Abstract
Introduction
Long-term clinical outcomes of microendoscopic laminotomy (MEL) for patients with multilevel radiographic lumbar spinal canal stenosis (LSS) have not been widely explored. The clinical significance and natural progression of additional untreated levels (e.g., remaining radiographic (RR)-LSS not addressed by selective MEL) remain unknown. This retrospective study aimed to investigate the long-term clinical outcomes of selective MEL in LSS patients and compare outcomes between patients with and without remaining RR-LSS to determine the efficacy of this procedure.
Methods
Forty-nine patients at a single center underwent posterior spinal microendoscopic decompression surgery for neurogenic claudication or radicular leg pain in moderate-to-severe spinal stenosis. The patients were categorized into the RR-LSS-positive and RR-LSS-negative cohorts based on unaddressed levels of stenosis. Pre-operative and 10-year follow-up evaluations, including the Japanese Orthopedic Association (JOA) score, visual analog scale (VAS) score for low back pain and leg pain, Oswestry Disability Index (ODI), and satisfaction, were compared between the groups. Additionally, the need for reoperation was determined.
Results
MEL significantly improved JOA scores, lumbar VAS, and ODI over the 10-year postoperative period. Pre-operative characteristics and postoperative outcomes were not significantly different between the cohorts. Overall, 18.4% (9/49) of patients required reoperation during the follow-up period. The reoperation rate in the RR-LSS-positive (13.8%; 4/29) group was similar to that in the RR-LL-negative (15.0%; 3/20) group.
Conclusions
MEL is effective for lumbar stenosis, with improved clinical outcomes up to 10 years following surgery. Selective MEL, addressing only symptomatic levels in multilevel stenosis, with residual remaining lumbar stenosis, is similarly effective without increased reoperation rates. Surgeons may consider more limited selective decompression in patients with multilevel stenosis, avoiding the risk and invasiveness of extensive procedures.
Level of Evidence
Level III.
Keywords: lumbar spinal stenosis, microendoscopic laminotomy, multilevel stenosis, selective decompression
Introduction
Lumbar spinal stenosis (LSS) is increasingly common1), resulting from increased longevity in an already aging demographic, prolongation of working years, and increased surveillance from medical diagnostics2,3). While surgery is an effective remedy for patients with symptomatic spinal stenosis, numerous investigations have demonstrated that symptoms do not always correlate with the severity of the stenosis4,5). For example, in the Wakayama Spine Study, only 17.5% of patients with severe radiographic stenosis presented with symptoms5).
In determining whether to approach patients with multilevel stenosis via selective versus multilevel inclusive decompression, the following factors drive decision making: (1) number of levels involved, (2) perceived confidence in identifying responsible symptomatic level(s), (3) relative risk profile and invasiveness of selective versus inclusive procedural options, and (4) expected natural progression of untreated levels. There is limited information on the longer-term natural progression of asymptomatic and untreated stenosis. Furthermore, techniques for lumbar neural decompression are rapidly evolving. Therefore, surgeons cannot yet understand the relative downstream effects of less invasive endoscopic decompressions that conceptually minimize collateral tissue damage.
Less invasive techniques for neural decompression have evolved from the use of smaller retractors and microscopic visualization to endoscopic visualization to minimize the size of spinal access systems further. Microendoscopic laminotomy (MEL), consisting of bilateral decompression, uses a unilateral approach to decompress the central canal and bilateral lateral recesses6,7). This procedure enlarges the spinal canal, providing safe relief of pressure on the involved spinal nerves while maximizing the preservation of posterior stabilizing structures, including the facet joints, the posterior ligament complex, and soft tissues. Despite the substantial body of literature demonstrating the short-term efficacy of MEL, the long-term clinical outcomes of selective MEL in patients with multiple-level radiographic LSS are unclear, as is the natural progression of untreated remaining radiographic LSS (RR-LSS).
In this context, we sought to: (1) investigate the long-term clinical outcomes of MEL in patients with multilevel lumbar stenosis and (2) evaluate the natural progression in patients after MEL with untreated remaining radiographic stenosis. We hypothesized that selective MEL is effective for LSS patients with RR-LSS. There would be no difference in clinical outcomes or the frequency of reoperation between individuals with and without RR-LSS.
