Abstract
The purpose of this study was to investigate whether an age-associated impaired acute-phase response exists. Nine healthy elderly volunteers (median, 66 years; range, 61 to 69 years) and eight young controls (median, 24 years; range, 20 to 27 years) were given an intravenous bolus of endotoxin (2 ng/kg). The rectal temperature was monitored continuously, and blood samples for cytokine measurements were obtained before endotoxin administration as well as 0.5, 1, 1.5, 2, 3, 4, 8, 12, and 24 h after the injection. The elderly subjects showed a more prolonged fever response compared to the young controls. Levels of tumor necrosis factor alpha (TNF-α), soluble TNF receptors (sTNFR-I), interleukin-6 (IL-6), IL-8, IL-10, and IL-1 receptor antagonist (IL-1ra) in plasma increased markedly following endotoxin administration in both groups. The elderly group showed larger initial increases in TNF-α and sTNFR-I levels and prolonged increased levels of sTNFR-I. Monocyte concentrations decreased in both groups, with the elderly group showing a more rapid decrease and a slower subsequent increase than did the young group. Furthermore, the elderly group had a more rapid increase in C-reactive protein levels than did the young group. In conclusion, ageing is associated with an altered acute-phase response including initial hyperreactivity, prolonged inflammatory activity, and prolonged fever response.
It has been suggested that there exists an age-related defective acute-phase response (11). This is supported by reports of afebrile bacteremia in elderly patients (10). In other studies, lack of fever and of leukocytosis were associated with a poor outcome of community-acquired pneumonia (15) and elderly patients had decreased levels of inflammatory cytokines in plasma in the acute phase compared to those in young patients (11). The purpose of the present study was to examine if the acute-phase response in a human sepsis model differed between old and young individuals.
It is possible that gram-positive and gram-negative bacteria may induce different patterns of cytokine response. However, the only experimental sepsis model currently established in humans is the endotoxin model (6). We therefore applied this model to groups of healthy young people and of healthy elderly people. In this model, a standard reference Escherichia coli endotoxin is injected (2 to 4 ng/kg). We chose a dose of 2 ng/kg taking into account the fact that elderly individuals may not tolerate the same dosages as young subjects. As an expression of the acute-phase response, we measured changes in concentrations of a series of cytokines, including tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), soluble TNF receptors (sTNFR-I), IL-8, IL-10, and IL-1 receptor antagonist (IL-1ra), as well as C-reactive protein (CRP), in plasma. We also measured changes in body temperature.
MATERIALS AND METHODS
Volunteers.
Eight healthy young volunteers (five men, three women) with a median age of 24 years (range, 20 to 27 years) were compared to a group of nine healthy elderly individuals (seven men, two women) with a median age of 66 years (range, 61 to 69 years). All subjects had a negative medical history, and physical examination revealed no abnormalities. Blood analyses showed a normal white blood cell count (WBC), WBC differential count, and CRP and blood glucose levels, as well as normal kidney function, normal liver function, and normal coagulation system. All had a normal electrocardiogram (ECG). Furthermore, the old subjects underwent an exercise ECG that in all cases was found to be normal. The volunteers did not use any medication, and they did not have any febrile illness in the fortnight preceding the study.
Study design.
The study was performed in an Intensive Care Unit setting under the continuous supervision of an anesthesiologist, with emergency and resuscitation equipment immediately available. Rectal temperature, heart rate, intra-arterial blood pressure (disposable transducer [Baxter]), oxygen saturation, and lead II of the ECG were recorded continuously for at least 7 h after endotoxin administration (Hewlett-Packard eight-channel recorder). Isotonic saline solution was infused during the first 7 h of the study through an intravenous line at a rate of 15 ml/kg/h during the first hour and then at 7 ml/kg/h. The subjects were given an intravenous bolus of E. coli endotoxin 2 ng/kg of body weight. The study was approved by the regional scientific ethical committee, and written informed consent was obtained from each volunteer.
Blood sampling.
Blood was drawn before and 0.5, 1, 1.5, 2, 3, 4, 8, 12, and 24 h after injection for differential WBC counts and hemoglobin as well as for isolation of serum and plasma. Blood for other chemical analyses of liver and kidney function was drawn before and 4, 8, 12, and 24 h after injection.
Measurement of cytokine levels.
