.2) | 0.6 (0.1–1.2) | 0.7 (0.3–1.3) | 0.5 (0.2–1.1) | 0.7 (0.1–1.1) | 0.7 (0.2–1.2) | 0.8 (0.2–1.3) | 0.6 (0.1–1.3) | 0.8 (0.3–1.4) |
| PHA | 9.9 (2.2–18.5)b | 14.8 (8.8–28.7)b | 5.4 (1.5–8.6)b | 3.2 (2.0–5.8)b | 6.8 (3.4–9.2)b | 1.8 (0.7–3.8) | 4.5 (2.8–6.5)b | 9.7 (5.5–13.2)b | 1.3 (0.6–2.5) |
| PMA-ionomycin | 33.2 (19.1–53.7)b | 47.3 (31.4–60.5)b | 30.1 (16.9–52.9)b | 9.8 (6.2–24.3)b | 5.4 (2.4–10.9)b | 6.5 (3.0–12.9)b | 2.1 (1.1–4.9) | 5.1 (2.8–7.8)b | 1.8 (0.9–2.9) |
Values are medians and ranges for five different donors.
P < 0.05.
TABLE 3.
TNF-α production by CD33+ cells and by subpopulations of CD14+ and CD14− monocytes after 6 h of stimulation with PMA-ionomycin or PHA
| Treatment | Frequency of TNF-α-producing cells (%)a
|
||
|---|---|---|---|
| CD33+ | CD14+ | CD14− | |
| Medium | 2.2 (1.5–3.7) | 3.2 (1.8–5.2) | 1.4 (0.6–2.8) |
| PHA | 63.7 (43.3–82.0)b | 75.4 (51.3–91.2)b | 12.5 (5.1–20.9)b |
| PMA-ionomycin | 50.5 (33.8–68.3)b | 69.6 (43.5–95.8)b | 50.7 (28.2–75.1)b |
Values are medians and ranges for five different donors.
P < 0.05.
Frequency of TNF-β- and IL-10-producing CD3+ and CD33+ cells after PMA-ionomycin or PHA stimulation.
Optimal intracellular detection of TNF-β and IL-10 in the population of PBMCs was observed after 48 h of culture (Table 1). To define the population of cells responsible for the production of these cytokines, PBMCs cultured for 48 h in the presence of PMA-ionomycin or PHA were labeled for CD3 and CD33. CD33 was chosen, as during culture for longer than 24 h, monocytes lose the surface expression of CD14 (data not shown). Results of one representative experiment of five experiments performed are shown in Fig. 6 and 7. Production of both cytokines could be observed only following PHA stimulation and not PMA-ionomycin stimulation. TNF-β was detected in CD3+ lymphocytes, whereas IL-10 was detected in both CD3+ and CD33+ cells, but mainly among the latter cell type.
FIG. 6.
Quantification of TNF-β- and IL-10-producing CD3+ cells by flow cytometry. PBMCs cultured for 48 h in the medium (left set of panels under “control”) or stimulated with PMA-ionomycin (middle set of panels) or PHA (right set of panels) in the presence of monensin were labeled for surface CD3 (PE/Cy5) and intracellular TNF-β or IL-10 (PE) expression. Cells were gated according to CD3 expression and SSC (region R1). Histogram overlays show FL2 (orange fluorescence) intensity corresponding to a given cytokine (solid line) compared to the intensity for the isotype-specific control (dotted line).
FIG. 7.
Flow cytometry analysis of TNF-β- and IL-10-producing CD33+ cells. PBMCs cultured for 48 h in medium (left set of panels under “control”) or stimulated with PMA-ionomycin (middle set of panels) or PHA (right set of panels) in the presence of monensin were labeled for surface CD33 (PE/Cy5) and intracellular TNF-β or IL-10. Cells were gated according to CD33 expression and SSC (region R1). Histogram overlays show FL2 (orange fluorescence) intensity corresponding to a given cytokine (solid line) compared to the intensity for the isotype-specific control (dotted line). Results from one representative experiment of five experiments performed are shown.
DISCUSSION
In this report we describe an alternative method for detection of intracellular cytokines in human PBMCs. In our study we compared PHA stimulation to the standard protocol using PMA-ionomycin for stimulation of TNF-α, IFN-γ, IL-4, IL-10, and TNF-β production. By the use of three-color flow cytometry analysis we were able to define precisely the population of lymphocytes and monocytes (among them, CD4+ and CD14+ cells, respectively) and to associate the cell phenotype with the production of certain cytokines.