Materials and Methods
Study population
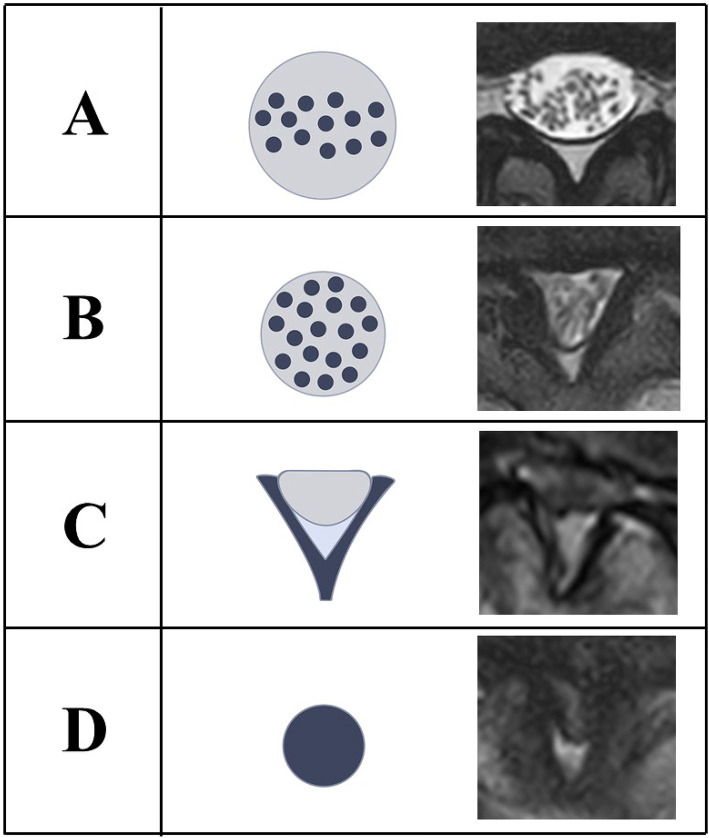
We retrospectively reviewed the medical records of all patients who underwent spinal microendoscopic decompression surgery for symptomatic LSS at our institution between January 1 and December 31, 2010. The inclusion criteria were neurogenic claudication or radicular leg pain with associated neurologic signs, moderate-to-severe spinal stenosis on cross-sectional imaging (such as magnetic resonance imaging [MRI]), and failure of conservative treatment for at least three months. Based on Schizas' classification8), Grade C was defined as moderate stenosis, and Grade D severe stenosis; moderate-to-severe stenosis was radiographic LSS (Fig. 1). Only the responsible stenotic level was targeted for surgery, based on neurologic and electrophysiological examinations and selective nerve root block9). Exclusion criteria included tumors, rheumatoid arthritis, arterial insufficiency of the leg, polyneuropathy, destructive spondyloarthropathy, other combined spinal lesions, and previous back surgery. To effectively evaluate MEL outcomes of degenerative LSS, cases with lumbar foraminal stenosis, lumbar disc herniation, lumbar spondylolisthesis, degenerative lumbar scoliosis with coronal curvature of more than 10° measured by Cobb's method (with the apex between L1 and L4), and cervical and/or thoracic neuropathy were excluded10). There were 127 patients who met the initial inclusion criteria. Patients with the following conditions were subsequently excluded from the study: tumor (n=1), rheumatoid arthritis (n=2), arterial insufficiency of the leg (n=2), polyneuropathy (n=3), destructive spondyloarthropathies (n=1), lumbar foraminal stenosis (n=5), lumbar disc herniation (n=1), lumbar spondylolisthesis (n=4), degenerative lumbar scoliosis (n=2), and cervical and/or thoracic neuropathy or previous back surgery (n=9). The remaining 87 patients were included in the final analysis (Fig. 2).
Figure 1.
Schizas’ classification.
Grade A: Cerebrospinal fluid (CSF) is clearly visible inside the dural sac.
Grade B: CSF is reduced but still present. The nerves can still be individualized inside the dural sac.
Grade C: Moderate stenosis. CSF disappears, and nerves cannot be individualized within the dural sac. Epidural fat is abundant posteriorly.
Grade D: Severe stenosis. Nerves cannot be individualized, and epidural fat is also significantly reduced.
Figure 2.
Flow diagram of the present study.
Ethics approval
All procedures performed in this study with human participants complied with the ethical standards of the relevant institutional research committee and the principles of the Declaration of Helsinki. Our institutional research committee approved this study and all participants provided written informed consent.
Surgical procedures
MEL11,12) was performed in all cases. The Japanese Orthopedic Association (JOA) certified endoscopic spine surgeons performed the procedures.
A skin incision approximately 16 mm in length was made to target the interlaminar space. Serial tubal dilators (METRx endoscopic system; Medtronics Sofamor Danek, Memphis, TN) were inserted through the incision. The microendoscopic procedure allows bilateral decompression of the central canal and bilateral lateral recesses via a unilateral approach11,12). To preserve the facet joint's integrity, we developed custom-designed instruments, including a high-speed drill with a long, curved endoscopic bit (e.g., Midas Rex Institute, Fort Worth, TX) and curved Kerrison rongeurs to undercut the facet joint. Laminotomy was performed using the long, curved-bit, high-speed drill. Decompression of the bilateral lateral recess was achieved via a medial trumpet facetectomy. The facet joint integrity was preserved with curved Kerrison rongeurs.
