Abstract
Interferons (IFN), first described 1957 by Isaacs and Lindemann, are antiviral proteins generated in cells after viral infections. One of several interferon-induced effector mechanisms is the so called 2-5A / RNaseL system: Interferon is produced in the virus-affected cells and released. After binding to cell membrane receptors of adjacent cells, 2-5 A synthetase (oligoadenylate synthetase, OAS) is generated, attaches to dsRNA section areas of the viral RNA and catalyses the production of 2-5 oligoadenylates from ATP. In 2-5 oligoadenylates, several adenosine residues (3–4 and more) are combined via phosphodiester binding in the unusual 2′-5′ positions of the riboses. 2-5 oligoadenylates activate a RNaseL which degrades the viral RNA. Recently, characteristic gene mutations and other disturbances concerning the interferon system were detected in patients with severe COVID-19, leading to problems of 2-5 oligoadenylate synthesis and the activation of RNAseL. In order to circumvent these problems, we hypothesize that a direct application of 2-5 oligoadenylates, included in an inhalation spray, may be effective in treatment of severe COVID-19 infections of the respiratory system. In contrast to some other anti-COVID-19 drugs, oligoadenylates act inside the cells (like e.g. Paxlovid) and are therefore independent of cell surface mutations of the virus. For confirmation of our hypothesis, proof of concept investigations in vitro are suggested, before a possible clinical application can be considered.
Keywords: COVID-19, SARS-CoV-2, Interferon, 2-5 Oligoadenylates, 2-5A/RNaseL system
Introduction
Several investigations are in progress in order to find new drugs for treatment of COVID-19. Among them are, e.g., monoclonal antibodies directed against different target structures as well as already established or new drugs. Moreover, it is hypothesized that some substances, not directly related to COVID-19, could be useful in a complementary fashion [1], [2], [3]. Interferons (IFN) are the classical antiviral drugs, but clinical results in COVID-19 are so far rather disappointing [4], [5]. With a better knowledge of the IFN-associated processes, the explanation of this failure in COVID-19 patients can be easier understood. We hypothesize that so called 2-5A oligoadenylates, small molecules physiologically associated with the interferon system, could be effective in patients suffering from severe COVID-19, and can bypass disturbances of the interferon system.
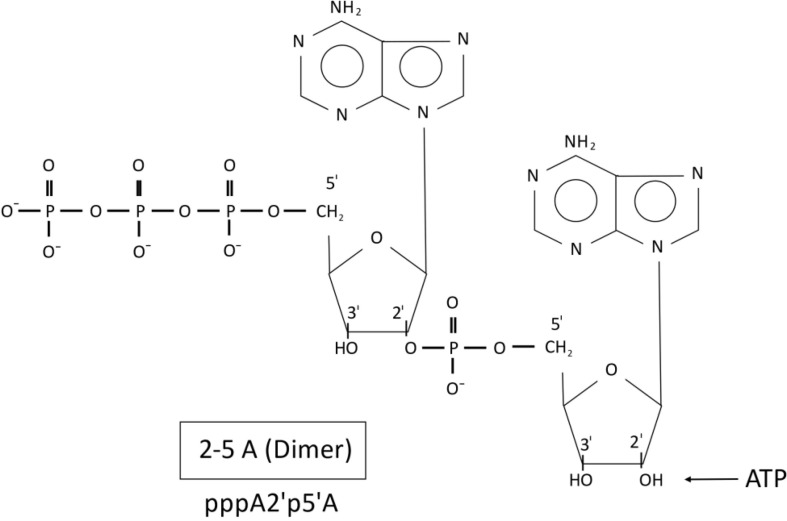
Interferon (IFN) was discovered by Isaacs and Lindemann [6] who observed that this protein is produced in cells in response to a viral infection. After its release and binding to adjacent cells a number of protecting anti-viral processes take place. One of the most important effector mechanisms is the so called 2-5A / RNaseL system [7], [8], [9], as shown in Fig. 1 . Interferon induces the synthesis of an enzyme, 2′-5′ oligoadenylate synthetase (OAS, 2-5A synthetase; mostly written without apostrophe) in almost all cells. OAS binds to double stranded RNA (dsRNA) section areas of the viral RNA and produces oligoadenylates from ATP. 2-14 adenosine molecules are connected by phosphodiester bonds in the unusual 2‘-5‘formation. Fig. 2 shows exemplarily the dimeric form; the trimeric molecule is generated by the connection of the next AMP (after splitting of PP from ATP) to the 2′ OH-group of the dimeric form, and so on. The 2-5 oligoadenylates (particularly the trimeric and tetrameric oligoadenylates) activate a latent (L) RNase which degrades preferentially the viral RNA [10]. However, the RNaseL (its gene was identified as the hereditary prostate cancer I gene [11], [12]) is able to destroy other RNA molecules as well. Finally, a phosphodiesterase (PDE) degrades the 2-5 oligoadenylates, thereby inactivating the RNaseL.
