Abstract
Introduction
The optimization of oral health before allogeneic hematopoietic cell transplantation (HCT) is important for preventing infectious complications during treatment.
Objective
The objective of this study was to evaluate the oral health condition and dental treatments performed in patients in pre-allogeneic HCT.
Method
The records of patients treated during 2018 at a Brazilian HCT service were reviewed.
The following oral health data were obtained: 1. Decayed, missing and filled teeth / correlated index for primary dentition (DMFT/dmft); 2. Quality of oral hygiene and 3. Dental pathologies: 3.1 Periodontal infectious focus, 3.2 Endodontic infectious focus and 3.3 Carie incidence. All dental procedures performed were surveyed.
Results
Thirty-three patients were included, with a mean age of 28.42 (±16.37), 20 male (60%) and 13 female. The average DMFT/dmft found in this study was 10.24 (± 8.37), similar to the index found in the population in southeastern Brazil. The younger study population presented a DMFT/dmft considered high, when compared to the general population. A total of 27.2% of the patients had active caries lesions, 33.3%, foci of periodontal infection, 15.1%, endodontic infectious focus and 40%, poor oral hygiene. Almost half of the patients (48.4%) had to undergo dental intervention, 24.2% needing periodontal scaling, 21.2%, fillings and 12.1%, tooth extractions.
Conclusion
We conclude that the studied population had an important incidence of dental pathologies and infectious conditions that could complicate throughout HCT, especially in younger patients, therefore presenting a high demand for dental treatment in the pre-HCT. Studies that assess the impact of dental conditioning on the outcomes of HCT with an emphasis on dental infectious complications, days of hospitalization and survival are necessary.”
Keywords: Bone marrow transplantation, Oral health, Focal infection, Dentistry
Introduction
Hematopoietic cell transplantation (HCT) is a consolidated therapy for the treatment of several spinal disorders, autoimmune diseases, solid tumors and hematological conditions.1 The disintensification of conditioning protocols, the development of new immunosuppressive drugs, antibiotics and the increased availability of donors, especially haploidentical donors, has contributed to the increase in transplant recipients.2,3
Infectious complications are the main causes of death in HCT.4 The literature has highlighted the importance of oral health conditions in pre-HCT patients due to systemic repercussions of local infection during the neutropenia. Bacteremia during neutropenia in patients undergoing HCT is often positive for Viridans streptococci, a common bacteria in odontogenic infections.5, 6, 7
Graber et al.8 identified a correlation between poor oral health and bacteremia by Viridans streptococci during HCT, especially in patients with endodontic infectious foci. Another study showed that 32% of the patients with leukemia had acute oral infection associated with fever during chemotherapy. A total of 58% of these patients had no other identifiable sources of infection.9 Laine et al.10 described an association between febrile episodes and odontogenic infections during chemotherapy for lymphoma. A recent study showed a low rate of complications due to oral infectious focus in patients who had undergone a dental preparation protocol prior to transplantation.11
Nausea and mucositis decrease the quality of oral hygiene because they cause discomfort when manipulating the oral cavity. This condition combined with reduced salivary flow is often related to rapid progression of periodontal disease and caries.12, 13, 14 Studies have shown that patients undergoing oral care have a lower incidence of oral mucositis15,16 and a lower risk of gingival bleeding.12
In this context, eliminating dental foci of infection before HCT has been suggested.5, 6, 7, 8,12 Nevertheless, there is limited information about the oral health status of these patients. The aim of the present study was to assess the oral health condition in patients with an indication for allogeneic hematopoietic stem cell transplantation (allo-HCT) at a Brazilian tertiary level hospital.
Methods
This was a prospective study conducted at a public Brazilian tertiary hospital. This institution has 28 years of experience in dental care for HCT recipients. All study participants signed an informed consent form. The study was conducted in accordance with the Helsinki Declaration, as revised in 2008. After approval by the local Research Ethics Committee, Certificado de Apresentação de Apreciação Ética (CAAE) no. 81213917.0.0000.5440, patients submitted to the first allogeneic HCT during 2018 were included for convenience. Individuals who had not undergone dental evaluation before the HCT or who had incomplete or inadequate filing in medical and dental records were excluded. All patients enrolled underwent dental evaluation, including clinical and radiographic examination. Infection and inflammatory conditions were treated before initiating the HCT.