Clinical measurements
Outcomes were evaluated using the JOA scoring system13), a visual analog scale (VAS) for lower back and leg pain (full score=100 mm), and the Oswestry Disability Index (ODI)14) for health-related quality of life before, 1 year, and 10 years after surgery. Patient satisfaction, reoperation rate, and causes of reoperation were evaluated in a survey conducted 1 year and 10 years after the surgery. We asked patients to answer this questionnaire survey by mail. Since the survey only examined patient-reported items, the JOA score was a maximum of 23 points (excluding objective findings). The JOA recovery rate was calculated as 100×(postoperative JOA score − pre-operative JOA score) / (23 − pre-operative JOA score). We used the Japanese version of the ODI15) that excluded components related to sex life; limited information is expected for this component within Japanese culture. Thus, the final score was calculated as total score / (5×9 questions excluding “sex life”)×100%. Improvement in the ODI score was calculated as pre-operative ODI% − postoperative ODI%. We used the VAS to evaluate patient satisfaction. A 10-point VAS included the descriptors “very dissatisfied” to “very satisfied,” and all scales were evaluated to obtain numeric values for patient satisfaction scores16).
Statistical analysis
The Wilcoxon rank-sum test was used to compare pre-operative JOA scores, VAS (low back pain and leg pain), and ODI with the same scores assessed at the 1-year and 10-year postoperative survey. The JOA recovery rate, VAS score improvement (lower back and leg pain), ODI, patient satisfaction, reoperation rate, and causes of reoperation were compared between the RR-LSS positive and RR-LSS negative groups. Reoperation rates between the groups were compared using the chi-square test. All statistical analyses were performed using JMP version 14 (SAS Inc., Cary, NC, USA), with statistical significance defined as a two-sided p-value <0.05.
Results
Participant characteristics and outcomes
The 87 patients included 47 men and 40 women, with a mean age of 72.3±7.8 years (range 46-87 years). The follow-up rate was 70.1% (61/87 patients), with 26 patients lost to the 10-year final follow-up. Twelve patients (7 men and 5 women, mean age 81.2±3.4 years) were excluded from the analysis due to death (10 patients) or because of difficulty answering the questionnaire (two patients had cerebrovascular accidents within 10 years post-operatively). Thus, 49 patients were completely followed up for 10 years after surgery and included in the final analysis (Fig. 2).
Table 1 demonstrates the baseline characteristics of the 49 patients (21 men and 28 women, mean age 69.3±6.0 years) who completed the 10-year postoperative survey. Patients were classified into one of three groups based on the pathological characteristics of intermittent neurogenic claudication (NIC): 5 patients in the cauda equina type, 23 in the radicular type, and 21 in the mixed type groups. Approximately 60% underwent MEL for one segment, and the rest underwent MEL for multiple segments. Many surgeries were performed at the L4/5 and L3/4 stenotic levels (accounting for approximately 90% of the study population). RR-LSS was present in 59.2% (29/49) of patients, with approximately half involving the L2/3 level.
Table 1.
Characteristics of Patients Who Underwent MEL for LSS.
| Characteristics | Patients (n=49) |
|---|---|
| Male/Female | 21/28 |
| Age (years) | 69.3±6.0 |
| BMI (kg/m2) | 23.3±4.8 |
| Type of NIC | |
| Cauda equina (%) | 10.2 (5/49) |
| Radicular (%) | 46.9 (23/49) |
| Mixed (%) | 42.9 (21/49) |
| Number of MELs | |
| 1 segment (%) | 57.1 (28/49) |
| 2 segments (%) | 40.8 (20/49) |
| 3 segments (%) | 2.1 (1/49) |
| Level of MEL | |
| L2/3 (%) | 4.2 (3/71) |
| L3/4 (%) | 36.6 (26/71) |
| L4/5 (%) | 52.1 (37/71) |
| L5/S (%) | 7.1 (5/71) |
| RR-LSS | |
| L2/3 (%) | 51.7 (15/29) |
| L3/4 (%) | 37.9 (11/29) |
| L4/5 (%) | 10.4 (3/29) |
MEL: microendoscopic laminotomy, LSS: lumbar spinal stenosis, BMI: body mass index, NIC: intermittent neurogenic claudication, RR-LSS: remained radiographic lumbar spinal canal stenosis. Data are presented as n, mean±standard deviation (range), or number.
We compared the clinical outcomes between pre-MEL and 1 year after MEL and between 1 year and 10 years after MEL for LSS. Table 2 shows the overall results of the 1-year postoperative survey. Each evaluation item showed considerable improvement in comparing the pre-operative and 1-year postoperative surveys, and the degree of patient satisfaction was high. No patients required reoperation at that time. Table 3 compares clinical outcomes 1 year and 10 years after MEL for LSS. Each evaluation item was maintained at a good level even 10 years after surgery. However, 18.4% (9/49) of patients needed reoperation.
Table 2.
