Abstract
Background
Considering the limitations of cell therapy, in case of adequate treatment efficacy, conditioned media (CM) may be a desirable alternative to cell therapy. Hence, the present systematic review and meta-analysis aims to evaluate the efficacy of mesenchymal stem cell-derived conditioned media (MSC-CM) in movement resolution following spinal cord injury (SCI) in animal models.
Methods
A comprehensive search in the databases of Medline, Scopus, Web of Science, and Embase was completed until the end of March 2021. Animal studies that evaluate the efficacy of MSC-CM on movement resolution following SCI were defined as the inclusion criteria. Lack of an SCI-untreated group, CM derived from a source other than MSC, not assessing motor function, failure to report CM administered dose, a follow-up period of less than 4 weeks, duplicates, and review articles were counted as the exclusion criteria. Final results are presented as overall standardized mean difference (SMD) with a 95% confidence interval (CI).
Results
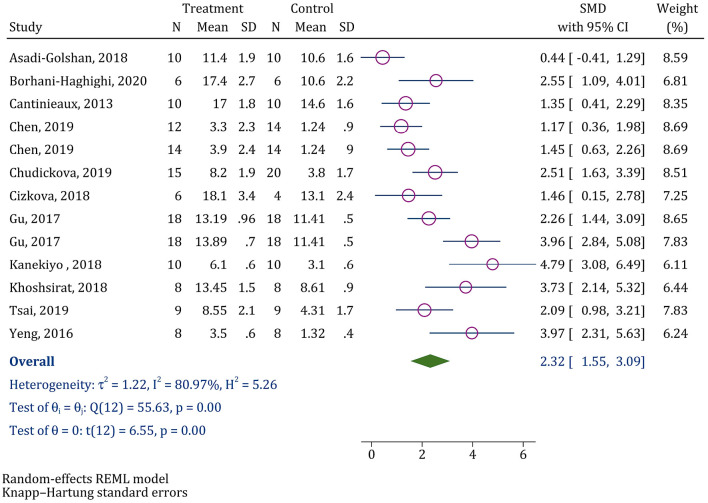
From the 361 nonduplicate articles, data from 11 articles were entered into the present meta-analysis. The analyses showed that MSC-CM administration in SCI animal models promotes motor recovery (SMD=2.32; 95% CI: 1.55, 3.09; p<0.0001). Subgroup analysis was performed because of the noticeable heterogeneity between the studies (I2=80.97%, p<0.0001), depicting that antibiotic administration, delivery amount, delivery type, and follow-up time were the possible sources of heterogeneity. Moreover, multiple meta-regression demonstrated that in cases of delivery amount of more than 120 μL, the efficacy of MSC-CM administration in motor recovery is more than that of delivery amount of less than 120 μL (regression coefficient=3.30; 95% CI: 0.72, 5.89; p=0.019).
Conclusions
Based on the results of the present study, it can be concluded that MSC-CM administration in SCI models improves motor recovery. The efficacy of this treatment strategy significantly increases at doses higher than 120 μL.
Keywords: Conditioned media, Mesenchymal stem cells, Motor function, Spinal cord injury, Systematic review
Introduction
Spinal cord injury (SCI) is one of the most devastating nervous injuries, most commonly occurring in the young population and causing life-span disabilities in patients. Approximately 78% of SCI patients suffer from moderate to severe pains. SCI and its complications impose major direct and indirect financial costs, both on the affected families and health systems, in such a way that the annual treatment cost of these patients is estimated to be approximately $262701).
Following SCI, a cascade of reactions occurs in the injured area, all of which lead to tissue destruction and the loss between cellular connections. This nerve destruction causes dissociation between upper-level neurons and lower-level ones. Thus, it seems that the disabling symptoms will not resolve until the injured area is repaired and efficient connections develop between the neurons proximal to the injured area and the ones distal to the area2,3). Therefore, researchers seek new approaches to be taken to promote the reconstruction of the damaged cells and tissue. Nowadays, it is widely hypothesized that cell transplantation can be an appropriate candidate in the treatment of SCI. Some researchers hypothesized that cell transplantation to the injured spinal cord may restore new neural connections, attenuating the debilitating symptoms4). Various pieces of evidence exist, indicating that mesenchymal stem cells (MSCs) transplantation may improve motor function following an injury to the central nervous system (CNS)5-7). MSCs secrete several cellular factors, promoting a desirable environment for the neural tissue to regenerate itself. It is known that only 1% of the transplanted MSCs survive after 1 week following transplantation8-10). Therefore, it can be concluded that their efficacy is mostly attributed to their paracrine responses and the various growth factors that they secrete11,12). Consequently, the excreted solution following the paracrine activity of these cells is called conditioned media, which effectively enhances neuronal tissue survival after the injury through activating several molecular pathways, such as the phosphoinositide 3-kinase/Akt pathway13-15). Consequently, in the case of MSCs derived conditioned medium (MSC-CM) transplantation being as effective as MSCs transplantation in the treatment of SCI, MSC-CM may be a desirable alternative for MSCs in the treatment of SCI, since it does not have the limitations of MSCs.
Nevertheless, there exist contradictions between the current studies over the subject. For instance, in 2018, Asadi-golshan et al. demonstrated that MSC-CM is ineffective in motor recovery following SCI16), whereas in 2017, Gu et al. reported considerable motor recovery following the MSC-CM treatment in SCI animal models17). Similarly, countless other studies are available, all of which present different results for the treatment. Thus, a consensus over the matter is yet to be achieved, and the factors causing this diversity should be identified. Thus, the present systematic review and meta-analysis aims to investigate the efficacy of MSC-CM transplantation in the treatment motor deteriorations in animal models of SCI.
