Abstract
Physical counter pressure maneuvers (CPM) are movements that are recommended to delay or prevent syncope (fainting) by recruiting the skeletal muscle pump to augment cardiovascular control. However, these recommendations are largely based on theoretical benefit, with limited data evaluating the efficacy of CPM to prevent syncope in the real-world setting. We conducted a semi-systematic literature review and meta-analysis to assess CPM efficacy, identify literature gaps, and highlight future research needs. Articles were identified through a literature search (PubMed, April 2022) of peer-reviewed publications evaluating the use of counter pressure or other lower body maneuvers to prevent syncope. Two team members independently screened records for inclusion and extracted data. From 476 unique records identified by the search, 45 met inclusion criteria. Articles considered various syncopal conditions (vasovagal = 12, orthostatic hypotension = 8, postural orthostatic tachycardia syndrome = 1, familial dysautonomia = 2, spinal cord injury = 1, blood donation = 10, healthy controls = 11). Maneuvers assessed included hand gripping, leg fidgeting, stepping, tiptoeing, marching, calf raises, postural sway, tensing (upper, lower, whole body), leg crossing, squatting, “crash” position, and bending foreword. CPM were assessed in laboratory-based studies (N = 28), the community setting (N = 4), both laboratory and community settings (N = 3), and during blood donation (N = 10). CPM improved standing systolic blood pressure (+ 14.8 ± 0.6 mmHg, p < 0.001) and heart rate (+ 1.4 ± 0.5 bpm, p = 0.006), however, responses of total peripheral resistance, stroke volume, or cerebral blood flow were not widely documented. Most patients experienced symptom improvement following CPM use (laboratory: 60 ± 4%, community: 72 ± 9%). The most prominent barrier to employing CPM in daily living was the inability to recognize an impending faint. Patterns of postural sway may also recruit the skeletal muscle pump to enhance cardiovascular control, and its potential as a discrete, proactive CPM needs further evaluation. Physical CPM were successful in improving syncopal symptoms and producing cardiovascular responses that may bolster against syncope; however, practical limitations may restrict applicability for use in daily living.
Keywords: syncope, orthostatic intolerance, postural sway, counter pressure maneuvers, blood pressure, cardiovascular control, skeletal muscle pump
Introduction
Syncope (fainting; a transient loss of consciousness due to inadequate cerebral perfusion) (1) is common; the cumulative lifetime incidence of syncope is estimated to be greater than 35% in the general population (2), although the true prevalence rate is hard to identify as episodes are often under-reported in the general public (3). The age distribution for syncope is bimodal, with episodes most commonly observed in adolescents and the elderly (2). Syncope accounts for up to 2% of emergency department visits, of which 12–85% of patients are subsequently admitted to the hospital, with admission rates varying depending on the country and respective healthcare system considered (4). This presents a substantial healthcare burden, with an annual cost to the healthcare system of up to $2.4 billion annually in the United States (5, 6).
Up to 30% of those who have fainted will experience recurrent and severe episodes (7). Living with recurrent syncope presents many challenges to daily living, and can profoundly impact patient independence, and quality of life (8–12). Patients with syncope also face greater fatigue in daily living (13), and an increased risk of fall-related injury associated with their loss of consciousness, particularly in older adults with increased frailty (14, 15). Up to 30% of patients with syncope will experience injury or physical trauma secondary to their loss of consciousness (16, 17) with approximately 5% of individuals experiencing a significant injury that leads to further impairments in quality of life (18). This heightened risk increases both morbidity and mortality in older adults with syncope (19).
The final common pathway for syncope is a reduction in cerebral blood flow, which is exacerbated by orthostatic or gravitational stress, and as such syncope is typically associated with prolonged standing. When upright, gravitational fluid shifts redistribute blood into the lower limbs (venous pooling), which promotes capillary filtration and subsequent edema (Figure 1) (20–23). This effect can be substantial; as much as 700 ml of fluid will accumulate in the lower body with just 10 min of 60° head upright tilting (22, 24). As fluid is lost to the periphery, there will be a concordant decrease in effective circulating volume, leading to decreased blood pressure and venous return, with reductions in cardiac output (CO) that ultimately reduce cerebral perfusion (25, 26). This reduction in blood pressure initiates baroreflex-mediated vascular resistance and capacitance responses, combined with increases in heart rate and force of contraction, to increase blood pressure and regain circulatory homeostasis (27). However, in the presence of a sufficiently severe orthostatic stress, the baroreflex response may be overwhelmed, with the responses evoked insufficient to regain cardiovascular homeostasis. This can result in marked cerebral hypoperfusion, often associated with symptoms of presyncope, and ultimately, syncope (20, 21, 28). In many types of recurrent syncope, these reflex responses may be impaired or compromised, further predisposing to syncopal events. This is most notable with neurally-mediated syncope (vasovagal), postural orthostatic tachycardia syndrome (POTS), or neurological causes of syncope (e.g., autonomic failure) (29).
FIGURE 1.
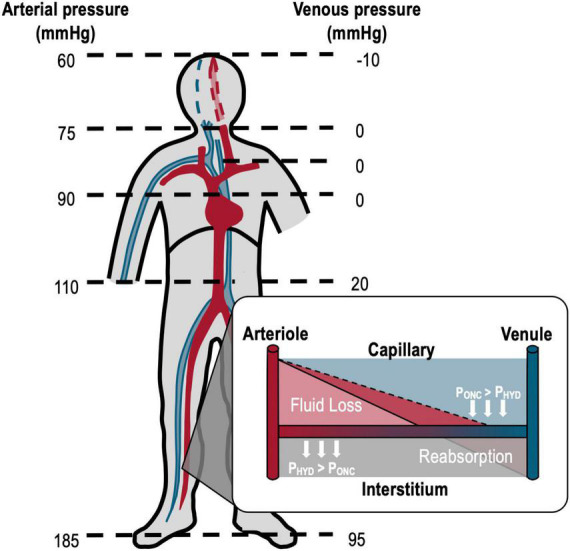
Effects of gravity on arterial and venous pressures in an upright, motionless human. Orthostatic arterial and venous pressures are shown on the left and right, respectively. With orthostasis, arterial and venous pressures above heart level decrease while pressures below the heart increase due to the gravitational redistribution of blood (venous pooling). The white inset diagram shows Starling forces of hydrostatic pressure (PHYD, light red shading; generated by the circulating blood pressure, which force fluid out of the vessel) and oncotic pressure (PONC, solid line; generated by plasma proteins, which promote fluid reabsorption) at the capillaries. In orthostasis, the increased lower extremity hydrostatic pressure (dark red shading, dashed line) promotes fluid loss at the capillaries and thus the accumulation of edema in the tissue. The combined venous pooling and capillary filtration reduce the effective circulating blood volume compromising cardiovascular control. Adapted from Hainsworth et al. (78).
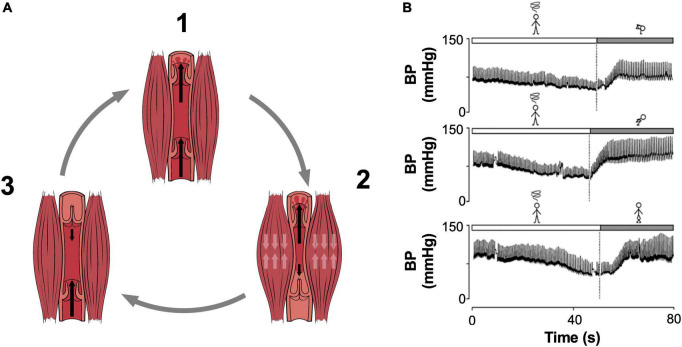
The skeletal muscle pump (Figure 2) also helps to counter the effects of orthostatic stress, by facilitating the ejection of pooled blood from the lower extremities back to the heart, and by aiding fluid movement through lymphatic vessels. This pump incorporates any lower body musculature (abdominal, gluteal, thigh, calf) with a role in protecting central blood volume. When recruited, their contraction increases intramuscular pressure to mechanically compress nearby veins, propelling any pooled blood past one-way valves to improve venous return, and subsequently, CO. The benefit of this pumping mechanism in cardiovascular control is clear; populations unable to recruit the skeletal muscle pump, such as patients with paraplegia or tetraplegia, experience increased susceptibility to postural hypotension that can be reversed with electrically stimulated contractions of the lower limb (30, 31). Further, the pumping action induced by intermittent calf compression (32, 33), but not sustained calf compression (34), delays the onset of presyncope and prevents venous pooling in healthy individuals.
FIGURE 2.