Fig. 1.
The Interferon-induced 2-5A / RNaseL system.
Fig. 2.
Structure and formation of 2-5 oligoadenylates from ATP by the 2-5A synthetase. Shown is the dimeric form which was generated by connection of ATP with AMP (after splitting of PP from the second ATP molecule) in 2‘-5‘position of the ribose(s).
Soon after discovery it was supposed that interferons could also be used as anticancer drugs. In fact, one of the first successfully treated patients was a child with a final nasopharyngeal carcinoma in the children’s university hospital in Tübingen [13]. Encouraged by this result a subsequent clinical study on neuroblastoma patients was started: A protocol with cytostatic drugs was compared with a protocol including interferon β (Fiblaferon, Rentschler,Laupheim, Germany). In connection with these clinical studies, laboratory experiments were carried out: Neuroblastoma cells expressing the N-myc oncogene were treated with an antisense vector against N-myc RNA for generation of double stranded (ds)RNA sectors for binding the 2-5A synthetase. In the presence of interferon proliferation of the neuroblastoma cells could be stopped, probably by the induced 2-5A system [14]. Unfortunately, the inclusion of IFN in the clinical treatment protocol did not improve the outcome of neuroblastoma patients and, therefore, the clinical and experimental IFN-studies were stopped.
Hypothesis: utilization of the 2-5A / RNaseL system for COVID-19 treatment
After an intensive research period in 1980/1990 only a small number of scientists continued research on the interferon-induced 2-5A / RNaseL system [9]. During the corona pandemic 30 years later, we remembered our former interest on this system and considered whether it could be used somehow for therapy. In analogy to our experiments with neuroblastoma cells just described, our first idea was to use interferon in combination with small (∼50 nucleotides) antisense RNA against SARS-CoV-2 RNA for attachment of the 2-5A synthetase on the thereby formed dsRNA structures. However, it was shown that SARS-CoV-2 RNA (ca 30.000 nucleotides) itself contains dsRNA stretches on which 2-5A synthetase could bind, making the combination antisense RNA and interferon no longer a preferential concept. Moreover, some confusing aspects concerning the role of interferon in SARS-CoV-2 defense were observed in the course of the pandemic: Ziegler et al, e.g., recently showed, that the expression of the ACE2 SARS-CoV-2 receptor, an important docking structure for the virus, can even be stimulated by interferon, thereby facilitating the virus entry into the target cells [24]. Furthermore, several reports were published showing that disorders in the context with interferon are associated with severe courses of COVID-19, e.g. occurrence of autoantibodies in patients [15] or mutations of interferon -stimulated genes (ISGs) [16]. Interestingly, among these mutated ISGs, 2-5A synthetase (oligoadenylate synthetase, OAS) plays an important role: Wickenhagen et al [17], [18], [19] showed that among the two isoforms of OAS1 (p42 and p46) p46, but not p42, could inhibit SARS-CoV-2 replication. Patients who did not express the p46 isoform suffered more frequently on severe COVID-19. In contrast to p42, p46 contains a four amino acids motif (CAAX-box) serving as binding structure for prenylation and fixation of OAS p46 on membranes of double membrane vesicles (DMV) where the coronavirus RNA is replicated [19]. RNA hairpins of SARS-CoV-2 bind on this membrane-bound OAS and, by generation of oligoadenylates, RNaseL is activated and subsequently degrades the viral RNA. In contrast, in corona patients expressing OAS p42 (lacking the four amino acid motif and prenylation) membrane fixation and generation of 2-5 oligoadenylates is disturbed, leading to severe outcomes.
Based on these data, we hypothesize that chemically synthesized (“pre-formed”) 2-5 oligoadenylates, delivered as inhalation drugs, are also able to activate the RNaseL and can prevent the severe acute respiratory syndrome.
The application of pre-formed 2-5 oligoadenylates (omitting the endogenous IFN-induced synthesis) could circumvent potential disturbances and/or counterproductive effects in the interferon system observed in patients with severe COVID-19. However, the pre-formed 2-5 oligoadenylates directly activate the RNaseL and the viral RNA is no more preferentially degraded. Temporarily, cellular RNA can also be affected, until the oligoadenylates are destructed by the phosphodiesterase (PDE) (Fig. 3 ); however, the transcription process is not influenced. In addition, 2-5 oligoadenylates should not only be suitable for treatment of SARS-CoV-2, but also for other RNA-viruses.
