Abstract
Candida albicans, an opportunistic pathogen, has the ability to form biofilms in the host or within medical devices in the body. Biofilms have been associated with disseminated/invasive disease with increased severity of infection by disrupting the host immune response and prolonging antifungal treatment. In this study, the in vivo virulence of three strains with different biofilm formation strengths, that is, non-, weak-, and strong biofilm formers, was evaluated using the zebrafish model. The survival assay and fungal tissue burden were measured. Biofilm-related gene expressions were also investigated. The survival of zebrafish, inoculated with strong biofilms forming C. albicans,, was significantly shorter than strains without biofilms forming C. albicans. However, there were no statistical differences in the burden of viable colonogenic cell number between the groups of the three strains tested. We observed that the stronger the biofilm formation, the higher up-regulation of biofilm-associated genes. The biofilm-forming strain (140 and 57), injected into zebrafish larvae, possessed a higher level of expression of genes associated with adhesion, attachment, filamentation, and cell proliferation, including eap1, als3, hwp1, bcr1, and mkc1 at 8 h. The results suggested that, despite the difference in genetic background, biofilm formation is an important virulence factor for the pathogenesis of C. albicans. However, the association between biofilm formation strength and in vivo virulence is controversial and needs to be further studied.
Keywords: biofilm, Candida albicans, gene, virulence, zebrafish
1. Introduction
The yeast Candida albicans is part of the microbiota of healthy individual’s oral, genital, and gastrointestinal tracts. The rate of Candida carriage on the mucosal surface in healthy individual ranges from 30% to 70% [1]. However, several factors, such as extreme age, pregnancy, chemotherapy, and cancer or prolonged use of broad-spectrum antibiotics and antifungal agents, which cause immune suppression, enable Candida to invade host tissue and cause mild superficial to severe systemic infection [2,3,4]. During this process, virulence characteristics, namely, the transition between yeast and hyphal forms; biofilm formation; the secretion of enzymes such as phospholipases and proteinases; the expression of adhesins and invasins on the cell surface; and phenotypic switching, are crucial to establishing infection [5].
The biofilm formed by Candida spp. is a complex community of yeast cells, hyphal forms, and pseudohyphal forms surrounded by an extracellular matrix of polysaccharides, proteins, lipids, and DNA [6]. Previous studies reported that approximately 80% of C. albicans infections in humans are directly or indirectly involved with biofilm formation on the host body or abiotic surfaces, including indwelling medical devices, such as vascular catheters, prosthetic heart valves, joint replacements, or dental implants. This biofilm mostly results in high morbidity and mortality [7,8].
Biofilm formation involves a series of events that begin with attachment/adhesion, the formation of basal microcolonies, and hyphal filamentation to release cells from the complex [9]. Host immune cells respond differently to Candida spp. when under planktonic conditions [10]. The biofilm matrix is partially protected from neutrophil activities, including phagocytosis, degranulation, and the formation of neutrophil extracellular traps (NET) [11]. Furthermore, cells that detach from such biofilms are more cytotoxic and increase host mortality, as seen in the murine model study [12].
In addition, biofilm formation is one of the main factors mitigating the effectiveness of antifungal treatments. As biofilm is typically a group of densely populated yeast cells surrounded by an extracellular matrix, this structure leads to a reduction in the effective drug concentration for the cells within the biofilm. A previous study found that the sessile cells within biofilm structure is more resistant to antifungal drugs than their planktonic cells [13].
Previous studies reported that among the different clinical isolates of Candida, biofilm formation can vary [14,15]. However, the effect on virulence of the ability to form biofilms between different strains of C. albicans using the zebrafish infection model is not known. Therefore, this study aims to compare virulence between biofilm and non-biofilm forming clinical C. albicans isolates using the zebrafish model of infection with associated gene expression for biofilm production.
2. Materials and Methods
2.1. Fungal Strains and Their In Vitro Virulence Study
The commercially available laboratory strain of C. albicans (ATCC 90028) was used as a positive control for biofilm formation. Other strains of C. albicans 104, 57, and 140, used in this study, were isolated from hemoculture in the Department of Microbiology, Faculty of Medicine Siriraj Hospital, Mahidol University, Thailand. Species identification was confirmed by both phenotypic and genotypic methods.
At the beginning, all C. albicans strains collected from positive hemocultures during April 2016 and November 2017 were evaluated for their four in vitro virulence activities, comprising phospholipase activity, proteinase activity, hemolytic activity, and biofilm formation, as previously described [16]. Of 46 C. albicans blood strains, the three isolates, C. albicans 104, 57, and 140, producing different biofilm levels but the same pattern of the other three virulence results, were divided into four in vitro virulence factors.
To confirm the four in vitro virulence factors, i.e., comprising phospholipase activity, proteinase activity, hemolytic activity, and biofilm formation. The isolates were pre-grown on Sabouraud Dextrose Agar (SDA) at 37 °C for 48 h before being suspended in a phosphate buffer saline (PBS) pH 7.4 to produce a 0.5 McFarland standard (1–5 × 106 cells/mL of yeast suspension). The 5 µL suspension was inoculated onto egg yolk medium, bovine serum albumin, and blood agar to determine phospholipase, proteinase, and hemolytic activity, respectively.
