Abstract
Background:
Transcranial doppler (TCD) ultrasonography can be used to identify stroke risk in children with sickle cell anemia. Previous studies have reported mixed findings on neurocognitive outcomes in children with elevated TCD. This study examined associations between TCD velocity and neurocognitive outcomes in children and adolescents without prior history of stroke.
Procedure:
Participants were selected from the Sickle Cell Clinical Research Intervention Program cohort. The highest recorded mean maximum TCD velocity was selected for analysis, along with participant’s most recent data from serial neurocognitive surveillance.
Results:
A total of 200 children with sickle cell anemia completed neurocognitive testing (109 males, 91 females; mean age 12.7 years [SD=3.56]). Most participants were prescribed hydroxyurea (72%) at the time of neurocognitive testing and nearly 16% had a history of chronic transfusions prior to neurocognitive evaluation. Mean age at time of highest TCD value was 6.6 years (SD=2.5) and 13.5% of screenings were abnormal (≥200 cm/sec). Mean interval between TCD and most recent neurocognitive evaluation was 6.1 years (±3.5). There were no significant differences in the interval between TCD and neurocognitive testing across normal, conditional, and abnormal groups. Maximum TCD velocity was not significantly associated with neurocognitive outcomes in multivariate models.
Conclusions:
History of elevated TCD in the absence of overt stroke should not be considered a risk factor for poor neurocognitive outcomes in children and adolescents with sickle cell anemia on modern disease-modifying therapy.
Keywords: sickle cell disease, neurocognitive, transcranial doppler, children
Introduction
Sickle cell disease (SCD) is an inherited hemoglobin disorder that disrupts delivery of oxygen throughout the body including the brain. Silent cerebral infarct and overt ischemic stroke are the leading neurological complications of SCD.1 The risk of overt stroke is 200–400 times greater for untreated children with SCD compared to the general population.2 Ischemic events occur more often than hemorrhagic stroke and are most likely to occur between the ages of two and five years old in children with the HbSS genotype.3,4
Transcranial doppler (TCD) ultrasonography measures blood flow velocity through major cerebral arteries and has become a core component of primary stroke management.5 The Stroke Prevention Trial in Sickle Cell Anemia study demonstrated that TCD ultrasonography was effective in identifying patients at risk for a primary stroke and that red blood cell transfusions were effective interventions for stroke prevention.6 These findings prompted development of guidelines by the National Heart, Lung, and Blood Institute (NHLBI)7,8, which recommend annual TCD ultrasonography for children with HbSS beginning at age 2 years. The incidence rate for abnormal TCDs is 6–10 % and they are more prevalent in children younger than 12 years old.5,9
Previous studies of associations between TCD and neurocognitive functioning have yielded inconsistent results. Importantly, these studies took place before established guidelines from the NHLBI on hydroxyurea use in children.7 The largest study on TCD and neurocognitive outcomes (n=156; 7.7% receiving chronic transfusion, 17.4% on hydroxyurea) reported that abnormal TCD velocity was associated with poor nonverbal intelligence during a moderate latency period (<1.5 years); however, this relationship was no longer significant when participants with history of stroke were excluded.9 A smaller study (n=60; 33% on chronic transfusion) reported abnormal TCD was associated with lower verbal intelligence compared to the conditional group, whereas the conditional group exhibited worse sustained attention and executive functioning compared to the abnormal TCD group.10 These findings appeared to reflect variability in duration of time between TCD and neurocognitive testing for those in the conditional (6 months) and abnormal (5 years) groups, with the latter group potentially demonstrating neurocognitive decline in verbal skills that is typically associated with SCD. Studies with shorter latency periods between TCD and neurocognitive testing (i.e., medians of 63 and 150 days) failed to find associations between TCD and neurocognitive functioning.11,12 Only verbal memory was associated with TCD results during an extended interval between ultrasonography and testing (i.e., 5 years) (n=27; 48.1% on chronic transfusion).13
This study addresses inconsistencies in the literature regarding the relationship between abnormal TCD examinations and neurocognitive functioning in children with sickle cell anemia (HbSS and HbSβ0-thalassemia). We aimed to overcome limitations of previous studies, including variability in latency periods between neurocognitive testing and TCD examinations, inclusion of children with a history of stroke, limited neuropsychological measures, and small sample size. Our results provide insight into risk for long-term neurocognitive deficits among children with a history of conditional or abnormal TCD.
Methods
This research was approved by the Internal Review Board at St. Jude Children’s Research Hospital. Written informed consent was obtained from legal guardians since all participants were minors. Adolescents gave assent according to the study requirements.
