Abstract
The prognostic value of health status metrics in patients with adult congenital heart disease (ACHD) and atrial arrhythmias is unclear. In this retrospective cohort study of an ongoing national, multicenter registry (PROTECT-AR, NCT03854149), ACHD patients with atrial arrhythmias on apixaban are included. At baseline, health metrics were assessed using the physical component summary (PCS), the mental component summary (MCS) of the Short-Form-36 (SF-36) Health Survey, and the modified European Heart Rhythm Association (mEHRA) score. Patients were divided into groups according to their SF-36 PCS and MCS scores, using the normalized population mean of 50 on the PCS and MCS as a threshold. The primary outcome was the composite of mortality from any cause, major thromboembolic events, major/clinically relevant non-major bleedings, or hospitalizations. Multivariable Cox-regression analyses using clinically relevant parameters (age greater than 60 years, anatomic complexity, ejection fraction of the systemic ventricle, and CHA₂DS₂-VASc and HAS-BLED scores) were performed to examine the association of health metrics with the composite outcome. Over a median follow-up period of 20 months, the composite outcome occurred in 50 of 158 (32%) patients. The risk of the outcome was significantly higher in patients with SF-36 PCS ≤ 50 compared with those with PCS > 50 (adjusted hazard ratio (aHR), 1.98; 95% confidence interval [CI], 1.02–3.84; p = 0.04) after adjusting for possible confounders. The SF-36 MCS ≤ 50 was not associated with the outcome. The mEHRA score was incrementally associated with a higher risk of the composite outcome (aHR = 1.44 per 1 unit increase in score; 95% CI, 1.03–2.00; p = 0.03) in multivariable analysis. In ACHD patients with atrial arrhythmias, the SF-36 PCS ≤ 50 and mEHRA scores predicted an increased risk of adverse events.
Keywords: atrial arrhythmia, ACHD, congenital heart disease, quality of life, SF-36, mEHRA
1. Introduction
The resounding progress in diagnostic and surgical methods over the last years has contributed to the considerably enhanced life expectancy of patients with adult congenital heart disease (ACHD) [1,2]. As this population ages, the attention of care shifts towards factors associated with long-term morbidity, along with mortality. Atrial arrhythmias are the most common cardiovascular comorbidity encountered in the course of ACHD, affecting up to 20% of these patients [3]. In the ACHD setting, atrial arrhythmias have been described as a major source of impaired quality of life (QoL) and burdening symptoms [3,4,5,6].
Patient-reported and physician-assessed health metrics commonly utilized in ACHD and atrial arrhythmias include the Short-Form-36 (SF-36) Health Survey and the modified European Heart Rhythm Association (mEHRA) score, respectively. These metrics provide information on QoL, physical and mental functional status, and perceived arrhythmia-related symptoms. They reflect overall well-being, quantify disease burden, and may guide further therapeutic options [7]. To date, few studies have investigated these health metrics in the particular population of ACHD with comorbid atrial arrhythmias [8,9,10]. Furthermore, the association of health status metrics with cardiovascular clinical outcomes remains obscure.
The current study explores data from a multicenter registry, including patients with atrial arrhythmias who receive oral anticoagulation treatment with apixaban. Our aim is to evaluate the association of health status metrics with morbidity and mortality.
2. Materials and Methods
2.1. Study Design
This is a retrospective cohort of the PReventiOn of ThromboEmbolism in Adults with Congenital HearΤ disease and Atrial aRrhythmias (PROTECT-AR, ClinicalTrials.gov: NCT03854149) registry. Briefly, PROTECT-AR is a multicenter, prospective registry that investigates the safety and efficacy of apixaban for thromboembolism prevention in patients with ACHD and atrial arrhythmias [11]. The PROTECT-AR was launched in July 2019 and continues to enroll patients in 4 centers across Greece. The protocol has been approved by independent ethics committees or the institutional review boards of all involved centers. Written and informed consent was given by all study participants.
2.2. Study Population
We included patients of the PROTECT-AR study for whom baseline health status metrics (SF-36 Health Survey or mEHRA score) and follow-up data were accessible. Patients were adults (≥18 years old) with ACHD and physician-documented atrial arrhythmia (atrial fibrillation, atrial flutter, intra-atrial, re-entrant tachycardia) who routinely received apixaban for primary or secondary thromboembolism prevention. Exclusion criteria were the presence of moderate/severe mitral stenosis or mechanical valves and conditions that could obstruct the follow-up.
2.3. Outcomes
The primary outcome in the current study was the composite of mortality due to any cause and morbidity. Morbidity was defined as major thromboembolic events (i.e., ischemic stroke, systemic/pulmonary embolism, intracardiac thrombus, or myocardial infarction), major or clinically relevant non-major bleedings, or hospitalizations due to any cause. Bleeding events were classified based on the International Society on Thrombosis and Hemostasis [12]. The definition of events is displayed in Table 1.
Table 1.