Method
Study design and search strategy
The present study was designed to investigate the efficacy of MSC-CM treatment on motor recovery following SCI in animal models. For this purpose, a comprehensive search was conducted on the electronic databases of Medline (through PubMed), Scopus, Web of Science, and Embase completed until the end of March 2021. The search strategy was designed using keywords related to MSCs, conditioned media, and SCI. The search strategy in the Medline database is presented in Supplementary table 1. Besides the systematic search, a manual search in the related articles' reference and through search engines Google and Google Scholar was performed.
Selection criteria
The definition of PICO in the present is as follows: Problem or study population (P): animal models of SCI; intervention or index (I): MSC-CM administration; Comparison (C): comparison between the treated and nontreated SCI animals; and Outcome (O): motor recovery evaluation based on standard tests.
Accordingly, the inclusion criteria were studies being conducted to investigate the efficacy of CM administration in SCI animal models. Studies that lacked a control nontreated SCI group, studies in which the source of CM was non-MSC stem cells, studies that transplanted MSCs instead of CM, studies that did not report the desired outcome or the CM preparation method, studies in which the follow-up period was less than 4 weeks (since motor recovery requires a time of at least four weeks following the injury), duplicate studies, and review studies were excluded.
Data collection
Two independent reviewers performed article screening and summarized the data. First, titles and abstracts of the searched articles were screened and related articles were selected. Then, full texts of the potentially related articles were reviewed, and based on the inclusion and exclusion criteria, included articles were selected. Afterward, data, including the study design, animals' characteristics (weight, sex, species, and number of animals in each group), injury mechanism, injury site, interval time between injury and MSC-CM administration, type of the stem cell used (autograft, allograft, and xenograft), source of the MSC-CM, number of the MSCs in the medium, the medium type used for cell culture, dosage and administration route of the CM, number of administrations, use of immunosuppressants, and antibiotics and follow-up period were extracted from the selected articles. Regarding the follow-up period, the eventual follow-up time was extracted from the articles. Moreover, since most of the selected studies reported their findings within their graphs, Sistorm and Mergo's method was adopted to gather the mean and standard deviation (SD) of the articles18). In all of the reported steps, any disagreements were resolved using a third reviewer's opinion.
Quality assessment of the articles
The risk of bias was assessed using the proposed method by Hassannejad et al19). The method is a checklist used for quality assessment of animal studies on SCI. This tool encompasses 15 questions regarding the characteristics of the studied animals, injury method, animal care, sampling method, definitions of the nontreated control group and treatment group, statistical analyses performed, the number of the excluded animals, and the reason for their exclusion. Each reviewer was assigned an independent risk of bias evaluation of the articles' presented data and responded low risk, high risk, and not reported to the checklist's questions. Similar to the data collection, any disagreements were resolved using a third reviewer's opinion.
In cases of fatal errors, the study was considered as having a high risk of bias, and in case of having a low risk of bias in all of the items, the study was considered as having a low risk of bias. Furthermore, in cases of not having fatal errors but at least one question was responded to as high risk of bias or at least two were responded to as not reported, the study would have been considered as a concern in the risk of bias evaluation. In the present study, fatal errors included lack of blinding of the assessor, not using the standard test for assessment of locomotion, and not reporting the severity and spinal level of SCI.
Statistical analysis
Data were analyzed using STATA 14.0 statistical program. All of the included studies were classified on the basis of the extent of motor recovery. Data were entered into the program as means and SDs. Since a number of the included studies reported standard errors (SEs) instead of SD, SD was calculated by multiplying SE to the square root of the number of animals in the group. Moreover, since the methodologies differed (for instance, in terms of administration route and the administration dosage of MSC-CM) in the original studies, based on the performed pilot study, diversity among the included articles was anticipated, and the analyses were performed using random effect model. Also, heterogeneity was evaluated using the Chi-square test and I2 statistics. In cases of heterogeneity, a subgroup analysis was performed to identify the source of the heterogeneity. Eventually, the study results were pooled together, and the overall effect size was reported. This effect size was reported as standardized mean difference (SMD) and 95% confidence interval (CI). Noteworthily, meta-analyses were performed in cases that were reported in at least three studies.
To identify the independent factors, multivariate meta-regression was performed to investigate the effect of methodology differences (for instance in the transplantation route and the administered dose of MSC-CM) on the motor recovery. For this purpose, the variables identified in the subgroup analysis as being potential sources of heterogeneity were included in this multivariate model. Furthermore, to evaluate the individual study effect, a sensitivity analysis based on the leave-one-out approach was performed. Additionally, a sensitivity analysis based on the overall risk of bias was performed. To evaluate the publication bias, a funnel plot and Egger's test were adopted20).