Participants
Participants in the Sickle Cell Clinical Research Intervention Program (SCCRIP) cohort, aged 8–17 years, and with HbSS or HbSβ0-thalassemia, were eligible for this analysis. The SCCRIP study is a longitudinal lifetime cohort that collects data on children, adolescents, and adults with SCD, including clinical, neurocognitive, psychosocial, and health outcomes.14 TCD ultrasonography was provided as standard of care for patients with sickle cell anemia beginning at age 2 years. Neurocognitive assessments were provided every four years beginning at age 8 years. Timepoints for testing were school-age (8–9 years), early adolescence (12–13 years), and late adolescence (16–17 years). These serial evaluations were provided as standard clinical care for patients with SCD and were not associated with clinical referrals, history of neurological complications, or disease severity. Children and adolescents had access to an academic advocacy coordinator who was available to help establish educational supports (i.e., Section 504 Plan or individual education plan [IEP]) to support identified neurocognitive deficits and/or accommodations for disease management.
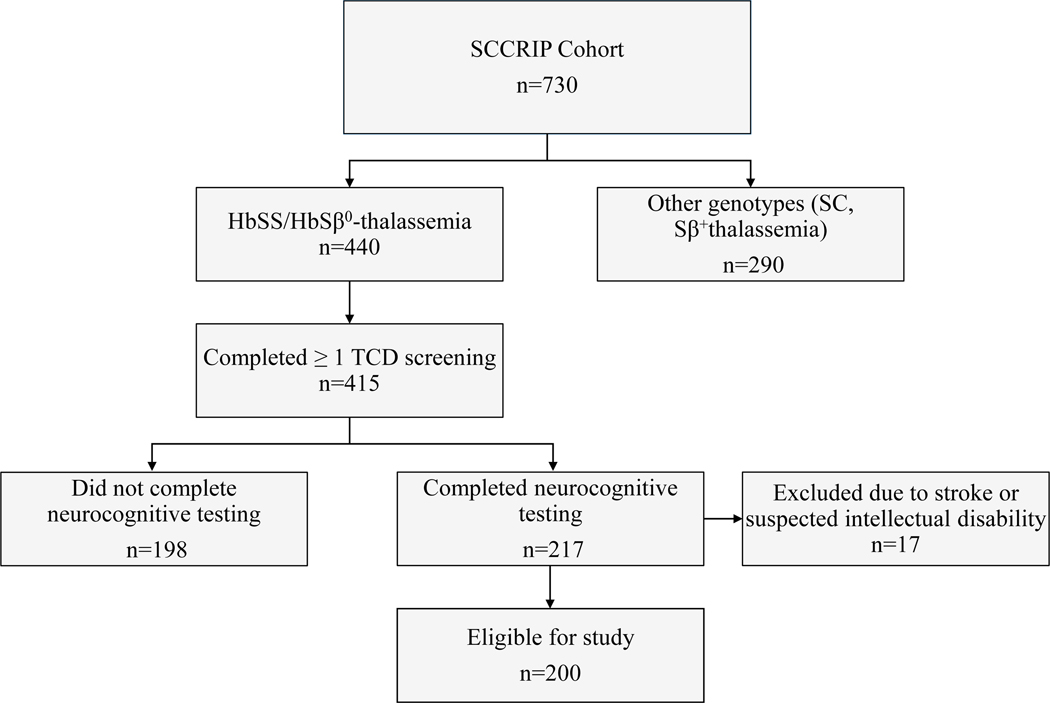
Of the 730 SCCRIP participants, 440 had the HbSS or HbSβ0-thalassemia genotype and 415 received at least one TCD screening. Over half (52%) of the 415 participants also completed neurocognitive testing (n=217). Potential reasons for participants not completing neurocognitive testing include missing a scheduled appointment or being referred for a clinical neuropsychological evaluation. We excluded patients with a history of confirmed or suspected intellectual disability (FSIQ ≤ 70; n=11) and stroke (n=6) leaving 200 eligible participants for the analysis. See Fig. 1 for further details on participant selection. Group differences between participants who did and did not complete neurocognitive testing were minimal (Supplemental Table S1).
FIGURE 1.
Eligibility criteria for participant selection. SCCRIP= sickle cell clinical research intervention program; TCD= transcranial doppler.
200 participants gave 80% statistical power to detect a linear correlation coefficient of 0.2 at a significance level of 0.05 based on a two-sided correlation test. In addition, out of 200 eligible participants, there were 27 participants with abnormal TCD (>200cm/sec). Based on the published data, 9 the effect size to compare neurocognitive function between abnormal TCD and normal TCD (<200cm/sec) was approximately 0.55. 200 participants (27 participants with abnormal TCD plus 173 participants with normal TCD) gave 75% power to detect the effect size of 0.55 at a significance level of 0.05 based on a two-sided t-test.
