Abstract
Background
Several studies have reported suboptimal efficacy of direct-acting antivirals (DAAs) to treat hepatitis C virus (HCV) subtypes endemic to sub-Saharan Africa (SSA) and Southeastern Asia (SEA). The extent of this issue in individuals with human immunodeficiency virus (HIV)/HCV from SSA or SEA residing in Europe is unknown.
Methods
We retrospectively analyzed data from several prospective European cohorts of people living with HIV. We included individuals with HIV/HCV who originated from SSA or SEA, were treated with interferon-free DAAs, and had an available HCV RNA result ≥12 weeks after the end of treatment. The primary outcome was sustained virological response at least 12 weeks after the end of treatment (SVR12).
Results
Of the 3293 individuals with HIV/HCV treated with DAA and with available SVR12 data, 142 were from SSA (n = 64) and SEA (n = 78). SVR12 was achieved by 60 (94% [95% confidence interval {CI}, 86%–98%]) individuals from SSA and 76 (97% [95% CI, 92%–99%]) from SEA. The genotypes of the 6 individuals failing DAA treatment were 2, 3a, 3h, 4a, 4c, and 6j. For 2 of the 4 unsuccessfully treated individuals with available sequence data at treatment failure, NS5A resistance-associated substitutions were present (30R/93S in an individual with genotype 4c and 31M in an individual with genotype 6j).
Conclusions
SVR12 rates were high in individuals with HIV/HCV residing in Europe and originating from regions where intrinsically NS5A-resistant HCV strains are endemic. HCV elimination for this population in Europe is unlikely to be hampered by suboptimal DAA efficacy.
Keywords: coinfection, elimination, hepatitis C virus, human immunodeficiency virus
We observed reassuring rates of sustained virological response at least 12 weeks after the end of treatment among individuals with HIV/hepatitis C virus from Africa/Asia, suggesting that reduced direct-acting antiviral efficacy is uncommon in this population.
The majority of hepatitis C virus (HCV) infections worldwide are found in low- and middle-income countries (LMICs), namely for sub-Saharan Africa (SSA) and Southeastern Asia (SEA) [1]. Trials evaluating the efficacy of current direct-acting antivirals (DAAs) have been predominately conducted in high-income countries. The HCV genotypes/subtypes studied in these trials were common to those of the global epidemic (ie, 1a/b, 2a/b, 3a, 4a/d), but not to those specific to SSA and SEA [2]. Clinical trials from LMICs and real-world studies from Western countries have recently shown suboptimal DAA efficacy for several HCV subtypes, termed “nonepidemic” (ie, other than 1a/b, 2a/b, 3a, 4a/d) [2–6]. These included a trial in SEA where 76% (32/42) of individuals with genotype 3b achieved sustained virological response at least 12 weeks after the end of treatment (SVR12), a trial in Rwanda where 56% (27/48) of individuals with genotype 4r achieved SVR12, and a real-world study from London, United Kingdom, where the SVR12 rate among African patients with nonepidemic genotype 1 subtypes was 75% (21/28) [3, 5, 7]. The most recent European Association for the Study of the Liver (EASL) HCV guidelines has therefore recommended NS5B sequencing to determine genotype and subtype for migrants from countries where nonepidemic subtypes are prevalent [8].
DAA efficacy for nonepidemic HCV subtypes has been rarely examined in individuals with HIV/HCV [3–6]. This is concerning given that the prevalence of HIV/HCV coinfection is high for several countries in SSA and SEA [9–11]. Furthermore, migrants from these regions comprise a substantial part of individuals living with HIV in Europe [12, 13]. Suboptimal DAA efficacy in individuals harboring HCV genotypes from these regions could then impede HCV elimination goals for the entire population with HIV/HCV in Europe. Real-world data on the prevalence of HCV genotypes/subtypes and DAA treatment outcomes would help assess the population-level risk of treatment failure and in establishing whether NS5B sequencing should be broadened, particularly for HIV/HCV coinfection.
The objective of this study was therefore to investigate the real-world efficacy of DAA treatment in individuals with HIV/HCV originating from SSA and SEA in multiple European cohorts of people living with HIV.
METHODS
Study Design and Population
Data were obtained from several longitudinal, observational, prospective cohorts of persons living with HIV in Europe. These were from EuroSIDA (including data from clinics from Southern, Western, Northern, Central, and Eastern Europe, last data extraction: July 2020) [14], AIDS Therapy Evaluation in the Netherlands (ATHENA) [15] (the Netherlands, last data extraction: May 2021), and the Swiss HIV Cohort Study (SHCS) [13] (Switzerland, last data extraction: June 2021). All participating cohorts included individuals with confirmed HIV-1 infection who were over the age of 18, without restrictions on HIV RNA or CD4+ cell count levels. HCV RNA testing was conducted according to routine clinical practice. To account for overlap between cohorts, data from any participant included in ATHENA or SHCS who also participated in EuroSIDA were only considered once.
