Abstract
Currently, there is no specific pharmaceutical agent for treating acute pancreatitis (AP). Somatostatin and its analogues have been used to prevent the autolysis of the pancreas in AP, however, their effectiveness has not been confirmed. This investigation aimed to examine the efficacy of ulinastatin, a protease inhibitor, combined with somatostatin analogues in the treatment of AP. We conducted a systematic database search in 4 databases to identify randomized controlled trials in which the efficacy of ulinastatin in combination with somatostatin analogue was compared to somatostatin analogue alone in patients with AP. Since the patient populations of analysed papers were slightly different, we used random effect models to pool odds ratios (OR) and mean differences (MD) and the corresponding 95% confidence intervals (CI). A total of 9 articles comprising 1037 patients were included in the meta-analysis. The combination therapy significantly reduced the complication rates for acute respiratory distress syndrome, acute kidney injury, and multiple organ dysfunction. Symptoms were relieved threefold with the combination therapy compared to somatostatin alone, and combination therapy significantly shortened the length of hospital stay. The decrease in mortality was not statistically significant.
Subject terms: Diseases, Endocrine system and metabolic diseases
Introduction
Acute pancreatitis (AP) is the sudden inflammation of the pancreas of various aetiologies, mainly alcohol and gallstones1. The incidence rate of AP ranges between 4.6 and 100 cases per 100,000 patients, however, its frequency has steadily increased in the past decade, especially in western countries2,3. The overall mortality rate is approximately 5%, but it is highly dependent on the disease severity4. Based on the Atlanta classification, AP can be categorized as mild, moderate, or severe depending on local and systemic complications5. Mild cases are primarily self-limiting and resolve within a week, but in severe cases the mortality can reach 20–40%6. Early identification and management of AP are crucial to achieve better patient outcomes. Treatment delay could lead to life-threatening complications even in cases of mild AP at onset. Currently, no specific pharmacological agents are targeting the pathophysiological mechanisms in AP. Only supportive therapies are available. International guidelines recommend early oral or enteral nutrition support, fluid therapy, and pain management4,7–9.
Somatostatin, and its more potent analogue octreotide, reduce pancreatic enzyme secretion, allowing the pancreas to rest and avoid further autodigestion10. However, clinical studies show no statistical difference in patient outcomes when comparing octreotide or somatostatin to placebo11. Even though international guidelines do not recommend somatostatin or octreotide, their use is common practice in the therapy of AP, especially in Asian countries12. Ulinastatin is a broad-spectrum serine protease inhibitor currently recommended by the Chinese authoritative guidelines and broadly used in many Asian countries for the treatment of acute pancreatitis12. However, a recent meta-analysis investigating mortality and adverse events of ulinastatin prescribed in AP did not find sufficient evidence to support its use11. Nevertheless, in theory, ulinastatin in combination with other agents might be useful in improving therapeutic efficiency.
The combination of ulinastatin with somatostatin or its analogue octreotide was tested in several clinical trials with promising results13–15, however the level of evidence is still low. Our systematic review and meta-analysis aimed to investigate the efficacy and safety of ulinastatin combined with somatostatin or octreotide in comparison with somatostatin derivatives alone in the management of acute pancreatitis.
Methods
Search strategy
For this systematic review and meta-analysis, we followed recommendations of the Cochrane collaboration16 and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement17. The review was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database (registration number: CRD42021282614).
To answer the clinical question, we used the PICO framework. The population consisted of adult patients (> 18 years old) with acute pancreatitis; the intervention group included patients who received the combination treatment (ulinastatin therapy with somatostatin or octreotide) besides other supportive measures; the control or comparator group included cases treated with somatostatin or octreotide monotherapy besides other supportive measures. The primary outcomes were mortality, complications—Acute Respiratory Distress Syndrome (ARDS), shock, Acute Kidney Injury (AKI), Multiple Organ Dysfunction Syndrome (MODS), and length of hospital stay. As secondary outcomes, we evaluated symptom reduction rate, changes in laboratory parameters, and adverse events of the intervention.
The search was performed on 15 November 2021 in four databases (PubMed, Embase, Web of Science, and the Cochrane Central Register of Controlled Trials) to identify the randomized clinical trials meeting the previously mentioned eligibility criteria. The search key was: pancreatitis AND ulinastatin AND (octreotide or octreotid* or somatostatin) AND random*, and we did not use restrictions or filtering options. We used Google Translate® for translation of articles in languages other than English or German. Plot Digitizer (2015) was used to transform graphical values into numerical form. We additionally searched the reference list of the included studies.
Selection and data collection process
The search results were exported to the EndNote X9 citation manager (Clarivate Analytics, Philadelphia, PA, USA). After the automatic and manual duplicate removal (ILH), the title and abstract, and full-text selection processes were done by two independent authors according to the inclusion criteria (ILH and DK). A third author (DC) made the final decision in case of disagreements. Cohen’s kappa coefficient was calculated at each selection step to evaluate the level of agreement between the authors. Two independent investigators (ILH and DK) manually extracted the data from the eligible articles and cross-checked each other’s data sets to ensure precision. The following data were extracted: study characteristics (first author, year of publication, country, number of centres, setting), population description (sample size, percentage of female participants, age, AP severity), therapy details (drug type, dose, regimen, duration), and outcomes as reported in each article. Microsoft Excel (Microsoft, Office 365, Redmond, WA, USA) was used for data collection.