Clinical Outcomes of MEL for LSS after 1 Year.
| Outcomes | Patients (n=49) | p-value |
|---|---|---|
| JOA score (lumbar spine) | ||
| Preop./Postop. 1 yr. (_/23) | 9.5±4.1/16.9±3.9 | <0.001* |
| Recovery rate (%) | 54.0±28.3 | |
| VAS (low back pain) | ||
| Preop./Postop. 1 yr. (mm) | 50.1±30.7/26.4±30.6 | <0.001* |
| VAS (leg pain) | ||
| Preop./Postop. 1 yr. (mm) | 60.7±28.7/28.3±29.6 | <0.001* |
| ODI | ||
| Preop./Postop. 1 yr. (%) | 35.1±12.1/17.7±8.2 | <0.001* |
| Satisfaction (_/10) | 8.1±2.9 | |
| Reoperation (%) | 0 (0/49) |
MEL: microendoscopic laminotomy, LSS: lumbar spinal stenosis, JOA score: Japanese Orthopaedic Association scoring system (full score=23 points excluding objective findings), Recovery rate:=100× (postoperative JOA score – pre-operative JOA score)/(23 – pre-operative JOA score), Preop.: pre-operation, Postop. 1 yr.: post-operation 1 years, VAS: visual analog scale, ODI: Oswestry Disability Index.
*: significance defined as p<0.05. Data are presented as n, mean±standard deviation (range), or number.
Table 3.
Comparison of Clinical Outcomes 1 Year and 10 Years after MEL for LSS.
| Outcomes | Patients (n=49) | p-value |
|---|---|---|
| JOA score (lumbar spine) | ||
| Postop. 1 yr./Postop. 10 yr. (_/23) | 16.9±3.9/15.7±3.3 | 0.099 |
| JOA Recovery rate | ||
| Postop. 1 yr./Postop. 10 yr. (%) | 54.0±28.3/44.9±24.6 | 0.125 |
| VAS (low back pain) | ||
| Postop. 1 yr./Postop. 10 yr. (mm) | 26.4±30.6/35.1±30.2 | 0.105 |
| VAS (leg pain) | ||
| Postop. 1 yr./Postop. 10 yr. (mm) | 28.3±29.6/33.9±30.4 | 0.255 |
| ODI | ||
| Postop. 1 yr./Postop. 10 yr. (%) | 17.7±8.2/25.2±19.0 | 0.091 |
| Satisfaction | ||
| Postop. 1 yr./Postop. 10 yr. (_/10) | 8.1±2.9/7.9±2.9 | 0.289 |
| Reoperation | ||
| Postop. 1 yr./Postop. 10 yr. (%) | 0 (0/49)/18.4 (9/49) | 0.002* |
MEL: microendoscopic laminotomy, LSS: lumbar spinal stenosis, JOA score: Japanese Orthopaedic Association scoring system (full score=23 points excluding objective findings), Recovery rate:=100× (postoperative JOA score – pre-operative JOA score)/(23 – pre-operative JOA score), Postop. 1 yr.: post-operation 1 year, Postop. 10 yr.: post-operation 10 years, VAS: visual analog scale, ODI: Oswestry Disability Index.
*: significance defined as p<0.05. Data are presented as n, mean±standard deviation (range), or number.
Pre-operative characteristics in the RR-LSS positive and negative groups
Twenty-nine patients were RR-LSS positive; 18 were male, and 11 were female (Table 4). Pre-operative age, body mass index (BMI), type of NIC, MEL procedures and levels, lumbar VAS, JOA score, and percent ODI were not significantly different between the RR-LSS positive and RR-LSS negative groups (Table 4).
Table 4.
Pre-operative Comparison of RR-LLS (+) and RR-LSS (−).
| Characteristics | RR-LSS (+) | RR-LSS (−) | p-value |
|---|---|---|---|
| Patients (male/female) | 29 (18/11) | 20 (10/10) | 0.401 |
| Age (years) | 69.9±6.3 | 68.4±5.5 | 0.384 |
| BMI (kg/m2) | 22.4±5.1 | 24.5±4.3 | 0.273 |
| Type of NIC | 0.653 | ||
| Cauda equina (%) | 6.9 (2/29) | 15.0 (3/20) | |
| Radicular (%) | 48.3 (14/29) | 45.0 (9/20) | |
| Mixed (%) | 44.8 (13/29) | 40.0 (8/20) | |
| Number of MELs | 0.106 | ||
| 1 segment (%) | 58.6 (17/29) | 55.0 (11/20) | |
| 2 segments (%) | 41.4 (12/29) | 40.0 (8/20) | |
| 3 segments (%) | 0 (0/29) | 5.0 (1/20) | |
| Decompression level of MEL | 0.852 | ||
| L2/3 (%) | 4.9 (2/41) | 3.3 (1/30) | |
| L3/4 (%) | 36.6 (15/41) | 36.7 (11/30) | |
| L4/5 (%) | 53.6 (22/41) | 50.0 (15/30) | |
| L5/S (%) | 4.9 (2/41) | 10.0 (3/30) | |
| JOA score (lumbar spine) (_/23) | 10.1±3.9 | 8.8±4.3 | 0.244 |
| VAS (low back pain) (mm) | 48.2±29.6 | 52.7±32.8 | 0.622 |
| VAS (leg pain) (mm) | 63.4±26.2 | 56.8±32.2 | 0.434 |
| ODI (%) | 35.7±11.5 | 34.3±13.0 | 0.721 |
RR-LSS: remaining radiographic lumbar spinal stenosis, BMI: body mass index, NIC: neurogenic intermittent claudication, MEL: microendoscopic laminotomy, JOA score: Japanese Orthopaedic Association scoring system (full score=23 points excluding objective findings), VAS: visual analog scale, ODI: Oswestry Disability Index. *: significance defined as p<0.05. Data are presented as n, mean±standard deviation (range), or number.
