Abstract
Polyethylene glycol (PEG) was used to produce whole-virus antigen derived from tissue culture cells infected with a Canadian strain of caprine arthritis-encephalitis virus. PEG antigen batches were obtained after precipitation and concentration of infected tissue culture material with PEG 8000 and final treatment with sodium dodecyl sulfate. The optimum time of harvest of tissue culture extracted material to produce the maximum amount of viral proteins was determined in roller bottles, after cocultivation of infected and noninfected fetal lamb corneal cells. Samples from day 9 to day 25 postculture were collected and processed. By Western blotting, the optimum time of harvest was found to be day 25 following the coculture. Two large batches of PEG antigen were prepared at the optimum time of harvest. Both batches gave similar results when tested by Western blotting and enzyme-linked immunosorbent assay (ELISA), using reference control sera from infected and noninfected goats. For further testing in ELISA, cutoff values and ratios were determined for PEG batch 1, using 200 known serum samples from goats free of the disease. The PEG antigen batch was compared with an in-house ELISA antigen in a kinetic mode, using 498 serum samples from field goats. The in-house ELISA antigen was produced following two rounds of ultracentrifugation and treatment with sodium dodecyl sulfate (R. A. Heckert, W. B. McNab, S. M. Richardson, and M. R. Briscoe, Can. J. Vet. Res. 56:237–241, 1992). The PEG antigen batch was found suitable for ELISA, with a relative specificity of 100% and a relative sensitivity of 99.4% compared to the in-house ELISA antigen. This method of antigen production for ELISA was found to be rapid, inexpensive, and reliable for the diagnosis of caprine-arthritis encephalitis, without requiring the use of sophisticated laboratory equipment.
Caprine arthritis-encephalitis virus (CAEV) belongs to the Retroviridae family and the subfamily Lentivirinae. The virus is distributed worldwide and causes leuko-encephalomyelitis in goat kids (13), chronic progressive arthritis-synovitis, indurative mastitis, and chronic interstitial pneumonia in adult goats (6, 9, 12). The development of clinical disease takes a few months to a few years, with infections in most animals remaining subclinical (7, 15). For both the clinical and asymptomatic forms, the CAEV causes a lifetime infection despite humoral and cellular immune responses (8), with no known effective treatment; the infected animals remain reservoirs of the virus for their entire lives. Transmission between infected and susceptible animals occurs mainly through colostrum and milk consumption (1, 15). Contact transmission between goats of all ages has also been demonstrated (15). The early detection of the infected animals and their segregation and/or eradication from the flock form an efficient practice to limit the spread of the virus (17). The infection is primarily detected by the demonstration of specific CAEV antibodies in the body fluids of the infected goats (15). The agar gel immunodiffusion (AGID) assay and the enzyme-linked immunosorbent assay (ELISA) are routine serological test methods used for the serologic detection of CAEV antibodies (2, 10, 11, 14, 20, 25). Antigens used for these laboratory methods are commonly extracted and purified from tissue culture (TC) infected cell material, using complex manipulation and/or expensive equipment for the purification steps (2, 4, 11, 20, 25). Recombinant technology is also used to produce recombinant viral proteins (5, 16, 18). Even though this modern approach is usually excellent for producing large amounts of recombinant viral proteins that are useful in ELISA, their production and purification require sophisticated approaches that are not always available to a diagnostic laboratory. This paper describes a very simple, rapid, and reliable method for the optimal production of TC-derived antigens suitable for ELISA for the diagnosis of CAEV.
MATERIALS AND METHODS
Virus isolate.