Statistics
We used the methods recommended by the working group of the Cochrane Collaboration23 for data synthesis. Only outcomes reported in at least three studies were considered for including in the meta-analysis. The pooled results were reported as ORs (odds ratios) for binary outcomes calculated with the Mantel–Haenszel method, and as mean differences (MDs) for continuous outcomes and the corresponding 95% confidence intervals (CI). In case of binary outcomes, ORs were used for the effect measure, while for continuous outcomes MDs with corresponding standard deviations (SDs) were used. In the latter case when only before-and-after treatment group means and SDs were reported, we used the difference in means, and the sum of within-group before-and-after SDs as a conservative estimate for SDs of the differences. For binary outcomes, raw data from the selected studies were pooled with the Mantel–Haenszel method, while for continuous outcomes mean differences were calculated. Random models were used for pooling in case of both outcome types. Subgroup comparisons were carried out following the description in Harrer et al.18. To estimate τ2 we used the Paule-Mandel method and the Q profile method for calculating the confidence interval of τ2 18,19. A funnel plot of the logarithm of effect size and comparison with the standard error for each trial was used to evaluate publication bias. Statistical heterogeneity across trials was assessed by means of the Cochrane Q test and the I2 statistic values20. I2 values of 25, 50, and 75% were identified as low, moderate, and high estimates, respectively. Outlier and influence analyses were carried out following the recommendations of Harrer et al. and Viechtbauer and Cheung18,21. Forest plots were used to graphically summarize results22,23. Where applicable, we reported the prediction intervals (i.e., the expected range of effects of future studies) of results following the recommendations of IntHout et al.23.
All analyses were carried out in R version 4.1.3 (R Core Team, Vienna, Austria) using the meta24 and dmetar18 packages.
Risk of bias assessment
The risk of bias assessment was performed by two independent authors (ILH and DK) using the revised Cochrane risk-of-bias tool (RoB2)25, while disagreements were solved by consensus. The domains evaluate the bias arising from the randomization process, deviations from the intended intervention, missing data, the measurement of the outcome, and the selection of the reported results. The final conclusion of the risk assessment could be characterized as ‘low’, ‘some concerns’, or ‘high’.
GRADE
We used the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework to evaluate the level of evidence for our findings26. Each outcome was rated for risk of bias, inconsistency, indirectness, imprecision, publication bias, and the presence of a large effect, dose-dependent response, and plausible confounders as ‘not serious’, ‘serious’, or ‘very serious’. The final certainty of the evidence was categorized as ‘very low’, ‘low’, ‘moderate’, or ‘high’.
Ethical approval
No ethical approval was required for this systematic review with meta-analysis, as all data were already published in peer-reviewed journals. No patients were involved in the design, conduct or interpretation of our study.
Results
Description of included studies
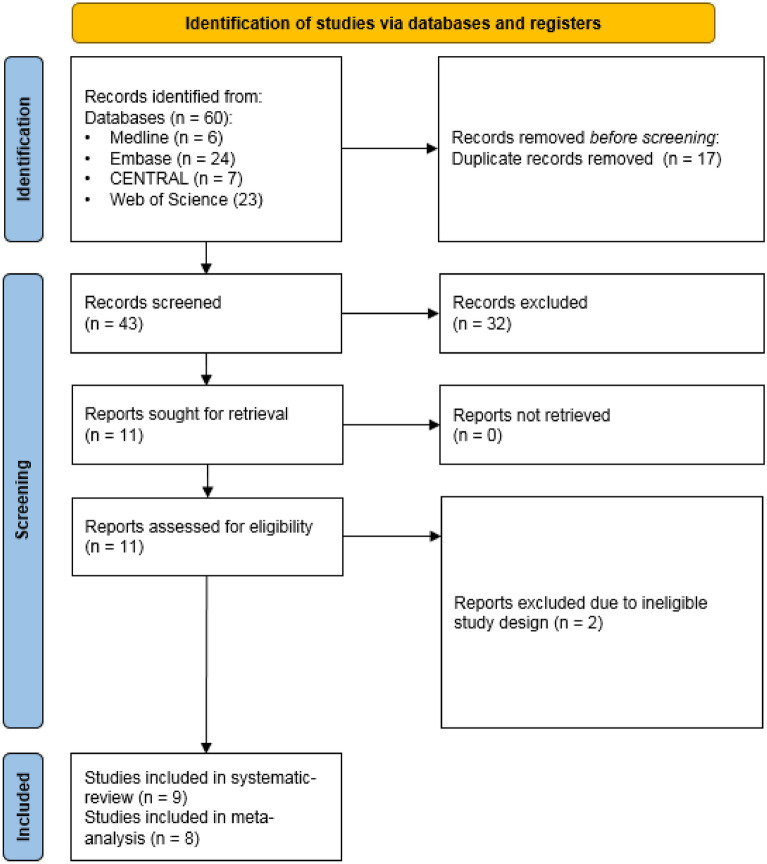
The database search identified 60 records. After duplicate removal, and title and abstract selection (Cohen’s Kappa 0.93), we identified 9 eligible articles during the full-text article analysis (Cohen’s Kappa 1.00). All included reports were available as peer reviewed journal articles. The search results and the selection process are summarized in Fig. 1.
