Abstract
Proprioception is a deep sensation that perceives the position of each part of the body, state of movement and muscle contraction, and resistance and mass applied to the body. Proprioceptive feedback influences movement and positional accuracy, resulting in key somatosensory functions for human postural control. Proprioception encompasses signals received from proprioceptors located in the skin, subcutaneous tissue, muscles, tendons, and joint capsules, commonly known as mechanoreceptors. The muscle spindle, a crucial proprioceptor, is stretched during eccentric contraction of muscle, thus generating an action potential on afferent fibers to convey a proprioceptive information to the sensorimotor cortex in the brain. For exercise therapy in patients with locomotor disease, proprioception serves an essential function for motor control; thus, this should be considered to obtain effective muscle output. As postural control is achieved by proprioceptive function according to the balance between the lower limb and trunk, relative proprioceptive weighting ratio can help clarify proprioceptive control using muscle response to mechanical vibration. The absence of proprioceptive information congruent with motor intention activates cortical center monitoring incongruence of sensation, leading to pathological pain. Therapeutic procedures may aim to restore the integrity of cortical information processing in musculoskeletal chronic pain. Poor proprioception is one of the main causes of decreased postural balance control in elderly patients with low back pain (LBP). It has been hypothesized that proprioception of the lower limbs deteriorates with age-related muscle mass loss (sarcopenia), which increases the proprioceptive burden on the lumbar spine. Accurate diagnosis of the proprioceptive function is important for establishing a treatment procedure for proprioceptive recovery, and further prospective research is required to clarify the relationship between proprioception and LBP improvement.
Keywords: proprioception, low back pain, sarcopenia, aging, elderly patient
Introduction
In Japan, the prevalence of chronic musculoskeletal pain is 15.4%, and it mostly occurs in the low back1). The prevalence and associated burden of low back pain (LBP) increase with age2), which is a major problem that cannot be overlooked considering Japan's super-aging society and the decline in quality of life associated with LBP. In particular, the incidence of chronic LBP (CLBP) increases with age, with more than 1 in 3 community-dwelling older adults experiencing LBP3). Furthermore, it presents one of the most disabling and therapeutic challenges affecting older adults4). For 40 years, the mechanical model of geriatric LBP has been based on pathoanatomical spinal changes; however, spinal degeneration is highly prevalent in old age and is weakly associated with pain intensity5,6). As older adults are susceptible to LBP, mechanisms other than spinal degeneration such as systemic senescence may also be implicated. Recently, it has been reported that age-related muscle mass loss (sarcopenia), which occurs with increasing age, may cause pain7-9) with changes due to systemic aging. Moreover, sarcopenia and/or sarcopenic obesity (age-related intramuscular fat deposition), which are associated with geriatric LBP10,11), are considered a result of systemic inflammation12,13). However, age-related skeletal muscle mass loss occurs in type II fibers14); therefore, trunk muscles, which contain a greater number of type I fibers, develop sarcopenic changes later than muscles in the lower extremities15). There is conflicting evidence for the relationship between morphological changes in the lumbar muscles and LBP16-18). At the other extreme, activation of local muscles is essential for segmental stability of the lumbar spine; therefore, local and global muscles, which were initially believed to function independently for stabilization and moment generation, respectively, are both crucial for the stability and mobility of the lumbar spine19,20). Clinical instability of the spine, as a potential cause of LBP, is defined as failure of the motor control system. Motor control adaptations are inevitably linked to somatosensory feedback, namely proprioception. There is growing scientific evidence that proprioceptive functional decline is one of the main causes of failure in postural balance control in patients with nonspecific LBP21-23). Thus, several studies have been conducted on the relationship between the muscle spindle function in proprioception and LBP24). In this review, we identify a unique mechanism of proprioceptive dysfunction via a sensory input disturbing postural control, resulting in LBP in elderly patients.
What Is Proprioception?
Proprioception, also referred to as deep sensation, is defined as afferent information that contributes to the detection of joint motion, limb position, muscle movement, and resistance applied to the body, whereas neuromuscular control is the efferent motor response to sensory information. Proprioceptive feedback influences movement and positional accuracy even in the absence of the visual sense, resulting in the key somatosensory functions for human postural control. For the response mechanism in the standing posture, the ankle strategy involves the distal proprioceptive reaction pattern while standing on a static or normal surface. On the other hand, the hip strategy, which is a more proximal proprioception function with little or no motion on the ankle strategy, is used when performing a task or when being unstable25). Accordingly, the ankle strategy is incorporated when being unperturbed or in the presence of low-amplitude perturbations, whereas the hip strategy is for fast and large-amplitude perturbations, wherein multiple strategies are used to maintain the standing balance26). A kyphotic flexed posture, with or without a vertebral fracture, in older adults has relatively unstable balance control because the center of mass in the body is shifted anteriorly. Hyperkyphotic spinal alignment changes the joint position sense27) and could influence the ability to recover from balance perturbation28), with decreased postural stability caused by ankle strategy dysfunction29). Alternations in proprioceptive signals impede the ability to reduce fall risk. Therefore, older adults with spinal malalignment are more susceptible to falls on an unstable surface.
Proprioception encompasses signals from mechanoreceptors, which are proprioceptors located in the skin, subcutaneous tissue, muscles, tendons, and joint capsules. Information from cutaneous and subcutaneous proprioceptors-such as Meissner's and Vater-Pacini corpuscles, which act as tactile and stretch receptors, respectively-is considered an additional sensory source that completes proprioceptive inputs30). Golgi tendon organs encode variations in the muscle force induced by the contraction of muscle fibers and contribute to the sense of force31), whereas feedback from joint receptors provides relevant information on limb position and joint movement32). The muscle spindle, one of the most important proprioceptors, consists of a bundle of differentiated muscle fibers called intrafusal fibers, which are surrounded by a fusiform capsule filled with a viscous fluid. Muscle spindles are stretched during eccentric muscle contraction, resulting in the generation of action potential on afferent fibers to convey the proprioceptive information to the sensorimotor cortex in the brain. Muscle proprioception can be altered by vibration, which activates the primary endings of the muscle spindle and produces a sensation of displacement of the body segment33-35). Vibratory stimulation to muscles generates illusory movement and muscle-tendon vibration is a powerful stimulus for muscle spindle primary afferents with different frequency vibrations for postural responses36). Therefore, local muscle vibration is often used to evaluate the proprioceptive function, which contributes to balance and postural stability.