From each cohort, we initially selected HCV RNA–positive individuals who were treated with an interferon-free DAA regimen. Subsequently, individuals originating from either SSA or SEA were selected (list of countries provided in Supplementary Data), while those originating from an unspecified country or region in Africa or Asia were not considered further. Finally, individuals who had an available HCV RNA result at least 12 weeks after the end of interferon-free DAA treatment and before starting any new HCV regimen were included in the analysis. Both primary HCV infection and reinfections were considered in the analysis.
Data Collection
Data were collected on the number of individuals with HIV/HCV treated with an interferon-free DAA regimen, the number of DAA-treated individuals with an available SVR12, and of these, the number who achieved SVR12. These data were collected for the complete cohort and for individuals originating from SSA and SEA.
Aggregated data of DAA-treated individuals who had an SVR12 result and originated from SSA or SEA were retrieved and included HCV genotypes and subtypes, age, sex, routes of HIV acquisition, presence of cirrhosis (based on the cohort-specific definition), region of origin, detectable or undetectable HIV RNA status, CD4+ cell count, and DAA regimen used. Nonepidemic HCV genotypes/subtypes were defined as those other than 1a/b, 2a/b, 3a, and 4a/d. Genotype results with missing or multiple subtypes were labeled as unassigned genotypes, except for those belonging to genotype 5 (no assigned subtypes known) or genotype 6 (no epidemic subtypes). HCV genotypes were determined via commercially available or in-house assays, yet data on the type of assay used for each participant were unavailable.
Finally, limited, anonymous, individual data were collected on included individuals who failed DAA treatment and included region of origin, HCV treatment history, presence of advanced fibrosis or cirrhosis, presence of renal insufficiency (defined as an estimated glomerular filtration rate <30 mL/minute/1.73 m2 according to formulas used in the cohort), HCV genotype/subtype, failed DAA regimen, resistance-associated substitutions (RASs), and retreatment data. Advanced fibrosis or cirrhosis was defined as a Fibroscan measurement ≥9.5 kPa for ATHENA and by a cohort-specific definition for EuroSIDA [16]. The EuroSIDA definition of advanced fibrosis or cirrhosis was Metavir F3/F4 from liver biopsy, Fibroscan measurement ≥9.5 kPa, AST to Platelet Ratio Index (APRI) score >1.75, or hyaluronic acid >160 ng/mL. If multiple variables were available, biopsy data were used, followed by Fibroscan, APRI, and hyaluronic acid. Liver fibrosis data were not available for SHCS.
Patient Consent Statement
For enrollment in EuroSIDA and SHCS, written informed consent was obtained from each participant. Enrollment in the ATHENA cohort is based on an opt-out principle. For all cohorts, institutional review boards of all participating centers approved the cohort protocols. Since the data for current study were supplied aggregated and anonymized, no additional consent was obtained.
Statistical Analysis
Descriptive data are reported as either percentage or mean with standard deviation (SD). For SVR12 rates, 95% confidence intervals (CIs) were calculated using the Jeffreys method. Means and standard deviations were recalculated from aggregated data to represent the study population including all cohorts. All characteristics are reported for the time at which the first interferon-free DAA treatment regimen was commenced, or until 1 year after for laboratory values. SVR12 rates were calculated for the complete SSA and SEA groups and stratified for nonepidemic, epidemic, and unassigned genotypes. In sensitivity analyses, SVR12 rates were calculated by assuming that all unassigned genotypes were either nonepidemic or epidemic genotypes and by assuming that individuals from SSA harboring genotype 4 with an unknown subtype had nonepidemic genotypes, as these are unlikely to be genotype 4a/d infections if acquired in SSA [17, 18].