Figure 1.
PRISMA flowchart.
Overall, 9 studies were included in our systematic review. There were no overlapping populations in the meta-analyses. All studies were single centre. Treatment arm allocation ratios were 1:1 in each study. The baseline characteristics of eligible studies are summarized in Table 1. The posology for each therapeutic regimen is detailed in Table 2.
Table 1.
Baseline characteristics of the included trials.
| Study | Country | Population | Sample size (% female) |
Intervention group | Sample size [intervention group] (% female) |
Mean age (years) ± SD [intervention group] |
Control group | Sample size [control group] (% female) | Mean age (years) ± SD [control group] |
Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Wang et al. (2013)27 | China | Severe acute pancreatitis | 123 (49.6) | Ulinastatin + octreotide | 62 (50.0) | 41.8 ± 13.9 | Somatostatin | 61 (49.2) | 42.6 ± 12.6 | Mortality; MODS |
| Tu et al. (2014)28 | China | Acute pancreatitis | 110 (47.3) | Ulinastatin + octreotide | 55 (45.5) | 37.3 ± 6.1 | Octreotide | 55 (49.1) | 38.7 ± 5.8 | LOH; SR; APR |
| Guo et al. (2015)13 | China | Severe acute pancreatitis | 120 (46.7) | Ulinastatin + octreotide | 60 (48.3) | 46.6 ± 4.1 | Octreotide | 60 (45.0) | 46.3 ± 4.3 | Mortality; LOH; MODS; ARDS AKI; shock; SR; APR |
| Wang et al. (2016)14 | China | Severe acute pancreatitis | 246 (48.8) | Ulinastatin + octreotide | 124 (49.2) | 40.8 ± 11.6 | Somatostatin | 122 (48.4) | 41.9 ± 12.8 | Mortality; LOH; MODS; SR; APR |
| Wang et al. (2017)30 | China | Moderateliy severe and severe acute pancreatitis | 42 (40.5) | Ulinastatin + octreotide | 21 (42.9) | 47.3 ± 11.1 | Somatostatin | 21 (38.1) | 48.6 ± 10.0 | ARDS; AKI; shock; APR |
| Yang et al. (2017)32 | China | Severe acute pancreatitis | 88 (39.8) | Ulinastatin + octreotide | 44 (40.9) | 42.1 ± 9.8 | Octreotide | 44 (38.6) | 43.2 ± 9.2 | N/A |
| Yang et al. (2018)15 | China | Severe acute pancreatitis | 94 (37.2) | Ulinastatin + octreotide | 46 (41.3) | 46.2 ± 10.6 | Octreotide | 48 (33.3) | 47.7 ± 11.8 | Mortality; LOH; ARDS; AKI; shock; SR; APR |
| Meng et al. (2019)31 | China | Acute pancreatitis | 108 (45.4) | Ulinastatin + octreotide | 54 (N/A) | N/A | Octreotide | 54 (N/A) | N/A | SR |
| Xu et al. (2019)29 | China | Severe acute pancreatitis | 106 (49.1) | Ulinastatin + octreotide | 53 (50.9) | 57.0 ± 6.9 | Somatostatin | 53 (47.2) | 57.5 ± 7.4 | LOH; SR |
SD, standard deviation; N/A, not reported; MODS, multiple organ dysfunction syndrome; LOH, length of hospital stay; SR, symptom reduction; APR, abdominal pain relief; ARDS, acute respiratory distress syndrome; AKI, acute kidney injury.
Table 2.
Summary of the applied therapies as reported in each eligible article.
| Study | Intervention group | Dose | Regime | Duration (days) | Control group | Dose | Regime | Duration (days) |
|---|---|---|---|---|---|---|---|---|
| Wang et al. (2013)27 | Ulinastatin + somatostatin | 100000 U | q12h | 10 | Somatostatin | 250 mcg/h | Continuous | 10 |
| Tu et al. (2014)28 | Ulinastatin + octreotide | 200000 U | qd | 14 | Octreotide | 0.5 g/(kg x h) | N/A | 14 |
| Guo et al. (2015)13 | Ulinastatin + octreotide |
(1) 100000 U (2) 50000 U |
(1) q12h (2) q12h |
(1) for 3 (2) then 7–14 |
Octreotide | 0.1 mg | q8h | 7–14 |
| Wang et al. (2016)14 | Ulinastatin + somatostatin | 100000 U | q12h | 10 | Somatostatin | 3 mg | Continuous | 10 |
| Wang et al. (2017)30 | Ulinastatin + somatostatin | 100000U |
(1) q12h (2) q24h |
(1) for 3 (2) then 7 |
Somatostatin | 6 mg | Continuous | 10 |
| Yang et al. (2017)32 | Ulinastatin + octreotide | 100000 U | q12h | 10 | Octreotide | 0.1 mg | q6h | 7 |
| Yang et al. (2018)15 | Ulinastatin + octreotide | 200000 U | qd | 14 | Octreotide | 0.1 mg bolus + 25 mcg/h | Continuous | 14 |
| Meng et al. (2019)31 | Ulinastatin + octreotide | 100000U | q12h | 7 | Octreotide | 0.6 mg | Continuous | 7 |
| Xu et al. (2019)29 | Ulinastatin + somatostatin | 100000 U | q24h | 7 | Somatostatin | 6 mg | Continuous | 7 |
U, unit; q, every; h, hour; d, day; mcg, microgram; mg, milligram, N/A, not reported.