A pure Saanen adult goat from New Brunswick, Canada, with a CAEV-positive serological status (determined by AGID) and a story of severe arthritis in all legs, swollen knees, and lameness was euthanatized after cesarian delivery of triplets. Synovial fluid and various tissues (synovial membranes, lungs, kidney, and mammary gland) were aseptically collected for TC isolation and were also fixed in formaldehyde for confirmation of CAE disease by histopathology. A virus producing giant cells and syncytia with a slow-evolving cytopathic effect was isolated from the knees, mammary gland, and lungs, at the third or fourth passages following cocultivation of explant tissues with fetal lamb corneal (FLCor) or fetal goat synovial membrane (FGSm) culture cells. The viral stock SK167a was derived from the infected FGSm culture cells cocultured with lung explants. The infected cells were found to be free of bovine diarrhea virus, parainfluenza-3 virus, and mycoplasma, as determined by indirect immunofluorescence (IFA) and a Mycoplasma detection kit (Canadian Life Technologies Inc., Burlington, Ontario, Canada) (11). CAEV was confirmed in infected cells by IFA staining and AGID specific to CAEV (11). Free viruses in clarified supernatant were frozen at −80°C, while infected cells were frozen in liquid nitrogen.
Virus growth.
FLCor cells were grown in low-glucose Dulbecco's modified Eagle medium (Sigma Chemical Co., Oakville, Ontario, Canada) supplemented with 2 mM l-glutamine (Canadian Life Technologies Inc.), 10% fetal bovine serum (FBS) (Canasera International), 1% antibiotic solution (10,000 U of penicillin G and streptomycin per ml) (Canadian Life Technologies Inc.), folic acid (4 μg/ml) dissolved in sodium bicarbonate (Sigma Chemical Co.), and 0.1 mM modified Eagle medium nonessential amino acid (Canadian Life Technologies Inc.). At an estimate of 80 to 90% confluence, 175-cm2 flasks of FLCor cells were infected with the SK167a strain of CAEV. The virus was propagated following normal TC procedures. Briefly, the growth medium was removed, the adherent cells were carefully washed, and then the cells were infected with the thawed virus in the presence of Polybrene (8 μg/ml; Sigma Chemical Co.) to facilitate adsorption of the virus to the cells. Cells were incubated at 37°C in a 5% CO2 atmosphere for 2 h before the addition of fresh supplemented Dulbecco's modified Eagle maintenance medium (with 2% FBS). Noninfected FLCor cells were maintained in a similar manner. Cells were checked daily for morphological changes. The establishment of infection was manifested by syncytium formation and appearance of enlarged nucleated cells in the infected flasks, which was more evident after 2 weeks postinfection.
Time course study.
A time course study was carried out to determine the optimal time of antigen production in 850-cm2 roller bottles, using the infected and noninfected cells grown as described above. Briefly, cells from each group of infected and noninfected 175-cm2 flasks were trypsinized, pooled, and counted. For each of the infected roller bottles, 5,000,000 noninfected cells were cocultured with 1,000,000 infected cells in the presence of growth medium. For each of the noninfected roller bottles, 6,000,000 noninfected cells were used. At 8 days post-TC split (post-TCS), when the cells were near confluence, the growth medium was replaced by 100 ml of the maintenance medium containing 2% FBS. One roller bottle of each infected and noninfected group was frozen at −70°C the following day and then every second day, until all roller bottles were frozen. The experiment was completed at 25 days, while the adherent cells were still in good condition, although many cells were in suspension due to the cytopathic effect of the virus with time. The best time for the optimum production of CAEV proteins in mixture of medium and cells, the optimum time of harvest (OTH), was determined following total protein concentration with polyethylene glycol (PEG). The procedure above was followed to produce two distinct larger batches of viral antigen for ELISA. For this purpose, more roller bottles were grown and harvested all together, at the predetermined OTH.
Precipitation and concentration of viral proteins with PEG.