Demographic, medical, and treatment variables
Demographic, medical, and treatment variables were extracted from the SCCRIP database. Mean maximum TCD values were calculated from available data for the left and right distal internal carotid, carotid bifurcation, and anterior, middle, and posterior arteries. The highest time-averaged mean velocity (TAMV) TCD value on record preceding neurocognitive testing was selected for analysis. There was no latency cutoff between TCD and neurocognitive testing. TAMV values were categorized as normal (< 170 cm/sec), conditional (170–199 cm/sec), and abnormal (> 200 cm/sec). Follow-up TCD ultrasonography was completed for children with conditional or abnormal findings. Packed red blood cell transfusion was initiated for participants with abnormal TCD findings. Transfusion was provided monthly and hemoglobin values were monitored regularly. Most non-transfused participants received hydroxyurea in accordance with guidelines from the NHLBI.7 Hematological measures including hemoglobin, fetal hemoglobin, white blood cell count, and platelet count were performed at steady state on the day of neurocognitive testing or were the average value of measurements within three months prior to neurocognitive testing.
Neurocognitive battery
We selected the participant’s most recent neurocognitive assessment data for analysis. Neurocognitive evaluations were supervised by a licensed psychologist. Neurocognitive variables associated with lateralized skills and localized anatomical regions were selected for analysis. The Wechsler Abbreviated Scale of Intelligence, Second Edition (WASI-II) provided an estimated Full-Scale Intelligence Quotient (4-subtest; FSIQ) and measurement of verbal comprehension and visual-spatial reasoning.15 Depending on the participant’s age, attention and working memory were measured with Digit Span subtests from the Wechsler Adult Intelligence Scale, Fourth Edition (WAIS-IV) or Wechsler Intelligence Scale for Children, Fourth and Fifth Editions (WISC-IV and WISC-V).16,17 The Wide Range Assessment of Memory and Learning, Second Edition (WRAML-2) was used to measure verbal memory via the Story Memory subtest.18 The Beery-Buktenica Visual Motor Integration Test, Sixth Edition (VMI) was used to assess visual-motor integration.19 Fine motor dexterity and speed were measured with the Grooved Pegboard Test.20
Statistical Analysis
Summaries and comparisons of patient characteristics, labs, treatments, TCD values, and neurocognitive variables were stratified by age group and TCD category. For two-group comparisons, continuous variables were compared by either the Wilcoxon-Mann-Whitney test or t-test. For three-group comparisons, continuous variables were compared by either the Kruskal-Wallis test or analysis of variance. Shapiro-Wilk’s test was used to test whether continuous data followed a normal distribution. For categorical variables, either the Pearson’s chi-square or Fisher’s exact test was used to compare their distributions among different groups. Linear regression models were used to assess the associations of neurocognitive outcomes with categorical and continuous TCD. The TCD variable was forced into the model. Other candidate predictors were eliminated using backwards selection until remaining p-values were less than 0.10. All models were fit in SAS version 9.4.
Results
Clinical and Demographic Characteristics
Table 1 displays clinical and demographic characteristics of the sample. Males accounted for 54.5% of the sample. The frequency of conditional TCDs was 25.5% and 13.5% of participants had abnormal TCD results. Mean age at time of highest TAMV was 6.6 years (SD=2.5) and there were no statistically significant differences in age at TCD ultrasonography between normal, conditional, and abnormal groups (p>0.05). Mean age at time of neurocognitive testing was 12.7 years (SD=3.56). Mean interval between TCD screening and most recent neurocognitive assessment was 6.1 years (SD= 3.5 years). There were no statistically significant differences in the interval between TCD and neurocognitive testing across normal, conditional, and abnormal groups. The majority of participants were prescribed hydroxyurea (72%) at the time of neurocognitive testing and nearly 16% had a history of chronic transfusions prior to neurocognitive evaluation.
TABLE 1.