Blood samples were drawn into ice-cold tubes containing EDTA and Trasylol and centrifuged immediately thereafter. Plasma for cytokine detection was stored at −80°C until analyzed. The following cytokines were determined by enzyme-linked immunosorbent assay: TNF-α (detection limit, 0.5 pg/ml), sTNFR-I (7.8 pg/ml), IL-6 (0.156 pg/ml), IL-1ra (46.9 pg/ml), IL-8 (31.2 pg/ml), and IL-10 (0.781 pg/ml). All enzyme-linked immunosorbent assay kits were from R&D Systems, Minneapolis, Minn. All cytokine determinations were run as duplicates, and mean values were calculated.
Clinical chemistry tests.
Standard laboratory procedures were employed.
Statistics.
Statistical calculations were performed using SYSTAT statistical software version 7.0.1 (SYSTAT, Evanston, Ill.). Initial analyses revealed that concentrations of cytokines, monocytes, and CRP were not normally distributed. Therefore, these parameters were log transformed, and geometrical means are given. Absolute changes in parameters following endotoxin administration were evaluated by an analysis of variance (ANOVA) for repeated measurements (model parameter = time + age + age × time). If a significant interaction (age × time) was found, a two-sample t test for independent groups was used to detect age-related differences in absolute changes from baseline levels. In all tests, P < 0.05 was considered significant.
RESULTS
Temperature.
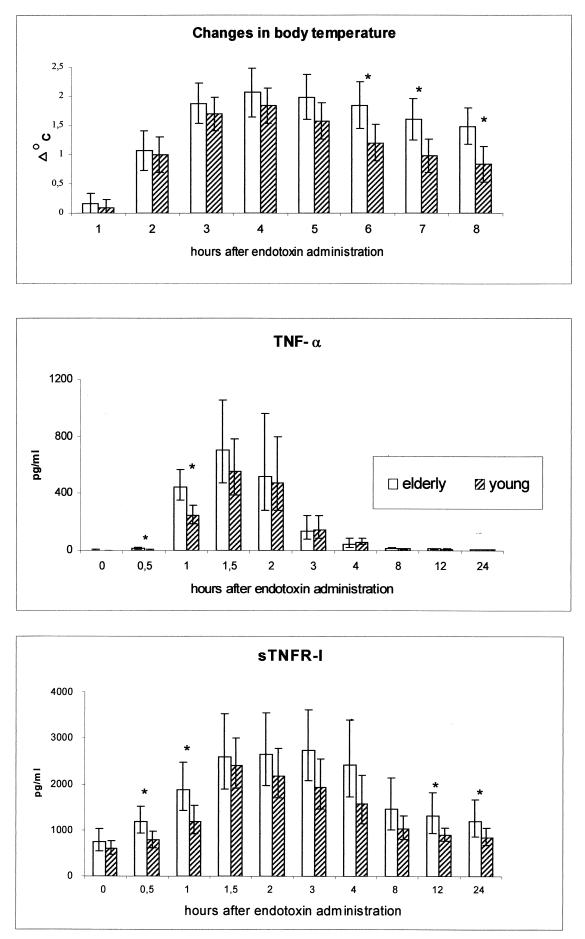
The temperature of the old versus young subjects did not differ at baseline. Furthermore, the maximal increase in temperature did not differ between the two groups. However, the old subjects had a prolonged fever response compared to the young (ANOVA, time × age, P = 0.006). Therefore, when the increase in temperature was compared at 6, 7, and 8 h postinjection, a significant difference was found (P = 0.027, 0.018, and 0.027, respectively) (Fig. 1).
FIG. 1.
Body temperature and circulating levels of TNF-α and sTNFR-I during human endotoxemia in young versus elderly subjects. Changes in body temperature from baseline in elderly subjects (n = 9) and young controls (n = 8) are shown at the top. Averages and 95% confidence intervals are shown. Concentrations of TNF-α in young (n = 8) and elderly (n = 8); subjects and concentrations of sTNFR-I in young (n = 8), elderly (n = 8) subjects are shown below. Geometric means and 95% confidence intervals are shown. ∗, significant difference between age groups in the changes from baseline values (p < 0.05).
TNF-α and sTNFR-I.