Significance of Proprioception in Motor Exercise
For musculoskeletal disorders, especially lumbar spinal disorders, evidence of their dysfunction has led to a new paradigm of exercise therapy to address motor control problems in local and global muscles. Panjabi proposed a stability-based spinal model based on the motor control approach, in which the spinal column is the passive subsystem; the neural elements, the control subsystem; and the motor system, the active subsystem19). Impairment in any one subsystem leads to chronic dysfunction and pain. Therefore, a spinal column robust enough to compensate for impairment in any system should be tolerant to other subsystems from a practical perspective. In this respect, LBP patients stiffen their spine by recruiting superficial spinal muscles, which provides robustness against perturbations37,38). In a critical element of motor control, which is responsible for spinal posture and stability, there is a constant interplay between motor outputs, such as trunk muscles, and sensory inputs, such as proprioception30,39). Motor control, which is particularly significant in exercise therapy for LBP, affects trunk posture, instability, and lumbar spine movement. In motor control adaptations induced in locomotor disorders, including LBP, emphasis is placed on responses to active systems such as muscle strengthening, endurance, and/or skillful motor function in rehabilitation medicine. The potential to adequately control posture and movement accompanied by muscle activation is limited without an optimal sensory input. Thus, consideration of a sensory component is required for most aspects of the motor control approach. Proprioception, which plays a central role in the maintenance of posture and control of voluntary movement, is the key somatosensory feedback system.
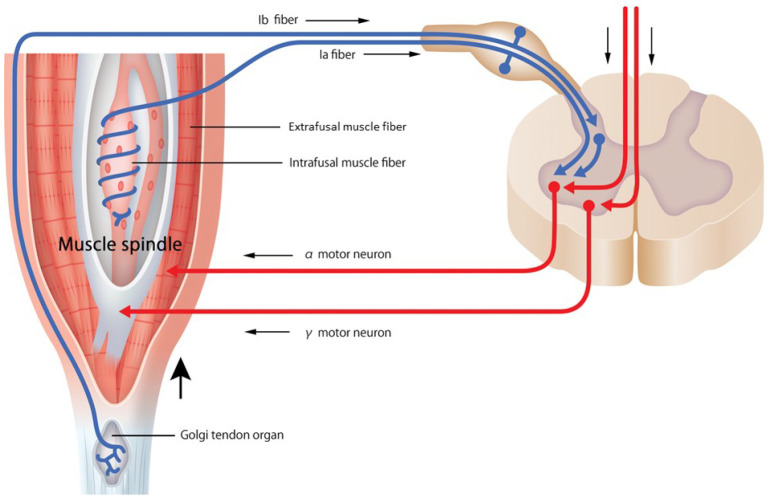
Sensory inputs serve as stimuli for reflexive movement organized at the spinal cord of the central nervous system, modulating the movement from commands originating from higher centers of the nervous system. Therefore, sensory information plays an important role in adjusting motor output that results from spinal cord activity. Peripheral mechanoreceptors receive sensory input, and the spinal stretch reflex and the long-loop or transcortical reflex activate synergistic muscles (Fig. 1). The harmony of muscle contraction by coactivation of both α and γ motor neurons indicates that sensory input and motor output are equally significant. It must be recognized that skeletal muscle is both an effector and a sensory organ with the proprioceptive function being a result of the neuromuscular activity of the nervous system. Especially with exercise therapy for older adults, improving motor output due to sarcopenia that occurs with aging is challenging. Therefore, beneficial changes in the proprioceptive sensory input should result in efficient improvement of the motor output40). However, there is no established method for the accurate quantitative evaluation of the proprioceptive function. Thus, the development of an evaluation method is essential for improving the sensory input in motor control.
Figure 1.
Proprioceptor and stretch reflex loop.
The muscle spindle or tendon organs react to muscle stretches, exciting the Ia and Ib afferent neurons. The spinal stretch reflex is activated by excitatory monosynaptic connections from group I afferent neurons to α motor neurons, which activate muscle contraction. Impulse from higher centers of the nervous system excites γ motor neurons, and intrafusal muscle fibers of the muscle spindle are activated. Activation of γ motor neurons enhances the dynamic responses of Ia afferent neurons, increasing muscle tone through the α motor neurons (γ loop). There is coactivation of both α motor neurons (activating the main muscle; the extrafusal muscle fiber) and γ motor neurons (activating the muscle spindle; the intrafusal muscle fiber). This coactivation allows group I and II afferents to detect unexpected stretches during contraction and compensate (α-γ linkage).
Evaluation of the Proprioceptive Function
Classically, the identification and repositioning of joint position angles and vibration sensing using a tuning fork (128 Hz) were developed as a method for evaluating the proprioceptive function; however, in addition to lacking objective quantification, these methods are dependent on the cognitive function and memory ability of older adults. Most studies on proprioception for spinal disorders have focused on repositioning accuracy in tests of position sense24,41-43). However, the reliability of measurements should often be affected by a learning effect, which substantially improves with repeated testing. Thus, a holistic approach using postural control or postural balance tasks is needed, instead of a separate assessment of individual components of proprioception, such as position, movement, and velocity sense44-46). This objective evaluation method is based on the displacement of the center of pressure (COP) using a force plate while maintaining constant signals from the visual and vestibular systems and incorporates the effects of sensory weighting using vibration. Mok et al. demonstrated a reduced hip strategy in postural control in LBP patients44). Subsequently, many studies on LBP and proprioception have been conducted using this evaluation method. However, the concept that proprioceptive function evaluation with COP should be performed in a more subconscious state led to the development of a method in which dual vibration stimulation was applied to the lower legs and the trunk. Muscle-tendon vibration is a powerful stimulus to muscle spindle primary afferents34,35), and the effect of vibration is to extract the proprioceptive output. Therefore, Brumagne et al. reported a proprioceptive weighting change from the trunk to the ankles in older adults with LBP21). Furthermore, Claeys et al. appraised the relative proprioceptive weighting (RPW) ratio, which was analyzed as COP displacements in the calf (triceps surae; TS) and trunk (lumbar multifidus; LM) muscle vibration trials using the next equation46). The use of muscle vibration can help clarify proprioceptive control more directly. Afference of stimulated muscles to the central nervous system for postural control expects direction-specific responses.