The Skeletal Muscle Pump. (A) Pumping cycle of the skeletal muscle pump is shown. (1) Prior to contraction, venous valves are open, and blood freely enters the vessel. (2) Muscular contraction compresses the vessel, forcing blood through the superior valves, meanwhile inferior valves close to prevent backflow. (3) With relaxation, negative pressure closes the superior valves and blood is drawn into the vessel through the inferior valve in preparation for the next cycle. (B) Representative sample tracings of pressure responses to the crash position (top; squatting with the head placed between the knees), squatting (middle), and leg crossing performed with buttock and abdominal tensing (bottom). The patient performed these maneuvers following a vasovagal reaction accompanied by prodromal pallor and sweating. All three maneuvers successfully restored blood pressure. BP, blood pressure. Adapted from (A) OpenStax College, Anatomy and Physiology (133) (licensed under the Creative Commons Attribution 3.0 Unreported license) and Boron and Boulpaep, Medical Physiology (27), (B) Krediet et al. (134).
Physical counter pressure maneuvers (CPM) are movements that promote cardiovascular stability and boost blood pressure through exploitation of the skeletal muscle pump, often accompanied by increases in sympathetic drive (1, 35–37). Movements such as leg crossing, arm tensing, and squatting are a commonly recommended component of syncope management for patients with recurrent fainting (29, 38), or those prone to blood-injection-injury phobia (e.g., vaccination, blood draws, blood donation) (39–41). Other finer lower body movements such as postural sway have also been linked to fluid volume shifts (42), while enhanced postural sway has been detected in populations susceptible to fainting such as Alzheimer’s disease (43), or astronauts after prolonged space flight (44). At the onset of presyncope, (near-fainting; marked by prodromal symptoms such as light headedness, “cold” sweating, nausea, visual tunneling, and disorientation) (29, 45, 46), patients are often advised to perform CPM to prevent the progression of their symptoms into a frank syncopal episode. Reports indicate that 79–95% of patients with vasovagal syncope (VVS; the “common” faint) experience some form of prodrome (37, 47–49), and 69% feel they are able to identify an impending faint (37). This may permit the deployment of CPM to act as a final line of defense against loss of consciousness once cardiovascular instability is apparent.
While CPM are often recommended to patients, these are based largely on clinical experience with a paucity of direct evidence for their benefit. Systematic reviews performed by Dockx et al. (50), and Jensen et al. (51), provided an important assessment of the quality of evidence to date, with both concluding that the evidence for CPM is of low, or very low quality. In addition, these systematic reviews employed strict inclusion criteria, which aids comparison of similar studies, but at the cost of limited scope, and accordingly, both considered only a narrow selection of articles. To date, full consideration of the responses to CPM and their efficacy is still an emerging field, and comprehensive evaluation of the heart rate (HR) responses to CPM has not been performed; this is of particular interest to patients with POTS who experience debilitating symptoms of orthostatic tachycardia associated with presyncopal symptoms. Further, responses of the cerebral circulation to CPM have not been evaluated; cerebral hypoperfusion is the final common pathway for syncope, therefore identifying cerebral responses is key to understanding the potential of CPM to provide a benefit for syncope prevention. Finally, prior works have focused on CPM that are most commonly recommended by clinicians. While this is a key area of research interest, there is also value in considering non-traditional maneuvers (e.g., neck flexion, seated maneuvers, marching, calf raises, abdominal compression, postural sway) in discussing future research needs as the field progresses. A more exhaustive summary of the diverse investigations performed to date is therefore needed in order to adequately consolidate current understanding of the utility of CPM for syncope management.
Aims and scope
In the present review, we take a semi-systematic approach to exploring the utility of CPM in syncope prevention. First, we provide an overview of the cardiovascular responses to these movements and explore plausible mechanisms for their utility. We also review the evidence for the efficacy and practical applicability of CPM that are currently recommended. Finally, we explore the role of postural sway in the maintenance of cardiovascular homeostasis, as this movement may provide a discrete, versatile, and accessible adjunct to currently recommended CPM.
Methodology
A semi-systematic approach was used to retrieve and review the current literature, as this is recommended for topics that have been conceptualized differently by various groups of researchers within diverse disciplines (52). This methodology uses the unbiased and rigorous search technique of a systematic review to identify a pool of literature, while allowing for flexibility in the dissemination of findings. Of note, a semi-systematic method permits the narrative review of emerging themes and knowledge gaps, and can highlight the complementary and contrasting techniques that have been used to study a given topic (53).
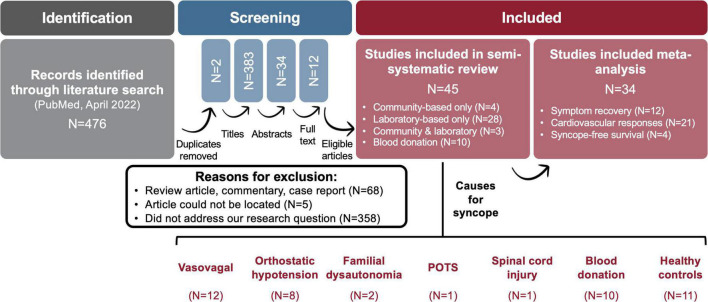
In our literature search, we identified works evaluating the role of CPM and other lower body maneuvers in preventing syncope (Figure 3). The initial search was conducted in the MEDLINE (PubMed) database (April 28, 2022) using the pre-determined search terms, and two reviewers (E. L. W. and F. M. K.) screened results using agreed-upon inclusion/exclusion criteria (Table 1). Our search originally uncovered 476 articles, and through the systematic screening of titles, abstracts, and full-text articles, 45 works were selected for inclusion. Articles with common themes were included in various meta-analyses (N = 34), while articles that did not evaluate these common outcomes were discussed in narrative form (N = 45).
FIGURE 3.
Semi-systematic method for literature review. The above flow diagram depicts our article selection process. Articles were screened at various levels (titles, abstracts, full text) to identify those that were relevant. Works were included if they evaluated the role of counter pressure and other lower body maneuvers in preventing syncope. Articles were selected for meta-analysis based on common themes amongst the article pool. Articles identified for our review that did not evaluate outcomes considered for meta-analysis were discussed in narrative form. POTS, postural orthostatic tachycardia syndrome.
TABLE 1.
Semi-systematic search strategy.
| Database | Search Terms | Inclusion Criteria | Exclusion Criteria |
| MEDLINE (PubMed) | [(syncope) OR (orthostatic tolerance) OR (postural syncope) OR (autonomic failure) OR (sympathetic failure) OR (orthostatic hypotension) OR (lower body negative pressure)] AND [(postural sway) OR (muscle pump) OR (counter pressure) OR (counter pressure) OR (leg crossing) OR (tensing) OR (muscle tension) OR (squatting)] |
Investigated cardiovascular, cerebrovascular, or other physiological responses to CPM. Investigated the efficacy of a given CPM to reduce symptoms of presyncope or presyncopal events or contribute to recurrent syncope management more broadly (e.g., improve quality of life). Investigated underlying mechanisms for the ability of muscular movement recruiting the skeletal muscle pump to contribute to cardiovascular and/or cerebrovascular stability. CPM defined as voluntary movements, either sustained or repetitive, performed for the purpose of maintaining or regaining cardiovascular stability. |
Used body movement as a means to induce presyncope (e.g., rapid squat to stand) for the sake of evaluating a different (non-CPM) intervention. Evaluated CPM in combination with additional treatments (outside those typically prescribed as part of usual care) that may also provide a benefit in syncope management. Movements were not considered as CPM if they were: 1. better classified as exercise (e.g., resistance or cardiovascular training); 2. required external equipment (e.g., passive movement, external compression); or 3. were not performed voluntarily (e.g., electrical stimulation). |
Agreed upon search terms, inclusion, and exclusion criteria for our systematic article search are reported above. CPM, counter pressure maneuver.
Counter pressure maneuvers
Cardiovascular responses
Cardiovascular responses to CPM have been studied most widely, with 21 articles considering responses in the laboratory setting (30, 36, 54–73). A summary of the cerebrovascular and hemodynamic responses to various CPM is reported in Table 2. While the movements performed during CPM constitute a small exercise dose, these may still be sufficient to initiate a physiological exercise response concurrent with any muscle-pumping effect (36, 54). In addition, while most CPM involve an isometric (sustained) contraction, some require dynamic movements with oscillating contraction and relaxation of major muscle groups. In our literature pool, nearly all maneuvers were sustained, however, cardiovascular responses to dynamic movements (tiptoeing and marching) were considered in two studies (60, 64). Further investigation into dynamic maneuvers would improve our understanding of these CPM and how they might compare to sustained movements, particularly since rhythmic contraction/relaxation could be easier to maintain and may better facilitate muscle pumping activity (32, 33, 74, 75).
TABLE 2.
