Abstract
Recent advancements in the field of cancer have established that the process of metastasis is organ-specific with tumor cell dissemination occurring in the very early stages of disease. Pre-metastatic niches are actively remodeled and transformed by both primary tumor specific factors and by influences from the extracellular matrix.Although improvements in cancer therapies have significantly improved outcomes in patients with early stage disease, the risk of recurrence and relapse leading to mortality remains high. Recent studies have emerged highlighting the influence of dormant tumor cells and exosomes as key players in cancer relapse. In this review we discuss the critical mediators of tumor progression and their link to cancer dormancy, while also exploring possible therapeutics for targeting relapse.
Keywords: Cancer, Dormancy, Relapse, Microenvironment, Extracellular matrix
1. Introduction
Considerable advancements in cancer treatments have evolved over the years due to improved diagnostic and therapeutic strategies. However, much more needs to be done to target metastatic progression of tumor cells which is the primary cause of cancer-related deaths. Until recently, most of our knowledge on metastases indicated that spreading of tumor cells, a hallmark of metastasis, occurred in later stages of disease progression. However, we now know that the dissemination of tumor cells occurs in the early stages of the disease [1,2], and it appears to be organ-specific, with a number of studies describing the incidence of early tumor cell spreading, in some cases, long before a primary tumor is even palpable [1,3–7]. Furthermore, in roughly 5–10 % of cases, a metastatic lesion is detected with an unknown primary origin [8,9]. More recent foundational studies have revealed the formation of a pre-metastatic niche (PMN)10 at distant organ sites, which essentially cultivates a microenvironment that is conducive to tumor cell seeding and survival. This is in contrast to the metastatic niche which is largely dependent on the arrival of circulating cancer cells (CTCs). Cancer cells are noticeably absent in PMNs, and instead, the preparation of PMNs for tumor cell survival is heavily influenced by remodelling of the extracellular matrix (ECM) and tumor-cell secreted factors [10]. Cardiac and skeletal muscle, for example, rarely provide a growth promoting environment for cancer; however, the perivascular niche has been shown to support the survival of breast disseminated tumor cells (DTCs). Additionally, laminin-111, which is present in the basement membrane of glandular organs, provides an environment especially conducive to the survival and growth of mammary epithelial cells while also promoting quiescence and resistance to cytotoxic therapies [7,11]. In contrast, the presence of collagen-I has been attributed with increased cancer invasion and metastatic potential [12–14]. In breast and prostate cancers, adjuvant therapies targeting hormone signalling have dramatically improved patient outcomes, however, in a meta-analysis of over 60,000 breast cancer patients treated with early-stage disease treated with endocrine therapy experienced breast cancer recurrences from 5 to 20 years after initial diagnosis [15]. Understanding the mechanisms responsible for cancer recurrence, most notably, tumor cell dormancy, and developing improved therapeutic strategies is essential to improving patient outcomes.
2. Tumor microenvironment
Cancer is a complex and systemic disease, which incorporates numerous components of both tumor and stromal cells embedded within the extracellular matrix (ECM) [16–19]. The microenvironment of a developing tumor is composed of proliferating tumor cells, tumor stroma, angiogenic interactions and immune responses and it is defined as the complex and dynamic interactions between cancer cells and the ECM [20]. The evolution and function of the cells in the tumor microenvironment mirror many of the processes of wound healing and inflammation, however, in cancer, these processes become aberrant [3, 21–23]. Of important consideration, tumor associated macrophages (TAMs) play an important role in the secretion of growth factors, angiogenesis, tissue remodeling and the suppression of adaptive immunity. Additionally, bone marrow derived stem cells (BMDSCs) also secrete growth factors that can promote the differentiation of BMDSCs to osteoblasts, fibroblasts, chondrocytes and adipocytes. Primary and metastatic tumors are known to recruit BMDSCs to the microenvironment where they differentiate into cancer-associated fibroblasts (CAFs), promoting tumor survival and persistence [24].
Several decades of in-depth cancer research has been focused on a tumor-cell autonomous view of cancer, however, more recently it has become apparent that tumor cells do not act alone, but rather, they persist in a fertile tumor microenvironment which is governed by tumor-stroma interactions to promote metastasis and cancer progression [17, 23,25–29]. As the cancer continues to evolve, the surrounding tumor stroma and ECM is also transformed into an activated state which is maintained by continuous paracrine signaling between the tumor cells and the host stroma, thereby, cultivating an environment permissive to cancer progression [20,23].
Fibroblasts were first identified by Virchow and Duvall in 1858, as cells that function to synthesize collagen in connective tissues [30,31]. Phenotypically, fibroblasts are spindle-shaped, which can become polarized with migratory signaling. In normal tissues, fibroblasts are thought to be in a dormant state because of their relatively low metabolic activity [32]. Quiescent fibroblasts are generally found in the interstitial stromal layers between the parenchyma and mature tissues [32]. These quiescent cells become activated by influences from their environmental stimuli, thus promoting proliferation, migration, and deposition of ECM proteins [23,32,33]. Once activated, these fibroblasts display significantly increased contractile and metabolic activity, which are critical components of wound repair and connective tissue production; in cancer, however, these typically normal processes become dysregulated [32]. With chronic inflammation or wound insults, either in the context of physical, toxic, autoimmune or metabolic disorders, the repair response will continue unrestricted, resulting in a condition called tissue fibrosis [23,32,34]. In the context of cancer, the tumor stroma is mainly comprised of fibroblasts; many studies to date have established that fibroblasts residing in the tumor microenvironment have a significant influence on cancer progression and invasion [19,25–27], [29, 35–37]. Fibroblasts in the tumor microenvironment which become activated are termed cancer-associated fibroblasts (CAFs) [26,34,38, 39]. Dysregulation of wound healing results from signaling responses which remain elevated and sustained even once the wound is resolved; this is typically observed in tissue fibrosis and cancer progression [33, 40]. CAFs are generally identified by their expression of α-smooth muscle actin (α-SMA), a cytoskeletal protein typically found in smooth muscle cells [32]. Early studies in the 1970s described a mechanism by which cancer cells recruit activated fibroblasts that are functionally similar to myofibroblasts associated with wound healing [41,42]. This recruitment is largely regulated by growth factors secreted by the primary cancer cells and immune cells. TGF-β, PDGF and FGF-2 are the main mediators of activation in fibroblasts in both acute and chronic tissue damage and repair [32]. Of importance when examining ECM matrix influences on metastasis, TGF-β, has been shown to increase the activity of lysyl oxidase (LOX), the primary enzyme responsible to collagen crosslinking and remodeling [43]. The mechanism responsible for fibroblast activation is still not completely understood; however, it has been shown that reversion of phenotype and behavior of malignant cells can be modified by altering the tumor ECM [44,45], suggesting that tumor cells do not act autonomously; rather, they are heavily influenced by the signaling and composition of the ECM in the tumor microenvironment.
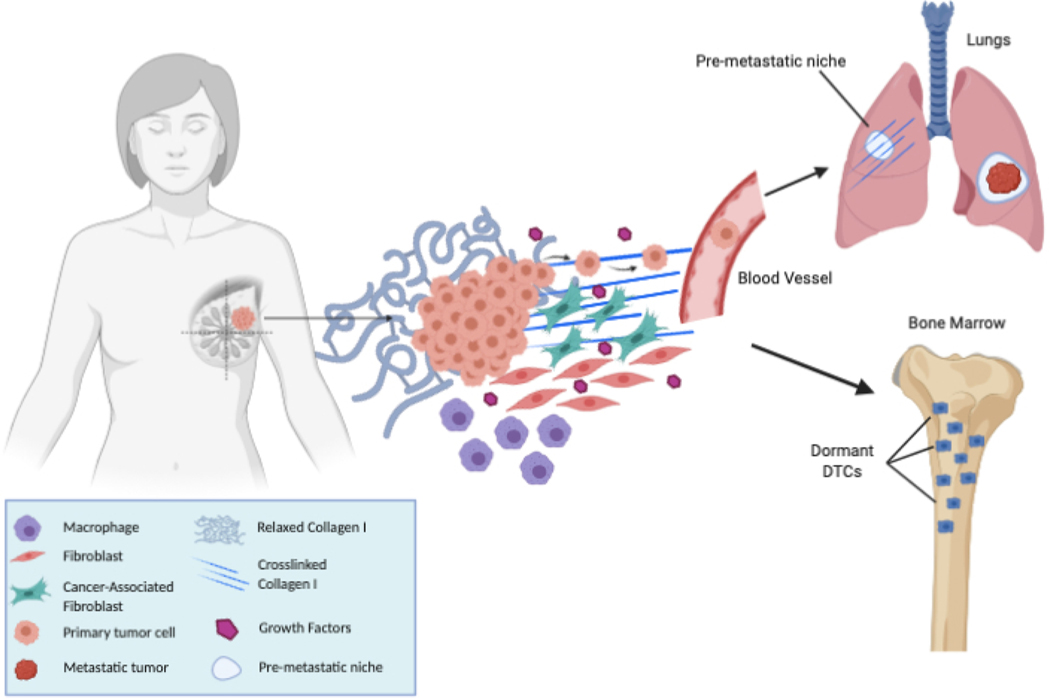
3. Pre-metastatic niche (PMN) and the metastatic niche
In recent years, studies have emerged highlighting the importance of primary-tumor derived soluble factors which function to prime distant tissue sites for tumor cell homing and adhesion. In response to these primary-tumor derived growth factors, macrophages and progenitor cells congregate at tissue sites called premetastatic niches (PMNs). PMN formation is largely dependent on modifications by the primary tumor, usually via soluble secreted factors. These future metastatic sites are actively formed via careful curation of microenvironments which are conducive to both survival and outgrowth of tumor cells long before their arrival at these distant sites [10]. Once these CTCs arrive at these secondary sites, colonization and engraftment occur, hence creating the metastatic niche. To cultivate an environment supportive of tumor cell growth and expansion, the PMN is noted in many studies as being formed first [10,46–50]. Bone marrow derived haematopoietic stem cells expressing VEGFR-1, have been noted as key contributors of the premetastatic niche, localizing to premetastatic sites long before the arrival of tumor cells [49]. Interestingly, these cells, of myeloid lineage, also express VLA-4, a fibronectin receptor [49]. Moreover, fibronectin expression is also increased in these premetastatic sites, suggesting that this increased stromal deposition paired with the localization of myeloid cells provide attractive disembarking sites for disseminating tumor cells [51]. At the PMN, recruited myeloid cells interact with various other cell types such as stromal and endothelial cells providing an array of chemokines, growth factors and matrix modifying factors, all favoring tissue remodelling and thereby, accelerating the assembly of the metastatic niche [51].