Summary of descriptive statistics for demographic, medical, and clinical variables
| Total | TCD Normal | TCD Conditional | TCD Abnormal | P | |
|---|---|---|---|---|---|
|
| |||||
| N (%): | N (%): | N (%): | N (%): | ||
| Sex | 0.24 | ||||
| Female | 91 (45.5%) | 50 (41.0%) | 28 (54.9%) | 13 (48.1%) | . |
| Male | 109 (54.5%) | 72 (59.0%) | 23 (45.1%) | 14 (51.9%) | . |
| Genotype | - | ||||
| HbSS/HbSβ0− | 200 (100%) | 122 (100%) | 51 (100%) | 27 (100.0%) | . |
| Race | . | ||||
| Black | 199 (99.5%) | 121 (99.2%) | 51 (100%). | 27 (100%) | 1 |
| Other | 1 (0.5%) | .1 (0.8%) | . | . | . |
| Maximum TCD Classification | 200 | 122 (61%) | 51 (25.5%) | 27 (13.5%) | . |
| Taking HU | 0.094 | ||||
| No | 56 (28.0%) | 33 (27.0%) | 11 (21.6%) | 12 (44.4%) | . |
| Yes | 144 (72.0%) | 89 (73.0%) | 40 (78.4%) | 15 (55.6%) | . |
| Chronic transfusions prior to evaluation | <.001 | ||||
| No | 169 (84.5%) | 116 (95.1%) | 47 (92.2%) | 6 (22.2%) | . |
| Yes | 31 (15.5%) | 6 (4.9%) | 4 (7.8%) | 21 (77.8%) | . |
| Chronic transfusions prior to TCD | 0.009 | ||||
| No | 185 (92.5%) | 117 (95.9%) | 47 (92.2%) | 21 (77.8%) | . |
| Yes | 15 (7.5%) | 5 (4.1%) | 4 (7.8%) | 6 (22.2%) | . |
| Receiving school servicesa | 0.687 | ||||
| No | 60 (31.1%) | 40 (33.3%) | 13 (27.7%) | 7 (26.9%) | |
| Yes | 133 (68.9%) | 80 (66.7%) | 34 (72.3%) | 19 (73.1%) | |
|
| |||||
| Total | TCD Normal | TCD Conditional | TCD Abnormal | P | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Social Vulnerability Rankingb | 0.66 (0.25) | 0.67 (0.25) | 0.64 (0.24) | 0.67 (0.28) | 0.535 |
| Max TCD | 163.8 (30.0) | 144.1 (15.4) | 183.4 (8.6) | 216.1 (14.7) | <.001 |
| Age at TCD, years | 6.67 (2.50) | 6.42 (2.51) | 6.90 (2.40) | 7.36 (2.59) | 0.182 |
| Age at evaluation, years | 12.75 (3.56) | 12.37 (3.52) | 12.98 (3.51) | 14.02 (3.61) | 0.065 |
| Interval between TCD and evaluationc | 2220 (1266) | 2173 (1214) | 2218 (1297) | 2432 (1454) | 0.670 |
| Total days on HU | 2187 (1418) | 2195 (1360) | 2166 (1476) | 2189 (1686) | 0.950 |
| Fetal Hemoglobin, % | 15.68(9.52) | 17.28 (9.24) | 15.01 (8.02) | 9.76(11.08) | <.001 |
| Hemoglobin, g/dL | 9.18 (1.23) | 9.31 (1.26) | 8.85 (1.13) | 9.23 (1.15) | 0.080 |
| Platelet count, × 109/L | 398.8 (173.3) | 392.7 (184.5) | 395.2 (154.5) | 433.4 (155.8) | 0.386 |
| White blood cell × 109/L | 9.94 (4.26) | 9.60 (4.04) | 9.52 (3.84) | 12.28 (5.30) | 0.019 |
Note. Values are presented as mean (standard deviation) or frequency (group %) unless otherwise noted. HU = hydroxyurea; TCD = transcranial doppler. P values based on chi-square or Fisher’s Exact test for categorical variables and Kruskal-Wallis for continuous variables.
Includes parent report of unspecified school services, 504 Plan, or Individual Education Plan (IEP).
Classifies individuals based on social vulnerabilities at the neighborhood level (e.g., housing data, poverty, and education); higher values indicate higher social vulnerability.
interval based on number of days.
Univariate associations between TCD velocities and neurocognitive functioning
Univariate models were used to examine TCD as a covariate with neurocognitive performance as the outcome variable (Table 2). When analyzed as a continuous variable, TCD was associated with visual-motor integration (estimate = −0.082, standard error [SE] = 0.04, p=.043). TCD was also analyzed categorically according to 1) binary categories of normal (<200 cm/sec) and abnormal (≥200 cm/sec), and 2) normal, conditional, and abnormal. When TCD velocity was collapsed into binary categories (normal and abnormal), statistically significant differences were found in vocabulary (estimate = −1.431, SE = 0.636, p=.026), working memory (estimate = −1.158, SE = .555, p=.038), and visual-motor integration (estimate = −8.543, SE = 3.751, p=.024) (Table 2). Neurocognitive outcomes did not vary significantly between TCD groups when analyzed according to normal, conditional, or abnormal classifications, except for vocabulary skills (p=.046) (Supplemental Table S2).
TABLE 2.
Univariate TCD models with continuous and binary categorical analysis
| Continuous TCD1 | Binary TCD2 | |||||
|---|---|---|---|---|---|---|
| Outcome / Dependent variable | Estimate | SE | P | Estimate | SE | P |
| FSIQa | −0.028 | 0.035 | 0.416 | −5.178 | 3.521 | 0.144 |
| Block Designa | −0.008 | 0.007 | 0.242 | −0.390 | 0.555 | 0.483 |
| Matrix Reasoninga | 0.002 | 0.007 | 0.731 | −0.563 | 0.603 | 0.352 |
| Vocabularya | −0.008 | 0.007 | 0.260 | −1.431 | 0.636 | 0.026 |
| Similaritiesa | −0.006 | 0.009 | 0.521 | −1.155 | 0.883 | 0.193 |
| Digit Span Forwardb | −0.003 | 0.007 | 0.714 | −0.442 | 0.610 | 0.470 |
| Digit Span Backwardb | −0.008 | 0.006 | 0.240 | −1.158 | 0.555 | 0.038 |
| Story Memory Recognitionc | 0.014 | 0.007 | 0.059 | 0.085 | 0.748 | 0.910 |
| Motor Dominant Handd | 0.003 | 0.005 | 0.598 | 0.367 | 0.470 | 0.436 |
| Motor Non-dominant Handd | 0.004 | 0.004 | 0.333 | 0.222 | 0.431 | 0.607 |
| Visual-Motor Integratione | −0.082 | 0.040 | 0.043 | −8.543 | 3.751 | 0.024 |
Abbreviations. TCD = transcranial doppler; SE = standard error.