For each roller bottle collected at the indicated time intervals and for a lot of roller bottles harvested at the predetermined OTH, the viral proteins were extracted, precipitated, and concentrated using PEG 8000 (Fisher Scientific Ltd., Nepean, Ontario, Canada). Briefly, frozen roller bottles (cells and medium) were freeze-thawed consecutively three to four times. The collected material was transferred to 50-ml centrifuge tubes and was centrifuged at 2,000 rpm for 20 min to clarify the supernatant. The supernatant was collected and measured prior to being transferred to 250-ml centrifuge bottles. It was mixed with 30% PEG 8000 in 0.4 M NaCl at the ratio of 2:1 (supernatant-PEG). The mixtures were incubated overnight at 4°C with constant gentle agitation or rocking. The precipitated proteins were recovered the following day by centrifugation at 2,000 rpm for 20 min. The supernatant obtained after PEG 8000 precipitation was discarded, and the pellets were dried by inverting the tubes on absorbent paper towels for 5 to 10 min. The pellets were dissolved in phosphate-buffered saline (PBS) containing 0.15 M NaCl, using 1/100 of the starting supernatant volume, in which the protease inhibitor phenylmethylsulfonyl fluoride (Canadian Life Technologies Inc.) was added at a final concentration of 0.1 mmol/ml. For the time course study, the content of only one roller bottle was used for each time point. For the production of the larger batches of viral antigens, the contents of the roller bottles were pooled after PEG 8000 precipitation and before dialysis. The dialysis tubing (12,000 to 14,000 molecular weight [MW]) (Spectapor; VWR Scientific Canada Ltd, Quebec, Canada) were prepared by heating at 60°C in 0.1 M sodium bicarbonate and 0.01 M EDTA, pH 7.00, prior to being filled with a preparation of the dissolved proteins. The tubes were dialyzed against type 1 Milli-Q water during the day and overnight at 4°C, with several changes of the water every 2 h for at least four to five times. The process was then repeated overnight in the presence of PBS. The material in each piece of the dialysis tubing was further concentrated by placing the dialysis tubes at 4°C for at least 1 to 2 h, depending on the initial volume; on a bed of PEG 8000; and by covering them with more PEG 8000, until the volume was reduced by around 50%. The content of each dialysis bag was transferred to 10-ml centrifuge tubes and centrifuged at 15,000 rpm for 5 min. The supernatant was removed and saved for further analysis of its protein content. The resulting pellet was dissolved in 1/200 of the starting TC supernatant volume with PBS–0.15 M NaCl for further analysis. Both the supernatant and the dissolved pellets were treated with a final concentration of 0.1% sodium dodecyl sulfate (SDS). They were checked by running them on an SDS–15% polyacrylamide gel electrophoresis (PAGE) followed by Coomassie blue staining and Western blotting before being tested by ELISA.
SDS-PAGE and WB.
SDS-PAGE and Western blotting (WB) were carried out following conventional techniques (19). Briefly, the dissolved proteins were heated at 95°C for 4 min and loaded in the presence of Laemmli sample buffer and β-mercaptoethanol. The samples were run on an SDS–15% PAGE in Tris-glycine-SDS electrophoresis buffer at a constant voltage. The protein bands were detected by staining with Coomassie blue and by conventional WB. For this technique, an AGID CAEV-positive reference serum from an infected goat was used as the primary antibody, and a rabbit anti-goat antibody alkaline phosphatase (whole-molecule immunoglobulin G; Sigma Chemical Co.) was used as the secondary antibody.
i-ELISA.
A conventional indirect ELISA (i-ELISA) was used for this study (11, 22). The PEG antigen batches produced in the present study were compared to a whole-virus antigen preparation that was previously produced at our laboratory, using two rounds of ultracentrifugation and final treatment with SDS (11), which, for the purpose of the present study, was identified as the in-house ELISA antigen. Preliminary experiments were carried out with the PEG antigen batches to determine the concentration of the viral antigen able to detect adequately a positive reference goat serum and the antiserum dilution of a negative reference goat serum necessary to minimize nonspecific background. For each antigen preparation, the i-ELISA was standardized by using 200 CAEV known negative goat serum samples to set up the cutoff values. These samples were from a proficiency panel used for the standardization of the CAEV ELISA as part of our quality assurance program. In the screening assay, cutoff values were established at the mean optical density at 414 nm (OD414) of the 200 negative samples tested on the positive antigen-coated wells plus 3 standard deviations (x̄ + 3SD) or x̄ + 5SD, respectively. A sample was declared negative if its OD414 was less than the cutoff value of 3SD. It was declared reactive if its OD414 was higher than or equal to the cutoff value of 3SD. Reactive samples were retested in a confirmatory assay in which series of wells were also coated with noninfected TC obtained in parallel with the antigen batches. In the confirmatory assay, a cutoff ratio was established at the mean of the ratio of the positive antigen OD414 to the negative antigen OD414 (P/N) of the 200 negative samples plus 3 SD (x̄P/N + 3SD). A sample with a P/N ratio higher than the cutoff ratio was declared suspicious if the OD414 was higher than or equal to the cutoff value of 3SD but lower than the cutoff value of 5SD. It was declared positive if the P/N ratio was higher than the cutoff ratio and the OD414 was equal to or higher than the cutoff value of 5SD. Using these cutoffs, a total of 498 field serum samples from goats submitted for diagnosis purposes were tested in a kinetic mode. In this setup, plates were read at a time determined by an ELISA program (developed by Walter Kelly, Software ELISA version 2.0; Canadian Food Inspection Agency, Nepean, Ontario, Canada), when a CAEV-positive target reference control added in each test plate was reaching an OD414 of 1.00. For validity of the test, each plate also contained duplicate samples of weakly positive and negative reference controls which were required to be within acceptable predetermined OD414 ranges at the target time of reading with a spectrophotometer (Multiscan, Titertek, Flow Laboratories, Irvine, Ayrshire, Scotland). Relative sensitivity and specificity were determined for the PEG antigen batch, as compared to the in-house ELISA antigen.