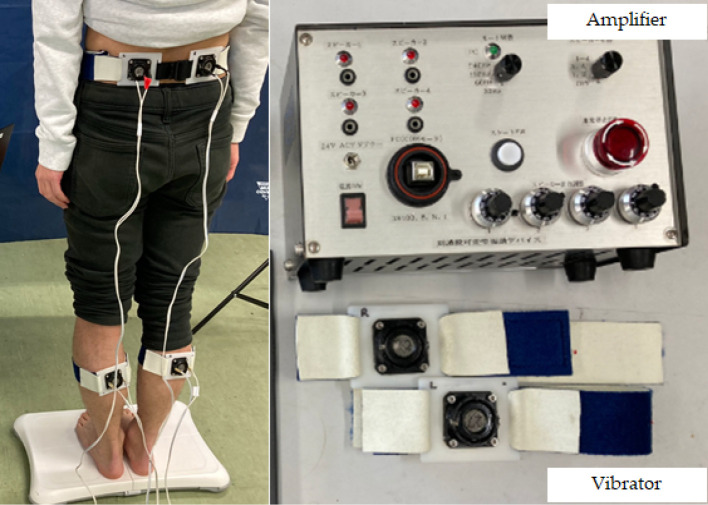
A score of 1 corresponds to complete reliance on the lower extremity muscle afference, whereas a score of 0 corresponds to reliance on trunk muscle afference. Claeys et al. demonstrated less reliance on back muscle proprioceptive inputs for postural control in young patients with nonspecific LBP46). Decreased sensitivity for muscle vibration indicates hyposensitivity of proprioceptive afference24). As postural control by proprioceptive function is performed according to the balance between the lower limb and trunk46), the larger the RPW, the more dominant the lower limb; the smaller the RPW, the more dominant the trunk; and the closer the RPW is to 50%, the more ideal the balance control. This is an excellent model for evaluating how proprioception is handled incorporating the effects of sensory weighting by comparing the ankle and hip strategies. However, the vibration stimulus was limited to 60 Hz, which is the frequency corresponding to the muscle spindle in the previous investigation. As described above, because the organ that controls proprioception does not depend only on muscle spindles, there exists a drawback that not all proprioceptors used for perception by humans have been evaluated. Although muscle spindles are considered to be the most important mechanism involved in kinesthesia, other proprioceptors, such as cutaneous and joint receptors, also play a role. The afferent response of the muscle spindle ranges from 20 to 220 Hz depending on muscle conditions34), and tactile stimulation in the neck improves the position sense in the cervical spine47). The significance of a Pacinian corpuscle function in response to high frequencies of around 250 Hz in the occurrence of LBP was demonstrated in our recent clinical study48). Evaluable proprioceptive diagnosis at various responsible frequencies is particularly important in older adults who are highly expected to have proprioceptive decline49). Therefore, to diagnose the proprioceptive function in older adults, we have developed a vibrating device that can change diverse frequencies ranging from 30 to 250 Hz, responsible for almost all proprioceptors48) (Fig. 2).
Figure 2.
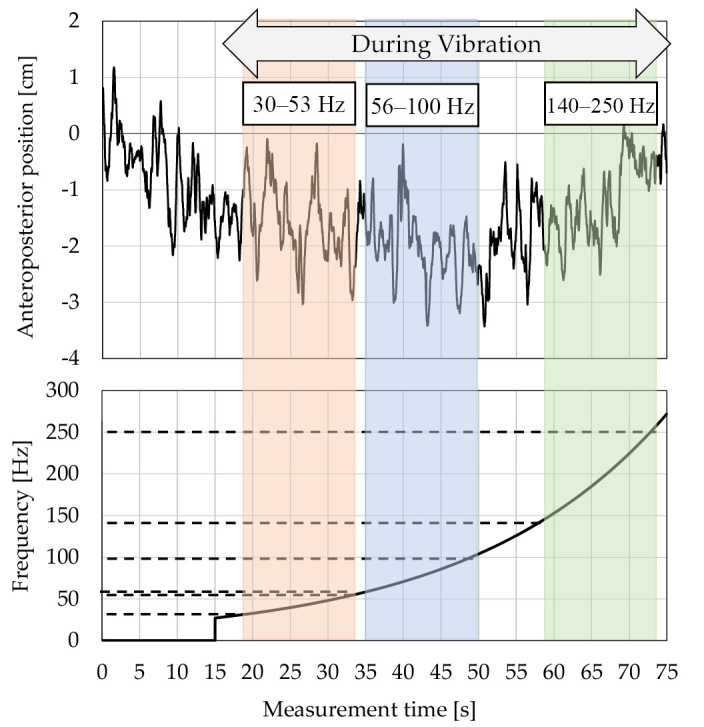
Wave form of center of pressure (COP) using sweep frequency of vibratory stimulation.
Vibratory stimulation, which can change a wide range of frequencies from 30 to 250 Hz, responsible for almost all proprioceptors, is given for the purpose of diagnosing the proprioceptive function in older adults. The COP displacement was continuously analyzed as a waveform using a stabilometer.
Aging and Proprioception
It is well known that age-associated functional decline occurs in the neuromuscular and sensorimotor systems. Proprioceptive declines also occur with age, which contributes to changes in postural stability, leading to increased fall risk in older adults50). With the occurrence of age-related changes in proprioception, alterations in muscle spindles, along with the integration of the neural signal, at the supraspinal level influence proprioceptive perception. Aged human muscle spindles exhibit increased spindle capsule thickness and loss of total intrafusal fibers per spindle, as a result of denervation51). The most characteristic age-related change in the muscle spindle may be the reduction in spindle diameter; however, this morphological modification was specific to certain muscles52). Muscle spindles innervation in the anterior tibial nerve dramatically decreased with age, suggesting an age-related decrease in the number of Ia afferents in the muscle spindles53). Degeneration of the proprioceptive sensory nerve with aging starts earlier than a morphological change in the intrafusal muscle fibers54). Aged muscle spindle function appears to exhibit impaired sensitivity due to denervation. The afferent response of the muscle spindles for applied stretch is lower in aged rats, implying a decline in spindle sensitivity55). In humans, the vibration-related muscle spindle activity and reflex-induced force generation have been reported to be reduced in older adults56,57). Age-related proprioceptive decline contributes to major changes in postural control. Response to proprioception in the standing posture is determined by the complicated interaction between the ankle strategy, which is the distal function centered on the lower limbs, and the hip strategy, which is the proximal function centered on the trunk and hip25). To achieve proprioceptive improvement for an age-related decline, the effect of age on trunk and leg proprioceptive function and its implication in postural control strategy are of paramount importance.
