Abstract
Functional foods such as mushrooms are rich in polyphenolic compounds and secondary metabolites with health-promoting properties such as antioxidant, antimicrobial, antidiabetic and immunostimulatory effects. The present study is aimed to investigate the ethanolic extracts of three varieties of mushrooms, namely, G. lucidum, G. tropicum, and C. indica grown in Bangladesh for phenolic and flavonoid content and their antioxidant properties. Moreover, the phenolic composition of the extracts was analyzed by using the HPLC-DAD system. G. lucidum extract exhibited the highest antioxidant potential as evidenced by its lowest IC50 value in all the tested assay models (40.44 ± 2.09 μg/mL, 151.32 ± 0.35 μg/mL, 137.89 ± 1.85 μg/mL in DPPH, H2O2, and NO scavenging assay, respectively) along with the highest phenolic content (81.34 ± 0.68 GAE g−1 extract). G. tropicum and C. indica extracts also showed significant antioxidant properties and a good amount of phenolic content, 52.16 ± 0.25 GAE g−1 extract, and 47.1 ± 0.26 GAE g−1 extract, respectively. The scavenging activity increased with the increasing concentration of extracts in all cases. The total phenolic content of the ethanolic extracts of mushroom species was highly correlated with antioxidant effects with Pearson's correlation coefficient (r) values ranging from 0.8883–0.9851. The α-amylase inhibitory and antibacterial activity of G. lucidum was evaluated by using 3,5-dinitrosalicylic acid and disc diffusion method, respectively. The maximum inhibitory activity recorded against α-amylase was 70.98 ± 0.042% at a concentration of 500 μg/mL. G. lucidum extract exhibited the highest antibacterial activity against Pseudomonas aeruginosa with 23.00 ± 1.00 mm clear zone of inhibition and an MIC value of 3.5 mg/mL. The results indicate that the mushroom species tested in this study could serve as a potential source of natural antioxidants in the development of nutraceuticals and herbal drugs for the management of oxidative stress-associated diseases as well as infectious diseases.
1. Introduction
Reactive oxygen species (ROS) are crucial for cellular activities at physiological concentration. However, excess production of reactive oxygen species and other free radicals may lead to oxidative stress—the imbalance between the generation and accumulation of reactive oxygen species. In addition, human beings are continuously exposed to free radicals produced from chemicals, radiation, and other environmental toxins [1]. Oxidative stress is responsible for the structural modification and the disruption of physiological function of cell components which may even lead to cell death [2]. Antioxidants convert the ROS to nonreactive oxygen species, interrupt the propagation of free radicals, and inhibit their formation to break radical chain reactions for the prevention of oxidative stress-related damage [3]. Endogenous antioxidant enzymes such as glutathione peroxidase, catalase, and superoxide dismutase also deactivate free radicals and maintain normal cellular functions [4]. However, under certain conditions, the endogenous biological antioxidant defense and repair systems become inadequate to prevent oxidative damage [4, 5] and contribute to diverse diseases such as cancer, atherosclerosis, diabetes, rheumatoid arthritis, and neurodegenerative disorders like Alzheimer's and Parkinson's disease, along with early aging process [3].
The consumption of exogenous antioxidants is pivotal to support the antioxidant defense system by maintaining a sufficient level of antioxidants. Synthetic antioxidants such as butylated hydroxy anisole (BHA), butylated hydroxytoluene (BHT), and propyl gallate (PG) have been used extensively. However, over time, safety concerns have been raised due to their associated adverse effects such as skin allergies, GI issues, and cancer [6]. This has necessitated the search for effective and safe natural antioxidants which can replace synthetic ones. Numerous studies have revealed the close association between polyphenol-rich food consumption and reduced risks of heart diseases, stroke, diabetes, and cancer [7–9].
In recent times, diverse antioxidant compounds have been detected in the Fungi kingdom [10, 11]. Mushrooms are widely consumed worldwide as a functional food in fresh as well as processed forms [12]. Apart from the rich aroma, taste, and high nutritional value, some varieties of mushrooms have been reported to possess anti-inflammatory, cholesterol-lowering, hepatoprotective, antibacterial, antiviral, antidiabetic immunomodulatory, and anticancer activities [13–15]. These properties are mainly attributed to the presence of a wide range of secondary metabolites including phenolics [1, 6, 7].
Mushrooms are cultivated and consumed worldwide due to their high nutritional content and therapeutic benefits [15]. Reports indicate that crude polysaccharides of a variety of mushroom C. indica, commonly known as the milky mushroom, might be therapeutically effective against oxidative stress and immunodeficiency disorders [16]. G. lucidum or Reishi mushroom is a popular medicinal mushroom due to its health-promoting effects such as antitumor, antimicrobial, anti-inflammatory, antidiabetic, and antioxidant activities [17, 18]. G. tropicum is one of the major wild Ganoderma species recorded in Chinese Pharmacopeia to treat cardiovascular diseases [19]. It is known that the active constituents and therapeutic potential of plants might vary among species [20, 21] and the cultivation conditions may also contribute to such variation [21, 22]. As commercial production of mushrooms is very recent in Bangladesh, very little information is available about the nutritional benefits and therapeutic potential of the varieties of mushrooms grown in Bangladesh [15]. Against this background, the current study aimed to assess the phytochemical constituents such as phenolic and flavonoid contents and evaluate the in vitro antioxidant activity of three locally grown varieties of mushroom—Calocybe indica, Ganoderma lucidum, and Ganoderma tropicum. The antioxidant potential has been evaluated using multiple in vitro models combined with HPLC analysis. In addition, two in vitro assays, the α-amylase inhibitory activity and the antibacterial activity, were performed to assess the biological activity of G. lucidum extract. Results obtained from the free radical scavenging activities have been examined for their correlation with phenolic contents towards the exploration of possible uses of this plant in the development of herbal drugs and nutraceuticals.
2. Materials and Methods
2.1. Sample Collection
Three species of mushrooms used in this study were collected from the National Mushroom Development & Extension Center, Sobhanbag, Dhaka-Aricha Highway, Savar, Bangladesh. The samples were identified and authenticated by the experts in the National Mushroom Development & Extension center. The Accession no. of the three species, C. indica, G. tropicum, and G. lucidum are ARMT 08, ARMT 04, and ARMT 018, respectively.
2.2. Preparation of Extracts
The fruiting bodies of mushrooms were cleaned and washed with distilled water to remove any residual compost. These were then air-dried to constant weight and ground into a coarse powder using a laboratory scale mill and blender. Dried and powdered samples (50.0 g) were extracted with 250 mL ethanol. The extraction was carried out for 7 days accompanied by occasional shaking and stirring. The extracts were then filtered first through a piece of clean, cotton material and finally through Whatman No. 1 filter paper. The residues were extracted again with 100 mL of ethanol. The combined extracts were evaporated to dryness under reduced pressure by a rotary vacuum evaporator. The dried extracts were stored at 4°C in a refrigerator until used for analysis.