The concentrations of TNF-α and sTNFR-I are shown in Fig. 1. Both cytokines were detectable at baseline. The concentrations of TNF-α and sTNFR-I increased significantly in response to endotoxemia (P < 0.0005). The TNF-α level increased 256-fold (geometric mean) (range, 58- to 774-fold), reaching a maximum at 1.5 h after injection, whereas the sTNFR-I level increased 3.9-fold (geometric mean) (range, 2- to 5-fold) and reached a plateau at 1.5 to 3 h. The concentrations of TNF-α and sTNFR-I did not differ among groups at baseline, but the rate at which the concentration of both increased was different between groups (ANOVA, time × age; P < 0.0005 and P = 0.013, respectively). When the increase in TNF-α concentration was compared at 0.5 and 1 h, the elderly group had larger elevations than the young subjects did (P = 0.002 and 0.005, respectively). At 24 h there was a tendency toward higher levels of TNF-α in the elderly group (P = 0.07). The elderly group had significantly higher increases in the sTNFR-I level at 0.5, 1, 12, and 24 h (P = 0.007, 0.05, 0.02, and 0.038, respectively).
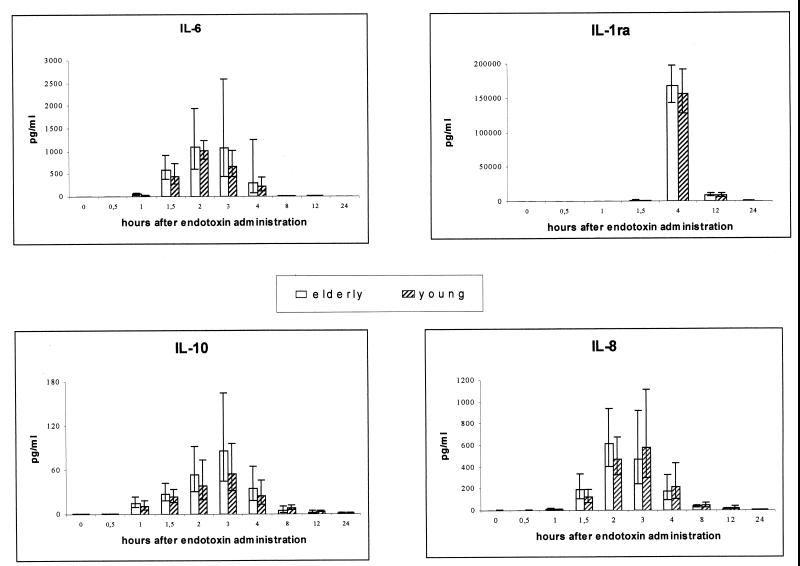
IL-6, IL-8, IL-10, and IL-1ra.
The concentrations of IL-6, IL-8, IL-10, and IL-1ra were detectable before endotoxin injection and peaked between 1.5 and 4 h after injection. Baseline levels of IL-1ra were higher in the elderly group than in the young group (geometric mean, 192.8 and 99.2 pg/ml, respectively; P = 0.002). The same tendency was found for IL-6 (geometric mean, 2.2 and 1.2 pg/ml, respectively; P = 0.059). The baseline levels of IL-8 and IL-10 did not differ between groups. There were no significant differences in the changes in the concentration of any of these cytokines between groups, although a graphical representation shows the same trend as described for TNF-α and s-TNFR-1 (Fig. 2).
FIG. 2.
Circulating levels of IL-6, IL-1ra, IL-10, and IL-8 during human endotoxemia in young versus elderly subjects. Abolute concentrations of IL-6, IL-1ra, IL-10, and IL-8 are shown in young (n = 8) and elderly (n = 8) humans during endotoxemia. Geometric means and 95% confidence intervals are shown.