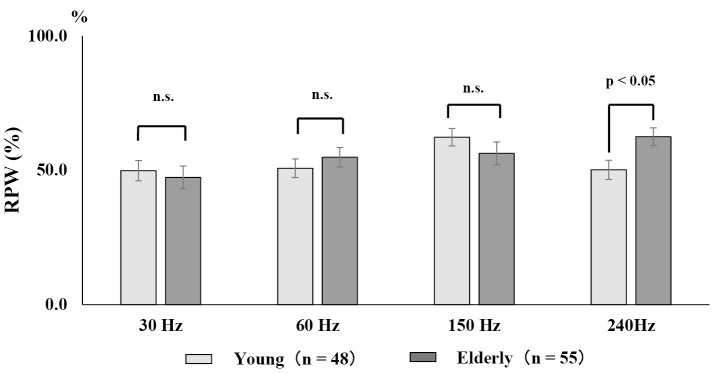
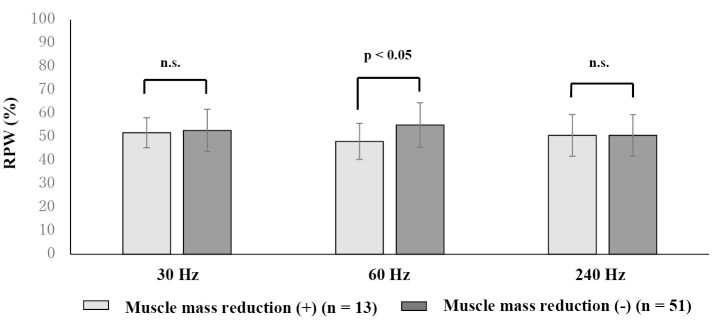
To evaluate the proprioceptive function in the trunk and lower limbs in older adults, a wide range of vibratory stimulation of 30-240 Hz from low to high frequencies was applied to the TS and the LM muscles measure COP displacement (Fig. 3). A comparison between healthy young and older adults revealed that older adults had a predominant lower leg balance control at 240 Hz, which is a high-frequency range, suggesting a decline in proprioception in the trunk with age40,58)(Fig. 4). Skeletal muscle mass loss is one of the causes of age-related proprioceptive dysfunction. When the relationship between proprioception and skeletal muscle mass in older adults was evaluated using physique correction by skeletal muscle mass index (kg/m2), balance control of the trunk was dominant in the middle frequency range in older adults with decreased muscle mass59) (Fig. 5). Decreased skeletal muscle mass in the lower limbs reduces RPW, that is, decreased proprioceptive function in the lower limbs, resulting in trunk-dependent proprioceptive control. Since age-related sarcopenia occurs mainly in type II fibers14), the decrease in lower limb muscles is more remarkable than that in the trunk. Thus, it is conceivable that the effect of sarcopenia in older adults with low muscle mass for the proprioceptive function is significant. The proprioceptive function of the trunk has also been identified in older adults with trunk muscle atrophy60). From the above, proprioception in humans reflects not only muscle function but also skeletal muscle volume. The relationship between age-related muscle mass reduction and proprioception is supported by the presence of chronic inflammation associated with senescence, which is one of the mechanisms of sarcopenia61), and recent research on the involvement of systemic inflammation in proprioceptive dysfunction62). There is also a relationship between proprioception and fall risk in older adults. Older people with fall risks show a proprioceptive decline in the trunk in the low-frequency range63), leading to dependence on the ankle strategy on an unstable surface for the tendency to fall promoted by muscle mass loss in the lower limb.
Figure 3.
Diagnostic system responsible for a wide range of frequencies of vibratory stimulation to evaluate the proprioceptive function.
Vibration was applied to the bilateral lumbar multifidus and triceps surae muscles, and mechanical vibrations from low to high frequency were applied continuously. The displacement of the center of pressure during vibration at the trunk and calf was automatically recorded by the stabilometer to evaluate the proprioceptor function.
Figure 4.
Comparison of proprioceptive function in each frequency band between healthy young and older adults.
A relative proprioceptive weighting ratio (RPW) score of 100% corresponds to complete reliance on the lower extremity muscle afference, and a score of 0 corresponds to reliance on the trunk muscle afference. In vibration-induced proprioceptive function evaluation, an RPW of <50% indicated trunk dominance and an RPW of >50% indicated lower limb dominance. Older adults (average 78.3 years) exhibit predominant lower leg balance control, that is, decreased trunk proprioceptive function, at 240 Hz, which is a higher frequency range than younger people (modified from Reference 58).
RPW: relative proprioceptive weighting
Figure 5.
Skeletal muscle mass and proprioception in older adults.
Older adults with skeletal muscle mass loss show trunk-dominant proprioception, that is, lower limb proprioceptive dysfunction, in the 60 Hz frequency range, which corresponds to the muscle spindle function. Skeletal muscle mass reduction is determined by skeletal muscle mass index (kg/m2), which has a cutoff value of <6.87 kg/m2 for men and <5.46 kg/m2 for women in the Japanese population (modified from Reference 59).
RPW: relative proprioceptive weighting
Proprioceptive Pain
Regarding the relationship between LBP and postural control, the first study in 1991 by Nies et al. showed instability while standing on one leg in patients with LBP64). With increasing attention given to the significance of proprioceptive function in humans, a more detailed evaluation of postural balance control using stabilometry, focusing on proprioception in LBP, has been reported. The delayed response of skeletal muscles to a sudden trunk load, which is a characteristic of LBP patients, is due to the impaired proprioceptive function in the lumbar spine37,65,66); this has been supported by the study result of the impairment of trunk position sense in patients with LBP41,67). Research on proprioceptive function evaluation has subsequently progressed, and a method using vibratory stimulation has been developed as a more objective procedure to evaluate the function of differences in the biological response to the vibratory stimulus in the trunk and lower limbs considering that muscle spindle is activated through muscle vibration inducing afferent nerve activity34). The study results using this method have made a possible comparison of proprioceptive functions of the lower limbs and trunk, and patients with LBP seem to use the proprioceptive postural strategy with strong reliance on ankle proprioceptive signals21,22,45,68). However, little is known about the mechanism by which the trunk proprioceptive function declines in patients with LBP46), whereas the muscle spindles that tend to concentrate in the deeper and central portion of muscles69) are presumed to be reduced in the trunk muscle. A new concept in the relationship between proprioception and pain is sensory-motor incongruity pain. This hypothesis, proposed by Harris in 1999, is the notion that discordance between awareness of motor intention and muscle and joint proprioception results in pathological chronic pain70). The source of pain from sensory-motor incongruity is thought to arise in the brain in the absence of tissue injury and/or peripheral pathology. Motor intention generation does not activate proprioceptors, and proprioceptive feedback is limited, leading to pathological pain in parts of the body. A condition used to explain this mechanism is the phantom limb. In an amputee, the absence of proprioceptive information congruent with motor intention activates cortical center monitoring incongruence of sensation; thus, most amputees experience pathological phantom pain71). A bimanual coordination experiment involving an artificial sensorimotor conflict via a mirror demonstrated a mismatch between motor output and sensory input trigger incongruence within the motor control system and could contribute to pain in some healthy individuals and ongoing pain in patients with musculoskeletal pain72). Prolonged limb immobilization affects body ownership73), and the illusion of body ownership leads to the sensory discrepancy and decreases the pain threshold74). Thus, elucidation of the relationship between proprioception and sarcopenic pain in older adults is also expected from the viewpoint of sensory-motor incongruity. Interestingly, watching a virtual image of a phantom limb moving synchronously with motor commands relieves phantom limb pain71). This indicates that therapy is best directed at restoring the integrity of cortical information processing in the presence of musculoskeletal chronic pain without pathology, instead of the use of analgesics and antiinflammatory agents70).
In view of these findings, proprioception is important in the use of motor control theory as an exercise therapy in LBP. An important part of the therapeutic exercise programs for LBP is the motor control approach, which focuses on the activation of deep trunk muscles independent of global muscles activity75). Lack of spinal intersegmental deep muscle control when the global muscle is working unwantedly could contribute to lumbar dysfunction, causing LBP. LM muscles are as important for segmental stability of the lumbar spine as deep trunk muscles, whereas the erector spinae muscles are torque generators for spinal motion and like guy ropes to control spinal orientation, and balance the external loads applied to the trunk20). When local muscles are not functioning enough to control lumbar segmental motion while global muscles are working appropriately, muscle stiffness results in reduced lumbar stabilization. If the nervous system interprets that spinal stability is already achieved by excessive contraction of the global muscles to stiffen the lumbar spine, a local muscle response is not initiated. This mechanism could explain some of the findings in patients with LBP from an electrophysiological perspective76,77). Proprioceptive dysfunction provokes a mismatch between motor output and sensory input78). Proprioceptive pain is relatively new and the range of sensory incongruities that can give rise to pain are still unknown78,79).