In a recent in vivo study, loss of the hydrogen peroxide scavenger, Peroxiredoxin-1 (PRDX1), resulted in a significant increase in both collagen remodelling and deposition in mammary ECM [52]. Furthermore, primary tumor-derived secreted factors resulted in a SRC-induced phosphorylation of PRDX1 in mammary fibroblasts, resulting in its inactivation [52]. Phosphorylated PRDX1 fails to bind lysyl oxidase (LOX), leading to the accumulation of LOX in the ECM and enhanced collagen remodelling, promoting the pre-metastatic niche and enhancing breast cancer progression [52]. Earlier studies have also shown that inhibition of LOX synthesis in human breast cancer cells decreased the accumulation of myeloid cells [53].
Local tissue modification is essential for supporting and promoting tumor cell invasion and metastasis. Several studies have emerged over the years emphasizing the importance of MMPs in the PMN because of their ability to degrade ECM during inflammatory responses. MMP9 has been established as a key player in the formation of the PMN, specifically in endothelial cells and in VEGFR1 expressing myeloid cells in the lung [54]. The mechanical properties of the ECM along with its stiffness and tensile strength has been shown to have a significant impact on promoting tumor cell dissemination in the mammary gland, while also being preserved even after transition to the metastatic niche of softer microenvironments such as bone marrow [55]. The evolution of the metastatic niche initially begins in response to growth factors secreted by the primary tumor leading to recruitment and accumulation of haematopoietic progenitor cells. Stromal-derived growth factor secreted by platelets function to recruit metastatic tumor cells allowing them to engraft the niche, creating micrometastases. Location specific adhesion integrins such a P-selectin and E-selectin function to promote metastatic tumor cell adhesion and extravasation at metastatic sites. Progression to a macrometastasis is partly dependent on recruitment of endothelial progenitor cells which mediate the angiogenic switch necessary to promote the progression to metastatic disease [7,10,49,51].
4. Tumor dormancy
Tumor dormancy is classically described as the delay of tumor growth between the formation of a primary tumor and the incidence of metastatic growths [56]. From a clinical perspective, tumor dormancy is described as the metastatic recurrence of disease, during a time period where the patient remains asymptomatic with the appearance of minimal residual disease following either surgical or therapeutic intervention of the primary tumor [2,56,57]. Tumor dormancy uncovers the ability of circulating tumor cells (CTCs), disseminated tumor cells (DTCs) and other micrometastases to remain viable in small populations following the resection of the primary tumor [2]. These cells remain undetected for extended periods of time, for even years to decades, allowing for extended periods of asymptomatic residual disease and resistance to treatment. These dormant cells, however, will not remain as such, and they will eventually initiate the progression of metastatic disease to secondary sites in the body [2,56–58]. Traditional chemotherapy is targeted towards proliferating cells; however, these therapies are ineffective on dormant cells [59,60]. Breast cancer is particularly noted for having extended periods of tumor dormancy, with metastases occurring at times, many years after initial treatment. In a study using a murine mouse model for dormancy in breast cancer, it was shown that cytotoxic chemotherapies, such as doxorubicin, spared the non-dividing, dormant cells [60,61]. In metastatic latency, these cells that have survived chemotherapy remain undetectable by conventional diagnostics, nor do patients manifest symptoms. As a result, when CTCs and DTCs are detected in either the blood or bone marrow, it is evidence of residual disease in latency and it is a risk factor for recurrence of disease in these patients [59]. CTCs isolated from the blood of cancer patients can provide critical information about the progression of the cancer, in addition to better informed treatment design and selection. In over 200 clinical trials, CTC counts and surface marker expression have uncovered their prognostic importance with respect to the progression of metastatic disease, by utilizing these characteristics to evaluate therapeutic response and treatment driven clonal selection [62].
In a significant percentage of breast cancer patients, metastases to distant sites in the body occur after years of latency. Many studies have attributed this phenomenon to DTCs which are kept dormant, until they receive a cue to wake up. This cue or signal remains a poorly understood aspect of cancer biology. However, a recent study revealed that dormant DTCs take up occupancy on the microvasculature of the lung, bone marrow and brain—all sites that are well accepted as typical locations for breast cancer metastases [7,22,63]. Using organotypic microvascular niches, the study demonstrated that endothelial thrombospondin-1 induced sustained breast cancer cell quiescence [7]. Since the early seventies, studies have shown that tumor dormancy could be induced in vivo by preventing neovascularization [64]. However, in angiogenic vasculature, sprouting vessels were shown to accelerate breast cancer cell growth, revealing that stable vasculature creates a dormant niche, while neovasculature initiates metastatic growth [7]. Although currently, the cues and signals responsible for disrupting tumor dormancy are largely unknown, more recent studies have examined the influence of certain patient lifestyle exposures, such as alcohol, on disrupting the tumor microenvironments which maintain the DTC dormant niche. Such environmental exposures injure the dormant niche, resulting in its reactivation, hence leading to metastatic progression and growth [65]. Tumors are known to secrete diffusible substances that stimulate and promote neovascularization [66]. Using a model of diabetic retinopathy for carcinoma implantation, a prolonged avascular state resulted in restricted tumor size, suggesting that normal vitreous functions to inhibit capillary proliferation [66]. Furthermore, in breast cancer, tumor-intrinsic signalling and specific bone marrow niches have been recognized as playing a key role in dictating the dormancy state of bone disseminated tumor cells [48].
Two models of tumor dormancy have emerged, cellular dormancy and tumor mass dormancy. Cellular dormancy is characteristic of single, non-proliferative DTCs, which enter a quiescent state in response to either intrinsic signals or signals from the external microenvironment. Contrary to this theory, tumor mass dormancy is classified as a state of equilibrium between proliferating DTCs and those undergoing apoptosis [67]. This dynamic is heavily controlled and regulated by stromal cells within the microenvironment, angiogenic dormancy and immunologic dormancy [56,59,67]. The enhanced secretion of angiogenic suppressors and the inhibition of factors promoting angiogenesis, in addition to immune clearance of certain DTC populations and immune escape of other DTC populations, describe angiogenic and immunological dormancy, respectively [2,7,68]. The dual role of the immune system functions to both identify and eliminate tumor cells, while also shaping the immunogenicity of tumor cells. The tumor microenvironment plays a critical role in the regulation of both tumor development and progression, and it has been implicated in tumorigenesis in a wide array of cancers [69]. This environment cultivates the integration of tumor cells with their surrounding cells via circulatory and lymphatic systems, in addition to influencing neighbouring adipocytes, fibroblasts, dendritic cells and CAFs. Consequently, the tumor microenvironment houses the capability to determine whether the tumor will persist and manipulate cells in its vicinity [21,34,69].
5. Tumor microenvironment, therapeutic resistance and relapse
Tumor relapse, treatment resistance and metastasis remain the major etiology responsible for the poor survival rates in cancer patients with advanced disease. Even with surgical resection and treatments, a primary cause of relapse and metastatic progression is due to cancer stem cells (CSCs) which are resistant to chemotherapy, allowing them to persist in the body, relatively undetected and unphased [70]. Studies of acquired relapse in cancer patients have indicated that tumor cells, upon treatment, experience a strong selective pressure resulting in clonal evolution of treatment resistant tumor cells. These cells which are neoplastic in origin, acquire somatic mutations and phenotypic alterations which vary greatly from their original cellular state.
Distant or local tumor recurrence is predominantly influenced by the tumor microenvironment, which provides a rich and adaptive atmosphere, cultivating cellular growth and proliferation of relapsed, highly resistant tumor cells. Merging of tumor cells with proteins and stromal cells of the extracellular matrix, such as collagen I and CAFs, respectively, have been well described in the promotion of metastatic disease [24,34,62,71]. Adhesion of tumor cells to the matrix influences the activation of gene signatures which play a critical role in disseminating cancer cells to distant sites while also activating tissue-dependent dormancy [62]. Patients with chemotherapeutic resistance or radiation resistance have been noted as having significantly higher numbers of CTCs transformed into CSCs via epithelial to mesenchymal transition (EMT) [62,72]. Clinical studies have linked observed EMT phenotypes in advanced malignancies to poor patient survival [73]. In studies of ductal carcinoma in situ (DCIS), alterations in the architecture of the extracellular matrix leading to crosslinking and linearization of collagen fibers surrounding the duct perimeter were notably more frequent in DCIS lesions with characteristics typically associated with poor prognosis and survival [12,74–76].
6. Chronic inflammation, microenvironment and dormancy
In the extracellular matrix (ECM), chronic inflammation is one of the main drivers of fibrosis, whereby continuous immune responses occur simultaneously with tissue repair and remodelling. Cancers have often been described as wounds that do not heal, precisely because the cellular and biochemical processes that occur in wound healing are characteristically similar to those that are observed in the growth and development of the tumor stroma. However, once the reactive processes of wound healing are complete, the system reinstates its equilibrium, while in cancer, these processes continue and progress, unchecked. Radio-therapy, often a first line of treatment for many cancers, has been well described as promoting reactive oxygen species (ROS) mediated proliferation through DNA damage and inflammation, and in some instances, compromising organ function. According to a recent study, elevated levels of CCL5 was associated with a poor prognosis because it was associated with a decrease in recurrence-free survival. Furthermore, CCL5 functions as a chemoattractant for collagen-depositing macrophages to the residual tumor, leading to elevated concentrations of crosslinked collagen, which has been well-described to increase tissue stiffness and promote cell migration [12,13,63,77]. In colorectal cancer, depletion of CCR2+ macrophages were found to inhibit tumor growth by decreasing CCR2+-mediated collagen deposition [78]. CCL5 has been described as a key player in many components of tumor progression, including invasion, metastasis and recurrence [79]. In glioblastoma, upregulation of CCL5 was correlated with recurrence following treatment [80], and in triple negative breast cancer, following neoadjuvant chemotherapy, expression of CCL5 was correlated with residual tumor size [81]. In Her2+ or hormone receptor positive breast cancers, elevated CCL5 expression was observed in residual tumor cells which persisted post-therapy, promoting recurrence of disease [77]. Several studies have emerged suggesting that tumor associated macrophages serve a protective role for cancer cells from immune cells in the microenvironment [77,82–84]. In a model of chemotherapy treated breast cancer, depletion of macrophages enhanced CD8 + T cells [85], and in a study of over 100 luminal-like tumors, high levels of CD204 were correlated with a decrease in relapse-free survival [86].