Cardiovascular responses to counter pressure maneuvers.
| Maneuver | Population | ΣN | SAP mmHg |
DAP mmHg |
MAP mmHg |
CO L.min–1 |
HR Bpm |
SV % |
TPR % |
CBFv cm.s–1 |
| Neck flexion | OH (64) | 9 | ↑ | |||||||
| Upper limb tensing | Control (58, 67, 73) | 80 | ↑↑↑ – | ↑↑ – | ↑↑↑ | ↑ – | ↑ | |||
| VVS (67, 73) | 69 | ↑ – – | ↑↑ – | – | – – | |||||
| Abdominal contraction | Control (94) | 10 | – | – | ↑ | |||||
| OH (64) | 9 | – | ||||||||
| FD (62, 94) | 23 | ↑ – | – | ↑ | ↑ | – ↑ | – | |||
| Lower body tensing | Control (55, 58) | 13 | ↑ | ↑↑ | ↑ – | ↑ | ↑ | ↑↓ | ||
| VVS (36, 56) | 30 | ↑↑ | ↑↑ | ↑ | ↑ | – – | ||||
| OH (55, 64) | 22 | ↑ | ↑ | – | – | |||||
| Leg crossing | Control (54, 60, 65, 71) | 27 | – –* | – –* | – – – | ↑ – – | ↑↓↓ | ↑↑↑ | – – – | ↑ |
| VVS (63) | 88 | ↑ | ↑ | ↑ | ↑ | – | ↑ | ↓ | ||
| OH (60, 64, 65, 71) | 29 | ↑↑↑* | ↑↑* | ↑↑↑* | ↑↑ | – – | ↑↑ | ↑– | ↑ | |
| FD (62) | 12 | ↑ | ↑ | – | – | – | – | |||
| Leg crossing (seated) | Control (69) | 43 | ↑ | ↑ | ↑ | |||||
| Leg crossing and tensing | Control (61, 66, 70, 73) | 54 | ↑ | – | ↑↑ – | ↑ | ↓↓ – | ↑ | ||
| VVS (36, 63, 72, 73) | 137 | ↑↑↑↑ | ↑↑↑ – | ↑↑ | ↑↑ | ↑↑↓ – – | ↑↑ | ↓ – | ||
| OH (61, 97) | 34 | ↑↑ | – | ↓ | ↑ | – | ↑ | |||
| Leg crossing and tensing (seated) | Control (69) | 43 | ↑ | ↑ | ↑ | |||||
| Tiptoeing | Control (60) | 6 | ↑ | – | ↑ | ↑ | – | ↑ | ↓ | |
| OH (60) | 7 | – | – | – | ↑ | – | ↑ | ↓ | ||
| Marching | OH (64) | 9 | ↑ | |||||||
| Calf raises | OH (64) | 9 | ↑ | |||||||
| Leg fidgeting | OH (68) | 6 | ↑* | ↑* | ↑* | |||||
| Kneeling | OH (64) | 9 | ↑ | |||||||
| Squatting | Control (65, 73) | 27 | ↑↑* | ↑–* | – | |||||
| VVS (36, 73) | 64 | ↑↑ | ↑↑ | ↑ | ↑ | – – – | ↑ | – | ||
| OH (64, 65) | 16 | ↑↑* | ↑* | ↑* | ||||||
| FD (62) | 12 | ↑ | ↑ | ↑ | ↑ | – | ↑ | |||
| Bending Forward | FD (62) | 13 | ↑ | ↑ | ↑ | ↑ | – | |||
| Whole body tensing | VVS (36) | 9 | ↑ | ↑ | – | ↑ | – | |||
| Crash position | VVS (36) | 9 | ↑ | ↑ | ↓ | ↑ | – |
The assessments summarized considered healthy controls (Control), as well as patients with vasovagal syncope (VVS), orthostatic hypotension (OH: including initial OH, classical OH, and hypoadrenergic OH secondary to autonomic failure), and familial dysautonomia (FD). Each entry denotes reports from a single study, with increases (↑) or decreases (↓) in physiological outcome measures reported where significance criteria of p < 0.05 were met. The horizontal line (–) indicates that no significant change in the outcome measure was detected. Blank spaces indicate that the outcome was not considered by a given study. Note that where an individual study performed more than one evaluation of a counter pressure maneuver, or provided analyses across multiple subgroups of patients or controls, more than one outcome was generated. Upper limb tensing includes both arm tensing, and hand grip maneuvers. Lower body tensing includes instruction to contract any combination of musculature of the lower body (abdomen, buttocks, thigh, calf, entire leg) without any additional instruction for movement or position change. *Significance was determined by student’s t-test using data reported in publication. SAP, systolic arterial pressure; DAP, diastolic arterial pressure; MAP, mean arterial pressure, CO, cardiac output; HR, heart rate; SV, stroke volume; TPR, total peripheral resistance (measures retrieved included total peripheral resistance, systemic vascular resistance, and peripheral vascular resistance); CBFv, cerebral blood flow velocity; ΣN, cumulative sample size.
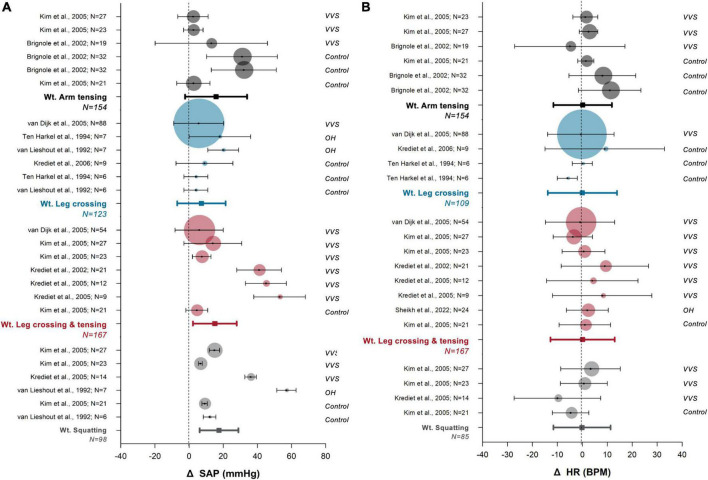
Most studies reported that CPM were successful in improving both systolic (SAP) and diastolic (DAP) arterial pressure, as expected during sustained CPM. Isometric muscle contraction leads to concurrent increases in SAP and DAP, driven by a net increase in total peripheral resistance (TPR) which occurs largely due to the mechanical compression of the vasculature by working muscle (36, 76, 77). Following tiptoeing (a dynamic maneuver), an increase in SAP with no change in DAP was reported, which is expected during dynamic exercise. In our combined analysis of SAP responses to the most commonly studied CPM (Figure 4A) SAP increased by + 14.8 ± 0.6 mmHg (N = 628, p < 0.0001), demonstrating evidence of benefit of CPM for syncope prevention.
FIGURE 4.
Meta-analysis of cardiovascular responses to commonly prescribed counter pressure maneuvers. Responses of systolic arterial pressure [SAP, (A)] and heart rate [HR, (B)] are shown. The most highly studied counter pressure maneuvers were arm tensing (black), leg crossing (blue), leg crossing with muscle tensing (red), and squatting (gray). Data for each study are presented as the mean (circle) and standard deviation (whiskers), with the size of the circle proportional to the study sample size. Bolded whisker plots show the weighted (wt.) mean and standard deviation of group analyses. Vertical dotted lines indicate zero effect. The population considered is indicated to the right of each plot: vasovagal syncope (VVS), orthostatic hypotension (OH, included classical OH, and hypoadrenergic OH), and healthy controls (control).
A small but significant HR response to CPM was detected (+ 1.4 ± 0.5 bpm; N = 539, p = 0.006) (Figure 4B) and this may reflect contradicting regulatory mechanisms. Baroreflex-mediated bradycardia would be expected in response to recovered blood pressure, and this would be augmented by increased stroke volume following increased preload (54). However, any concurrent exercise response caused by CPM would lead to increased HR (36, 78, 79). The combination of both stimuli likely explains why we detected only a small net effect on the HR response.
In almost all studies that reported CO responses, CO was increased following CPM. Given the small HR response to CPM identified in our combined analysis, this suggests that improvements in CO are likely driven by the well documented increase in SV (observed in every study that examined SV responses), reflecting improved venous return, and ultimately, effective skeletal muscle pumping (1, 35, 36). Due to a lack of uniformity in reporting methods for SV responses (with different units, and variations in reporting of absolute responses, absolute changes, or percentage changes) we were unable to perform meta-analyses on SV data.
TPR responses during sustained (isometric) CPM were variable, with some studies reporting increases and some reporting decreases in TPR. This likely also reflects contradictory regulatory mechanisms with different CPM approaches. As with SV data, a combined analysis on TPR was not possible due to a lack of uniformity in reporting measures. During exercise, muscle mechanoreflexes and chemoreflexes promote increased TPR through increased sympathetic nerve activity. Of note, chemoreflex responses may not contribute to all findings reported, as they only become prominent after approximately 1 min of sustained muscular contraction (36, 63, 79). The mechanical compression of vasculature by sustained isometric contraction also contributes to increased TPR (36, 76, 77), but this may be countered by baroreflex-mediated decreases in TPR as blood pressure recovers due to successful skeletal muscle pumping. Interestingly, in patient populations with reduced sympathetic drive (e.g., pure autonomic failure, multiple system atrophy), the mechanical increases in TPR may be even more beneficial by countering the vasodilatory effects caused by reduced sympathetic vascular control (71). In one study that evaluated dynamic CPM (tiptoeing), a net decrease in TPR was reported as expected. Further investigation is required in both sustained, and dynamic CPM to clarify the TPR response, and tease apart the many regulatory mechanisms at play.
