Abstract
Background:
Reward system dysfunction is evident across neuropsychiatric conditions. Here we present data from a double-blinded pharmaco-fMRI study investigating the triggering of anhedonia and reward circuit activity in women.
Methods:
The hormonal states of pregnancy and parturition were simulated in euthymic women with a history of postpartum depression (PPD+; n=15) and those without such a history (PPD-; n=15) by inducing hypogonadism, adding back estradiol and progesterone for 8 weeks (“addback”), and then withdrawing both steroids (“withdrawal”). Anhedonia was assessed using the Inventory of Depression and Anxiety Symptoms (IDAS) during each hormone phase. Those who reported a 30% or greater increase in IDAS anhedonia, dysphoria, or ill temper during addback or withdrawal, compared with pre-treatment, were identified as hormone sensitive (HS+) and all others were identified as non-hormone sensitive (HS-). The monetary incentive delay (MID) task was administered during fMRI sessions at pre-treatment and during hormone withdrawal to assess brain activation during reward anticipation and feedback.
Results:
On average, anhedonia increased during addback and withdrawal in PPD+ but not PPD-. During reward feedback, both HS+(n=10) and HS-(n=18) showed decreased activation in clusters in the right putamen (p<.031, FWE-corrected) and left postcentral and supramarginal gyri (p<.014, FWE-corrected) at the withdrawal scans, relative to pre-treatment scans.
Limitations:
A modest sample size, stringent exclusion criteria, and relative lack of diversity in study participants limit the generalizability of results.
Conclusion:
Although results do not explain differential hormone sensitivity in depression, they demonstrate significant effects of reproductive hormones on reward-related brain function in women.
Keywords: Anhedonia, fMRI, depression, reward, postpartum, estrogen, progesterone
Aberrant reward processing has been implicated in numerous, fundamentally diverse neuropsychiatric disorders characterized by anhedonia, a symptomatic deficit in positive affect and dysregulation in motivation (Craske et al., 2016; Dichter et al., 2012a), including depression (Keren et al., 2018), post-traumatic stress disorder (Mehta et al., n.d.), bipolar disorder (Rizvi et al., 2018; Whitton et al., 2015), schizophrenia (Segarra et al., 2016; Whitton et al., 2015), autism (Greene et al., 2019; Han et al., 2019), anorexia (Wierenga et al., 2015), bulimia (Grob et al., 2012; Simon et al., 2016), and substance use disorder (Cho et al., 2019; Huhn et al., 2016). Nevertheless, the neurobiological mechanisms contributing to onset and severity of anhedonia remain poorly understood (Gordon et al., 2018; Husain and Roiser, 2018). Activation within fronto-striate neural circuits defining the reward network and associated behavior, is regulated by gonadal steroids (Dreher et al., 2007; Schiller et al., 2016), shown to play a role in triggering postpartum depression (PPD) (Bloch et al., 2000), a disorder characterized by anhedonia (Putnam et al., 2017). Given the regulatory control of gonadal steroids on approach-avoidance motivation (Numan, 2007) and the predictable, marked steroid changes during pregnancy and the postpartum period, depression triggered by childbirth offers a unique context to examine the physiology underlying both the triggering of, and susceptibility to, reward system dysfunction.
In order to assess the role of gonadal steroids in PPD directly, independent of the profound biological and psychosocial changes that accompany childbirth, Bloch and colleagues (Bloch et al., 2000) simulated the hormonal changes of pregnancy and parturition in non-pregnant, euthymic women, half with a history of PPD and half without such a history. Participants were administered a GnRH agonist to induce hypogonadism, then administered estradiol and progesterone for 8 weeks, and both steroids were withdrawn. Despite identical hormonal manipulations, women with a history of PPD reported increased depressive symptoms during both hormone addback and withdrawal, whereas women lacking a history of PPD experienced no change in mood. Some have therefore hypothesized that PPD results from an abnormal neural response to normative perinatal hormonal changes (Schiller et al., 2016). PPD is characterized by the same core symptoms (i.e., dysphoria and anhedonia) and abnormal activations of extended reward network nodes associated with major depressive disorder (MDD) more broadly: the amygdala, insula, ventral striatum, orbitofrontal cortex, and dorsomedial prefrontal cortex (Dichter et al., 2012b; Kumar et al., 2018; Moses-Kolko et al., 2011, 2008; Schiller et al., 2013). Although reduced striatal responsiveness to rewards has been reported in both current and remitted MDD (Dichter et al., 2012b; Pizzagalli et al., 2009; Schiller et al., 2013; Smoski et al., 2009; Zhang et al., 2013), which is associated with severity of self-reported anhedonia (Pizzagalli et al., 2009), the role of neural circuit dysfunction in the vulnerability to and triggering of anhedonia specifically remains poorly understood.
The purpose of the current project was to use functional magnetic resonance imaging (fMRI) to characterize neurobiological mechanisms underlying the vulnerability to, and onset of, anhedonia by capitalizing on the ability to “turn on and off” depressive symptoms using the Bloch et al (Bloch et al., 2000) reproductive hormone challenge paradigm. We used the hormone challenge to select a single, homogeneous, and experimentally confirmed “hormone sensitive” phenotype (HS+), which we hypothesized would increase the likelihood of identifying meaningful neurophysiological biomarkers of anhedonia and help identify the source of differential sensitivity to hormone change in PPD. While we evaluated functional activation during monetary reward processing across the whole brain, we were most interested in activation in regions comprising the striatum (i.e., nucleus accumbens, caudate, and putamen) given its central role in reward-network function and links to anhedonia (Borsini et al., 2020). We aimed to: 1) characterize changes in anhedonia and other affective symptoms provoked by estradiol and progesterone in euthymic women with a history of PPD (PPD+), compared with those without such a history (PPD-); 2) to explore whether patterns of striatal activation could identify PPD+ vulnerable to hormone related affective dysfunction (i.e., “hormone sensitivity”) at pre-treatment, prior to the hormone challenge; and 3) to characterize changes in striatal activation relative to pre-treatment in HS+, compared with those who were not hormone sensitive (HS-), irrespective of their PPD history. We hypothesized that the hormone challenge (both hormone addback and withdrawal) would provoke increased anhedonia in PPD+ but not in PPD-. We further hypothesized that the hormone challenge would decrease activity in the striatum during anticipation of and following the receipt of monetary rewards in HS+ but not HS- (primary hypothesis), and that activity in these regions at pre-treatment may distinguish HS+ from HS- (exploratory hypothesis).
Methods and Materials
Participants
Healthy, euthymic, unmedicated, non-pregnant, 22–45-year-old women (N=36) with regular menstrual cycles were recruited from the community using social media, flyers, mass emails, and print ads. All women had at least one biological child and were at least one year postpartum. To maximize the likelihood of selecting a HS+ phenotype of PPD, those in the PPD+ group were required to have had at least one past episode of major depression with an onset within four weeks postpartum, and no history of major depression with an onset outside of the postpartum period or any other psychiatric disorder, except for premenstrual dysphoric disorder (PMDD), which was not exclusionary. The PPD- group comprised parous women without a history of any past or present psychiatric disorder. Additional exclusionary criteria are listed in the Appendix.
The study [NCT 01762943] was pre-registered at https://clinicaltrials.gov/ct2/show/NCT01762943 and approved by the UNC Biomedical Institutional Review Board. The informed consent process comprised a descriptive overview of the study and discussion with the study team (CES and LCL), a questionnaire to ensure comprehension of the protocol and risks of participation, and written consent. Six women (4 PPD+ and 2 PPD-) dropped out of the study prior to completion for various reasons: two experienced traumatic life events, one was worried about the effects of leuprolide acetate, two started new jobs that prevented them from attending clinic visits, and one fell asleep during the first fMRI session and declined to repeat it. Thus, 30 women completed the protocol: 15 PPD+ and 15 PPD-. Participant demographics are detailed in Table 1. PPD+ were more likely to be divorced and of lower income status than PPD-; the groups did not differ on any of the other demographic variables measured. Of the 30 women who completed the protocol, 2 were excluded from fMRI analyses. One HS+ participant was removed due to substantial motion and the other was a woman with PPD-identified as HS+. The final sample for fMRI analyses included 10 women with HS+ and 18 women who were HS-, whether PPD+ (n=4) or PPD- (n=14).
