Abstract
Background
COVID-19 medicines delivery units (CMDU) were established in late December 2021 to deliver early antiviral therapy to patients classified as at risk with the aim of preventing hospitalization.
Methods
We performed a service evaluation at 4 CMDUs in England. We assessed demographics and triage outcomes of CMDU referral, uptake of antiviral therapy, and the rate of subsequent hospitalizations within 2 weeks of CMDU referral.
Results
Over a 3-week period, 4788 patients were referred and 3989 were ultimately assessed by a CMDU. Overall, 832 of the patients referred (17%) were judged eligible for treatment and 628 (13%) were ultimately prescribed an antiviral agent. The overall rate of admission within 14 days was 1%. Patients who were admitted were significantly older than those who did not require hospitalization. Of patients prescribed molnupiravir and sotrovimab, 1.8% and 3.2%, respectively, were admitted.
Conclusions
There was a high volume of referrals to CMDU service during the initial surge of the Omicron wave in the United Kingdom. A minority of patients were judged to be eligible for therapy. In a highly vaccinated population, the overall hospitalization rate was low.
Keywords: COVID-19, sotrovimab, molnupiravir
In a multi-centre study we found most patients with COVID-19 referred for consideration of anti-viral therapy ultimately were not eligible for therapy. In a highly vaccinated population the overall rate of admission was low.
Clinical trials have demonstrated efficacy of anti–severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antiviral therapies in prevention of hospitalization among higher-risk patients [1–3]. These studies however, recruited patients who were unvaccinated, included few or no immunosuppressed patients, and did not necessarily relate to contemporaneously prevalent variants with differing risks of severe illness and hospitalization.
An access program for antiviral therapies was launched in the United Kingdom (UK) soon after Medicines and Healthcare Products Regulatory Agency licensing in December 2021. This focused access predominantly on those deemed eligible on the basis of degree of immunosuppression, or a set list of other conditions that had previously been associated with an increased risk of severe disease. These groups of patients had already been prioritized for vaccination, with triple-dose coverage in the general population in mid-December being >50% [4], but in the absence of outcome data in vaccinated eligible cohorts there remained concerns regarding their ongoing risk of hospitalization after infection. COVID-19 medicines delivery units (CMDUs) across the UK were stood up to deliver therapies according to standardized national policies regarding eligibility and preferred antiviral drug [5].
As CMDUs were established, a new wave of transmission occurred in the UK driven by the B1.1.529 Omicron variant [6, 7]. This created a significant pressure on CMDU capacity but also created further uncertainty as to the efficacy of antiviral therapies, due to both the lower reported hospitalization rate following infection with B1.1.529 and evidence on the differential efficacy of neutralizing monoclonal antibodies against Omicron compared to previous variants of concern (VOCs) [8–10]. CMDUs were therefore faced with significant capacity challenges to establish effective and comprehensive services during this time.
We describe the characteristics of patients referred to CMDUs in 4 regions across England and present both service-level and clinical outcomes of referral during the initial 3 weeks of peak transmission of the Omicron wave in the United Kingdom.
METHODS
This service evaluation was performed at 4 CMDUs, serving North Central London, South East London, Sheffield and Humber, and Coast and Vale (North and East Yorkshire and North Lincolnshire). CMDUs were launched nationally on 16 December 2021, but access to neutralizing monoclonal antibody therapy was delayed for 4 days pending national access to sotrovimab. We therefore focus this service evaluation on CMDU referrals triaged between 20 December 2021 through 9 January 2022.
Patients were referred to their local CMDU predominantly via an automated electronic referral system that triangulated positive SARS-CoV-2 polymerase chain reaction (PCR) results with National Health Service (NHS) numbers listed on a central national general practitioner database. In addition, referrals could be made by primary and secondary care providers via email or electronic referral systems and via the NHS 111 telephone advice service. Each day this system was used to generate a list of patients who required evaluation by a member of the CMDU team.