Geriatric LBP Associated with Proprioceptive Dysfunction
Numerous studies have shown that individuals with LBP have decreased lumbosacral proprioception in various postures, including standing and sitting, compared with healthy subjects without LBP21,24,41,43). Regarding the mechanism of lumbar proprioceptive change, the question remains whether local dysfunction of proprioceptors affects the quality or quantity of sensory reception or changes in the central processing of proprioceptive signals. Muscle spindles in the paraspinal muscles are more sensitive to position and movement sense than those in the arm and leg muscles80). The number of muscle spindles in the lumbar region is higher than in other regions due to the muscle bulk in the lumbar spine81). Afferent input spinal ligaments are integrated into the proprioceptive input arising from muscle spindle afferents and initiate reflex muscle activation via the γ motoneuron-muscle spindle system82). In particular, the specific ligament-muscular reflex is induced by stretching of the supraspinous ligament in anesthetized patients83). As mentioned above, both muscle spindle and afferent tract dysfunction occur with age-related changes and affect proprioceptive function in older adults; however, the mechanism by which the decrease in position and movement sense is seen in patients with LBP has not been elucidated.
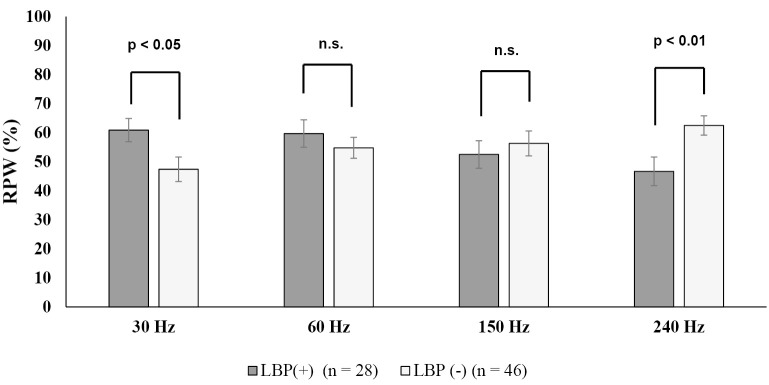
There is inconsistent evidence of an association between proprioceptive impairment and LBP; therefore, it is unclear whether proprioceptive exercises affect pain and disability. Recent systematic reviews with meta-analyses have provided different conclusions that there is a relationship between proprioception and LBP84,85), that there is insufficient evidence86), that there is a relationship but its correlation is weak87), and that there is no relationship88). One factor that causes such disagreement is that the measurement approach varies considerably among studies85,86). Different testing positions, number of repetitions, movement instructions, and measurement systems render a comparison of findings difficult. Thus, most studies using the position and/or movement sense evaluated proprioceptive function in LBP; however, these methods are less objective and measurement errors are unavoidable86). On the other hand, the evaluation of proprioception using vibration sensation enables accurate and objective procedures by targeting receptors that respond to a wide range of vibration frequencies. Although various research reports on LBP and proprioception exist in recent years, most are studies on single-frequency vibration stimuli for LBP in young adults. Recent studies in older LBP patients evaluating proprioceptive responses to all frequency stimuli that humans can perceive has shown trunk-dependent proprioceptive control due to functional decline of high-frequency responsive receptors (240 Hz) at the lower extremity89,90) (Fig. 6). Lasting hyperactivation of proprioceptors continuously stimulates the reflex arc in the spinal cord, further inducing microglial activation, leading to the initiation and maintenance of pain91). Previous studies have shown that proprioceptive sensitivity in the trunk muscles, afferent information transmission decrease in older adults21,40,58), and the ankle strategy are dominant in older individuals whose trunk proprioceptive function declines. Thus, it is hypothesized that the proprioception of the lower limbs deteriorates with age-related sarcopenia, which increases the proprioceptive burden on the lumbar spine (Fig. 7). Establishing a diagnosis for proprioceptive function in patients with LBP could be a new approach to LBP treatment.
Figure 6.
Evaluation of the proprioceptive function in the trunk and lower limbs in older adults with low back pain (LBP).
Older LBP patients (>65 years) show trunk-dependent proprioceptive control due to functional decline of high-frequency responsive receptors (240 Hz) at the lower extremity (figure modified from Reference 90).
LBP: low back pain, RPW: relative proprioceptive weighting
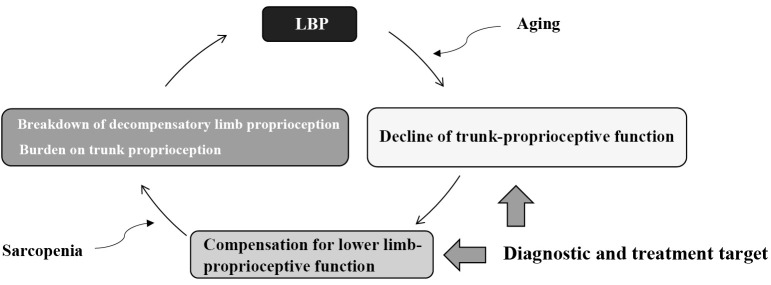
Figure 7.
Proprioceptive function and low back pain in older adults (hypothesis).
When the trunk proprioceptive function (hip strategy) declines with aging, older adults become dependent on lower limb-proprioceptive function (ankle strategy). Furthermore, when the skeletal muscle mass of the lower limbs decreases due to sarcopenia, decompensation superimposed on trunk proprioception leads to a burden on the lumbar spine and low back pain (LBP). If an accurate diagnosis of a proprioceptive function of the trunk and lower limbs is possible, determining a therapeutic target will be an exit strategy as a new treatment procedure for LBP.
Conclusion
While proprioception is important in older adults, there is insufficient evidence for its contribution to LBP and its role in pain management. To enable accurate diagnosis of proprioceptive function, it is necessary to establish a treatment procedure for recovering proprioception decline due to aging, and further prospective research is also needed to present a relationship with the improvement of LBP.
Conflicts of Interest: The device(s)/drug(s) is/are FDA approved or approved by the corresponding national agency for this indication.
No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Sources of Funding: None
Author Contributions: Yoshihito Sakai: Conception, design and creation of the manuscript.
Norimitsu Wakao, Hiroki Matsui, Naoaki Osada, Tsuyoshi Watanabe, and Takaya Sugiura: Acquisition of data and revision of the manuscript for important intellectual content.
Yoshifumi Morita, and Keitaro Kawai: Providing diagnostic equipment and acquisitional data, and approval of the final version of the manuscript.