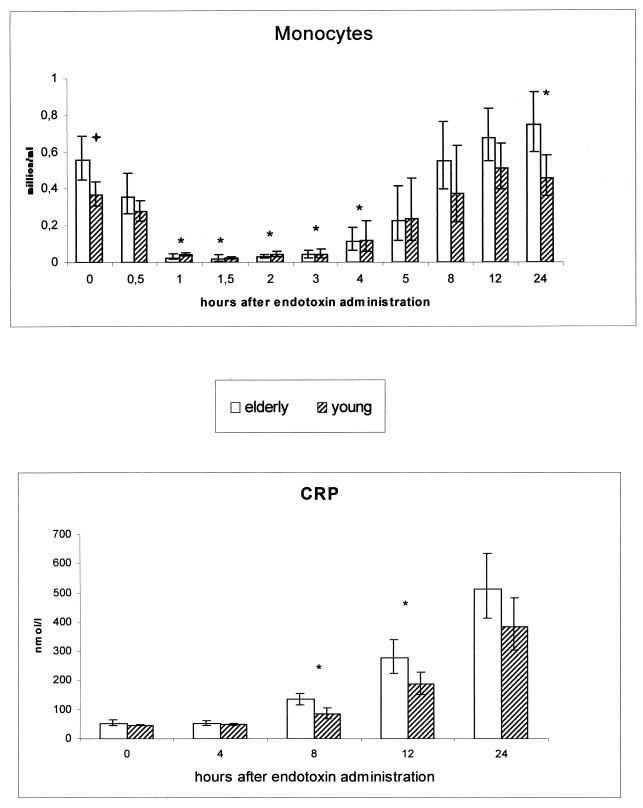
Monocytes and CRP.
The elderly subjects showed higher concentrations of monocytes at baseline than did the young persons (P = 0.012) (Fig. 3). The monocyte concentration decreased after the endotoxin infusion in both groups, to reach minimal levels at 1.5 h after injection. However, the elderly subjects showed a more rapid decrease and a slower subsequent increase (ANOVA, time × age; P = 0.057) in monocyte concentration than the young subjects did; significant differences in the changes in monocyte concentration were found at 1 to 4 h. Furthermore, 24 h after the endotoxin injection, both groups exhibited monocyte levels above baseline (elderly, P < 0.0005; young, P = 0.021).
FIG. 3.
Monocyte count and levels of CRP in young versus elderly subjects during human endotoxemia Absolute monocyte concentrations in young (n = 8) versus elderly (n = 9) humans during endotoxemia are shown at the top. Geometric means and 95% confidence intervals are given. Absolute concentrations of CRP in young (n = 8) and elderly (n = 9) subjects are shown at the bottom. Averages and 95% confidence intervals are shown. ∗, significant difference between age groups in the changes from baseline values (p < 0.05);  , difference between groups in baseline value.
, difference between groups in baseline value.
An increase in the level of CRP was not detectable until 8 h after injection. The CRP level also increased differently in the two age groups (ANOVA, time × age; P = 0.005). At 8 and 12 h, higher levels were found in the elderly subjects than in the young subjects (P = 0.011 and 0.048, respectively). Peak values at 24 h did not differ between the groups (Fig. 3).
DISCUSSION
The present study is the first to report the influence of ageing on the acute-phase response to an in vivo endotoxin challenge in humans. The two major findings were as follows. (i) Healthy elderly humans showed prolonged inflammatory activity compared to young subjects in response to endotoxemia in vivo. Thus, in these subjects, ageing was associated with a slower normalization of body temperature and lower rates of decrease in concentrations of TNF-α (borderline) as well as sTNFR-I in plasma. (ii) During the first hour after endotoxin administration, the elderly showed larger increases in the levels of TNF-α and sTNFR-I, concomitant with a more pronounced decrease in the number of monocytes. Furthermore, the initial production of CRP was more pronounced in the elderly.
Thus, these results indicate that the acute-phase response of healthy elderly humans varies from that of the young, showing initial hyperreactivity and a delayed termination of the response.
It should be stated that the elderly subjects in our study were selected to be exceptionally healthy and that their chronological age was not extreme. Therefore, our conclusions should not be extended to older age groups or elderly persons with medical disorders. Previous clinical studies have included older age groups of patients with underlying diseases, which may influence the immune response to infection. Furthermore, the present study includes eight plus nine subjects, which is a rather high n value for human endotoxin studies (6). However, elderly individuals demonstrate heterogeneity, and it cannot be excluded that this may skew our results.
Elderly patients with pneumococcal infections also have prolonged increases in the levels of TNF-α and sTNFR-I in plasma compared to those in young patients (5), in accordance with the present results. The slower decline in TNF-α and sTNFR-I levels in the elderly in our study was accompanied by prolonged fever and could in principle be due to a decreased clearing of endotoxin or proinflammatory cytokines. It is also possible that this phenomenon is due to insensitivity to feedback-inhibitory mechanisms or to an imbalance between the inflammatory and anti-inflammatory response. Interestingly, a tendency to a difference in the TNF-α/IL-10 ratio was found between age groups after 8 h (P = 0.06) and 12 h (P = 0.07); the elderly subjects showed higher ratios than the young subjects (data not shown). This would indicate a preponderance of inflammatory over anti-inflammatory activity in the elderly group.