RESULTS
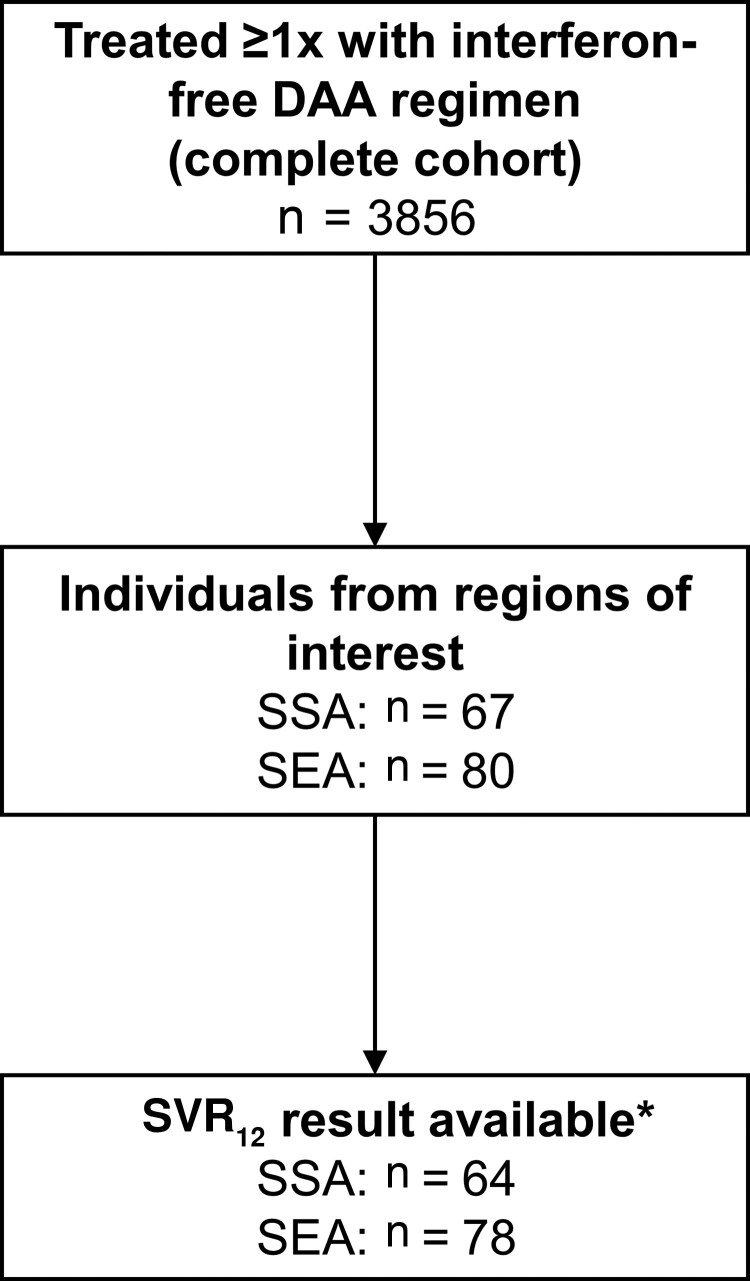
In total, 3856 individuals with HIV/HCV coinfection were treated at least once with an interferon-free DAA regimen, of whom 3293 (85%) had an available SVR12 HCV RNA result (Figure 1). This included 64 and 78 individuals from SSA and SEA, respectively. Among the 142 individuals from SSA or SEA (Table 1), HIV was mainly acquired through heterosexual contact (n = 52 [37%]) or among men who have sex with men (n = 51 [36%]). Of the 122 individuals with known liver fibrosis status, 28 (23%) had evidence of advanced fibrosis or cirrhosis.
Figure 1.
Flowchart. *Reason for hepatitis C virus (HCV) RNA sustained virological response at least 12 weeks after the end of treatment results being unavailable: awaiting SVR12 HCV RNA measurement (n = 2), HCV RNA missing (n = 2), discontinued cohort participation soon after HCV treatment (n = 1). Abbreviations: DAA, direct-acting antiviral; SEA, Southeastern Asia; SSA, sub-Saharan Africa; SVR, sustained virological response at least 12 weeks after the end of treatment.
Table 1.
Demographic and Clinical Description of Individuals With Human Immunodeficiency Virus/Hepatitis C Virus From Sub-Saharan Africa or Southeastern Asia Treated With Interferon-Free Direct-Acting Antivirals and With Available Data on Sustained Virological Response at Least 12 Weeks After the End of Treatment
| Characteristic | (N = 142) |
|---|---|
| Age, y, mean (SD) | 47 (10) |
| Female sex | 44 (31) |
| Route of HIV acquisition | |
| Men who have sex with men | 51 (36) |
| Injection drug use | 25 (18) |
| Heterosexual contact | 52 (37) |
| Other | 6 (4) |
| Unknown | 8 (6) |
| Advanced fibrosis or cirrhosis | |
| No | 94 (66) |
| Yes | 28 (20) |
| Missing | 20 (14) |
| Region of origin | |
| Eastern Africa | 19 (13) |
| Western Africa | 11 (8) |
| Southern Africa | 7 (5) |
| Middle Africa | 27 (19) |
| Eastern Asia | 7 (5) |
| Southeastern Asia | 53 (37) |
| Southern Asia | 18 (13) |
| Undetectable HIV RNA (<50 copies/mL) | |
| No | 6 (4) |
| Yes | 134 (94) |
| Missing | 2 (1) |
| CD4+ count, cells/µL, mean (SD) | 633 (286) |
| DAA regimen | |
| Sofosbuvir/ledipasvir | 64 (45) |
| Sofosbuvir/daclatasvir | 28 (20) |
| Sofosbuvir/velpatasvir | 13 (9) |
| Elbasvir/grazoprevir | 13 (9) |
| Glecaprevir/pibrentasvir | 10 (7) |
| Sofosbuvir/simeprevir | 9 (6) |
| Sofosbuvir/ribavirin | 3 (2) |
| Dasabuvir/ombitasvir/paritaprevir/ritonavir | 2 (1) |
Data are presented as No. (%) unless otherwise indicated. All characteristics are reported for the time at which the first interferon-free DAA treatment regimen was commenced, or until 1 year after for laboratory values. Data were obtained from EuroSIDA (including data from clinics from Southern, Western, Northern, Central, and Eastern Europe) [14], AIDS Therapy Evaluation in the Netherlands (ATHENA) [15] (the Netherlands), and Swiss HIV Cohort Study [13] (Switzerland).