Patients who were eligible for treatment were not hospitalized, had symptomatic SARS-CoV-2 with onset within the last 5 days, and were a member of preidentified risk groups including certain malignancies, organ transplants, and conditions or therapies associated with immunosuppression [11]. Patients were contacted by telephone by a CMDU clinician on receipt of referral and assessed against eligibility criteria that included confirming the patient had a condition on the eligible list for treatment, evaluating the presence or absence of symptoms, and establishing the day of illness both with regard to symptom onset and time since a positive SARS-CoV-2 PCR test. Where information accompanying the referral made clear that patients were outside symptom onset or PCR date, a telephone call was often not made.
Patients who were found to meet all eligibility criteria were offered appropriate therapy. Molnupiravir and sotrovimab were offered according to site local protocols and policies. Pregnant individuals and individuals aged <18 years were only able to receive sotrovimab.
Each CMDU developed its own templates and tools for recording information relating to CMDU triage and assessment. We extracted fully anonymized data from each local system to assess service and clinical outcomes of CMDU referral. We obtained the index of multiple deprivation (IMD) decile for each patient's postcode [12]. Patients were categorized as uncontactable, if a minimum of 2 attempts to contact by phone were ineffective. Patients were categorized as not eligible if they were (1) asymptomatic, (2) beyond 5 days since symptom onset, (3) their symptoms had resolved, (4) beyond 5 days since PCR result, or (5) not in an at-risk group. Eligible patients were categorized according to the therapy they received as either receipt of sotrovimab, receipt of molnupiravir, or declined treatment.
Clinical outcomes were assessed by collating data on hospitalizations for COVID-19, within 14 days of CMDU referral, from all acute hospitals within the catchment area of each CMDU. Hospitalizations were only recorded if a lead clinician at each site, blinded to CMDU referral outcome, deemed the admission being for management of COVID-19 after reviewing the patients’ medical records. Patients admitted for other reasons, for example, elective admissions or acute presentations unrelated to COVID-19, were excluded and we did not collect detailed information on their hospital stay.
Patient Consent Statement
This work meets the NHS definition of a service evaluation and therefore ethics approval and patient consent were not required. Audit approval was obtained at each site and appropriate data sharing agreements were arranged within each sites Integrated Care System (ICS). No confidential data were shared cross-ICS.
RESULTS
A total of 4788 referrals were received across the 4 CMDU sites between 20 December 2021 and 9 January 2022. The median age of patients was 51 (IQR, 38–62) years and 2408 (50%) referrals were female. Vaccination data were available for 1802 patients (37.6%); of these, 93.1% had received at least 1 dose of an approved SARS-CoV-2 vaccine (Table 1).
Table 1.
Demographics of Patients Referred for Treatment Between 20 December and 9 January 2022
| Characteristic | No. (%) |
|---|---|
| Sex | |
| Female | 2408 (50) |
| Male | 2234 (47) |
| Unknown | 146 (3) |
| Age, y, median (IQR) | 51 (38–62) |
| Vaccination status, doses | |
| None | 124 (2) |
| 1 | 55 (1) |
| 2 | 316 (7) |
| ≥3 | 1273 (26) |
| Vaccinated but doses unknown | 34 (1) |
| Data not available | 2986 (62.4) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviation: IQR, interquartile range.