The increased concentration of monocytes at baseline in the elderly subjects in our study might be responsible for the initially high endotoxin-induced cytokine levels in this group. However, when TNF-α levels were adjusted for the concentration of monocytes in blood at baseline, the elderly group still showed a pronounced elevation of TNF-α levels at 0.5 h compared to the young (data not shown).
One could speculate that the larger initial production of cytokines after endotoxin administration in the elderly might be a result of preceding in vivo activation of monocytes in the blood. This could be related to the decreased rate of termination of the inflammatory response, which could result in a constant low-grade activation of cytokine-producing cells, thus causing the observed hyperreactivity.
This contention is further supported by the finding that healthy elderly humans show low-grade inflammatory activity in the blood in vivo, including increased concentrations of IL-1ra (8) and IL-6 (3) circulating in the blood, as substantiated by the present study. Furthermore, studies have reported increased circulating levels of neopterin (8), TNF-α (4), sTNFR (4, 8), and acute-phase proteins (1) in elderly persons as well as increased unstimulated production of IL-1β (13), IL-6, and IL-1ra (14) in vitro.
It should be mentioned that there is an inverse relationship between levels of TNF-α in plasma and the concentration of monocytes in the blood. Thus, it cannot be excluded that other blood cells such as lymphocytes may contribute to cytokine production: it is well known that endotoxin causes polyclonal activation of B cells, and it has recently been shown that it induces strong stimulation of T cells in mice (7, 16). Alternatively, cells outside the blood may contribute to the increased initial production of cytokines in elderly subjects, for example due to arterial wall atherosclerosis, since increased release of TNF-α and IL-6 from the arterial walls of aged rats has been demonstrated in response to endotoxin in vitro (2).
In the present study we found no evidence of a decreased ability to produce fever or leukocytosis and detected no age-related differences with regard to peak elevations of body temperature or cytokine or CRP levels. Consistent with this, a study on mice did not show any age-associated differences in peak levels of TNF-α from two age groups of mice after administration of a sublethal endotoxin dose (9). Following lethal LPS doses, enhanced peak levels of TNF-α were found in old rodents; however, young animals showed even higher cytokine levels at endotoxin doses that were lethal for the old mice (9). Thus, it is possible that the lack of age-related differences in the peak response seen in the present human study was due to the use of a relatively low endotoxin dose. On the other hand, it is difficult to ascertain how well septic shock in mice imitates human septic shock, since mice are relatively resistant to bacterial toxins (12).
In conclusion, the acute-phase response to endotoxemia is altered in old subjects. The age-associated larger initial production of proinflammatory cytokines may be related to the well-documented low-grade inflammatory activity in the elderly, resulting in a state of increased hyperreactivity. The prolonged elevation of proinflammatory cytokine levels and body temperature in the elderly subjects may in turn be linked to an impaired anti-inflammatory response, which again may partly account for the constant low-grade inflammatory activity. The clinical significance of this remains unclear; e.g., it is not known whether a causal relationship exists between the cytokine and fever response and the increased mortality due to bacterial sepsis in aged patients (10, 15).
Elderly subjects maintain the ability to generate fever and to produce peak levels of cytokines comparable to the young. We therefore suggest that the phenomenon of afebrile bacteremia in elderly humans is connected to the presence of underlying diseases or is limited to the very old.
ACKNOWLEDGMENTS
The excellent assistance of Hanne Willumsen, Ruth Rousing, and Leila Jacobsen is acknowledged.