7. Extracellular matrix and tumor dormancy in breast Cancer
The ECM is a critical component of tumor tissue, and this microenvironment mimics a wound healing state by maintaining itself in a state of perpetual fibrotic repair. Alterations to the architecture, and stromal integrity contributing to stiffness in the mammary gland has been recognized as a characteristic leading to poor prognosis and disease progression in breast cancer [26,87]. Normal mammary gland is a soft and flexible tissue, mostly composed of relaxed, helical collagen fibers. In malignant progression, the architecture of the mammary gland undergoes collagen crosslinking via lysyl oxidase (LOX) family enzymes, contributing to increased stiffness of the gland. A biophysical and histological analysis showed that that stromal stiffness is highest at the invasive front of highly aggressive breast cancers, such as HER2 amplified, and triple-negative breast cancer compared to luminal tumors. Moreover, collagen fibril orientation has been correlated with poor survival regardless of tumor size, grade, subtype and nodal status [12,16,75]. In estrogen-receptor negative breast cancer, stroma-related gene-signatures were found to predict resistance to neoadjuvant chemotherapy [88]. Such studies, which have emerged more recently have further identified the contributions of the tumor microenvironment, specifically, the ECM as key regulators in the progression of metastatic disease. Strong evidence has emerged in recent years supporting the notion that specific ECM proteins function to promote cellular dormancy. In breast cancer, endothelial-derived thrombospondin-1, which is present on the microvasculature, induces continuous breast cancer cell quiescence, however, in sprouting neovasculature, this suppressive signal is lost [7]. Further analysis has revealed that sprouting vasculature accelerates breast cancer cell growth and progression via the presence of enhanced tumor promoting factors, TGF-β1 and periostin7. Primary tumors are known to condition premetastatic sites for tumor cell dissemination by secreting a milieu of proteins and lipids which function to remodel the distal tissue, cultivating a microenvironment encouraging the retention, growth and survival of DTCs. These alterations in tissue architecture for the growth of metastases are characterized as the premetastatic niche; remodelling of the ECM is particularly important in identifying the metastatic potential of a tumor [10,89]. Given the crucial role that the ECM plays in the progression of metastatic disease clinical approaches targeting fibrosis and integrin signalling create an alternative strategy in the reduction of aggressive, metastatic cancers, and ultimately improving patient outcomes. Currently, multiple therapies have been developed for the reduction of tissue fibrosis. Pirfenidone, an anti-fibrotic drug originally used in the treatment of idiopathic pulmonary fibrosis, has recently been suggested to function as an anti-fibrotic drug targeting tumor progression to treat metastatic progression in solid cancers.
Although the leading therapies in the treatment of cancers are chemotherapy and radiotherapy, multidrug resistance has increased over the years, leading to cancer relapse and poor overall outcomes in these patients. Recent research has shown that the tumor microenvironment plays a critical role in cancer progression and in multidrug resistance [90]. The tumor microenvironment is highly complex and heterogenous and gaining a thorough understanding of how the microenvironment supports tumor growth and metastatic progression is critical to develop effective therapeutic strategies for the treatment of cancer (Fig. 1). The ECM is a 3D structure composed various proteins, such as collagen, elastin, laminins, fibronectin and proteoglycans, which provide support to tissues. As a tumor grows, a variety of factors including hypoxia, inflammation, and tumor cell heterogeneity all induce modifications to the proteins of the ECM resulting in elevated collagen deposition and ECM disorganization, resulting in a stiff matrix which has been shown to promote tumor progression. Moreover, the ECM has also been shown to support tumors by providing growth factors and signals for proliferation, and enabling evasion of growth suppressors, while also inducing neoangiogenesis [7,13,91]. Use of FDA approved angiotensin II receptor agonists such as Losartan, Olmesartan, Valssartan or Candersartan, which are typically utilized for the treatment of hypertension, reduced mortality in gastro-esophageal cancer patients [92]. This study suggests that Losartan functions by inhibiting TGF-β signalling, resulting in a reduction of secreted collagen I and desmoplasia, facilitating improved delivery of chemotherapeutics to the tumor [92–95].
Fig. 1.
Tumor Microenvironment, Dormancy and the Pre-metastatic Niche.
8. Lung microenvironment and dormancy
Multiple cancer types utilize the lungs as a prime target organ for metastatic dissemination of cancer cells, and it is the surrounding microenvironment and premetastatic niches that provide a fertile ground for metastatic growth by reawakening quiescent cells. Conditions of hypoxia and inflammation in the lung microenvironment promote the development of metastases. The lysyl oxidase (LOX) family of enzymes have been well described as collagen crosslinking enzymes which facilitate ECM remodelling, and the promotion of EMT. The expression of the enzyme, lysyl oxidase like 2 (LOXL2), is elevated under hypoxic conditions of the tumor microenvironment, and it has been associated with metastatic progression and poor prognosis in lung, breast and hepatocellular carcinoma [89,96–98]. Furthermore, inflammation in the lungs may enhance the proliferation of dormant metastatic breast cancer cells by activation of Tank-binding kinase-1 (TBK1). TBK1 promotes EMT and invasion of lung cancer cells via phosphorylation of synthase kinase-3β (GSK-3β) and expression of zinc finger E-box-binding homeobox 1 (ZEB1) [99,100]. In the lungs, microenvironments with low levels of TGF-β2 were shown to activate dormant DTCs, allowing for metastatic growth [101,102]. In normal tissue, periostin (POSTN), is a secretory protein expressed in bone tissues, and in the ECM, where it functions as an adhesion protein, regulating cell adhesion and differentiation of osteogenic cells. However, in cancer, it is often upregulated and specifically, in lung cancer, studies have shown that POSTN interacts with Wnt1 and Wnt3A, enhancing Wnt signalling and promoting tumor cell growth. Because the lungs are leukocyte-rich, macrophages express α4 integrins inducing protein kinase B (Akt) which interact with vascular cell adhesion molecule 1 (VCAM-1) promoting lung metastases. VCAM-1 serves to aid the attachment of metastases-associated macrophages to cancer cells by α4 integrin, hence, protecting cancer cells from cytokine-mediated apoptosis such as TNF-Related Apoptosis Inducing Ligand (TRAIL) [103].
The lung ECM is composed of a wide variety of matrisomal proteins which provide structural support to the tissue paired with soluble factors and remodelling enzymes which associate with the matrix. The functions of the central airways and parenchyma are largely influenced by the composition and architecture of the ECM. Recent studies have identified circulating ECM-related proteins in non-small cell lung cancer (NSCLC) patient plasma [104]. Further associations have been made linking the ECM composition of NSCLC tumors with an increased risk of recurrence, highlighting the critical role of the ECM in regulating metastatic progression, dormancy and relapse [105]. Mechanisms regulating tumor cell dormancy have been described as being mediated via mitogenic and stress response pathways such as, TGFβ/BMP, Src, FAK, EGFR and integrin, paired with ERK/p38, JNK and cyclin and other downstream regulators of the cell cycle, mediating cell cycle activity. Crosstalk between integrin, EGFR and uPAR influence downstream signalling of FAK which mediates dormancy via p38 and p27-dependent signalling [106]. Quiescent signalling, typically found in stem cells, have been found to promotes dormancy and metastasis in cancer. These signals originate from the ECM and interact with Wnt and Notch signalling to mediate these pathways of dormancy in breast cancer [107]. Similar transcriptional signatures between breast metastases in the lung and aggressive metastatic lung cancer, reveal that similar pathways of ECM-mediated dormancy and metastatic progression exist across differing cancer types [48,108].
In NSCLC, stiff ECMs have been shown to promote FAK activation and increased Wnt signalling, driving NSCLC viability. Moreover, LOX and LOXL2 enzymes are upregulated in NSCLC, increasing the metastatic progression of lung tumor cells. The activity of LOX-family proteins in lung cancer depends on the histological subtype and genomic profile of the tumor, however, studies have emerged identifying the loss of LKB1 as a key regulator in activating mTOR-HIF1α signalling, causing LOX expression in the tumor microenvironment, leading to LOX-mediated collagen crosslinking and increased stiffness of the tumor tissue [109]. Cancer-associated fibroblasts (CAFs) display increased LOX secretion and elevated glycolytic and autophagic activity in comparison to normal lung fibroblasts. CAFs have been well-described in both breast and lung cancers for their role in promoting metastatic progression. Collagen turnover is partly an autophagic process, suggesting that activation of autophagy by CAFs and collagen fibrillogenesis, may promote quiescence and dormancy in lung cancer [110]. The lungs are comprised of a highly heterogeneous fibroblast population which can contribute to ECM remodelling in lung tumors when specific fibroblast phenotypes are allowed to expand unregulated. Compounding the effects of increased stiffness of the ECM, cancer cell-secreted growth factors such as TGFβ, PDGF, and FG2, in addition to immune cells function to both recruit and promote fibroblasts to a myofibroblast-like state which drive proliferation and inhibition of apoptosis. These specific features of the ECM that characterize NSCLC may provide key information for use in clinical practice when deciding which therapies are the most effective.
9. Brain
The first barrier against cancer cell infiltration into the brain are endothelial cells. However, the unique microenvironment of the brain including microglia, astrocytes and paracrine growth factors all contribute to the development of metastatic disease [111]. In breast cancer metastasis to the brain, cancer cells have been found to exclusively extravasate via capillaries and they were also found to overexpress the metalloproteinase-9 (MMP-9) which has been well described as promoting angiogenesis and growth in brain tumors. Moreover, activated astrocytes also secrete MMP-9 aiding the extravasation of cancer cells into the brain [54]. Elevated expression of Glial fibrillary acidic protein (GFAP) and nestin maintain the cellular phenotype of astrocytes while also cultivating a supportive microenvironment for growth and proliferation. Together, such research has shown that activated astrocytes in the tumor microenvironment may promote the progression of metastatic disease in the brain [54,111].