Tadashi Ito, and Kazunori Yamazaki: Statistical analysis and approval of the final version of the manuscript.
Ethical Approval: Ethical approval was given by the National Center for Geriatrics and Gerontology Ethics Committee (IRB # 1405).
Informed Consent: Not applicable
Acknowledgement
We would like to thank Editage (www.editage.com) for English language editing.
The authors would like to thank Y. Sato, J. Suzuki and M. Morita for their assistance with data preparation.
References
- 1.Nakamura M, Nishiwaki Y, Ushida T, et al. Prevalence and characteristics of chronic musculoskeletal pain in Japan. J Orthop Sci. 2011;16(4):424-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoy D, March L, Brooks P, et al. The global burden of low back pain: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73(6):968-74. [DOI] [PubMed] [Google Scholar]
- 3.Weiner DK, Haggerty CL, Kritchevsky SB, et al. How does low back pain impact physical function in independent, well-functioning older adults? Evidence from the Health ABC cohort and implications for the future. Pain Med. 2003;4(4):311-20. [DOI] [PubMed] [Google Scholar]
- 4.Rudy TE, Weiner DK, Lieber SJ, et al. The impact of chronic low back pain on older adults: a comparative study of patients and controls. Pain. 2007;131(3):293-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hicks GE, Morone N, Weiner DK. Degenerative lumbar disc and facet disease in older adults: prevalence and clinical correlates. Spine. 2009;34(12):1301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brinjikji W, Luetmer PH, Comstock B, et al. Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. AJNR Am J Neuroradiol. 2015;36(4):811-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koca I, Savas E, Ozturk ZA, et al. The evaluation in terms of sarcopenia of patients with fibromyalgia syndrome. Wien Klin Wochenschr. 2016;128(21-22):816-21. [DOI] [PubMed] [Google Scholar]
- 8.Scott D, Blizzard L, Fell J, et al. Prospective study of self-reported pain, radiographic osteoarthritis, sarcopenia progression, and falls risk in community-dwelling older adults. Arthritis Care Res. 2012;64(1):30-7. [DOI] [PubMed] [Google Scholar]
- 9.Silva TAA, Junior AF, Pinherio MM, et al. Sarcopenia and aging: ethiological aspects and therapeutic options. Rev Bras Reumatol. 2006; 46(6):391-7. [Google Scholar]
- 10.Sakai Y, Matsui H, Ito S, et al. Sarcopenia in elderly patients with chronic low back pain. Osteoporos Sarcopenia. 2017;3(4):195-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanishima S, Hagino H, Matsumoto H, et al. Association between sarcopenia and low back pain in local residents: prospective cohort study from the GAINA study. BMC Musculoskelet Disord. 2017;18(1):452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsujinaka T, Fujita J, Ebisui C, et al. Interleukin 6 receptor antibody inhibits muscle atrophy and modulates proteolytic systems in interleukin 6 transgenic mice. J Clin Invest. 1996;97(1):244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haddad F, Zaldivar F, Cooper DM, et al. IL-6-induced skeletal muscle atrophy. J Appl Physiol. 2005;98(3):911-7. [DOI] [PubMed] [Google Scholar]
- 14.Lexell J, Taylor CC, Sjöström M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-years-old men. J Neurosci. 1988; 84(2-3):275-94. [DOI] [PubMed] [Google Scholar]
- 15.Abe T, Loenneke JP, Thiebaud RS, et al. Age-related site-specific muscle wasting of upper and lower extremities and trunk in Japanese men and women. Age. 2014;36(2):813-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hultman G, Nordin M, Saraste H, et al. Body composition, endurance, strength, cross-sectional area, and density of MM erector spinae in men with and without low back pain. J Spinal Disord. 1993;6(2):114-23. [PubMed] [Google Scholar]
- 17.Parkkola R, Rytökoski U, Kormano M. Magnetic resonance imaging of the discs and trunk muscles in patients with chronic low back pain and healthy control subjects. Spine. 1993;18(7):830-6. [DOI] [PubMed] [Google Scholar]
- 18.Ranger TA, Cicuttini FM, Jensen TS, et al. Are the size and composition of the paraspinal muscles associated with low back pain? A systematic review. Spine J. 2017;17(11):1729-48. [DOI] [PubMed] [Google Scholar]
- 19.Panjabi M. The stabilizing system of the spine. Part II. Neutral zone and instability hypothesis. J Spinal Disord. 1992;5(4):390-7. [DOI] [PubMed] [Google Scholar]
- 20.Bergmark A. Stability of the lumbar spine: a study in mechanical engineering. Acta Orthop Scand Suppl. 1989;230:1-54. [DOI] [PubMed] [Google Scholar]
- 21.Brumagne S, Cordo P, Verschueren S. Proprioceptive weighting changes in persons with low back pain and elderly persons during upright standing. Neurosci Lett. 2004;366(1):63-6. [DOI] [PubMed] [Google Scholar]
- 22.Johanson E, Brumagne S, Janssens L, et al. The effect of acute back muscle fatigue on postural control strategy in people with and without recurrent low back pain. Eur Spine J. 2011;20(12):2152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henry SM, Hitt JR, Jones SL, et al. Decreased limits of stability in response to postural perturbations in subjects with low back pain. Clin Biomech. 2006;21(9):881-92. [DOI] [PubMed] [Google Scholar]
- 24.Brumagne S, Cordo P, Lysens R, et al. The role of paraspinal muscle spindles in lumbosacral position sense in individuals with and without low back pain. Spine. 2000;25(8):989-94. [DOI] [PubMed] [Google Scholar]
- 25.Horak FB, Henry SM, Shumway-Cook A. Postural perturbations: new insights for treatment of balance disorders. Phys Ther. 1997;77(5):517-33. [DOI] [PubMed] [Google Scholar]
- 26.Colobert B, Crétual A, Allard P, et al. Force-plate based computation of ankle and hip strategies from a double-inverted pendulum model. Clin Biomech. 2006;21(4):427-34. [DOI] [PubMed] [Google Scholar]
- 27.Granito RN, Aveiro MC, Renno AC, et al. Comparison of thoracic kyphosis degree, trunk muscle strength and joint position sense among healthy and osteoporotic elderly women: a cross-sectional preliminary study. Arch Gerontol Geriatr. 2012;54(2):e199-202. [DOI] [PubMed] [Google Scholar]
- 28.Hsu WL, Chou LS, Woollacott M. Age-related changes in joint coordination during balance recovery. Age. 2013;35(4):1299-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang LY, Liaw MY, Huang YC, et al. Static and dynamic balance performance in patients with osteoporotic vertebral compression fracture. J Back Musculoskelet Rehabil. 2013;26(2):199-205. [DOI] [PubMed] [Google Scholar]