Abbreviations: DAA, direct-acting antiviral; HIV, human immunodeficiency virus; SD, standard deviation.
Nonepidemic genotypes were identified in 20% (n = 13/64) of individuals from SSA and 4% (n = 3/78) from SEA (Table 2). For individuals originating from SSA, the most common genotypes were genotype 4 (n = 26 [41%]) and genotype 1 (n = 23 [36%]), with the most common subtype being 1a (n = 18 [28%]). Twenty-one (33%) individuals from SSA had a genotype with unassigned subtype, mainly those harboring genotype 4 (n = 15% [23%]) or genotype 2 (n = 4 [6%]). For individuals originating from SEA, the most common genotypes were genotype 1 (n = 58 [74%]) and genotype 3 (n = 10 [13%]), with the most common subtypes being 1a (n = 42 [54%]), 1b (n = 10 [13%]), and 3a (n = 7 [9%]). Ten (13%) individuals from SEA had a genotype with unassigned subtype. Among individuals in the parent cohorts that were not included in the study, 128 of 3660 (3%) had a nonepidemic genotype. These were mainly subtypes 4c (n = 94 [73%]), 2c (n = 8 [6%]), and 1c (n = 7 [5%]).
Table 2.
Distribution of Hepatitis C Virus Genotypes of Individuals With Human Immunodeficiency Virus/Hepatitis C Virus From Sub-Saharan Africa or Southeastern Asia Treated With Interferon-Free Direct-Acting Antivirals and With Available Data on Sustained Virological Response at Least 12 Weeks After the End of Treatment
| Genotype and Subtype | Sub-Saharan Africa (n = 64), No. (%) |
Southeastern Asia (n = 78), No. (%) |
|---|---|---|
| Genotype 1 | 23 (36%) | 58 (74%) |
| a | 18 | 42 |
| b | 2 | 10 |
| c | 1 | 0 |
| Unknown | 2 | 6 |
| Genotype 2 | 8 (13%) | 1 (1%) |
| a | 2 | 0 |
| c | 2 | 0 |
| Unknown | 4 | 1 |
| Genotype 3 | 2 (3%) | 10 (13%) |
| a | 1 | 7 |
| h | 1 | 0 |
| Unknown | 0 | 3 |
| Genotype 4 | 26 (41%) | 3 (4%) |
| a | 2 | 1 |
| c | 2 | 0 |
| d | 1 | 2 |
| e | 2 | 0 |
| f | 2 | 0 |
| g | 1 | 0 |
| v | 1 | 0 |
| Unknown | 15 | 0 |
| Genotype 5 | 1 (2%) | 0 |
| Genotype 6 | 0 | 3 (4%) |
| a | 0 | 2 |
| j | 0 | 1 |
| Unknown | 4 (6%) | 3 (4%) |
The most commonly prescribed DAA regimen was sofosbuvir/ledipasvir (n = 63 [44%]), while 23 (16%) individuals were treated with pan-genotypic regimens (ie, either sofosbuvir/velpatasvir or glecaprevir/pibrentasvir). Among those with an available SVR12 result, the SVR12 rates were 94% (n = 60/64; 95% CI, 86%–98%) for individuals originating from SSA and 97% (n = 76/78; 95% CI, 92%–99%) for those from SEA. SVR12 rates were 98% (n = 86/88; 95% CI, 93%–100%) for individuals with epidemic genotypes, 81% (n = 13/16; 95% CI, 58%–94%) for individuals with nonepidemic genotypes, and 97% (n = 30/31; 95% CI, 86%–100%) for those with genotypes with unassigned subtype. When assuming that all unassigned genotypes were either epidemic or nonepidemic genotypes, SVR12 rates were 97% (n = 116/119; 95% CI, 93%–99%) and 91% (n = 43/47; 95% CI, 81%–97%), respectively. When assuming that individuals from SSA harboring genotype 4 infection with an unknown subtype had an nonepidemic genotype, the SVR12 rate for individuals from SSA with a nonepidemic genotype was 93% (n = 26/28; 95% CI, 79%–98%). The different DAA regimens all resulted in SVR12 rates ≥92% (Table 3).