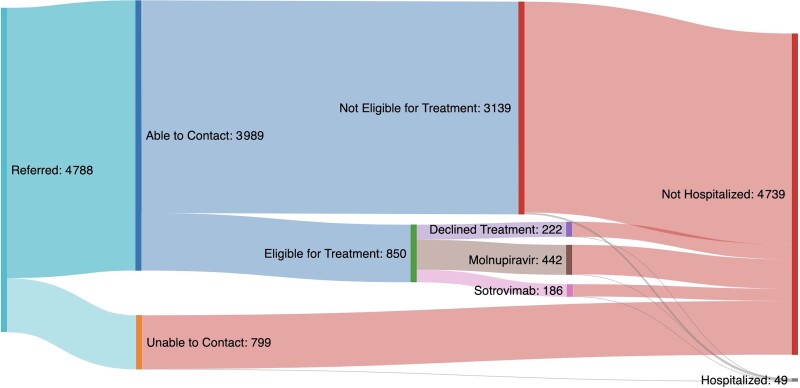
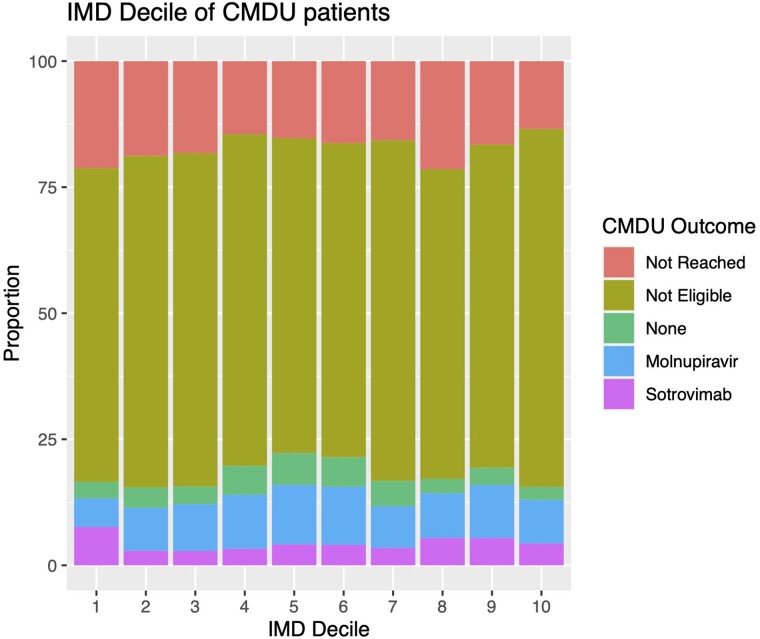
We were able to reach a total of 3989 (83.3%) patients referred to the CMDUs. Overall, 850 of the patients referred (17.7%) were judged eligible for treatment and 3139 patients were judged ineligible for treatment. Where information was available on eligibility, the most common reason was not being in an at-risk clinical group (1155 patients), followed by being either asymptomatic or clinically improved (706 patients). Being outside the PCR or symptom onset window was a reason for exclusion in only 420 and 205 patients, respectively. Granular data on why patients were deemed not eligible were not available on the remaining patients. Of 832 patients judged eligible for treatment, a total of 627 were ultimately prescribed an antiviral agent and a further 222 were deemed eligible but declined treatment (Figure 1). Outcomes of the referral process were similar across all IMD deciles (Figure 2).
Figure 1.
Sankey diagram showing the COVID-19 medicines delivery units (CMDUs) and hospitalization outcomes for patients referred to CMDUs.
Figure 2.
COVID-19 medicines delivery unit (CMDU) treatment outcomes by index of multiple deprivation (IMD) decile.
There were a total of 49 admissions due to COVID-19 within 2 weeks of referral to a CMDU. Overall, 27 patients who were admitted were female (55%), and the median age was 71 (IQR, 48–78) years. The median time to admission was 8 (IQR, 3.5–10.5) days. Hematological malignancies and immune-mediated diseases were the most common underlying diagnosis and 78% of patients were receiving some form of immunosuppression. VOC data were available for 65% of admissions, the majority of which were due to Omicron B1.1.529. Among patients who were admitted, 3 (6%) were unvaccinated (Table 2). Inpatient mortality was 18% in this cohort. The number of patients admitted by treatment status is shown in Table 3. Among patients who were ultimately admitted, patient characteristics did not vary between those who had and had not received therapy.
Table 2.