The following outcomes were eligible for meta-analysis: mortality in 4 trials13–15,27; length of hospital stay in 5 trials13–15,28,29; multiple organ dysfunction syndrome in 3 trials13,14,27; acute respiratory distress syndrome in 3 trials13,15,30; acute kidney injury in 3 trials13,15,30; shock in 3 trials13,15,30; symptom reduction in 6 trials13–15,28,29,31; and abdominal pain relief in 5 trials13–15,28,30; CRP change in 6 trials13,15,28–30,32. We reported the results of Yang et al.32 in the systematic review since they only assessed laboratory parameters, which were insufficient for further statistical analysis.
Primary outcomes
Complication rates
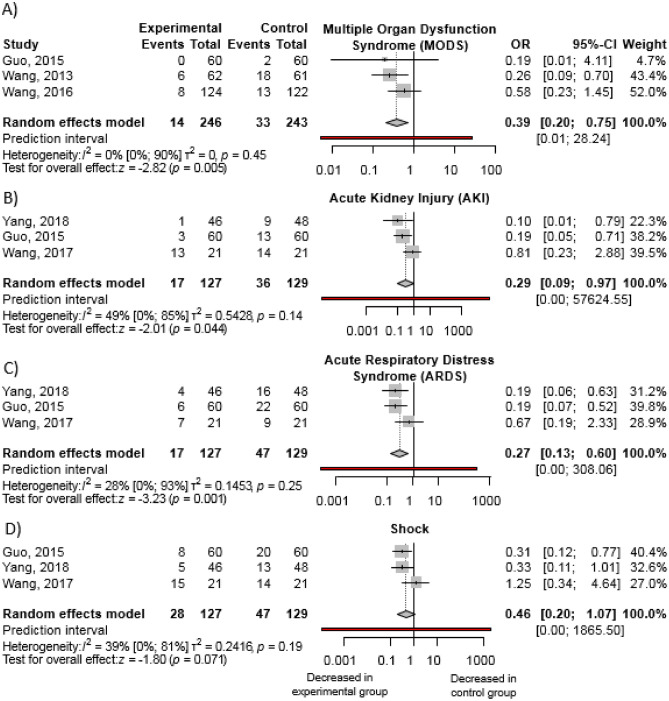
Our pooled results revealed decreased complication rates in the intervention group (Fig. 2). With the combination therapy, rates of ARDS [OR 0.27; 95% CI 0.13–0.60; I2 = 28%] and AKI [OR 0.29; 95% CI 0.09.-0.97; I2 = 49%] were reduced by approximately 70%, while MODS could be prevented in around 60% of cases [OR 0.39; 95% CI 0.20–0.75; I2 = 0%]. Reduction of shock incidence was not statistically significant [OR 0.46; 95% CI 0.20–1.07; I2 = 39%]. The associated heterogeneity for the results was not important or moderate, however, due to the low number of trials, interpretation has to be treated with caution.
Figure 2.
Ulinastatin in combination with somatostatin analogue decreases rates of: (a) MODS, (b) AKI, and (c) ARDS, but not of (d) shock, compared to somatostatin analogue monotherapy when administered besides standard of care in acute pancreatitis. (OR, odds ratio; CI, confidence interval).
Mortality
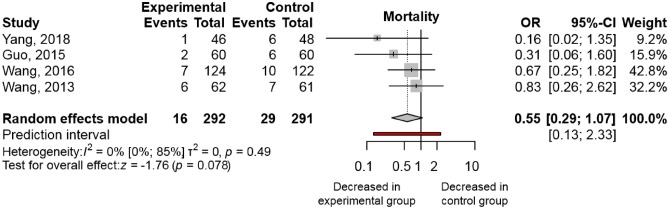
Analysis of pooled data from 4 trials13–15,27, including 583 patients, shows a tendency for a decreased mortality rate with the combination therapy [OR 0.55; 95% CI 0.29–1.07; I2 = 0%]; however, the result was not statistically significant (Fig. 3). These studies yielded homogenous results. All studies reported on in-hospital mortality.
Figure 3.
Ulinastatin in combination with somatostatin analogue is associated with decreasing trends in mortality when compared to somatostatin analogue monotherapy. (OR, odds ratio; CI, confidence interval).
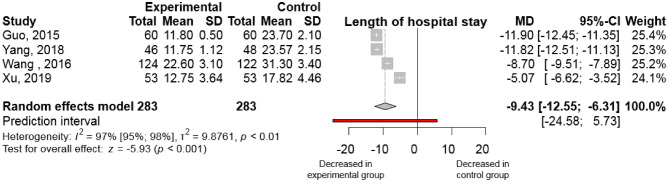
Length of hospital stay
Four studies13–15,28,29 reported the length of hospital stay, measured in days. In the intervention group, admission duration was shortened by 9.43 days [95% CI (-12.55)-(-6.31); I2 = 97%] by comparison with the control group (Fig. 4). The results showed substantial heterogeneity. The effect was similar for severe AP cases [MD (− 8.10); 95% CI (− 11.64) to (− 4.56); I2 = 99%; Fig. 3S].