The phospholipase activity was performed by measuring the size of the precipitation zone after growth on egg yolk agar at 37 °C for 48 h [17]. The value of phospholipase activity (Pz) was determined by the ratio of the diameter of the colony to the total diameter of the colony plus the visible precipitation zone. A Pz value of 1.00 indicates no phospholipase activity, and less than 1.00 shows phospholipase activity. The lower the Pz value, the higher the enzymatic activity. Reference strains of C. albicans (ATCC 24433) served as a positive control.
Proteinase activity was analyzed using the presence of a clear halo zone around a five-days colony grown at 37 °C on a bovine serum albumin medium. The clear halo zone around the colony was recorded as evidence of proteinase activity, and the level of activity was determined by the ratio of the colony diameter to the total diameter of the colony plus the halo zone, Pr value. A Pr value of 1.00 indicates no proteinase activity, and less than 1.00 shows proteinase activity [18]. Reference strains of C. albicans (ATCC 10231) served as a positive control.
For determination of hemolytic activity, the tested and control strains on SDA supplemented with 3% enriched glucose and 6.5% fresh sheep blood were examined after incubation at 37 °C for 48 h. The presence of a distinct translucent halo around the yeast colony indicated positive hemolytic activity. The ratios of the diameter of the colony to the total diameter of the colony plus the translucent halo zone (Hz value) were used to represent the level of hemolytic activity as follows: Hz < 0.64, strongly positive; 0.64 ≤ Hz < 1.00, positive; and Hz = 1. Reference strains of C. albicans (ATCC 90028) served as a positive control [17,18].
For the assessment of biofilm formation, 2,3-Bis-(2-Methoxy-4-Nitro-5-Sulfophenyl)-2H-Tetrazoilum-5-Carboxanilide (XTT) reduction assay (Thermo Scientific, Waltham, MA, USA) using 96-well microplates was used. Briefly, an 18 h culture of C. albicans strains was adjusted to a 0.5 McFarland standard with yeast extract peptone dextrose (YPD) medium. The yeast suspensions (200 µL) were seeded in flat bottom 96-well plates [19]. After sealing, the plates were incubated at 37 °C for 48 h. The wells were washed with 200 μL PBS three times followed by the addition of a 100 µL mixture of 1 mg/mL of XTT and 10 µL of activation reagent, phenazine methosulfate, to the wells. The plates were further incubated in the dark for 3 h, and the biofilms were quantified at A450 and A630 using a plate reader. Microtitre wells containing only YPD medium that were not inoculated were used as negative controls. The cut-off optical density (ODc) was defined as three standard deviations above the mean OD of the negative control, and the strains were classified as follows: OD ≤ ODc = no biofilm producer; ODc < OD ≤ 2 × ODc = weak biofilm producer; 2 × ODc < OD ≤ 4 × ODc = moderate biofilm producer; and 4 × ODc < OD = strong biofilm producer [20,21,22,23]. Reference strains of C. albicans (ATCC 90028) served as a positive control strain, while C. glabrata (ATCC 90030) was used as a negative control strain.
2.2. Microinjection in Zebrafish Larvae
C. albicans strains were grown on YPD agar at 30 °C. The liquid cultures were grown for 18 h in 5 mL of YPD broth in a shaking incubator at 250 rpm at 30 °C for injections. Yeast cells were centrifuged at 3000× g, washed twice with PBS, and resuspended in 2 mL of PBS. The concentration of the yeast suspension was diluted to provide an OD 600 nm of 0.7 (~107 cells/mL). This diluted suspension was resuspended in autoclaved 5% polyvinylpyrrolidone 40 (PVP40) with 0.5% phenol red in PBS [24]. The PBS containing 5% PVP40 and 0.5% phenol red was used as a control.
Five days after fertilization, larvae of the wild-type zebrafish strain (AB strain) were used for Candida infection experiments. The larvae were anesthetized in E3-MB, containing 4 mg/mL of tricaine (ethyl-1,3-aminobenzoate; Sigma-Aldrich, St. Louis, MO, USA). The yeast cells were inoculated into zebrafish larvae using a microinjector. Two to three nanoliters of the yeast suspension was injected into the swim bladder of the larvae. The control and yeast-infected larvae were kept in an E3 buffer at 30 °C. This study was approved by the Siriraj Animal Care and Use Committee (SiACUC) (020/2562; 1 March 2022).
2.3. Survival Study
The survival study of zebrafish larvae was carried out in the control group and the yeast-infected group using 25 larvae per group. The larvae from each group were daily checked for their heartbeat under the light stereomicroscope. No heartbeat of zebrafish larvae observed was considered as lethal endpoint. Survival was recorded daily for up to nine days. The data were subsequently evaluated with the Mantel–Cox test using GraphPad Prism 8.
2.4. Fungal Tissue Burden/CFU Assay
The infectivity in the larvae was assessed by quantification of the colony forming unit (CFU) using the spread plate technique in biological triplications. Five representative larvae were pooled and homogenized at 8 hpi (hours post infection), 24 hpi, and 96 hpi in 100 μL of 0.85% normal saline. For plating, 100 μL of homogenate from each group was plated on SDA agar supplemented with 50 mg/L chloramphenicol. Undiluted homogenate, 1:10, 1:100, and 1:1000 dilutions were plated for the sample in duplicate plates at each time point. The plates were incubated at 30 °C for 48 h for the counting of CFU [24,25].