Fig. 3.
Preferred degradation of viral RNA by activated RNaseL according to the model of Nilsen and Baglioni [10].
Proposal for the evaluation of the hypothesis
Fig. 4 gives a summary about the suggested experiments for evaluation of our hypothesis.
Fig. 4.
Proposed experiments for evaluation of our hypothesis to use 2-5 oligoadenylates for treatment of severe COVID-19. More detailed information: see text.
Description of the planned experiments presented in Fig. 4 in more detail:
1. Synthesis of 2-5 oligoadenylates (b: Contract synthesis by a company).
This can be done in two ways:
-
a)
Enzymatic synthesis using OAS: The classical method uses OAS isolated from rabbit reticulocyte or interferon-treated HeLa / MNBC cells which increases the basic OAS-levels 10–10.000 fold [25]; OAS of the extracts is bound to dsRNA Poly(I):Poly(C)- Sepharose. Starting with unlabeled or radiolabeled ATP ([3H]-or alpha-[32P]ATP), mg-amounts of 2-5 oligoadenylates can be prepared in this way.
-
b)
Alternatively, several companies offer chemical RNA syntheses (2-5 oligoadenylates) by contract synthesis.
2. Separation and characterization of the generated 2-5 oligoadenylates.
Mixtures of 2-5 oligoadenylates (Dimeric, Trimeric, Tetrameric….oligoadenylates) obtained by the enzymatic synthesis with OAS can be separated by DEAE-Sephadex column chromatography or HPLC. The trimeric and tetrameric 2-5 oligoadenylates are biologically highly active (with respect to activation of RNaseL) [25]. The different 2-5 oligoadenylates can be characterized by isotachophoresis [20], [21], [22], [23], or by standard procedures like HPLC or capillary electrophoresis.
3. Drug targeting of the 2-5 oligoadenylates using lipid nano particles (LNPs) and preparation of an inhalation spray containing packed 2-5 oligoadenylates (Contract manufacturing by a company).
2-5 oligoadenylates [pppA2′p5′A2′p5′A(trimer), pppA2′p5′A2′p5′A2′p5′A (tertramer)] are short anionic RNA molecules with unusual 2′-5′ phosphodiester-binding. Therefore, similar package materials (kationic nanolipids) as used in RNA vaccine preparations e.g. by Biontech or Moderna, could be considered for the oligoadenylates [26], [27], [28]. Next, the LNP/2-5 oligoadenylates are integrated in suitable inhalation sprays. This manufacturing should be performed in collaboration with a partner with acknowledged expertise in this field.
4. Cell culture experiments with 2-5 oligoadenylates using uninfected and COVID-19 infected cells of the respiratory system.
Ciliated cells of the nose and alveolar type II epidermal cells in the lung are the most important target cells of the respiratory system for SARS-CoV-2 [29]. For cell culture experiments, cells of the respiratory system can be ordered by ATCC (American Type Culture Collection) or other companies. Different SARS-CoV-2 virus strains are available [30], for establishment of the tests, especially the plaque test for virus titration [31], [32].
-
a)
Plaque test after Infections of the unprotected cells (Titration)
-
b)
Virus infection after IFN-treatment on the target cells (time and concentration-dependent experiments)
-
c)
Infection after application of the oligoadenylates (LNP/2-5 oligoadenylates and inhalation-spray formulation). Concentration- and time-dependent experiments (application shortly before, simultaneously and after incubation with the viral strains)
-
d)
Uptake experiments of radiolabeled 2-5 oligoadenylates into the target cells
5. Influence of β-Interferon on the induction of OAS in mononuclear blood cells (MNBC) of patients after mild and severe COVID-19 infection.
Patients with severe COVID-19 infection are often characterized by mutations in the interferon stimulated gene (ISG) coding for oligoadenylate synthetase (OAS) [16]. Therefore, retrospective measurements of OAS activity in isolated MNBC before and after incubation with Interferon from patients with severe – compared to mild – infections could provide valuable predictive parameters for future decisions whether a therapy with interferon or 2-5 oligoadenylates can be recommended or not for an individual patient. MNBC are isolated by Ficoll-separation and incubated with IFN. OAS activity is measured according to the protocols described [21], [23].
Outlook
If the in vitro experiments will be successful, mouse experiments will be considered next in order to clarify risk and benefit in vivo, followed by clinical studies and evaluation.