Figure 4.
Ulinastatin combination with somatostatin analogue administered besides standard of care decreases the length of hospital stay in severe acute pancreatitis cases by comparison with somatostatin alone. (MD, mean difference; CI, confidence interval).
Secondary outcomes
The definition of treatment effectiveness varied across the included studies. The common elements of these definitions were (a) reduction of pancreatitis symptoms; abdominal pain, nausea, vomiting, (b) normalization of laboratory parameters evaluated at certain time intervals after treatment initiation. The time of evaluation varied among the included studies. The therapy was considered ineffective if the patients’ symptoms or laboratory parameters were not improved. A summary of effectiveness definitions in each study is available in Table 1S.
Symptom reduction
Six trials13–15,28,29,31, including 651 patients, reported symptom reduction. Among the assessed symptoms were gastrointestinal manifestations and abdominal pain, as well as laboratory parameters. They were evaluated at 7–17 days from treatment start. Pooled analysis shows 3.51 times higher odds of symptoms reduction in the combined therapy group than in the monotherapy group [OR 3.51; 95% CI 2.30–5.37; I2 = 0%; Fig. 4S]. This effect is similar in the subgroup analysis of the severe cases [OR 3.32; 95% CI 2.07–5.33; I2 = 0%; Fig. 4S].
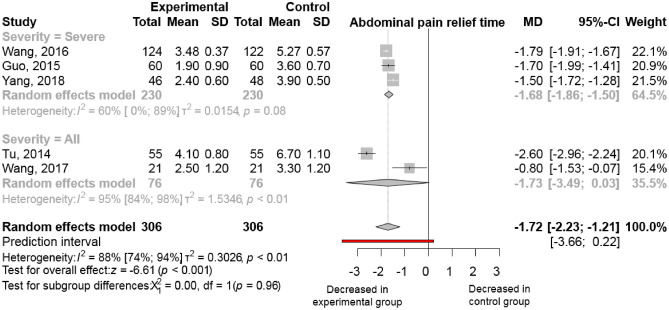
Abdominal pain relief
Duration until abdominal pain relief was specifically reported in 5 trials13–15,28,30, including 612 patients. It was measured as the number of days patients reported abdominal pain. Ulinastatin combined with somatostatin analogue led to significantly faster pain relief than somatostatin derivates monotherapy. The mean difference is − 1.72 days [95% CI (− 2.23) to (− 1.21); I2 = 88%, Fig. 5]. The results were similar in the severe form of acute pancreatitis [MD − 1.68; 95% CI (− 1.86) to (− 1.50); I2 = 60%; (Fig. 5)].
Figure 5.
Ulinastatin in combination with somatostatin analogue decreases time to abdominal pain relief. (MD, mean difference; CI, confidence interval).
Additional outcomes
Some of the studies reported on variations from baseline in several laboratory parameters, of which we were able to meta-analyse the results for C-reactive protein (CRP). There was a significant difference between the two groups regarding the reduction in CRP values from baseline to the end of treatment [MD 13.73 mmol/L, 95% CI 4.44–23.02; I2 = 73%], favouring the intervention group (Fig. 5S). Although we could not include the results for the other laboratory parameters (amylase, white blood cell count, TNFα, interleukins (Il-6, -8, -10), diamine oxidase) in our meta-analysis, the identified trends favoured the combination therapy. These results are summarized in the supplementary material (Tables 2S–10S).
Risk of bias assessment and quality of evidence
The overall risk of bias was moderate, mainly due to inaccurate reporting of blinding, imprecise measure reporting, and lack of available study protocols. The quality of evidence was low to moderate because of the small sample sizes and the overall moderate bias. The detailed results of the risk of bias assessment and the summary of findings table for GRADE are presented in the supplementary material (Figs. 1S, 2S, and Table 11S, respectively).
Publication bias could not be assessed due to an insufficient number of studies.
Discussion
Principal findings
Our meta-analysis assessed the clinical advantage of the combination therapy of ulinastatin with somatostatin analogues compared to somatostatin alone besides standard of care in acute pancreatitis. The ulinastatin combined with somatostatin or octreotide therapy significantly reduced the majority of systemic complications rates, the systemic inflammation as reflected by the significant improvement in the laboratory parameters, the length of hospital stay and the time to abdominal pain relief compared to somatostatin alone. Data about mortality and shock rates are limited.
Our results indicate that the intervention determines a threefold symptom reduction compared with monotherapy, which is consistent in severe acute pancreatitis. The better response rate might be a contributing factor to a faster recovery and to avoid complications. It could alleviate abdominal pain almost 2 days earlier than monotherapy. Abdominal pain is the leading symptom of AP; adequate management has a great impact on patients’ perspectives33. Moreover, the combination therapy could significantly reduce CRP, thus decreasing the inflammation. With fewer days of hospital stay and lower complication rates, it is a clinically effective therapy. Additional health care expenses could be spared in both short- and long-term.