During pre-allo-HCT dental appointments, data related to the oral health condition and dental procedures performed were registered by the same dentist for all patients. These data were used to compose the variables of this study. The outcomes of interest for the assessment of oral health condition were: decayed, missing and filled teeth index (DMFT); decayed, extraction indicated and filled deciduous teeth index (dmft), and; quality of oral hygiene, infectious focus (periodontal and endodontic) and dental carie incidence identified.
The DMFT is an index that had been formulated in 1937 by Klein and Palmer and is still recommended by the World Health Organization (WHO) to measure and evaluate the experience of dental caries in groups of individuals. Its value represents the average of decayed, missing and filled teeth in a given studied population.17 The dmft index was used to evaluate patients with primary dentition. It is an adaptation of the DMFT proposed by Gruebbel in 1944.18 In this study, the dmft record also followed the parameters indicated by the WHO.17
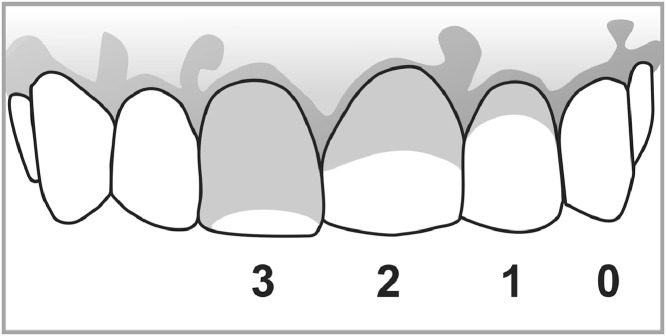
The quality of oral hygiene was assessed using the Silness and Löe plaque index,19 which measures the presence of biofilm on the surface of index teeth. For the analysis, the following scores are attributed: 0 - Absence of plaque in the gingival area recognized by running a probe across the tooth surface; 1- A film of plaque adhering to the free gingival margin and adjacent area of the tooth. The plaque may only be recognized by running a probe across the tooth surface; 2- Moderate accumulation of soft deposits within the gingival pocket, on the gingival margin and/or adjacent tooth surface, which can be seen by the naked eye; 3- Abundance of soft matter within the gingival pocket and/or on the gingival margin and adjacent tooth surface (Figure 1). The scores of the four tooth areas are added and divided by four. The plaque index is obtained by adding all teeth scores and dividing by the number of teeth examined.19 In our study, patients with final indexes of 0 or 1 were classified as having satisfactory oral hygiene, while patients with indexes 2 or 3 were classified as having poor oral hygiene.
Figure 1.
Silness and Löe plaque index scores.
Gray in the tooth surface represents plaque
Cases of gingivitis and/or active periodontitis, confirmed by active bleeding on periodontal probing, were defined as periodontal infectious foci. Symptomatic teeth that showed radiographic periapical lesions and cases of pulp necrosis, without the presence of treatment and/or temporary root canal filling, were considered foci of endodontic infection. The requirement for dental treatment was obtained from the registered dental procedures in the first pre-HCT appointment prior to the dentist release for transplantation.
The data found were submitted to descriptive evaluation. The Brazilian Oral Health Survey performed in 2010 was used as a reference to discuss our results. Therefore, we used the same classification by age group as recommended by the survey. This classification follows the criteria established by the WHO in its manuals for surveys in oral health, the latest version having been published in 2013.17 Patients not belonging to the age groups used in the survey were allocated to intermediate groups.