Maximum TCD velocity
Normal (< 200 cm/sec) and Abnormal (≥ 200 cm/sec).
Wechsler Abbreviated Scale of Intelligence, Second Edition (scaled score)
Wechsler Intelligence Scale for Children, Fourth and Fifth Editions and Wechsler Adult Intelligence Scale, Fourth Edition (scaled score)
The Wide Range Assessment of Memory and Learning, Second Edition (scaled score)
The Grooved pegboard Test (z score)
Beery-Buktenica Visual Motor Integration Test, Sixth Edition (standard score)
Multivariate associations between TCD velocities and neurocognitive functioning
In multivariate models, TCD values were not significantly associated with visual-motor integration, vocabulary, or working memory skills when analyzed as a continuous or categorical variable (Table 3) after controlling for demographic and treatment factors. After reducing the full models using backward selection (p<0.10), TCD values (continuous or binary) continued to demonstrate no significant associations with visual-motor integration, vocabulary, or working memory.
TABLE 3.
Multivariable models for TCD and neurocognitive functioning
| Continuous TCD Models | Categorical TCD Models | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Full model | Reduced Model | Full model | Reduced Model | |||||||||
| Beta | SE | P | Beta | SE | P | Beta | SE | P | Beta | SE | P | |
|
| ||||||||||||
| Visual-Motor Integration a | ||||||||||||
|
| ||||||||||||
| Intercept | 109.3 | 12.4 | <.0001 | 111.7 | 7.5 | <.0001 | 101.5 | 9.7 | <.0001 | 100.6 | 4.8 | <.0001 |
| TCD | −0.046 | 0.045 | 0.310 | −0.070 | 0.040 | 0.086 | ||||||
| Normal+ | 0.000 | . | . | 0.000 | . | . | ||||||
| Conditional | −0.637 | 2.597 | 0.807 | −0.653 | 2.504 | 0.795 | ||||||
| Abnormal | −2.586 | 5.152 | 0.617 | −6.378 | 3.981 | 0.111 | ||||||
| Age, years | −0.733 | 0.356 | 0.041 | −0.612 | 0.300 | 0.043 | −0.742 | 0.362 | 0.042 | −0.588 | 0.305 | 0.056 |
| Female | −0.346 | 2.178 | 0.874 | −0.469 | 2.191 | 0.831 | ||||||
| Chronic transfusions | −5.444 | 3.962 | 0.172 | −5.731 | 4.607 | 0.216 | ||||||
| Using HU | −1.095 | 3.481 | 0.754 | −1.138 | 3.527 | 0.747 | ||||||
| Time on HU, years | 0.315 | 0.371 | 0.396 | 0.322 | 0.375 | 0.392 | ||||||
| Socioeconomic Vulnerabilityc | −8.750 | 4.193 | 0.039 | −9.234 | 4.072 | 0.025 | −8.548 | 4.253 | 0.046 | −8.699 | 4.106 | 0.036 |
| Hemoglobin, g/dL | 0.198 | 1.003 | 0.844 | 0.281 | 1.013 | 0.782 | ||||||
| Fetal Hemoglobin, % | −0.103 | 0.136 | 0.451 | −0.102 | 0.137 | 0.458 | ||||||
|
| ||||||||||||
| Vocabulary b | ||||||||||||
|
| ||||||||||||
| Intercept | 9.60 | 2.35 | <.0001 | 10.88 | 1.52 | <.0001 | 11.08 | 1.83 | <.0001 | 11.93 | 0.96 | <.0001 |
| TCD | 0.008 | 0.008 | 0.323 | 0.007 | 0.008 | 0.410 | ||||||
| Normal+ | 0.000 | . | . | 0.000 | . | . | ||||||
| Conditional | −0.024 | 0.485 | 0.961 | −0.076 | 0.470 | 0.871 | ||||||
| Abnormal | 0.500 | 0.922 | 0.588 | 0.386 | 0.904 | 0.670 | ||||||
| Age, years | −0.222 | 0.067 | 0.001 | −0.248 | 0.064 | 0.000 | −0.220 | 0.068 | 0.001 | −0.246 | 0.065 | 0.0002 |
| Female | −0.246 | 0.413 | 0.553 | −0.196 | 0.414 | 0.636 | ||||||
| Chronic transfusions | −1.284 | 0.709 | 0.072 | −1.434 | 0.666 | 0.033 | −1.268 | 0.843 | 0.135 | −1.430 | 0.807 | 0.078 |
| Using HU | 0.448 | 0.637 | 0.483 | 0.490 | 0.642 | 0.447 | ||||||
| Time on HU, years | 0.161 | 0.071 | 0.024 | 0.211 | 0.052 | <.0001 | 0.159 | 0.071 | 0.027 | 0.211 | 0.053 | <.0001 |
| Socioeconomic Vulnerabilityc | −1.497 | 0.811 | 0.067 | −1.424 | 0.806 | 0.079 | −1.532 | 0.820 | 0.063 | −1.448 | 0.814 | 0.077 |
| HgB | 0.034 | 0.193 | 0.861 | 0.009 | 0.194 | 0.964 | ||||||