Results
Characteristics of the included studies
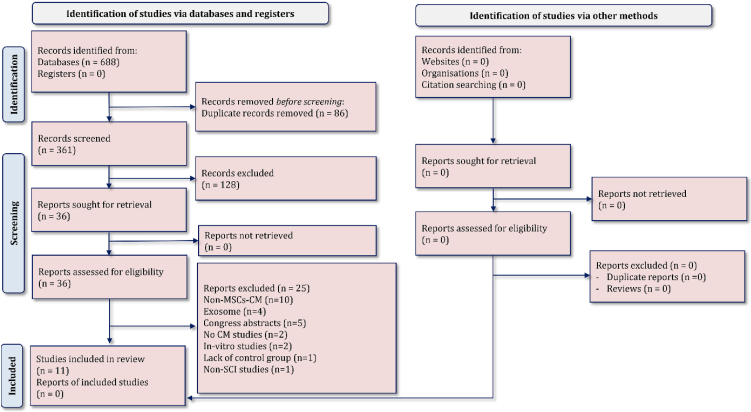
The initial search resulted in 361 nonduplicated articles. After the initial screening, full texts of 36 articles were studied, and finally, 11 articles were entered into the present meta-analysis14-17,21-27) (Fig. 1). The reasons for exclusion of the articles were the use of CM derived from sources other than MSCs (n=10), exosome administration instead of CM (n=4), no CM administration (n=2) in vitro studies (n=2), lacking a nontreated SCI control group (n=1), non-SCI study (n=1), and not reporting the required variables due to the study being presented in a congress (n=5). Worth mentioning is that the authors of congress published studies were contacted, and their names with relevant keywords were searched in electronic databases so that no articles would be neglected. However, no result regarding the last five excluded articles was found.
Figure 1.
PRISMA flow diagram of the present meta-analysis.
All of the included articles were performed on rats. The injury method was contusion in six articles, compression in four articles, and transection in one article. The severity of injury was moderate in seven articles and severe in the other four. The Basso, Beattie, and Bresnahan test was used in all of the included studies for motor function evaluation. The score ranges from 0 (no movement) to 21 (no impairment). Only data gathered using this test was extracted from the studies since it was the only test used in all of the studies. Nine studies administered the CM immediately after the injury, one study started treatment one day after the injury, and one other study started their treatment seven days post-SCI. The MSCs were from the bone marrow in six articles, umbilical cord in two studies, dental pulp in one study, and olfactory ensheathing cells and breast milk stem cells in one other study. Only two studies reported that they had not used immunosuppressive agents, and the other nine articles did not report whether or not they had used immunosuppressive. Seven articles administered antibiotics, and four other articles did not. Ten studies applied a single-dose treatment regimen. The single-dose injections varied between 3 and 1400 μL, and the multidose varied between 42000 and 84000 μL. Transplantation route was intrathecal in five studies, intraperitoneal in two studies, intravenous in two studies, intraventricular in one study, and in situ in one other study. The follow-up period ranged from 1 to 12 weeks (1 week in one study, 4 weeks in one study, 6 weeks in five studies, 8 weeks in one study, 9 weeks in one study, 10 weeks in one study, and 12 weeks in one other study) (Table 1).
Table 1.
Characteristics of Included Studies.
| Study | Animals | n SCI, n treat | SCI model and location | Injury to treatment (days) | Cell source, type of graft | Medium | Immunosuppressive, antibiotic | Delivery amount (μL) and type | Follow- up (weeks) |
|---|---|---|---|---|---|---|---|---|---|
| Asadi-Golshan, 201816) | Male, Rat, SD, 250–280 | 10, 10 | Compression, Moderate, T7 | 0 | Dental Pulp, Xenogeneic | DMEM | No, Yes | 3, Single-dose, In situ | 6 |
| Borhani-Haghighi, 202021) | Male, Rat, SD, 250–280 | 6, 6 | Compression, Moderate, T7 | 2 | Breast milk stem cell, Xenogeneic | DMEM | NR, No | 3, Single-dose, IT | 6 |
| Cantinieaux, 201314) | Female, Rat, Wistar, 200 | 10, 10 | Contusion, Severe, T11 | 0 | BMSCs, Allogenic | DMEM | NR, No | 10, Single-dose, IT | 6 |
| Chen, 201922) | Female, Rat, SD, 250–270 | 14, 26 | Transection, Severe, T8 | 0 | BMSCs, Xenogeneic | DMEM and NRLM | NR, Yes | 120, Single-dose, IT | 8 |
| Chudickova, 201923) | Male, Rat, Wistar, 250–300 | 20, 15 | Compression, Moderate, T8 | 7 | UCMSCs, Xenogeneic | CCM | NR, Yes | 50, Single-dose, IT | 9 |
| Cizkova, 201815) | Male, Rat, Wistar, 300–320 | 4, 6 | Compression, Moderate, T8–T9 | 0 | BMSCs, Allogenic | DMEM | NR, No | 120, Single-dose, IT | 10 |
| Gu, 201717) | Male, Rat, SD, 250–300 | 18, 36 | Contusion, Moderate, T10 | 0 | OEC, Allogenic | DMEM | NR, Yes | 42000 and 84000, Multidose, IP | 6 |
| Kanekiyo, 201824) | Female, Rat, SD, 200 | 10, 10 | Contusion, Severe, T8–T9 | 0 | BMSCs, Allogenic | DMEM | NR, Yes | 1400, Single-dose, Intraventricular | 4 |
| Khoshsirat, 201825) | Female, Rat, Wistar, 180–200 | 8, 8 | Contusion, Moderate, T9–T10 | 0 | BMSCs, Allogenic | DMEM | No, Yes | NR, Single-dose, IP | 12 |
| Tsai, 201926) | Female, Rat, SD, 250–350 | 9, 9 | Contusion, Moderate, T10 | 0 | BMSCs, Allogenic | DMEM | NR, No | 450, Single-dose, IV | 6 |
| Yeng, 201627) | Male, Rat, SD, 206–230 | 8, 8 | Contusion, Severe, T8–T10 | 0 | UCMSCs, Xenogeneic | DMEM | NR, Yes | 500, Single-dose, IV | 1 |
BMSCs: Bone marrow mesenchymal stem cell; CCM: Complete culture medium; DMEM: Dulbecco’s Modified Eagle’s medium; ECGM: Endothelial cell growth medium; IP: Intraperitoneal; IT: Intrathecal; IV: Intravenous; NR: Not reported; NRLM: neural regeneration laboratory medium; OEC: Olfactory ensheathing cell; SD: Sprague-Dawley; UCMSCs: Umbilical cord mesenchymal stem cells
Risk of bias
Items of species, using appropriate tests, severity of injury, level of injury, designation of strain, definition of control, description of statistical analysis, regulation and ethics, genetic background, and method of allocation to treatments were at low risk in all of the studies. Moreover, age/weight, bladder expression, and the number of the animals per group were each not reported in one study. Finally, a description of the reasons to exclude animals from the experiment during the study was reported in only one study (Table 2).