- 30.Riemann BL, Lephart SM. The sensorimotor system, part I: the physiologic basis of functional joint stability. J Athl Train. 2002;37(1):71-9. [PMC free article] [PubMed] [Google Scholar]
- 31.Proske U, Gandevia SC. The proprioceptive sense: their role in signaling body shape, body position and movement, and muscle force. Physiol Rev. 2021;92(4):1651-97. [DOI] [PubMed] [Google Scholar]
- 32.Macefield VG. Physiological characteristics of low-threshold mechanoreceptors in joints, muscle, and skin in human subjects. Clin Exp Pharmacol Physiol. 2005;32(1-2):135-44. [DOI] [PubMed] [Google Scholar]
- 33.Goodwin GM, McCloskey DI, Matthews PB. Proprioceptive illusions induced by muscle vibration: contribution by muscle spindles to perception? Science. 1972;175(4028):1382-4. [DOI] [PubMed] [Google Scholar]
- 34.Burke D, Hagbarth KE, Löfstedt L, et al. The responses of human muscle spindle endings to vibration of non-contracting muscles. J Physiol. 1976;261(3):673-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roll JP, Vedel JP. Kinaesthetic role of muscle afferents in man, studied by tendon vibration and microneurography. Exp Brain Res. 1982;47(2):177-90. [DOI] [PubMed] [Google Scholar]
- 36.Polónyová A, Hlavacka F. Human postural responses to different frequency vibrations of lower leg muscles. Physiol Res. 2001;50(4):405-10. [PubMed] [Google Scholar]
- 37.Hodges PW, Richardson CA. Altered trunk muscle recruitment in people with low back pain with upper limb movement at different speeds. Arch Phys Med Rehabil. 1999;80(9):1005-12. [DOI] [PubMed] [Google Scholar]
- 38.van Dieén JH, Kingma I, van der Burg JCE. Evidence for a role of antagonistic contraction in controlling trunk stiffness during lifting. J Biomech. 2003;36(12):1829-36. [DOI] [PubMed] [Google Scholar]
- 39.Lee AS, Cholewicki J, Reeves NP, et al. Comparison of trunk proprioception between patients with low back pain and healthy controls. Arch Phys Med Rehabil. 2010;91(9):1327-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ito T, Sakai Y, Ito Y, et al. Association between back muscle strength and proprioception or mechanoreceptor control strategy in postural balance in elderly adults with lumbar spondylosis. Healthcare. 2020;8(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gill KP, Callaghan MJ. The measurement of lumbar proprioception in individuals with and without low back pain. Spine. 1998;23(3):371-7. [DOI] [PubMed] [Google Scholar]
- 42.Swinkels A, Dolan P. Regional assessment of joint position sense in the spine. Spine. 1998;23(5):590-7. [DOI] [PubMed] [Google Scholar]
- 43.O'Sullivan PB, Burnett A, Floyd AN, et al. Lumbar repositioning deficit in a specific low back pain population. Spine. 2003;28(10):1074-9. [DOI] [PubMed] [Google Scholar]
- 44.Mok NW, Brauer SG, Hodges PW. Hip strategy for balance control in quiet standing is reduced in people with low back pain. Spine. 2004;29(6):E107-12. [DOI] [PubMed] [Google Scholar]
- 45.Brumagne S, Janssens L, Knapen S, et al. Persons with recurrent low back pain exhibit a rigid postural control strategy. Eur Spine J. 2008;17(9):1177-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Claeys K, Brumagne S, Dankaerts W, et al. Decreased variability in postural control strategies in young people with non-specific low back pain is associated with altered proprioceptive reweighting. Eur J App Physiol. 2011;111(1):115-23. [DOI] [PubMed] [Google Scholar]
- 47.Pinsault N, Bouvier B, Sarrazin Y, et al. Effects of vision and tactile stimulation of the neck on postural control during unperturbed stance and cervical joint position sense in young asymptomatic adults. Spine. 2010;35(17):1589-94. [DOI] [PubMed] [Google Scholar]
- 48.Nishio R, Ito Y, Morita Y, et al. Investigation of the functional decline in proprioceptors for low back pain using the sweep frequency method. Appl Sci. 2019;9(23):4988. [Google Scholar]
- 49.Pyykkö I, Jäntti P, Aalto H. Postural control in elderly subjects. Age Aging. 1990;19(3):215-21. [DOI] [PubMed] [Google Scholar]
- 50.Shaffer SW, Harrison AL. Aging of the somatosensory system: a translational perspective. Phys Ther. 2007;87(2):193-207. [DOI] [PubMed] [Google Scholar]
- 51.Swash M, Fox KP. The effect of age on human skeletal muscle studies of the morphology and innervation of muscle spindles. J Neuro Sci. 1972;16(4):417-32. [DOI] [PubMed] [Google Scholar]
- 52.Kararizou E, Manta P, Kalfakis N, et al. Morphometric study of the human muscle spindle. Anal Quant Cytol Histol. 2005;27(1):1-4. [PubMed] [Google Scholar]
- 53.Swallow M. Fibre size and content of the anterior tibial nerve of the foot. J Neurol Nuerosurg Psychiatry. 1966;29(3):205-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vaughan SK, Stanley OL, Valdez G. Impact of aging on proprioceptive sensory neurons and intrafusal muscle fibers in mice. J Gerontol A Biol Sci Med Sci. 2017;72(6):771-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miwa T, Miwa Y, Kanda K. Dynamic and static sensitivities of muscle spindle primary endings in aged rats to ramp stretch. Neurosci Lett. 1995;201(2):179-82. [DOI] [PubMed] [Google Scholar]
- 56.Burke JR, Schutten MC, Koceja DM, et al. Age-dependent effects of muscle vibration and the Jendrassik maneuver on the patellar tendon reflex response. Arch Phys Med Rehabil. 1996;77(6):600-4. [DOI] [PubMed] [Google Scholar]
- 57.Chung SG, Van Rey EM, Bai Z, et al. Aging-related neuromuscular changes characterized by tendon reflex system properties. Arch Phys Med Rehabil. 2005;86(2):318-27. [DOI] [PubMed] [Google Scholar]
- 58.Ito T, Sakai Y, Nishio R, et al. Postural sway in adults and elderly individuals during local vibratory stimulation of the somatosensory system. SN Compr Clin Med. 2020;2:753-8. [Google Scholar]
- 59.Yamazaki K, Ito T, Sakai Y, et al. Postural sway during local vibratory stimulation for proprioception in elderly individuals with pre-sarcopenia. Phys Ther Res. 2020;23(2):149-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ito T, Sakai Y, Nakamura E, et al. Relationship between paraspinal muscle cross-sectional area and relative proprioceptive weighting ratio of older persons with lumbar spondylosis. J Phys Ther Sci. 2015;27(7):2247-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(1):S4-9. [DOI] [PubMed] [Google Scholar]