Individuals with advanced melanoma often experience metastatic disease in the brain, and unfortunately with poor prognosis. The mechanisms responsible for the development of melanoma originating brain metastases are still not well understood, however, a recent study has shown that brain micrometastases composed of dormant melanoma cells contain specific transcriptional signatures. In a panel of differential gene expression of 35 genes, levels of cysteine-rich protein 61 (CYR61) and of preferentially expressed antigen in melanoma (PRAME), have been shown to regulate the response to microenvironmental cues in the brain by disseminated melanoma cells [112–114]. Although mechanisms of angiogenic dormancy in cancer remains largely unknown, dormant metastatic cells have been shown to have higher expression of angiomotin which serves as a regulator of antiangiogenic activity [115]. In brain metastases, in addition to melanoma, lung and breast, cancers cells are found in perivascular niches which provide an optimal environment for survival and dormancy of DTCs [7,116]. In brain metastases, elevated levels of vascular endothelial growth factor A (VEGF-A) re-awaken dormant cells, intimating the critical role of angiogenesis in the development of metastatic disease in the brain [111,113,115,116].
10. Bone microenvironment
One of the most common target organs for metastasis, particularly in patients with advanced prostate and breast cancers, is the bone [117, 118]. The development of bone metastases characterizes one of the most complex components of oncogenic progression, due to the multifaceted and intricate functions of haematopoiesis, osteogenesis and osteolysis, which all characterize the bone microenvironment [48,118]. During systemic dissemination of cancer cells, the spongy tissue of the red bone marrow accumulates DTCs from practically all types of cancers, with its rich microenvironment appropriated by cancers cells, cultivating its progression into further metastatic disease [117]. Over 30 years ago, it was observed that metastasis to the bone was only observed in locations of high hematopoietic activity of red bone marrow [119]. Furthermore, some cancers are able to thrive in bone by their ability to destroy calcified bone matrix while in other cases initiating the generation of new bone tissue [120]. DTCs detected in the bone marrow have been characterized as one of the only sites in the body which houses minimal residual disease from almost every type of cancer without developing bone metastases in most instances. In the incidence that bone metastases do develop, such as in breast or prostate cancer, DTCs are detected in the bone marrow at much higher frequencies compared to the rate in which metastatic disease will develop [121,122]. Collectively, this points to the notion that although many types of cancer have the ability to enter dormancy in the bone, it is the regulation of dormancy and its awakening that is the principle behind relapse and progression of metastatic disease. Within the bone marrow microenvironment, a variety of cell types have been shown to promote the dormant state. Secreted factors from osteoblasts promote quiescence of hematopoietic stem cells (HSCs) [118]. Prostate DTCs are known to bind to Annexin II, serving to mediate HSC adhesion within the microenvironmental niche. Moreover, this binding upregulates both the AXL family of receptors and the TGF-β receptors which are known to enhance the dormant state [123,124]. Breast cancer cells localized to the microvasculature of the bone marrow are facilitated to a quiescent state by thrombospondin-1 (TSP-1) secreting endothelial cells [7]. TSP-1, which functions as a tumor suppressing and anti-angiogenic factor, is found to accelerate micrometastatic growth when its expression is lost [7]. Osteolytic metastases in the bone marrow from originating breast cancer cells, have been shown to secrete parathyroid hormone-related protein (PTHRP), tumor necrosis factor (TNFα) and interleukin 6 (IL-6) [125]. These factors have been well-described in osteoclast formation, further promoting their metastatic capabilities in the bone.
Accumulating evidence has emerged suggesting that spreading of metastatic cells occurs much earlier than what was initially thought. Evidence of early tumor cell dissemination was shown by the detection of DTCs in the bone marrow of MMTV-PyMT and MMTV-HER2 transgenic mice and in patients with ductal carcinoma in situ [126]. Mounting clinical evidence has described the presence of DTCs in the bone marrow of patients with early-stage cancer. Hypoxia in the primary tumor has been described as influencing the generation of a subpopulation of DTCs in breast cancer that disseminate and highly express NR2F1 mediated pro-dormancy genes which enable cancer cells to escape the lethal effects of chemotherapeutic treatment [126]. In the bone microenvironment, the CXCL12/CXCR4 signalling cascade, functions to attract tumor cells to the bone. The chemoattractant CXCL12 is expressed throughout the bone microenvironment, on osteoblasts, endothelial, bone marrow stromal cells and mesenchymal stem cells [127]. Through its receptor, CXCR4, hematopoietic stem cells are recruited to the bone marrow, promoting invasion and metastasis in melanoma, breast cancer and prostate cancers. Studies have suggested that phosphatidylinositol 4-kinase III localizes to lipid rafts on CXCR4, enhancing invasion of tumor cells to distant metastatic sites [128]. Interestingly, upon inhibition of CXCL12-CXCR4 in prostate cancer, formation of metastatic bone tumors was diminished, while pre-existing tumors in the bone structures remained uncompromised [129]. The creation of a premetastatic niche was observed in LOX-secreting breast tumors. In breast cancer, the secretion of LOX disrupted the normal homeostasis of the bone by forming osteolytic lesions in the bone, further enhancing and promoting metastatic growth [48]. Once DTCs localize to specific niches supporting their survival in the endosteal bone microenvironment, tumor cells can evade the immune system, and they can acquire a dormant phenotype rendering them resistant to chemotherapy. Dormancy within the bone has been attributed to GAS6 binding to the protein tyrosine kinase receptor MER in lymphoblastic leukemia124, [130], and in prostate cancer, the interaction between GAS6/AXL and osteoblasts was shown to induce dormancy [131]. In vitro studies have shown that conditioned media collected from differentiated osteoblasts induced quiescence in prostate cancer cells. This suggests the critical role of secreted factors in signalling-induced dormancy, specifically, through osteoblast-secreted factors growth differentiation factor 10 (GDF10) and transforming growth factor-beta 2 (TGFβ2) [124,130,132]. In prostate cancer, these secreted factors induced tumor cell dormancy in the bone via activation of the TGFβRIII-p38MAPK-pS249/pT252-RB pathway. Clinically, low levels of TGFβRIII correlate with an overall poor prognosis and increased metastatic progression in prostate cancer patients [132]. The unique characteristics of the bone microenvironment function to induce dormancy in cancer cells, and increased osteoclast activity which is typically observed in conditions of high bone turnover have been suggested to play a critical role in the reactivation of dormant cancer cells residing in the bone. In multiple myeloma, DTCs colonizing the bone were shown to increase osteoclastic bone resorption via the soluble receptor activator of nuclear factor kappa-B ligand (sRANKL). This resulted in the release of dormant tumor cells from the niche of endosteal bone, while at the same time, leaving DTCs in soft tissue environments largely unaffected [133].
11. Exosomes and tumor progression
Exosomes are small, membrane bound vesicles ranging in size from 50−100 nm in diameter [134]. Increasing evidence suggests that exosomes play a critical role in cancer cell communication, both locally and systemically. Specifically, cancer-derived exosomes may participate in the recruitment and remodelling of the tumor microenvironment [134,135]. Tumor cells of epithelial origin have been shown to secrete exosomes with epithelial cell adhesion molecules (EpCAM) as part of its cargo [135,136]. Exosomes from breast, gastric and pancreatic cancers have been shown to express proteins of the human epidermal receptor (HER) family of proteins. Moreover, proinflammatory environments which enhance the expression of membrane receptor proteins such as ICAM-1, has been well-established as increasing the adhesion of exosomes to target cells, thereby, facilitating tumor progression [137]. Interestingly, exosomes have also been shown to fuse with the plasma membrane of target cells, resulting in the release of exocytic cargo directly into to the cytoplasm. More recently, studies have emerged highlighting the importance of organ-specific cell uptake of tumor-derived exosomes in the preparation of the pre-metastatic niche [134]. Specifically, tumor-derived exosomes have been shown to attribute to vascular permeability, inflammation and bone marrow progenitor cell recruitment in metastatic progression and in the formation of the PMN. Recent studies have also revealed the prognostic potential of exosomal protein signatures in identifying which melanoma patients were most at-risk for metastatic progression of their cancer [10,134]. It has been suggested that exosomal integrins on tumor-derived exosomes, are key mediators of PMN formation and organ-specific colonization of distant sites by enhancing fusion with target cells [134]. Quantitative mass spectrometry of liver-, lung- and brain-tropic metastatic exosomes, revealed six members of the integrin family among the most abundant adhesion molecules present on these tumor-derived exosomes; this further highlighted a critical link between the presence of exosomal integrins and metastatic tropism [134]. Furthermore, integrin alpha 6 and integrins beta 1 and 4, were significantly expressed in lung-tropic exosomes, while integrin beta 5 was expressed in liver-tropic exosomes [134]. In lung cancer PMN formation, exosomal integrin alpha 6 beta 4 activate the Src-S100A4 axis. Collectively, these data indicate that exosomal integrins are not only critical in adhesion, but they also activate signalling and inflammatory pathways in target cells at distant sites, reprogramming the organ and cultivating the growth of metastatic cells [134]. Exosomes have also been shown to promote drug resistance. CAF-secreted exosomal miR-21 taken up by ovarian cancer cells was found to decrease the expression of APAF1, a pro-apoptotic gene, leading to chemotherapeutic resistance and reduced apoptosis of ovarian cancer cells [138]. Additionally, M2-polarized macrophage derived exosomes diminished sensitivity to cisplatin and exosomal miR-21 reduced apoptosis while also enhancing the PI3K/AKT signalling pathway in gastric cancer cells [139]. Gemcitabine treated CAFs increased secretion of Snail and miR-146a-enriched exosomes, thereby, facilitating tumor cell and CAFs survival [140].
Exosomes also play a critical role in lymphangiogenesis, influencing the immune system and its lymphatic organs. Since most cancers metastasize via lymphatic organs by dissemination to distant organs, exosome-mediated metastasis to sentinel lymph nodes in melanoma, promoted PMN formation via increasing expression of gene profiles contributing to angiogenesis, ECM remodelling and tumor-cell recruitment. Exosomes from MDA-MB-231 breast cancer cells influenced growth of the primary tumor and lymph metastasis by activation of macrophage polarization to M2 tumor-supporting macrophages in axillary lymph nodes. These studies reveal the critical roles of exosomes not only in preparing the PMN, but also as promoters and regulators of tumor cell dissemination through the lymphatic system [134,138]. The interest in exosomes has grown as they emerge as promising targets for diagnostic and therapeutic strategies. More recently, non-modified, proinflammatory exosomes derived from immune cells were utilized as a therapeutic in cancer. These exosomes induced the secretion of pro-inflammatory cytokines, resulting in the inhibition of tumor growth and proving to be a potent stimulator of the immune system [141]. Similarly, in B16F10 cells, natural killer cell- derived exosomes were found to induce apoptosis and inhibit tumor growth in vitro and in vivo, respectively [142]. PEGylated liposome nanoparticles, modelled after exosome-like particles, were found to accumulate within metastatic sites in the lung. Although the use of exosomes and exosome-like particles have potential for enhancing the targeting of metastases in cancer therapies, challenges still remain regarding the optimization of mechanisms of exosome delivery and uptake at metastatic sites.