Table 2.
Risk of Bias Assessment of Included Studies.
| Study | Item 1 | Item 2 | Item 3 | Item 4 | Item 5 | Item 6 | Item 7 | Item 8 | Item 9 | Item 10 | Item 11 | Item 12 | Item 13 | Item 14 | Item 15 | Overall |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Asadi-Golshan, 2018 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Low |
| Borhani-Haghighi, 2020 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | NR | ✓ | ✓ | NR | High |
| Cantinieaux, 2013 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | NR | Low |
| Chen, 2019 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | NR | Low |
| Chudickova, 2019 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | NR | Low |
| Cizkova, 2018 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | NR | ✓ | ✓ | NR | High |
| Gu, 2017 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | NR | Low |
| Kaneliyo, 2018 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | NR | ✓ | ✓ | ✓ | NR | Some concern |
| Khoshsirat, 2018 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | NR | Low |
| Tsai, 2019 | ✓ | ✓ | ✓ | ✓ | NR | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | NR | Some concern |
| Yeng, 2016 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | NR | Low |
1. Species; 2. Using appropriate tests; 3. Severity of injury; 4. Level of injury; 5. Age/weight; 6. Number of animals per group; 7. Designation of strain; 8. Definition of control; 9. Description of statistical analysis; 10. Regulation and ethics; 11. Bladder expression; 12. Blindness of assessor; 13. Genetic background; 14. Method of allocation to treatments; 15. Description of the reasons to exclude animals from the experiment during the study (attrition).
✓: Low risk of bias; NR: Not reported
Since two studies did not report the blinding status of the assessor, they had fatal errors and were considered as having a high risk of bias. Furthermore, two studies were scored to have some concern status and seven studies were classified as having a low risk of bias.
Meta-analysis
Motor recovery
Data from all of the 11 articles were evaluated in this section. Analyses demonstrated that MSC-CM administration in SCI animal models improves motor recovery (SMD=2.32; 95% CI: 1.55, 3.09); nevertheless, considerable heterogeneity was observed among the studies (I2=80.97%, p<0.0001) (Fig. 2). Consequently, the studies were classified on the basis of the severity of SCI, cell source, antibiotic administration, treatment protocol, delivery type, medium type, type of graft, delivery amount, and follow-up duration, and separate analyses were performed for each of the subgroups (Table 3). Subgroup analysis showed that heterogeneity among the studies in which antibiotic was not administered (I2=0.00%; p=0.494) was lower than that of the other studies (I2=87.12%; p<0.001). Moreover, nearly all of the studies that used an intrathecal route were homogenous (I2=32.47%; p=0.205). Eventually, calculations show that the possible sources of heterogeneity were the delivery amount (I2=56.46%; p=0.032) and the follow-up duration (I2=67.14%; p=0.022).
Figure 2.
Forest plot for the effect of conditioned media on locomotor recovery after spinal cord injury.
SMD: Standardized mean difference; CI: Confidence interval
Table 3.
Subgroup Analysis.
| Subgroups | No. of experiments | SMD (95% CI) | P value | Heterogeneity (p value) |
|---|---|---|---|---|
| Severity of SCI | ||||
| Moderate | 8 | 2.32 (1.36, 3.28) | 0.001 | 76.98% (<0.0001) |