2.3. Preliminary Phytochemical Screening
The ethanolic extracts of C. indica, G. tropicum, and G. lucidum were screened for the presence of phytoconstituents such as phenolic compounds, flavonoids, tannins, saponins, and alkaloids according to standard phytochemical screening methods [23]. Wagner's reagent and Molisch's reagent were utilized to test the presence of alkaloids and carbohydrates, respectively. Flavonoids and tannins were detected with concentrated HCl and FeCl3, respectively [20]. Salkowski's test was used to screen for saponins and the frothing test was employed for the detection of terpenoids [20]. Unless mentioned differently in individual tests, 10% (w/v) solution of the ethanolic extract was used in each test [24].
2.4. Estimation of Total Phenolic Content (TPC)
Total phenolic content in the extracts was determined by the Folin-Ciocalteu assay [23]. Briefly, 0.3 mL of the extract was mixed with 1.2 mL of the Folin-Ciocalteau reagent solution (10%, v/v) and 1.5 mL of sodium carbonate solution (7.5% w/v). The absorbance was measured at 765 nm after incubation of the reaction mixture for 1 hour. A calibration curve was constructed with varying concentrations (60-150 μg/mL) of gallic acid as standard. The results were expressed as mg of gallic acid equivalents (GAE) per gram extract.
2.5. Estimation of Total Flavonoid Content
The AlCl3 colorimetric method was used to estimate the total flavonoid content of the extracts [25]. Briefly, 0.5 mL of extract was mixed with 1.5 mL methanol, 0.1 mL 1 M potassium acetate solution, 2.8 mL distilled water, and 0.1 mL 10% aluminum chloride solution. To complete the reaction, the mixtures were incubated for 30 minutes at room temperature. The absorbance of the solutions was measured at 415 nm using a spectrophotometer. Total flavonoid content was calculated using quercetin standard calibration curve. Results were expressed as mg of Quercetin Equivalents (QE) per gram extract.
2.6. DPPH Radical Scavenging Assay
The scavenging activity of extracts was determined using DPPH free radical-scavenging assay [26]. Briefly, 0.1 mL of the extract at various concentrations was added to 2.9 mL of a 0.002% methanolic solution of DPPH. The mixture was incubated for 30 minutes in a dark place to complete the reaction at room temperature. The absorbance of the mixture at 517 nm was then measured. The capability to scavenge the DPPH radical was calculated using the following equation:
| (1) |
A 0 is the absorbance of the control and A1 is the absorbance in the presence of the sample.
2.7. H2O2 Scavenging Assay
The H2O2 scavenging activity of the extracts was evaluated as described by Reddy et al. [27]. In brief, a solution of H2O2 (40 mM) was prepared in 0.1 M phosphate buffer (pH 7.4), and 3.4 mL of each extract at varying concentrations was mixed with 0.6 mL H2O2 solution. The mixtures were incubated at room temperature and the absorbance of these mixtures was recorded at 230 nm. Ascorbic acid was used as the positive control. The H2O2 scavenging activity of the extracts and standard compounds was calculated as follows:
| (2) |
A 0 was the absorbance of the control, and A1 was the absorbance in the presence of the sample of extract/standard.
2.8. Nitric Oxide Scavenging Assay
Nitric oxide scavenging activity of the extracts was estimated based on the Griess Illosvoy reaction [28]. Briefly, a reaction mixture (3 mL) containing 10 mM sodium nitroprusside and the extract solutions at varying concentrations were prepared in phosphate buffer and incubated at 25°C for 2.5 h. This was followed by the addition of 1 mL of sulfanilic acid reagent to 0.5 mL of the incubated solution and allowed to stand for 5 min. The mixture was then incubated at room temperature for 30 min after the addition of 1 mL of naphthyl ethylene diamine dihydrochloride (0.1%). Absorbance of the reaction mixtures at 540 nm was recorded. Ascorbic acid was used as a standard. The nitric oxide radical scavenging activity of the extracts was expressed as % inhibition.
| (3) |
A 0 was the absorbance of the control, and A1 was the absorbance in the presence of the sample of extract/standard.
2.9. High Performance Liquid Chromatography (HPLC) System
HPLC-DAD analysis method was utilized to determine the phenolic composition of C. indica, G. lucidum, and G. tropicum extracts as described previously [29]. It was carried out on a Dionex UltiMate 3000 system equipped with a quaternary rapid separation pump (LPG-3400RS) and photodiode array detector (DAD-3000RS). Separation was performed using Acclaim® C18 (5 μm) Dionex column (4.6 x 250 mm) at 30°C with a flow rate of 1 mL/min and an injection volume of 20 μl. The mobile phase consisted of acetonitrile (solvent A), acetic acid solution pH 3.0 (solvent B), and methanol (solvent C) with the gradient elution program of 5%A/95%B (0-5 min), 10%A/90%B (6-9 min), 15%A/75%B/10%C (11-15 min), 20%A/65%B/15%C (16-19 min), 30%A/50%B/20%C (20-29 min), 40%A/30%B/30%C (30-35 min), and 100%A (36-40 min). The UV detector was set to 280 nm for 22.0 min, changed to 320 nm for 28.0 min, again changed to 280 nm for 35 min, and finally to 380 nm for 36 min and held for the rest of the analysis period while the diode array detector was set at an acquisition range from 200 nm to 700 nm. For the preparation of calibration curve, a standard stock solution was prepared in methanol containing arbutin (AR), (-)-epicatechin (ECA) (5 μg/mL each), gallic acid (GA), hydroquinone (HQ), vanillic acid (VA), rosmarinic acid (RA), myricetin (MC) (4 μg/mL each), caffeic acid (CA), Syringic acid (SA), vanillin (VL), trans-ferulic acid (FA) (3 μg/mL each), p-coumaric acid (PCA), quercetin (QU), kaempferol (KF) (2 μg/mL each), (+)-catechin hydrate (CH), ellagic acid (EA) (10 μg/mL each), trans-cinnamic acid (TCA) (1 μg/mL), rutin hydrate (RH) (6 μg/mL), and benzoic acid (BA) (8 μg/mL). A solution of the extract (10 mg/mL) was prepared in ethanol prior to HPLC analysis and all the solutions (mixed standards, sample, and spiked solutions) were filtered through 0.20 μm syringe filter (Sartorius, Germany) and then degassed in an ultrasonic bath (Hwashin, Korea) for 15 min. Data acquisition, peak integration, and calibrations were calculated with Dionex Chromeleon software (Version 6.80 RS 10).
2.10. Alpha Amylase Enzyme Inhibition Assay
The in vitro alpha amylase inhibitory activity of G. lucidum extract was determined by a modified Bernfeld's method [30]. Briefly, 200 μL of the test extract at concentrations ranging from 31.25-500 μg/mL was mixed with 200 μL of α-amylase enzyme solution and 200 μL of 2 mM of phosphate buffer (pH −6.9). After 20 minutes of incubation at room temperature, 200 μL of 1% starch solution was added to the reaction mixture and incubated further for 10 minutes at room temperature. To terminate the reaction, 200 μL of 3,5-dinitrosalicylic acid (DNSA) reagent was added and the tubes are incubated for 5 minutes in a boiling water bath. The mixture was allowed to cool at room temperature and diluted with 5 mL of distilled water. The absorbance was measured by a UV-spectrophotometer at 540 nm and the α-amylase inhibitory activity was calculated as percent inhibition using the following formula:
| (4) |
A 0 is the absorbance of the control, and A1 is the absorbance of the sample of extract/standard. Commercially available acarbose (Sugatrol 50 mg) was used as a standard.