Cerebrovascular responses
The final common pathway for syncope is a reduction in cerebral perfusion (80, 81), therefore cerebral blood flow is a primary outcome of interest; however, cerebrovascular responses to CPM have not been studied widely.
Both cerebral blood flow velocity (CBFv) (61, 66, 71, 82, 83) and cerebral oxygenation (66, 69, 71) are reported to increase following sustained lower body CPM. This likely reflects improved cerebral perfusion in light of the increases in blood pressure, SV and CO that accompany CPM (66, 71). However, one study also reported that bolstering of CBFv with CPM occurred alongside increased PaCO2 and PETCO2 [and therefore ameliorated the orthostatic hypocapnia-mediated cerebral vasoconstriction (66)]. This may be attributable to an exercise response increasing carbon dioxide production (84), or perhaps to reduced reliance on the respiratory muscle pump when cerebrovascular control is bolstered by CPM, reducing postural hyperventilation and associated hypocapnia.
Treatment efficacy
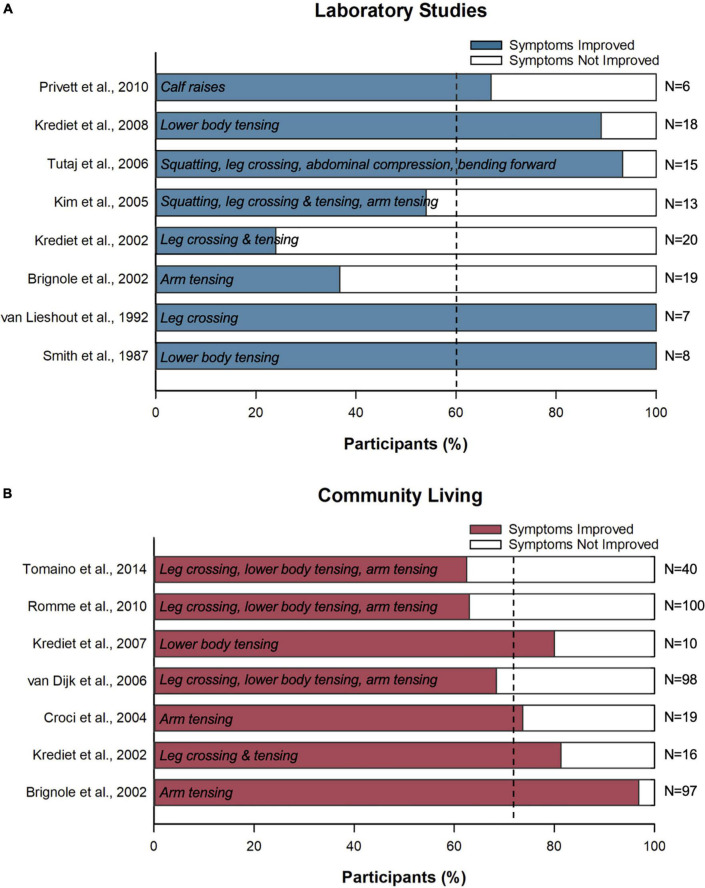
CPM are considered a low-cost, first-line management strategy for syncope prevention (35, 36, 65, 67, 72, 73, 85). The European Society of Cardiology guidelines for the diagnosis and treatment of syncope list CPM as a Class I intervention for patients with reflex syncope if they typically experience a prodrome prior to an episode (29). These maneuvers are recommended alongside mainstay treatments of syncope of patient education and lifestyle and management advice (29, 38). They may also provide additional benefit alongside other preventative measures such as the avoidance of known triggers for syncope, blood volume expansion by means of raised water and salt intake, and adjustment of aggravating medications (37, 38, 86, 87). Current CPM recommendations are based primarily on physiological evidence and clinical experience (29, 38), however, clear evidence for the efficacy of these maneuvers in mitigating symptoms is lacking. While CPM are a low-cost prevention strategy with a minimal risk of adverse side effects, an accurate evaluation of efficacy is needed to properly prescribe this treatment, identify populations most likely to benefit, and inform best practice. In our literature pool, we identified 13 studies where symptom recovery following CPM use in combination with usual care was evaluated through patient reports of sensations of presyncope, or the occurrence of a syncopal event (Figure 5).
FIGURE 5.
Effects of counter pressure maneuvers on symptoms of syncope and presyncope. Bars show the proportion of patients (%) who reported recovered symptoms following counter pressure maneuver implementation in (A) the laboratory and (B) the community setting. White text indicates the counter pressure maneuvers investigated in each study. Vertical dotted lines indicate the pooled mean.
Our combined analysis revealed that CPM diminished symptoms of presyncope and syncope for 62 ± 4% of patients in the laboratory, and 72 ± 9% of patients when used in combination with usual care during activities of daily living (Table 3). Alizadeh et al. also reported improvement in 65% of patients with use of hand grip, and 75% with use of squatting (compared to 43% with the control treatment of increased hydration and salt consumption). While this study was not identified in our original search, it does align with the findings of our meta-analysis (88). During activities of daily living, while many patients do appear to experience relief from CPM use, the current findings are limited by a lack of control or placebo condition (55, 65, 67, 72, 89), and a small sample size (57, 58, 65). As such, it was difficult to account for episodes of presyncope that would have resolved spontaneously even without CPM use, or the improvement in syncopal events reported relative to the patient’s baseline, or over and above improvements associated with usual care. This is important because, based on follow-up studies that included a control group, 48% of patients experienced improvement in syncopal episodes with usual care alone, compared to 65% experiencing improvement with usual care and CPM use (35, 90). The relatively high improvement rates in the control arm make the efficacy of CPM harder to ascertain and may reflect regression to the mean, improvements in syncopal symptoms in response to standard care and lifestyle advice, as well as the benefit of patient education or the relief associated with receiving diagnostic testing or an adequate diagnosis, all of which are known to contribute to improved patient outcomes (91, 92). A strong placebo effect has also been noted in syncope research, with patient expectancy for improvement possibly playing a further role in episode reduction (93). While this highlights the need for placebo controlled studies, it also suggests that perhaps the reassurance and confidence that accompanies training with a “safety-net” type strategy such as CPM may contribute to a positive benefit (35).
TABLE 3.
Improvement of symptoms of syncope and presyncope with counter pressure maneuvers.