| Severe | 5 | 2.40 (0.34, 4.45) | 0.032 | 88.54% (<0.0001) |
| Cell source | ||||
| BMSCs | 7 | 2.14 (0.92, 3.36) | 0.005 | 79.64% (0.001) |
| Other | 6 | 2.54 (1.16, 3.92) | 0.005 | 83.50% (<0.0001) |
| Antibiotic | ||||
| Yes | 9 | 2.57 (1.44, 3.71) | 0.001 | 87.12% (<0.0001) |
| No | 4 | 1.76 (0.92, 2.60) | 0.007 | 0.00% (0.494) |
| Treatment protocol | ||||
| Single dose | 11 | 2.17 (1.30, 3.04) | <0.0001 | 80.07% (<0.0001) |
| Multi dose | 2 | NA | NA | NA |
| Delivery type | ||||
| IT | 6 | 1.68 (1.05, 2.31) | 0.001 | 32.47% (0.205) |
| IP | 3 | 3.22 (0.83, 5.61) | 0.029 | 67.33% (0.035) |
| IV | 2 | NA | NA | NA |
| In situ | 1 | NA | NA | NA |
| Intraventricular | 1 | NA | NA | NA |
| Medium | ||||
| DMEM | 11 | 2.41 (1.48, 3.34) | <0.0001 | 82.49% (<0.0001) |
| Other | 2 | NA | NA | NA |
| Type of graft | ||||
| Allogenic | 7 | 2.71 (1.50, 3.92) | 0.002 | 78.39% (<0.001) |
| Xenogeneic | 6 | 1.88 (0.62, 3.14) | 0.012 | 80.69% (0.001) |
| Delivery amount | ||||
| ≤120 μL | 7 | 1.49 (0.81, 2.18) | 0.002 | 56.46% (0.032) |
| >120 μL | 6 | 3.32 (2.20, 4.45) | 0.001 | 56.67% (0.012) |
| Follow-up duration | ||||
| <8 weeks | 8 | 2.57 (1.35, 3.79) | 0.002 | 84.38% (<0.0001) |
| ≥8 weeks | 5 | 1.94 (0.74, 3.14) | 0.011 | 67.15% (0.022) |
| Risk of bias | ||||
| Low risk | 9 | 2.21 (1.21, 3.20) | 0.001 | 84.48% (<0.001) |
| High risk and some concern | 4 | 2.63 (0.42, 4.85) | 0.032 | 72.72% (0.020) |
BMSCs: Bone marrow mesenchymal stem cell; DMEM: Dulbecco’s Modified Eagle’s medium; IP: Intraperitoneal; IT: Intrathecal; IV: Intravenous; NA: Not applicable due to the limited number of included studies in the subgroup
Meta-regression
Multivariate meta-regression showed that among the studied factors, only the administered dosage of MSC-CM affects the efficacy of the treatment. In other words, in doses higher than 120 μL, the efficacy of MSC-CM treatment is significantly higher than that of doses lower than 120 μL (coefficient=3.30; 95% CI: 0.72, 5.89; p=0.019) (Table 4).
Table 4.
Multiple Meta-regression for Identification of the Source of Heterogeneity.
| Variables | Coefficient | 95% CI | P |
|---|---|---|---|
| Delivery amount | |||
| ≤120 μL | Ref. | Ref. | Ref. |
| >120 μL | 3.30 | 0.72 to 5.89 | 0.019 |
| Delivery type | |||
| IT | Ref. | Ref. | Ref. |
| IP | −2.33 | −5.93 to 1.28 | 0.171 |
| Other | −1.89 | −4.77 to 0.99 | 0.165 |
| Antibiotic | |||
| Yes | Ref. | Ref. | Ref. |
| No | −0.79 | −2.56 to 0.99 | 0.329 |
| Follow-up duration | |||
| <8 weeks | Ref. | Ref. | Ref. |
| ≥8 weeks | −0.38 | −2.28 to 1.51 | 0.645 |
CI: Confidence interval; IP: Intraperitoneal; IT: Intrathecal; Ref.: Reference category
Sensitivity analysis
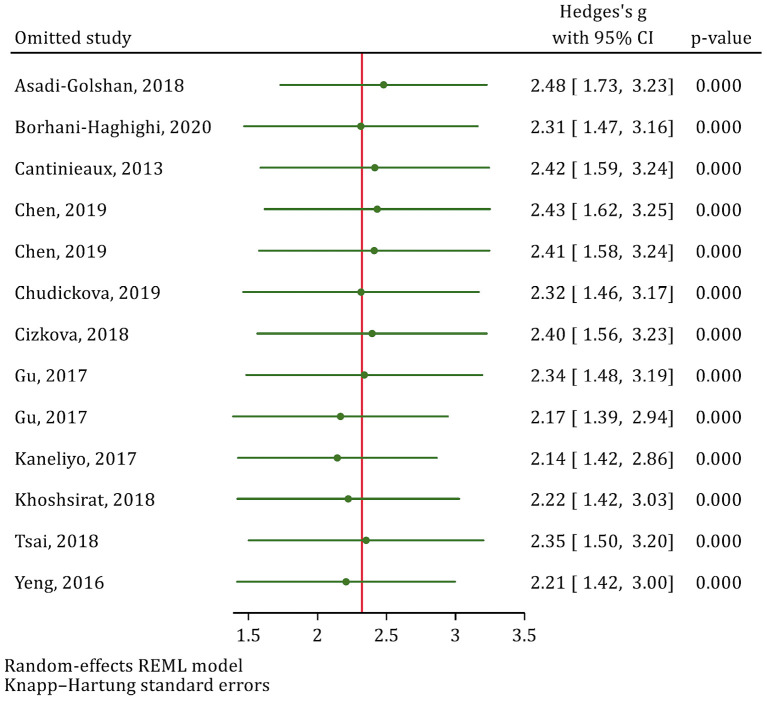
Since two studies were reported as having a high risk of bias and two other studies were considered as some concern in terms of risk of bias, these two groups of studies were combined in the sensitivity analysis. Consequently, the analyses revealed that the reported efficacy for MSC-CM in the studies having low risk of bias (SMD=2.1; 95% CI: 1.21, 3.20; p=0.001) did not significantly vary with that of studies having some concern and high risk of bias (SMD=2.63; 95% CI: 0.42, 4.85; p=0.032). Moreover, the leave-one-out sensitivity analysis showed that eliminating none of the articles significantly affected the pooled SMD (Fig. 3).
Figure 3.
Leave-one-out sensitivity analysis to explore single study’s effect on overall effect size.