Table 1.
Participant demographics.
| PPD+ n=15 |
PPD− n=15 |
|||||
|---|---|---|---|---|---|---|
|
| ||||||
| M | SD | M | SD | t df=28 | p | |
| Age | 35.27 | 4.061 | 36.33 | 3.658 | 0.76 | .456 |
| Education (in Years) | 16.60 | 2.098 | 16.80 | 1.699 | 0.29 | .776 |
| Number of pregnancies | 3.33 | 1.676 | 2.80 | 1.474 | −0.93 | .363 |
| Number of children | 2.47 | .915 | 2.07 | .884 | 0.47 | .234 |
| Age of youngest child | 4.13 | 4.454 | 3.40 | 4.421 | 0.17 | .654 |
|
|
||||||
| n | % | n | % | χ 2 | p | |
|
|
||||||
| Ethnicity | ||||||
| Hispanic or Latino | 2 | 13.3 | 0 | 0.0 | 3.33 | .189 |
| Not Hispanic or Latino | 12 | 80.0 | 15 | 100.0 | ||
| Declined | 1 | 6.7 | 0 | 0.0 | ||
| Racial Ancestry | ||||||
| American Indian or Alaskan Native | 2 | 13.3 | 0 | 0.0 | 5.18 | .159 |
| Asian | 0 | 0.0 | 2 | 13.3 | ||
| Black or African American | 1 | 6.7 | 3 | 20.0 | ||
| White | 12 | 80.0 | 10 | 66.7 | ||
| Marital Status | ||||||
| Married | 11 | 73.3 | 13 | 86.7 | 6.17 | .046 |
| Divorced | 4 | 26.7 | 0 | 0.0 | ||
| Single, never married | 0 | 0.0 | 2 | 13.3 | ||
| Household Income | ||||||
| $0–49,999 | 5 | 33.3 | 0 | 0.0 | 6.04 | .049 |
| $50,000–99,999 | 7 | 46.7 | 8 | 53.3 | ||
| $100,000+ | 3 | 20.0 | 6 | 40.0 | ||
| Declined | 0 | 0.0 | 1 | 6.7 | ||
| Current Psychotherapy | ||||||
| Yes | 2 | 13.3 | 0 | 0.0 | 2.14 | .143 |
| No | 13 | 86.7 | 15 | 100.0 | ||
| Trauma History Questionnaire | ||||||
| Any Trauma | ||||||
| Yes | 3 | 20.0 | 3 | 23.1 | 0.04 | .843 |
| No | 12 | 80.0 | 10 | 76.9 | ||
| Early Life Trauma | ||||||
| Yes | 7 | 46.7 | 8 | 61.5 | 0.62 | .431 |
| No | 8 | 53.3 | 5 | 38.5 | ||
Study Design
The study design is depicted in Figure 1. After providing written informed consent and prior to beginning the hormone protocol, participants completed a screening process, including a psychiatric assessment, gynecological exam, and self-ratings of mood and physical symptoms for one full menstrual cycle. The hormone administration protocol, detailed below, was initiated to mimic the hormone profile of pregnancy and parturition.
Figure 1.
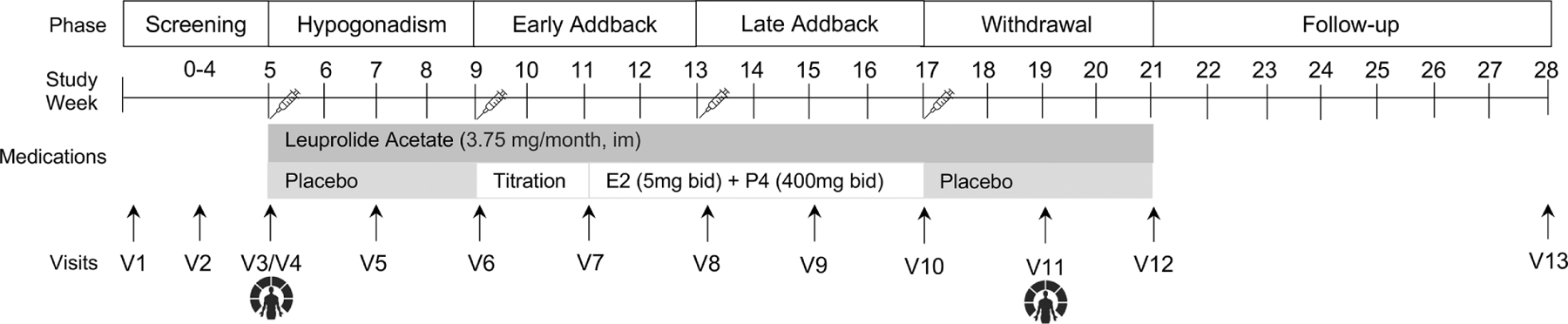
Study Design.
Hormone Administration
The hormone administration protocol was administered in a double-blind manner and replicated the methods of Bloch et al. (Bloch et al., 2000). Participants received monthly injections of the gonadotropin-releasing hormone (GnRH) agonist leuprolide acetate (Lupron) 3.75 mg/month via intramuscular injection to produce a stable hypogonadal condition (after the initial “flair”). The first leuprolide acetate injection was administered on day six of the participants’ first menstrual cycle, and monthly thereafter, for a total of 4 months. During the first month of leuprolide acetate administration, all participants received placebo capsules and were told that at some point the placebo would be switched to active medication. In order to maintain blinding, participants took the same number of capsules throughout the protocol, medications were dispensed by the Investigational Drug Service at study visits, which occurred every two weeks during the hormone protocol, and clinical ratings were made by staff blinded to participant group and hormone phase.
After one month of leuprolide acetate-alone treatment (i.e., hypogonadal state), micronized progesterone and estradiol tablets were administered for eight weeks with continued leuprolide acetate administration. Micronized estradiol (Estrace, Allergan USA, Inc., Irvine, CA) was started at a dose of 2mg bid for two weeks, then increased up to 5mg bid for six weeks; micronized progesterone was started at 200mg bid for two weeks and then increased up to 400mg bid for six weeks (see Figure 1) (Bloch et al., 2000).
After eight weeks of hormone replacement, active hormone capsules were replaced with placebo capsules to induce a precipitous drop in plasma estradiol and progesterone levels (“withdrawal”). Leuprolide acetate was used to maintain hypogonadal levels for the four-week withdrawal phase. Participants were followed for an additional eight more weeks (i.e., the “follow-up” phase).