Clinical events definitions.
| Stroke | Determined as a new-onset neurological deficit provoked by central nervous system injury due to haemorrhage or infarction (symptoms lasting at least 24 h or clear matching CT or MRI lesion for symptoms lasting <24 h), excluding any other nonvascular cause. Strokes will be categorized as “ischemic“, “primary hemorrhagic”, or “undetermined cause” if there are no available imaging data, according to the following definitions. Ischemic stroke
|
| Systemic embolism | Acute blood stoppage in a peripheral artery or embolism from other causes (e.g., surgical specimens, angiography, vascular imaging), localized in the lower or upper limb, intraocular, intra-abdominal viscera or elsewhere. |
| Pulmonary embolism | PE symptoms accompanied by one of the following:
|
| Intracardiac thrombus | Determined as a distinguished echo-dense mass in one of the cardiac chambers that is discovered by echocardiography or cardiac MRI, has well-demarcated borders, and is present both in systole and diastole. |
| Major bleeding (isth) | Determined as apparent bleeding accompanied by:
|
| Clinically relevant non-major bleeding | Determined as overt bleeding not satisfying the aforementioned criteria for major bleeding requiring intervention:
|
| Minor bleeding | Determined as other overt bleeding not satisfying the criteria of a major bleeding event or a clinically relevant non-major bleeding event. |
| Transient ischemic attack | Determined as new neurologic symptoms or deficit of <24 h in the absence of acute infarction on CT or MRI. |
| Myocardial infarction (4th universal definition) | A rise and/or fall of cardiac Troponin levels (≥1 value above the 99th percentile URL) and accompanied by at least one of the above:
|
| Death | The cause of death will be classified as follows: Cardiovascular
|
CT, computer tomography; CTPA, computed tomography pulmonary angiography/angiogram; ECG, electrocardiogram; ISTH, international society on thrombosis and haemostasis; MI, myocardial infarction; MRI, magnetic resonance imaging; PE, pulmonary embolism; URL, upper reference limit; VQ scan; ventilation/perfusion lung scintigraphy.
2.4. Data Sources and Measures
ACHD type, atrial arrhythmia type, baseline characteristics, laboratory and echocardiographic measures, and health status metrics were available in the study database.
Health status metrics were evaluated according to the SF-36 Health Survey and the mEHRA classification. The SF-36 Health Survey was completed via paper-and-pen versions by the included patients at baseline. It is a standardized self-report questionnaire which assesses the health-related QoL regarding physical, mental, and social life aspects. It accumulates scores from 0 (worst) to 100 (best) [13]. The SF-36 consists of 36 items which encompass 8 domains of health, including physical functioning (10 items), limitations due to physical health problems (4 items), bodily pain (2 items), energy/fatigue (4 items), social functioning (2 items), limitations due to emotional problems (3 items), and psychological distress and well-being (5 items). Items belonging to the same domain are averaged together to assemble one score in each one of the 8 health domains. These 8 multi-item scales can be assembled into 2 summary clusters, the physical component summary (PCS) and mental component summary (MCS), using an oblique model that enables the physical and mental components to be correlated [14]. Using the normalized population mean of 50 on the PCS of the SF-36 score as a threshold, patients were divided into two groups: those with PCS ≤ 50 and those with PCS > 50. The same procedure was repeated for the MCS of SF-36; patients were grouped into those with MCS ≤ 50 and those with MCS > 50. This structure has been used to simplify the SF-36 score in clinical practice.
The mEHRA score is a patient-reported score and quantifies the effect of arrhythmia-related symptoms on patients’ daily activity. Symptoms are classified into a scale from class 1 to class 4, with higher scores corresponding to more severe symptoms. A mEHRA score of 1 indicates the absence of arrhythmia-related symptoms. A score of 2a represents the presence of mild symptoms not annoying to the patient and which do not affect daily activity. A score of 2b represents the presence of moderate symptoms annoying to the patient that do not affect daily activity. A score of 3 indicates severe symptoms and affected normal daily activity. A score of 4 is consistent with disabling symptoms, while normal daily activity is impossible [15].
Prospective outcome data from the PROTECT-AR study were also used. Follow-up data were tracked directly from in-person or telephone interviews 1 month after each patient enrolment in the PROTECT-AR study and every 6 months afterwards. The last follow-up visit took place in January 2022.
2.5. Statistical Analysis
Variables were presented as numbers with percentages for categorical variables and means with standard deviation or medians with interquartile range (IQR) for continuous variables. Comparisons between groups were made with the Student’s t-test or the Wilcoxon–Mann–Whitney test for continuous variables and the Pearson chi-square or the Fisher’s exact test for categorical variables. Cumulative incidence curves were constructed for the composite outcome of all-cause mortality and morbidity using a log-rank test for comparisons between the groups. Cox regression analyses were applied to identify the independent association of PCS, MCS, and mEHRA scores with the composite outcome after adjusting for the following clinically relevant parameters: age greater than 60 years, anatomic complexity, ejection fraction (EF) of the systemic ventricle, CHA₂DS₂-VASc score (Congestive heart failure, Hypertension, Age ≥ 75, clinical history of Diabetes mellitus, prior Stroke/ transient ischemic attack /thromboembolism, Vascular disease, Age 65–74, Sex (Female category)), and HAS-BLED (Hypertension, Abnormal liver or renal function, Stroke, Bleeding tendency or predisposition, Labile international normalized ratio (INR), Elderly: age > 65 years old, Drugs: concomitant antiplatelet agents, non-steroidal anti-inflammatory drugs or alcohol) score. The corresponding adjusted hazard ratios (aHR) with 95% confidence intervals (CIs) are provided. Follow-up was censored in the event of death. The Z-score transformation was applied to standardize the SF-36 score for each domain to each component summary, and factor score coefficients were used. The SF-36 PCS was calculated by multiplying each SF-36 scale Z-score with its respective factor score coefficient, and then the eight domains were aggregated. SF-36 MCS was also calculated similarly. Finally, normalized scores for each summary component were created. A two-tailed p-value of less than 0.05 was regarded as statistically significant. SPSS software, version 24.0 (IBM Corp, Armonk, New York, NY, USA), and Stata software, release 13.1 (StataCorp), were used to perform the statistical analyses.