12. Targeting cancer dormancy
The two clinical models available for targeting dormant DTCs are, 1) to maintain a state of dormancy throughout the patient’s life, or 2) to eliminate dormant DTCs [143]. To date, successful execution of either avenue has proven difficult because each requires the identification and utilization of unique window of time, allowing for the identification of dormant tumor cells and selection of therapeutic treatments for desired clinical outcomes.
Therapies targeting the maintenance of tumor dormancy in breast cancer was described in oestrogen-receptor (ER) positive patients who were treated with tamoxifen, an ER antagonist, for five to ten years after initial diagnosis. In patients who were treated with tamoxifen for five years, rates of cancer recurrence in early ER + breast cancer was roughly halved [144]. Maintenance of tamoxifen therapy for ten years after initial diagnosis further reduced the rate of cancer reccurence [145]. These studies suggest that continuation of tamoxifen therapy restricts DTC proliferation, thereby, preventing metastatic progression. However, even with sustained tamoxifen treatment, a little over 20 % of patients experienced disease reccurence [145], indicating that even in extended therapies, maintaining disease dormancy proves to be a challenge. In NSCLC, senescent-like dormant cells are established by the upregulation of YAP/TEAD activity. Studies have suggested that inhibition of YAP/TEAD could eradicate dormancy, however, this approach has its complications because it may enable the recurrence of chemoresistant tumors [146].
In 79 % of breast cancer patients treated with docetaxel following treatment with adjuvant fluorouracil, epirubicin and cyclophosphamide (FEC) chemotherapy, enduring DTCs were eliminated, allowing for improved metastasis-free survival [147]. Interestingly, patients with DTC elimination experienced similar rates of metastasis-free survival to those who were DTC negative at the initiation of the study. This strongly suggests that DTCs are critical players in metastatic progression, and elimination of these cell populations indeed enhances overall survival in breast cancer patients [147]. Currently, the major frontline treatment for approximately 60 % of cancers is radiotherapy. In breast cancer, irradiation was shown to induce phenotypic transformation of myofibroblasts in the microenvironment to CAFs [26]. CAFs are activated by irradiation, and they have been well-described as secreting a variety of growth factors and chemokines, and also as regulating architectural remodelling of the stromal microenvironment via production and processing of collagen [148]. This microenvironmental remodelling and increased collagen deposition results in a stiff extracellular matrix, which in breast cancer has been well-described as contributing to increased metastatic potential [12,75]. Crosslinked collagen fiber angles have been used as a diagnostic feature typically attributed to more advanced breast cancer [12]. Furthermore, irradiation leads to vascular destruction in the tumor microenvironment contributing to hypoxia, a key contributor to radioresistance. Hypoxia has been shown to enhance cancer stem cell dormancy, correlating with increased incidence of tumor recurrence and overall poor prognosis [149].
Because dormant tumor cells are able to evade chemotherapy and/or radiotherapies by means of their state of quiescence, recent advances in immunotherapies have shown promise for the treatment of dormancy in cancer. More recently, dormant tumor cells were shown to be more sensitive to immunotherapy if targeted during the critical window prior to clinical recurrence of cancer [150]. Multiple studies have shown that upregulation of UPR-associated genes play a critical role in regulating tumor dormancy [146,151,152]. Furthermore, in prostate cancer, the expression of survival receptors such as endothelial receptor A (ETA) induces the expression of antiapoptotic genes, allowing tumor cells to evade cancer therapies [153,154]. Melanoma, compared to prostate and ovarian cancers, displays increased sensitivity to immunotherapy possibly due to lower expression of ETA, hence, reduced tumor cell evasion of therapeutics. Recent studies describing neoantigens, which are random somatic mutations in tumor cells that are not characteristic of normal cells, may provide a tumor-specific target for immune attack. Various clinical trials have described antitumor immune responses via recognition of tumor neoantigens by CD8+ and CD4 + T cells [155]. In advanced melanoma [156] and glioblastoma [157], neoantigen tumor vaccines have been developed and tested; neoantigen targeted vaccines for cancers are currently in clinical trials [158,159]. Another point to take into consideration, however, is that DTCs have fewer mutations and neoantigens, in addition to poor antigen presentation, in comparison to primary tumors and metastases. These characteristics make targeting quiescent DTCs via enhanced T cell response or vaccine, challenging [2, 143]. Therapies which do not depend on MHC antigen presentation such as chimeric antigen receptors (CAR), may provide an alternative treatment option. However, CAR T cell therapy does have its own set of obstacles because it requires the identification of homogenously expressed DTC-specific surface antigens; in the past, this has hindered its utilization in the treatment of solid tumors [160].
13. Concluding remarks
The work described here highlights the critical role of the tumor microenvironment in regulating tumor dormancy and relapse. The field of cancer has made many advancements in the development of therapeutics for targeted treatment of cancer, with renewed focus on gaining a more comprehensive understanding of tumor cell dormancy and its link with relapse of disease. In this review, we have addressed the key components of the tumor microenvironment and its role in dictating the progression to metastatic disease. Specifically, to ensure metastasis-free survival in cancer patients, it is critical to understand the biology of DTC survival via maintenance of dormancy and triggered reawakening of these cells leading to relapse, in addition to designing therapeutics blocking or eliminating the intricate signalling involved in dormancy. In the near future it will be essential to establish a thorough understanding of exosomes and their biology, specifically in the realm of exosome-mediated metastatic progression and dormancy. Answers to key questions regarding how these cancer cells are able to evade immune recognition and action are crucial to enhancing our ability to prevent metastatic progression of disease and to improve patient outcomes
Funding sources
NIH R01- Definition of the Microenvironment in Breast Cancer 2R01CA064786-20A1 (Bissell), NIH R01- Diversity Supplement (Attaran).
Footnotes
Declaration of Competing Interest
The authors declare that there are no conflicts of interest.
References
- [1].A pooled analysis of bone marrow micrometastasis in breast cancer, N. Engl. J. Med 353 (8) (2005). [DOI] [PubMed] [Google Scholar]
- [2].Ghajar C, Circulating and disseminated tumor cells: harbingers or initiators of metastasis?, - Dasgupta - 2019 −. Molecular Oncology Wiley Online Library, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bissell MJ, Kenny PA, Radisky DC, Microenvironmental regulators of tissue structure and function also regulate tumor induction and progression: the role of extracellular matrix and its degrading enzymes, Cold Spring Harb. Symp. Quant. Biol 70 (2005) 343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fiore A, Spencer VA, Mori H, Carvalho HF, Bissell MJ, Bruni-Cardoso A, Laminin-111 and the level of nuclear actin regulate epithelial quiescence via Exportin-6, Cell Rep. 19 (10) (2017) 2102–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Furuta S, Ren G, Mao JH, Bissell MJ, Laminin signals initiate the reciprocal loop that informs breast-specific gene expression and homeostasis by activating NO, p53 and microRNAs, Elife 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ghajar CM, Bissell MJ, Extracellular matrix control of mammary gland morphogenesis and tumorigenesis: insights from imaging, Histochem. Cell Biol 130 (6) (2008) 1105–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].The perivascular niche regulates breast tumour dormancy, Nat. Cell Biol 15 (7) (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Klein CA, Parallel progression of primary tumours and metastases, Nat. Rev. Cancer 9 (4) (2020) 302–312. [DOI] [PubMed] [Google Scholar]
- [9].JL A; MC A; KR H; MN R; R L; P F, Unknown primary carcinoma: natural history and prognostic factors in 657 consecutive patients. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 1994, 12 (6). [DOI] [PubMed] [Google Scholar]
- [10].H P; H Z; IR M; B C-S; A H; G R; B P; RN K; JF B; Y K; MJ B; TR C; AJ G; JT E; S H; CM G; D L, Pre-metastatic niches: organ-specific homes for metastases. Nature reviews. Cancer 2017, 17 (5). [DOI] [PubMed] [Google Scholar]
- [11].Paszek MJ, Weaver VM, The tension mounts: mechanics meets morphogenesis and malignancy, J. Mammary Gland Biol. Neoplasia 9 (4) (2004) 325–342. [DOI] [PubMed] [Google Scholar]
- [12].Conklin MW, Eickhoff JC, Riching KM, Pehlke CA, Eliceiri KW, Provenzano PP, Friedl A, Keely PJ, Aligned collagen is a prognostic signature for survival in human breast carcinoma, Am. J. Pathol 178 (3) (2011) 1221–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Egeblad M, Rasch MG, Weaver VM, Dynamic interplay between the collagen scaffold and tumor evolution, Curr. Opin. Cell Biol 22 (5) (2010) 697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gilkes DM, Chaturvedi P, Bajpai S, Wong CCL, Wei H, Pitcairn S, Hubbi ME, Wirtz D, Semenza GL, Collagen prolyl hydroxylases are essential for breast cancer metastasis, Cancer Res. 73 (11) (2013) 3285–3296. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [15].20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years, N. Engl. J. Med 377 (19) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bredfeldt JS, Liu Y, Conklin MW, Keely PJ, Mackie TR, Eliceiri KW, Automated quantification of aligned collagen for human breast carcinoma prognosis, J. Pathol. Inform 5 (2014) 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shekhar MP, Pauley R, Heppner G, Host microenvironment in breast cancer development: extracellular matrix–stromal cell contribution to neoplastic phenotype of epithelial cells in the breast, Breast Cancer Res. 5 (2003) 130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yan Mao ETK, David H. Garfield, Shen Kunwei, Stromal cells in tumor microenvironment and breast cancer | SpringerLink. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Orimo A, Weinberg RA, Stromal Fibroblasts in Cancer: A Novel Tumor-Promoting Cell Type, 2006, 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