Table 3.
Rates of Sustained Virological Response at Least 12 Weeks After the End of Treatment, by Treatment Regimen
| DAA Regimen | Included, No. | Achieved SVR12, No. | SVR12 Rate |
|---|---|---|---|
| Sofosbuvir/ledipasvir | 64 | 62 | 97% |
| Sofosbuvir/daclatasvir | 28 | 26 | 93% |
| Sofosbuvir/velpatasvir | 13 | 12 | 92% |
| Elbasvir/grazoprevir | 13 | 12 | 92% |
| Glecaprevir/pibrentasvir | 10 | 10 | 100% |
| Sofosbuvir/simeprevir | 9 | 9 | 100% |
| Sofosbuvir/ribavirin | 3 | 3 | 100% |
| Dasabuvir/ombitasvir/paritaprevir/ritonavir | 2 | 2 | 100% |
Abbreviations: DAA, direct-acting antiviral; SVR12, sustained virological response at least 12 weeks after the end of treatment.
Of the 6 individuals with unsuccessful DAA treatment (Table 4), 3 had a nonepidemic HCV genotype (3h, 4c, 6j), 2 had genotypes commonly encountered in the global epidemic (3a, 4a), and 1 had a genotype with unassigned subtype (2). Four individuals were successfully retreated, 1 has not yet received retreatment, and 1 individual with cirrhosis was unsuccessfully retreated with sofosbuvir/glecaprevir/pibrentasvir. Posttreatment RAS data were available for 4 unsuccessfully treated individuals. Two of these individuals had clinically relevant NS5A RASs (ie, 30R/93S and 31M).
Table 4.
Individuals With Human Immunodeficiency Virus/Hepatitis C Virus From Sub-Saharan Africa or Southeastern Asia Failing Interferon-Free Direct-Acting Antiviral Treatment
| Characteristic | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Region of origin | Middle Africa | Southeastern Asia | East Africa | South East Asia | Middle Africa | East Africa |
| HCV treatment history | No | No | 1 treatment | 1 treatment | No | 1 treatment |
| Cirrhosis | No | Unknown | No | Yes | No | Yes |
| eGFR <30 mL/min/1.73 m2 | No | Unknown | No | No | No | No |
| Genotype/subtype | 4c | 6j | 3h | 3a | 2a | 4a |
| Failed DAA regimen | SOF/LDV | ELB/GRZ | SOF/VEL | SOF/DAC | SOF/LDV | SOF/DAC |
| Treatment adherenceb | NA | 100% | Good | Good | Good | Good |
| Pre-DAA RAS | NA | NA | NA | NA | NA | NA |
| Post-DAA RAS | NS5A: 30R, 93S | NS3: 170V NS5A: 31M |
NS3: 166S, 175M NS5A, NS5B: Nonec |
NS3, NS5A, NS5B: None | NA | NA |
| Successful retreatment | Yes | Yes | Yes | No | Yes | Not retreated |
| DAA regimen used for retreatment | GLE/PIB | SOF/VEL/VOX | SOF/VEL/VOX | SOF/GLE/PIB | SOF/DAC/RBV | … |
Abbreviations: DAA, direct-acting antiviral; DAC, daclatasvir; eGFR, estimated glomerular filtration rate; ELB, elbasvir; GLE, glecaprevir; GRZ, grazoprevir; HCV, hepatitis C virus; LDV, ledipasvir; NA, not available; PIB, pibrentasvir; RAS, resistance-associated substitution; RBV, ribavirin; SOF, sofosbuvir; VEL, velpatasvir; VOX, voxilaprevir.
Subtype was missing for this individual.
As reported by the treating physician.
NS5B sequence was available from position 217 to 346. Presence of RASs was therefore not assessed for several NS5B positions where genotype 3 RASs can occur (eg, 150, 159, and 206).
DISCUSSION
Previous studies reporting decreased DAA efficacy for certain HCV subtypes endemic to SSA and SEA have included very few individuals with HIV/HCV [3–6]. In this large European study of individuals with HIV/HCV treated with interferon-free DAAs and originating from regions where nonepidemic genotypes are prevalent, we observed high SVR12 rates similar to those observed in other patient populations [8]. This result alone would suggest that reduced efficacy of DAAs is not common in this setting.