RESULTS
Time course study of harvest.
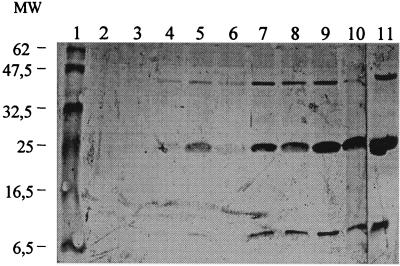
For the time course study, dissolved proteins of the supernatant and pellet fractions obtained following precipitation, dialysis, and concentration with PEG 8000 were tested by WB at some time points for comparison. A positive goat serum recognizing the capsid antigen of CAEV (gag p28) was used. Samples were from TCS day 19 of the negative control roller bottle and TCS days 19, 23, and 25 of infected roller bottles. WB showed no reactivity of a mix of pellet and supernatant material for the negative control (Fig. 1, lane 6). In contrast, a strong immune reaction was detected with the infected material. An expected specific band of around 25 kDa was evident (Fig. 1, lanes 1 to 5). The dissolved pellet fractions showed stronger reactivity against gag CAEV for all tested infected TCS, as compared to their corresponding supernatant fractions (Fig. 1, compare lanes 2 and 3 with lanes 4 and 5). Interestingly, dissolved pellet of TCS day 25 (Fig. 1, lane 1) showed stronger reaction than dissolved pellet of TCS day 19 (Fig. 1, lane 3), although there were fewer infected cells in the system due to higher cytolysis at that time. To determine the OTH of the pellet fraction, the infected dissolved pellet fractions at all the time points were tested in WB following a similar approach. Figure 2 presents the results for the infected pellet fractions of TCS days 9, 11, 13, 15, 17, 19, 21, 23, and 25 and TCS day 17 of the negative control pellet. No signal was found in the pellet fraction of the noninfected cells at TCS day 17 (Fig. 2, lane 2). Weak specific CAEV bands were seen in WB on TCS day 9 (Fig. 2, lane 3). The intensity of the bands increased gradually from this time point until TCS day 25, where the strongest signal was observed (Fig. 2, lane 11). The CAEV polyclonal goat serum detected three specific bands, at approximately 10, 25, and 42 kDa. The protein of ∼25 kDa was the most prominent band (Fig. 2).
FIG. 1.
WB of supernatant and dissolved pellet fractions of PEG-derived CAEV whole-virus antigens at different TCS days, following precipitation, dialysis, and concentration with PEG 8000. Lanes 1 to 3, PEG-derived antigen fractions from the infected dissolved pellet of TCS days 25 (lane 1), 23 (lane 2), and 19 (lane 3); lanes 4 to 5, PEG-derived antigen fractions from the infected supernatant of TCS days 23 (lane 4) and 19 (lane 5); lane 6, PEG-derived fraction from TCS day 19 of noninfected cells. A reference CAEV-positive goat serum recognizing the capsid antigen of the virus was used. A specific band of around 25 kDa was detected in lanes 1 to 5.
FIG. 2.