Publication bias
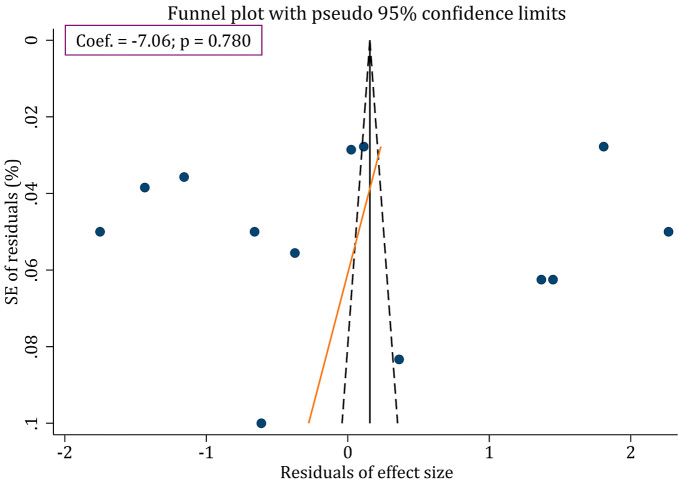
Egger's test revealed that there existed no publication bias regarding the efficacy of MSCs derived CM in motor recovery following SCI in animal models (p=0.780) (Fig. 4).
Figure 4.
Funnel plot for assessment of publication bias in the efficacy of conditioned media on locomotor recovery after spinal cord injury.
Discussion
Findings of the present meta-analysis revealed that MSC-CM administration promotes motor recovery in animal models following SCI. Since a 0.2 score for the effect size shows a weak efficacy, 0.5 score shows a moderate efficacy and a score of ≥0.8 shows a strong efficacy28,29), our findings present a strong efficacy for MSC-CM treatment following SCI in animal models.
Several systematic reviews have been published on the efficacy of MSC-CM treatment on CNS repair, and the reported efficacy on motor function enhancement is attributed to the growth factors (such as Nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), matrix metalloproteinase, Vascular endothelial growth factor (VEGF), and Hepatocyte growth factor (HGF)) secreted from these cells resulting in the repair and maintenance of the nervous tissue30-33). However, no systematic review has been published on the efficacy of MSC-CM on the brain or spinal cord tissue. The existing systematic review studies were found to be conducted on the administration efficacy of CM on bone regeneration (on animal and clinical studies)34), pulmonary fibrosis35), and lung disease36). All these studies have confirmed the efficacy of CM administration in tissue repair attributed this repair to the anti-inflammatory properties of the factors found in CM. The anti-inflammatory effects of CM prevent TNFa and IL-6 rises in the nervous tissue of the spinal cord, which may lead to tissue protection and promotes functional recovery following SCI14).
Another nervous tissue protection, eventually causing functional recovery improvement, occurs as a result of angiogenesis. Angiogenesis provides oxygen and nutritional factors, preventing nervous tissue and regrowth of the axons37,38). Conversely, VEGF, as one of the key elements in CM, is essential in angiogenesis, besides its neuroprotective effects39,40). Osteopontin41,42), fibroblast growth factor-binding protein43,44), and matrix metalloproteinase-1345), which are present in the CM, are also responsible for the initiation and progression of angiogenesis and consequently motor function recovery.
Besides the stimulants of angiogenesis, antiapoptotic factors are present in the CM, which prevent tissue destruction and protect the nervous tissue resulting in motor recovery following SCI. These factors include NGF (protecting sympathetic and sensory neurons)46), TIMP-1 and CINC-3 (protecting motor neurons)47) and BDNF (reducing astroglia scar formation)48).
The findings of the present study demonstrated that MSC-CM administered volumes of more than 120 μL promote the efficacy of the treatment in motor recovery following SCI. Nonetheless, although the studies administering higher doses of CM observed greater efficacies, their quality assessment revealed that all of the studies entered in the present meta-analysis lacked sample size calculations and optimal dose assessments. Thus, as a determinant in the process of translating the results into clinical applications49), the mentioned quality shortcomings are considered to be among the limitations of the present study. Furthermore, as a disadvantage for the CM treatment in comparison with stem cell therapy, cytokines and growth factors present in the CM have shorter half-lives, resulting in multiple drug administrations when CM therapy is intended50). Thus, it is suggested that further animal studies be performed to determine the optimal administered dose.
The primary goal in animal studies is to evaluate the treatment efficacy, whereas in clinical studies, the first step to approve a treatment strategy is to determine the safety of the treatment. Therefore, as another limitation of the present study, the safety and adverse effects of the CM treatment were not evaluated in the animal studies included in our meta-analysis. Consequently, it is recommended that the safety of CM treatment be assessed in future studies. It is worth mentioning that no clinical trials exist regarding the application of MSC-CM in SCI, whereas clinical trials are on their way in the application of MSCs-CM in SCI, while clinical trials are on their way regarding the application of CM in other conditions51), as well as the application of MSCs in SCI30,52). This highlights the need for further animal studies and clinical trials for researchers to be able to investigate the clinical application and translation of this treatment strategy.
Conclusion
The findings of the present study revealed that MSC-CM administration promotes motor recovery in SCI animal models. The efficacy of this treatment strategy enhances in volumes higher than 120 μL. Since MSC-CM treatment has fewer limitations in comparison with MSC administration, the treatment can be considered as an alternative treatment approach in translational studies.
Conflicts of Interest: The authors declare that there are no relevant conflicts of interest.
Sources of Funding: Iran University of Medical Sciences has supported this research (Grant number: 98-2-37-15667).
Author Contributions: MY and AS designed the study. AS, AT, AM, HGN, and AMN gathered the data. MY analyzed the data. All authors interpreted the findings. AS and MY wrote the first draft, and other authors critically revised the manuscript.