| Hgb_F | 0.026 | 0.027 | 0.333 | 0.026 | 0.027 | 0.342 | ||||||
|
| ||||||||||||
| Working Memory d | ||||||||||||
|
| ||||||||||||
| Intercept | 8.51 | 2.22 | 0.0002 | 9.16 | 1.36 | <.0001 | 9.03 | 1.73 | <.0001 | 9.64 | 0.75 | <.0001 |
| TCD | 0.005 | 0.008 | 0.542 | 0.004 | 0.007 | 0.619 | ||||||
| TCD Normal+ | 0 | . | . | 0.000 | . | . | ||||||
| TCD Conditional | 0.461 | 0.456 | 0.313 | 0.436 | 0.436 | 0.319 | ||||||
| TCD Abnormal | 0.671 | 0.890 | 0.452 | 0.295 | 0.775 | 0.704 | ||||||
| Age, years | −0.097 | 0.065 | 0.133 | −0.090 | 0.054 | 0.098 | −0.101 | 0.065 | 0.121 | −0.093 | 0.055 | 0.090 |
| Female | 0.222 | 0.392 | 0.573 | 0.197 | 0.391 | 0.616 | ||||||
| Chronic transfusions | −1.556 | 0.657 | 0.019 | −1.395 | 0.599 | 0.021 | −1.725 | 0.820 | 0.037 | −1.357 | 0.726 | 0.063 |
| Using HU | 1.344 | 0.608 | 0.028 | 1.034 | 0.417 | 0.014 | 1.314 | 0.611 | 0.033 | 1.010 | 0.421 | 0.018 |
| Time on HU, years | −0.021 | 0.067 | 0.752 | −0.018 | 0.067 | 0.789 | ||||||
| Socioeconomic Vulnerabilityc | 0.385 | 0.737 | 0.601 | 0.348 | 0.740 | 0.639 | ||||||
| HgB | 0.048 | 0.182 | 0.790 | 0.066 | 0.182 | 0.717 | ||||||
| Hgb_F | −0.022 | 0.027 | 0.421 | −0.021 | 0.027 | 0.434 | ||||||
Abbreviations: HU = hydroxyurea; TCD = transcranial doppler. Note: TCD was forced into the models. Neurocognitive variables selected based on statistical significance in univariate models. Candidate predictors were eliminated using backward selection until remaining p values were less than 0.10.
reference group for comparison
Beery-Buktenica Visual Motor Integration Test, Sixth Edition (standard score)
Wechsler Abbreviated Scale of Intelligence, Second Edition Vocabulary (scaled score)
Classifies individuals based on social vulnerabilities at the neighborhood level (e.g., housing data, poverty, and education); higher values indicate higher social vulnerability
Wechsler Intelligence Scale for Children, Fourth and Fifth Editions and Wechsler Adult Intelligence Scale, Fourth Edition Digit Span Backward (scaled score)
Discussion
Findings from the largest study to-date on TCD and neurocognitive outcomes provide little evidence to suggest that neurodevelopmental trajectory is associated with the results of prior TCD examinations. In our study, the average duration between TCD screening and neurocognitive testing was 6.1 years, which represents the longest reported follow-up of TCD outcomes. Our results indicate that highest recorded TAMV was not significantly associated with future neurocognitive performances in a sample with high rates of hydroxyurea use and school support services (e.g., 504 Plan or IEP). Consistent with our prior work, aging and treatment factors (i.e., hydroxyurea and chronic transfusions) were significant predictors of visual-motor integration, vocabulary, and working memory skills.21
In contrast to previous studies, we did not find evidence that TCD predicted outcomes in areas of nonverbal intelligence, language, working memory/executive functioning, or memory.10,13,22 Our study excluded children with overt stroke, thus confirming the findings reported by Bernaudin and colleagues when stroke patients were removed from their analysis.9 Previous studies identifying differences in language and working memory/executive functioning between TCD groups have been limited by variability in duration of time between TCD and testing (for example, 6 months vs. 5 years for conditional and abnormal groups, respectively).10 In our study, there were no statistically significant differences in duration between TCD groups and neurocognitive testing, allowing us to overcome this limitation.