WB of the dissolved pellet fractions of PEG-derived CAEV whole virus antigens at increasing TCS days and following precipitation, dialysis, and concentration with PEG 8000. Lane 2, PEG-derived fraction from TCS day 17 of noninfected dissolved pellet; lanes 3 to 11, PEG-derived antigen fractions from infected dissolved pellet at TCS days 9 (lane 3), 11 (lane 4), 13 (lane 5), 15 (lane 6), 17 (lane 7), 19 (lane 8), 21 (lane 9), 23 (lane 10), and 25 (lane 11). A reference polyclonal CAEV-positive goat serum was used. Specific bands were detected at around 10, 25, and 42 kDa (refer to molecular weight [MW] markers [in thousands], lane 1).
Antigen batches for ELISA.
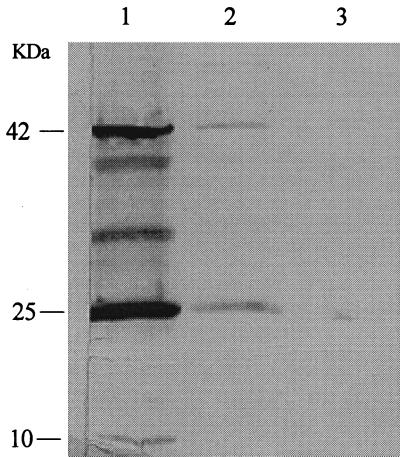
From the above results, two large antigen-derived PEG batches from eight (batch 1) and six (batch 2) roller bottles of virus-infected and corresponding noninfected FLCor cells were produced. The roller bottles were frozen at TCS days 23 to 25 for PEG batches 1 and 2. An identical approach using PEG 8000 to precipitate and concentrate the viral proteins was followed. To ensure the PEG batches were comparable to the smaller scale antigens produced at TCS days 23 to 25 from the previous experiment, WB were set up. The pellet fraction of the PEG batch 1 revealed 3 bands of similar molecular weight found with smaller batches at TCS days 23 to 25 (compare Fig. 2 and 3). However, 2 minor bands between 25 and 42 kDa, which were not detected previously, were found. The band at ∼25 kDa was still the most abundant antigenic viral protein in this viral stock. Again, weaker signals were detected with the supernatant fraction (Fig. 3, lane 2). PEG batch 2 gave similar results (data not shown).
FIG. 3.
WB of supernatant and dissolved pellet fractions of PEG-derived CAEV whole-virus antigen batch 1 following precipitation, dialysis, and concentration with PEG 8000. Lanes 1 and 2, dissolved pellet (lane 1) and supernatant (lane 2) fractions of PEG-derived antigens from eight roller bottles at TCS day 23 of infected cells; lane 3, dissolved pellet fraction of noninfected cells. A reference polyclonal CAEV-positive goat serum was used. Specific bands were detected at approximately 10, 25, and 42 kDa. Also, two other bands were detected between 25 and 42 kDa.
i-ELISA.
Both PEG batches of viral antigens were used in an i-ELISA following a conventional procedure (11, 22). They were tested at different dilutions against known positive and negative reference sera from CAEV-infected and noninfected goats respectively, with a confirmatory assay in a static mode. Table 1 indicates the OD414 readings obtained for the positive reference serum tested against a positive antigen batch and its corresponding negative antigen control. Both PEG batches were found suitable in ELISA, giving good discrimination between the positive and the negative antigen wells, and were found comparable to each other (Table 1). For further in-depth comparison and validation, PEG batch 1 was tested with 498 serum samples from field goats in a kinetic mode, after the cutoff values and ratio were determined (refer to Material and Methods). Table 2 presents the data obtained for this PEG-antigen batch, as opposed to the in-house preparation. The relative specificity and relative sensitivity for the PEG-Batch 1 were, respectively, 100 and 99.4% (Table 2).
TABLE 1.