Postoperative outcomes in the RR-LSS positive and negative groups
We compared the postoperative outcomes after 1 year as a short-term evaluation and 10 years as a long-term evaluation between the RR-LSS positive and negative groups.
Table 3 shows the overall results of the 10-year postoperative survey. In the 1-year postoperative survey, all evaluated outcomes, including the JOA score, the VAS scores for lower back and leg pain, and the ODI, significantly improved compared to baseline values in both groups. These items were not significantly different between the two groups (Table 5). Similar results were observed in the 10-year postoperative survey, and both groups maintained good results (Table 6). Furthermore, the degree of satisfaction was maintained at a relatively high level, even 10 years after the operation in both groups (Table 6). However, 18.4% (9/49) of patients needed reoperation. There was no significant difference in the reoperation rate between the two groups (Table 6). The following reasons for reoperation were reported in the RR-LSS positive group: one patient underwent surgery for de novo stenosis at the adjacent segment 5 years after the first surgery, one patient underwent surgery for re-stenosis at the decompressed segment 2 years after the first surgery, and four patients underwent surgery for RR-LSS (2, 3, 5, and 8 years after surgery, respectively) (Table 6). The two patients who underwent reoperation for RR-LSS relatively early, 2 and 3 years after the first surgery, had been classified as having the “cauda equina type” at the time of the first surgery. The reoperation rate for RR-LSS was 13.8% (4/29), which was equivalent to the reoperation rate for de novo stenosis in the RR-LSS negative group (15.0%; 3/20) (Table 6). On the other hand, the following reasons for reoperation were reported in the RR-LSS negative group: three patients underwent surgery for de novo stenosis (3, 6, and 7 years after surgery, respectively). Two of three patients had de novo stenosis in the adjacent segment, and the remaining patient had de novo stenosis two segments away (Table 6).
Table 5.
Comparison of Outcomes 1 Year after Surgery Between RR-LSS (+) and RR-LSS (−).
| Outcomes | RR-LSS (+) | RR-LSS (−) | p-value |
|---|---|---|---|
| JOA score (lumbar spine) (_/23) | 17.4±3.8 | 16.1±4.0 | 0.312 |
| recovery rate (%) | 56.2±30.0 | 50.8±26.1 | 0.410 |
| VAS (low back pain) (mm) | 26.8±24.7 | 25.8±38.4 | 0.230 |
| improvement in VAS score (low back pain) | 21.4±26.5 | 27.0±31.9 | 0.706 |
| VAS (leg pain) (mm) | 28.2±29.7 | 28.5±30.2 | 0.967 |
| improvement in VAS score (leg pain) | 35.2±36.3 | 28.3±29.6 | 0.416 |
| ODI (%) | 17.3±7.1 | 18.2±9.9 | 0.861 |
| improvement in ODI | 17.0±11.5 | 18.4±11.2 | 0.654 |
| Satisfaction (_/10) | 8.1±2.7 | 8.1±3.4 | 0.708 |
| Reoperation (%) | 0 (0/29) | 0 (0/20) |
RR-LSS: remaining radiographic lumbar spinal stenosis, JOA score: Japanese Orthopaedic Association scoring system (full score=23 points excluding objective findings), recovery rate: =100× (postoperative JOA score – pre-operative JOA score)/(23 – pre-operative JOA score), VAS: visual analog scale, improvement of VAS: =pre-operative VAS – postoperative VAS, ODI: Oswestry Disability Index, improvement of ODI: =pre-operative ODI – postoperative ODI, MEL: microendoscopic laminotomy. *: significance defined as p<0.05. Data are presented as n, mean±standard deviation (range), or number.
Table 6.