Clinical Assessments
The Structured Clinical Interview for DSM-IV-TR Axis-I Disorders (SCID-IV) (First et al., 2002) was administered at pre-treatment to assess past and present psychiatric illness and the Trauma History Questionnaire (Hooper et al., 2011) was administered to characterize the sample. Each of the following measures were administered at pre-treatment and at bi-monthly clinic visits. The Inventory of Depression and Anxiety Symptoms (IDAS) (Watson et al., 2007), validated in both non-pregnant and postpartum women, was used to assess anxiety and depression symptoms along nine separate dimensions. Anhedonia was operationally defined as the inverse of the IDAS Wellbeing scale score and was the primary mood outcome variable (Jimenez et al., 2022; Khoo et al., 2020). The IDAS Wellbeing Scale is highly correlated with standard measures of anhedonia (e.g., the Mood and Anxiety Questionnaire (MASQ) Anhedonic Depression Scale, r=−.89) and taps low positive affectivity (Watson et al., 2017)—a defining feature of major depressive disorder in quantitative hierarchical models of internalizing psychopathology (Simms et al., 2022). We also included gold-standard self-report and clinician-rated measures of perinatal depression symptom severity, the Edinburgh Postnatal Depression Scale (EPDS) (Watson et al., 2017) and the Hamilton Rating Scale for Depression (HRSD) (Hamilton, 1960), respectively.
fMRI Data Acquisition and Preprocessing
Following the screening process, participants completed the first (“pre-treatment”) fMRI session within menstrual days 4–6, the early to mid-follicular phase, during a period of relatively low and stable reproductive hormone levels to promote consistency in hormone profiles across participants and provide the optimal contrast for affective symptoms during the period of reproductive hormone withdrawal. The second (“withdrawal”) fMRI session occurred two weeks following the last active hormone dose (i.e., midway through the month-long withdrawal phase).
fMRI Task
Participants completed the Monetary Incentive Delay (MID) task to engage reward circuitry during monetary reward anticipation and outcome (Knutson et al., 2001), described in detail in the appendix (p. 2).
fMRI Monetary Incentive Delay Task
Participants completed the Monetary Incentive Delay (MID) task to engage reward circuitry during monetary reward anticipation and outcome (Knutson et al., 2001). Participants completed one practice run outside the scanner followed by two functional imaging runs consisting of 72 trials. During each trial, participants saw one of nine cue shapes (cue; 2000 ms), fixated on a crosshair for a variable interval (anticipation; 2000–2500 ms), viewed target of variable duration indicating to press a button as quickly as possible (target; up to 500 ms), shown in Figure S1 (Supplementary Material, p. 2 (Knutson et al., 2001). Following the target presentation, feedback was presented indicating the amount of money they had gained or lost during that trial and the total amount of money they had gained during the run (outcome; 3000 ms). During incentive trials, participants could either gain or avoid losing money by pressing the button during target presentation. The task was set up to reward participants regardless of their reaction time on two thirds of their target responses. FMRI volume acquisitions were time-locked to cue offset and outcome presentations were therefore acquired during anticipation and outcome periods (Knutson et al., 2001).
As described previously (Knutson et al., 2008) and shown in Figure S2 (Supplementary Material, p. 3), cues identified potential gains (n=28, indicated by circles), potential losses (n=26, indicated by squares), or no response required (n=18, indicated by triangles). The number of lines within each shape denoted the magnitude of the gain or loss: $0 (no lines), $1 (one line), or $5 (two lines). No response trials (triangles) indicated that the participant should not respond to that trial and wait for the next trial (Knutson et al., 2008). For the current study, we examined responses to Gain cues, and Loss cues were not analyzed. Each 9-minute run included ten $5 (“high”), nine $1 (“low”), and nine $0 (“non-win”) Gain trials. Stimuli were presented using E-Prime (Psychology Software Tools Inc. Pittsburgh, PA) using a mirrored screen. Participants were trained for at least 10 minutes, demonstrated cue comprehension, and told they would be given cash based on their performance before entering the scanner.
fMRI Image Acquisition
Scans were performed using a Siemens Magnetom 3T TIM Trio scanner. High-resolution, T1-weighted anatomical images were acquired using an MPRAGE sequence (TR=2400 ms; TE=3.16 ms; FOV=256 mm; acquisition matrix = 256 × 224 × 160 mm; flip angle = 8°; voxel size = 1 × 1 × 1 mm; 160 axial slices). This structural image was aligned in a near axial plane defined by the anterior and posterior commissures. Whole-brain functional images were acquired using a multi-slice, interleaved pulse sequence (TR = 2000 ms; TE = 25 ms; FOV = 256 mm; acquisition matrix = 256 × 232 × 144 mm; flip angle = 80°; voxel size = 4 4 × 4 mm; 36 axial slices) sensitive to blood-oxygen-level-dependent (BOLD) contrast.
fMRI Data Preprocessing and Motion Correction
Data were preprocessed using FSL version 6.0.4 (FMRIB Software Library; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/; (Jenkinson et al., 2012). Oxford University, U. K.), ANTs version 2.3.1 (http://stnava.github.io/ANTs/), and AFNI version 17.2.09 (https://afni.nimh.nih.gov/). Standard preprocessing in FEAT v.6.00 included motion correction with MCFLIRT, slice timing correction, BET brain extraction, pre-whitening with the FILM tool (Woolrich et al., 2001) co-registration of functional and anatomical images using the boundary based registration algorithm (Greve and Fischl, 2009), registration to a standard stereotaxic space (Montreal Neurological Institute; MNI152 2mm) using nonlinear transformation (FNIRT; 12 DOF, 10mm warp resolution), and high-pass filtering (cutoff of 100sec). We corrected for a susceptibility artifact in the left orbito-frontal cortex by using ANTS to run a cross-modality deformable registration with mutual information as our metric between the functional images and each subject’s T1 image. Because the ANTS warping algorithm resulted in some spatial smoothing, we conducted smoothing to FWHM 5 mm using AFNI’s “3dBlurToFWHM”. To control for excessive motion, participants with motion greater than 3 mm along any of six axes (i.e., x, y, z, pitch, yaw, and roll) were excluded from analyses (n=1 HS+). We also excluded volumes that exceeded a framewise displacement threshold of 0.5mm (Siegel et al., 2013). Runs with greater than 20% of volumes censored were discarded. T-tests were used to compare differences in motion between groups: there were no significant differences in mean and maximum values along any of the six axes (all p values > .10).
Plasma Hormone Levels
Serum estradiol and progesterone levels were assessed with enzyme linked immunosorbent assay (ELISA). Estradiol ultrasensitive assays (supplier: ALPCO) had an intra-assay coefficient of variation (CV) of 2.02%, inter-assay CV of 4.19%, and sensitivity of 2.2 pg/ml. Progesterone assays (supplier: ALPCO) had an intra-assay CV of 4.92, inter-assay CV of 7.03%, and sensitivity of 100 pg/ml.
Statistical Analysis
Effects of Hormone Protocol on Mood Symptoms
To examine whether PPD+ would report increased anhedonia during hormone addback and withdrawal, compared with PPD-, we used multilevel models. The following study phases were examined within subjects: (1) Pre-treatment was defined as the first fMRI session (visit 3), which was timed to occur on day 4–6 of the menstrual cycle and occurred prior to the administration of leuprolide acetate; (2) Early hormone addback, defined as the first 4 weeks of hormone addback (visits 7–8) after leuprolide acetate administration when hormone levels were being titrated up to their full dose; (3) Late hormone addback, defined as the second 4 weeks of hormone addback (visits 9–10) when hormone levels were held constant at the full dose; (4) Withdrawal, defined as the 4 weeks (visits 11–12) when participants were maintained on leuprolide acetate but were taking placebo only, and thus, hormone levels were falling; and (5) Follow-Up, defined as visit 13, which occurred approximately 8 weeks after the cessation of all medications. The primary outcome measured was the IDAS Wellbeing scale, the inverse of which was defined as anhedonia (Jimenez et al., 2022; Khoo et al., 2020). Additional outcome measures explored included the EPDS, HRSD, and IDAS subscales.
Multilevel models (structured as 8 visits nested within each participant), conducted using PROC MIXED in SAS 9.5 (SAS Institute, Cary, NC), were used to test the interactive effects of diagnostic Group (PPD- = 0, PPD+ = 1; between-subjects factor) and study phase (within-subjects factor). Categorical coding of the phase variable with pre-treatment specified as the reference phase allowed for examination of group differences in phase contrasts (i.e., Pre-treatment vs. all other phases). We used a restricted maximum likelihood (REML) approach and specified the Kenward-Roger method for denominator degrees of freedom. An autoregressive (phase-1) covariance structure for within-person error was considered but rejected because it did not improve model fit. In each model, we retained random effects for each phase contrast only where doing so improved model fit.