| Paper | N | Population | Setting | Follow Up (Mo) | Endpoint | Description | Maneuver | Improvement (%) |
| Privett et al., (57) | 6 | Control | Post-exercise | – | OI Reoccurrence | Control: 6/6 (100%) had OI CPM: 2/6 (33%) had OI |
Calf raises | 67 |
| Krediet et al., (56) | 13 | VVS | Sit-stand | – | Presyncope | Control: 13/18 (72%) reported presyncope CPM: 2/18 (11%) reported presyncope |
Lower body tensing | 89 |
| Tutaj et al., (62) | 15 | FD | Standing | – | Presyncope | 14/15 (93%) verbally reported reduced symptoms with CPM | Squat, leg cross, abdominal compression, bend forward | 93 |
| Kim et al., (73) | 13 | VVS | Tilt (Passive) | – | Tilt Endpoint | Control tilt: 13/13 (100%) positive Tilt + CPM: 6/13 (46%) positive |
Leg crossing | 54 |
| Brignole et al., (67) | 19 | VVS | Tilt (Passive) | – | Tilt Endpoint | Control tilt: 19/19 (100%) positive Tilt + CPM: 12/19 (63%) positive |
Hand grip | 37 |
| Krediet et al., (72) | 21 | VVS | Tilt (Passive) | – | Tilt Endpoint | Reaction averted with CPM in 5/21 patients (24%), 15/21 (71%) had delayed syncope by median 2.5 min (30 s–11 min) | Leg crossing and tensing | 24 |
| van Lieshout et al., (65) | 5 | OH | Sit-stand | – | Presyncope | Control: 5/5 (100%) reported presyncope Stand + CPM: 0/5 (0%) reported presyncope |
Leg crossing | 100 |
| Smith et al., (58) | 8 | Control | Tilt (LBNP) |
– | Tilt Endpoint | Control tilt: 6/8 (75%) positive Tilt + CPM: 0/8 (0%) positive |
Lower body tensing | 100 |
|
| ||||||||
| Weighted Mean: | 62 ± 4 | |||||||
|
| ||||||||
| Tomaino et al., (90) | 95 | VVS | Community | 16 | Syncopal Reoccurrence | Reoccurred in 15/40 (37%, CPM), 24/45 (53%, control at follow up | Leg crossing, lower body tensing, arm tensing | 63 |
| Romme et al., (96) | 100 | VVS | Community | 12 | Syncopal Reoccurrence | 63/100 (63%) using CPM experienced a decreased syncope burden (episodes/year) at follow up | Squatting, leg-crossing, lower body tensing, arm tensing | 63 |
| Krediet et al., (36) | 10 | OH | Community | 2 | Presyncope | 8/10 (80%) patients reported reduced symptoms with CPM | Lower body tensing | 80 |
| van Dijk et al., (35) | 85 | VVS | Community | 12 | Syncopal Reoccurrence | Reoccurred in 31/98 (32%, CPM), 56/110 (51%, control) at follow up | Leg crossing, lower body tensing, arm tensing | 68 |
| Croci et al., (89) | 19 | VVS | Community | 14 | Syncopal Reoccurrence | 5/29 (17%) patients experienced syncopal reoccurrence at follow up | Arm tensing | 74 |
| Krediet et al., (72) | 16 | VVS | Community | 10 | Syncopal Reoccurrence | 13/16 (81%) with recurrence experienced fewer episodes than in previous year | Leg crossing and tense | 81 |
| Brignole et al., (67)* | 97 | VVS | Community | 9 | Presyncope | Among 11 patients (median 3 syncopal episodes/year), 94/97 (97%) cases of presyncope resolved. | Arm tensing | 97 |
| Weighted Mean: | 72 ± 9 | |||||||
Reports from studies evaluating counter pressure maneuvers (CPM) in the laboratory (top) and community setting (bottom, employment of counter pressure maneuvers in daily living) are summarized. The weighted mean ± standard deviation (%) of participants improved is reported under each respective list. These assessments considered populations of healthy controls (control), as well as patients with vasovagal syncope (VVS), orthostatic hypotension (OH, included initial OH and classical OH), and familial dysautonomia (FD). The follow up periods of community-based studies are given in months (Mo). Improvement was characterized by reports of the onset and subsequent alleviation of symptoms via counter pressure maneuvers, as indicated by the endpoint of the study. The endpoint for improvement varied across studies: OI reoccurrence, symptoms of orthostatic intolerance detected; presyncope, detected with cardiovascular cut-offs or patient reports; tilt endpoint, detected as per experimental protocol; syncopal reoccurrence, episode of syncope reported by patient over follow up period. Individuals that did not experience symptom onset initially or over the trial period were excluded from analyses. Individuals that were unable to employ counter pressure maneuvers for any reason were included in the analysis. Because most studies did not incorporate a control, or crossover design, improvement was calculated based on intervention group only. *Denotes study that used pseudo-replication in reporting of results; episodes of syncope experienced by 11 participants were pooled, and the improvement of individual participants was not evaluated.
There was also a lack of standardized methodology to document patient symptoms, with those used often relying on patient reports or extended recall of events (57, 58, 65). Of note, Brignole et al. (67) evaluated CPM success by pooling total syncopal episodes experienced among a small sample of patients. While this group reports one of the highest success rates (97% recovered), their methods involve pseudo replication, and fail to account for any episodes of presyncope that would not have progressed into syncope even in the absence of deployment of CPM. With this cohort reporting a median of 3 (1–11) syncopal episodes in the prior year, the majority of presyncopal episodes assessed would have presumably resolved spontaneously (67). For a clearer understanding of the efficacy of CPM in managing syncope symptoms, more rigorous and unbiased assessments including a placebo or control treatment are needed.
Following the release of CPM, symptoms may return (55, 56, 71, 73); although complete recovery is most beneficial, delaying symptoms can be valuable in preventing a fall (and related injuries). In some patients that did not experience complete symptom recovery, CPM were still successful in delaying syncope by approximately 2.5 min (67, 72). Further, patients using CPM also experienced quicker cardiovascular recovery when an orthostatic stress was introduced following maneuver release (69, 94). In practice, this could be enough time to assume a more protective position (lying down, sitting down), or call for assistance. Combining CPM can be particularly beneficial in this regard, with one movement serving to dampen symptoms, and the second aborting symptoms altogether (56). For example, squatting produces a large cardiovascular response, and was successful in aborting symptoms while the maneuver was sustained (alongside a practical benefit of being less socially embarrassing than lying down); however, when participants return to the standing position, symptoms often resume (55, 56, 73). Symptom recurrence is common with rising from squatting in particular, due to the removal of relative ischemia (due to mechanical compression during the maneuver, the “post tourniquet effect”) (95) and an abrupt increase in gravitational stress upon standing. Therefore, the duration of time spent in a squat may affect the syncope recurrence (55). Patients have found success in combining the rise from squatting with an additional maneuver (e.g., leg tensing, lower body tensing) to abort the milder presyncope they experienced upon orthostasis. In future investigations, the evaluation of the symptom recurrence following termination of the maneuver would be helpful in better stratifying currently recommended movements, as interventions that most often terminate symptoms at first use are more practical than those that permit a return of presyncope when terminated and must be repeated.
Practical application
Thus far, much of the documented benefit of CPM comes from laboratory studies, and few studies have included follow-up assessments considering their use in daily living (35, 55, 67, 72, 89, 90, 96). Only four studies have directly evaluated the real-world efficacy of CPM; while two of these provide high level evidence (35, 90), two are limited by a lack of comparator group (89, 96). There is, therefore, a need for more randomized controlled trials considering the efficacy of various CPM in terminating impending syncope.
We conducted a combined survival analysis (time to first syncopal recurrence in months) for the available data reporting efficacy of CPM in the community setting (Figure 6). When comparing combined analyses (35, 90) between patients using CPM (N = 121) and those treated with usual care (N = 141), we found no significant differences in time to first syncopal recurrence with the adoption of CPM (Figure 6). However, the combined relative risk reduction in syncopal occurrence with CPM was 34% (relative risk with CPM: 0.67, CI: 0.51 to 0.88, p = 0.004), which was associated with a number needed to treat of 5.75 patients (p = 0.004), and an odds ratio of 0.49 (CI: 0.31 to 0.78, p = 0.003). Of note, in our combined analyses, we did not include studies that did not incorporate a control group (89, 96) (although they are provided for interest in Figure 6); the findings of Croci et al. were not significantly different from our combined CPM results, but those reported by Romme et al. reported more limited success of CPM, with a significantly shorter time to first syncopal recurrence (p = 0.005). The reasons for this discrepancy are unclear; all four studies recruited a similar patient population with a similar frequency of syncopal events prior to enrollment in the study. In summary, as noted previously, there was a clear benefit of CPM on cardiovascular responses during laboratory testing. However, the efficacy of CPM in the community setting is less clear, and this may reflect challenges with adopting CPM in the real-world.
FIGURE 6.
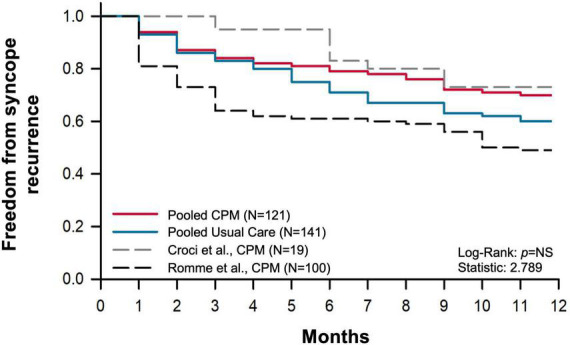
Kaplan–Meier syncope-free survival curve. Pooled time to first syncopal recurrence over a 1-year follow up in groups provided with usual care and trained in counter pressure maneuvers (CPM), and controls (usual care only) were compared (9, 87). Each study followed patients with vasovagal syncope who experienced prodromal symptoms prior to syncope. Data reported by Croci et al. (86) and Romme et al. (93), (dashed) did not include a control group and therefore were not included in combined analysis.
Evaluating practical efficacy is complex, as there are many factors outside physiological benefits at play. In CPM, consideration of prodromal history is of interest, as a patient must be able to recognize and employ CPM effectively prior to losing consciousness in order for them to be effective (72). While most follow-up assessments considered only patients with a significant prodrome (35, 89, 96), approximately 47% of participants assessed by Tomaino et al. (90) had a history of some sudden episodes without presyncope. In their complete analyses, this group did not detect a significant difference in 21-month syncope free survival between CPM and usual care. However, when considering only patients with a recognizable prodrome, although there was still a lack of significant difference in syncope-free survival, relative risk reduction was slightly improved. When evaluating the true efficacy of CPM among all patients, a wider population should be considered to avoid over estimation of benefit, as many patients with syncope do not experience a prodrome (approximately one-third of patients with VVS) and this obviously limits their ability to adopt CPM (48).
In many studies, even those considering only patients with a known history of syncopal prodromes, insufficient time to enable execution of CPM prior to loss of consciousness was a prominent barrier to their use in daily living (35, 55, 72, 89, 90, 96). To address this barrier, discrete and proactive use of maneuvers in provocative situations may improve CPM accessibility to a wider range of patients. Muscle tensing prior to a stand can bolster blood pressure more than tensing after standing and was beneficial in preventing initial orthostatic hypotension; this could perhaps be combined with a classical counter maneuver to provide a further benefit (97). Dynamic maneuvers may also be of particular benefit as they could better facilitate muscle pumping and can be maintained for longer periods of time (as they incorporate cyclical contraction and relaxation) (32, 34, 74, 75). In this case, the maneuvers could be performed proactively when one knows they are in a provocative situation (e.g., for occupations requiring sustained motionless standing, or experiencing known triggers for patients).