2.11. Antibacterial Activity
2.11.1. Disc Diffusion Assay
Antibacterial activity of G lucidum extract was determined by the disc diffusion method against five bacterial species. The strains were maintained on agar slants at 4°C and activated by subculturing at 37°C for 24 h before screening [21]. The overnight cultures grown in broth were adjusted to an inoculum size of approximately 106CFU/mL following a 0.5 McFarland turbidity standard. Using a cotton swab, a suspension of the test microorganisms was swabbed on the Muller Hinton Agar (MHA) medium and the plates were allowed to dry for 5 min. For screening, sterile 6 mm diameter filter paper discs were impregnated with filter sterilized test extracts of G. lucidum (2 mg/disc) and placed on the surface of the seeded agar plates followed by incubation at 37°C for 24 h. Commercially available kanamycin discs (30 μg/disc) were used as reference standard. Discs prepared using the solvent (DMSO) served as the negative control. Antibacterial activity was evaluated by measuring the diameters of zones of inhibition.
2.11.2. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
The MIC of G. lucidum extract was determined by the broth dilution method in the Mueller Hinton broth supplemented with 10% glucose and 0.5% phenol red [22, 31]. The stock solution of the test extract was diluted to obtain concentrations from 1.0 mg/mL to 10 mg/mL. Each tube was then inoculated with an overnight culture of the appropriate bacterial strain diluted to give a final concentration of 106CFU/mL. The culture tubes were incubated aerobically at 37°C for 24 h. The MIC values were taken as the lowest concentration of the extract that inhibited bacterial growth or change in color from red to yellow, resulting from the formation of acidic metabolites that corresponds to microbial growth [20, 31]. Culture media without the sample extract and the others without bacteria served as controls. For determining the MBC value, a loopful of the culture medium from the broth MIC assay sample was spread on fresh MHA plates. After incubation at 37°C for 24 h, the MBC was recorded as the lowest concentration of the test sample with no bacterial growth [22].
2.12. Statistical Analysis
All analyses were carried out in triplicates and the data are expressed as mean ± standard deviation (SD). GraphPad Prism software and Microsoft Excel 2007 were used for statistical analyses. One-way ANOVA and appropriate post hoc tests were performed and differences were considered significant when p < 0.05. For evaluation of the possible correlation between phenolic content and the free radical activities of the extracts, Pearson's correlation analysis was carried out. The IC50 values were determined by linear regression analysis.
3. Results and Discussion
Qualitative phytochemical screening of the mushroom extracts showed the presence of various secondary metabolites in ethanolic extracts (Table 1).
Table 1.
Phytochemical analysis of the ethanolic extracts of the three mushroom species.
| Species | Flavonoid | Carbohydrate | Phenol | Tannin | Steroid | Saponin | Terpenoid | Alkaloid |
|---|---|---|---|---|---|---|---|---|
| C. indica | ++ | — | ++ | ++ | + | ++ | + | — |
| G. tropicum | ++ | + | ++ | + | + | — | + | — |
| G. lucidum | ++ | + | +++ | ++ | + | + | — | + |
‘++' denotes present in high concentration; ‘+' denotes present in small concentration; ‘-' denotes absent.
Total phenolic and flavonoid contents in the mushroom extracts, expressed as mg GAEs/g of extract and mg QEs/g of extract, respectively, are shown in Table 2. The total phenolic content (TPC) in G. lucidum extract (81.34 ± 0.68 mg GAE/g) was significantly higher than that in C. indica (52.16 ± 0.03 mg GAE/g) and G. tropicum (47.13 ± 0.23 mg GAE/g). TPC found in the ethanolic extract of locally grown G. lucidum was higher than that reported by Sheikh et al. (71.43 ± 0.94 mg GAE/g) [32] and by Celik et al. (49.52 ± 0.68 mg GAE/g) [33]. This suggests that G. lucidum could be used as a potential source of natural antioxidants. Total flavonoid content in the ethanolic extract of G. tropicum (29.31 ± 0.38 mg QE/g) was slightly higher than that in C. indica and G. lucidum (Table 2). Polyphenolics are one of the major contributors to the antioxidant properties of mushrooms [7–12]. HPLC-DAD system was used for the identification and quantification of polyphenols in the ethanolic extracts of the examined mushrooms species. The chromatographic separations of polyphenols in the tested extracts are shown in Figure 1. The content of each compound was calculated from the corresponding calibration curve and presented as the mean of five determinations as shown in Table 3. Compounds such as catechin, epicatechin, trans-ferulic acid, rosmarinic acid, and myricetin were detected from the fruiting bodies of C. indica (Table 3; Figure 1). High amount of myricetin (11.47 mg/100 g extract), a natural flavanol of significant pharmacological importance, was observed in C. indica extract. Antioxidant and free radical scavenging activities have been widely studied by researchers and interestingly, myricetin was reported to be more effective than α-tocopherol as an antioxidant in liposomes [34, 35]. Trans-ferulic acid (TFA) content was highest in G. lucidum extract (11.93 mg/100 g extract), followed by C. indica (8.85 mg/100 g extract) and G. tropicum (8.09 mg/100 g extract) extracts. TFA is known to exhibit anti-inflammatory, cytoprotective, and antioxidant effects and also prevents lipid peroxidation in biological systems [36]. Catechin was found in the highest amount in G. tropicum extract (10.53 mg/100 g extract) whereas epicatechin was highest in G. lucidum extract (8.67 mg/100 g extract). Due to their potent antioxidant properties, catechins are found to be beneficial in preventing and protecting from pathologies caused by oxidative stress [37]. These results demonstrate that the tested mushroom species contain several important polyphenols, which possess important pharmacological effects. Hence, these locally grown varieties of mushrooms could find potential use in the development of herbal medicines, nutraceuticals, and natural preservatives.
Table 2.
Total phenolic and flavonoid content in ethanolic extracts of the three mushroom species.
| Species | Total phenolic content Mg GAE/g extract |
Total flavonoid content Mg QEE/g extract |
|---|---|---|
| C. indica | 47.13 ± 0.23a | 29.17 ± 0.3a |
| G. tropicum | 52.16 ± 0.03b | 29.31 ± 0.38b |
| G. lucidum | 81.34 ± 0.68c | 29.2 ± 0.55c |
Results are expressed as mean ± SD (n = 3). Values in the same column followed by a different letter superscript (a-c) are significantly different (p < 0.05).
Figure 1.