Ethical Approval: This study received ethics approval from the ethics committee of Iran University of Medical Sciences (Ethical Approval Code: IR.IUMS.REC.1398.572).
Consent for Publication: Not applicable.
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplementary Material
References
- 1.Mann R, Schaefer C, Sadosky A, et al. Burden of spinal cord injury-related neuropathic pain in the United States: retrospective chart review and cross-sectional survey. Spinal Cord. 2013;51(7):564-70. [DOI] [PubMed] [Google Scholar]
- 2.Finnerup NB, Otto M, McQuay HJ, et al. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain. 2005;118(3):289-305. [DOI] [PubMed] [Google Scholar]
- 3.Backonja M-M, Irving G, Argoff C. Rational multidrug therapy in the treatment of neuropathic pain. Curr Pain Headache Rep. 2006;10(1):34-8. [DOI] [PubMed] [Google Scholar]
- 4.Hama A, Sagen J. Behavioral characterization and effect of clinical drugs in a rat model of pain following spinal cord compression. Brain Res. 2007;1185:117-28. [DOI] [PubMed] [Google Scholar]
- 5.Fang K-M, Chen J-K, Hung S-C, et al. Effects of combinatorial treatment with pituitary adenylate cyclase activating peptide and human mesenchymal stem cells on spinal cord tissue repair. PloS One. 2010;5(12):e15299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang R, Liu Y, Yan K, et al. Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. J Neuroinflammation. 2013;10(1):871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarveazad A, Babahajian A, Bakhtiari M, et al. The combined application of human adipose derived stem cells and chondroitinase ABC in treatment of a spinal cord injury model. Neuropeptides. 2017;61:39-47. [DOI] [PubMed] [Google Scholar]
- 8.Lee RH, Pulin AA, Seo MJ, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5(1):54-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noiseux N, Gnecchi M, Lopez-Ilasaca M, et al. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther. 2006;14(6):840-50. [DOI] [PubMed] [Google Scholar]
- 10.Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lalu MM, McIntyre L, Pugliese C, et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PloS One. 2012;7(10):e47559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maguire G. Stem cell therapy without the cells. Commun Integr Biol. 2013;6(6):e26631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheibe F, Klein O, Klose J, et al. Mesenchymal stromal cells rescue cortical neurons from apoptotic cell death in an in vitro model of cerebral ischemia. Cell Mol Neurobiol. 2012;32(4):567-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cantinieaux D, Quertainmont R, Blacher S, et al. Conditioned medium from bone marrow-derived mesenchymal stem cells improves recovery after spinal cord injury in rats: an original strategy to avoid cell transplantation. PloS One. 2013;8(8):e69515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cizkova D, Cubinkova V, Smolek T, et al. Localized intrathecal delivery of mesenchymal stromal cells conditioned medium improves functional recovery in a rat model of spinal cord injury. Int J Mol Sci. 2018;19(3):870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asadi-Golshan R, Razban V, Mirzaei E, et al. Sensory and motor behavior evidences supporting the usefulness of conditioned medium from dental pulp-derived stem cells in spinal cord injury in rats. Asian Spine J. 2018;12(5):785-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu M, Gao Z, Li X, et al. Conditioned medium of olfactory ensheathing cells promotes the functional recovery and axonal regeneration after contusive spinal cord injury. Brain Res. 2017;1654(Part A):43-54. [DOI] [PubMed] [Google Scholar]
- 18.Sistrom CL, Mergo PJ. A simple method for obtaining original data from published graphs and plots. Am J Roentgenol. 2000;174(5):1241-4. [DOI] [PubMed] [Google Scholar]
- 19.Hassannejad Z, Sharif-Alhoseini M, Shakouri-Motlagh A, et al. Potential variables affecting the quality of animal studies regarding pathophysiology of traumatic spinal cord injuries. Spinal Cord. 2016;54(8):579-83. [DOI] [PubMed] [Google Scholar]
- 20.Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borhani-Haghighi M, Navid S, Mohamadi Y. The therapeutic potential of conditioned medium from human breast milk stem cells in treating spinal cord injury. Asian Spine J. 2020;14(2):131-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y-T, Tsai M-J, Hsieh N, et al. The superiority of conditioned medium derived from rapidly expanded mesenchymal stem cells for neural repair. Stem Cell Res Ther. 2019;10(1):390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chudickova M, Vackova I, Machova Urdzikova L, et al. The effect of Wharton jelly-derived mesenchymal stromal cells and their conditioned media in the treatment of a rat spinal cord injury. Int J Mol Sci. 2019;20(18):4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanekiyo K, Wakabayashi T, Nakano N, et al. Effects of intrathecal injection of the conditioned medium from bone marrow stromal cells on spinal cord injury in rats. J Neurotrauma. 2018;35(3):521-32. [DOI] [PubMed] [Google Scholar]
- 25.Khoshsirat S, Abbaszadeh HA, Ahrabi B, et al. Evaluation of the effect of BMSCs condition media and methylprednisolone in TGF-β expression and functional recovery after an acute spinal cord injury. Bratisl Lek Listy. 2018;119(11):684-91. [DOI] [PubMed] [Google Scholar]