Many studies evaluating the practical applicability of CPM are also limited by a lack of a placebo control or comparison to baseline syncope frequency (55, 67, 72, 89). This is especially noteworthy, as the role of CPM in syncope management is not necessarily to eliminate episodes all together. Patient quality of life is inversely related to episode frequency (12), therefore reducing episodes could still lower syncope burden, bringing a substantial benefit to the patient (90, 96). Over a 1-year follow up, Romme et al. reported that while 49% of patients experienced a syncopal recurrence, disease-specific quality of life improved most in patients with the largest reductions in syncope burden (as quantified by episode frequency over time) (96). In future studies, additional metrics beyond time to first syncopal recurrence should be considered to evaluate treatment benefit, especially those evaluating a change in symptom frequency or further investigations into quality of life improvement (90, 96).
Proper CPM training is also imperative to effective execution in daily living (64). Syncope onset can be frightening, and even patients with an identifiable prodrome may experience panic at the moment of presyncope onset, causing them to forget the specific movements, or forget to employ them altogether (35, 96). Particularly in patients with a short prodrome, longer training periods may be required to improve patient comfort and confidence in using CPM independently. Training is often achieved using a combination of instruction, visual cues (demonstration, video), and biofeedback during CPM performance by the patient (35, 89, 96). Additionally, patients also must avoid straining (i.e., Valsalva maneuver) during the maneuver as this increases intrathoracic pressure, thereby reducing blood flow to the thorax (63, 72). Given the importance of prodromal recognition, introducing CPM training following a tilt table test may bring a further benefit, as patients will have a safe opportunity to become familiar with their presyncopal symptoms, and better identify these in daily living (67, 72).
Neurally mediated syncope and orthostatic hypotension are particularly prevalent among elderly individuals (15, 98), and elderly patients with syncope that are both frail and have impaired orthostatic blood pressure control also experience a heightened risk of falling (15). While this population would theoretically benefit from employment of CPM should they become symptomatic, CPM appear to be less effective in older patients for whom mobility and strength may be limited (89, 90). Populations with reduced muscularity may require additional time (and therefore a greater prodromal warning sign) to employ the maneuvers properly, and may therefore require more rigorous training, or additional practice at home (35). Actions such as squatting involve difficult transitions such as lowering one’s center of gravity or returning to an upright position from the ground, which could be challenging for patients with limited mobility, strength, or those that experience presyncopal reoccurrence upon standing (55, 56, 73). While leg crossing with tensing has been suggested as a useful alternative to squatting (as it does not involve bending or crouching), leg crossing does, however, reduce the base of support, which could also predispose a fall and subsequent injury in patients with limited balance (73). Patient populations with severe orthostatic hypotension and associated impairments in postural control may face similar difficulties accompanying more challenging movements such as squatting, leg crossing, or bending forward (62). Given the range of complications that could arise in vulnerable populations, it is advisable to teach patients several different CPM, allowing the patient to decide which is the best fit relative to their comfort, mobility, balance, and needs in specific social situations (62).
Ideal population
CPM have been studied most widely in patients in whom their syncope episodes present with a prominent vascular component; this includes VVS (36, 56, 63, 67, 72, 73), initial orthostatic hypotension (55, 97), and populations with neurological causes for syncope such as autonomic failure (60, 61, 64, 65, 68, 71, 96) or familial dysautonomia (62, 94). Of note, community-based trials documenting syncope free survival with CPM have only been performed in VVS (35, 89, 90, 96). To our knowledge, CPM have not been widely considered for cardiac causes of syncope (e.g., arrhythmia, structural abnormalities), presumably because improvements in venous return would have a limited benefit with this pathology. However, there may be a particular benefit for patients with VVS that are fitted with a pacemaker (90). Cardiac pacing for uncomplicated VVS is controversial but is sometimes employed as a last resort for syncope that is refractory to other treatments, accompanied by severe symptoms, and associated with asystole. However, as many as one-quarter of patients with syncope that also have an implanted pacemaker continue to experience recurrent episodes (90, 99), presumably because the pacemaker does not address the vasodepressor component of their syncope; in these patients CPM may be of benefit to combat this hypotensive component of their episodes. However, this needs to be investigated further as patients with asystolic faints often have minimal prodrome (99), and the complexity of a mixed pathology may reduce the ability of CPM to adequately recover cerebral perfusion and blood pressure (35).
The benefit of CPM in pediatric syncope or POTS has also not yet been widely evaluated, with the latter perhaps also driven by the lack of evidence for meaningful benefit of CPM on orthostatic HR responses. Static hand grip was reported to terminate symptoms in patients aged 15–22 years with initial orthostatic hypotension, however, no further investigations in pediatric patients have been performed to date (51, 100). Neuropathic or “partial dysautonomic” POTS is the most commonly diagnosed subtype and occurs due to an inability to vasoconstrict peripheral vasculature upon standing (101, 102). In response, as blood pools in the lower extremities, excessive increases in HR occur in order to maintain CO and cerebral perfusion (102, 103). Since CPM can reduce venous pooling, and supplement venous return through initiating muscle pumping, it may be that CPM could dampen orthostatic tachycardia in some forms of POTS. This is supported by the observation that some patients with POTS have diminished calf venous capacity, circumference and ejection fraction (59); while the etiology of this observation is still unknown, it does support a role for targeting improvements in skeletal muscle pumping in this population.
Ultimately, more extensive investigation considering CPM use in a wider variety of syncope patients, including both POTS and cardioinhibitory forms of VVS, is needed to better understand the applicability of this intervention, and inform clinical practice. Additionally, evaluations in both adult and pediatric populations should be performed, as children may not respond in the same manner as adults, given their complex physiology and common occurrence of “dysautonomia of adolescence,” dysregulation of the autonomic nervous system during puberty (11).
CPM have also been evaluated for use during syncope associated with blood-injection-injury stimuli, especially during blood donation and intravenous catheter insertion. Multiple studies report that CPM are effective at reducing vasovagal reactions and presyncopal symptoms in this setting (104–110). Responses were more favorable when tensing was applied specifically when the needle was inserted, when the needle was removed, and when getting up from the chair after the procedure (106). Thus far, muscle tensing of the calf, thigh, abdomen, buttock, arm, and chest have been considered (104–113). Applying CPM during or immediately preceding blood donation significantly increases HR (105, 111) and CBFv (111), although responses may vary in patients with heightened anxiety, or those that experience blood-injection-injury phobia (105). Interestingly, when simulated blood draws were performed on a cohort of individuals that were highly fearful of needles, CPM were still effective in producing cerebrovascular benefits (104). Donors using CPM reported greater feelings of confidence and control during the procedure (106), and reduced anxiety (105). Blood donor rate was also greater in those using CPM, particularly for women (112), or those who had a pre-disposing fear to needles (40). Accordingly, CPM appear to be a simple and effective intervention to reduce vasovagal reactions and improve patient comfort during invasive procedures.
Postural sway
Sway as a counter pressure maneuver
Through our literature search, a small subset (N = 6) of papers were retrieved providing preliminary evidence for a potential role of postural sway as a CPM (114–119). Postural sway describes the fine, unconscious movements that are generated in response to neural feedback in order to maintain balance in the upright position (120, 121). Throughout this process, the musculature of the lower limbs and torso initiate stochastic movements of the body’s many segments to ultimately keep the center of mass [average position of all parts of a system, weighted according to their mass (122)] in motion, within the bounds of their base of support [the area beneath an object or person, encompassed by every point of contact between them and their supporting surface (123)] (124). As the center of mass moves, its two dimensional path within this base can be traced and reduced to movement in the anteroposterior (predominantly controlled by muscles of the calf) and mediolateral (predominantly controlled by muscles of the thigh) directions (125).
The fine movements initiated by postural sway, while small, recruit lower body musculature and may be sufficient to bolster against syncope through skeletal muscle pumping. The oscillatory nature of postural sway would yield benefits in comparison to classically recommended counter maneuvers, as postural sway is dynamic; thus, it may better promote pumping in the lower limbs, and could be performed proactively. Further, it can be performed in resting upright posture without a change in body position (i.e., lowering of center of mass, or reducing base of support), and could therefore be more accessible to mobility limited patients. Indeed, postural movements correspond with blood fluid volume shifts, indicating that sway may reduce orthostatic pooling associated with capillary filtration (22, 42). Additionally, in comparison to quiet (free) standing, DAP increases during supported standing, which is perhaps reflective of a reliance on vasoconstriction in the absence of skeletal muscle pumping produced from postural sway (126). However, it is unclear whether these responses are sufficiently pronounced to produce a clinically meaningful effect in syncope prevention through a role in blood pressure stability, and ultimately, increasing cerebral perfusion.