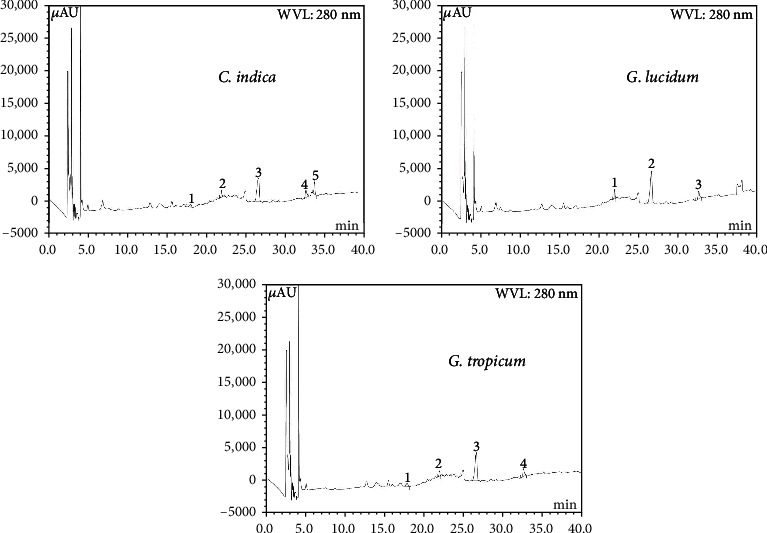
HPLC chromatogram of CI, GL, and GT extract. CI extract: Peaks: 1, (+)-catechin (CH); 2, (-)-epicatechin (ECA); 3, trans-ferulic acid (FA); 4, rosmarinic acid (RA). GL extract: Peaks: 1, (-)-epicatechin (ECA); 2, trans-ferulic acid (FA); 3, rosmarinic acid (RA). GT extract: Peaks: 1, (+)-catechin (CH); 2, (-)-epicatechin (ECA); 3, trans-ferulic acid (FA); 4, rosmarinic acid (RA).
Table 3.
Contents of polyphenolic compounds in the three mushroom extracts (n = 5).
| Polyphenolic Compound |
C. indica extract | G. lucidum extract | G. tropicum extract | |||
|---|---|---|---|---|---|---|
| Content (mg/100 g of dry extract) | % RSD | Content (mg/100 g of dry extract) | % RSD | Content (mg/100 g of dry extract) | % RSD | |
| CH | 9.03 | 0.07 | 10.53 | 0.08 | ||
| ECA | 7.61 | 0.05 | 8.67 | 0.06 | 5.66 | 0.03 |
| FA | 8.85 | 0.07 | 11.93 | 0.13 | 8.09 | 0.05 |
| RA | 2.72 | 0.02 | 2.75 | 0.02 | 2.61 | 0.01 |
| MC | 11.47 | 0.10 | ||||
CH (catechin), ECA (Epicatechin), FA (trans-ferulic acid), RA (rosmarinic acid), MC (myricetin).
Investigation of antioxidant activity through multiple methods accounts for various mechanisms for antioxidant action, and thereby provides a strong assessment of the antioxidant potential of any sample. Antioxidants differ in their chemical nature as well as scavenging mechanisms such as elimination of peroxide, free radical-mediated chain termination, and hydrogen donation [38]. Hence, the use of multiple methods is required for evaluation of the antioxidant potential of plant extracts. In this study, three complimentary test systems, DPPH radical scavenging assay, H2O2 scavenging assay, and nitric oxide scavenging assay, have been utilized to assess the antioxidant capacity of ethanolic extracts of C. indica, G. lucidum, and G. tropicum. The scavenging potential of the extracts was expressed in terms of IC50 values (Table 4). A stronger ability to scavenge the free radical is indicated by a lower IC50 value.
Table 4.
IC50 (μg/mL) values of various ethanolic mushroom extracts and standard in different antioxidant assays.
| Extracts | DPPH | H2O2 | NO |
|---|---|---|---|
| C. indica | 135.17 ± 1.67a | 221.96 ± 1.42a | 219.96 ± 1.41a |
| G. tropicum | 81.34 ± 0.79b | 183.27 ± 1.25b | 315.74 ± 2.27b |
| G. lucidum | 40.44 ± 2.1c | 151.32 ± 0.35c | 137.89 ± 1.85c |
| Standard∗ | 10.78 ± 0.65d | 61.01 ± 1.59d | 83.58 ± 1.51d |
∗Positive reference standard (ascorbic acid). Results are expressed as mean ± SD (n = 3).
Values in the same column with different letter superscript letter (a-c) indicate that they are significantly different (p < 0.05).
DPPH scavenging assay is based on the ability of DPPH to react with proton donors. DPPH, the stable free radical, possesses a characteristic absorption at 517 nm which decreases when it reacts with the antioxidants. This decrease in absorption is determined as a measure of the extent of free radical scavenging capability. The use of DPPH radical is advantageous as this free radical is unaffected by side reactions including enzyme inhibition [39]. Figure 2 shows the concentration-dependent DPPH scavenging activity of the mushroom extracts (R2 = 0.7836 − 0.8086). The scavenging activity of the extracts followed the sequence G. lucidum > G. tropicum > C. indica. At a concentration of 500 μg/mL, the scavenging ability of the G. lucidum extract is 93.78 ± 0.42%, whereas the value was 81.43 ± 0.67% for C. indica and 87.01 ± 0.30% for G. tropicum. The lowest activity was observed in G. tropicum having 30.76 ± 0.24% scavenging at the concentration of 31.25 μg/mL. G. lucidum extract also showed lowest IC50 value (40.44 ± 2.1 μg/mL) among the mushroom extracts tested (Table 4), indicating the strongest scavenging potential. Standard compound ascorbic acid showed IC50 value of 10.78 ± 0.65 μg/mL indicating stronger scavenging activity as compared to the mushroom extracts (p < 0.05). Varied scavenging effectiveness of G. lucidum extracts has been reported previously. Sheikh et al. from India reported DPPH scavenging efficiency for ethanolic extract of G. lucidum with IC50 of 13.615 μg/mL [32] while the methanolic extract from G. lucidum [40] was reported to have 142.84 ± 71.84 μg/mL. Celik et al. [33] from Turkey reported an IC50 value of 7.03 ± 0.07 μg/mL with ethanolic extract of G. lucidum. Such variations in scavenging activity could be attributed to geographical origin, environmental growth conditions and the type of solvents used for extraction.
Figure 2.
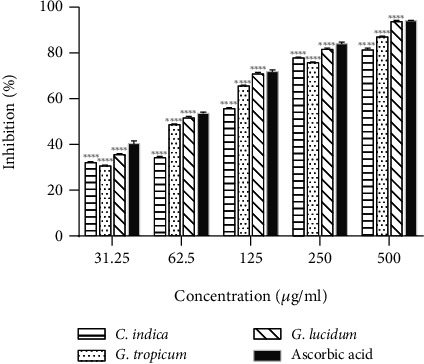
DPPH radicals scavenging effects of various ethanolic mushroom extracts in comparison with ascorbic acid. Results are expressed as mean ± SD (n = 3). ∗∗∗∗p < 0.0001 vs. standard (ascorbic acid) at each concentration.