Identification of a HS+ Phenotype
In order to define a relatively “pure” HS+ phenotype for the fMRI analyses, we operationally defined hormone sensitivity as a 30% increase in affective symptoms (Schmidt et al., 1998) during hormone addback or withdrawal compared with pre-treatment as measured by the IDAS. Because some of the IDAS scales assess physical symptoms of depression that are also known side effects of estradiol and progesterone administration (e.g., fatigue, appetite change) that affect non-depressed women, we included scales focused on the emotional experience of depression to determine hormone sensitivity. Thus, a 30% increase (i.e., percent change from pre-treatment to withdrawal=(average withdrawal score-pre-treatment score)/pre-treatment score)) in the dysphoria, anhedonia (i.e., reverse scored wellbeing), and ill temper scale scores was used to define the HS+ phenotype (Schmidt et al., 1998). All participants, both PPD+ and PPD-, who did not show a 30% increase in any mood symptom during either hormone addback or withdrawal were classified as HS-. Because we were interested in the HS+ phenotype specifically and not in PPD history more generally, we did not attempt to disambiguate the effects of a past PPD in HS- and no history of PPD.
fMRI Task Analysis
We examined the neural correlates of reward anticipation and reward outcomes using a whole brain voxel-wise general linear model (GLM). The GLM for the MID task contained separate regressors for each anticipation and outcome period: reward trials [win $1 or 5], non-eward trials [win $0], and neutral trials [no response required and no chance of winning or losing]. Contrasts of interest included (1) reward vs. non-reward anticipation, (2) reward win vs. non-reward win outcome, and (3) reward win vs. reward miss outcome. Although this version of the MID task contained loss trials that were included as regressors in the GLM (to minimize impact on the implicit baseline), results from loss-specific contrasts are not reported here. Prior to performing group-level or mixed-model analyses, task runs were combined in a fixed-effects model.
Whole-brain task-based functional activation maps were generated using the permutation analysis of linear models (PALM) toolbox (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/PALM/) (Winkler et al., 2015). For our application of PALM, data were permuted 5000 times and significant effects were identified through threshold-free cluster enhancement method (TFCE) (Smith and Nichols, 2009), controlling for family-wise error (FWE) rate of P < 0.05. To further reduce risk of false positives, we only report clusters with a minimum voxel size of 50. To evaluate group differences at pre-treatment (prior to hormone administration), we conducted a two-sample unpaired t-test, with (demeaned) age included as a nuisance covariate (i.e., HS+ versus HS-). Next, a 2×2 mixed-effect ANOVA was used to examine the effect of condition (pre-treatment versus hormone withdrawal) and the interaction of group (HS+ versus HS-) by condition (pre-treatment versus hormone withdrawal). For the 2×2 model, exchangeability block structure was specified such that observations could be shuffled both within-person (“within-block”) and between-person (“whole-block”) (Winkler et al., 2015).
For activation maps that yielded significant clusters, cluster size and peak activations are reported in MNI coordinate space. In order to understand the extent to which condition-related changes in brain activation were related to symptom changes, for each participant and condition, Blood-Oxygen Level Dependent (BOLD) percent-signal change was calculated and extracted from significant data-derived clusters using FSL featquery. We then examined associations between percent-signal change in significant clusters and self-reported ratings of anhedonia. Furthermore, given the central relevance of Dysphoria (sadness) and Ill-Temper (irritability) in both reproductive-related mood disorders (de Wit et al., 2021; Kaiser et al., 2018; Williamson et al., 2015) and mood disorders (Bell et al., 2021) more generally, we also explored associations between BOLD responses in significant clusters and these IDAS symptom scales. We view these brain-behavior correlations as post-hoc and exploratory.
Results
Effects of Hormone Protocol on Mood Symptoms
Results of hierarchical linear models comparing early and late hormone addback, withdrawal, and follow-up with pre-treatment across PPD+ (n=15) and PPD- (n=15) revealed significant group by hormone phase differences in anhedonia and other psychiatric symptoms in response to the hormone challenge (Appendix p. 2). Compared with PPD-, PPD+ showed increased anhedonia as measured by the IDAS during early hormone addback, late hormone addback, and withdrawal (Figure 2). Exploratory analyses also showed increased IDAS Dysphoria, Ill Temper (Irritability), and EPDS total scores during both addback and withdrawal, whereas HAM-D total scores were elevated during addback but not during withdrawal in PPD+ compared with PPD- (Appendix p. 2). There were no significant group differences at pre-treatment or follow-up.
Figure 2.
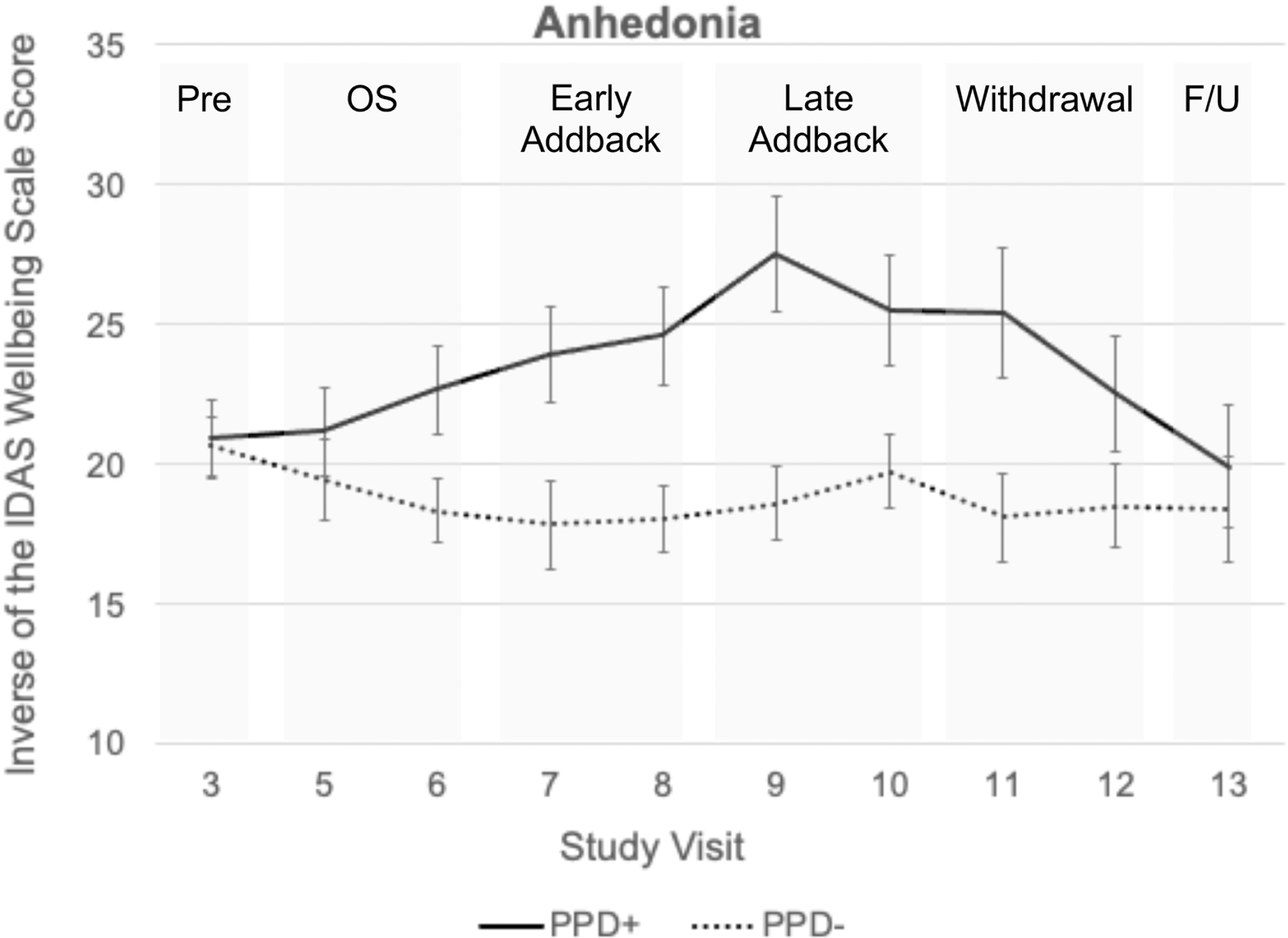
Anhedonia ratings in PPD+ and PPD- groups during each hormone phase.