Clinical Characteristics of 49 Patients Admitted to Hospital Within 14 Days of COVID-19 Medicines Delivery Unit Referral
| Characteristic | No. (%) |
|---|---|
| Admitted to hospital within 14 d | 49 |
| Days to admission, median (IQR) | 8 (3.5–10.5) |
| Age at admissions, y, median (IQR) | 71 (48–78) |
| Genotype of admissions | |
| Delta | 5 (10) |
| Omicron | 27 (55) |
| Not known/unable to sequence | 15 (35) |
| Underlying CEV risk category | |
| Hematological malignancy and/or treatment | 10 (20) |
| Solid organ cancer and/or treatment | 7 (15) |
| Immune-mediated disease | 10 (20) |
| Solid organ transplant | 3 (6) |
| Other priority group | 19 (39) |
| Medications | |
| No immunosuppression | 21 (43) |
| Steroids | 14 (29) |
| Chemotherapy | 4 (8) |
| B-cell–depleting agent | 3 (6) |
| Other immunosuppression | 10 (20) |
| No data available | 2 (5) |
| Vaccine status, doses | |
| None | 3 (6) |
| 1 | 3 (6) |
| 2 | 4 (8) |
| ≥3 | 33 (67) |
| Unknown | 6 (12) |
| CMDU outcome | |
| Sotrovimab | 6 (12) |
| Molnupiravir | 8 (16) |
| Eligible and declined treatment | 2 (4) |
| Ineligible for treatment | 27 (55) |
| Uncontactable | 6 (12) |
| Survival to discharge | |
| No | 9 (18) |
| Yes | 37 (76) |
| Unknown | 3 (6) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: CEV, clinically extremely vulnerable; CMDU, COVID-19 medicines delivery unit; IQR, interquartile range.
Table 3.
COVID-19 Medicines Delivery Unit Referral Outcomes
| Triage Outcome | No. of individuals (%) | Admitted Within 14 d, No. (%) |
|---|---|---|
| Prescribed molnupiravir | 442 | 8 (1.8) |
| Prescribed sotrovimab | 186 | 6 (3.2) |
| Eligible but declined treatment | 222 | 2 (1) |
| Ineligible for treatment | 3139 (66) | 27 (0.9) |
| Uncontactable | 799 (17) | 6 (0.8) |
DISCUSSION
This service evaluation presents the first real-world detailed assessment of the rollout of antiviral therapy for COVID-19 in the UK. We demonstrate that a minority of patients referred to the service were ultimately judged eligible for treatment and that the overall hospitalization rate in this highly vaccinated population was low. Scale-up of CMDUs occurred during a period of significant challenges for health services associated with a wave of transmission driven by the Omicron variant [6, 7]. Significant resources were required to cope with the high volume of referrals received during this period. Our data may inform future iterations of antiviral pathways for vulnerable individuals.
Our 4 CMDU sites managed >1500 referrals a week during the first 3 weeks of the service but initiated <1000 patients on antiviral therapy during this time. We observed that patients were frequently ineligible for therapy either because the time from onset of disease was >5 days or because they had been incorrectly identified as being from an at-risk population. The overall hospitalization rate in our cohort was 1%, which is markedly lower than in the pivotal trials underpinning the use of antiviral therapy to prevent SARS-CoV-2–associated hospitalization [1–3]. Patients who were admitted were significantly older than the overall CMDU population, consistent with the strong age-related risk of severe disease that has been observed with SARS-CoV-2 [3, 13]. Among younger individuals, even those whose comorbidities confer a large relative risk of hospitalization or adverse outcome, the risk of an adverse outcome is still much lower than for older adults [14, 15]. Age per se was not used as a criterion for eligibility for early antiviral therapy, but our data suggest that such a change may be appropriate. We saw higher rates of admission among individuals who ultimately were prescribed molnupiravir and sotrovimab, but this may represent confounding by indication, as the highest-risk patients were both more likely to receive treatment and be admitted regardless of treatment status. A study by the OpenSafely consortium suggested that patients receiving sotrovimab were at lower risk of hospitalization than those who received molnupiravir [16]; however, this study did not have data on patients referred but deemed not eligible for treatment, and in keeping with our study cannot easily control for confounding by indication. Data from randomized controlled trials, including those with and without underlying immunosuppression or other comorbidities, are urgently needed to better inform our understanding of the effectiveness of these therapies in a highly vaccinated population.
We found that the majority of admissions were due to the Omicron variant. Although UK vaccination coverage overall is high, it is lower among disadvantaged groups [17] and there has been particular concern about equitable access to service. Across our study period we found that the majority of referrals lived in an area of higher than average deprivation, with >50% of patients living in 1 of the bottom 4 IMD deciles.