Comparison of Outcomes 10 Years after Surgery Between RR-LSS (+) and RR-LSS (−).
| Outcomes | RR-LSS (+) | RR-LSS (−) | p value |
|---|---|---|---|
| JOA score (lumbar spine) (_/23) | 16.1±2.9 | 15.1±3.7 | 0.282 |
| recovery rate (%) | 46.2±22.2 | 43.0±28.3 | 0.661 |
| VAS (low back pain) (mm) | 33.0±24.9 | 40.7±39.5 | 0.405 |
| improvement in VAS score (low back pain) | 15.3±30.8 | 12.0±35.8 | 0.733 |
| VAS (leg pain) (mm) | 32.3±27.2 | 36.2±35.2 | 0.665 |
| improvement in VAS score (leg pain) | 31.1±30.8 | 20.6±30.2 | 0.200 |
| ODI (%) | 26.8±16.6 | 22.6±22.7 | 0.460 |
| improvement in ODI | 9.8±16.0 | 15.0±18.9 | 0.367 |
| Satisfaction (_/10) | 8.2±2.5 | 7.4±3.4 | 0.357 |
| Reoperation (%) | 20.6 (6/29) | 15.0 (3/20) | 0.613 |
| MEL for de novo stenosis | 3.4 (1/29) | 15.0 (3/20) | |
| MEL for re-stenosis | 3.4 (1/29) | - | |
| MEL for RR-LSS | 13.8 (4/29) | - |
RR-LSS: remaining radiographic lumbar spinal stenosis, JOA score: Japanese Orthopaedic Association scoring system (full score=23 points excluding objective findings), recovery rate: =100× (postoperative JOA score – pre-operative JOA score)/(23 – pre-operative JOA score), VAS: visual analog scale, improvement of VAS: =pre-operative VAS – postoperative VAS, ODI: Oswestry Disability Index, improvement of ODI: =pre-operative ODI – postoperative ODI, MEL: microendoscopic laminotomy. *: significance defined as p<0.05. Data are presented as n, mean±standard deviation (range), or number.
Discussion
Spine specialists frequently encounter patients with multilevel lumbar stenosis, driven by an aging population and increased surveillance with imaging modalities, such as MRI; despite this, most cases of radiographic LSS may be asymptomatic1,4,5,17,18). In this context, surgeons must decide whether to provide selective solutions to limited symptomatic levels or more inclusive treatments to address all levels of significant stenosis. As less invasive solutions such as endoscopic decompressions continue to evolve and are more frequently used for focal neural decompressions at 1 or 2 vertebral segments, selective MEL may be an alternative to more inclusive open multilevel lumbar laminectomies. To determine when to deploy these solutions in patients with multilevel lumbar stenosis, surgeons will require more information than is currently available about the durability of these solutions and the natural progression of treated and untreated levels in multilevel lumbar stenosis patients.
Our study aimed to demonstrate the long-term clinical outcomes for MEL in patients with LSS with and without RR-LSS; additionally, we aimed to perform comparative analytics between these cohorts to determine whether MEL was an effective treatment in patients with RR-LSS. Our results demonstrate that patients who underwent MEL for LSS significantly improved all measured clinical outcome metrics (JOA, VAS, and ODI) relative to their pre-operative status, and improvements were sustained up to the 10-year follow-up. Moreover, comparing the 10-year postoperative outcomes between the RR-LSS positive and negative groups demonstrated that these items were not significantly different between the two groups. The total reoperation rate for all patients at 10 years was 18.4% (9/49), which consisted of reoperations at index levels, reoperations for de novo stenosis, and reoperations at previously identified stenotic levels that were not addressed at the index operation (RR-LSS). To determine the efficacy of selective MEL for patients with RR-LSS, we compared the reoperation rate for remaining stenosis in the RR-LSS positive group (13.8%; 4/29) to the reoperation rate for de novo stenosis in the RR-LSS negative group (15.0%; 3/20) and found no significant difference. Hence, the long-term clinical outcomes of selective MEL for patients with multilevel radiographic LSS were found to be very good, and RR-LSS was not a risk factor for poor performance or reoperation.
To the best of our knowledge, this is the first study to characterize MEL's long-term (10-year) results for LSS patients. Long-term results for conventional open lumbar decompression surgery have been reported previously for comparative purposes16,19). Specifically, studies have reported a 40-55% JOA recovery rate 10 years after surgery11,19-24), lumbar VASs of 30-35 mm19-22), ODIs of 25%-35%, and mean patient satisfaction of 7.0-8.0 points20,21) following conventional lumbar decompression. Thus, conventional lumbar decompression procedures and MEL have similar long-term clinical outcomes associated with symptoms at the index level. However, we found that the 10-year reoperation rate following MEL was 18.4%, which was relatively lower than in the previous open lumbar decompression studies (18%-25%)11,20,23,24). Perhaps of even greater significance, no patients in this study required revision with spinal fusion surgery (Table 6). Previous larger population studies have shown that approximately 30% of reoperations after conventional open decompressions involve spinal fusion for symptoms associated with postoperative spinal instability11,21-24). We hypothesize that the relative maintenance of stabilizing structures with microendoscopic approaches likely mitigates the risk of postoperative instability and avoids the need for fusion procedures7,9-12,25).