2.5. RNA Extraction and Gene Expression Analysis
RNA was isolated from larvae 4, 8, 12, 24, and 96 h post infection (hpi) using TRIzol reagent. To synthesize cDNA, a total of 2 µg of RNA was used in a reverse transcription reaction using Super Script III RT (Life Technologies, Carlsbad, CA, USA) and a random decamer according to the manufacturer’s instructions or iScript Transcriptase (Bio-Rad, Hercules, CA, USA). The genes of interest and the primer pairs used are provided in the table below (Table 1). Real-time PCR was performed on a Roche Light Cycler-480. cDNA amplification was performed with an initial denaturation at 95 °C for 10 min followed by 40 cycles of denaturation (95 °C for 10 s), annealing (50–54 °C for 30 s), and extension (72 °C for 20 s). This was followed by a melting curve generation and cooling time. The determination of gene expression was made by calculating the normalized expression ratio (2−ΔΔCt). In brief, data from the comparative expression approach were produced as fold changes in gene expression relative to a control or baseline condition, normalized to the constitutive reference gene act1. In this case, the baseline condition was mRNA isolated from planktonically grown C. albicans of the corresponding strains [24,26].
Table 1.
Primers and their sequences used in this study.
| Sequence Name | Gene Function | Sequence (5′-3′) | Ta (°C) | References |
|---|---|---|---|---|
| Act1 | Housekeeping gene (Internal control) | F: GCTGGTAGAGACTTGACCAACCA R: GACAATTTCTCTTTCAGCACTAGTAGTGA |
54 | [27] |
| Eap1 | Cell–cell adhesion, mediates adhesion to biotic and abiotic surfaces | F: CTGCTCACTCAACTTCAATTGTCG R: GAACACATCCACCTTCGGGA |
54 | [27] |
| Als3 | Mediates attachment to host cells and matrix proteins | F: GGTTATCGTCCATTTGTTGA R: TTCTGTATCCAGTCCATCTT |
54 | [28] |
| Hwp1 | Cell adherence | F: ACAGGTAGACGGTCAAGG R: GGGTAATCATCACATGGTTC |
50 | [28] |
| Bcr1 | Transcription factor controls the als1, als3, hwp1, and hyr1 expressions | F: AATGCCTGCAGGTTATTTGG R: TTTTAGGTGGTGGTGGCAAT |
50 | [29] |
| Mkc1 | Induced invasive filamentation and cell growth under stressed condition | F: AATGGGTCCAAAAAGGTTCC R: TTATGGCCCCTGAAGAACTG |
50 | This study |
2.6. Statistical Analysis
The statistical analyses were performed using GraphPad Prism 8. CFU quantification and normalized gene expressions at different time points and were compared by ordinary one-way or two-way ANOVA with Tukey’s multiple comparison test according to the requirements. In all cases, a p-value less than 0.05 was considered significant.
3. Results
3.1. In Vitro Virulence Assessment
Among the isolates of C. albicans strains obtained from hemoculture in the hospital laboratory, the three isolates with mainly differences in biofilm activity were selected for the study of biofilm formation in vitro and in vivo animal models. C. albicans strains 104, 57, and 140 with similar phospholipase activity, proteinase activity, and hemolytic activity (Table 2) were selected.
Table 2.
Determination of in vitro virulence factors.
| C. albicans Strains Used | Phospholipase Activity | Proteinase Activity | Hemolytic Activity | Biofilm Activity | ||||
|---|---|---|---|---|---|---|---|---|
| Pz Value | Interpretation | Pr Value | Interpretation | Hz Value | Interpretation | OD | Interpretation | |
| No. 104 | 1.00 | - | 1.00 | - | 0.59 | ++ | 0.031 | No |
| No. 57 | 1.00 | - | 1.00 | - | 0.52 | ++ | 0.073 | Weak |
| No. 140 | 1.00 | - | 1.00 | - | 0.53 | ++ | 0.396 | Strong |
Pz, Pr, Hz values were calculated by the ratio of colony diameter to the diameter of activity zone produced by phospholipase, proteinase, and hemolytic enzymes on the tested media, respectively; -, no activity; ++, strong activity; and OD, optical density, measured at 450 nm and subtracted background at 630 nm using cut-off = 0.064.