3. Results
3.1. Study Population
A total of 171 patients with ACHD and atrial arrhythmia on anticoagulation therapy with apixaban were screened from the PROTECT-AR study. The current study included 158 patients (mean age 51.5 years, standard deviation 16.8, 46% male) after excluding 13 patients with missing data on health status metrics (Figure S1 in the Supplementary Material). The median CHA2DS2-VASc score was 2 (IQR 1 to 2), whereas the median HAS-BLED score was 1 (IQR 0 to 1.75). Repaired tetralogy of Fallot (17%) was the most prevalent ACHD type. The most common atrial arrhythmia was atrial fibrillation (77%), followed by atrial flutter (15%) and intra-atrial, re-entrant tachycardia (8%), whereas the most common temporal pattern was paroxysmal atrial arrhythmia (58%).
Of the included patients, 89 (56%) had PCS ≤ 50, and 77 (49%) had MCS ≤ 50. The baseline characteristics of the patients according to SF-36 PCS and MCS are depicted in Table 2 and Table 3, respectively. In general, no significant differences were detected regarding demographics, past medical history, medication, and stroke and bleeding risks. Of note, patients with PCS ≤ 50 had a more severe NYHA class compared to those with PCS > 50 (p < 0.001). On the other hand, in patients with MCS ≤ 50, CHA2DS2-VASc was significantly higher than in patients with MCS > 50 (p = 0.04). Additionally, of the included patients, 44 (30.8%) were asymptomatic (mEHRA score 1) at baseline. Among symptomatic patients, 43 (30.1%) had mild symptoms (mEHRA score 2a), 37 (25.9%) had moderate (mEHRA score 2b), 16 (11.2%) severe (mEHRA score 3), and 3 (2.1%) disabling symptoms (mEHRA score 4).
Table 2.
Descriptive and clinical characteristics by physical component summary of SF-36.
| Overall n = 158 |
PCS ≤ 50 n = 89 |
PCS > 50 n = 69 |
p-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years), mean ± SD | 52 ± 17 | 52 ± 17 | 51 ± 17 | 0.62 |
| Female sex | 85 (54%) | 49 (55%) | 36 (53%) | 0.92 |
| BMI, (kg/m2), median (IQR) | 26.2 (5.7) | 26.4 (7.2) | 25.9 (4.9) | 0.32 |
| Systolic blood pressure (mmHg), median (IQR) | 120 (16) | 120 (20) | 115 (10) | 0.07 |
| Diastolic Blood pressure (mmHg), median (IQR) | 70 (15) | 70 (15) | 70 (11) | 0.56 |
| Smoking | 11 (8.0%) | 4 (4.9%) | 7 (13%) | 0.12 |
| Atrial arrhythmia | 1 | |||
| Atrial fibrillation | 101 (77%) | 61 (77%) | 40 (77%) | |
| Atrial flutter | 20 (15%) | 12 (15%) | 8 (15%) | |
| Intra-atrial reentry tachycardia | 10 (7.6%) | 6 (7.6%) | 4 (7.7%) | |
| Pattern of arrhythmia | 0.4 | |||
| First diagnosed | 10 (7.9%) | 5 (6.6%) | 5 (10%) | |
| Paroxysmal | 73 (58%) | 43 (57%) | 30 (60%) | |
| Persistent | 7 (5.6%) | 3 (3.9%) | 4 (8.0%) | |
| Permanent | 36 (29%) | 25 (33%) | 11(22%) | |
| Medical history | ||||
| Major bleeding | 5 (3.6%) | 2 (2.4%) | 3 (5.5%) | 0.38 |
| Clinically relevant non-major bleeding | 19 (14%) | 14 (17%) | 5 (9.1%) | 0.30 |
| Minor bleeding | 41 (31%) | 29 (36%) | 12 (23%) | 0.13 |
| Thromboembolism | 3 (2.2%) | 2 (2.5%) | 1 (1.8%) | 1 |
| Dyslipidemia | 26 (19%) | 17 (21%) | 9 (16%) | 0.51 |
| Hypertension | 40 (29%) | 26 (32%) | 14 (25%) | 0.48 |
| Diabetes | 22 (16%) | 14 (17%) | 8 (15%) | 0.92 |
| Heart failure | 74 (54%) | 48 (59%) | 26 (46%) | 0.14 |
| Chronic kidney disease | 9 (6.5%) | 6 (7.1%) | 3 (5.6%) | 1 |
| NT-proBNP (pg/mL), median (IQR) | 756 (1611) | 725 (1585) | 924 (1020) | 0.34 |
| NYHA functional class | <0.001 | |||
| 1 | 44 (33%) | 15 (18%) | 29 (59%) | |
| 2 | 59 (45%) | 44 (53%) | 15 (31%) | |
| 3 | 26 (20%) | 21 (25%) | 5 (10%) | |
| 4 | 3 (2.3%) | 3 (3.6%) | 0 (0%) | |
| Stroke–bleeding risk | ||||
| CHA2DS2-VASc score, median (IQR) | 2 (1) | 2 (2) | 1 (1) | 0.07 |
| HAS-BLED score, median (IQR) | 1 (1.75) | 1 (2) | 1 (1) | 0.07 |
| Echocardiography | ||||
| Systemic ventricular fraction (%), median (IQR) | 53 (8) | 50 (8) | 55 (12) | 0.07 |
| LA diameter (cm), median (IQR) | 4.45 (1.15) | 4.50 (0.77) | 4.30 (1.20) | 0.15 |
| Electrocardiogram | ||||
| QRS, median (IQR) | 110 (25) | 109 (30) | 116 (22) | 0.34 |
| Health status metrics | ||||
| mEHRA score, mean ± SD | 1.24 ± 1.07 | 1.33 ± 1.13 | 1.10 ± 0.98 | 0.20 |
| Medication | ||||
| Class I antiarrhythmic | 10 (6.3%) | 4 (4.5%) | 6 (8.7%) | 0.3 |
| Class III antiarrhythmic | 41 (26%) | 23 (26%) | 18 (26%) | 1 |
| beta-blocker | 82 (60%) | 52 (65%) | 30 (54%) | 0.2 |
Table 3.