Consent statement/Ethical approval:
Not required.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Bai X., Hippensteel J., Leavitt A., Maloney J.P., et al. Hypothesis: Alpha-1-antitrypsin is a promising treatment option for COVID-19. Med Hypotheses. 2021;146 doi: 10.1016/j.mehy.2020.110394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taghavi-Farahabadi M., Mahmoudi M., Soudi S., Mahmoud Hashemi S. Hypothesis for the management and treatment of the COVID-19-induced acute respiratory distress syndrome and lung injury using mesenchymal stem cell-derived exosomes. Med Hypotheses. 2020;144 doi: 10.1016/j.methy.2020.109865. Epub 2020 May 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deloncle R., Guillard O., Pineau A., Lesage G. Copper acetate aerosols: A possible tool complemantary to vaccination in fight against SARS-CoV-2 and variants replication. Med Hypotheses. 2022;160 doi: 10.1016/j.methy.2022.110775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monk P.D., Marsden R.J., Tear V.J., et al. Safety and efficacy of inhaled nebulised interferon beta 1a (SNG001) for treatment of SARS-CoV-2 infection: a randomized, double blind – controlled phase 2 trial. Lancet Resp. Med. 2021;9:196–2006. doi: 10.1016/S2213-2600(20)30511–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalil A.C., Metha A.K., Patterson T.F., Erdmann N., et al. Efficacy of interferon beta-1a plus remdesivir compared with remdesivir alone in hospitalized adults with COVID-19: a double-blind, randomized, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2021;9:1365–1376. doi: 10.1016/S2213-2600(21)00384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isaacs A., Lindemann J. Virus interference I. The interferon. Proc R Soc B. 1957;147:258. doi: 10.1098/rspb.1957.0048. https://royalsocietypublishing.org/doi/10.1098/rspb.1957.0048 [DOI] [PubMed] [Google Scholar]
- 7.Williams B.R.G., Silverman R.H. Progress in Clinical and Biological Research. Vol. 202, Alan R. Liss, INC; New York: 1985. The 2-5A System: Molecular and clinical aspects of the interferon regulated pathway. [PubMed] [Google Scholar]
- 8.Ball L.A. In: The enzymes. Vol X.V., editor. Academic Press; 1982. 2'-5' Oligoadenylate synthetase; pp. 281–313. [Google Scholar]
- 9.Silverman R.H. A scientific journey through the 2-5A / RNase L system. Cytokine Growth Factor Rev. 2007;18:381–388. doi: 10.1016/j.cytogfr.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nilsen T.W., Baglioni C. A mechanism for discrimination between viral and host mRNA in interferon treated cells. Proc Natl Acad Sci USA. 1979;76:2600–2604. doi: 10.1007/978-3-642-78549-8-10.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silverman R.H. Implications of RNaseL in prostate cancer biology. Biochemistry. 2003;42:1805–1812. doi: 10.1021/bi027147i. [DOI] [PubMed] [Google Scholar]
- 12.Meyer M.S., Penney K.L., Stark J.R., et al. Genetic variation in RNaseL associated with prostate cancer risk and progression. Carcinogenesis. 2010;31:1597–1603. doi: 10.1093/carcin/bgg132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Treuner J. Successful treatment of nasopharyngeal carcinoma with interferon. Lancet. 1980;315(8172):817–818. doi: 10.1016/s0140-6736(80)91308-2. [DOI] [PubMed] [Google Scholar]
- 14.Schilbach K., Pollwein P., Schwab M., Handgretinger R., Treuner J., Niethammer D., et al. Reduction of N-myc expression by antisese RNA is amplified by Interferon: possible involvement of the 2–5A system. Biochem Biophys Res Commun. 1990;170:1242–1248. doi: 10.1016/0006-291X(90)90527-T.CorpusID:6109177. [DOI] [PubMed] [Google Scholar]
- 15.Bastard P., Rosen L.B. et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19, Science 370 (2020) 423, eabd4585 (2020). Doi:10.1126/science. abd4585; pmid:32972996. [DOI] [PMC free article] [PubMed]
- 16.Callaway E. The quest to find genes that drive severe COVID. Nature. 2021;595(7867):346–348. doi: 10.1038/d41586-021-01827-w. [DOI] [PubMed] [Google Scholar]