Despite the substantial strengths of this study, which had a long duration of longitudinal follow-up, it has several limitations. First, the retrospective nature of this investigation bears the limitations associated with this study design, including the potential for selection, indication, and expertise bias. Patients were retrospectively classified into RR-LSS positive or negative groups in this study without pre-operative blinding or randomization. Therefore, we could not completely deny that the surgeon's diagnostic ability to determine the responsible lesion and the relationship of trust between the surgeon and the patient may have affected the results. Additionally, there is some uniformity in the patient population and technical cohesion of the surgeons coming from a single high-volume endoscopic center that may limit generalizability to other clinical contexts. The relatively limited sample may impair some secondary comparisons, and we recognize that the study may be underpowered to detect some interactions. Last, this study did not measure or account for the potential effects of global radiographic parameters and sagittal balance. The patients were deemed not to have deformity-related symptoms or focal radiographic features and, thus, did not uniformly undergo surgery full-length radiographic assessments. Given our increased understanding of the role of global balance, it is certainly conceivable that these features may have had some undetected influence on clinical outcomes. However, we would not expect the role of these factors to be systematically disparate between the two analyzed cohorts25-27).
This is the first study to reveal the long-term clinical outcomes of MEL for patients with multilevel lumbar stenosis, demonstrating significant improvements in clinical outcome metrics that may last beyond 10 years following surgery. Further, we found selective MEL in the context of RR-LSS to be effective in patients with lumbar stenosis. Reoperation rates after MEL were not different between the two cohorts. Furthermore, the overall reoperation rate was lower than that reported by most studies published in the literature for open lumbar decompression over similar periods. The need for revision with spinal fusion was minimal in our population. Therefore, surgeons should consider these data when making decisions about less invasive selective, as opposed to more extensive, approaches in patients with multilevel lumbar stenosis.
Conflicts of Interest: The authors declare that there are no relevant conflicts of interest.
Hiroshi Yamada and Hiroshi Hashizume are some of the Editors of Spine Surgery and Related Research and on the journal's Editorial Committee. They were not involved in the editorial evaluation or decision to accept this article for publication at all.
Sources of Funding: None.
Author Contributions: K.N., H.I., and H.Y. designed the study; H.H., Y.Y., M.A., T.R., T.K., and N.Y. performed the experiments and analyzed the data; S.T. and M.T. provided critical reagents; AJ.S., AK.S., and M.Y. supervised the experiments; S.M. wrote the manuscript.
Ethical Approval: All procedures performed in this study about human participants complied with the ethical standards of the relevant institutional research committee and the principles of the Declaration of Helsinki. The Research Ethics Committee approved this study of Wakayama Medical University (approval number: 3242).
Informed Consent: All participants provided written informed consent.
Acknowledgement
The authors wish to thank Ms. Ayaka Shimazaki and Ms. Yukako Hashimoto for their assistance with data processing and administration. We also thank Ms. Maya Ueda for medical writing.
References
- 1.Verbiest H. A radicular syndrome from developmental narrowing of the lumbar vertebral canal. 1954. Clin Orthop Relat Res. 2001;384(384):3-9. [DOI] [PubMed] [Google Scholar]
- 2.Oeppen J, Vaupel JW. Demography. Broken limits to life expectancy. Science. 2002;296(5570):1029-31. [DOI] [PubMed] [Google Scholar]
- 3.Yabuki S, Fukumori N, Takegami M, et al. Prevalence of lumbar spinal stenosis, using the diagnostic support tool, and correlated factors in Japan: a population-based study. J Orthop Sci. 2013;18(6):893-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boden SD, Davis DO, Dina TS, et al. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72(3):403-8. [PubMed] [Google Scholar]
- 5.Ishimoto Y, Yoshimura N, Muraki S, et al. Associations between radiographic lumbar spinal stenosis and clinical symptoms in the general population: the Wakayama Spine Study. Osteoarthr Cartil. 2013;21(6):783-8. [DOI] [PubMed] [Google Scholar]