Descriptive and clinical characteristics by mental component summary of SF-36 Health Survey.
| Overall N = 158 |
MCS ≤ 50 N = 89 |
MCS > 50 N = 69 |
p-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years), mean ± SD | 52 (17) | 52 (17) | 51 (17) | 0.77 |
| Female sex | 85 (54%) | 39 (51%) | 46 (57%) | 0.48 |
| BMI, (kg/m2), median (IQR) | 26.2 (5.7) | 26.6 (5.7) | 26.0 (5.1) | 0.49 |
| Systolic blood pressure (mmHg), median (IQR) | 120 (16) | 120 (20) | 120 (14) | 0.30 |
| Diastolic Blood pressure (mmHg), median (IQR) | 70 (15) | 70 (15) | 70 (14) | 0.88 |
| Smoking | 11 (8.0%) | 5 (7.1%) | 6 (9.0%) | |
| Atrial arrhythmia | 0.37 | |||
| Atrial fibrillation | 101 (77%) | 53 (82%) | 48 (73%) | |
| Atrial flutter | 20 (15%) | 7 (11%) | 13 (20%) | |
| Intra-atrial reentry tachycardia | 10 (7.6%) | 5 (7.7%) | 5 (7.6%) | |
| Pattern of arrhythmia | 0.28 | |||
| First diagnosed | 10 (7.9%) | 4 (6.6%) | 6 (9.2%) | |
| Paroxysmal | 73 (58%) | 43 (57%) | 30 (60%) | |
| Persistent | 7 (5.6%) | 3 (3.9%) | 4 (8.0%) | |
| Permanent | 36 (29%) | 25 (33%) | 11 (22%) | |
| Medical history | ||||
| Major bleeding | 5 (3.6%) | 1 (1.4%) | 4 (5.8%) | 0.21 |
| Clinically relevant non-major bleeding | 19 (14%) | 8 (12%) | 11 (16%) | 0.62 |
| Minor bleeding | 41 (31%) | 24 (35%) | 17 (26%) | 0.34 |
| Thromboembolism | 3 (2.2%) | 2 (3.0%) | 1 (1.4%) | 0.62 |
| Dyslipidemia | 26 (19%) | 13 (19%) | 13 (19%) | 1 |
| Hypertension | 40 (29%) | 24 (35%) | 16 (24%) | 0.15 |
| Diabetes | 22 (16%) | 13 (18%) | 9 (13%) | 0.56 |
| Heart failure | 74 (54%) | 43 (61%) | 31 (46%) | 0.08 |
| Chronic kidney disease | 9 (6.5%) | 7 (9.9%) | 2 (3.0%) | 0.17 |
| NT-proBNP (pg/mL), median (IQR) | 756 (1611) | 642 (1131) | 782 (1756) | 0.61 |
| NYHA functional class | 0.16 | |||
| 1 | 44 (33%) | 18 (25%) | 26 (43%) | |
| 2 | 59 (45%) | 34 (48%) | 25 (41%) | |
| 3 | 26 (20%) | 17 (24%) | 9 (15%) | |
| 4 | 3 (2.3%) | 2 (2.8%) | 1 (1.6%) | |
| Stroke–bleeding risk | ||||
| CHA2DS2-VASc score, median (IQR) | 2 (1) | 2 (2) | 1 (1) | 0.04 |
| HAS-BLED score, median (IQR) | 1 (1.75) | 1 (2) | 1 (1) | 0.31 |
| Echocardiography | ||||
| Systemic ventricular fraction (%), median (IQR) | 53 (8) | 54 (8) | 53 (7) | 0.78 |
| LA diameter (cm), median (IQR) | 4.45 (1.15) | 4.55 (1.20) | 4.30 (1.00) | 0.31 |
| Electrocardiogram | ||||
| QRS, Median (IQR) | 110 (25) | 114 (32) | 110 (20) | 0.79 |
| Health status metrics | ||||
| mEHRA score, mean ± SD | 1.24 ± 1.07 | 1.41 ± 1.47 | 1.06 ± 0.97 | 0.05 |
| Medication | ||||
| Class I antiarrhythmic | 10 (6.3%) | 4 (5.2%) | 6 (7.4%) | 0.75 |
| Class III antiarrhythmic | 41 (26%) | 20 (26%) | 21 (26%) | 1 |
| beta-blocker | 82 (60%) | 41 (59%) | 41 (61%) | 0.8 |
BMI, body mass index; CHA2DS2-Vasc (Congestive heart failure, Hypertension, Age ≥ 75 years, Diabetes mellitus, Stroke/transient ischemic attack, Vascular disease, Age 65 to 74 years, Sex category); HAS-BLED (Hypertension, Abnormal renal/ liver function, Stroke, Bleeding history or predisposition, Labile INR, Elderly, Drugs/alcohol); IQR, interquartile range; LA, left atrium; mEHRA, modified European Heart Rhythm Association; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; NYHA, New York Heart Association; SD, standard deviation; SF-36, Short Form-36.