- [20].Whiteside T, The tumor microenvironment and its role in promoting tumor growth, Oncogene 27 (45) (2008) 5904–5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Balkwill FR, Capasso M, Hagemann T, The Tumor Microenvironment at a Glance, 2012. [DOI] [PubMed] [Google Scholar]
- [22].Chan N, Willis A, Kornhauser N, Ward MM, Lee SB, Nackos E, Seo BR, Chuang E, Cigler T, Moore A, Donovan D, Vallee Cobham M, Fitzpatrick V, Schneider S, Wiener A, Guillaume-Abraham J, Aljom E, Zelkowitz R, Warren JD, Lane ME, Fischbach C, Mittal V, Vahdat L, Influencing the tumor microenvironment: a phase II study of copper depletion using tetrathiomolybdate in patients with breast cancer at high risk for recurrence and in preclinical models of lung metastases, Clin. Cancer Res 23 (3) (2017) 666–676. [DOI] [PubMed] [Google Scholar]
- [23].Pietras K, Ostman A, Hallmarks of cancer: interactions with the tumor stroma, Exp. Cell Res 316 (8) (2010) 1324–1331. [DOI] [PubMed] [Google Scholar]
- [24].Karagiannis GS, Schaeffer DF, Cho C-KJ, Musrap N, Saraon P, Batruch I, Grin A, Mitrovic B, Kirsch R, Riddell RH, Diamandis EP, Collective migration of cancer-associated fibroblasts is enhanced by overexpression of tight junction-associated proteins claudin-11 and occludin, Mol. Oncol 8 (2) (2014) 178–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shiga K, Hara M, Nagasaki T, Sato T, Takahashi H, Takeyama H, Cancer-Associated Fibroblasts: Their Characteristics and Their Roles in Tumor Growth, Cancers (Basel) 7 (2015) 2443–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cirri P, Chiarugi P, Cancer-associated-fibroblasts and tumour cells: a diabolic liaison driving cancer progression, Cancer Metastasis Rev. 31 (1–2) (2012) 195–208. [DOI] [PubMed] [Google Scholar]
- [27].Ronnov-Jessen L, Petersen OW, Bissell MJ, Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction, Physiol. Rev 76 (1) (1996) 69–125. [DOI] [PubMed] [Google Scholar]
- [28].Park JE, Lenter MC, Zimmermann RN, Garin-Chesa P, Old LJ, Rettig WJ, Fibroblast activation protein, a dual specificity serine protease expressed in reactive human tumor stromal fibroblasts, J. Biol. Chem 274 (51) (1999) 36505–36512. [DOI] [PubMed] [Google Scholar]
- [29].Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR, Reactive Stroma in Human Prostate Cancer, 2002. [PubMed] [Google Scholar]
- [30].Virchow R, Die Cellularpathologie in lhrer, in: Hirschwald A.(Ed.), Begruendung auf Physiologische und Pathologische Gewebelehre, 1858. Berlin, Germany. [Google Scholar]
- [31].Duvall M, in: Masson G.(Ed.), Atlas d’Embryologie, 1879. Paris, France. [Google Scholar]
- [32].Kalluri R, The biology and function of fibroblasts in cancer, Nat. Rev. Cancer 16 (9) (2016) 582. [DOI] [PubMed] [Google Scholar]
- [33].Kalluri R, Zeisberg M, Fibroblasts in cancer, Nat. Rev. Cancer 6 (5) (2006) 392–401. [DOI] [PubMed] [Google Scholar]
- [34].Erdogan B, Ao M, White LM, Means AL, Brewer BM, Yang L, Washington MK, Shi C, Franco OE, Weaver AM, Hayward SW, Li D, Webb DJ, Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin, J. Cell Biol 216 (11) (2017) 3799–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Friedl P, Locker J, Sahai E, Segall JE, Classifying collective cancer cell invasion, Nat. Cell Biol 14 (2012) 777–783. [DOI] [PubMed] [Google Scholar]
- [36].Sappino AP, Schurch W, Gabbiani G, Differentiation repertoire of fibroblastic cells: expression of cytoskeletal proteins as marker of phenotypic modulations, Lab. Invest 63 (2) (1990) 144–161. [PubMed] [Google Scholar]
- [37].Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, Sahai E, Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells, Nat. Cell Biol 9 (12) (2007) 1392. [DOI] [PubMed] [Google Scholar]
- [38].L.; Department of Medical Oncology, J. H., Medical School of Nanjing University, Nanjing, Jiangsu 210002, P.RL, Department of Medical Oncology, J. H, Medical School of Nanjing University, Nanjing, Jiangsu 210002, P.R. China, Cancer associated fibroblasts: An essential role in the tumor microenvironment (Review), Oncol. Lett 14 (3) (2018) 2611–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Buchheit CL, Weigel KJ, Schafer ZT, Cancer cell survival during detachment from the ECM: multiple barriers to tumour progression, Nat. Rev. Cancer 14 (2014) 632–641. [DOI] [PubMed] [Google Scholar]
- [40].Flier JS, Underhill LH, Dvorak HF, Tumors: wounds that do not heal, N. Engl. J. Med (1986). [DOI] [PubMed] [Google Scholar]
- [41].Ryan GB, Cliff WJ, Gabbiani G, Irle C, Statkov PR, Majno G, Myofibroblasts in an avascular fibrous tissue, Lab. Invest 29 (2) (1973) 197–206. [PubMed] [Google Scholar]
- [42].Ryan GB, Cliff WJ, Gabbiani G, Irle C, Montandon D, Statkov PR, Majno G, Myofibroblasts in human granulation tissue, Hum. Pathol 5 (1) (1974) 55–67. [DOI] [PubMed] [Google Scholar]
- [43].Shanley CJ, Gharaee-Kermani M, Sarkar R, Welling TH, Kriegel A, Ford JW, Stanley JC, Phan SH, Transforming growth factor-β1 increases lysyl oxidase enzyme activity and mRNA in rat aortic smooth muscle cells, J. Vasc. Surg 25 (3) (1997) 446–452. [DOI] [PubMed] [Google Scholar]
- [44].Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ, Reversion of the Malignant Phenotype of Human Breast Cells in Three- Dimensional Culture and In Vivo by Integrin Blocking Antibodies, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kenny PA, Bissell MJ, Tumor reversion: correction of malignant behavior by microenvironmental cues, Int. J. Cancer 107 (5) (2003) 688–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche, Cancer Cell 15 (1) (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].PLOS ONE, The Lysyl Oxidase Inhibitor, Î2-Aminopropionitrile, Diminishes the Metastatic Colonization Potential of Circulating Breast Cancer Cells. 10.1371/journal.pone.0005620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Cox TR, Rumney RM, Schoof EM, Perryman L, Hoye AM, Agrawal A, Bird D, Latif NA, Forrest H, Evans HR, Huggins ID, Lang G, Linding R, Gartland A, Erler JT, The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase, Nature 522 (7554) (2015) 106–110. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [49].RN K; RD R; S Z; AH B; L V; C C; DD M; DK J; K S; SA K; Z Z; D H; Y W; JL P; N A; ER P; D R; SV S; KK J; S R; D L, VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 2005, 438 (7069). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Tano K, Mizuno R, Okada T, Rakwal R, Shibato J, Masuo Y, Ijiri K, Akimitsu N, MALAT-1 enhances cell motility of lung adenocarcinoma cells by influencing the expression of motility-related genes, FEBS Lett. 584 (22) (2010) 4575–4580. [DOI] [PubMed] [Google Scholar]
- [51].Psaila B, Lyden D, The metastatic niche: adapting the foreign soil, Nat. Rev. Cancer (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Attaran S, Skoko JJ, Hopkins BL, Wright MK, Wood LE, Asan A, Woo HA, Feinberg A, Neumann CA, Peroxiredoxin-1 Tyr194 phosphorylation regulates LOX-dependent extracellular matrix remodelling in breast cancer, Br. J. Cancer (2021) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le Q-T, J Giaccia A, Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche, Cancer Cell 15 (1) (2009) 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion, Cancer Cell 13 (3) (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Breast Tumor Stiffness Instructs Bone Metastasis Via Maintenance of Mechanical Conditioning: Cell Reports, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ronai Ze., Senft Daniela, Immunogenic, cellular, and angiogenic drivers of tumor dormancy-a Melanoma view - Senft - 2016 - Pigment Cell & Melanoma Research. Pigment Cell and Melanoma Research, Wiley Online Library, 2015. [DOI] [PubMed] [Google Scholar]
- [57].Ghajar CM, Metastasis prevention by targeting the dormant niche, Nat. Rev. Cancer 15 (4) (2015) 238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Gawrzak S, Gomis, Roger Tumor cell dormancy, 2021, 10.1016/j.molonc.2016.09.009. [DOI] [PMC free article] [PubMed]
- [59].Aguirre-Ghiso J, Models, mechanisms and clinical evidence for cancer dormancy. Nature Reviews Cancer, 2007. Nature Reviews Cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Naumov GN, Townson JL, MacDonald IC, Wilson SM, Bramwell VHC, Groom AC, Chambers AF, Ineffectiveness of doxorubicin treatment on solitary dormant mammary carcinoma cells or late-developing metastases, Breast Cancer Res. Treat 82 (3) (2003) 199–206. [DOI] [PubMed] [Google Scholar]
- [61].Karagiannis GS, Pastoriza JM, Wang Y, Harney AS, Entenberg D, Pignatelli J, Sharma VP, Xue EA, Cheng E, D’Alfonso TM, Jones JG, Anampa J, Rohan TE, Sparano JA, Condeelis JS, Oktay MH, Neoadjuvant chemotherapy induces breast cancer metastasis through a TMEM-mediated mechanism, Sci. Transl. Med 9 (397) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].A M; L M; S L, EMT, CTCs and CSCs in tumor relapse and drug-resistance. Oncotarget 2015, 6 (13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Fenner J, Stacer AC, Winterroth F, Johnson TD, Luker KE, Luker GD, Macroscopic stiffness of breast tumors predicts metastasis, Sci. Rep 4 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Tumor dormancy in vivo by prevention of neovascularization, J. Exp. Med 136 (2) (1972). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].CA G; CM G, Metastasis’ systems’ biology: how are macro-environmental signals transmitted into microenvironmental cues for disseminated tumor cells? Current opinion in cell biology 2017, 48. [DOI] [PubMed] [Google Scholar]
- [66].Prolonged tumor dormancy by prevention of neovascularization in the vitreous, Cancer Res. 36 (8) (1976). [PubMed] [Google Scholar]