In our hands, both stimulators used were effective for induction of intracellular cytokine production. The kinetics of occurrence of various intracellular cytokines were different; e.g., for TNF-α, IFN-γ, and IL-4, culture for 6 h was optimal, but stimulation of IL-10 and TNF-β production required 48 h. PHA was superior for induction of IL-4 in CD3+ and CD3+-CD4+ cells and of TNF-α in CD33+ monocytes at 6 h. Our data confirm previous results that showed that PMA-ionomycin is a potent stimulator of IFN-γ (18), although in comparison to PHA, it is less effective for stimulation of IL-4. As PHA was also effective for TNF-β and IL-10 stimulation, this is in agreement with data suggesting that PMA-ionomycin induces Th1 cytokine profiles (18), whereas PHA stimulated both the Th1 and the Th2 cytokines profiles, although with different kinetics. Very few data are available regarding the frequency of IL-10 and TNF-β producing cells (1–3, 5, 11, 20, 21). Most of them come from one group and are related to antigen (tetanus toxoid, Mycobacterium bovis BCG)-driven cytokine production (1, 5, 20, 21). In our hands, the maximum frequency of IL-10-producing cells was observed within CD33+ monocytes after 48 h of PHA stimulation. Production of TNF-α was observed in both T cells and monocytes, while production of TNF-β was observed in T cells only. PMA-ionomycin was ineffective in the stimulation of significant levels of production of intracellular TNF-β and IL-10. Thus, paradoxically, PHA was the most potent activator of monocytes for the production of TNF-α and IL-10.
After 24 and 48 h of cell culture without any stimulus, we noticed a high frequency of cells expressing TNF-α and IL-4 (13.3% ± 7.2% and 11.7% ± 5.4%, respectively), but this was not increased further in the stimulated cultures. The reason for this phenomenon remains unknown. This was not due to endotoxin contamination, as cells cultured in the presence of polymyxin B showed similar levels of expression of these cytokines and the expression was not due to nonspecific staining, as it was inhibited by recombinant cytokines (data not shown). It was most likely due to nonspecific activation by the culture alone.
PMA-ionomycin stimulation induced a dramatic decrease in the level of CD4 expression, which is in keeping with other data (7, 13). Moreover, using these activators we have also noticed the significant decrease in the level of CD14 expression on cultured monocytes and detected a lot of dead cells even during 6 h of culture (data not shown). Dead cells can introduce serious artifacts into cytokine measurements by flow cytometry, as they may possess a high-level binding capacity for some anticytokine antibodies. These phenomena were much less pronounced or even not detectable when PHA was used as a stimulator. There are several ways to circumvent downregulation of CD4 after PMA-ionomycin stimulation. The most often used is analysis of CD3+ and CD8− T cells (18). However, this population can contain natural killer cells and “double-negative” T cells that are present at especially high levels in patients with certain infections, e.g., patients with human immunodeficiency virus type 1 infection. A large fraction of these cells produce IFN-γ, few cells make IL-2, and none produces IL-4. Therefore, the CD3+ and CD8− gate, regarded as representing CD4+ T cells, may significantly overestimate the level of IFN-γ production by CD4+ T cells (M. Roederer, the Purdue cytometry CD, vol 5 [cytometry@flowcyt.cyto.purdue.edu]). Another approach is the use of isolated CD4+ T cells (11). This, however, allows the analysis of cytokine produced only by a single homogeneous cell population, and it is not suitable for analysis of samples from patients for whom the amount of blood available for study is limited. Due to its rapid and robust activation of lymphocytes, PMA-ionomycin may be an activator of choice in situations in which the precise analysis of lymphocyte subpopulations is not important and the only criterion used for cell definition is CD3 expression.
In conclusion, PHA stimulates a wider range of intracellular cytokines in human PBMCs than does PMA-ionomycin and is particularly effective for activation of monocytes for cytokine production. Moreover, PHA does not cause downregulation of CD4 and CD14 expression and makes much less pronounced alterations in FSC and SSC signals during flow cytometry analysis. The current approach may limit in vitro artifacts and may allow more precise analysis of intracellular cytokine production in various disease states. This procedure is used for the study of cytokine patterns in children with different forms of hypogammaglobulinemia, in which we have previously observed abberrant patterns of TNF-α, TNF-β, and IL-10 release by PBMCs (9, 10).
ACKNOWLEDGMENT
This study was supported by the State Committee for Scientific Research (grant 4 P05E 03513).