- 6.Yamada H, Yoshida M, Hashizume H, et al. Efficacy of novel minimally invasive surgery using spinal microendoscope for treating extraforaminal stenosis at the lumbosacral junction. J Spinal Disord Tech. 2012;25(5):268-76. [DOI] [PubMed] [Google Scholar]
- 7.Schizas C, Theumann N, Burn A, et al. Qualitative grading of severity of lumbar spinal stenosis based on the morphology of the dural sac on magnetic resonance images. Spine (Phila Pa 1976). 2010;35(21):1919-24. [DOI] [PubMed] [Google Scholar]
- 8.Aebi M. The adult scoliosis. Eur Spine J. 2005;14(10):925-48. [DOI] [PubMed] [Google Scholar]
- 9.Minamide A, Yoshida M, Yamada H, et al. Clinical outcomes after microendoscopic laminotomy for lumbar spinal stenosis: a 5-year follow-up study. Eur Spine J. 2015;24(2):396-403. [DOI] [PubMed] [Google Scholar]
- 10.Minamide A, Yoshida M, Yamada H, et al. Endoscope-assisted spinal decompression surgery for lumbar spinal stenosis. J Neurosurg Spine. 2013;19(6):664-71. [DOI] [PubMed] [Google Scholar]
- 11.Murata S, Minamide A, Takami M, et al. Microendoscopic decompression for lumbar spinal stenosis caused by facet-joint cysts: a novel technique with a cyst-dyeing protocol and cohort comparison study. J Neurosurg Spine. 2021;34(4):573-9. [DOI] [PubMed] [Google Scholar]
- 12.Izumida S, Inoue S. Assessment of treatment for low back pain. J Jpn Orthop Assoc. 1986;60:391-4. [Google Scholar]
- 13.Fairbank JCT, Pynsent PB. The Owestry Disability Index. Spine. 2000;25(22):2940-52; discussion 2952. [DOI] [PubMed] [Google Scholar]
- 14.Fujiwara A, Kobayashi N, Saiki K, et al. Association of the Japanese Orthopaedic Association score with the Oswestry Disability Index, Roland-Morris Disability Questionnaire, and short-form 36. Spine. 2003;28(14):1601-7. [PubMed] [Google Scholar]
- 15.Zhang XM, Shi JY, Gu YX, et al. Clinical investigation and patient satisfaction of short implants versus longer implants with osteotome sinus floor elevation in atrophic posterior maxillae: a pilot randomized trial. Clin Implant Dent Relat Res. 2017;19(1):161-6. [DOI] [PubMed] [Google Scholar]
- 16.Iguchi T, Kurihara A, Nakayama J, et al. Minimum 10-year outcome of decompressive laminectomy for degenerative lumbar spinal stenosis. Spine. 2000;25(14):1754-9. [DOI] [PubMed] [Google Scholar]
- 17.Porter RW, Ward D. Cauda equina dysfunction. The significance of two-level pathology. Spine. 1992;17(1):9-15. [PubMed] [Google Scholar]
- 18.Sato K, Kikuchi S. Clinical analysis of two-level compression of the cauda equina and the nerve roots in lumbar spinal canal stenosis. Spine. 1997;22(16):1898-903; discussion 1904. [DOI] [PubMed] [Google Scholar]
- 19.Atlas SJ, Keller RB, Wu YA, et al. Long-term outcomes of surgical and nonsurgical management of lumbar spinal stenosis: 8 to 10 year results from the Maine Lumbar Spine Study. Spine. 2005;30(8):936-43. [DOI] [PubMed] [Google Scholar]
- 20.Tuomainen I, Aalto T, Pesonen J, et al. Unfolding the outcomes of surgical treatment of lumbar spinal stenosis-a prospective 5- and 10-year follow-up study. Eur Spine J. 2020;29(9):2231-42. [DOI] [PubMed] [Google Scholar]
- 21.Jung JM, Chung CK, Kim CH, et al. The long-term reoperation rate following surgery for lumbar stenosis: a nationwide sample cohort study with a 10-year follow-up. Spine. 2020;45(18):1277-84. [DOI] [PubMed] [Google Scholar]
- 22.Martin BI, Mirza SK, Comstock BA, et al. Reoperation rates following lumbar spine surgery and the influence of spinal fusion procedures. Spine. 2007;32(3):382-7. [DOI] [PubMed] [Google Scholar]
- 23.Atlas SJ, Deyo RA, Keller RB, et al. The Maine lumbar spine study, Part III. 1-year outcomes of surgical and nonsurgical management of lumbar spinal stenosis. Spine (Phila Pa 1976). 1996;21(15):1787-94; discussion 1794. [DOI] [PubMed] [Google Scholar]
- 24.Jansson KA, Németh G, Granath F, Blomqvist P. Spinal stenosis re-operation rate in Sweden is 11% at 10 years-a national analysis of 9,664 operations. Eur Spine J. 2005;14(7):659-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiaochuan L, Zhong CF, Tang JH, et al. The effectiveness and safety of selective lumbar decompression in diagnostic doubt patients: a retrospective control study. Pain Phys. 2017;20(4):E541-50. [PubMed] [Google Scholar]
- 26.Endo K, Suzuki H, Tanaka H, et al. Sagittal spinal alignment in patients with lumbar disc herniation. Eur Spine J. 2010;19(3):435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Everett CR, Patel RK. A systematic literature review of nonsurgical treatment in adult scoliosis. Spine. 2007;32(19Suppl):S130-4. [DOI] [PubMed] [Google Scholar]