Differences in design methodology likely account for the contrast in our results and previously reported findings. Our study represents the most robust sample of participants with TCD and neurocognitive testing and includes the most comprehensive assessment of neurocognitive abilities to date. We opted to select participant’s highest recorded TAMV in order to capture the most significant marker of risk for neurological injury. Our decision to select participant’s most recent neurocognitive test data allowed us to examine future neurocognitive risk, thus providing a better understanding of the long-term impact of TCD on neurocognitive development.
This study has several limitations. MRI was not available for all participants, and we are unable to consider the impact of silent infarcts on neurocognitive outcomes. Although the highest-recorded TAMV on record was not associated with most neurocognitive outcomes, future studies may consider examining multiple TAMV timepoints. Academic measures were not included in our analysis, thus this study did not assess the potential impact of TCD on learning skills. While our sample is representative of the sickle cell anemia population in the southern United States, our study lacked diversity with regard to other racial and ethnic groups who experience sickle cell anemia worldwide.
In summary, these results indicate that elevated TCD velocities have minimal influence on long-term neurocognitive outcomes in children without history of stroke who receive modern disease-modifying therapies, such as hydroxyurea and chronic transfusion. Elevated TCD velocity in the absence of stroke should not be considered a risk-factor for future neurocognitive outcomes for children on disease modifying therapy. Other measures of cerebral blood flow, such as functional magnetic resonance imaging, that demonstrate concordance with neurocognitive performance may be better predictors of neurocognitive outcomes for children and adolescents with sickle cell anemia.23 Additionally, emerging research with single-and multi-inflow time arterial spin labeling sequences in sickle cell anemia suggests that this technique may be better equipped to identify hemodynamic stress and associations with cognition, including overall intellectual functioning, processing speed, and executive functioning. 24,25
Supplementary Material
Supplemental Table S1: Comparison of SCCRIP participants who did and did not complete cognitive testing
Supplemental Table S2: Neurocognitive outcomes according to TCD categories
Acknowledgements
We would like to thank SCCRIP collaborator Jeremie H. Estepp for his contributions to the project. We are also appreciative of Erin MacArthur and Pei-Lin Chen for their efforts in data management.
J.S.H received funding from U01HL133996 during the conduct of this study. A.A.K received funding from R01HL129241, K24HL148305, K12HL137942, U01HL143477, and 5U01HL133994 during the time of his study. This research was supported by the American Lebanese Syrian Associated Charities (ALSAC).
Abbreviations
- FSIQ
Full scale intellectual quotient
- NHLBI
National Heart, Lung, Blood Institute
- SCCRIP
Sickle Cell Clinical Research and Intervention Program
- SCD
Sickle cell disease
- TAMV
Time-averaged mean velocity
- TCD
Transcranial doppler
- VMI
Beery-Buktenica Visual Motor Integration Test, Sixth Edition
- WAIS-IV
Wechsler Adult Intelligence Scale, Fourth Edition
- WASI-II
Wechsler Abbreviated Scale of Intelligence, Second Edition
- WISC-IV and WISC-V
Wechsler Intelligence Scale for Children, Fourth and Fifth Editions
- WRAML-2
Wide Range Assessment of Memory and Learning, Second Edition
Footnotes
Conflict of Interest
A.A.K . receives research funding from Global Blood Therapeutics. J.S.H. receives consultancy fees from Global Blood Therapeutics, VForma Therapeutics, UpToDate and bluebird bio. A.M.H. receives consultancy fees from Global Blood Therapeutics. There are no other conflicts of interest to report.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.DeBaun MR, Derdeyn CP, McKinstry Iii RC. Etiology of strokes in children with sickle cell anemia. Mental Retardation and Developmental Disabilities Research Reviews. 2006;12(3):192–199. [DOI] [PubMed] [Google Scholar]
- 2.Lynch JK, Hirtz DG, DeVeber G, Nelson KB. Report of the National Institute of Neurological Disorders and Stroke Workshop on Perinatal and Childhood Stroke. Pediatrics. 2002;109(1):116–123. [DOI] [PubMed] [Google Scholar]
- 3.Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: Rates and risk factors. Blood. 1998;1(91):288–294. [PubMed] [Google Scholar]