- 26.Tsai M-J, Liou D-Y, Lin Y-R, et al. Attenuating spinal cord injury by conditioned medium from bone marrow mesenchymal stem cells. J Clin Med. 2019;8(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeng C-H, Chen P-J, Chang H-K, et al. Attenuating spinal cord injury by conditioned medium from human umbilical cord blood-derived CD34+ cells in rats. Taiwan J Obstet Gynecol. 2016;55(1):85-93. [DOI] [PubMed] [Google Scholar]
- 28.Schulz KF, Chalmers I, Hayes RJ, et al. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273(5):408-12. [DOI] [PubMed] [Google Scholar]
- 29.Cohen J. Statistical power analysis for the behavioral sciences. New York: Routledge; 1988. 567 p [Google Scholar]
- 30.Xu P, Yang X. The efficacy and safety of mesenchymal stem cell transplantation for spinal cord injury patients: a meta-analysis and systematic review. Cell Transplant. 2019;28(1):36-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yousefifard M, Shamseddin J, Babahajian A, et al. Efficacy of adipose derived stem cells on functional and neurological improvement following ischemic stroke: a systematic review and meta-analysis. BMC Neurol. 2020;20(1):294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muthu S, Jeyaraman M, Gulati A, et al. Current evidence on mesenchymal stem cell therapy for traumatic spinal cord injury: systematic review and meta-analysis. Cytotherapy. 2021;23(3):186-97. [DOI] [PubMed] [Google Scholar]
- 33.Antonic A, Sena ES, Lees JS, et al. Stem cell transplantation in traumatic spinal cord injury: a systematic review and meta-analysis of animal studies. PLoS Biol. 2013;11(12):e1001738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benavides-Castellanos MP, Garzón-Orjuela N, Linero I. Effectiveness of mesenchymal stem cell-conditioned medium in bone regeneration in animal and human models: a systematic review and meta-analysis. Cell Regen. 2020;9(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ali KM, Fattah FHR, Omar SH, et al. Mesenchymal stromal cells derived conditioned medium in pulmonary fibrosis: a systematic review and meta-analysis. Arch Iran Med. 2020;23(12):870-9. [DOI] [PubMed] [Google Scholar]
- 36.Emukah C, Dittmar E, Naqvi R, et al. Mesenchymal stromal cell conditioned media for lung disease: a systematic review and meta-analysis of preclinical studies. Respir Res. 2019;20(1):239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hobson MI, Green CJ, Terenghi G. VEGF enhances intraneural angiogenesis and improves nerve regeneration after axotomy. J Anat. 2000;197(4):591-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dray C, Rougon G, Debarbieux F. Quantitative analysis by in vivo imaging of the dynamics of vascular and axonal networks in injured mouse spinal cord. Proc Natl Acad Sci U S A. 2009;106(23):9459-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun Y, Jin K, Xie L, et al. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Investig. 2003;111(12):1843-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Storkebaum E, Lambrechts D, Carmeliet P. VEGF: once regarded as a specific angiogenic factor, now implicated in neuroprotection. Bioessays. 2004;26(9):943-54. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Yan W, Lu X, et al. Overexpression of osteopontin induces angiogenesis of endothelial progenitor cells via the avβ3/PI3K/AKT/eNOS/NO signaling pathway in glioma cells. Eur J Cell Biol. 2011;90(8):642-8. [DOI] [PubMed] [Google Scholar]
- 42.Dai J, Peng L, Fan K, et al. Osteopontin induces angiogenesis through activation of PI3K/AKT and ERK1/2 in endothelial cells. Oncogene. 2009;28(38):3412-22. [DOI] [PubMed] [Google Scholar]
- 43.Li W-M, Chen W-B. Effect of FGF-BP on angiogenesis in squamous cell carcinoma. Chin Med J. 2004;117(4):621-3. [PubMed] [Google Scholar]
- 44.Harris VK, Coticchia CM, Kagan BL, et al. Induction of the angiogenic modulator fibroblast growth factor-binding protein by epidermal growth factor is mediated through both MEK/ERK and p38 signal transduction pathways. J Biol Chem. 2000;275(15):10802-11. [DOI] [PubMed] [Google Scholar]
- 45.Lederle W, Hartenstein B, Meides A, et al. MMP13 as a stromal mediator in controlling persistent angiogenesis in skin carcinoma. Carcinogenesis. 2010;31(7):1175-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y, Luo W, Reiser G. Activation of protease-activated receptors in astrocytes evokes a novel neuroprotective pathway through release of chemokines of the growth-regulated oncogene/cytokine-induced neutrophil chemoattractant family. EurJ Neurosci. 2007;26(11):3159-68. [DOI] [PubMed] [Google Scholar]
- 47.Tejima E, Guo S, Murata Y, et al. Neuroprotective effects of overexpressing tissue inhibitor of metalloproteinase TIMP-1. J Neurotrauma. 2009;26(11):1935-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jain A, McKeon RJ, Brady-Kalnay SM, et al. Sustained delivery of activated Rho GTPases and BDNF promotes axon growth in CSPG-rich regions following spinal cord injury. PloS One. 2011;6(1):e16135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wardlaw J, Warlow C, Sandercock PAG, et al. Neuroprotection disappointment yet aGAIN. Lancet. 2000;356(9229):P597. [DOI] [PubMed] [Google Scholar]
- 50.Pawitan JA. Prospect of stem cell conditioned medium in regenerative medicine. BioMed Res Int. 2014;2014:965849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katagiri W, Watanabe J, Toyama N, et al. Clinical study of bone regeneration by conditioned medium from mesenchymal stem cells after maxillary sinus floor elevation. Implant Dent. 2017;26(4):607-12. [DOI] [PubMed] [Google Scholar]
- 52.Cofano F, Boido M, Monticelli M, et al. Mesenchymal stem cells for spinal cord injury: current options, limitations, and future of cell therapy. Int J Mol Sci. 2019;20(11):2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.