All the mushroom extracts were efficient in scavenging hydrogen peroxide (Figure 3) in a dose-dependent manner (R2 = 0.7953 − 0.8975). As shown in Figure 3, G. lucidum showed the highest free radical inhibition at all the tested concentrations whereas C. indica extract exhibited the lowest efficiency. Hydrogen peroxide results in tissue damage in living systems [21] due to its rapid conversion into hydroxyl free radicals (OH) [24]. The ability of mushroom extracts to scavenge H2O2 indicates their cytoprotective activity, which could be of potential use in the development of herbal medicines. IC50 value of G. lucidum extract (151.32 ± 0.35 μg/mL, Table 4) obtained in this study was significantly lower than a previously reported value of 822.188 μg/mL by Radhika et al. from India [41] for ethanol extract of G. lucidum. The strong hydrogen peroxide scavenging efficiency of G. lucidum as demonstrated in the present study could be attributed to its highest total phenolic content (Table 2).
Figure 3.
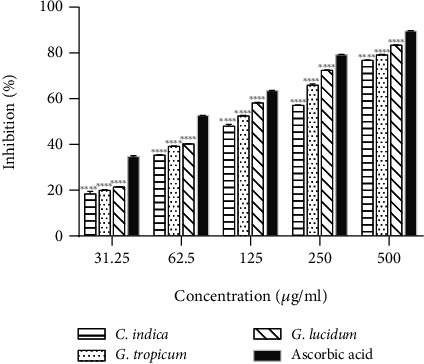
H2O2 radicals scavenging effects of various ethanolic mushroom extracts in comparison with ascorbic acid. Results are expressed as mean ± SD (n = 3). ∗∗∗∗p < 0.0001, ∗∗p < 0.01 vs. standard (ascorbic acid) at each concentration.
Nitric oxide, an unstable free radical, is known to induce various inflammatory responses leading to tissue toxicity, carcinoma, and pathological conditions such as multiple sclerosis, arthritis, and ulcerative colitis. Nitric oxide reacts with oxygen and generates stable nitrate and nitrites in cells. The free radical scavengers compete with nitric oxide for oxygen and inhibit the overproduction of nitrite ions which mitigates the propagation of inflammation [42]. All the mushroom extracts tested in this study efficiently scavenged nitric oxide radical in a dose-dependent manner (R2 = 0.8168 − 0.9346). The highest NO scavenging activity was exhibited by G. lucidum extract (Figure 4) with IC50 value of 137.89 ± 1.85 μg/mL (Table 4), which is significantly lower than that reported by Radhika et al. from India (860.184 μg/mL) [41] for NO scavenging by ethanolic extract of G. lucidum. The results demonstrate that G. lucidum possesses strong NO scavenging property. In this study, G. lucidum extract scavenged 52% nitric oxide radical at 100 μg/mL, which is lower than that reported by Sheikh et al. (63%) [32] from India at the same concentration. These variations in scavenging efficiency in different studies may be attributed to the difference in growth conditions, source, handling, and time of extraction.
Figure 4.
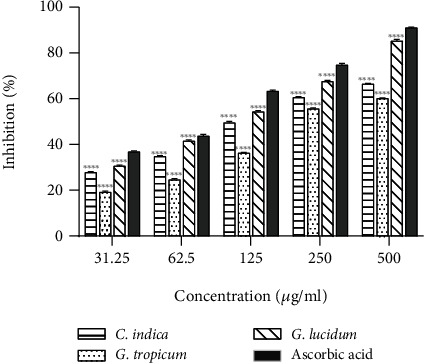
NO radicals scavenging effects of various ethanolic mushroom extracts in comparison with ascorbic acid. Results are expressed as mean ± SD (n = 3). ∗∗∗∗p < 0.0001 vs. standard (ascorbic acid).
Total phenols are considered as the major natural antioxidant components and contributors to the biological activities of medicinal plants including mushrooms [20, 32, 41]. In this study, the correlation between total phenolic content in the mushroom extracts and antioxidant activity was evaluated. Pearson's correlation coefficient (r) and coefficient of determination (R2) were determined for each assay model. As shown in Figure 5, a positive correlation was observed between total phenolic content (TPC) and 1/IC50 values of extracts in antioxidant assays with the Pearson value (r) ranging from 0.8883 to 0.9851. Strong positive correlation was found between TPC and DPPH scavenging (r = 0.9851, R2 = 0.9705) and H2O2 scavenging (r = 0.9449, R2 = 0.8928) activity. Relatively lower correlation was observed with NO scavenging (r = 0.8883, R2 = 0.7890) activity.
Figure 5.
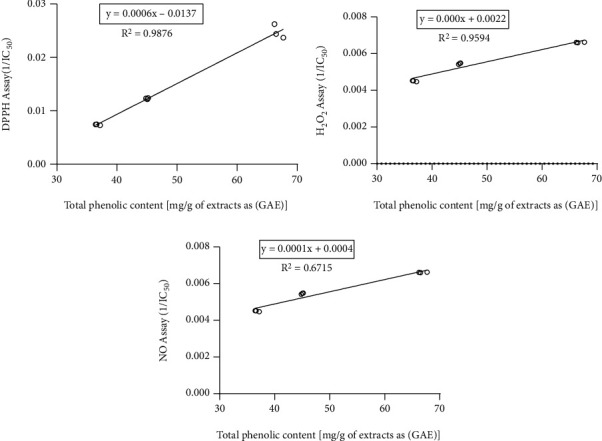
Correlation between total phenolic content and (a) DPPH radical scavenging activity of the extracts. Correlation coefficient, r = 0.9851 and coefficient of determination, R2 = 0.9705. (b) H2O2 radical scavenging activity of the extracts. Correlation coefficient, r = 0.9449 and coefficient of determination, R2 = 0.8928. (c) NO radical scavenging activity of the extracts. Correlation coefficient, r = 0.8883 and coefficient of determination, R2 = 0.7890.
The results obtained in this study are in line with published studies that report correlation between the antioxidant activities and total phenolics, thereby suggesting phenolics as the major contributor towards the antioxidant activity [32, 43, 44] in mushrooms. G. lucidum extract demonstrated the highest scavenging activity compared to other extracts in all assay models used in this study, which could be attributed to its high total phenolic content. In order to further explore the medicinal effects of G. lucidum extract, two in vitro biological assays were performed, namely, the α-amylase inhibitory activity and antibacterial activity. Use of antioxidants has been found effective in reducing the severity of complications linked with oxidative stress-associated diseases such as diabetes [45]. G. lucidum supplementation has exhibited reduced fasting blood sugar, glycosylated hemoglobin, and improved insulin resistance in animal models [46]. Previously, some studies have reported aldose reductase, α-glucosidase, and α-amylase inhibitory activities of G. lucidum [18, 40, 46–48]. The α-amylase enzyme is normally found in the pancreatic juice and saliva which helps to break down starch into absorbable molecules. Inhibitors of this enzyme delay the breakdown of carbohydrates in the small intestine and help to control the postprandial blood glucose elevation [49], hence are valuable to diabetic patients. In this study, ethanolic extract of G. lucidum showed 70.98 ± 0.042% of α-amylase inhibition (Figure 6) at the highest test concentration (500 μg/mL). The extract exhibited a dose-dependent increase in the percentage of enzyme inhibition. The standard, acarbose, showed an IC50 value of 88.05 ± 2.96 μg/mL, whereas the G. lucidum extract inhibited the enzyme with an IC50 value of 124.41 ± 3.40 μg/mL. This value found for G. lucidum is lower than that reported by Uddin et al. [50] for methanolic mushroom extract (386.04 μg/mL) indicating stronger α-amylase inhibitory activity of the ethanolic extract. The results obtained from α-amylase inhibition assay suggest that G. lucidum can be used as a functional food for diabetics and also serve as a natural source of antidiabetic drugs.