Eleven percent of individuals with HIV/HCV included in our study had a nonepidemic HCV genotype (SSA 20%, SEA 4%). This prevalence is almost certainly underestimated given that NS5B sequencing is not commonly performed in clinics across Europe and commercial HCV genotyping assays often result in missing or incorrect subtypes, especially for genotypes 2, 4, and 6 [19]. An additional 22% (SSA 33%, SEA 13%) had an unassigned subtype, of whom a substantial proportion was likely harboring a nonepidemic genotype. In comparison, another study among individuals with mainly HCV monoinfection (9% HIV/HCV) from SSA living in London, United Kingdom, demonstrated a much higher prevalence of nonepidemic genotypes as determined by a commercial assay (56%) [3]. Additionally, our study sample did not include HCV subtypes that intrinsically harbor NS5A-resistant mutations (eg, 1l, 3b, 3g, 4r, 6u, 6v) [6, 8]. These observations, alongside the high SVR12 rate observed in our study, could indicate a lower frequency of intrinsically resistant HCV strains among individuals with HIV/HCV. This suggests that decreased DAA efficacy due to intrinsically resistant HCV strains, as observed in individuals from SSA or SEA with HCV monoinfection, might not be pervasive in individuals with HIV/HCV from SSA or SEA living in Europe.
It is difficult to determine whether included individuals acquired HCV in the country of origin or after moving to Europe, as we lacked sufficient virological data and data on risk behavior of participants while residing in Europe. Although data on HCV transmission in migrant populations are not available, a study modeling HIV transmission in migrants from Asia and Africa demonstrated that HIV is acquired in Europe for 32%–45% of cases [20]. We did observe several nonepidemic genotypes that are common to SSA or SEA in our study population, suggesting that some HCV transmission occurred prior to migration or within specific communities of similar origin in Europe. Nevertheless, a substantial part of our study population harbored genotypes and subtypes frequently circulating in Europe and not in SSA or SEA (eg, genotype 1a was the most common subtype in DAA-treated individuals from SSA, while this genotype is uncommon in large parts of this region); thus, many were likely to have acquired HCV in Europe [1]. This finding should be kept in mind when considering the high SVR rates observed in our study, and it should be stressed that our results are by no means representative for individuals with HIV/HCV living in SSA or SEA.
Nevertheless, these results have important clinical and public health implications for the European setting. DAA failure was uncommon in our study and many individuals had an epidemic genotype that was likely acquired in Europe. The 81% SVR rate in individuals harboring nonepidemic genotypes might potentially suggest lower effectiveness of DAA therapy in this population; however, this rate only reflects a small number of individuals and would require more data to confirm its clinical relevance. Moreover, only 16% of our study population, which includes 1 patient failing DAA treatment, were given the pangenotypic DAA regimens that are currently standard of care in Europe and contain more potent NS5A inhibitors. A study from Rwanda almost exclusively including individuals with HCV monoinfection reported a 91% (n = 10/11) SVR rate for individuals with subtype 4r treated with sofosbuvir/velpatasvir [21]. The SVR12 rates observed in current clinical practice might therefore be even higher than those reported in our study. Our results imply that HCV elimination for this population in Europe is unlikely to be hampered by suboptimal efficacy of DAAs to strains harboring naturally occurring NS5A-resistant RASs.
Currently, the EASL recommends using NS5B sequencing as the standard method to determine baseline HCV genotypes in all individuals with HCV originating from SSA or SEA [8]. However, as we observed high SVR12 rates in our study, it can be questioned whether this method is uniformly required for individuals with HIV/HCV from SSA or SEA. With an SVR12 rate >95%, approximately 1 in 20 of these individuals could potentially benefit from a tailored DAA treatment regimen if a nonepidemic genotype were detected. NS5B sequencing might be worthwhile in settings where it is already part of standard care, but not for those where sequencing methods are not readily available and additional costs or time are required. Deciding to use NS5B sequencing for individuals with HIV/HCV originating from SSA or SEA should be considered in light of the characteristics of the patient (eg, transmission route and assumed location of acquiring HCV) and resources of the healthcare structure (eg, availability of sequencing methods).
To our knowledge, this study provides the first real-world data on DAA efficacy for individuals with HIV/HCV living in Europe and originating from countries where HCV strains intrinsically resistant to NS5A inhibitors are endemic. Nevertheless, our study has several limitations. Due to a lack of available samples, we were unable to sequence all HCV strains of included participants to more accurately determine genotype/subtype and presence of resistance-associated substitutions. Genotyping assays based on the 5′UTR strand of HCV regularly lead to missing or incorrect classification of subtype or occasionally even genotype [19]. Additionally, SVR results were missing for 5 DAA-treated individuals. The SVR12 rate would then be lower if considering these individuals as failing DAA treatment (ie, 93% of those treated with DAA achieved SVR12). Furthermore, due to privacy regulations, we were unable to collect data on the specific countries of origin, thereby limiting the interpretability of our results. In addition, since only aggregated data were available, we were unable to assess differences in SVR12 rate between individuals with different HIV transmission routes or specifically for individuals with cirrhosis. Finally, individuals included in this real-world cohort were treated with a very heterogeneous array of DAA regimens. To conclude definitively on DAA efficacy in individuals with HIV/HCV from SSA and SEA living in Europe, future research should focus on more accurately determining genotypes/subtypes and within which groups HCV transmission is occurring.