3.2. Survival Assay
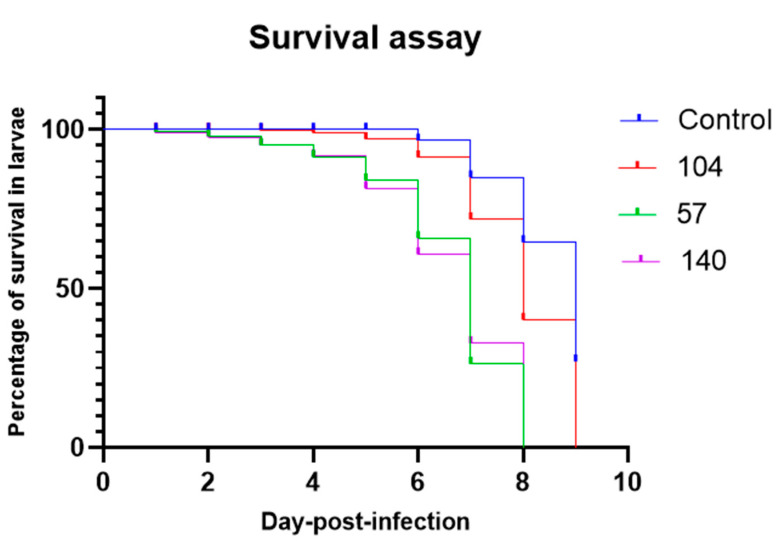
The survival of zebrafish larvae infected with different strains of C. albicans was monitored for nine days post infection (Figure 1). Deaths in the group of zebrafish larvae infected with all three strains of C. albicans were statistically significantly different (p < 0.0001) when compared to the control group. Similarly, there was statistically significant (p < 0.0001) more death in zebrafish infected with biofilm-forming strain (140 and 57) compared to non-biofilm strain (104). However, there were no significant differences (p > 0.05) between larvae infected with weak biofilm strains (57) and strong biofilm strains (140).
Figure 1.
Survival of zebrafish larvae injected with either C. albicans strains 104 (non-biofilm former), 57 (weak biofilm former), or 140 (strong biofilm former). The control group was injected with PBS without an organism. Each experiment was performed in duplicate.
3.3. Tissue Burden Assessment
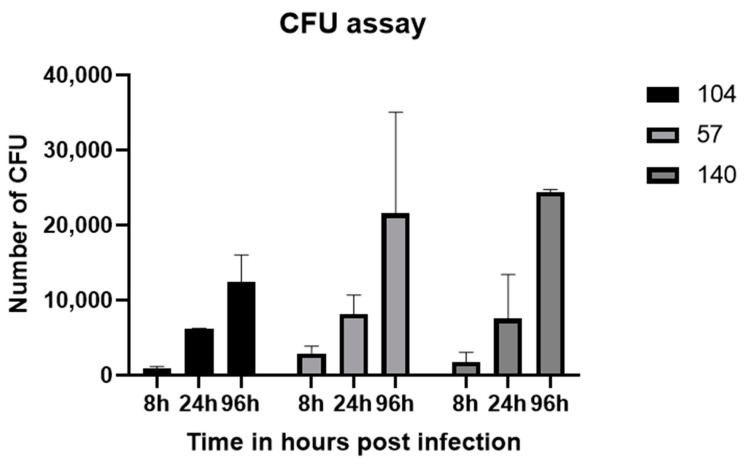
As demonstrated in Figure 2, the number of C. albicans cells showed an increasing pattern from 8 to 96 hpi, which indicated cell proliferation. However, no significant differences were observed between strains at any time point.
Figure 2.
Fungal tissue burden in zebrafish larvae that were infected with yeast cells of C. albicans strains 104, 57, or 140. Each experiment was performed in triplicate. CFU, colony forming unit.
3.4. Gene Expression Related to Biofilm Genes
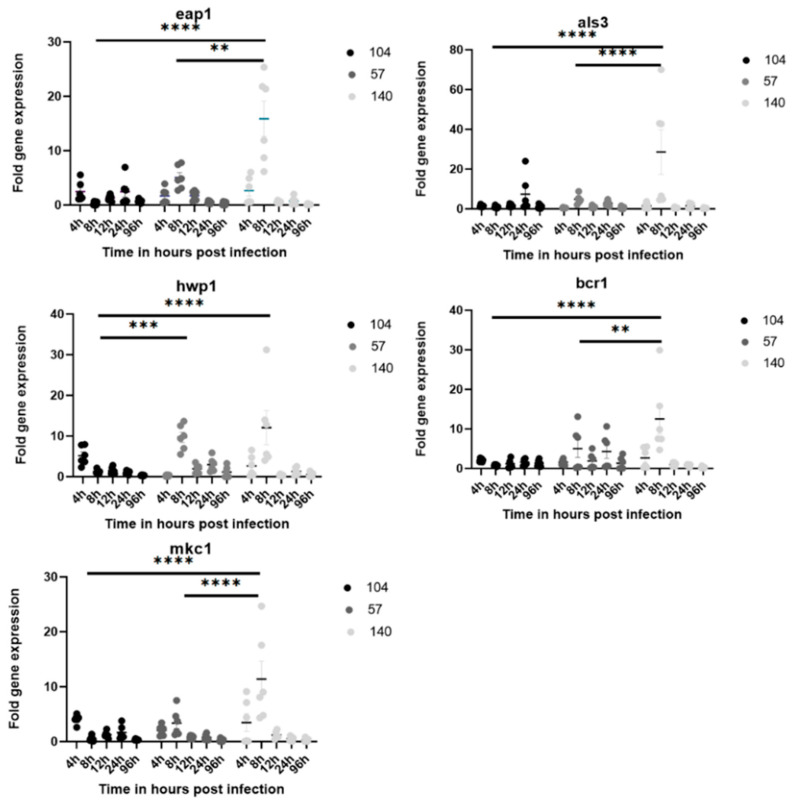
For the determination of biofilm activity within host cells, the expression profiles of the genes that played an important role in biofilm formation during C. albicans infection were monitored by real-time quantitative PCR (qPCR) 4 hpi (very early), 8 hpi (early), 12 hpi (intermediate), 24 hpi (late), and 96 hpi (very late). Generally, gene up-regulations were mostly seen at 8 hpi (Figure 3). It was observed that all genes (eap1, als3, hwp1, bcr1, and mkc1) in this study were significantly up-regulated in the strong biofilm-forming strain (140) compared to the non-biofilm strain (104) at 8 hpi. Similarly, there was a significant difference in gene expression between the weak biofilm strain (57) and the strong biofilm strain (140) for four of the above genes, except hwp1. No significant differences were observed in gene expression between different strains at the other corresponding time points (4, 12, 24, and 96 hpi).