Eleven out of 15 (73%) PPD+ met criteria for HS+. One out of 15 PPD- met criteria for HS+, and she was excluded from fMRI analyses. In total, 18 participants were classified at HS-(4 PPD+ and 14 PPD-).
Effects of Hormone Withdrawal on Brain Function in Response to Monetary Reward
The final sample for fMRI analyses included 10 women with HS+ (one of the 11 HS+ was removed due to substantial motion) and 18 women who were HS-. At pre-treatment (prior to hormone administration), there were no significant between-group differences in functional brain activation during any MIDT reward anticipation and outcome phases. Further, we did not observe any significant Group (HS+ versus HS-) x Condition interaction effects (pre-treatment versus hormone withdrawal) of functional brain activation during anticipation or receipt of rewards on the MIDT. However, there was a significant main effect of condition: During hormone withdrawal, compared to pre-treatment, all participants (irrespective of HS status) showed decreased activation in clusters within the right putamen (Figure 3A; Table 1) and left postcentral and supramarginal gyri (Figure 3B; Table 2) during successful reward (vs. non-reward) feedback (i.e., winning $1 or $5 vs. winning $0).
Figure 3.
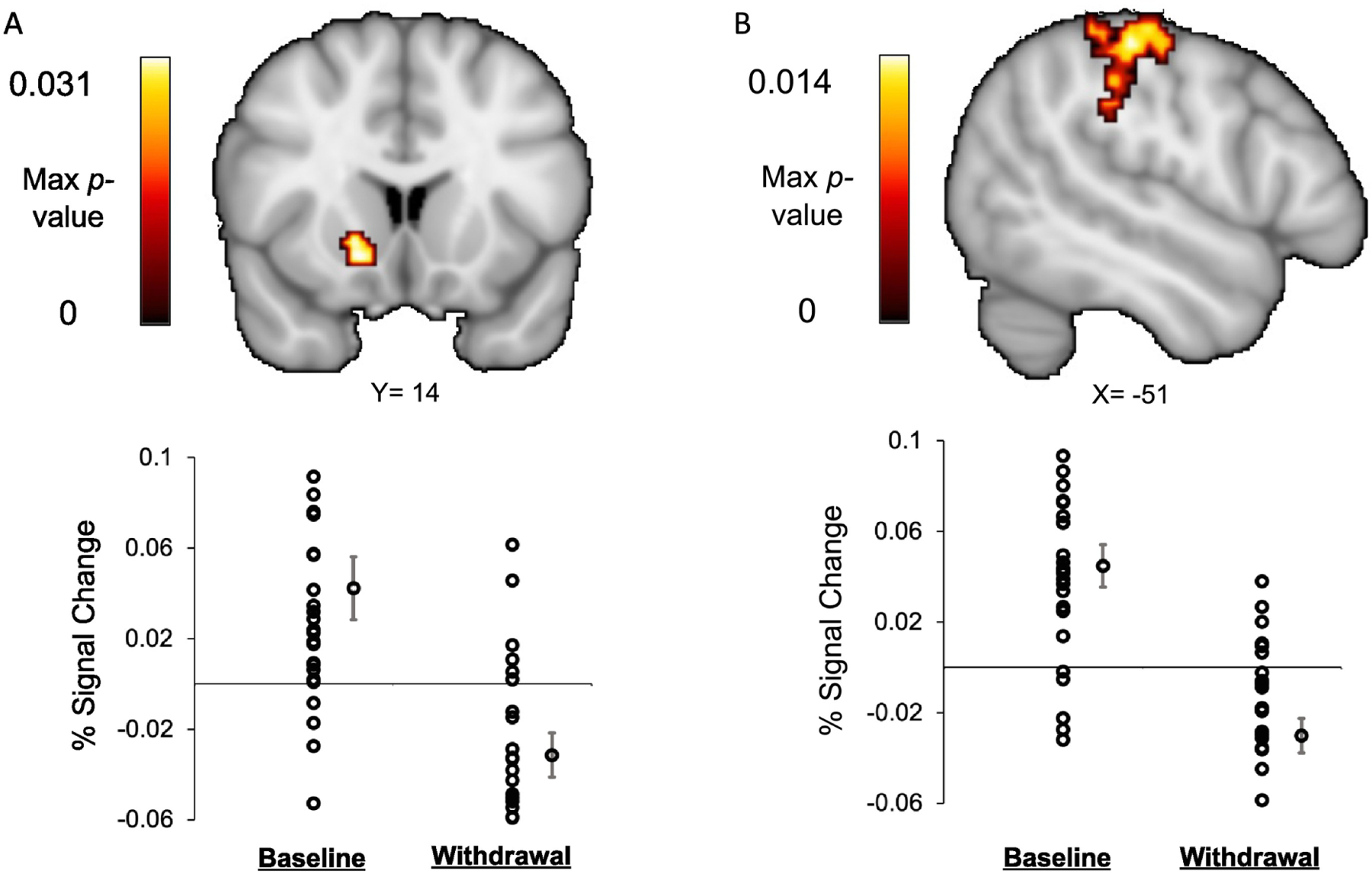
Activation of the (A) right putamen and (B) left postcentral and supramarginal gyri at pre-treatment relative to hormone withdrawal during successful reward feedback (Reward Win > Non-Reward Win) on the MID task MID in women (N=28), 10 of whom were HS+ and 18 of whom were HS-. Clusters significant at p<0.05 (FWE-corrected) using Threshold Free Cluster Enhancement. Max p-value represents p-value at peak MNI coordinates.
Table 2.
Effect of condition (pre-treatment > hormone withdrawal) on whole-brain functional activation during successful reward feedback (Reward Win > Non-Reward Win) in all participants.
| Region | MNI coordinates | ||||
|---|---|---|---|---|---|
| Baseline > Withdrawal | X | Y | Z | k | Peak p-value, FWE-corrected |
| L Postcentral Gyrus / Supramarginal Gyrus | −50 | −26 | 52 | 428 | 0.014 |
| R Putamen | 16 | 14 | −8 | 97 | 0.031 |
Note: Clusters are significant at p<0.05 (whole-brain FWE-corrected) using Threshold Free Cluster Enhancement; k = number of voxels; R: right, L: left.
Correlations Between Brain Activation and Anhedonia
Neither activation of the right putamen (r=.04, p>.85; n=28 ) nor activation of the left postcentral or supramarginal gyri during reward outcome (r=−.10, p>.63; n=28 ) at the hormone withdrawal scan were significantly associated with anhedonia. The change in activation within each brain area was also not associated with the change in anhedonia between the pre-treatment and withdrawal time points (p’s>.10). However, analyses revealed a significant correlation between activation of the left supramarginal gyrus with IDAS Ill Temper scores measured during hormone withdrawal such that lower activation was associated with higher Ill Temper (r=−.536, p<.005, n=28), with Bonferroni correction.
Plasma Hormone Levels
Plasma estradiol and progesterone levels were sufficiently elevated during the addback phase without significant differences between HS+ (estradiol mean =1212 pg/ml, SD=549; progesterone mean =62 ng/ml, SD=42) and HS- (estradiol mean =1423 pg/ml, SD=589; progesterone mean =69 ng/ml, SD=100). Similarly, plasma estradiol or progesterone levels fell as expected during withdrawal phase without significant differences between HS+ (estradiol mean =78 pg/ml, SD=38; progesterone mean =0.53 ng/ml, SD=0.76) and HS- (estradiol mean =195 pg/ml, SD=204; progesterone mean =0.83 ng/ml, SD=1.4). The ANOVA comparing the main effect of group (HS+ and HS-) and group by phase interaction were nonsignificant for both hormones. Participants showed return of endogenous hormone production during the follow-up phase and resumed regular menses following the end of the trial.