- [67].Clements ME, Johnson RW, Breast cancer dormancy in bone, Curr. Osteoporos. Rep 17 (5) (2019) 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].CN B; SA P, Cancer Dormancy: A Regulatory Role for Endogenous Immunity in Establishing and Maintaining the Tumor Dormant State. Vaccines 2015, 3 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].B A, Tumor Microenvironment. Medicina (Kaunas, Lithuania) 2019, 56 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].ML DA; F F; A Z, Breast Cancer Stem Cells as Drivers of Tumor Chemoresistance, Dormancy and Relapse: New Challenges and Therapeutic Opportunities. Cancers 2019, 11 (10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].A J-D; S A; BL H; JJ S; SA R; CA N, The peroxidase PRDX1 inhibits the activated phenotype in mammary fibroblasts through regulating c-Jun N-terminal kinases. BMC cancer 2019, 19 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].HE B; JT P; AA K; KJ H, The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nature reviews. Cancer 2015, 15 (7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].E W; MB R; C K; J T; E B; EA H; S M; HM W; M M; IM S; AM O; KH K; LA A; HB S, Lack of estrogen receptor-α is associated with epithelial-mesenchymal transition and PI3K alterations in endometrial carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research 2013, 19 (5). [DOI] [PubMed] [Google Scholar]
- [74].Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ, Collagen reorganization at the tumor-stromal interface facilitates local invasion, BMC Med. 4 (1) (2006) 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Conklin MW, Gangnon RE, Sprague BL, Gemert LV, Hampton JM, Eliceiri KW, Bredfeldt JS, Liu Y, Surachaicharn N, Newcomb PA, Friedl A, Keely PJ, Trentham-Dietz A, Collagen Alignment As a Predictor of Recurrence After Ductal Carcinoma in Situ, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Sanati S, Morphologic and molecular features of breast ductal carcinoma in situ, Am. J. Pathol (2018). [DOI] [PubMed] [Google Scholar]
- [77].Walens A, DiMarco AV, Lupo R, Kroger BR, Damrauer JS, Alvarez JV, CCL5 Promotes Breast Cancer Recurrence Through Macrophage Recruitment in Residual Tumors, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Tumor macrophages are pivotal constructors of tumor collagenous matrix, J. Exp. Med 213 (11) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].D A; A C, The inflammatory chemokine CCL5 and cancer progression. Mediators of inflammation 2014, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].AL H; NR P; P K; JF P; T D; RJ I; Y Z; J C; HR W; VM H, Glioblastoma Recurrence Correlates With Increased APE1 and Polarization Toward an Immuno-Suppressive Microenvironment. Frontiers in oncology 2018, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Effect of CCL5 expression in the recruitment of immune cells in triple negative breast cancer, Sci. Rep 8 (1) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Williams CB, Yeh ES, Soloff AC, Tumor-associated macrophages: unwitting accomplices in breast cancer malignancy, NPJ Breast Cancer (2016) 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, Segall JE, Pollard JW, Condeelis J, Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors, Cancer Res. 67 (6) (2007) 2649–2656. [DOI] [PubMed] [Google Scholar]
- [84].Macrophages and therapeutic resistance in cancer, Cancer Cell 27 (4) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy, Cancer Discov. 1 (1) (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].High density of CD204-positive macrophages predicts worse clinical prognosis in patients with breast cancer, Cancer Sci. 108 (8) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Ng MR, Brugge JS, A stiff blow from the stroma: collagen crosslinking drives tumor progression, Cancer Cell 16 (6) (2009) 455–457. [DOI] [PubMed] [Google Scholar]
- [88].A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer, Nat. Med 15 (1) (2009). [DOI] [PubMed] [Google Scholar]
- [89].Peinado H, Moreno-Bueno G, Hardisson D, Pérez-Gómez E, Santos V, Mendiola M, Diego J.Id., Nistal M, Quintanilla M, Portillo F, Cano A, Lysyl Oxidase–Like 2 As a New Poor Prognosis Marker of Squamous Cell Carcinomas, 2008. [DOI] [PubMed] [Google Scholar]
- [90].MJ T; WA C; MS H; PL K, Tumor microenvironment: a new treatment target for cancer. ISRN biochemistry 2014, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Lareu RR; Tissue Modulation Laboratory, D. o. B., Faculty of Engineering, National University of Singapore, Singapore; NUS Tissue Engineering Program, D. o. O. S., Yong Loo Lin School of Medicine, National University of Singapore, Singapore; Subramhanya, K. H.; Tissue Modulation Laboratory, D. o. B., Faculty of Engineering, National University of Singapore, Singapore; Peng, Y.; Tissue Modulation Laboratory, D. o. B., Faculty of Engineering, National University of Singapore, Singapore; Benny, P.; Tissue Modulation Laboratory, D. o. B., Faculty of Engineering, National University of Singapore, Singapore; Chen, C.; Tissue Modulation Laboratory, D. o. B., Faculty of Engineering, National University of Singapore, Singapore; Wang, Z.; Tissue Modulation Laboratory, D. o. B., Faculty of Engineering, National University of Singapore, Singapore; Rajagopalan, R.; Department of Chemical and Biomolecular Engineering, F. o. E., National University of Singapore, Singapore; Raghunath, M.; Tissue Modulation Laboratory, D. o. B., Faculty of Engineering, National University of Singapore, Singapore; Department of Biochemistry, Y. L. L. S. o. M., National University of Singapore, Division Office Block EA# 03–12, 9 Engineering Dr. 1, Singapore 117576, Singapore, Collagen matrix deposition is dramatically enhanced in vitro when crowded with charged macromolecules: The biological relevance of the excluded volume effect. FEBS Letters 2017, 581 (14), 2709–2714. [DOI] [PubMed] [Google Scholar]
- [92].Angiotensin receptor blocker use and gastro-oesophageal cancer survival: a population-based cohort study, Aliment. Pharmacol. Ther 47 (2) (2018). [DOI] [PubMed] [Google Scholar]
- [93].Löhr M, Schmidt C, Ringel J, Kluth M, Müller P, Nizze H, Jesnowski R, Transforming Growth Factor-β1 Induces Desmoplasia in an Experimental Model of Human Pancreatic Carcinoma, 2001. [PubMed] [Google Scholar]
- [94].R C; SH; AA C; NS Y; S D; B K; AC V; SA OT; AC P; A P; T P; RJ M; M A; KA L; EFW Y; KL W; CA H; NJ H; AJ G; CD C; M E; AM A; AL C, The angiotensin receptor blocker, Losartan, inhibits mammary tumor development and progression to invasive carcinoma. Oncotarget 2017, 8 (12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].B D-F; VP C; S K; Y B; RK J, Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proceedings of the National Academy of Sciences of the United States of America 2011, 108 (7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Canesin G, Cuevas EP, Santos V, López-Menéndez C, Moreno-Bueno G, Huang Y, Csiszar K, Portillo F, Peinado H, Lyden D, Cano A, Lysyl oxidase-like 2 (LOXL2) and E47 EMT factor: novel partners in E-cadherin repression and early metastasis colonization, Oncogene (2014). [DOI] [PubMed] [Google Scholar]
- [97].Choi J, Chung T, Rhee H, Kim YJ, Jeon Y, Yoo JE, Noh S, Han DH, Park YN, Increased expression of the matrix-modifying enzyme lysyl oxidase-like 2 in aggressive hepatocellular carcinoma with poor prognosis, Gut Liver (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Salvador F, Martin A, López-Menéndez C, Moreno-Bueno G, Santos V, Vázquez-Naharro A, Santamaria PG, Morales S, Dubus PR, Muinelo-Romay L, López-López R, Tung JC, Weaver VM, Portillo F, Cano A, Lysyl oxidase-like protein LOXL2 promotes lung metastasis of breast cancer, Cancer Res. 77 (21) (2017) 5846–5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Inhibition of TBK1 attenuates radiation-induced epithelial-mesenchymal transition of A549 human lung cancer cells via activation of GSK-3β and repression of ZEB1, Lab. Invest 94 (4) (2014). [DOI] [PubMed] [Google Scholar]
- [100].ZEB1 drives epithelial-to-mesenchymal transition in lung cancer, J. Clin. Invest 126 (9) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].TGF-β2 dictates disseminated tumour cell fate in target organs through TGF-β-RIII and p38α/β signalling, Nat. Cell Biol 15 (11) (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Ronnov-Jessen L, Petersen OW, Induction of alpha-smooth muscle actin by transforming growth factor-beta 1 in quiescent human breast gland fibroblasts. Implications for myofibroblast generation in breast neoplasia, Lab. Invest 68 (6) (1993) 696–707. [PubMed] [Google Scholar]
- [103].Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs, Cancer Cell 20 (4) (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Diagnostic role of circulating extracellular matrix-related proteins in non-small cell lung cancer, BMC Cancer 18 (1) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].AL P; TR C, The Role of the ECM in Lung Cancer Dormancy and Outgrowth. Frontiers in oncology 2020, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].<au>D, B.</au>; <au>AF, C.</au>, β1-integrin: a potential therapeutic target in the battle against cancer recurrence. Clinical cancer research : an official journal of the American Association for Cancer Research 2011, 17 (23). [DOI] [PubMed] [Google Scholar]
- [107].Clinical and biological significance of circulating tumor cells in cancer, Mol. Oncol 10 (3) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].AJ M; GP G; PM S; PD B; W S; DD G; A V; AB O; WL G; J M, Genes that mediate breast cancer metastasis to lung. Nature 2005, 436 (7050). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Y G; Q X; H M; L L; J L; Y F; Z F; J W; X H; J Z; Y S; G W; R P; H C; KK W; G G; H J, LKB1 inhibits lung cancer progression through lysyl oxidase and extracellular matrix remodeling. Proceedings of the National Academy of Sciences of the United States of America 2010, 107 (44). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].MS S; P B; JA A-G, Mechanisms of disseminated cancer cell dormancy: an awakening field. Nature reviews. Cancer 2014, 14 (9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Capturing changes in the brain microenvironment during initial steps of breast cancer brain metastasis, Am. J. Pathol 176 (6) (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].The metastatic microenvironment: brain-residing melanoma metastasis and dormant micrometastasis, Int. J. Cancer 131 (5) (2012). [DOI] [PubMed] [Google Scholar]