Results
Thirty-three patients were included in the study, 20 (60.6%) being male. The average age was 28.4 years (±16.37). The underlying diseases in the studied patients were: 8 cases of acute myeloid leukemia (34.7%); 7, acute lymphocytic leukemia (21.2%); 7, sickle cell anaemia (21.2%); 4, myelodysplastic syndrome (12.1%); 3, severe aplastic anaemia (9%); 2, Fanconi's anaemia (6%); 1, chronic myeloid leukemia (3%), and; 1, non-Hodgkin's lymphoma (3%). Table 1 shows data of underlying disease and oral health conditions. Table 2 shows the results of DMFT/dmft by age group and those found in the survey of the general population in southeastern Brazil, the same region where the study was developed.20 The average DMFT/dmft in the study patients was 10.24 (±8.37), similar to the value of 10.3 in the last Brazilian Oral Health Survey for the same region of the study. In the 15-to-19-year age group, there was a predominance of the “decayed” component (85.7% of the DMFT), while in the National Survey, filled teeth were more frequent (59.5% of the DMFT). In the 35-to-44-year age group, there was a predominance of the filled component in pre-HCT patients (82.7% of DMFT) and filled and lost in the index obtained from the national survey (48.2% and 41.2% of DMFT, respectively).
Table 1.
Underlying disease and oral health conditions.
| Underlying desease (n) | Age | Poor oral hygiene (n/%) | DMFT/dmft | Infection Focus n (%) |
|---|---|---|---|---|
| Fanconi's anemia (2) | 11 (10–12) | 2 (100%) | 7(±1.41) | 2 (100%) |
| Sickle cell anemia (7) | 16 (11–30 | 2 (28.5%) | 4.42(±4.82) | 2 (28.5%) |
| Acute lymphocytic leukemia (7) | 20 (4–53) | 2 (28.5%) | 5.42(±4.79) | 3 (42.8%) |
| Severe aplastic anemia (3) | 26 (21–30) | 1 (33.3%) | 10(±2.64) | 0 (0%) |
| Acute myeloid leukemia (8) | 31 (14–63) | 4 (50%) | 12.6(±8.07) | 4 (50%) |
| Chronic myeloid leukemia (1) | 45 | 0 (0%) | 18 | 1 (100%) |
| Myelodysplastic syndrome (4) | 49.5 (46–58) | 2 (50%) | 20.25(±8.34) | 1 (25%) |
| non-Hodgkin's lymphoma (1) | 56 | 0 (0%) | 17 | 1 (100%) |
Table 2.
DMFT/dmft by age group
| Age group | n | % | Study patient data |
Southeast parameter Brazilian oral health survey 2010* |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DMFT/dmft | D(%) | M(%) | F(%) | DMFT/dmft | D(%) | M(%) | F(%) | |||
| Equal or less than 5 years | 1 | 3.00% | 0.0 | 0.0 | 0.0 | 0.0 | - | - | - | - |
| 6 to 11 years | 3 | 9.00% | 4.33(±3.21) | 29.1 | 0 | 70.8 | - | - | - | - |
| 12 years | 3 | 9.00% | 4(±3.46) | 66.6 | 33.3 | 0.0 | 1.72 | 49.4 | 6.4 | 44.8 |
| 13 to 14 years | 2 | 6.00% | 5(±1.41) | 25.0 | 0 | 75.0 | - | - | - | - |
| 15 to 19 years | 3 | 9.00% | 3(±3.60) | 85.7 | 7.14 | 7.14 | 3.83 | 32.4 | 8.1 | 59.5 |
| 20 to 34 years | 10 | 30.30% | 8.1(±3.34) | 11.5 | 10.7 | 77.7 | - | - | - | - |
| 35 to 44 years | 3 | 9.00% | 15.33(±4.50) | 0.0 | 17.2 | 82.77 | 16.36 | 10.6 | 41.2 | 48.2 |
| 45 to 64 years | 8 | 24.20% | 20.87(±8.14) | 0.8 | 48.9 | 51.1 | - | - | - | - |
(20) D: decayed teeth; M: missing teeth; F: filled teeth.
Table 3 shows the results for the quality of oral hygiene, focus of infection, number of patients undergoing procedures and number/types of procedures performed. Table 4 shows the percentage of dental procedures performed and infectious pathologies found in the oral cavity of the patients studied by age group. Overall, 27.2% of the patients had caries lesions, 33.3%, foci of periodontal infection, 30.3%, gingivitis and 3%, periodontitis; 15.1% had endodontic infectious foci, 24.2% underwent periodontal scaling and 21.2% were submitted to dental restorative treatment.