OD414s of PEG antigens at various dilutionsa
| PEG antigen batch | OD414 at indicated dilution:
|
|||
|---|---|---|---|---|
| 1:200 | 1:400 | 1:600 | 1:800 | |
| 1 | ||||
| Infected | 0.915 | 0.927 | 0.932 | 0.611 |
| Noninfected | 0.124 | 0.117 | 0.100 | 0.059 |
| 2 | ||||
| Infected | 1.201 | 1.026 | 0.853 | 0.683 |
| Noninfected | 0.109 | 0.110 | 0.092 | 0.078 |
Batches were diluted from 1:200 to 1:800 and tested by i-ELISA with a CAEV polyclonal-positive goat serum. Serum was tested on the PEG antigen batch of the infected and noninfected dissolved pellet fractions.
TABLE 2.
Comparison between CAEV PEG antigen (batch 1) and CAEV in-house antigen in i-ELISA using a kinetic modea
| CAEV PEG antigen result | No. with indicated CAEV in-house antigen result:
|
Total no. | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 155 | 0 | 155 |
| Negative | 1 | 340 | 341 |
| Suspicious | 2 | 0 | 2 |
| Total | 158 | 340 | 498 |
Relative specificity = number of negative results obtained/number of negative results expected × 100 = 100%. Relative sensitivity = Number of positive and suspicious results obtained/number of positive results expected × 100 = 99.4%.
DISCUSSION
This work describes a very simple and rapid method of antigen production suitable in i-ELISA for the serodiagnosis of CAEV. Whole virus antigen obtained from TC was simply pelleted with PEG, dialyzed against water and PBS, and concentrated with PEG again, prior to being treated with SDS. The optimum time of antigen production was determined by a time course study, following cocultivation of CAEV-infected and noninfected cells. The OTH, using the CAEV Canadian isolate (SK167a), was determined to be at TCS days 23 to 25. Several strains of CAEV exist, which differ in virulence and antigenicity (15) and which do not have the same behavior in a culture system in vitro (3). It is recommended that each laboratory determine the best time to harvest the viral stock that will be used to produce the antigen batch, prior to initiating the production of a larger batch of antigen production suitable in ELISA. It is possible that a slow cytopathic strain, as used in this study, will perform better than a highly cytopathic strain which induces a faster destruction of TC cells.
The production of the PEG antigen did not require the use of expensive equipment such as an ultracentrifuge or complex manipulations involved in pressure filtration, chromatography, and/or gradient centrifugation required for the production of other reported TC-derived CAEV antigen batches suitable for ELISA (2, 11, 20). In fact, the PEG antigen was found to be comparable to our in-house antigen used previously at our laboratory for the diagnosis of CAEV (11). Morever, the PEG antigen batches were found stable at −70°C for many months, in suspension or when the ELISA plates were coated with them. The benefits of the PEG antigen were its rapidity, simplicity of production, reproducibility, and lower cost of production. A single batch of eight infected roller bottles provided a capacity of screening of around 24,000 serum samples.
We were the first to report the use of SDS for the final treatment of antigens used in ELISA for Maedi-visna virus, a closely related retrovirus (21). This ionic detergent was also used to produce our in-house CAEV ELISA antigen (11), as well as our CAEV PEG antigen. The same final treatment has been used also by others and found to be the best approach in the treatment of CAEV whole-virus antigens from TC, although they were obtained using different purification approaches (4, 23). SDS at a low concentration seems mainly to expose linearized antigenic CAEV or MV epitopes recognized by specific antibodies. Using WB and a polyclonal serum from an infected goat, at least three major immunogenic viral proteins in the PEG antigen were detected, at approximately 10, 25, and 42 kDa, representing most probably the reported trans-membrane envelope protein (40 kDa), the capsid protein (28 kDa), and another minor (p15) viral protein (24). Two other minor protein bands between 25 and 42 kDa were also detected, which may represent degradation subproducts of higher-molecular-weight proteins. The membrane envelope gp 130 (24) was not demonstrated in this system. This might be because of the lack of the corresponding specific antibody in the reference polyclonal goat serum used in WB, or most probably because of degradation or loss of conformational epitopes during the antigen production and/or WB procedures. ELISAs based on whole-virus antigens have been shown to be effective for the diagnosis of CAEV-infected animals (2, 11, 20, 25). Recombinant ELISAs have also been developed successfully with recombinant proteins derived from the trans-membrane or capsid genomic sequences regions (5, 16, 18). Although the latter are using single antigenic proteins (5, 16, 18) or a mix of two (16), whole-virus antigens usually comprise a variety of viral proteins for which animals develop antibodies; thus, whole virus antigens might be more sensitive in ELISA than a single protein or the mix of two recombinant proteins.