Our study has a number of limitations. Most notably, we relied on routine clinical data and therefore were missing data for a number of variables. This commonly occurred either because the patient was not reachable or because once a patient was judged ineligible, further data were not collected. In addition, each site used a slightly different template for data collection, which limited comparison of outcome and process data across site. Because of missing data, we are not able to provide highly granular data on why patients were judged ineligible for treatment or for why some eligible individuals declined treatment. An important variable for which we had missing data was vaccination status; however, where available the vaccination rate was >93%, in keeping with the high levels of vaccination reported nationally in the UK by December 2021. It is possible that we may have missed some COVID-19–related admissions, in particular, if patients were admitted to a hospital outside of the catchment area of their CMDU, or occurred >14 days after referral. Given the overall extremely low hospitalization rate, it is unlikely this would have significantly influenced our findings.
CMDUs were established at a time of considerable uncertainty about the scale of anticipated peak of ongoing SARS-CoV-2 transmission, the impact of vaccination on hospitalizations, and the efficacy of antiviral therapy and how emerging VOCs would impact both treatment efficacy and overall disease severity. Both the ultimate uptake of treatment and the hospitalization rate were relatively low in this population. Logistical improvements may help reduce the number of ineligible patients referred to CMDUs and therefore improve the conversion rate of referrals to uptake of treatment. Larger-scale, ideally randomized data are urgently needed to better understand the impact of antiviral therapy in settings with high vaccination coverage.
Contributor Information
Michael Brown, Division of Infection, University College London Hospitals NHS Foundation Trust, London, United Kingdom; Clinical Research Department, London School of Hygiene and Tropical Medicine, London, United Kingdom.
Jasjot Saund, Division of Infection, University College London Hospitals NHS Foundation Trust, London, United Kingdom.
Azka Qureshi, Department of Infection, Guys and St Thomas’s NHS Foundation Trust, London, United Kingdom; South East London Covid Prevention and Intervention Service, Guys and St Thomas’s NHS Foundation Trust, London, United Kingdom.
Megan Plowright, Department of Infectious Diseases and Tropical Medicine, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, United Kingdom; Department of Infection, Immunity and Cardiovascular Disease, University of Sheffield, Sheffield, United Kingdom.
Katie Drury, Infection Research Group, Hull University Teaching Hospitals NHS Foundation Trust, Hull, United Kingdom.
Joshua Gahir, Division of Infection, University College London Hospitals NHS Foundation Trust, London, United Kingdom.
Tom Simpson, Department of Respiratory Medicine, Lewisham Hospital, London, United Kingdom.
Thomas Newman, Department of Infectious Diseases and Tropical Medicine, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, United Kingdom; Department of Infection, Immunity and Cardiovascular Disease, University of Sheffield, Sheffield, United Kingdom.
Kate Adams, Infection Research Group, Hull University Teaching Hospitals NHS Foundation Trust, Hull, United Kingdom.
James Galloway, Centre for Rheumatic Disease, Kings College London, London, United Kingdom.
Kezia Durairaj, Department of Infection, Guys and St Thomas’s NHS Foundation Trust, London, United Kingdom; South East London Covid Prevention and Intervention Service, Guys and St Thomas’s NHS Foundation Trust, London, United Kingdom.
Kamla Elgizouli, Infection Research Group, Hull University Teaching Hospitals NHS Foundation Trust, Hull, United Kingdom.
Tommy Rampling, Division of Infection, University College London Hospitals NHS Foundation Trust, London, United Kingdom.
Joby Cole, Department of Infectious Diseases and Tropical Medicine, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, United Kingdom; Department of Infection, Immunity and Cardiovascular Disease, University of Sheffield, Sheffield, United Kingdom.
Nicholas Easom, Infection Research Group, Hull University Teaching Hospitals NHS Foundation Trust, Hull, United Kingdom.
Anna L Goodman, Department of Infection, Guys and St Thomas’s NHS Foundation Trust, London, United Kingdom; South East London Covid Prevention and Intervention Service, Guys and St Thomas’s NHS Foundation Trust, London, United Kingdom; Medical Research Council Clinical Trials Unit, University College London, London, United Kingdom.