- [113].Y D; J R; M R; ND S-K; J G; BP M; J B; R A; D M, Brain metastases in melanoma: roles of neurotrophins. Neuro-oncology 2004, 6 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Mechanisms of cancer cell dormancy–another hallmark of cancer, Cancer Res. 75 (23) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].GN N; E B; D Z; SY K; D S; E F; RS W; O S; LA A; J F; N A, A model of human tumor dormancy: an angiogenic switch from the nonangiogenic phenotype. Journal of the National Cancer Institute 2006, 98 (5). [DOI] [PubMed] [Google Scholar]
- [116].Real-time imaging reveals the single steps of brain metastasis formation, Nat. Med 16 (1) (2010). [DOI] [PubMed] [Google Scholar]
- [117].Bone metastasis: interaction between cancer cells and bone microenvironment, J. Oral Biosci 61 (2) (2019). [DOI] [PubMed] [Google Scholar]
- [118].The biology of bone metastasis, Cold Spring Harb. Perspect. Med 8 (6) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Red-yellow marrow conversion: its effect on the location of some solitary bone lesions, Skeletal Radiol. 14 (1) (1985). [DOI] [PubMed] [Google Scholar]
- [120].GR M, Metastasis to bone: causes, consequences and therapeutic opportunities. Nature reviews. Cancer 2002, 2 (8). [DOI] [PubMed] [Google Scholar]
- [121].N H; M U; L P; W E, Tumour cell detection in the bone marrow of breast cancer patients at primary therapy: results of a 3-year median follow-up. British journal of cancer 1994, 69 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].SW M; E C; WJ E; AA R; TJ L; MM O; PH L; RL V, Early tumor cell dissemination in patients with clinically localized carcinoma of the prostate. Clinical cancer research : an official journal of the American Association for Cancer Research 1997, 3 (2). [PubMed] [Google Scholar]
- [123].HD A; KC V; SR A; JL H; P P; G T; AM D; KJ P, AXL Is a Putative Tumor Suppressor and Dormancy Regulator in Prostate Cancer. Molecular cancer research : MCR 2019, 17 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Y S; EA P; LR P; AM Z; AM H; Y J; J W; S Z; RD L; KJ P; RS, GAS6/AXL axis regulates prostate cancer invasion, proliferation, and survival in the bone marrow niche. Neoplasia (New York, N.Y.) 2010, 12 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Mechanisms of metastatic tumor dormancy and implications for cancer therapy, Int. J. Mol. Sci 20 (24) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Breast cancer dormancy in bone, Curr. Osteoporos. Rep 17 (5) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Y J; J W; A S; YX S; AJ K-P; NI O; LK M; RS T, Regulation of SDF-1 (CXCL12) production by osteoblasts; a possible mechanism for stem cell homing. Bone 2006, 38 (4). [DOI] [PubMed] [Google Scholar]
- [128].D S; L S; B G; Y L; NJ C; PM S; ML C; S S; U V; A S; SR C, A novel cross-talk between CXCR4 and PI4KIIIα in prostate cancer cells. Oncogene 2019, 38 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Pharmacological targeting of CXCL12/CXCR4 signaling in prostate cancer bone metastasis, Mol. Cancer 15 (1) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].GAS6/Mer axis regulates the homing and survival of the E2A/PBX1-positive B- cell precursor acute lymphoblastic leukemia in the bone marrow niche, Exp. Hematol 38 (2) (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].K Y; MR E; J W; FC C; AM D; E L; AR N; JA A-G; Y J; RS T, Axl is required for TGF-β2-induced dormancy of prostate cancer cells in the bone marrow. Scientific reports 2016, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Osteoblast-secreted factors mediate dormancy of metastatic prostate cancer in the bone via activation of the TGFβRIII-p38MAPK-pS249/T252RB pathway, Cancer Res. 78 (11) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].MA L; MM M; N K; W HK; RL T; J D; W K; J P-H; C F; JA P; T ND; E VV; PA B; MJ R; CL E; K V; AR P; JM Q; AC Z; TG P; PI C, Osteoclasts control reactivation of dormant myeloma cells by remodelling the endosteal niche. Nature communications 2015, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].A H; B C-S; TL S; G R; A H; M TM; H M; S K; A DG; S C; S S; C W; N S; K U; L P; T K; L B; AE D; Y A; T Z; H Z; J H; JM W; VD D-C; K K; LH W; A N; GK S; JH H; P S; KJ L; EH K; PM G; MA H; M d. S.; S K; M J; K M; SK B; WR J; MS B; O F; V M; K P; AJ M; MJ B; BA G; Y K; VK R; CM G; I M; H P; J B; D L, Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527 (7578). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].C K; R K, Exosomes in tumor microenvironment influence cancer progression and metastasis. Journal of molecular medicine (Berlin, Germany) 2013, 91 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Exosomes play roles in sequential processes of tumor metastasis, Int. J. Cancer 144 (7) (2019). [DOI] [PubMed] [Google Scholar]
- [137].J L; M T; Q X; Z Z; H Y, Thrombin-activated platelet-derived exosomes regulate endothelial cell expression of ICAM-1 via microRNA-223 during the thrombosis-inflammation response. Thrombosis research 2017, 154. [DOI] [PubMed] [Google Scholar]
- [138].I W; S D; CM K; D L, Exosome-Mediated Metastasis: Communication from a Distance. Developmental cell 2019, 49 (3). [DOI] [PubMed] [Google Scholar]
- [139].Zheng P, Chen L, Yuan X, Luo Q, Liu Y, Xie G, Ma Y, Shen L, Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells, J. Exp. Clin. Cancer Res 36 (1) (2017) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].KE R; AE Z; ML F; J W; LE L; R H, Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene 2017, 36 (13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].L C; Y W; L H, Exosomes from M1-Polarized Macrophages Potentiate the Cancer Vaccine by Creating a Pro-inflammatory Microenvironment in the Lymph Node. Molecular therapy : the journal of the American Society of Gene Therapy 2017, 25 (7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].L Z; S K; P G; JM O; HW L; SH B; SY J; SW L; J L; BC A, Exosomes Derived From Natural Killer Cells Exert Therapeutic Effect in Melanoma. Theranostics 2017, 7 (10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].ET G; I B; SR R; CM G, Dormant tumour cells, their niches and the influence of immunity. Nature cell biology 2018, 20 (11). [DOI] [PubMed] [Google Scholar]
- [144].(EBCTCG), E. B. C. T. C. G, Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials, Lancet (London, England) 365 (9472) (2005). [DOI] [PubMed] [Google Scholar]
- [145].C D; H P; J G; R G; R A; V R; M A; VH MA; A B; X B; J B; M C; R C; SR D; A D; JF F; P H; MF H; M I; H K; J K; WH K; BS M; I M; B M; A N; O P; F P; L P; T P; R R; B R; MT R; S T; G U; M V; Y W; R P, Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet (London, England) 2013, 381 (9869). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].MH M, The premise of personalized immunotherapy for cancer dormancy. Oncogene 2020, 39 (22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].B N; M S; RS F; G W; K W; T R; C K; I M; L V; HH S; AB S; MC R; C BS; EA W; E B, Clinical outcome with correlation to disseminated tumor cell (DTC) status after DTC-guided secondary adjuvant treatment with docetaxel in early breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2014, 32 (34). [DOI] [PubMed] [Google Scholar]
- [148].Jezierska-Drutel A, Rosenzweig SA, Neumann CA, Role of oxidative stress and the microenvironment in breast cancer development and progression, Adv. Cancer Res 119 (2013) 107–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Radiation-induced inflammatory cascade and its reverberating crosstalks as potential cause of post-radiotherapy second malignancies, Cancer Metastasis Rev. 36 (2) (2017). [DOI] [PubMed] [Google Scholar]
- [150].SA S; M Z; SH M; G G; XY W; MH M, Immunotherapy of cancer: targeting cancer during active disease or during dormancy? Immunotherapy 2017, 9 (11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Discovery of a novel unfolded protein response phenotype of cancer stem/ progenitor cells from the bone marrow of breast cancer patients, J. Proteome Res 9 (6) (2010). [DOI] [PubMed] [Google Scholar]
- [152].Functional coupling of p38-induced up-regulation of BiP and activation of RNA-dependent protein kinase-like endoplasmic reticulum kinase to drug resistance of dormant carcinoma cells, Cancer Res. 66 (3) (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].JB N; MS U; G G; BR P, Endothelin-1 inhibits apoptosis in prostate cancer. Neoplasia (New York, N.Y.) 2005, 7 (7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Emerging role of the endothelin axis in ovarian tumor progression, Endocr. Relat. Cancer 12 (4) (2005). [DOI] [PubMed] [Google Scholar]
- [155].C L; MM v. B.; L B; EM V; R S; JJ C; S B; A V; H H; DE A; M V; MR S; JB H; H S; SH v. d. B.; TN S, High-throughput epitope discovery reveals frequent recognition of neo-antigens by CD4+ T cells in human melanoma. Nature medicine 2015, 21 (1). [DOI] [PubMed] [Google Scholar]
- [156].PA O; Z H; DB K; SA S; J S; DJ B; W Z; A L; A G-H; L P; C C; O O; TA C; S L; DJ L; T E; E G; J S; WJ L; I J; K N; AM S; H D; M S; EI B; CH Y; M H; N L; S G; SJ R; DH B; JC A; G G; K W; D N; J R; ES L; EF F; N H; CJ W, An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017, 547 (7662). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].N H; S K-C; K F; V B; S S; C G; M P; G T; V D; SH v. d. B.; P TS; F M-R; B P; H O; U L; A A; CH O; A U; S K; A v. D.; M S; D M; JR K; M I; J R; J P; HS P; B S; K M; R M; M L; M S; CM B; D C; MJP W; J S; K K; E D; E R; L B; C S; S H; C W; A K-B; J L; JC C; O S; AD T; E G; J F; M M; N P; S D; F H; B R; D M; T W; C R; C H; HG R; H S-J; U S; PY D; W W, Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature 2019, 565 (7738). [DOI] [PubMed] [Google Scholar]
- [158].P Z; GP D; T L; M G; OL G; R U, Neoantigens in immunotherapy and personalized vaccines: Implications for head and neck squamous cell carcinoma. Oral oncology 2017, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Tumor neoantigens: building a framework for personalized cancer immunotherapy, J. Clin. Invest 125 (9) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].CH J; RS OC; OU K; S G; MC M, CAR T cell immunotherapy for human cancer. Science (New York, N.Y.) 2018, 359 (6382). [DOI] [PubMed] [Google Scholar]