Figure 3.
The expression levels of eap1, als3, hwp1, bcr1, and mkc1 in C. albicans strains 104, 57, and 140 infected zebrafish larvae, by time point. Each experimental group of 30 zebrafish was injected with 0.7 OD yeast cells of one of the C. albicans strains. The normalized ratios of expression were calculated by comparison with the level of expression of the act-1 gene in each group of planktonic cells. Each experiment was performed in duplicate. h, hour; **, p < 0.01; ***, p < 0.001; and ****, p < 0.0001.
4. Discussion
C. albicans is considered to be the strongest biofilm producer among Candida species [30,31]. The biofilm provides the fungus with greater adaptation in the host cell with protection against immune defenses and antifungal agents and is a reservoir for persistent infections [32,33]. In this study, more deaths in zebrafish infected with the strong biofilm former occurred as soon as 24 hpi, compared to late deaths in the strain without biofilm formation. However, zebrafish infected with biofilm-forming strains did not show significantly higher fungal burdens in tissue compared to non-biofilm-forming strains. Strong biofilm-forming strains showed more up-regulation of biofilm-related genes such as eap1, als3, hwp1, bcr1, and mkc1 eight hours after infection in the zebrafish model.
As expected, the strong biofilm former was more virulent in the zebrafish model of infection despite the difference in genetic background. A previous study by Pham LTT et al. showed different clinical outcomes (death or recovery) in the human patient infected with different strains of C. albicans with higher mortality for those infected with high biofilm-forming strains [16]. In another study with the Galleria mellonella model of infection, high biofilm formation mortality was significantly increased in comparison to low biofilm mortality (p = 0.0005) [14]. Similar studies in the past identified that the classification of clinical isolates as high or low biofilm formation abilities is a good predictor of clinical outcomes as high biofilm formers have been shown to correlate significantly with mortality [14,34].
Interestingly, despite the more in vivo virulence of the strong biofilm former, no statistically significant differences in fungal tissue burden were observed. This was similar to the CFU quantification assay we performed by scrapping the biofilm wells after washing at 48 h, where there were also no significant differences between CFU between biofilm and non-biofilm strains. This may be possible due to the increased proliferation of non-biofilm strains (lower doubling time) even when adhered to a low number compared to the biofilm, which is reciprocal to a study in which slow grower C. albicans exhibited higher biofilm formation [31].
During the steps of biofilm formation, several genes have been associated with each step that helps the fungus establish a well-organized biofilm system. Genes such as eap1 and als3 associated with the initial attachment of yeast cells to the surface were found to be significantly up-regulated in strains forming high biofilms during infection in zebrafish at the initial time point of 8 hpi. Hwp1, which encodes the cell wall mannoprotein of the hyphal forms and the germ tube and is found to be strongly induced during hyphal growth, was up-regulated at 8 hpi in our study.
This suggests that this time point is suitable for filamentation and increasing fungal biomass in the biofilm as per our study. In congruence with our study, als3 and hwp1 were found to be highly expressed in vaginal isolate biofilm strains in a previous study [35]. Similarly, a previous study by Nailis et al. reported that hwp1 and als3 are constitutively expressed in C. albicans biofilms in an in vivo subcutaneous catheter rat model and on mucosal surfaces in the model of reconstituted human epithelium [36].
Similarly, the transcription factor bcr1 that governs the adhesion process mediated by als3 [34], the filamentation mediated by hwp1 [37], and the development of persister cells in biofilm [38] was up-regulated at 8 hpi in our study in biofilm strains. The mkc1 gene is expressed during the adhesion phase and is involved in contact-induced invasive filamentation, as well as in the maturation of biofilms [8]. This gene was up-regulated in biofilm strain at very early (4 hpi) and early (8 hpi) time points and the non-biofilm strain at 4 hpi, suggesting its role in early adhesion and cell proliferation.
Maximum up-regulation of genes related to adherence and filamentation was at 8 hpi, implying that the adhesion and initiation of the biofilm complex occur at most at 8 hpi. At later time points, host immunity may perhaps be able to control the burden of Candida and therefore the reduced expression of adherence- and filamentation-related genes and at the later timepoints, that is, 24 and 96 hpi. A study using C. albicans as a pathogen in the zebrafish model (liver) showed early 2 hpi as the adhesion phase, 2–8 hpi as the invasion phase, and beyond 12 hpi as the damage phase, irrespective of biofilm formation [20]. The discrepancy of survival between biofilm-forming and non-biofilm-forming zebrafish larvae may be influenced by a mixture of Candida morphotypes, that is, yeast and filamentous forms, while the non-biofilm-forming strain has only the yeast form [39].