CONCLUSIONS
DAA efficacy in people with HIV/HCV originating from SSA or SEA and living in Europe is high. Although the limited number of participants with genotypes of concern and the lack of data on location of HCV acquisition limit conclusions on DAA efficacy for individuals with HIV/HCV residing in SSA or SEA, it seems unlikely that suboptimal response to DAAs specific to these individuals could become a complicating factor for overall HCV elimination in Europe in the near future.
Supplementary Material
Contributor Information
Cas Isfordink, Division of Infectious Diseases, Department of Internal Medicine, Amsterdam Institute for Infection and Immunity, Amsterdam University Medical Center, University of Amsterdam, Amsterdam, The Netherlands; Department of Gastroenterology and Hepatology, University Medical Centre Utrecht, Utrecht, The Netherlands.
Anders Boyd, Stichting HIV Monitoring, Amsterdam, The Netherlands; Department of Infectious Diseases, Research and Prevention, Public Health Service of Amsterdam, Amsterdam, The Netherlands.
Amanda Mocroft, Centre for Clinical Research, Epidemiology, Modelling and Evaluation, Institute for Global Health, University College London, London, United Kingdom; Centre of Excellence for Health, Immunity and Infections, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark.
Katharina Kusejko, Department of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich, Zurich, Switzerland; Institute of Medical Virology, University of Zurich, Zurich, Switzerland.
Colette Smit, Stichting HIV Monitoring, Amsterdam, The Netherlands.
Stephane de Wit, Division of Infectious Diseases, St Pierre Hospital, Université Libre de Bruxelles, Brussels, Belgium.
Tabitha Mahungu, Department of Infectious Diseases, Royal Free Hospital London NHS Foundation Trust, London, United Kingdom.
Karolin Falconer, Department of Infectious Diseases/Venhälsan, Södersjukhuset, Stockholm, Sweden.
Gilles Wandeler, Department of Infectious Diseases, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland.
Matthias Cavassini, Division of Infectious Diseases, Lausanne University Hospital, University of Lausanne, Lausanne, Switzerland.
Marcel Stöckle, Division of Infectious Diseases and Hospital Epidemiology, University Hospital Basel, University of Basel, Basel, Switzerland.
Janke Schinkel, Section of Clinical Virology, Department of Medical Microbiology and Infection Prevention, Amsterdam Infection and Immunity Institute, Amsterdam University Medical Center, University of Amsterdam, Amsterdam, The Netherlands.
Andri Rauch, Department of Infectious Diseases, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland.
Lars Peters, Centre of Excellence for Health, Immunity and Infections, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark.
Marc van der Valk, Division of Infectious Diseases, Department of Internal Medicine, Amsterdam Institute for Infection and Immunity, Amsterdam University Medical Center, University of Amsterdam, Amsterdam, The Netherlands; Stichting HIV Monitoring, Amsterdam, The Netherlands.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. Concept and design: C. I., A. B., C. S., J. S., M. v. d. V. Data extraction and preparation/cleaning: A. M., K. K., C. S., L. P. Data analysis: C. I. All authors contributed to the interpretation of the data and preparation and review of the manuscript. All authors read and approved the final manuscript.
Financial support. The ATHENA cohort is managed by Stichting HIV Monitoring and supported by a grant from the Dutch Ministry of Health, Welfare and Sport through the Centre for Infectious Disease Control of the National Institute for Public Health and the Environment. The Swiss HIV Cohort Study (SCHS) is supported by the Swiss National Science Foundation (grant number 201369) and by the SHCS Research Foundation. The data are gathered by the 5 Swiss university hospitals, 2 cantonal hospitals, 15 affiliated hospitals, and 36 private physicians (listed at: http://www.shcs.ch/180-health-care-providers). EuroSIDA has received funding from ViiV Healthcare LLC, Janssen Scientific Affairs, Janssen R&D, Bristol-Myers Squibb, Merck Sharp & Dohme Corp, Gilead Sciences, and the European Union’s Seventh Framework Programme for research, technological development, and demonstration under EuroCoord grant agreement number 260694. The study is also supported by the Danish National Research Foundation (grant number DNRF126) and by the International Cohort Consortium of Infectious Disease (RESPOND).