Mortality showed a decreasing trend in the experimental group, but the results were not statistically significant. If we expect a reduction in mortality of 10% (from 12 to 2%) within the intervention group15,34 an optimal study sample size would be approximately 99 patients in each study arm (80% power, one-sided alpha level of 5% with continuity correction). None of the studies reached this threshold, so our results must be considered cautiously since we cannot strongly confirm the impact of the combination therapy on mortality.
The development of acute pancreatitis is initiated by excess Ca2+ signal generation, which leads to decreased mitochondrial ATP generation in the acinar cells, and promotes the activation of trypsin, resulting in necrosis35. An in vitro study by Kanayama36 suggests that ulinastatin might inhibit Ca2+ influx or mobilization, however, this effect has not been studied further. If given early, ulinastatin, a trypsin inhibitor, may suppress the trypsin autoactivation sequence. Furthermore, it also inhibits chymotrypsin, thrombin, kallikrein, neutrophil elastase, and cathepsin, thereby regulating systemic inflammation by reducing release of pro-inflammatory cytokines37. Moreover, ulinastatin inhibits necrosis by preventing mitochondrial damage, decreases endothelial dysfunction, normalizes coagulation disturbances, improves perfusion, and thereby restores organ functions37–40. This complex mechanism of action might complement those of somatostatin analogues explaining the increased efficacy of the combination treatment in acute pancreatitis. In hereditary pancreatitis, activation of trypsinogen has a pathogenic role in the development of chronic pancreatitis after an acute AP episode41,42. Further investigations are needed for the precise mechanism of action.
Several meta-analyses revealed positive effects of ulinastatin in many severe clinical scenarios: it can prevent postoperative bleeding in patients undergoing cardiac surgery43, it protects against ischemia–reperfusion injuries in hepatectomy44, in ARDS of various etiologies it decreases the mortality rates45, after cardiopulmonary bypass it reduces pulmonary injury and improves pulmonary function46, and decreases the duration of mechanical ventilation47. The clinical effects of ulinastatin observed in patients suffering from diseases that associate high risk of major complications come to support its potential in the management of acute pancreatitis.
Strength and limitations
To the best of our knowledge, this is the first meta-analysis on this topic. The strength of this review is its rigorous methodology. We strictly followed the Cochrane and PRISMA recommendations and ensured the study’s transparency through the prior publication of the review protocol on PROSPERO.
However, we identified several limitations. The conclusions are based on a limited number of trials performed only in China. Due to the small sample sizes, interpretation must be made carefully. There was no mention of sample size calculation in the trials. These factors resulted in high heterogeneity in some cases. Furthermore, variability in the population, and the differences in the applied treatment durations, doses, and follow-up times were also major contributing factors to the high heterogeneity. The included trials are of low to moderate quality, with the risks of bias resulting from a lack of proper reporting of blinding participants and investigators. Furthermore, there were no available study protocols to assess the intended and reported outcomes.
Implications for research and clinical practice
Somatostatin analogue monotherapy is not sufficiently effective in the therapy of AP. Although the results presented here suggest an improvement of efficacy when combined with ulinastatin, this combination should be further studied e.g., to overcome the limitation that all the available data are available from trials performed in China. Because of the differences in the applied treatments, outcome measures, and follow-up time, further multicentre, double-blind, randomized controlled clinical trials with greater sample sizes and well-defined outcomes are needed to assess the combination therapy's effect in acute pancreatitis. Moreover, data on the safety of the combination therapy in AP are missing. Because of the shorter hospital stay and decreased complications risk, cost-effectiveness and health technology assessment should be considered. The clinical efficacy and safety of further combination therapies should be assessed systematically.
This meta-analysis provides new insight into a possible drug therapy treatment for acute pancreatitis. This is especially important in severe cases, as there are limited treatment options and the mortality is high.
Conclusion
Ulinastatin combined with somatostatin analogue significantly decreased complication rates (ARDS, AKI, MODS) in AP in comparison with somatostatin analogue monotherapy. Moreover, combination therapy is associated with earlier symptoms relief and shorter hospital stay. Further RCTs of larger sample sizes would accurately evaluate the effect of this combination therapy.
Supplementary Information
Author contributions
I.L.H., S.B. and D.Cs. wrote the article I.L.H., K.D., D.Cs. carried out the title, abstract and full text selection, and the data extraction P.F. performed the statistics Sz.V., R.N., K.D., P.H., B.E., S.B. G.G. and D.Cs. contributed to the final version S.B. and D.Cs. supervised the project
Funding
Open access funding provided by Semmelweis University. Centre for Translational Medicine, Semmelweis University, 1085 Budapest, Üllői út 26, Hungary. All authors agreed on the final version of the manuscript and have no conflict of interest to disclose.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-22341-7.