3.2. Prognostic Significance of SF-36 PCS and MCS
During a median follow-up period of 20 months (IQR 9 to 24 months), the composite outcome occurred in 50 (32%) of the patients. More specifically, death due to any cause occurred in 6, major bleeding in 3, clinically relevant, non-major bleeding in 20, a thromboembolic event in 1, and hospitalization for any cause in 20 patients.
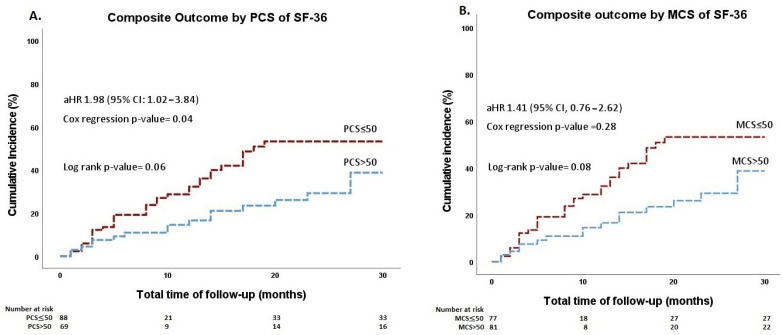
Cumulative incidence curves for the composite outcome are presented in Figure 1. No significant differences were found between patients with PCS ≤ 50 and patients with PCS > 50 with regard to the composite outcome in the univariate analysis (unadjusted HR= 1.76; 95% CI, 0.97 to 3.20; p = 0.06). After adjustment for clinically relevant baseline characteristics (age greater than 60 years, anatomic complexity, EF of the systemic ventricle, CHA₂DS₂-VASc score and HAS-BLED score), PCS ≤ 50 was associated with an increased risk of the composite outcome, as compared with PCS > 50 (aHR = 1.98; 95% CI, 1.02 to 3.84; p = 0.04). The differences between patients with PCS ≤ 50 and those with PCS > 50 regarding the individual components of the composite outcome were not significant (Table S1 in the Supplementary Material). On the other hand, the SF-36 MCS ≤ 50 was not significantly associated with the composite outcome in either univariate or multivariate analyses (unadjusted HR = 1.64; 95% CI, 0.93 to 2.88; p = 0.09 and aHR = 1.41; 95% CI, 0.76 to 2.62; p = 0.28, respectively).
Figure 1.
Cumulative incidence curves of the composite outcome stratified by (A) the physical component summary of the SF-36 and (B) the mental component summary of the SF-36 Health Survey. MCS, mental component summary; PCS, physical component summary; SF-36, 36-Item Short Form.
3.3. Prognostic Significance of mEHRA Score
The univariate regression analysis on the cumulative incidence of the composite outcome did not yield a significantly higher hazard for morbidity and mortality in patients with a higher mEHRA score (unadjusted HR = 1.26; 95% CI, 0.98 to 1.62; p = 0.07). In multivariate analysis, the presence of arrhythmia-related symptoms was associated with the composite outcome, with a unit increase of 1 in mEHRA corresponding to higher morbidity and mortality rates (aHR = 1.44; 95% CI, 1.03 to 2.00; p = 0.03).
4. Discussion
This post-hoc analysis of a prospective cohort including patients with ACHD and atrial arrhythmias on oral anticoagulation with apixaban revealed that poor physical health, namely SF-36 PCS ≤ 50, was associated with increased rates of the composite outcome of mortality due to any cause, thromboembolic events, major/clinically relevant non-major bleedings, or hospitalizations. This association appeared statistically significant after adjustment for age greater than 60 years, anatomic complexity, the EF of the systemic ventricle, HAS-BLED score, and CHA₂DS₂-VASc score. On the contrary, no significant association was found between the SF-36 MCS and the composite outcome. Furthermore, the mEHRA score was independently associated with an increased risk of the composite outcome. To our knowledge, this is the first study to examine the relation of health status metrics, measured by the SF-36 PCS and MCS, and the mEHRA score with hard outcomes in patients with ACHD and atrial arrhythmias.
More than half of the patients in our cohort had a PCS score below the normalized threshold of 50, underscoring the adverse impact of atrial arrhythmias on health status in ACHD. Two recent metanalyses of patients with Tetralogy of Fallot [16] and Fontan circulation [17], as well as other studies of mixed groups of congenital defects, have revealed low scores in the physical aspects of QoL in ACHD [18,19,20,21]. On the other hand, the majority of our patients had MCS > 50, reflecting acceptable mental health. This dissociation between the SF-36 PCS and MCS scores may be attributed to the chronicity of their disease, which, over time, renders them familiarized with their physical limitations. Subsequently, patients reported being satisfied with their own health status despite their impaired physical health. In concordance with our results, the mental health status of ACHD patients has been evaluated as good or even excellent in previous studies [21,22,23]. Nevertheless, these patients, and especially those with complex ACHD, sometimes face psychosocial or emotional issues due to the uncertainty of their illness. Indeed, the mental health of patients with Fontan circulation [17] and older patients [24] has been found to be significantly reduced.