Table 3.
Oral hygiene, presence of focus of infection and procedures performed in the pre-allo-HCT.
| Quality of oral hygiene (n = 33) | Infection / Infection Focus (n = 33) | Procedures performed (n = 33) |
|---|---|---|
| 60%: Satisfactory (n = 20) | Periodontal: 33% (n = 11) | Patients undergoing procedures: 48.48% (n = 16) |
| 40%: Poor (n=13) | Endodontic: 15% (n = 5) | Total procedures: 43: -18 Fillings (41.8%) -9 Scalings (20.9%) -9 Extractions (20.9%) -4 Professional teeth cleaning (9.3%) -1 Operculectomy (2.3%) -1 Removal of orthodontic appliances (2.3%) -1 Temporary root canal filling (2.3%) |
Table 4.
Procedures performed and oral pathologies found in the study patients.
| Age groups | Procedures % |
Oral pathologies % |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fillings | Scalings | Extractions | Professional tooth cleaning | Temporary root canal filling | Decay | Periodontal infectious focus | Endodontic infectious focus | ||||
| Equal or less than 5 years | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| 6 to 11 years | 33.3 | 0 | 33.3 | 33.3 | 0 | 33.3 | 33.3 | 33.3 | |||
| 12 years | 33.3 | 66.6 | 33.3 | 0 | 0 | 66.6 | 66.6 | 33.3 | |||
| 13 to 14 years | 50 | 0 | 0 | 100 | 0 | 50 | 100 | 0 | |||
| 15 to 19 years | 33.3 | 33.3 | 33.3 | 0 | 0 | 66.6 | 0 | 33.3 | |||
| 20 to 34 years | 30 | 30 | 10 | 10 | 0 | 40 | 30 | 10 | |||
| 35 to 44 years | 0 | 33.3 | 0 | 0 | 0 | 0 | 33.3 | 0 | |||
| 45 to 64 years | 0 | 25 | 0 | 0 | 12.5 | 12.5 | 25 | 12.5 | |||
| TOTAL | 21.21 | 24.24 | 12.12 | 12.12 | 3.03 | 27.27 | 33.33 | 15.15 | |||
Discussion
Although the overall DMFT/dmft found was similar to that in the last Brazilian Survey, our youngest patients (6-to-11 year and 12-year age groups) had an index considered high for their age. In the 12-year age group, patients had a DMFT 2.32 times greater than that in the Brazilian Oral Health Survey 2010, with 66.6% of decayed teeth, suggesting a lack of assistance. The oral health condition in our 6-to-11-year-old patients (dmft: 4.33) appears to be worse than that in the Brazilian population (dmft-5 years: 2.10 and DMFT-12 years: 1.75). We believe that this finding is due to the neglect of the dental caregiving priority to the systemic requirements presented by the patient. Another possible reason is that half of our patients in these age groups have Fanconi's anemia. Poor oral hygiene has been reported as a common finding in these patients; decreased salivary flow and reduced calcium and urea in the saliva have also been described.21, 22, 23 Furthermore, other underlying diseases, such as sickle cell anemia and leukemias, have been linked to increased oral pathology risks. The literature points to aspects of the pathophysiology of these diseases as an explanation.24, 25, 26 In this study, the variation of the DMFT/dmft seems to be more related to age than to the type of underlying diseases with the exception for Fanconi's anemia.
In agreement with our results, Lucas et al.27 evaluated pediatric patients and found that children in the pre-HCT had a significantly higher dmft index than the control group. Another study conducted at St. Jude Children's Research Hospital (Memphis, USA) with 259 pediatric patients undergoing HCT between 1990 and 2000 identified caries in 51% of patients, which resulted in a high demand for dental procedures.14
The high incidence of active oral pathologies with therapeutic requirements found in our study (33% of patients with periodontal focus, 15% with endodontic focus and 27% with active caries) is in agreement with the literature. Yamagata et al.28 found caries in 30% and gingivitis in 23.3% of the children in pre-HCT. A study carried out in Israel with 46 pre-HCT patients with an average age of 37 years (6 to 63 years) found caries in 50% and gingivitis in 15.2% of the patients.29 Another recent American study found an average DMFT of 17 in its sample of 375 patients.11 Such studies reinforce that these patients may have a high incidence of oral pathologies.