REFERENCES
- 1.Ballou S P, Lozanski F B, Hodder S, Rzewnicki D L, Mion L C, Sipe J D, Ford A B, Kushner I. Quantitative and qualitative alterations of acute-phase proteins in healthy elderly persons. Age Ageing. 1996;25:224–230. doi: 10.1093/ageing/25.3.224. [DOI] [PubMed] [Google Scholar]
- 2.Belmin J, Bernard C, Corman B, Merval R, Esposito B, Tedgui A. Increased production of tumor necrosis factor and interleukin-6 by arterial wall of aged rats. Am J Physiol. 1995;268:H2288–H2293. doi: 10.1152/ajpheart.1995.268.6.H2288. [DOI] [PubMed] [Google Scholar]
- 3.Blackwell T S, Christman J W. Sepsis and cytokines: current status. Br J Anaesth. 1996;77:110–117. doi: 10.1093/bja/77.1.110. [DOI] [PubMed] [Google Scholar]
- 4.Bruunsgaard H, Andersen-Ranberg K, Jeune B, Pedersen A N, Skinhoj P, Pedersen B K. A high plasma concentration of TNF-α is associated with dementia in centenarians. J Gerontol Ser A. 1999;54:M357–M364. doi: 10.1093/gerona/54.7.m357. [DOI] [PubMed] [Google Scholar]
- 5.Bruunsgaard H, Skinhoj P, Qvist J, Pedersen B K. Elderly humans show prolonged in vivo inflammatory activity during pneumococcal infections. J Infect Dis. 1999;180:551–554. doi: 10.1086/314873. [DOI] [PubMed] [Google Scholar]
- 6.Burrell R. Human responses to bacterial endotoxin. Circ Shock. 1994;43:137–153. [PubMed] [Google Scholar]
- 7.Castro A, Bemer V, Nobrega A, Coutinho A, Truffa-Bachi P. Administration to mouse of endotoxin from gram-negative bacteria leads to activation and apoptosis of T lymphocytes. Eur J Immunol. 1998;28:488–495. doi: 10.1002/(SICI)1521-4141(199802)28:02<488::AID-IMMU488>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 8.Catania A, Airaghi L, Motta P, Manfredi M G, Annoni G, Pettenati C, Brambilla F, Lipton J M. Cytokine antagonists in aged subjects and their relation with cellular immunity. J Gerontol Ser A. 1997;52:B93–B97. doi: 10.1093/gerona/52a.2.b93. [DOI] [PubMed] [Google Scholar]
- 9.Chorinchath B B, Kong L Y, Mao L, McCallum R E. Age-associated differences in TNF-alpha and nitric oxide production in endotoxic mice. J Immunol. 1996;156:1525–1530. [PubMed] [Google Scholar]
- 10.Finkelstein M S, Petkun W M, Freedman M L, Antopol S C. Pneumococcal bacteremia in adults: age-dependent differences in presentation and in outcome. J Am Geriatr Soc. 1983;31:19–27. doi: 10.1111/j.1532-5415.1983.tb06283.x. [DOI] [PubMed] [Google Scholar]
- 11.Gon Y, Hashimoto S, Hayashi S, Koura T, Matsumoto K, Horie T. Lower serum concentrations of cytokines in elderly patients with pneumonia and the impaired production of cytokines by peripheral blood monocytes in the elderly. Clin Exp Immunol. 1996;106:120–126. [PubMed] [Google Scholar]
- 12.Gutierrez-Ramos J C, Bluethmann H. Molecules and mechanisms operating in septic shock: lessons from knockout mice. Immunol Today. 1997;18:329–334. doi: 10.1016/s0167-5699(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 13.Riancho J A, Zarrabeitia M T, Amado J A, Olmos J M, Gonzalez M J. Age-related differences in cytokine secretion. Gerontology. 1994;40:8–12. doi: 10.1159/000213568. [DOI] [PubMed] [Google Scholar]
- 14.Roubenoff R, Harris T B, Abad L W, Wilson P F, Dallal G E, Dinarello C A. Monocyte cytokine production in an elderly population: effect of age and inflammation. J Gerontol Med Sci. 1998;53:M20–M26. doi: 10.1093/gerona/53a.1.m20. [DOI] [PubMed] [Google Scholar]
- 15.Torres J M, Cardenas O, Vasquez A, Schlossberg D. Streptococcus pneumoniae bacteremia in a Community Hospital. Chest. 1998;113:387–390. doi: 10.1378/chest.113.2.387. [DOI] [PubMed] [Google Scholar]
- 16.Tough D F, Sun S, Sprent J. T cell stimulation in vivo by lipopolysaccharide (LPS) J Exp Med. 1997;185:2089–2094. doi: 10.1084/jem.185.12.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]