Michael Marks, Division of Infection, University College London Hospitals NHS Foundation Trust, London, United Kingdom; Clinical Research Department, London School of Hygiene and Tropical Medicine, London, United Kingdom; Division of Infection and Immunity, University College London, London, United Kingdom.
Notes
Author contributions. M. B., A. G., M. M., J. C., N. E., J. G., and T. R. conceived and designed the study. M. B., A. G., J. C., N. E., and M. M. drafted the manuscript. M. M. performed the analysis. All authors contributed to data collection and manuscript revisions.
Acknowledgments. We are grateful for the immense contribution to establishment, delivery, and governance of all staff supporting the North Central London, South East London, Sheffield, and Hull COVID-19 medicine delivery units and associated acute hospitals, including nurses, doctors, pharmacists, administration, project management, information governance, and regional leadership teams. The work in Sheffield was supported by the Sheffield Teaching Hospital COVID-19 Research Database.
Financial support. M. M. is supported by the National Institute for Health and Care Research and the European and Developing Countries Clinical Trials Partnership.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med 2022; 386:509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gottlieb RL, Vaca CE, Paredes R, et al. Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med 2022; 386:305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med 2021; 385:1941–50. [DOI] [PubMed] [Google Scholar]
- 4. UK Government . Vaccinations in the UK. Coronavirus in the UK. https://coronavirus.data.gov.uk/details/vaccinations. Accessed 18 May 2022.
- 5. National Health Service . CAS-ViewAlert. https://www.cas.mhra.gov.uk/ViewandAcknowledgment/ViewAttachment.aspx?Attachment_id=103968. Accessed 18 May 2022.
- 6. Menni C, Valdes AM, Polidori L, et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of Omicron and Delta variant dominance: a prospective observational study from the ZOE COVID study. Lancet 2022; 399:1618–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 Omicron (B.1.1.529) and Delta (B.1.617.2) variants in England: a cohort study. Lancet 2022; 399:1303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carr EJ, Wu M, Harvey R, et al. Omicron neutralising antibodies after COVID-19 vaccination in haemodialysis patients. Lancet 2022; 399:800–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cheng SMS, Mok CKP, Leung YWY, et al. Neutralizing antibodies against the SARS-CoV-2 Omicron variant BA.1 following homologous and heterologous CoronaVac or BNT162b2 vaccination. Nat Med 2022; 28:486–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Planas D, Saunders N, Maes P, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature 2022; 602:671–5. [DOI] [PubMed] [Google Scholar]
- 11. National Health Service . Coronavirus. Interim clinical commissioning policy: neutralizing monoclonal antibodies or antivirals for non-hospitalised patients with COVID-19. https://www.england.nhs.uk/coronavirus/publication/interim-clinical-commissioning-policy-neutralising-monoclonal-antibodies-or-antivirals-for-non-hospitalised-patients-with-covid-19. Accessed 19 September 2022.
- 12. UK Government . English indices of deprivation 2019. https://www.gov.uk/government/statistics/english-indices-of-deprivation-2019. Accessed 18 May 2022.
- 13. Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ 2020; 369:m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Herrera-Esposito D, de los Campos G. Age-specific rate of severe and critical SARS-CoV-2 infections estimated with multi-country seroprevalence studies. BMC Infect Dis 2022; 22:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020; 584:430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zheng B, Green AC, Tazare J, et al. Comparative effectiveness of sotrovimab and molnupiravir for prevention of severe COVID-19 outcomes in non-hospitalised patients: an observational cohort study using the OpenSAFELY platform. medRxiv [Preprint]. Posted online 23 May 2022. doi: 10.1101/2022.05.22.22275417 [DOI] [Google Scholar]
- 17. Collaborative TO, Curtis HJ, Inglesby P, et al. Trends and clinical characteristics of COVID-19 vaccine recipients: a federated analysis of 57.9 million patients' primary care records in situ using OpenSAFELY. Br J Gen Pract 2021; 72:e51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]