Discussion
The goals of this study were as follows: 1) characterize the increase in anhedonia and other affective symptoms provoked by estradiol and progesterone in euthymic women with PPD+ relative to PPD-; 2) explore whether a neural signature could identify those vulnerable to hormone sensitivity at pre-treatment prior to the hormone challenge, and 3) characterize changes in neural reward circuit activation relative to pre-treatment in HS+ compared with HS-. The significant increase in anhedonia and other depressive symptoms provoked by both addback and withdrawal of estradiol and progesterone in PPD+, but not in PPD-, supported our primary hypothesis and replicated an earlier finding that PPD+ have a differential response to abrupt changes in plasma levels of gonadal steroids (Bloch et al., 2000). Results are also consistent with the epidemiologic studies of perinatal depression and anxiety (Stowe et al., 2005), demonstrating that PPD symptoms commonly begin during pregnancy.
Despite the robust effects of the steroid manipulation on mood in HS+, the source of the differential sensitivity to hormones remains unclear—contrary to our hypothesis, we did not find differences in neural activation between groups either at pre-treatment or during hormone withdrawal. The caudate, putamen, and nucleus accumbens are the three regions most reliably activated by the MID task (Wilson et al., 2018). Compared with euthymic participants, individuals with MDD show reduced activation of caudate, putamen, thalamus, insula, and anterior cingulate in response to rewards (Zhang et al., 2013). Thus, we hypothesized we would see differences between HS+ and HS- groups in the caudate, putamen, and nucleus accumbens. However, women in both HS+ and HS- groups demonstrated reduced activation during reward feedback in the right putamen and left postcentral and supramarginal gyri during hormone withdrawal compared with pre-treatment, and thus, the differential hormone regulation of these brain regions can be ruled out as a source of susceptibility to PPD. Nevertheless, the change in response to monetary reward as a result of hormone withdrawal across both groups is consistent with prior research demonstrating that gonadal steroids powerfully regulate response to reward in the putamen (Meitzen et al., 2018), regardless of predisposition to depression (Dreher et al., 2007). Prior studies also demonstrate a unilateral response of the left supramarginal gyrus in response to monetary reward, which is thought to reflect engagement of the central-executive network (CEN) (Wilson et al., 2018). Reduced activity in selective nodes of the CEN, such as the supramarginal gyrus, may be associated with dysfunction of goal directed cognitive tasks (Ge et al., 2019), which is a commonly reported postpartum phenomenon (Brown and Schaffir, 2019). Although reward-related activation was not associated with ratings of anhedonia during hormone withdrawal, the association between activation in the left supramarginal gyrus and self-reported irritability during hormone withdrawal may help explain irritability onset during the postpartum period (Williamson et al., 2015).
Strengths of the current study were the prospective, experimental confirmation of a homogeneous HS+ phenotype for the purpose of neuroimaging, conservative statistical methods to avoid false discovery in a small sample size, and repeated symptom and neural activation assessments under different hormone conditions. Albeit laborious, the selection of this relatively pure phenotype within the group of women with a history of PPD permitted assessment of the underlying neural substrates of both susceptibility to and triggering of hormone precipitated depression. Despite these important strengths, the study also had limitations. First, the sample size was modest. Contrary to prior findings (Wacker et al., 2009), we did not find any significant differences in nucleus accumbens activation over time or between HS+ and HS-, which may reflect limited power to detect effects in this small brain structure. Second, as a result of the stringent inclusion and exclusion criteria for undergoing the hormone protocol, the vast majority of women with a history of PPD whom we screened were excluded due to prescription medication use (most often hormonal contraceptives and antidepressant medication) or a personal or family history of a medical condition that precluded hormone treatment. Additionally, the majority of women who participated in this study were white, married, and middle-class. The population of women who experience PPD is more racially and ethnically diverse (Guintivano et al., 2018), more likely to be single and poor (Segre et al., 2007) and have a history of MDD outside the perinatal period (Guintivano et al., 2018). Additional research with a larger, more diverse sample and with less stringent inclusion criteria is needed to understand how results generalize the broader population of those at risk for PPD. Additionally, the use of the IDAS Wellbeing Scale as the primary measure of anhedonia is novel, although consistent with the literature defining anhedonia as a deficit in positive affect (Craske et al., 2016). Prior research examining anhedonia and reward responsiveness using the MID have employed the MASQ Anhedonic Depression Scale (Watson and Clark, 1991) or the Snaith-Hamilton Pleasure Scale (Snaith et al., 1995). Although the IDAS Wellbeing Scale is not regarded as a standard measure of anhedonia, it is highly correlated with the MASQ Anhedonic Depression Scale (Watson et al., 2017).
In this study, estradiol and progesterone administration and withdrawal were accompanied by reduced activation of the putamen and left postcentral and supramarginal gyri in response to a monetary reward task. Although these findings do not explain differential hormone sensitivity in depression, they demonstrate significant effects of reproductive hormones on reward-related brain function and mood. Future attempts to identify the source of differential hormone sensitivity in depression may enable the identification of new treatment targets and enable the prevention of perinatal depression.
Supplementary Material
Highlights.
The pregnancy hormones estradiol and progesterone triggered depression symptoms in women with a history of postpartum depression.
Pregnancy hormones decreased activity in brain areas that respond to rewards.
Changes in brain activity in the reward network caused by pregnancy hormones did not explain why some women experience postpartum depression while others do not.
Acknowledgements
Dr. Schiller acknowledges Dr. Samantha Meltzer-Brody and Dr. Susan Girdler for their mentorship; Dr. Kai Xia and Dr. Robert Hamer for statistical consulting; Russ Dean for data management; Anna Abate for assisting with data collection; Dr. Neil Fedarko for conducting hormone assays; Amber Leinwand for assisting with the imaging protocol; Dr. Martin Styner for assisting with artifact correction; and Dr. Mary Kimmel and Dr. Leeza Park for editing drafts of this manuscript.
Role of the Funding Source
This research was supported by funding from the Foundation of Hope (CES), Brain & Behavior Research Foundation (CES), NIH R21MH101409 (DRR and CES), NIH T32MH093315 (DRR), RF1MH120843 (TEM) and the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number UL1TR002489 (DRR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The sponsors had no input into the study design; collection, analysis or interpretation of the data; in writing the report; or in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Dr. Rubinow serves on the Scientific Advisory Board of the Foundation of Hope, one of the funding sources for this study. Dr. Eisenlohr-Moul receives consulting fees from the nonprofit International Association for Premenstrual Disorders. Dr. Schiller, Dr. Walsh, Dr. Dichter, Dr. Schiff, Mr. Bizzell, Dr. Slightom, Ms. Richardson, Dr. Belger, and Dr. Schmidt have no conflicts of interest to report.