- 17.Wickenhagen A., Sugrue E., Spyros L. et al, A prenylated dsRNA sensor protects against severe COVID 19 (Research article Summary) Science 374 (2021), Issue 6567. Doi:10.1126/science.abj3624.Epub2021. [DOI] [PMC free article] [PubMed]
- 18.Wickenhagen A., Sugrue E., Spyros L., et al. A prenylated dsRNA sensor protects against severe COVID 19. Science. 2021;374(6567) doi: 10.1126/scienceabj3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schoggins J. Defective viral RNA sensing linked to severe COVID 19. Genetic variation in a sensor of double-stranded RNA can exacerbate COVID-19. Science 374 (2021) 535-536, Issue 6567, 29. October 2021. https://www.science.org/doi/10.1126/science.abm3921. [DOI] [PubMed]
- 20.Bruchelt G., Buedenbender M., Treuner J., Niethammer D., Schmidt K.H. Investigations on the interferon-induced 2–5 oligoadenylate system using analytical capillary isotachophoresis. J Chromatogr. 1989;470:185–190. doi: 10.1016/s0021-9673(00)94211-0. [DOI] [PubMed] [Google Scholar]
- 21.Handgretinger R., Bruchelt G., Kimmig A., Lang P., Daurer B., Dopfer R., et al. In vitro and in vivo effects of IL-2 on the 2–5A synthetase activity of peripheral MNBC. J Interferon Res. 1990;10:75–82. doi: 10.1089/jir.1990.10.75. [DOI] [PubMed] [Google Scholar]
- 22.Bruchelt G., Buedenbender M., Schmidt K.H., Jopski B., Treuner J., Niethammer D. Isotachophoretic determination of 2–5A phosphodiesterase. J Chromatogr. 1991;545:407–412. doi: 10.1016/s0021-9673(01)88734-3. https://europepmc.org/article/MED/1885695 [DOI] [PubMed] [Google Scholar]
- 23.Bruchelt G., Buedenbender M., Schmidt K.H., Birk A., Treuner J., Niethammer D. Determination of 2–5A synthetase and 2–5A phosphodiesterase by capillary analytical isotachophoresis: Effects of cytokines and comparison with radioenzymatic assays. Electrophoresis. 1994;15:40–45. doi: 10.1002/elps.1150150107. [DOI] [PubMed] [Google Scholar]
- 24.Ziegler C.G.K., Allon S.J., Nyquist S.K., Mbano I.M., et al. SARS-CoV-2 Receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181:1016–1035.e19. doi: 10.1016/j.cell.2020. 04.0.35.Epub 2020 Apr27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hovanessian A.G., Brown E.R., Martin E.M., Roberts W.K., Knight M., Kerr I.M. Enzymatic synthesis, purification and fractionation of (2’-5’)-Oligoadenylic acid. In: Methods in Enzymology, Vol 79 Interferons, Part B, pp 184-199 (1981) Academic Press, New York, London, Toronto, Sydney, San Francisco. www.tailieudaihoc.com/vnua/33615.html. [DOI] [PubMed]
- 26.Naseri N., Valizadeh H., Zakeri-Milani P. Solid lipid nanoparticles and nanostructured lipid carriers: structure, preparation and application. Adv Pharm Bull. 2015;5:305–313. doi: 10.15171/apb.2015.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Viger-Gravel J., Schantz A., Pinon A.C., et al. Structure of lipid nanoparticles containing siRNA or mRNA by dynamic nuclear polarization-enhanced NMR spectroscopy. J Phys Chem B. 2018;122:2073–2081. doi: 10.1021/acs.jpcb.7b10795. Epub2018Feb. [DOI] [PubMed] [Google Scholar]
- 28.Ickenstein L.M., Gardiel P. Lipid-based nanoparticle formulations for small molecules and RNA drugs. Expert Opin Drug Deliv. 2019;16:1205–1226. doi: 10.1080/17425247.2019.1669558. [DOI] [PubMed] [Google Scholar]
- 29.Bridges J.P., Vladar E.K., Huang H., Mason R.J. Respiratory epithelial cell responses to SARS-CoV-2 in COVID-19. Thorax. 2022;77:203–209. doi: 10.1136/thoraxjnl-2021-21756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Svilenov H.L., Sacherl J., Reiter A., et al. Picomolar inhibition of SARS-CoV-2 variants of concern by an engineered ACE2-IgG4-Fc fusion protein. Antiviral Res. 2021;196 doi: 10.1016/j.antiviral.2021.105197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dulbecco R., Vogt M. Some problems of animal virology as studied by the plaque technique. Cold Spring Harbor Symp Quant Biol. 1953;18:273–279. doi: 10.1101/sqb1953.018.01.039. [DOI] [PubMed] [Google Scholar]
- 32.Baer A., Kehn-Hall K. Viral concentration determination through the plaque assays using traditional and novel overlay systems, J Vis Exp 2014 [93]e52065. doi: 10.3791/52065. [DOI] [PMC free article] [PubMed]