The i-ELISA can be automated, making this technique useful for the screening of large numbers of sera. The sensitivity and specificity of the i-ELISA depend, however, on the quality of antigens. According to the Office international des épizooties, the production of satisfactory antigen preparations has limited routine application of the ELISA for the diagnosis of CAEV (14). Serological methods are required in eradication and control programs for CAEV. For those, many laboratories across the world are still using the AGID test (14), mainly because of the ease and low cost of producing the crude antigen required in the AGID. However, this technique has been shown to be significantly less sensitive than the ELISA, both for CAEV and for Maedi-visna virus (2, 11, 22). Due to the lack of sensitivity of the AGID test method, a more sensitive assay, such as the ELISA, is strongly recommended in eradication campaigns. The use of recombinant protein antigens and of monoclonal antibodies has been developed to overcome the problems associated with the production and purification of whole-virus antigens suitable for ELISA. Although these newer technologies are reported to be highly sensitive and specific, they are not available to all laboratories involved in the diagnosis of CAEV. The development of a sensitive and specific assay based on whole-virus antigen that could be produced easily with a rapid and cheap methodology, such as the PEG approach, could be very useful for the diagnosis of CAEV.
ACKNOWLEDGMENTS
We acknowledge Phillip Maxwell for his training and technical help. A special thanks goes to Judith Bossé for revision of the manuscript.
REFERENCES
- 1.Adams D S, Klevjer-Anderson P, Carlson J L, McGuire T C, Gorham J R. Transmission and control of caprine arthritis-encephalitis virus. Am J Vet Res. 1983;44:1670–1674. [PubMed] [Google Scholar]
- 2.Archambault D, East N, Perk K, Dahlberg J E. Development of an enzyme-linked immunosorbent assay for caprine arthritis-encephalitis virus. J Clin Microbiol. 1988;26:971–975. doi: 10.1128/jcm.26.5.971-975.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blondin I, Grillet C, Thiogane Y. Syncytia formation in cultures and analysis of the protein composition of various strains of caprine arthritis-encephalitis virus. Ann Rech Vet. 1989;20:153–158. [PubMed] [Google Scholar]
- 4.Celer V, Zanoni R G, Paterhans E. Comparison of various antigens in the diagnosis of caprine arthritis-encephalitis virus using the ELISA test. Vet Med (Prague) 1993;38:237–244. [PubMed] [Google Scholar]
- 5.Clavijo A, Thorsen J. Serologic diagnosis of caprine arthritis-encephalitis by ELISA with two recombinant proteins in a parallel testing format. J Immunoassay. 1995;16:419–436. doi: 10.1080/15321819508013571. [DOI] [PubMed] [Google Scholar]
- 6.Crawford T B, Adams D S, Cheevers W P, Cork L C. Chronic arthritis in goats caused by a retrovirus. Science. 1980;207:997–999. doi: 10.1126/science.6153243. [DOI] [PubMed] [Google Scholar]
- 7.Crawford T B, Adams D S. Caprine arthritis-encephalitis: clinical features and presence of antibodies in selected goat populations. J Am Vet Med Assoc. 1981;178:713–719. [PubMed] [Google Scholar]
- 8.Dawson M. The caprine arthritis-encephalitis syndrome. Vet Annu. 1989;29:98–102. [Google Scholar]
- 9.Ellis T M, Robinson W F, Wilcox G E. The pathology and aetiology of lung lesions in goats infected with caprine arthritis-encephalitis virus. Aust Vet J. 1988;65:69–73. doi: 10.1111/j.1751-0813.1988.tb07361.x. [DOI] [PubMed] [Google Scholar]