In this study, three chosen strains possessed strong hemolysis activity, while other virulence activities, that is, phospholipase and proteinase activities, were negative. The hemolytic capability of Candida species is considered a virulence attribute as it can acquire host iron, facilitating cellular growth. In addition, C. albicans exploited iron from intracellular ferritin to promote biofilm formation though the hyphal associated adhesin and invasin (Als3), whose expression increased in strong biofilm former strains [40]. This interaction may contribute to virulence in our strong biofilm former strain.
In summary, our study emphasized that biofilm formation at the early phase with the hemolysin activity appeared to somewhat enhance the virulence of C. albicans in zebrafish. However, the association between biofilm-forming strength and in vivo virulence remains controversial. Genes related to adhesion and filamentation were also associated with biofilm-forming strength. For further study, gene expression related to biofilm associated with fungal metabolism and the study related to immune gene expression in the corresponding time frame of biofilm formation would be helpful in the demonstration of immune pathogenesis in the zebrafish model of infection.
5. Conclusions
The formation of biofilm by C. albicans is a strong strategy applied by the fungus to increase infectivity and mortality in the host, which is facilitated by earlier and higher up-regulation of genes related to biofilm formation. In practice, biofilm evaluation could be a good predictor of clinical outcomes in patients.
Acknowledgments
We are very grateful to all the members of the Zebrafish facility for Biomedical Research (ZeBR), Department of Biochemistry, Faculty of Medicine Siriraj Hospital, Mahidol University, as well as all the staff of the Mycobacteriology and Mycology Laboratory, Department of Microbiology, Faculty of Medicine Siriraj Hospital, Mahidol University for the reference and clinical strains used in our study. We are also thankful to Perapon Nitayanon and Rattapha Chinli for laboratory support and guidance.
Author Contributions
Conceptualization, C.M. and P.N.; methodology, S.P. (Sabi Pokhrel), C.M., P.N. and S.P. (Sujiraphong Pharkjaksu); software, S.P. (Sabi Pokhrel); validation, S.P. (Sabi Pokhrel), C.M. and P.N; formal analysis, S.P. (Sabi Pokhrel); investigation, S.P. (Sabi Pokhrel), O.T. and N.B.; resources, S.P. (Sabi Pokhrel), S.P. (Sujiraphong Pharkjaksu) and O.T.; data curation, S.P. (Sabi Pokhrel); writing of original draft, S.P. (Sabi Pokhrel); review and editing of manuscript, S.P. (Sabi Pokhrel), P.N. and C.M.; visualization, S.P. (Sabi Pokhrel); supervision, C.M., P.N., I.T. and P.C.; project administration, C.M. and P.N.; and funding acquisition, C.M. and P.N. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Faculty of Medicine Siriraj Hospital, Mahidol University (Si. 091/2016; 9 February 2022). The animal ethics was approved by the Siriraj Animal Care and Use Committee (SiACUC) of the Faculty of Medicine Siriraj Hospital, Mahidol University (020/2562; 1 March 2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funder had no any role in the design, data collection, analysis, or interpretation regarding the study or in the manuscript preparation.
Funding Statement
This research was supported by the Graduate Scholarship for International Students from Neighboring Countries, Faculty of Medicine Siriraj Hospital, Mahidol University, Mahidol University (Research Development Fund), and Mahidol University (Basic Research Fund fiscal year 2023).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hall R.A. Dressed to impress: Impact of environmental adaptation on the Candida albicans cell wall. Mol. Microbiol. 2015;97:7–17. doi: 10.1111/mmi.13020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almirante B., Rodríguez D., Park B.J., Cuenca-Estrella M., Planes A.M., Almela M., Mensa J., Sanchez F., Ayats J., Gimenez M., et al. Epidemiology and predictors of mortality in cases of Candida bloodstream infection: Results from population-based surveillance, Barcelona, Spain, from 2002 to 2003. J. Clin. Microbiol. 2005;43:1829–1835. doi: 10.1128/JCM.43.4.1829-1835.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klevay M.J., Ernst E.J., Hollanbaugh J.L., Miller J.G., Pfaller M.A., Diekema D.J. Therapy and outcome of Candida glabrata versus Candida albicans bloodstream infection. Diagn. Microbiol. Infect. Dis. 2008;60:273–277. doi: 10.1016/j.diagmicrobio.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Leroy O., Gangneux J.P., Montravers P., Mira J.P., Gouin F., Sollet J.P., Carlet J., Reynes J., Rosenheim M., Regnier B., et al. Epidemiology, management, and risk factors for death of invasive Candida infections in critical care: A multicenter, prospective, observational study in France (2005–2006) Crit. Care Med. 2009;37:1612–1618. doi: 10.1097/CCM.0b013e31819efac0. [DOI] [PubMed] [Google Scholar]