Unconscious postural sway enhancement
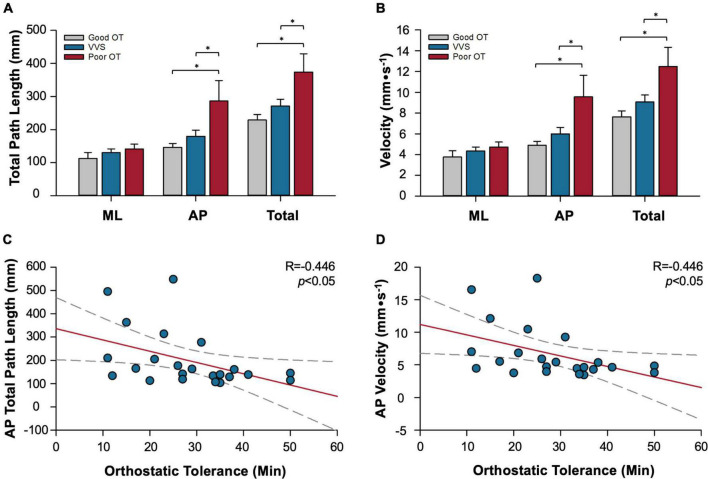
Enhanced postural sway during quiet standing has been observed in populations with poor cardiovascular control (Figure 7). Otherwise healthy participants that did not report symptoms of syncope and presyncope in daily living, but had poor orthostatic tolerance [OT, as determined by a head-upright tilt test with lower body negative pressure (127)], demonstrated increased anteroposterior and mediolateral postural sway (quantified in terms of both path length and velocity) upon standing (114, 115). In this cohort, OT was negatively correlated with postural sway behavior (Figure 7), whereby those with the poorest orthostatic tolerance adopted greater postural sway during standing. Skeletal muscle pumping is inactivated during the head up tilt test, however, during normal standing, postural muscles are continually and reciprocally activated (125, 128). In these asymptomatic individuals with poor OT, it seems that it is this skeletal muscle pumping strategy that supplements other mechanisms of cardiovascular control to prevent presyncope and syncope in daily living.
FIGURE 7.
Relationships between postural sway and susceptibility to syncope. Data summarize relationships between postural sway behavior (measured as total path length or sway velocity), and cardiovascular control (measured as orthostatic tolerance, OT, time to presyncope in minutes during standard head upright tilt test with lower body negative pressure). Participant groups studied: patients with vasovagal syncope (VVS), healthy participants with good orthostatic tolerance (good OT), healthy participants with poor orthostatic tolerance (poor OT). Significant increases in the distance (A) and velocity (B) of postural sway are observed during quiet standing in individuals with poor OT who do not experience syncope in daily life, particularly in the AP direction. In patients with VVS, there is no such enhancement of postural sway to compensate for their orthostatic intolerance. The distance (C) and velocity (D) moved in the AP direction was significantly correlated with OT, where those with the lowest OT had greater postural sway. *Denotes significant difference where p < 0.05. ML, mediolateral component of sway; AP, anteroposterior component of sway. Adapted from Claydon and Hainsworth 2005 (111), and Claydon and Hainsworth (110).
While a relationship between postural behavior and cardiovascular control exists in this cohort, any underlying cause is still unclear. It may be that enhanced postural sway is a subconscious adaptation, or other compensatory mechanism to combat reduced cardiovascular control. Habitual leg restlessness, another discrete leg movement, has been noted in patients with orthostatic hypotension secondary to autonomic failure, and was also thought to reduce symptomatic drops in blood pressure (68). Trends of sway enhancement have been observed in other populations experiencing poor cardiovascular control such as Alzheimer’s disease (43) or deconditioned astronauts following space flight (44); increased postural sway in these populations could also be explained as a response to poor cerebral perfusion (43, 116, 129), or a simple reflection of simultaneous impairments in postural and cardiovascular control driven by the multifaceted impacts of neurodegenerative diseases or anti-gravity deconditioning. Conversely, in the case that asymptomatic individuals with poor OT are “natural swayers,” it may be that a habit of increased sway (and therefore increased muscle pumping) reduced their dependence on neural regulatory mechanisms to produce a detraining-like effect that impaired the sensitivity and efficacy of those reflexes (115).
Postural sway and cardiovascular control
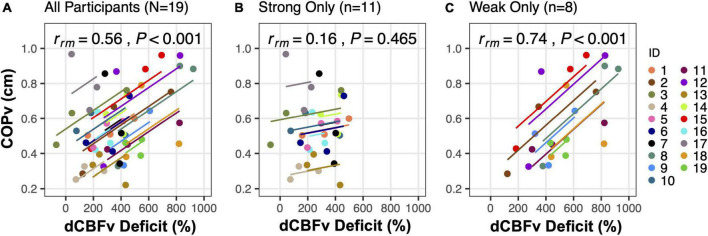
While the contribution of locally (e.g., autocrine, paracrine) and neurally-mediated (i.e., baroreflex) responses to orthostatic stress are well understood, the regulation of skeletal muscle pumping in upright standing is less clear. Decreased blood pressure (130) and cerebral hypoperfusion (117, 131) increase postural sway in quiet standing. Similarly, during a quiet stand following a supine-stand transition, the total path length of sway increased concordantly with progressive cerebral hypoperfusion (induced by bilateral thigh cuff deflation during the stand, and hyperventilation introduced prior to the stand) (Figure 8) (116). Perhaps the increased postural sway following the postural transition results from impaired neural control that is secondary to cerebral hypoperfusion, such that impairment sets in when cerebral blood flow drops below a threshold value (116).
FIGURE 8.
Postural sway response to cerebral hypoperfusion. Repeated measures within-participant correlations between diastolic cerebral blood flow velocity deficit (dCBFv, quantified as the difference between dCBFv in the middle cerebral artery following the supine-stand transition and its baseline value in the supine posture before any intervention) and mean center of pressure vector (COPv) are shown. Analyses were performed among (A) all participants, and groups of (B) strong regulators only, or (C) weak regulators only, as identified through an a posteriori cluster analysis (112). The number of participants (n) included in each condition are shown at the top of each respective plot. Parallel slopes are fitted for each participant with ANCOVA using data from the 3 postural transitions, such that the overall model error is minimized. The repeated measures correlation coefficient (rrm) is derived from a ratio of the sum of squares. Adapted from Fitzgibbon-Collins et al. (112).
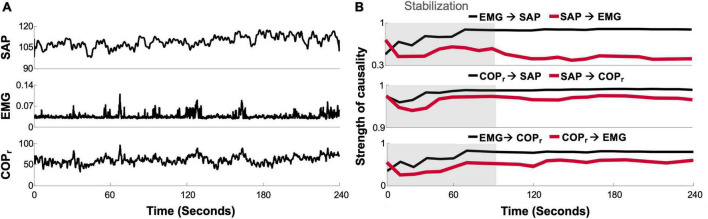
Integration between blood pressure and cardiovascular control has also been evaluated mathematically through identifying the coherence (132, 133) and causality (134) between signals of blood pressure, muscle activity, and center of pressure movements during quiet standing (Figure 9). Bidirectional coupling between fluctuations in SAP, electromyography activity, and center of pressure movement were uncovered, particularly in the period of standing after initial stabilization, such that sway was found to drive changes in SAP (presumably relating to the skeletal muscle pumping effect) but also that SAP was found to drive changes in sway (118, 119). While this provides further evidence that postural and cardiovascular systems may work together during orthostatic stress, this group also hypothesized the existence of a cardio-postural reflex whereby muscle pumping could be activated in response to hypotension (119); however, any physiological or anatomical evidence for such a mechanism is yet to be uncovered. While postural sway has been shown to increase during baroreceptor stimulation through neck suction (135), supporting an interaction between baroreflex and postural control, this stimulus simulates hypertension, and as such would be expected to be associated with a reduction in postural sway if a homeostatic mechanism between blood pressure and sway exists. Considerable evidence has also accumulated in support of a role for the vestibular system in cardiovascular control during body movement (termed the “vestibulo-sympathetic reflex”) (136), however, these are most active during pronounced postural changes, such as a sit-to-stand transition. Whether these reflexes would take effect during fine postural adjustments that occur during prolonged standing is unknown. Thus, while it is clear that the control of postural sway and cardiovascular homeostasis do overlap, the underpinnings of cause and effect, as well as our specific understanding of mechanisms, are still emerging.
FIGURE 9.
Cardio-postural causality. (A) Example tracing of simultaneously recorded systolic arterial pressure (SAP), calf electromyography (EMG), and center of pressure (COPr) signals. (B) Convergence plot of causality between signals over time during a quiet upright standing trial. Causality between signals, as calculated from the convergent cross mapping method (130, 135, 136), was measured on a scale between 0 and 1 (unitless). Gray shading indicates stabilization period following upright stance. Adapted from Verma et al. (137).