- 4.Balkaran B, Char G, Morris JS, Thomas PW, Serjeant BE, Serjeant GR. Stroke in a cohort of patients with homozygous sickle cell disease. The Journal of Pediatrics. 1992;120(3):360–366. [DOI] [PubMed] [Google Scholar]
- 5.Adams RJ, McKie VC, Hsu L, et al. Prevention of a First Stroke by Transfusions in Children with Sickle Cell Anemia and Abnormal Results on Transcranial Doppler Ultrasonography. N Engl J Med. 1998;339(1):5–11. [DOI] [PubMed] [Google Scholar]
- 6.Adams RJ. Lessons From the Stroke Prevention Trial in Sickle Cell Anemia (STOP) Study. J Child Neurol. 2000;15(5):344–349. [DOI] [PubMed] [Google Scholar]
- 7.National Heart, Lung, and Blood Institute. Evidence-based management of sickle cell disease. In: Expert pannel report, 2014: Guide to reocmmendations. 4 ed. Bethesda, MD: United States Department of Health Services; 2014: https://www.nhlbi.nih.gov/files/docs/guidelines/sc_mngt.pdf. [Google Scholar]
- 8.DeBaun MR, Jordan LC, King AA, et al. American Society of Hematology 2020 guidelines for sickle cell disease: Prevention, diagnosis, and treatment of cerebrovascular disease in children and adults. Blood Advances. 2020;4(8):1554–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernaudin F, Verlhac S, Fréard F, et al. Multicenter Prospective Study of Children With Sickle Cell Disease: Radiographic and Psychometric Correlation. J Child Neurol. 2000;15(5):333–343. [DOI] [PubMed] [Google Scholar]
- 10.Kral MC, Brown RT, Nietert PJ, Abboud MR, Jackson SM, Hynd GW. Transcranial doppler ultrasonography and neurocognitive functioning in children with sickle cell disease. Pediatrics. 2003;112(2):324–331. [DOI] [PubMed] [Google Scholar]
- 11.Hijmans CT, Grootenhuis MA, Oosterlaan J, Heijboer H, Peters M, Fijnvandraat K. Neurocognitive deficits in children with sickle cell disease are associated with the severity of anemia. Pediatr Blood Cancer. 2011;57(2):297–302. [DOI] [PubMed] [Google Scholar]
- 12.Wang WC, Gallagher DM, Pegelow CH, et al. Multicenter Comparison of Magnetic Resonance Imaging and Transcranial Doppler Ultrasonography in the Evaluation of the Central Nervous System in Children With Sickle Cell Disease. J Pediatr Hematol Oncol. 2000;22(4):335–339. [DOI] [PubMed] [Google Scholar]
- 13.Kral MC, Brown RT, Curé JK, Besenski N, Jackson SM, Abboud MR. Radiographic predictors of neurocognitive functioning in pediatric sickle cell disease. J Child Neurol. 2006;21(1):37–44. [DOI] [PubMed] [Google Scholar]
- 14.Hankins JS, Estepp JH, Hodges JR, et al. Sickle Cell Clinical Research and Intervention Program (SCCRIP): A lifespan cohort study for sickle cell disease progression from the pediatric stage into adulthood. Pediatr Blood Cancer. 2018;65(9):e27228. [DOI] [PubMed] [Google Scholar]
- 15.Wechsler D. Wechsler Abbreviated Scale of Intelligence, 2nd edn. Bloomington, MN: Pearson; 2011. [Google Scholar]
- 16.Wechsler D. Wechsler Adult Intellgience Scale, 4th edn. Bloomington, MN: Pearson; 2008. [Google Scholar]
- 17.Wechsler D. Wechsler Intelligence Scale for Children, 5th edn. Bloomington, MN: Pearson; 2014. [Google Scholar]
- 18.Sheslow D, Adams W. Wide range assessment of memory and learning, 2nd edn. Lutz, FL: Psychological Assessment Resources, Inc. ; 2003. [Google Scholar]
- 19.Beery KE, Buktenica NA, Beery NA. Beery-Buktenica Developmental Test of Visual-Motor Integration, 6th edn. Bloomington, MN: Pearson; 2010. [Google Scholar]
- 20.Lafayette Instrument. Grooved Pegboard Test: User’s Manuel. Lafayette, IN: Lafayette Instrument Company; 2021. [Google Scholar]
- 21.Heitzer AM, Longoria J, Okhomina V, et al. Hydroxyurea treatment and neurocognitive functioning in sickle cell disease from school age to young adulthood. Br J Haematol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanchez CE, Schatz J, Roberts CW. Cerebral blood flow velocity and language functioning in pediatric sickle cell disease. J Int Neuropsychol Soc. 2010;16(2):326–334. [DOI] [PubMed] [Google Scholar]
- 23.Roalf DR, Ruparel K, Gur RE, et al. Neuroimaging predictors of cognitive performance across a standardized neurocognitive battery. Neuropsychology. 2014;28(2):161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stotesbury H, Hales PW, Koelbel M, et al. Venous cerebral blood flow quantification and cognition in patients with sickle cell anemia. J Cereb Blood Flow Metab. 2022;42(6):1061–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stotesbury H, Hales PW, Hood AM, et al. Individual Watershed Areas in Sickle Cell Anemia: An Arterial Spin Labeling Study. Front Physiol. 2022;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1: Comparison of SCCRIP participants who did and did not complete cognitive testing
Supplemental Table S2: Neurocognitive outcomes according to TCD categories
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.