Almost half of our patients (48.4%) had to undergo dental procedures to prepare the oral environment; the most common procedures were scaling (23.53%), fillings (22.48%) and extractions (13.73%). The study carried out in Israel also involved children and adult patients and presented a similar demand for procedure: 47.8% of the patients needed scaling, 39.1%, filling and 19.5%, tooth extraction.29 Japanese children in pre-HCT also had a high demand for dental procedures, 20% needing filling, 13.2%, endodontic intervention and 10%, extraction.28 The American study pointed out the need for treatment in 32.6% of the patients.11
It is useful to know the profile of oral health and the dental care needs in patients who will undergo HCT. This knowledge can help the oncology and dentistry team to eliminate possible sources of oral infection. It has been shown that chronic foci of infection can become acute during antineoplastic treatment.5, 6, 7, 8, 9, 10 Schuurhuis et al., found that 4% of the patients undergoing stem cell transplantation had reactivation of previously identified chronic infectious foci.30 Skallsjöa et al. evaluated 213 patients treated with chemotherapy for lymphoma and found 86 patients with apical periodontitis (AP), 7 (8%) of whom developed local symptoms related to teeth with AP. No patients in the control group developed symptoms.31 We reported patients with caries, foci of periodontal and endodontic infection who were at risk of infectious exacerbation during antineoplastic therapy, as supported by the literature. Dental preparation protocols in patients who will undergo cancer therapy have been responsible for the decrease in infectious reactivation.11
The sample size and the variation of age and of underlying diseases in our study did not allow a statistical evaluation of the impact of the diseases and treatments on the oral health of our patients. Although we used the data from the local survey to discuss our finds, it was not possible to make a statistical comparison between the oral health status of our population and that of the general population. However, as the variation in age and underlying diseases is routine in HCT units, we believe that our results translate the oral health conditions and the need for dental intervention in these patients.
As far as we know, this is the first Brazilian study that evaluated the oral health condition of patients undergoing allo-HCT. We can conclude that the studied population had an important incidence of oral pathologies and infectious conditions that could complicate throughout HCT, especially among younger patients. Studies that assess the impact of dental preparation performed on the outcome of the transplant with an emphasis on infectious complications, days of hospitalization and survival are necessary.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
We thank the dentists, medical and nursing staffs of the Ribeirão Preto Clinical Hospital for their support.
References
- 1.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354(17):1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 2.Thomas ED, Lochte HL, Lu WC, Ferrebee JW. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257(11):491–496. doi: 10.1056/NEJM195709122571102. [DOI] [PubMed] [Google Scholar]
- 3.Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, et al. Guidelines for preventing infectious complications among hematopoietic cell transplant recipients: a global perspective. Bone Marrow Transplant. 2009;15(10):1143–1238. doi: 10.1038/bmt.2009.254. [DOI] [PubMed] [Google Scholar]