- 62.Cudejko T, van der Esch M, van der Leeden M, et al. Proprioception mediates the association between systemic inflammation and muscle weakness in patients with knee osteoarthritis: results from the Amsterdam osteoarthritis cohort. J Rehabil Med. 2018;50(1):67-72. [DOI] [PubMed] [Google Scholar]
- 63.Ito T, Sakai Y, Nishio R, et al. Relationship between postural stability and fall risk in elderly people with lumbar spondylosis during local vibratory stimulation for proprioception: a retrospective study. Somatosens Mot Res. 2020;37(3):133-7. [DOI] [PubMed] [Google Scholar]
- 64.Nies N, Snnott PL. Variations in balance and body sway in middle-aged adults. Subjects with healthy backs compared with subjects with low-back dysfunction. Spine. 1991;16(3):325-30. [DOI] [PubMed] [Google Scholar]
- 65.Luoto S, Taimela S, Hurri H, et al. Psychomotor speed and postural control in chronic low back pain patients. A controlled follow-up study. Spine. 1996;21(22):2621-7. [DOI] [PubMed] [Google Scholar]
- 66.Radebold A, Cholewicki J, Polzhofer GK, et al. Impaired postural control of the lumbar spine is associated with delayed muscle response times in patients with chronic idiopathic low back pain. Spine. 2001;26(7):724-30. [DOI] [PubMed] [Google Scholar]
- 67.Taimela S, Kankaanpää M, Luoto S. The effect of lumbar fatigue on the ability to sense a change in lumbar position. A controlled study. Spine. 1999;24(13):1322-7. [DOI] [PubMed] [Google Scholar]
- 68.Janssens L, Brumagne S, Polspoel K, et al. The effect of inspiratory muscles fatigue on postural control in people with and without recurrent low back pain. Spine. 2010;35(10):1088-94. [DOI] [PubMed] [Google Scholar]
- 69.Kokkorogiannis T. Two enigmas in proprioception: abundance and location of muscle spindles. Brain Res Bull. 2008;75(5):495-6. [DOI] [PubMed] [Google Scholar]
- 70.Harris AJ. Cortical origin of pathological pain. Lancet. 1999;354(9188):1464-6. [DOI] [PubMed] [Google Scholar]
- 71.Ramachandran VS, Hirstein W. The perception of phantom limbs: the D.O. Hebb lecture. Brain. 1998;121(9):1603-30. [DOI] [PubMed] [Google Scholar]
- 72.McCabe CS, Haigh RC, Halligan PW, et al. Simulating sensory-motor incongruence in healthy volunteers: implications for a cortical model of pain. Rheumatology. 2005;44(4):509-16. [DOI] [PubMed] [Google Scholar]
- 73.Burin D, Garbarini F, Bruno V, et al. Movements and body ownership: evidence from the rubber hand illusion after mechanical limb immobilization. Neuropsychologia. 2017;107:41-7. [DOI] [PubMed] [Google Scholar]
- 74.Osumi M, Imai R, Ueta K, et al. Negative body image associated with changes in the visual body appearance increases pain perception. PLoS One. 2014;9(9):e107376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Richardson C, Hodges P, Hides J. Therapeutic exercise for lumbopelvic stabilization. A motor control approach for the treatment and prevention of low back. 2nd ed. Oxford: Elsevier; c2004. Chapter 14, Local segmental control; p.185-219. [Google Scholar]
- 76.Sihvonen T, Partanen J, Hänninen O, et al. Electric behavior of low back muscles during lumbar pelvic rhythm in low back pain patients and healthy controls. Arch Phys Med Rehabil. 1991;72(13):1080-7. [PubMed] [Google Scholar]
- 77.Sakai Y, Matsui H, Ito S, et al. Electrophysiological function of the lumbar multifidus and erector spinae muscles in elderly patients with chronic low back pain. Clin Spine Surg. 2019;32(1):E13-19. [DOI] [PubMed] [Google Scholar]
- 78.McCabe CS, Haigh RC, Halligan PW, et al. Simulating sensory-motor incongruence in healthy volunteers: implications for a cortical model of pain. Rheumatology. 2005;44(4):509-16. [DOI] [PubMed] [Google Scholar]
- 79.Moseley GL, Gandevia SC. Sensory-motor incongruence and reports of ‘pain’. Rheumatology. 2005;44(9):1083-5. [DOI] [PubMed] [Google Scholar]
- 80.Cao DY, Khalsa PS, Pickar JG. Dynamic responsiveness of lumbar paraspinal muscle spindles during vertebral movement in the cat. Exp Brain Res. 2009;197(4):369-77. [DOI] [PubMed] [Google Scholar]
- 81.Amonoo-Kuofi HS. The number and distribution of muscle spindles in human intrinsic postvertebral muscles. J Anat. 1982;135(3):585-99. [PMC free article] [PubMed] [Google Scholar]
- 82.Sjölander P, Johansson H, Djupsjöbacka M. Spinal and supraspinal effects of activity in ligament afferents. J Electromyogr Kinesiol. 2002;12(3):167-76. [DOI] [PubMed] [Google Scholar]
- 83.Solomonow M, Zhou BH, Harris M, et al. The ligamento-muscular stabilizing system of the spine. Spine. 1998;23(23):2552-62. [DOI] [PubMed] [Google Scholar]
- 84.Laird RA, Gilbert J, Kent P, et al. Comparing lumbo-pelvic kinematics in people with and without back pain: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2014;15:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tong MH, Mousavi SJ, Kies H, et al. Is there a relationship between lumbar proprioception and low back pain? A systematic review with meta-analysis. Arch Phys Med Rehabil. 2017;98(1):120-36. [DOI] [PubMed] [Google Scholar]
- 86.Rausch Osthoff AK, Ernst MJ, Rast FM, et al. Measuring lumbar reposition accuracy in patients with unspecific low back pain: Systematic review and meta-analysis. Spine. 2015;40(2):E97-111. [DOI] [PubMed] [Google Scholar]
- 87.Lin J, Halak M, Rajan P, et al. Relationship between proprioception and pain and disability in people with non-specific low back pain. A systematic review with meta-analysis. Spine. 2019; 44(10):E606-17. [DOI] [PubMed] [Google Scholar]
- 88.Ghamkhar L, Kahlaee AH. Pain and pain-related disability associated with proprioceptive impairment in chronic low back pain patients: a systematic review. J Manipulative Physiol Ther. 2019;42(3):210-7. [DOI] [PubMed] [Google Scholar]
- 89.Ito T, Sakai Y, Yamazaki K, et al. Proprioceptive change impairs balance control in older patients with low back pain. J Phys Ther. 2017;29(10):1786-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ito T, Sakai Y, Morita Y, et al. Proprioceptive weighting ratio for balance control in static standing is reduced in elderly patients with non-specific low back pain. Spine. 2018;43(24):1704-9. [DOI] [PubMed] [Google Scholar]
- 91.Yasui M, Menjo Y, Tokizane K, et al. Hyperactivitivation of proprioceptors induces microglia-mediated long-lasting pain in a rat model of chronic fatigue syndrome. J Neuroinflammation. 2019;16(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]