Figure 6.
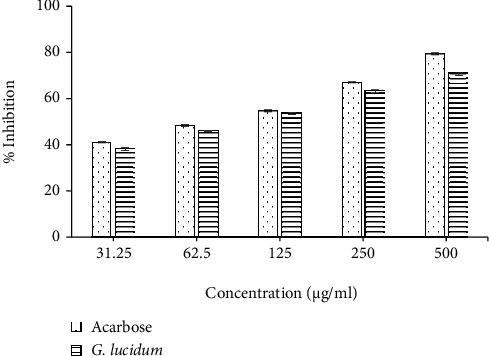
α-Amylase inhibitory activity of ethanolic extract of G. lucidum in comparison with the standard (Acarbose). Results are expressed as mean ± SD (n = 3).
The antibacterial activity of G. lucidum extract was evaluated against five bacterial strains (Table 5) by disc diffusion method. Maximum antibacterial activity was recorded against Pseudomonas aeruginosa and Escherichia coli showing clear zones of inhibition of 23.00 ± 1.00 and 22.67 ± 1.15 mm, respectively. The effect was similar to the standard drug kanamycin against Pseudomonas aeruginosa (Table 5), a gram-negative bacterium considered to be responsible for infections in the lung, skin, eye, and urinary tract [20]. This suggests that G. lucidum may find applications in treating infections caused by Pseudomonas aeruginosa. Significant activities were also recorded against Salmonella typhi (16.67 ± 1.15 mm) and Shigella flexneri (16.33 ± 1.15 mm), and the results were comparable with kanamycin (Table 5). However, no antibacterial effect was observed against Staphylococcus aureus in this study. The standard drug, kanamycin showed zones of inhibition ranging from 18.33 ± 0.58 to 27.00 ± 1.00 mm against all the test organisms. The negative control, solvent DMSO, showed no inhibitory effect on the organisms used in this study. Our results are in line with the previous studies [51, 52] that reported the antibacterial property of G. lucidum. In this study, ethanol extract of locally grown G. lucidum showed higher activity against bacteria S. aureus, E. coli, S. typhi, and P. aeruginosa compared to that in a study by Quereshi et. al, [51] which indicates the local variety of G. lucidum could serve as a potential source of antimicrobial agents. The significant antibacterial effect exhibited by G. lucidum extract could be attributed to the presence of polyphenolics and compounds such as alkaloids, tannins, and saponins as revealed through phytochemical screening. MIC and MBC values were determined against organisms for which antimicrobial activity was observed in the zone inhibition assay. G. lucidum extract exhibited the lowest MIC and MBC values of 3.5 and 4.5 mg/mL, respectively, against both E. coli. and P. aeruginosa. MIC and MBC values of the extract were found to be 4.0 and 5.5 mg/mL, respectively, against S. typhi while against S. flexneri, MIC and MBC values obtained were 4.5 and 6.5 mg/mL, respectively. Varying MIC values ranging between 0.20 mg/ml -8.0 mg/ml for different solvent extracts of G. lucidum have been reported previously [52, 53] against various organisms. A study on methanolic extracts of 3 edible mushrooms (Pleurotus ostreatus, Lentinula edodes, and Hypsizigus tessulatus) cultivated in Bangladesh reported MIC values ranging from 1 mg/mL to 9 mg/mL against test organisms [54]. Variation in source, species, and the choice of extracting solvent account for the differences in pharmacological activities [22]. Hence, further studies using different solvent extracts and a wider range of test organisms could provide more insights into the antibacterial potential of locally grown G. lucidum.
Table 5.
Antibacterial activity of G. lucidum extract.
| Tested bacteria | Zone of inhibition (mm) | |
|---|---|---|
| G. lucidum extract | Standard (kanamycin) | |
| Staphylococcus aureus (ATCC 25923) | 00 | 22.33 ± 0.58 |
| Escherichia coli (ATCC 25922) | 22.67 ± 1.15 | 27.00 ± 1.00 |
| Pseudomonas aeruginosa (ATCC 27853) | 23.00 ± 1.00 | 23.33 ± 0.57 |
| Salmonella typhi (ATCC 6539) | 16.67 ± 1.15 | 20.67 ± 0.58 |
| Shigella flexneri (ATCC 12022) | 16.33 ± 1.15 | 18.33 ± 0.58 |
4. Conclusion
The present study revealed that Calocybe indica, Ganoderma lucidum, and Ganoderma tropicum possess significant antioxidant and free radical scavenging activities which could be correlated to their phenolic content. Among the three local varieties of mushroom tested, G. lucidum exhibited the highest antioxidant potential and also demonstrated significant α-amylase inhibitory and antibacterial activities. This suggests that locally grown varieties of mushrooms especially, G. lucidum could serve as a potential source of natural antioxidants and antibacterial agents. Inclusion of these mushrooms in diet and as a functional food could help in alleviating pathologies linked with oxidative stress-associated diseases such as diabetes, degenerative disorders, and aberrant immune response. The significant antibacterial activity demonstrated by G. lucidum extracts against gram-negative bacteria used in this study indicates that it can be exploited as a natural drug in the treatment of several infectious diseases. However, isolation and identification of the specific compounds responsible for the biological activities and in vivo studies are needed for better clinical applications.
Acknowledgments
The authors sincerely thank North South University, Bangladesh for providing facilities to carry out this study (CTRG-20/SHLS/35).
Contributor Information
Hasan Mahmud Reza, Email: hasan.reza@northsouth.edu.