- 4.Sahin U, Toprak SK, Atilla PA, Atilla E, Demirer T. An overview of infectious complications after allogeneic hematopoietic cell transplantation. J Infect Chemother. 2016;22(08):505–514. doi: 10.1016/j.jiac.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Bochud PY, Eggiman P, Calandra T, Van Melle G, Saghafi L, Francioli P. Bacteremia due to viridans streptococcus in neutropenic patients with cancer: clinical spectrum and risk factors. Clin Infect Dis. 1994;18(1):25–31. doi: 10.1093/clinids/18.1.25. [DOI] [PubMed] [Google Scholar]
- 6.Bochud PY, Calandra T, Francioli P. Bacteremia due to viridans streptococci in neutropenic patients: a review. Am J Med. 1994;97(3):256–264. doi: 10.1016/0002-9343(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 7.Martino R, Manteiga R, Sánchez I, Brunet S, Sureda A, Badell I, et al. Viridans streptococcal shock syndrome during bone marrow transplantation. Acta Haematol. 1995;94(2):69–73. doi: 10.1159/000203976. [DOI] [PubMed] [Google Scholar]
- 8.Graber CJ, de Almeida K, Atkinson JC, Javaheri D, Fukuda CD, Gill VJ, et al. Dental health and viridans streptococcal bacteremia in allogeneic hematopoietic cell transplant recipients. Bone Marrow Transplant. 2001;27(5):537–542. doi: 10.1038/sj.bmt.1702818. [DOI] [PubMed] [Google Scholar]
- 9.Peterson DE, Overholser CD. Increased morbidity associated with oral infection in patients with acute nonlymphocytic leukemia. Oral Surg Oral Med Oral Pathol. 1981;51(4):390–393. doi: 10.1016/0030-4220(81)90148-1. [DOI] [PubMed] [Google Scholar]
- 10.Laine PO, Lindqvist JC, Pyrhönen SO, Strand-Pettinen IM, Teerenhovi LM, Meurman JH. Oral infection as a reason for febrile episodes in lymphoma patients receiving cytostatic drugs. Eur J Cancer B Oral Oncol. 1992;28B(2):103–107. doi: 10.1016/0964-1955(92)90036-z. [DOI] [PubMed] [Google Scholar]
- 11.Hansen HJ, Estilo C, Owosho A, Solano AK, Randazzo J, Huryn J, et al. Dental status and risk of odontogenic complication in patients undergoing hematopoietic stem cell transplant. Support Care Cancer. [Internet] 2020:1–8. doi: 10.1007/s00520-020-05733-1. https://www.researchgate.net/publication/344213667_Dental_status_and_risk_of_odontogenic_complication_in_patients_undergoing_hematopoietic_stem_cell_transplant cited 2020 sept 15Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elad S, Raber-Durlacher JE, Brennan MT, Saunders DP, Mank AP, Zadik Y, et al. Basic oral care for hematology–oncology patients and hematopoietic cell transplantation recipients: a position paper from the joint task force of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) and the European Society for Blood and Marrow Transplantation (EBMT) Support Care Cancer. 2015;23(1):223–236. doi: 10.1007/s00520-014-2378-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Epstein JB, Thariat J, Bensadoun R-J, Barasch A, Murphy BA, Kolnick L, et al. Oral complications of cancer and cancer therapy. CA Cancer J Clin. 2012;62(6):400–422. doi: 10.3322/caac.21157. [DOI] [PubMed] [Google Scholar]
- 14.Vaughan MD, Rowland CC, Tong X, Srivastava DK, Hale GA, Rochester R, et al. Dental abnormalities in children preparing for pediatric bone marrow transplantation. Bone Marrow Transplant. 2005;36(10):863–866. doi: 10.1038/sj.bmt.1705111. [DOI] [PubMed] [Google Scholar]
- 15.Kashiwazaki H, Matsushita T, Sugita J, Shigematsu A, Kasashi K, Yamazaki Y, et al. Professional oral health care reduces oral mucositis and febrile neutropenia in patients treated with allogeneic bone marrow transplantation. Support Care Cancer. 2012;20(2):367–373. doi: 10.1007/s00520-011-1116-x. [DOI] [PubMed] [Google Scholar]
- 16.Saito H, Watanabe Y, Sato K, Ikawa H, Yoshida Y, Katakura A, et al. Effects of professional oral health care on reducing the risk of chemotherapy-induced oral mucositis. Support Care Cancer. 2014;22(11):2935–2940. doi: 10.1007/s00520-014-2282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization . 5th ed. World Health Organization; Geneva: 2013. Oral health surveys: basic methods.https://apps.who.int/iris/bitstream/handle/10665/97035/9789241548649_eng.pdf?sequence=1 editors. Internetcited 2020 Sept 10Available from: [Google Scholar]