References
- Bell E, Boyce P, Porter RJ, Bryant RA, Malhi GS, 2021. Irritability in Mood Disorders: Neurobiological Underpinnings and Implications for Pharmacological Intervention. CNS Drugs 35, 619–641. 10.1007/s40263-021-00823-y [DOI] [PubMed] [Google Scholar]
- Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR, 2000. Effects of gonadal steroids in women with a history of postpartum depression. Am. J. Psychiatry 157, 924–930. [DOI] [PubMed] [Google Scholar]
- Borsini A, Wallis ASJ, Zunszain P, Pariante CM, Kempton MJ, 2020. Characterizing anhedonia: A systematic review of neuroimaging across the subtypes of reward processing deficits in depression. Cogn. Affect. Behav. Neurosci 20, 816–841. 10.3758/s13415-020-00804-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E, Schaffir J, 2019. “Pregnancy Brain”: A Review of Cognitive Changes in Pregnancy and Postpartum. Obstet. Gynecol. Surv 74, 178–185. 10.1097/OGX.0000000000000655 [DOI] [PubMed] [Google Scholar]
- Cho J, Stone MD, Leventhal AM, 2019. Anhedonia as a Phenotypic Marker of Familial Transmission of Polysubstance Use Trajectories across Mid-Adolescence. Psychol. Addict. Behav. J. Soc. Psychol. Addict. Behav 33, 15–25. 10.1037/adb0000427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JL, Holden JM, Sagovsky R, 1987. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry 150, 782–786. [DOI] [PubMed] [Google Scholar]
- Craske MG, Meuret AE, Ritz T, Treanor M, Dour HJ, 2016. Treatment for Anhedonia: A Neuroscience Driven Approach. Depress. Anxiety 33, 927–938. 10.1002/da.22490 [DOI] [PubMed] [Google Scholar]
- de Wit AE, Giltay EJ, de Boer MK, Nathan M, Wiley A, Crawford S, Joffe H, 2021. Predictors of irritability symptoms in mildly depressed perimenopausal women. Psychoneuroendocrinology 126, 105128. 10.1016/j.psyneuen.2021.105128 [DOI] [PubMed] [Google Scholar]
- Dichter GS, Damiano CA, Allen JA, 2012a. Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: animal models and clinical findings. J. Neurodev. Disord 4, 19. 10.1186/1866-1955-4-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Kozink RV, McClernon FJ, Smoski MJ, 2012b. Remitted major depression is characterized by reward network hyperactivation during reward anticipation and hypoactivation during reward outcomes. J. Affect. Disord 136, 1126–1134. 10.1016/j.jad.2011.09.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JC, Schmidt PJ, K ohn P, Furman D, Rubinow D, Berman KF, 2007. Menstrual cycle phase modulates reward-related neural function in women. Proc. Natl. Acad. Sci. U. S. A 104, 2465–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP) Biometrics Research, New York State Psychiatric Institute, New York. [Google Scholar]
- Ge R, Downar J, Blumberger DM, Daskalakis ZJ, Lam RW, Vila-Rodriguez F, 2019. Structural network integrity of the central executive network is associated with the therapeutic effect of rTMS in treatment resistant depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 92, 217–225. 10.1016/j.pnpbp.2019.01.012 [DOI] [PubMed] [Google Scholar]
- Gordon JA, Bellgowan JAF, Lawhorn C, Scheinert RB, 2018. Challenges and Opportunities in Psychiatric Neuroscience. Cold Spring Harb. Symp. Quant. Biol 83, 1–8. 10.1101/sqb.2018.83.037523 [DOI] [PubMed] [Google Scholar]
- Greene RK, Walsh E, Mosner MG, Dichter GS, 2019. A potential mechanistic role for neuroinflammation in reward processing impairments in autism spectrum disorder. Biol. Psychol 142, 1–12. 10.1016/j.biopsycho.2018.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve DN, Fischl B, 2009. Accurate and robust brain image alignment using boundary-based registration. NeuroImage 48, 63–72. 10.1016/j.neuroimage.2009.06.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grob S, Pizzagalli DA, Dutra SJ, Stern J, Mörgeli H, Milos G, Schnyder U, Hasler G, 2012. Dopamine-Related Deficit in Reward Learning After Catecholamine Depletion in Unmedicated, Remitted Subjects with Bulimia Nervosa. Neuropsychopharmacology 37, 1945–1952. 10.1038/npp.2012.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guintivano J, Sullivan P, Stuebe A, Penders T, Thorp J, Rubinow D, Meltzer-Brody S, 2018. Adverse Life Events, Psychiatric History, and Biological Predictors of Postpartum Depression in an Ethnically Diverse Sample of Postpartum Women. Psychol. Med 48, 1190–1200. 10.1017/S0033291717002641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M, 1960. A Rating Scale for Depression. J. Neurol. Neurosurg. Psychiatry 23, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han GT, Tomarken AJ, Gotham KO, 2019. Social and nonsocial reward moderate the relation between autism symptoms and loneliness in adults with ASD, depression, and controls. Autism Res 12, 884–896. 10.1002/aur.2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper LM, Stockton P, Krupnick JL, Green BL, 2011. Development, Use, and Psychometric Properties of the Trauma History Questionnaire. J. Loss Trauma 16, 258–283. 10.1080/15325024.2011.572035 [DOI] [Google Scholar]
- Huhn AS, Meyer RE, Harris JD, Ayaz H, Deneke E, Stankoski DM, Bunce SC, 2016. Evidence of anhedonia and differential reward processing in prefrontal cortex among post-withdrawal patients with prescription opiate dependence. Brain Res. Bull, Addiction: From Mouse to Man 123, 102–109. 10.1016/j.brainresbull.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain M, Roiser JP, 2018. Neuroscience of apathy and anhedonia: a transdiagnostic approach. Nat. Rev. Neurosci 19, 470–484. 10.1038/s41583-018-0029-9 [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM, 2012. FSL. NeuroImage 62, 782–790. 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- Jimenez A, McMahon TP, Watson D, Naragon-Gainey K, 2022. Dysphoria and well-being in daily life: Development and validation of ecological momentary assessment scales. Psychol. Assess 10.1037/pas0001117 [DOI] [PubMed] [Google Scholar]
- Kaiser G, Janda C, Kleinstäuber M, Weise C, 2018. Clusters of premenstrual symptoms in women with PMDD: Appearance, stability and association with impairment. J. Psychosom. Res 115, 38–43. 10.1016/j.jpsychores.2018.10.004 [DOI] [PubMed] [Google Scholar]
- Keren H, O’Callaghan G, Vidal-Ribas P, Buzzell GA, Brotman MA, Leibenluft E, Pan PM, Meffert L, Kaiser A, Wolke S, Pine DS, Stringaris A, 2018. Reward Processing in Depression: A Conceptual and Meta-Analytic Review Across fMRI and EEG Studies. Am. J. Psychiatry 175, 1111–1120. 10.1176/appi.ajp.2018.17101124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo S, Stanton K, Clark LA, Watson D, 2020. Facet-Level Personality Relations of the Symptom Dimensions of the Tripartite Model. J. Psychopathol. Behav. Assess 42, 160–177. 10.1007/s10862-019-09763-w [DOI] [Google Scholar]
- Knutson B, Bhanji JP, Cooney RE, Atlas LY, Gotlib IH, 2008. Neural responses to monetary incentives in major depression. Biol. Psychiatry 63, 686–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D, 2001. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport 12, 3683–7. [DOI] [PubMed] [Google Scholar]