Preeti Jain, Email: preti.jain@northsouth.edu.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Panthi M., Subba R. K., Raut B., Khanal D. P., Koirala N. Bioactivity evaluations of leaf extract fractions from young barley grass and correlation with their phytochemical profiles. BMC Complementary Medicine and Therapies . 2020;20(1):p. 64. doi: 10.1186/s12906-020-2862-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pizzino G., Irrera N., Cucinotta M., et al. Oxidative Stress: harms and benefits for human health. Oxidative Medicine and Cellular Longevity . 2017;2017:13. doi: 10.1155/2017/8416763.8416763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharifi-Rad M., Kumar N. V. A., Zucca P., et al. Lifestyle, oxidative stress, and antioxidants: back and forth in the pathophysiology of chronic diseases. Frontiers in Physiology . 2020;11(694) doi: 10.3389/fphys.2020.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aryal S., Baniya M. K., Danekhu K., Kunwar P., Gurung R., Koirala N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from western Nepal. Plants . 2019;8(4):p. 96. doi: 10.3390/plants8040096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Modi B., Koirala N., Aryal S. P., et al. Tinospora cordifolia (Willd.) Miers: phytochemical composition, cytotoxicity, proximate analysis and their biological activities. Cellular and Molecular Biology . 2021;67(1):50–57. doi: 10.14715/cmb/2021.67.1.8. [DOI] [PubMed] [Google Scholar]
- 6.Lourenço S. C., Moldão-Martins M., Alves V. D. Antioxidants of natural plant origins: from sources to food industry applications. Molecules . 2019;24(22, article 4132) doi: 10.3390/molecules24224132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gan C. H., Nurul A. B., Asmah R. Antioxidant analysis of different types of edible mushrooms (Agaricus bisporus and Agaricus brasiliensis) International Food Research Journal . 2013;20:1095–1102. [Google Scholar]
- 8.Khurana S., Venkataraman K., Hollingsworth A., Piche M., Tai T. Polyphenols: benefits to the cardiovascular system in health and in aging. Nutrients . 2013;5(10):3779–3827. doi: 10.3390/nu5103779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tressera-Rimbau A., Arranz S., Eder M., Vallverdú-Queralt A. Dietary polyphenols in the prevention of stroke. Oxidative Medicine and Cellular Longevity . 2017;2017:10. doi: 10.1155/2017/7467962.7467962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chun S., Gopal J., Muthu M. Antioxidant activity of mushroom extracts/polysaccharides—their antiviral properties and plausible antiCOVID-19 properties. Antioxidants . 2021;10(12, article 1899) doi: 10.3390/antiox10121899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yildiz O., Can Z., Laghari A., Şahin H., Malkoç M. Wild edible mushrooms as a natural source of phenolics and antioxidants. Journal of Food Biochemistry . 2015;39(2):148–154. doi: 10.1111/jfbc.12107. [DOI] [Google Scholar]
- 12.Ferrari G. P., Soares A. A., Bazanella G. C. D., et al. Mushrooms: Types, Properties and Nutrition . New York, USA: Nova Science Publishers, Inc.; 2012. Antioxidant properties of the most common edible mushrooms consumed in Brazil; pp. 285–297. [Google Scholar]
- 13.Blagodatski A., Yatsunskaya M., Mikhailova V., Tiasto V., Kagansky A., Katanaev V. Medicinal mushrooms as an attractive new source of natural compounds for future cancer therapy. Oncotarget . 2018;9(49):29259–29274. doi: 10.18632/oncotarget.25660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valverde M., Hernández-Pérez T., Paredes-López O. Edible mushrooms: improving human health and promoting quality life. International Journal of Microbiology . 2015;2015:14. doi: 10.1155/2015/376387.376387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferdousi J., Riyadh Z., Hossain M., Saha S., Zakaria M. Mushroom production benefits, status, challenges and opportunities in Bangladesh: a review. Annual Research & Review in Biology . 2020;34(6):1–13. doi: 10.9734/arrb/2019/v34i630169. [DOI] [Google Scholar]
- 16.Ghosh S., Khatua S., Dasgupta A., Acharya K. Crude polysaccharide from the milky mushroom, Calocybe indica, modulates innate immunity of macrophage cells by triggering MyD88-dependent TLR4/NF-κB pathway. Journal of Pharmacy and Pharmacology . 2020;73(1):70–81. doi: 10.1093/jpp/rgaa020. [DOI] [PubMed] [Google Scholar]
- 17.Cör D., Knez Ž., Hrnčič M. K. Antitumour, antimicrobial, antioxidant and antiacetylcholinesterase effect of Ganoderma lucidum terpenoids and polysaccharides: a review. Molecules . 2018;23(3):p. 649. doi: 10.3390/molecules23030649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma H., Hsieh J., Chen S. Anti-diabetic effects of _Ganoderma lucidum_. Phytochemistry . 2015;114:109–113. doi: 10.1016/j.phytochem.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 19.Zhang S., Wang Y., Ma Q., et al. Three new lanostanoids from the mushroom Ganoderma tropicum. Molecules . 2015;20(2):3281–3289. doi: 10.3390/molecules20023281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jain P., Hossain K. R., Mishu T. R., Reza H. M. Antioxidant and antibacterial activities of Spondias pinnata Kurz. leaves. European Journal of Medicinal Plants . 2014;4(2):183–195. doi: 10.9734/EJMP/2014/7048. [DOI] [Google Scholar]
- 21.Jain P., Hossain K. R., Islam T., Gias Z. T., Hossain M., Reza H. M. Antioxidant and antibacterial activities of different solvent extracts of Citrus acida leaf and correlation with phenolic content. Medicinal plants - International Journal of Phytomedicines and Related Industries . 2021;13(2):302–312. doi: 10.5958/0975-6892.2021.00034.4. [DOI] [Google Scholar]
- 22.Jain P., Haque A., Islam T., Alam M. A., Reza H. M. Comparative evaluation of Ziziphus mauritiana leaf extracts for phenolic content, antioxidant and antibacterial activities. Journal of Herbs, Spices & Medicinal Plants . 2019;25(3):236–258. doi: 10.1080/10496475.2019.1600627. [DOI] [Google Scholar]
- 23.Sánchez-Rangel J., Benavides J., Heredia J., Cisneros-Zevallos L., Jacobo-Velázquez D. The Folin–Ciocalteu assay revisited: improvement of its specificity for total phenolic content determination. Analytical Methods . 2013;5(21):p. 5990. doi: 10.1039/c3ay41125g. [DOI] [Google Scholar]
- 24.Jain P., Hossain M. S., Fatema K., et al. Anti-Inflammatory, analgesic and antioxidant activities of Allophylus cobbe leaves. American Journal of Pharmacology and Toxicology . 2014;9(4):223–231. doi: 10.3844/ajptsp.2014.223.231. [DOI] [Google Scholar]
- 25.Kumaran A., Karunakaran R. J. In vitro antioxidant activities of methanol extracts of five _Phyllanthus_ species from India. LWT-Food Science and Technology . 2007;40(2):344–352. doi: 10.1016/j.lwt.2005.09.011. [DOI] [Google Scholar]
- 26.Braca A., Tommasi N. D., Bari L. D., Pizza C., Politi M., Morelli I. Antioxidant principles from Bauhinia tarapotensis. Journal of Natural Products . 2001;64(7):892–895. doi: 10.1021/np0100845. [DOI] [PubMed] [Google Scholar]