- 18.Gruebbel AO. A measurement of dental caries prevalence and treatment service for deciduous teeth. J Dent Res. 1994;23(3):163–168. [Google Scholar]
- 19.Löe H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967;38(6):610–616. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 20.Ministério da Saúde . 1st ed. Ministério da Saúde; Brasília: 2012. Pesquisa Nacional de Saúde Bucal: resultados principais.http://bvsms.saude.gov.br/bvs/publicacoes/pesquisa_nacional_saude_bucal.pdf editors. Internetcited 2020 sept 15. Available from: [Google Scholar]
- 21.D'Agulham ACD, Chaiben CL, de Lima AAS, Torres-Pereira CC, Machado MAN. Fanconi Anemia: main oral manifestations. Rev Gaúcha Odontol. 2014;62(3):281–288. [Google Scholar]
- 22.Tekcicek M, Tavil B, Cakar A, Pinar A, Unal S, Gumruk F. Oral and dental findings in children with Fanconi anemia. Pediatr Dent. 2007;29(3):248–252. [PubMed] [Google Scholar]
- 23.Mattioli TMF, Koubik AC, de Oliveira Ribas M, França BHS, Brancher JA, de Lima AAS. Salivary flow rate, calcium, urea, total protein, and amylase levels in fanconi anemia. J Pediatr Hematol Oncol. 2010;32(2):46–49. doi: 10.1097/MPH.0b013e3181c29c11. [DOI] [PubMed] [Google Scholar]
- 24.Hsu LL, Fan-Hsu J. Evidence-based dental management in the new era of sickle cell disease: a scoping review. J Am Dent Assoc. 2020;151(9):668–677. doi: 10.1016/j.adaj.2020.05.023. [DOI] [PubMed] [Google Scholar]
- 25.Angst PDM, Maier J, Dos Santos Nogueira R, Manso IS, Tedesco TK. Oral health status of patients with leukemia: a systematic review with meta-analysis. [Internet] 2020:1–62. doi: 10.1016/j.archoralbio.2020.104948. cited 2021 jan 10. Available from: https://www.researchgate.net/publication/346296191_Oral_health_status_of_patients_with_leukemia_a_systematic_review_with_meta-analysis. [DOI] [PubMed] [Google Scholar]
- 26.Mazaheri R, Jabbarifar E, Ghasemi E, Akkafzadeh E, Poursaeid E. Oral health status, salivary pH status, and Streptococcus mutans counts in dental plaques and saliva of children with acute lymphoblastic leukemia. Dent Res J. 2017;14(3):188–194. doi: 10.4103/1735-3327.208764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lucas V, Roberts G, Beighton D. Oral health of children undergoing allogeneic bone marrow transplantation. Bone Marrow Transplant. 1998;22(1):801–808. doi: 10.1038/sj.bmt.1701415. [DOI] [PubMed] [Google Scholar]
- 28.Yamagata K, Onizawa K, Yoshida H, Yamagata K, Kojima Y, Koike K, et al. Dental management of pediatric patients undergoing hematopoietic cell transplant. Pediatr Hematol Oncol. 2006;23(7):541–548. doi: 10.1080/08880010600814187. [DOI] [PubMed] [Google Scholar]
- 29.Elad S, Garfunkel AA, Michaeli E, Galili D. Time limitations and the challenge of providing infection-preventing dental care to hematopoietic stem-cell transplantation patients. Support Care Cancer. 2003;11(10):674–677. doi: 10.1007/s00520-003-0499-8. [DOI] [PubMed] [Google Scholar]
- 30.Schuurhuis JM, Span LFR, Stokman MA, van Winkelhoff AJ, Vissink A, Spijkervet FKL. Effect of leaving chronic oral foci untreated on infectious complications during intensive chemotherapy. Br J Cancer. 2016;114(9):972–978. doi: 10.1038/bjc.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skallsjö K, Johansson J-E, Jonasson P, Hasséus B. Apical periodontitis as potential source of infection in patients with lymphoma treated with chemotherapy. Clin Oral Investig. 2020;24(1):133–140. doi: 10.1007/s00784-019-02909-w. [DOI] [PubMed] [Google Scholar]