Atrial arrhythmias constitute an additional burden for the health status of ACHD patients [9,10,25]. In APPROACH-IS, the greatest international registry of ACHD patients, atrial arrhythmias were related to poorer perceived physical and mental health status, higher levels of psychological distress, and an impaired illness perception [8]. Our study tried to expand these findings and associate the patient-reported health metrics with morbidity and mortality. In a non-ACHD population with comorbid atrial fibrillation, the association of impaired health-related QoL with adverse clinical events has already been identified. The AFFIRM study showed that a 5-point increase in SF-36 PCS score was associated with a 50% decrease in the risk of hospitalization or mortality [26]. The prognostic significance of patient-reported health metrics, as measured by the SF-36 PCS score in ACHD patients with atrial arrhythmias, would convert this questionnaire into a useful tool for identifying high-risk patients who need more frequent follow-up visits.
The mEHRA score, as a standardized physician-based assessment tool for the quantification of atrial fibrillation-related symptoms, has emerged as a useful adjunct to current clinical practice [27]. In patients with normally structured hearts, symptomatic atrial fibrillation has been shown to carry a significant risk for cardiovascular outcomes [28,29,30]. On the contrary, in AFFIRM [31] and EORP-AF [32] studies, asymptomatic patients had a similar or higher risk for mortality, respectively. Subsequently, there is no consensus about the association of arrhythmia-related symptom burden with adverse events. In our cohort, the presence of worsening arrhythmia-related symptoms, as implied by higher mEHRA scores, conferred a poorer prognosis. Should this finding be validated by future studies, it could bear considerable clinical significance.
5. Limitations
The current analysis was not determined at the beginning of the PROTECT-AR study and is therefore retrospective and nonrandomized. Although extensive adjustment for demographic and clinical characteristics was performed, residual confounders cannot be excluded. Furthermore, SF-36 and mEHRA scores were provided only at initial enrollment; therefore, there were no available data about score changes over time. Additionally, excluding patients that could not/refused to complete the SF-36 Health Survey may indicate a biased sample. Moreover, due to the limited number of patients included in our study, the association of each item of the SF-36 Survey with the composite outcome was not assessed; analyses were performed using the component summary scores. Finally, as the mEHRA score is a physician- and not a patient-assessed health metric, a possibility of misclassification exists.
6. Conclusions
In this cohort of ACHD patients with atrial arrhythmias, poor physical status, as indicated by SF-36 PCS ≤ 50, and higher arrhythmia-related symptom burden, as indicated by mEHRA score, were prospectively associated with an increased risk of morbidity and mortality. These results suggest the SF-36 Health Survey and mEHRA score to be of use in the risk stratification and personalized therapy of ACHD patients with atrial arrhythmias. Further longitudinal and larger studies are required to verify the significance of health status metrics as predictors of clinical outcomes in these patients.
Acknowledgments
The authors want to acknowledge the participants of the PROTECT-AR study who contributed to data collection during the conduction of the study. We also thank Boulmpou A., Dimos A., Ekklisiarxos D., Giamouzis G., Karantoumanis I., Papadopoulos C., Patrianakos A., Plevritaki A., Tzikas S.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11206181/s1, Figure S1: Flowchart of the study population; Table S1: Hazard ratios for outcomes at a median 20-month follow-up.
Author Contributions
Conceptualization, A.B., A.K. (Anastasios Kartas) and G.G.; Formal analysis, A.B.; Funding acquisition, I.D., G.P.R., D.N. and H.K.; Investigation, A.S.P., D.K., D.V.M., N.O., S.D., E.V., A.K. (Athanasios Koutsakis), S.L., E.K., T.T.N., S.A., A.F. and A.T.; Methodology, A.B. and A.K. (Anastasios Kartas); Project administration, H.K.; Resources, D.A., H.K. and G.G.; Software, A.B.; Supervision, I.D., H.K. and G.G.; Writing—original draft, A.B.; Writing—review and editing, A.K. (Anastasios Kartas), A.S.P. and G.G. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was approved by the Institutional Review Board of the involved centers: Institutional review board and ethics committee of AHEPA University Hospital, Thessaloniki, Greece (145/05.04.2019); Institutional review board of Attikon University Hospital, Athens, Greece (296 28.05.2019); Institutional review board and ethics committee of Onassis Cardiac Surgery Center, Athens, Greece (642/21.03.2019); Institutional review board of Mitera Childrens’ Hospital, Athens, Greece (05.03.2019).
Informed Consent Statement
Written informed consent was obtained from all study participants.
Data Availability Statement
The study’s data are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the study design, collection, management, analysis, and interpretation of data; writing of the report; and the decision to submit the report for publication.