- 27.Reddy G. M., Rao V., Sarma D., Reddy T. K., Subramanyam P., Naidu M. D. Evaluation of antioxidant activity index (AAI) by the 2, 2-diphenyl-1-picryl hydrazyl method of 40 medicinal plants. Journal of Medicinal Plants Research . 2012;6(24) [Google Scholar]
- 28.Johnson E. The quantitative analysis of drugs- by D. C. Garratt. Journal of Pharmacy and Pharmacology . 1964;16(11):772–772. [Google Scholar]
- 29.Jahan I. A., Akbar P. N., Khan N., et al. Comparative study of anti-nociceptive activity and phenolic content of the ethanol extracts of Piper nigrum and Piper longum fruits. International Journal of Pharmaceutical Sciences Review and Research . 2014;27(7):47–52. [Google Scholar]
- 30.Kwon Y., Apostolidis E., Shetty K. Inhibitory potential of wine and tea against α-amylase and α-glucosidase for management of hyperglycemia linked to type 2 diabetes. Journal of Food Biochemistry . 2008;32(1):15–31. doi: 10.1111/j.1745-4514.2007.00165.x. [DOI] [Google Scholar]
- 31.Mbosso E., Ngouela S., Nguedia J., Beng V., Rohmer M., Tsamo E. In vitro_ antimicrobial activity of extracts and compounds of some selected medicinal plants from Cameroon. Journal of Ethnopharmacology . 2010;128(2):476–481. doi: 10.1016/j.jep.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 32.Sheikh I. A., Vyas D., Ganaie M. A., Dehariya K., Singh V. HPLC determination of phenolics and free radical scavenging activity of ethanolic extracts of two polypore mushrooms. International Journal of Pharmacy and Pharmaceutical Sciences . 2014;6:679–684. [Google Scholar]
- 33.Celık G. Y., Onbaslı D., Altınsoy B., Allı H. In vitro antimicrobial and antioxidant properties of Ganoderma lucidum extracts grown in Turkey. European Journal of Medicinal Plants . 2014;4(6):709–722. doi: 10.9734/EJMP/2014/8546. [DOI] [Google Scholar]
- 34.Barzegar A. Antioxidant activity of polyphenolic myricetin in vitro cell- free and cell-based systems. Molecular Biology Research Communications . 2016;5(2):87–95. [PMC free article] [PubMed] [Google Scholar]
- 35.Gordon M., Roedig-Penman A. Antioxidant activity of quercetin and myricetin in liposomes. Chemistry and Physics of Lipids . 1998;97(1):79–85. doi: 10.1016/S0009-3084(98)00098-X. [DOI] [PubMed] [Google Scholar]
- 36.Rezaeiroshan A., Saeedi M., Morteza-Semnani K., et al. Vesicular formation of trans-ferulic acid: an efficient approach to improve the radical scavenging and antimicrobial properties. Journal of Pharmaceutical Innovation . 2022;17:652–661. doi: 10.1007/s12247-021-09543-8. [DOI] [Google Scholar]
- 37.Bernatoniene J., Kopustinskiene D. The role of catechins in cellular responses to oxidative stress. Molecules . 2018;23(4):p. 965. doi: 10.3390/molecules23040965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karadag A., Ozcelik B., Saner S. Review of methods to determine antioxidant capacities. Food Analytical Methods . 2009;2(1):41–60. doi: 10.1007/s12161-008-9067-7. [DOI] [Google Scholar]
- 39.Pal J., Ganguly S., Tahsin K. S., Acharya K. In vitro free radical scavenging activity of wild edible mushroom, Pleurotus squarrosulus (Mont.) Singer. Indian Journal of Experimental Biology . 2010;48(12):1210–1218. [PubMed] [Google Scholar]
- 40.Fatmawati S., Shimizu K., Kondo R. Ganoderol B: a potent α-glucosidase inhibitor isolated from the fruiting body of Ganoderma lucidum. Phytomedicine . 2011;18(12):1053–1055. doi: 10.1016/j.phymed.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 41.Radhika R., Sundar S. K., Anuradha R. Antioxidant property of fruiting bodies of Ganoderma lucidum. International Journal of Modern Agriculture . 2021;10(2) [Google Scholar]
- 42.Adebayo S., Ondua M., Shai L., Lebelo S. Inhibition of nitric oxide production and free radical scavenging activities of four South African medicinal plants. Journal of Inflammation Research . 2019;12:195–203. doi: 10.2147/JIR.S199377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Modi H. A., Shah P., Shukla M. D., Lahiri S. K. Determination of total phenolic content and antioxidant activity of Ganoderma lucidum collected from dang district of Gujarat. Natural Products: An Indian Journal . 2014;10(3):75–83. [Google Scholar]
- 44.Veljović S., Veljović M., Nikićević N., et al. Chemical composition, antiproliferative and antioxidant activity of differently processed Ganoderma lucidum ethanol extracts. Journal of Food Science and Technology . 2017;54(5):1312–1320. doi: 10.1007/s13197-017-2559-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wickramaratne M., Punchihewa J., Wickramaratne D. In-vitro alpha amylase inhibitory activity of the leaf extracts of Adenanthera pavonina. BMC Complementary and Alternative Medicine . 2016;16(1):p. 466. doi: 10.1186/s12906-016-1452-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qomi M. S. M., Hatami M. Effects of powder, extracts, and components of Ganoderma lucidum in treatment of diabetes. Journal of Guilan University of Medical Sciences . 2021;29(4):86–101. doi: 10.32598/JGUMS.29.4.1509.1. [DOI] [Google Scholar]
- 47.Fatmawati S., Shimizu K., Kondo R. Ganoderic acid Df, a new triterpenoid with aldose reductase inhibitory activity from the fruiting body of Ganoderma lucidum. Fitoterapia . 2010;81(8):1033–1036. doi: 10.1016/j.fitote.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 48.Sarnthima R., Khammaung S., Sa-ard P. Culture broth of Ganoderma lucidum exhibited antioxidant, antibacterial and α-amylase inhibitory activities. Journal of Food Science and Technology . 2017;54(11):3724–3730. doi: 10.1007/s13197-017-2839-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwon Y., Apostolidis E., Shetty K. Evaluation of pepper (Capsicum annuum) for management of diabetes and hypertension. Journal of Food Biochemistry . 2007;31(3):370–385. doi: 10.1111/j.1745-4514.2007.00120.x. [DOI] [Google Scholar]
- 50.Pk M. M. U., Islam M. S., Pervin R., et al. Enzyme inhibitory and antioxidant activity of combination of two edible mushrooms of Ganoderma lucidum and Pleurotus ostreatus. Tropical Journal of Natural Product Research . 2018;2(7):314–319. [Google Scholar]
- 51.Quereshi S., Pandey A. K., Sandhu S. S. Evaluation of antibacterial activity of different Ganoderma lucidum extracts. People’s Journal of Scientific Research . 2010;3(1):9–13. [Google Scholar]
- 52.Mustafin K., Bisko N., Blieva R., et al. Antioxidant and antimicrobial potential of Ganoderma lucidum and Trametes versicolor. Turkish Journal of Biochemistry . 2022;47(4):483–489. doi: 10.1515/tjb-2021-0141. [DOI] [Google Scholar]
- 53.Keypour S., Riahi H., Moradali M. F., Rafati H. Investigation of the antibacterial activity of a chloroform extract of ling zhi or reishi medicinal mushroom, Ganoderma lucidum (W. Curt.: Fr.) P. karst. (Aphyllophoromycetideae), from Iran. International Journal of Medicinal Mushrooms . 2008;10(4):345–349. doi: 10.1615/IntJMedMushr.v10.i4.70. [DOI] [Google Scholar]
- 54.Chowdhury M. M. H., Kubra K., Ahmed S. R. Screening of antimicrobial, antioxidant properties and bioactive compounds of some edible mushrooms cultivated in Bangladesh. Annals of Clinical Microbiology and Antimicrobials . 2015;14(1):p. 8. doi: 10.1186/s12941-015-0067-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