References
- 1.Parniczky A, et al. Prospective, multicentre, nationwide clinical data from 600 cases of acute pancreatitis. PLoS ONE. 2016;11:e0165309. doi: 10.1371/journal.pone.0165309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts SE, et al. The incidence and aetiology of acute pancreatitis across Europe. Pancreatology. 2017;17:155–165. doi: 10.1016/j.pan.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Iannuzzi JP, et al. Global incidence of acute pancreatitis is increasing over time: A systematic review and meta-analysis. Gastroenterology. 2022;162:122–134. doi: 10.1053/j.gastro.2021.09.043. [DOI] [PubMed] [Google Scholar]
- 4.Crockett SD, et al. American gastroenterological association institute guideline on initial management of acute pancreatitis. Gastroenterology. 2018;154:1096–1101. doi: 10.1053/j.gastro.2018.01.032. [DOI] [PubMed] [Google Scholar]
- 5.Banks PA, et al. Classification of acute pancreatitis–2012: Revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 6.Boxhoorn L, et al. Acute pancreatitis. Lancet. 2020;396:726–734. doi: 10.1016/S0140-6736(20)31310-6. [DOI] [PubMed] [Google Scholar]
- 7.Leppaniemi A, et al. 2019 WSES guidelines for the management of severe acute pancreatitis. World J. Emerg. Surg. 2019;14:27. doi: 10.1186/s13017-019-0247-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samarasekera E, Mahammed S, Carlisle S, Charnley R, Comm G. Pancreatitis: Summary of NICE guidance. BMJ-Br. Med. J. 2018;362:3443. doi: 10.1136/bmj.k3443. [DOI] [PubMed] [Google Scholar]
- 9.Tenner S, Baillie J, DeWitt J, Vege SS, American College of G American College of Gastroenterology guideline: Management of acute pancreatitis. Am. J. Gastroenterol. 2013;108(1400–1415):1416. doi: 10.1038/ajg.2013.218. [DOI] [PubMed] [Google Scholar]
- 10.Bang UC, Semb S, Nojgaard C, Bendtsen F. Pharmacological approach to acute pancreatitis. World J. Gastroenterol. 2008;14:2968–2976. doi: 10.3748/wjg.14.2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moggia E, et al. Pharmacological interventions for acute pancreatitis. Cochrane Database Syst. Rev. 2017 doi: 10.1002/14651858.CD011384.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mao X, Yang Z. Current usage status of somatostatin and its analogs and trypsin inhibitors: A real-world study of 34,654 Chinese adult patients with acute pancreatitis. Ann. Palliat. Med. 2021;10:1325–1335. doi: 10.21037/apm-19-363. [DOI] [PubMed] [Google Scholar]
- 13.Guo H, Chen J, Suo D. Clinical efficacy and safety of ulinastatin plus octreotide for patients with severe acute pancreatitis. Natl. Med. J. China. 2015;95:1471–1474. doi: 10.3760/cma.j.issn.0376-2491.2015.19.008. [DOI] [PubMed] [Google Scholar]
- 14.Wang GL, et al. Effect of somatostatin, ulinastatin and gabexate on the treatment of severe acute pancreatitis. Am. J. Med. Sci. 2016;351:506–512. doi: 10.1016/j.amjms.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Yang JF, Chen S, Xia HW. Efficacy of ulinastatin combined with octreotide for patients with severe pancreatitis: Effect on clinical symptoms, serological markers and safety. World Chin. J. Digestol. 2018;26:1778–1783. doi: 10.11569/wcjd.v26.i30.1778. [DOI] [Google Scholar]
- 16.Higgins, J. P. T. T. J., Chandler, J., Cumpston, M., Li, T., Page, M. J. et al. Cochrane Handbook for Systematic Reviews of Interventions (Cochrane, 2021).
- 17.Page MJ, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrer M CP, F. T., Ebert DD. Doing Meta-Analysis with R: A Hands-On Guide (CRC, 2021).
- 19.Paule RC, Mandel J. Consensus values and weighting factors. J. Res. Natl. Bur. Stand. 1982;1977(87):377–385. doi: 10.6028/jres.087.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 21.Viechtbauer W, Cheung MW. Outlier and influence diagnostics for meta-analysis. Res. Synth. Methods. 2010;1:112–125. doi: 10.1002/jrsm.11. [DOI] [PubMed] [Google Scholar]
- 22.Rucker G, Schwarzer G. Beyond the forest plot: The drapery plot. Res. Synth. Methods. 2021;12:13–19. doi: 10.1002/jrsm.1410. [DOI] [PubMed] [Google Scholar]
- 23.IntHout J, Ioannidis JP, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6:e010247. doi: 10.1136/bmjopen-2015-010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balduzzi S, Rucker G, Schwarzer G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health. 2019;22:153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sterne JAC, et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 26.GRADEpro GDT. GRADEpro Guideline Development Too. <Available from gradepro.org> (2021).