Funding Statement
The PROTECT-AR study received funding from Pfizer, through ERISTA (European Thrombosis Investigator-Initiated Research Program, WI246683). The funders had no role in the study design, collection, management, analysis, and interpretation of data; writing of the report; and the decision to submit the report for publication.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kempny A., Dimopoulos K., Uebing A., Moceri P., Swan L., Gatzoulis M.A., Diller G.P. Reference values for exercise limitations among adults with congenital heart disease. Relation to activities of daily lifesingle centre experience and review of published data. Eur. Heart J. 2012;33:1386–1396. doi: 10.1093/eurheartj/ehr461. [DOI] [PubMed] [Google Scholar]
- 2.Ntiloudi D., Giannakoulas G., Parcharidou D., Panagiotidis T., Gatzoulis M.A., Karvounis H. Adult congenital heart disease: A paradigm of epidemiological change. Int. J. Cardiol. 2016;218:269–274. doi: 10.1016/j.ijcard.2016.05.046. [DOI] [PubMed] [Google Scholar]
- 3.Koyak Z., Achterbergh R.C.A., De Groot J.R., Berger F., Koolbergen D.R., Bouma B.J., Lagrand W.K., Hazekamp M.G., Blom N.A., Mulder B.J.M. Postoperative arrhythmias in adults with congenital heart disease: Incidence and risk factors. Int. J. Cardiol. 2013;169:139–144. doi: 10.1016/j.ijcard.2013.08.087. [DOI] [PubMed] [Google Scholar]
- 4.Negishi J., Ohuchi H., Yasuda K., Miyazaki A., Norifumi N., Yamada O. Unscheduled hospitalization in adults with congenital heart disease. Korean Circ. J. 2015;45:59–66. doi: 10.4070/kcj.2015.45.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hernández-Madrid A., Paul T., Abrams D., Aziz P.F., Blom N.A., Chen J., Chessa M., Combes N., Dagres N., Diller G., et al. Arrhythmias in congenital heart disease: A position paper of the European Heart Rhythm Association (EHRA), Association for European Paediatric and Congenital Cardiology (AEPC), and the European Society of Cardiology (ESC) Working Group on Grown-up Congeni. Europace. 2018;20:1719–1720. doi: 10.1093/europace/eux380. [DOI] [PubMed] [Google Scholar]
- 6.Kartas A., Papazoglou A.S., Kosmidis D., Moysidis D.V., Baroutidou A., Doundoulakis I., Despotopoulos S., Vrana E., Koutsakis A., Rampidis G.P., et al. The Adult Congenital Heart Disease Anatomic and Physiological Classification: Associations with Clinical Outcomes in Patients with Atrial Arrhythmias. Diagnostics. 2022;12:466. doi: 10.3390/diagnostics12020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anker S.D., Agewall S., Borggrefe M., Calvert M., Caro J.J., Cowie M.R., Ford I., Paty J.A., Riley J.P., Swedberg K., et al. The importance of patient-reported outcomes: A call for their comprehensive integration in cardiovascular clinical trials. Eur. Heart J. 2014;35:2001–2009. doi: 10.1093/eurheartj/ehu205. [DOI] [PubMed] [Google Scholar]
- 8.Casteigt B., Samuel M., Laplante L., Shohoudi A., Apers S., Kovacs A.H., Luyckx K., Thomet C., Budts W., Enomoto J., et al. Atrial arrhythmias and patient-reported outcomes in adults with congenital heart disease: An international study. Heart Rhythm. 2021;18:793–800. doi: 10.1016/j.hrthm.2020.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Yang H., Kuijpers J.M., de Groot J.R., Konings T.C., van Dijk A., Sieswerda G.T., Post M.C., Mulder B.J.M., Bouma B.J. Impact of atrial arrhythmias on outcome in adults with congenital heart disease. Int. J. Cardiol. 2017;248:152–154. doi: 10.1016/j.ijcard.2017.06.073. [DOI] [PubMed] [Google Scholar]
- 10.Egbe A.C., Najam M., Banala K., Vojjini R., Bonnichsen C., Ammash N.M., Faizee F., Khalil F., Deshmukh A.J., Connolly H.M. Impact of atrial arrhythmia on survival in adults with tetralogy of Fallot. Am. Heart J. 2019;218:1–7. doi: 10.1016/j.ahj.2019.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Kartas A., Doundoulakis I., Ntiloudi D., Koutsakis A., Kosmidis D., Rampidis G., Apostolopoulou S., Frogoudaki A., Tzifa A., Avramidis D., et al. Rationale and design of a prospective, observational, multicentre study on the safety and efficacy of apixaban for the prevention of thromboembolism in adults with congenital heart disease and atrial arrhythmias: The PROTECT-AR study. BMJ Open. 2020;10:e038012. doi: 10.1136/bmjopen-2020-038012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulman S., Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 13.McHorney C., Ware J., Raczek A. The MOS 36-item short-form health survey (SF-36): II. Psychometric. Med. Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Farivar S.S., Cunningham W.E., Hays R.D. Correlated physical and mental health summary scores for the SF-36 and SF-12 Health Survey, V.1. Health Qual. Life Outcomes. 2007;5:54. doi: 10.1186/1477-7525-5-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wynn G.J., Todd D.M., Webber M., Bonnett L., McShane J., Kirchhof P., Gupta D. The European Heart Rhythm Association symptom classification for atrial fibrillation: Validation and improvement through a simple modification. Europace. 2014;16:965–972. doi: 10.1093/europace/eut395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malik M., Dawood Z.S., Janjua M., Chauhan S.S.B., Ladak L.A. Health-related quality of life in adults with tetralogy of Fallot repair: A systematic review and meta-analysis. Qual. Life Res. 2021;30:2715–2725. doi: 10.1007/s11136-021-02875-5. [DOI] [PubMed] [Google Scholar]