- 5.Talapko J., Juzbašić M., Matijević T., Pustijanac E., Bekić S., Kotris I., Škrlec I. Candida albicans-The Virulence Factors and Clinical Manifestations of Infection. J. Fungi. 2021;7:79. doi: 10.3390/jof7020079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall-Stoodley L., Stoodley P. Evolving concepts in biofilm infections. Cell Microbiol. 2009;11:1034–1043. doi: 10.1111/j.1462-5822.2009.01323.x. [DOI] [PubMed] [Google Scholar]
- 7.Fanning S., Mitchell A.P. Fungal biofilms. PLoS Pathog. 2012;8:e1002585. doi: 10.1371/journal.ppat.1002585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu Z., Huang T., Min D., Soteyome T., Lan H., Hong W., Peng F., Fu X., Peng G., Liu J., et al. Regulatory network controls microbial biofilm development, with Candida albicans as a representative: From adhesion to dispersal. Bioengineered. 2022;13:253–267. doi: 10.1080/21655979.2021.1996747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsui C., Kong E.F., Jabra-Rizk M.A. Pathogenesis of Candida albicans biofilm. Pathog. Dis. 2016;74:ftw018. doi: 10.1093/femspd/ftw018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nett J.E., Andes D.R. Contributions of the Biofilm Matrix to Candida Pathogenesis. J. Fungi. 2020;6:21. doi: 10.3390/jof6010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirschfeld J. Dynamic interactions of neutrophils and biofilms. J. Oral. Microbiol. 2014;6:26102. doi: 10.3402/jom.v6.26102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uppuluri P., Chaturvedi A.K., Srinivasan A., Banerjee M., Ramasubramaniam A.K., Köhler J.R., Kadosh D., Lopez-Ribot J.L. Dispersion as an important step in the Candida albicans biofilm developmental cycle. PLoS Pathog. 2010;6:e1000828. doi: 10.1371/journal.ppat.1000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zarnowski R., Sanchez H., Covelli A.S., Dominguez E., Jaromin A., Bernhardt J., Mitchell K.F., Heiss C., Azadi P., Mitchell A. Candida albicans biofilm–induced vesicles confer drug resistance through matrix biogenesis. PLoS Biol. 2018;16:e2006872. doi: 10.1371/journal.pbio.2006872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sherry L., Rajendran R., Lappin D.F., Borghi E., Perdoni F., Falleni M., Tosi D., Smith K., Williams C., Jones B., et al. Biofilms formed by Candida albicans bloodstream isolates display phenotypic and transcriptional heterogeneity that are associated with resistance and pathogenicity. BMC Microbiol. 2014;14:182. doi: 10.1186/1471-2180-14-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vitális E., Nagy F., Tóth Z., Forgács L., Bozó A., Kardos G., Majoros L., Kovács R. Candida biofilm production is associated with higher mortality in patients with candidaemia. Mycoses. 2020;63:352–360. doi: 10.1111/myc.13049. [DOI] [PubMed] [Google Scholar]
- 16.Pham L.T.T., Pharkjaksu S., Chongtrakool P., Suwannakarn K., Ngamskulrungroj P. A predominance of clade 17 Candida albicans isolated from hemocultures in a tertiary care hospital in Thailand. Front. Microbiol. 2019;10:1194. doi: 10.3389/fmicb.2019.01194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tulyaprawat O., Pharkjaksu S., Chongtrakool P., Ngamskulrungroj P. An Association of an eBURST group with triazole resistance of Candida tropicalis blood isolates. Front. Microbiol. 2020;11:934. doi: 10.3389/fmicb.2020.00934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sachin C., Ruchi K., Santosh S. In vitro evaluation of proteinase, phospholipase and haemolysin activities of Candida species isolated from clinical specimens. Int. J. Med. Biomed. Res. 2012;1:153–157. doi: 10.14194/ijmbr.1211. [DOI] [Google Scholar]
- 19.Oz Y., Dag I., Kiraz N. Efficacy of disinfectants on Candida biofilms at different concentrations and contact times. Br. Microbiol. Res. J. 2012;2:40–52. doi: 10.9734/BMRJ/2012/1281. [DOI] [Google Scholar]
- 20.Chen Y.Y., Chao C.C., Liu F.C., Hsu P.C., Chen H.F., Peng S.C., Chuang Y.J., Lan C.Y., Hsieh W.P., Wong D.S. Dynamic transcript profiling of Candida albicans infection in zebrafish: A pathogen-host interaction study. PLoS ONE. 2013;8:e72483. doi: 10.1371/journal.pone.0072483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X., Yan Z., Xu J. Quantitative variation of biofilms among strains in natural populations of Candida albicans. Microbiology. 2003;149:353–362. doi: 10.1099/mic.0.25932-0. [DOI] [PubMed] [Google Scholar]
- 22.Kadkhoda H., Ghalavand Z., Nikmanesh B., Kodori M., Houri H., Taghizadeh Maleki D., Karimi Bavandpour A., Eslami G. Characterization of biofilm formation and virulence factors of Staphylococcus aureus isolates from paediatric patients in Tehran, Iran. Iran J. Basic. Med. Sci. 2020;23:691–698. doi: 10.22038/ijbms.2020.36299.8644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X.F., Shi H.Q., Liang Y., Li J., Jiang B., Song G.B. Interaction of biofilm and efflux pump in clinical isolates of carbapenem resistant P. aeruginosa. Eur. Rev. Med. Pharmacol. Sci. 2022;26:1729–1737. doi: 10.26355/eurrev_202203_28242. [DOI] [PubMed] [Google Scholar]