- 27.Wang GL, et al. The effect of somatostatin, ulinastatin and salvia miltiorrhiza on severe acute pancreatitis treatment. Am. J. Med. Sci. 2013;346:371–376. doi: 10.1097/MAJ.0b013e31827aa2bc. [DOI] [PubMed] [Google Scholar]
- 28.Tu Y, Li GH. Octreotide injection combined with intravenous ulinastatin for treatment of acute pancreatitis: A controlled study. World Chin. J. Digestol. 2014;22:5009–5012. doi: 10.11569/wcjd.v22.i32.5009. [DOI] [Google Scholar]
- 29.Xu RS, Jiang S, Zhou HF, Jin WX. Clinical efficacy of ulinastatin combined with somatostatin for treatment of severe acute pancreatitis and effects on immune function. Int. J. Clin. Exp. Med. 2019;12:11333–11341. [Google Scholar]
- 30.Wang J, et al. Clinical value of the early use of ulinastatin in patients with moderately severe or severe acute pancreatitis. Zhonghua Yi Xue Za Zhi. 2017;97:1252–1255. doi: 10.3760/cma.j.issn.0376-2491.2017.16.015. [DOI] [PubMed] [Google Scholar]
- 31.Meng L, Wu Z, Zhang H. Effect of ulinastatin combined with octreotide on serum endothelin, endotoxin levels and immune function in acute pancreatitis. J. Coll. Phys. Surgeons-Pak. 2019;29:90–92. doi: 10.29271/jcpsp.2019.01.90. [DOI] [PubMed] [Google Scholar]
- 32.Yang ZY, et al. Effect of octreotide combined with ulinastatin on the serum endotoxin and intestinal mucosal permeability in patients with severe pancreatitis. Biomed. Res. (India) 2017;28:4062–4065. [Google Scholar]
- 33.Foldi M, et al. The characteristics and prognostic role of acute abdominal on-admission pain in acute pancreatitis: A prospective cohort analysis of 1432 cases. Eur. J. Pain. 2022;26:610–623. doi: 10.1002/ejp.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Besselink MG, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: A randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:651–659. doi: 10.1016/S0140-6736(08)60207-X. [DOI] [PubMed] [Google Scholar]
- 35.Petersen OH, Gerasimenko JV, Gerasimenko OV, Gryshchenko O, Peng S. The roles of calcium and ATP in the physiology and pathology of the exocrine pancreas. Physiol. Rev. 2021;101:1691–1744. doi: 10.1152/physrev.00003.2021. [DOI] [PubMed] [Google Scholar]
- 36.Kanayama N, et al. Kunitz-type trypsin inhibitor prevents LPS-induced increase of cytosolic free Ca2+ in human neutrophils and HUVEC cells. Biochem. Biophys. Res. Commun. 1995;207:324–330. doi: 10.1006/bbrc.1995.1191. [DOI] [PubMed] [Google Scholar]
- 37.Mehta Y, et al. Therapeutic approaches in modulating the inflammatory and immunological response in patients with sepsis, acute respiratory distress syndrome, and pancreatitis: An expert opinion review. Cureus. 2021;13:e18393. doi: 10.7759/cureus.18393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meng WT, et al. Ulinastatin: A potential alternative to glucocorticoid in the treatment of severe decompression sickness. Front. Physiol. 2020;11:273. doi: 10.3389/fphys.2020.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong WW. ICE family proteases in inflammation and apoptosis. Agents Actions Suppl. 1998;49:5–13. doi: 10.1007/978-3-0348-8857-8_2. [DOI] [PubMed] [Google Scholar]
- 40.Neumann IGB, Bdair F, Rada G. Antiproteases for acute pancreatitis. Cochrane Database Syst. Rev. 2011 doi: 10.1002/14651858.CD009426. [DOI] [Google Scholar]
- 41.Jancso Z, Sahin-Toth M. Mutation that promotes activation of trypsinogen increases severity of secretagogue-induced pancreatitis in mice. Gastroenterology. 2020;158:1083–1094. doi: 10.1053/j.gastro.2019.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hegyi E, Sahin-Toth M. Genetic risk in chronic pancreatitis: The trypsin-dependent pathway. Dig. Dis. Sci. 2017;62:1692–1701. doi: 10.1007/s10620-017-4601-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao YT, Fang NX, Liu DH, Li LH. Ulinastatin reduces postoperative bleeding and red blood cell transfusion in patients undergoing cardiac surgery: A PRISMA-compliant systematic review and meta-analysis. Med. (Baltim.) 2020;99:19184. doi: 10.1097/MD.0000000000019184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao H, Lyu Y, Yang Y, Li Y, Cao H. Perioperation ulinastatin intervention protects liver function in hepatectomy: A systematic review of randomized controlled trials and meta-analysis. Ann. Palliat. Med. 2020;9:774–787. doi: 10.21037/apm.2020.04.28. [DOI] [PubMed] [Google Scholar]
- 45.Zhang X, et al. Ulinastatin treatment for acute respiratory distress syndrome in China: A meta-analysis of randomized controlled trials. BMC Pulm. Med. 2019;19:196. doi: 10.1186/s12890-019-0968-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He G, et al. Effect of ulinastatin on interleukins and pulmonary function in bypass patients: A meta-analysis of randomized controlled trials. Herz. 2020;45:335–346. doi: 10.1007/s00059-018-4732-0. [DOI] [PubMed] [Google Scholar]
- 47.He S, Lin K, Ma R, Xu R, Xiao Y. Effect of the urinary tryptin inhibitor ulinastatin on cardiopulmonary bypass-related inflammatory response and clinical outcomes: A meta-analysis of randomized controlled trials. Clin. Ther. 2015;37:643–653. doi: 10.1016/j.clinthera.2014.12.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.