- 10.Grewal A S, Littlejohns I R, Smith J E. Two distinct gel diffusion precipitin tests for the diagnosis of retrovirus infection in goats. Aust Vet J. 1986;63:86–88. doi: 10.1111/j.1751-0813.1986.tb02937.x. [DOI] [PubMed] [Google Scholar]
- 11.Heckert R A, McNab W B, Richardson S M, Briscoe M R. Evaluation of an enzyme-linked immunosorbent assay for the detection of antibodies to caprine arthritis-encephalitis virus in goat serum. Can J Vet Res. 1992;56:237–241. [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy-Stroskopf S, Narayan O, Strandberg J D. The mammary gland as a target organ for infection with caprine arthritis-encephalitis virus. J Comp Pathol. 1985;95:609–617. doi: 10.1016/0021-9975(85)90030-1. [DOI] [PubMed] [Google Scholar]
- 13.Norman S, Smith M C. Caprine arthritis-encephalitis: review of the neurologic form in 30 cases. J Am Vet Med Assoc. 1983;182:1342–1345. [PubMed] [Google Scholar]
- 14.Office International des Epizooties. Manual of standards for diagnostic tests and vaccines. 3rd ed. Paris, France: Office International des Epizooties; 1996. pp. 369–373. [Google Scholar]
- 15.Phelps S L, Smith M C. Caprine arthritis-encephalitis virus infection. J Am Vet Med Assoc. 1993;203:1663–1666. [PubMed] [Google Scholar]
- 16.Rimstad E, East N, DeRock E, Higgins J, Pedersen N C. Detection of antibodies to caprine arthritis-encephalitis virus using recombinant gag proteins. Arch Virol. 1994;134:345–356. doi: 10.1007/BF01310572. [DOI] [PubMed] [Google Scholar]
- 17.Robinson W F, Ellis M. Caprine arthritis-encephalitis virus infection: from recognition to eradication. Austr Vet J. 1986;63:237–241. doi: 10.1111/j.1751-0813.1986.tb02983.x. [DOI] [PubMed] [Google Scholar]
- 18.Rosati S, Pittan M, Tolari F, Erre G, Kwang J. Genetic and antigenic characterization of CAEV (caprine arthritis-encephalitis virus) recombinant trans-membrane protein. Vet Microbiol. 1995;45:363–370. doi: 10.1016/0378-1135(94)00138-m. [DOI] [PubMed] [Google Scholar]
- 19.Sambrook J, Fritsh E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 20.Schroeder B A, Oliver R E, Cathcart A. The development and evaluation of an ELISA for the detection of antibodies to caprine arthritis-encephalitis virus in goat sera. N Zeal Vet J. 1985;33:213–215. doi: 10.1080/00480169.1985.35240. [DOI] [PubMed] [Google Scholar]
- 21.Simard C, Briscoe M. An enzyme-linked immunosorbent assay for detection of antibodies to Maedi-Visna virus in sheep. I. A simple technique for production of antigen using sodium dodecyl sulfate treatment. Can J Vet Res. 1990;54:446–450. [PMC free article] [PubMed] [Google Scholar]
- 22.Simard C, Briscoe M. An enzyme-linked immunosorbent assay for detection of antibodies to Maedi-Visna in sheep. II. Comparison to conventional agar gel immunodiffusion test. Can J Vet Res. 1990;54:451–456. [PMC free article] [PubMed] [Google Scholar]
- 23.Vander Schalie J, Bradway D S, Besser T E, Evermann J F. Evaluation of a kinetic enzyme-linked immunosorbent assay for detection of caprine arthritis-encephalitis virus specific antibodies. J Vet Diagn Investig. 1994;6:30–33. doi: 10.1177/104063879400600106. [DOI] [PubMed] [Google Scholar]
- 24.Zanoni R, Krieg A, Peterhans E. Detection of antibodies to caprine arthritis-encephalitis virus by protein G enzyme-linked immunosorbent assay and immunoblotting. J Clin Microbiol. 1989;27:580–582. doi: 10.1128/jcm.27.3.580-582.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zanoni R G, Vogt H R, Pohl B, Bottcher J, Bommeli W, Peterhans E. An ELISA based on whole virus for the detection of antibodies to small-ruminant lentiviruses. Zentbl Veterinarmed. 1994;41:662–669. doi: 10.1111/j.1439-0450.1994.tb00277.x. [DOI] [PubMed] [Google Scholar]