- 24.Pharkjaksu S., Boonmee N., Mitrpant C., Ngamskulrungroj P. Immunopathogenesis of Emerging Candida auris and Candida haemulonii Strains. J. Fungi. 2021;7:725. doi: 10.3390/jof7090725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergeron A.C., Seman B.G., Hammond J.H., Archambault L.S., Hogan D.A., Wheeler R.T. Candida albicans and Pseudomonas aeruginosa Interact To Enhance Virulence of Mucosal Infection in Transparent Zebrafish. Infect. Immun. 2017;85:e00475-17. doi: 10.1128/IAI.00475-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andes D., Nett J., Oschel P., Albrecht R., Marchillo K., Pitula A. Development and characterization of an in vivo central venous catheter Candida albicans biofilm model. Infect. Immun. 2004;72:6023–6031. doi: 10.1128/IAI.72.10.6023-6031.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Theberge S., Semlali A., Alamri A., Leung K.P., Rouabhia M.C. albicansgrowth, transition, biofilm formation, and gene expression modulation by antimicrobial decapeptide KSL-W. BMC Microbiol. 2013;13:246. doi: 10.1186/1471-2180-13-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morici P., Fais R., Rizzato C., Tavanti A., Lupetti A. Inhibition of Candida albicans Biofilm Formation by the Synthetic Lactoferricin Derived Peptide hLF1-11. PLoS ONE. 2016;11:e0167470. doi: 10.1371/journal.pone.0167470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ribeiro F.C., de Barros P.P., Rossoni R.D., Junqueira J.C., Jorge A.O. Lactobacillus rhamnosus inhibits Candida albicans virulence factors in vitro and modulates immune system in Galleria mellonella. J. Appl. Microbiol. 2017;122:201–211. doi: 10.1111/jam.13324. [DOI] [PubMed] [Google Scholar]
- 30.Kuhn D.M., Chandra J., Mukherjee P.K., Ghannoum M.A. Comparison of biofilms formed by Candida albicans and Candida parapsilosis on bioprosthetic surfaces. Infect. Immun. 2002;70:878–888. doi: 10.1128/IAI.70.2.878-888.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hasan F., Xess I., Wang X., Jain N., Fries B.C. Biofilm formation in clinical Candida isolates and its association with virulence. Microbes Infect. 2009;11:753–761. doi: 10.1016/j.micinf.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kernien J.F., Johnson C.J., Bayless M.L., Chovanec J.F., Nett J.E. Neutrophils From Patients with Invasive Candidiasis Are Inhibited by Candida albicans Biofilms. Front. Immunol. 2020;11:587956. doi: 10.3389/fimmu.2020.587956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Douglas L.J. Candida biofilms and their role in infection. Trends Microbiol. 2003;11:30–36. doi: 10.1016/S0966-842X(02)00002-1. [DOI] [PubMed] [Google Scholar]
- 34.Rajendran R., May A., Sherry L., Kean R., Williams C., Jones B.L., Burgess K.V., Heringa J., Abeln S., Brandt B.W., et al. Integrating Candida albicans metabolism with biofilm heterogeneity by transcriptome mapping. Sci. Rep. 2016;6:35436. doi: 10.1038/srep35436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohammadi F., Hemmat N., Bajalan Z., Javadi A. Analysis of Biofilm-Related Genes and Antifungal Susceptibility Pattern of Vaginal Candida albicans and Non-Candida albicans Species. Biomed. Res. Int. 2021;2021:5598907. doi: 10.1155/2021/5598907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nailis H., Kucharíková S., Ricicová M., Van Dijck P., Deforce D., Nelis H., Coenye T. Real-time PCR expression profiling of genes encoding potential virulence factors in Candida albicans biofilms: Identification of model-dependent and -independent gene expression. BMC Microbiol. 2010;10:114. doi: 10.1186/1471-2180-10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Desai J.V., Mitchell A.P. Candida albicans Biofilm Development and Its Genetic Control. Microbiol. Spectr. 2015;3:99–114. doi: 10.1128/microbiolspec.MB-0005-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aboualigalehdari E., Tahmasebi Birgani M., Fatahinia M., Hosseinzadeh M. Transcription Factors of CAT1, EFG1, and BCR1 Are Effective in Persister Cells of Candida albicans-Associated HIV-Positive and Chemotherapy Patients. Front. Microbiol. 2021;12:651221. doi: 10.3389/fmicb.2021.651221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seman B.G., Moore J.L., Scherer A.K., Blair B.A., Manandhar S., Jones J.M., Wheeler R.T. Yeast and Filaments Have Specialized, Independent Activities in a Zebrafish Model of Candida albicans Infection. Infect. Immun. 2018;86:e00415-18. doi: 10.1128/IAI.00415-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Almeida R.S., Brunke S., Albrecht A., Thewes S., Laue M., Edwards J.E., Filler S.G., Hube B. the hyphal-associated adhesin and invasin Als3 of Candida albicans mediates iron acquisition from host ferritin. PLoS Pathog. 2008;4:e1000217. doi: 10.1371/journal.ppat.1000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.