- 17.Marshall K.H., D’Udekem Y., Sholler G.F., Opotowsky A.R., Costa D.S.J., Sharpe L., Celermajer D.S., Winlaw D.S., Newburger J.W., Kasparian N.A. Health-Related Quality of Life in Children, Adolescents, and Adults with a Fontan Circulation: A Meta-Analysis. J. Am. Heart Assoc. 2020;9:e014172. doi: 10.1161/JAHA.119.014172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lane D.A., Lip G.Y.H., Millane T.A. Quality of life in adults with congenital heart disease. Heart. 2002;88:71–75. doi: 10.1136/heart.88.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamphuis M., Ottenkamp J., Vliegen H.W., Vogels T., Zwinderman K.H., Kamphuis R.P., Verloove-Vanhorick S.P. Health related quality of life and health status in adult survivors with previously operated complex congenital heart disease. Heart. 2002;87:356–362. doi: 10.1136/heart.87.4.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soufi A., Gouton M., Metton O., Mitchell J., Bernard Y.F., Bozio A., Ninet J., Di Filippo S. Quality of life of adult Fontan patients. Cardiol. Young. 2021;31:97–104. doi: 10.1017/S1047951120003431. [DOI] [PubMed] [Google Scholar]
- 21.Gratz A., Hess J., Hager A. Self-estimated physical functioning poorly predicts actual exercise capacity in adolescents and adults with congenital heart disease. Eur. Heart J. 2009;30:497–504. doi: 10.1093/eurheartj/ehn531. [DOI] [PubMed] [Google Scholar]
- 22.Müller J., Hess J., Hager A. Minor symptoms of depression in patients with congenital heart disease have a larger impact on quality of life than limited exercise capacity. Int. J. Cardiol. 2012;154:265–269. doi: 10.1016/j.ijcard.2010.09.029. [DOI] [PubMed] [Google Scholar]
- 23.Pravda N.S., Zusman O., Richter I., Blieden L., Vig S., Marchushamer I., Dadashev A., Razon Y., Kornowski R., Hirsch R. Self-Reported Mental and Physical Measures in Adult Fontan Patients. J. Clin. Med. 2022;11:3969. doi: 10.3390/jcm11143969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Müller J., Berner A., Ewert P., Hager A. Reduced health-related quality of life in older patients with congenital heart disease: A cross sectional study in 2360 patients. Int. J. Cardiol. 2014;175:358–362. doi: 10.1016/j.ijcard.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Van Den Bosch A.E., Roos-Hesselink J.W., Van Domburg R., Bogers A.J.J.C., Simoons M.L., Meijboom F.J. Long-term outcome and quality of life in adult patients after the Fontan operation. Am. J. Cardiol. 2004;93:1141–1145. doi: 10.1016/j.amjcard.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 26.Schron E., Friedmann E., Thomas S.A. Does health-related quality of life predict hospitalization or mortality in patients with atrial fibrillation? J. Cardiovasc. Electrophysiol. 2014;25:23–28. doi: 10.1111/jce.12266. [DOI] [PubMed] [Google Scholar]
- 27.Hindricks G., Potpara T., Dagres N., Arbelo E., Bax J.J., Blomström-Lundqvist C., Boriani G., Castella M., Dan G.-A., Dilaveris P.E., et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the Europe. Eur. Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 28.Schnabel R.B., Pecen L., Rzayeva N., Lucerna M., Purmah Y., Ojeda F.M., Caterina R., Kirchhof P. Symptom Burden of Atrial Fibrillation and Its Relation to Interventions and Outcome in Europe. J. Am. Heart Assoc. 2021;7:e007559. doi: 10.1161/JAHA.117.007559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rienstra M., Vermond R.A., Crijns H.J.G.M., Tijssen J.G.P., Van Gelder I.C., RACE Investigators Asymptomatic persistent atrial fibrillation and outcome: Results of the RACE study. Heart Rhythm. 2014;11:939–945. doi: 10.1016/j.hrthm.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 30.Vermond R.A., Crijns H.J.G.M., Tijssen J.G.P., Alings A.M., Van Den Berg M.P., Hillege H.L., Van Veldhuisen D.J., Van Gelder I.C., Rienstra M., Ii R. Symptom severity is associated with cardiovascular outcome in patients with permanent atrial fibrillation in the RACE II study. Europace. 2014;16:1417–1425. doi: 10.1093/europace/euu151. [DOI] [PubMed] [Google Scholar]
- 31.Flaker G.C., Belew K., Beckman K., Vidaillet H. Asymptomatic atrial fibrillation: Demographic features and prognostic information from the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Am. Heart J. 2005;149:657–663. doi: 10.1016/j.ahj.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 32.Boriani G., Laroche C., Diemberger I., Fantecchi E., Popescu M.I., Rasmussen L.H., Sinagra G., Petrescu L., Tavazzi L., Maggioni A.P., et al. Asymptomatic atrial fibrillation: Clinical correlates, management and outcomes in the EORP-AF Pilot General Registry. Am. J. Med. 2015;128:509–518.e2. doi: 10.1016/j.amjmed.2014.11.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study’s data are available on request from the corresponding author.