REFERENCES
- 1.Andersson J, Abrams J, Bjork L, Litton M, Agren K, Andersson U. Concomitant in vivo production of 19 different cytokines in human tonsils. Immunology. 1994;83:16–24. [PMC free article] [PubMed] [Google Scholar]
- 2.Assenmacher M, Schmitz J, Radbruch A. Flow cytometric determination of cytokines in activated murine T helper lymphocytes: expression of interleukin-10 in interferon-gamma and inteleukin-4-expressing cells. Eur J Immunol. 1994;24:1097–1101. doi: 10.1002/eji.1830240513. [DOI] [PubMed] [Google Scholar]
- 3.Bjork L, Fehninger T E, Andersson U, Andersson J. Computerized assessment of production of multiple human cytokines at the single-cell level using image analysis. J Leukoc Biol. 1996;59:287–295. doi: 10.1002/jlb.59.2.287. [DOI] [PubMed] [Google Scholar]
- 4.Carter L L, Swain S L. Single cell analysis of cytokine production. Curr Opin Immunol. 1997;9:177–182. doi: 10.1016/s0952-7915(97)80132-x. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez V, Andersson J, Andersson U, Troye-Blomberg M. Cytokine synthesis analyzed at the single-cell level before and after revaccination with tetanus toxoid. Eur J Immunol. 1994;24:1808–1815. doi: 10.1002/eji.1830240813. [DOI] [PubMed] [Google Scholar]
- 6.Gagro A, Rabatic S, Ivancic I, Bendelja K, Jelacic J, Sabioncello A, Misulic J, Buneta D, Dekaris D. Detection of intracellular cytokines in human lymphocytes and monocytes at the single cell level by flow cytometry. Period Biol. 1999;101:17–26. [Google Scholar]
- 7.Ito M, Watanabe M, Kamiya H, Sakurai M. Changes in intracellular cytokine levels in lymphocytes induced by measles virus. Clin Immunol Immunopathol. 1997;83:281–286. doi: 10.1006/clin.1997.4344. [DOI] [PubMed] [Google Scholar]
- 8.Jung T, Schauer U, Heusser C, Neumann C, Rieger C. Detection of intracellular cytokines by flow cytometry. J Immunol Methods. 1993;159:197–207. doi: 10.1016/0022-1759(93)90158-4. [DOI] [PubMed] [Google Scholar]
- 9.Kowalczyk D, Mytar B, Zembala M. Cytokine production in transient hypogammaglobulinaemia and isolated IgA deficiency. Clin Immunol. 1997;100:556–562. doi: 10.1016/s0091-6749(97)70150-7. [DOI] [PubMed] [Google Scholar]
- 10.Kowalczyk D, Pietrzyk J J, Zembala M. TNF production in children with humoral immunodeficiency. Acta Paediatr. 1994;83:1310–1311. doi: 10.1111/j.1651-2227.1994.tb13024.x. [DOI] [PubMed] [Google Scholar]
- 11.Muller F, Aukrust P, Lien E, Hang C J, Froland S S. Enhanced interleukin-10 production in response to Mycobacterium avium products in mononuclear cells from patients with HIV infection. J Infect Dis. 1998;177:586–594. doi: 10.1086/514222. [DOI] [PubMed] [Google Scholar]
- 12.Muller W, Sinigaglia F. Single cell analysis: sharpening the view through the magnifying glass. Curr Opin Immunol. 1995;7:255–257. [Google Scholar]
- 13.Munck Petersen C, Christenen E I, Andresen B S, Moller B K. Internalization, lysosomal degradation and new synthesis of surface membrane CD4 phorbol ester-activated T-lymphocytes and U937 cells. Exp Cell Res. 1992;201:160–173. doi: 10.1016/0014-4827(92)90360-k. [DOI] [PubMed] [Google Scholar]
- 14.Openshaw P, Murphy E E, Hosken N A, Maino V, Davis K, Murphy K, O'Garra A. Heterogeneity of intracellular cytokine synthesis at the single-cell level in polarized T helper 1 and T helper 2 populations. J Exp Med. 1995;182:1357–1367. doi: 10.1084/jem.182.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Picker L J, Singh M K, Zdraveski Z, Treer J R, Waldrop S L, Bergstresser P R, Maino V C. Direct demonstration of cytokine synthesis heterogeneity among human memory/effector T cells by flow cytometry. Blood. 1995;86:1408–1419. [PubMed] [Google Scholar]
- 16.Prussin C. Cytokine flow cytometry: understanding cytokine biology at the single cell level. J Clin Immunol. 1997;17:195–203. doi: 10.1023/a:1027350226435. [DOI] [PubMed] [Google Scholar]
- 17.Prussin C, Metcalfe D D. Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anti-cytokine antibodies. J Immunol Methods. 1995;188:117–128. doi: 10.1016/0022-1759(95)00209-x. [DOI] [PubMed] [Google Scholar]
- 18.Rostaing L, Tkaczuk J, Durand M, Peres C, Durand D, de Preval C, Ohayon E, Abbal M. Kinetics of intracytoplasmic Th1 and Th2 cytokine production assessed by flow cytometry following in vitro activation of peripheral blood mononuclear cells. Cytometry. 1999;35:318–328. doi: 10.1002/(sici)1097-0320(19990401)35:4<318::aid-cyto4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 19.Sander B, Andersson J, Andersson U. Assessment of cytokines by immunofluorescence and the paraformaldehyde-saponin procedure. Immunol Rev. 1991;119:65–93. doi: 10.1111/j.1600-065x.1991.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 20.Sander B, Damm O, Gustafsson B, Andersson U, Hakansson L. Localization of IL-1, IL-2, IL-4, IL-8 and TNF in superficial bladder tumors treated with intravesical bacillus Calmette-Guérin. J Urol. 1996;156:536–541. doi: 10.1097/00005392-199608000-00078. [DOI] [PubMed] [Google Scholar]
- 21.Sander B, Skansen-Saphir U, Hakansson L, Andersson J, Andersson U. Sequential production of Th1 and Th2 cytokines in response to live bacillus Calmette-Guérin. Immunology. 1995;86:512–518. [PMC free article] [PubMed] [Google Scholar]
- 22.Shauer U, Jung T, Krug N, Frew A. Measurement of intracellular cytokines. Immunol Today. 1996;17:305–307. doi: 10.1016/0167-5699(96)30020-0. [DOI] [PubMed] [Google Scholar]