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.References
References
- 1. Polaris Observatory HCV Collaborators . Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol 2017; 2:161–76. [DOI] [PubMed] [Google Scholar]
- 2. Pawlotsky JM. DAA Failures in African patients with “unusual” HCV subtypes: Hey! Didn’t you know there was another world? J Hepatol 2019; 71:1070–2. [DOI] [PubMed] [Google Scholar]
- 3. Childs K, Davis C, Cannon M, et al. Suboptimal SVR rates in African patients with atypical genotype 1 subtypes: implications for global elimination of hepatitis C. J Hepatol 2019; 71:1099–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wei L, Wang G, Alami NN, et al. Glecaprevir–pibrentasvir to treat chronic hepatitis C virus infection in Asia: 2 multicentre, phase 3 studies—a randomised, double-blind study (VOYAGE-1) and an open-label, single-arm study (VOYAGE-2). Lancet Gastroenterol Hepatol 2020; 5:839–49. [DOI] [PubMed] [Google Scholar]
- 5. Gupta N, Mbituyumuremyi A, Kabahizi J, et al. Treatment of chronic hepatitis C virus infection in Rwanda with ledipasvir–sofosbuvir (SHARED): a single-arm trial. Lancet Gastroenterol Hepatol 2019; 4:119–26. [DOI] [PubMed] [Google Scholar]
- 6. Isfordink CJ, Van De Laar TJW, Rebers SPH, et al. Direct-acting antiviral treatment for hepatitis C genotypes uncommon in high-income countries: a Dutch nationwide cohort study. Open Forum Infect Dis 2021; 8:ofab006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wei L, Lim SG, Xie Q, et al. Sofosbuvir–velpatasvir for treatment of chronic hepatitis C virus infection in Asia: a single-arm, open-label, phase 3 trial. Lancet Gastroenterol Hepatol 2019; 4:127–34. [DOI] [PubMed] [Google Scholar]
- 8. European Association for the Study of the Liver . EASL recommendations on treatment of hepatitis C—final update of the series. J Hepatol 2020; 73:1170–218. [DOI] [PubMed] [Google Scholar]
- 9. Platt L, Easterbrook P, Gower E, et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis 2016; 16:797–808. [DOI] [PubMed] [Google Scholar]
- 10. Rao VB, Johari N, du Cros P, Messina J, Ford N, Cooke GS. Hepatitis C seroprevalence and HIV co-infection in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Infect Dis 2015; 15:819–24. [DOI] [PubMed] [Google Scholar]
- 11. Martinello M, Amin J, Matthews GV, Dore GJ. Prevalence and disease burden of HCV coinfection in HIV cohorts in the Asia Pacific region: a systematic review and meta-analysis. AIDS Rev 2016; 18:69–80. [PubMed] [Google Scholar]
- 12. van Sighem A, Gras L, Smit C, Stolte I, Reiss P. Dutch HIV monitoring report 2020.2020. Available at: https://www.hiv-monitoring.nl/application/files/7716/0571/6500/Netherlands_HIV_Monitoring_Report_2020.pdf. Accessed 25 March 2021.
- 13. Scherrer AU, Traytel A, Braun DL, et al. Cohort profile update: the Swiss HIV Cohort Study (SHCS). Int J Epidemiol 2021; 51:33–34j. [DOI] [PubMed] [Google Scholar]
- 14. Laut K, Kirk O, Rockstroh J, et al. The EuroSIDA study: 25 years of scientific achievements. HIV Med 2020; 21:71–83. [DOI] [PubMed] [Google Scholar]
- 15. Boender TS, Smit C, van Sighem A, et al. AIDS Therapy Evaluation in the Netherlands (ATHENA) national observational HIV cohort: cohort profile. BMJ Open 2018; 8:e022516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peters L, Laut K, Resnati C, et al. Uptake of HCV treatment in HIV/HCV coinfected patients across Europe in the era of direct-acting antivirals. AIDS 2018; 32:1. [DOI] [PubMed] [Google Scholar]
- 17. Davis C, Mgomella GS, da Silva Filipe A, et al. Highly diverse hepatitis C strains detected in sub-Saharan Africa have unknown susceptibility to direct-acting antiviral treatments. Hepatology 2019; 69:1426–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Njouom R, Frost E, Deslandes S, et al. Predominance of hepatitis C virus genotype 4 infection and rapid transmission between 1935 and 1965 in the Central African Republic. J Gen Virol 2009; 90:2452–6. [DOI] [PubMed] [Google Scholar]
- 19. Welzel TM, Bhardwaj N, Hedskog C, et al. Global epidemiology of HCV subtypes and resistance-associated substitutions evaluated by sequencing-based subtype analyses. J Hepatol 2017; 67:224–36. [DOI] [PubMed] [Google Scholar]
- 20. Pantazis N, Rosinska M, van Sighem A, et al. Discriminating between premigration and postmigration HIV acquisition using surveillance data. J Acquir Immune Defic Syndr 2021; 88:117–24. [DOI] [PubMed] [Google Scholar]
- 21. Gupta N, Manirambona L, Shumbusho F, et al. Safety and efficacy of sofosbuvir-velpatasvir-voxilaprevir for re-treatment of chronic hepatitis C virus infection in patients with previous direct-acting antiviral treatment failure in Rwanda (SHARED-3): a single-arm trial. Lancet Gastroenterol Hepatol 2022;7:542–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.