- Kumar P, Goer F, Murray L, Dillon DG, Beltzer ML, Cohen AL, Brooks NH, Pizzagalli DA, 2018. Impaired reward prediction error encoding and striatal-midbrain connectivity in depression. Neuropsychopharmacology 43, 1581–1588. 10.1038/s41386-018-0032-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta ND, Stevens JS, Li Z, Gillespie CF, Fani N, Michopoulos V, Felger JC, n.d. Inflammation, reward circuitry and symptoms of anhedonia and PTSD in trauma-exposed women. Soc. Cogn. Affect. Neurosci 10.1093/scan/nsz100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzen J, Meisel RL, Mermelstein PG, 2018. Sex differences and the effects of estradiol on striatal function. Curr. Opin. Behav. Sci., Sex and Gender 23, 42–48. 10.1016/j.cobeha.2018.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses-Kolko EL, Fraser D, Wisner KL, James JA, Saul AT, Fiez JA, Phillips ML, 2011. Rapid habituation of ventral striatal response to reward receipt in postpartum depression. Biol. Psychiatry 70, 395–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses-Kolko EL, Wisner KL, Price JC, Berga SL, Drevets WC, Hanusa BH, Loucks TL, Meltzer CC, 2008. Serotonin 1A receptor reductions in postpartum depression: a positron emission tomography study. Fertil. Steril 89, 685–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M, 2007. Motivational systems and the neural circuitry of maternal behavior in the rat. Dev. Psychobiol 49, 12–21. 10.1002/dev.20198 [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Dougherty DD, Iosifescu DV, Rauch SL, Fava M, 2009. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am. J. Psychiatry 166, 702–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam KT, Wilcox M, Robertson-Blackmore E, Sharkey K, Bergink V, Munk-Olsen T, Deligiannidis KM, Payne J, Altemus M, Newport J, Apter G, Devouche E, Viktorin A, Magnusson P, Penninx B, Buist A, Bilszta J, O’Hara M, Stuart S, Brock R, Roza S, Tiemeier H, Guille C, Epperson CN, Kim D, Schmidt P, Martinez P, Di Florio A, Wisner KL, Stowe Z, Jones I, Sullivan PF, Rubinow D, Wildenhaus K, Meltzer-Brody S, 2017. Clinical phenotypes of perinatal depression and time of symptom onset: analysis of data from an international consortium. Lancet Psychiatry 4, 477–485. 10.1016/S2215-0366(17)30136-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi SJ, Lambert C, Kennedy S, 2018. Presentation and Neurobiology of Anhedonia in Mood Disorders: Commonalities and Distinctions. Curr. Psychiatry Rep 20, 13. 10.1007/s11920-018-0877-z [DOI] [PubMed] [Google Scholar]
- Schiller CE, Johnson SL, Abate AC, Schmidt PJ, Rubinow DR, 2016. Reproductive steroid regulation of mood and behavior. Compr. Physiol 6, 1135–1160. 10.1002/cphy.c150014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller CE, Minkel J, Smoski MJ, Dichter GS, 2013. Remitted major depression is characterized by reduced prefrontal cortex reactivity to reward loss. J. Affect. Disord 151, 756–762. 10.1016/j.jad.2013.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR, 1998. Differential Behavioral Effects of Gonadal Steroids in Women with and in Those without Premenstrual Syndrome. N. Engl. J. Med 338, 209–216. 10.1056/NEJM199801223380401 [DOI] [PubMed] [Google Scholar]
- Segarra N, Metastasio A, Ziauddeen H, Spencer J, Reinders NR, Dudas RB, Arrondo G, Robbins TW, Clark L, Fletcher PC, Murray GK, 2016. Abnormal Frontostriatal Activity During Unexpected Reward Receipt in Depression and Schizophrenia: Relationship to Anhedonia. Neuropsychopharmacology 41, 2001–2010. 10.1038/npp.2015.370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segre LS, O’Hara MW, Arndt S, Stuart S, 2007. The prevalence of postpartum depression: the relative significance of three social status indices. Soc. Psychiatry Psychiatr. Epidemiol 42, 316–21. [DOI] [PubMed] [Google Scholar]
- Siegel JS, Power JD, Dubis JW, Vogel AC, Church JA, Schlaggar BL, Petersen SE, 2013. Statistical improvements in functional magnetic resonance imaging analyses produced by censoring high‐motion data points. Hum. Brain Mapp 35, 1981–1996. 10.1002/hbm.22307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms LJ, Wright AGC, Cicero D, Kotov R, Mullins-Sweatt SN, Sellbom M, Watson D, Widiger TA, Zimmermann J, 2022. Development of Measures for the Hierarchical Taxonomy of Psychopathology (HiTOP): A Collaborative Scale Development Project. Assessment 29, 3–16. 10.1177/10731911211015309 [DOI] [PubMed] [Google Scholar]
- Simon JJ, Skunde M, Walther S, Bendszus M, Herzog W, Friederich H-C, 2016. Neural signature of food reward processing in bulimic-type eating disorders. Soc. Cogn. Affect. Neurosci 11, 1393–1401. 10.1093/scan/nsw049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Nichols TE, 2009. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage 44, 83–98. 10.1016/j.neuroimage.2008.03.061 [DOI] [PubMed] [Google Scholar]
- Smoski MJ, Felder J, Bizzell J, Green SR, Ernst M, Lynch TR, Dichter GS, 2009. fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. J. Affect. Disord 118, 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P, 1995. A Scale for the Assessment of Hedonic Tone the Snaith–Hamilton Pleasure Scale. Br. J. Psychiatry 167, 99–103. 10.1192/bjp.167.1.99 [DOI] [PubMed] [Google Scholar]
- Stowe ZN, Hostetter AL, Newport DJ, 2005. The onset of postpartum depression: Implications for clinical screening in obstetrical and primary care. Am. J. Obstet. Gynecol 192, 522–526. 10.1016/j.ajog.2004.07.054 [DOI] [PubMed] [Google Scholar]
- Wacker J, Dillon DG, Pizzagalli DA, 2009. The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: integration of resting EEG, fMRI, and volumetric techniques. NeuroImage 46, 327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, 1991. The Mood and Anxiety Symptoms Questionnaire Southern Methodist University, Dallas, TX. [Google Scholar]
- Watson D, O’Hara MW, Simms LJ, Kotov R, Chmielewski M, McDade-Montez EA, Gamez W, Stuart S, 2007. Development and validation of the Inventory of Depression and Anxiety Symptoms (IDAS). Psychol. Assess 19, 253–268. [DOI] [PubMed] [Google Scholar]
- Watson D, Stanton K, Clark LA, 2017. Self-report indicators of negative valence constructs within the research domain criteria (RDoC): A critical review. J. Affect. Disord., RDoC Constructs: Integrative reviews and empirical perspectives 216, 58–69. 10.1016/j.jad.2016.09.065 [DOI] [PubMed] [Google Scholar]
- Whitton AE, Treadway MT, Pizzagalli DA, 2015. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr. Opin. Psychiatry 28, 7–12. 10.1097/YCO.0000000000000122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CE, Bischoff-Grethe A, Melrose AJ, Irvine Z, Torres L, Bailer UF, Simmons A, Fudge JL, McClure SM, Ely A, Kaye WH, 2015. Hunger Does Not Motivate Reward in Women Remitted from Anorexia Nervosa. Biol. Psychiatry, Eating Disorders 77, 642–652. 10.1016/j.biopsych.2014.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson JA, O’Hara MW, Stuart S, Hart KJ, Watson D, 2015. Assessment of Postpartum Depressive Symptoms: The Importance of Somatic Symptoms and Irritability. Assessment 22, 309–318. 10.1177/1073191114544357 [DOI] [PubMed] [Google Scholar]
- Wilson RP, Colizzi M, Bossong MG, Allen P, Kempton M, Abe N, Barros-Loscertales AR, Bayer J, Beck A, Bjork J, Boecker R, Bustamante JC, Choi JS, Delmonte S, Dillon D, Figee M, Garavan H, Hagele C, Hermans EJ, ICCAM Consortium, Ikeda Y, Kappel V, Kaufmann C, Lamm C, Lammertz SE, Li Y, Murphy A, Nestor L, Pecina M, Pfabigan D, Pizzagalli D, Rademacher L, Admon R, Sommer T, Stark R, Suzuki H, Van Amelsvoort T, Van Hell E, Vink M, Votinov M, Wotruba D, Bhattacharyya S, MTAC, 2018. The Neural Substrate of Reward Anticipation in Health: A Meta-Analysis of fMRI Findings in the Monetary Incentive Delay Task. Neuropsychol. Rev 28, 496–506. 10.1007/s11065-018-9385-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Webster MA, Vidaurre D, Nichols TE, Smith SM, 2015. Multi-level block permutation. NeuroImage 123, 253–268. 10.1016/j.neuroimage.2015.05.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM, 2001. Temporal Autocorrelation in Univariate Linear Modeling of FMRI Data. NeuroImage 14, 1370–1386. 10.1006/nimg.2001.0931 [DOI] [PubMed] [Google Scholar]
- Zhang W-N, Chang S-H, Guo L-Y, Zhang K-L, Wang J, 2013. The neural correlates of reward-related processing in major depressive disorder: A meta-analysis of functional magnetic resonance imaging studies. J. Affect. Disord 151, 531–539. 10.1016/j.jad.2013.06.039 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