Discussion and conclusion
Counter pressure maneuvers
CPM are a mainstay component of syncope management recommendations, and offer a cheap and relatively risk-free means to abort or delay an episode of syncope that can be adopted in addition to other commonly used preventative management strategies (e.g., avoidance measures, behavioral interventions, salt and volume loading, medication adjustment). Physiological evidence suggests that CPM hold promise in syncope prevention, with demonstrable improvements in blood pressure, SV and CBFv; however, the effects of CPM on HR, CO and TPR are more complex, and require further investigation to assess their mechanistic underpinnings, as well as to ascertain whether the significant improvements reported hold practical significance. The real-world efficacy of CPM to prevent syncope is less clear and few studies have directly assessed CPM benefit in daily living. Community-based investigations are often limited by a lack of comparator group, and inconsistent quantification of treatment success.
Ultimately, there is a need for rigorous clinical trials that include a placebo or control group in order to properly evaluate CPM efficacy. While it is recommended that patients learn multiple maneuvers so they can combine them in daily living, or select based on their social setting, abilities, or preference, comparisons between different CPM (e.g., dynamic vs. static maneuvers, movement efficacy, patient preference) would best inform clinical practice. Future trials should also include more diverse patient populations including those without a prodrome, pediatric patients, patients with POTS, and patients with cardioinhibitory VVS. These should evaluate improvement with consideration of each patient’s baseline episode frequency, presyncopal episodes that would have resolved without intervention, and improvements in patient quality of life after CPM introduction.
Postural sway
There is emerging evidence that postural sway may contribute to the maintenance of cardiovascular homeostasis through activation of the skeletal muscle pump. Relationships between postural movements and cardiovascular responses have been identified that are suggestive of either a learned response of enhanced sway to combat orthostatic intolerance, or a more complex regulatory mechanism. While the oscillatory nature of postural sway has potential to serve as a dynamic CPM, more work is needed to identify the potential of this movement to enhance cardiovascular stability and prevent syncope.
Author contributions
VC and EW conceived the manuscript. EW and FK conducted the search, screened articles for inclusion, and extracted data from included studies. All authors performed data analysis and visualization, interpreted the data, contributed to writing the manuscript and approved the final version for submission.
Funding
This work was supported in part by a grant-in-aid from the Heart and Stroke Foundation of Canada awarded to VC (#G-18-0022174).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Shen W-K, Sheldon RS, Benditt DG, Cohen MI, Forman DE, Goldberger ZD, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope. J Am Coll Cardiol. (2017) 70:e39 LP–e110. 10.1016/j.jacc.2017.03.003 [DOI] [PubMed] [Google Scholar]
- 2.Ganzeboom KS, Mairuhu G, Reitsma JB, Linzer M, Wieling W, van Dijk N. Lifetime cumulative incidence of syncope in the general population: a study of 549 Dutch subjects aged 35-60 years. J Cardiovasc Electrophysiol. (2006) 17:1172–6. 10.1111/j.1540-8167.2006.00595.x [DOI] [PubMed] [Google Scholar]
- 3.Kenny RA, Bhangu J, King-Kallimanis BL. Epidemiology of syncope/collapse in younger and older Western patient populations. Prog Cardiovasc Dis. (2013) 55:357–63. 10.1016/j.pcad.2012.11.006 [DOI] [PubMed] [Google Scholar]
- 4.Sandhu RK, Sheldon RS. Syncope in the emergency department. Front Cardiovasc Med. (2019) 6:180. 10.3389/fcvm.2019.00180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCarthy F, McMahon CG, Geary U, Plunkett PK, Kenny RA, Cunningham CJ. Management of syncope in the Emergency Department: a single hospital observational case series based on the application of European Society of Cardiology Guidelines. Europace Eur Pacing Arrhythmias Card Electrophysiol J Work Groups Card Pacing Arrhythmias Card Cell Electrophysiol Eur Soc Cardiol. (2009) 11:216–24. 10.1093/europace/eun323 [DOI] [PubMed] [Google Scholar]
- 6.Geer B. Current best practices in emergency evaluation and management of syncope. Nurse Pract. (2021) 46:24–31. 10.1097/01.NPR.0000757080.85601.1e [DOI] [PubMed] [Google Scholar]
- 7.Providência R, Silva J, Mota P, Nascimento J, Leitão-Marques A. Transient loss of consciousness in young adults. Int J Cardiol. (2011) 152:139–43. 10.1016/j.ijcard.2011.07.064 [DOI] [PubMed] [Google Scholar]
- 8.Rose MS, Koshman ML, Spreng S, Sheldon R. The relationship between health-related quality of life and frequency of spells in patients with syncope. J Clin Epidemiol. (2000) 53:1209–16. 10.1016/S0895-4356(00)00257-2 [DOI] [PubMed] [Google Scholar]
- 9.van Dijk N, Sprangers MA, Colman N, Boer KR, Wieling W, Linzer M. Clinical factors associated with quality of life in patients with transient loss of consciousness. J Cardiovasc Electrophysiol. (2006) 17:998–1003. 10.1111/j.1540-8167.2006.00533.x [DOI] [PubMed] [Google Scholar]
- 10.Anderson JB, Czosek RJ, Knilans TK, Marino BS. The effect of paediatric syncope on health-related quality of life. Cardiol Young. (2012) 22:583–8. 10.1017/S1047951112000133 [DOI] [PubMed] [Google Scholar]
- 11.Armstrong KR, De Souza AM, Sneddon PL, Potts JE, Claydon VE, Sanatani S. Exercise and the multidisciplinary holistic approach to adolescent dysautonomia. Acta Paediatr. (2017) 106:612–8. 10.1111/apa.13750 [DOI] [PubMed] [Google Scholar]
- 12.Hockin BCD, Heeney ND, Whitehurst DGT, Claydon VE. Evaluating the impact of orthostatic syncope and presyncope on quality of life: a systematic review and meta-analysis. Front Cardiovasc Med. (2022) 9:834879. 10.3389/fcvm.2022.834879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu REY, Khan FM, Hockin BCD, Lobban TCA, Sanatani S, Claydon VE. Faintly tired: a systematic review of fatigue in patients with orthostatic syncope. Clin Auton Res. (2022) 32:185–203. 10.1007/s10286-022-00868-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw BH, Claydon VE. The relationship between orthostatic hypotension and falling in older adults. Clin Auton Res. (2014) 24:3–13. 10.1007/s10286-013-0219-5 [DOI] [PubMed] [Google Scholar]
- 15.Shaw BH, Borrel D, Sabbaghan K, Kum C, Yang Y, Robinovitch SN, et al. Relationships between orthostatic hypotension, frailty, falling and mortality in elderly care home residents. BMC Geriatr. (2019) 19:80. 10.1186/s12877-019-1082-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Dijk N, Sprangers MA, Boer KR, Colman N, Wieling W, Linzer M. Quality of life within one year following presentation after transient loss of consciousness. Am J Cardiol. (2007) 100:672–6. 10.1016/j.amjcard.2007.03.085 [DOI] [PubMed] [Google Scholar]
- 17.Sheldon RS, Amuah JE, Connolly SJ, Rose S, Morillo CA, Talajic M, et al. Effect of metoprolol on quality of life in the Prevention of Syncope Trial. J Cardiovasc Electrophysiol. (2009) 20:1083–8. 10.1111/j.1540-8167.2009.01518.x [DOI] [PubMed] [Google Scholar]
- 18.Bartoletti A, Fabiani P, Bagnoli L, Cappelletti C, Cappellini M, Nappini G, et al. Physical injuries caused by a transient loss of consciousness: main clinical characteristics of patients and diagnostic contribution of carotid sinus massage. Eur Heart J. (2008) 29:618–24. 10.1093/eurheartj/ehm563 [DOI] [PubMed] [Google Scholar]
- 19.Gupta V, Lipsitz LA. Orthostatic hypotension in the elderly: diagnosis and treatment. Am J Med. (2007) 120:841–7. 10.1016/j.amjmed.2007.02.023 [DOI] [PubMed] [Google Scholar]
- 20.Manyari DE, Rose S, Tyberg JV, Sheldon RS. Abnormal reflex venous function in patients with neuromediated syncope. J Am Coll Cardiol. (1996) 27:1730–5. 10.1016/0735-1097(96)00051-4 [DOI] [PubMed] [Google Scholar]
- 21.Mosqueda-Garcia R, Furlan R, Fernandez-Violante R, Desai T, Snell M, Jarai Z, et al. Sympathetic and baroreceptor reflex function in neurally mediated syncope evoked by tilt. J Clin Invest. (1997) 99:2736–44. 10.1172/JCI119463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown CM, Hainsworth R. Assessment of capillary fluid shifts during orthostatic stress in normal subjects and subjects with orthostatic intolerance. Clin Auton Res Off J Clin Auton Res Soc. (1999) 9:69–73. 10.1007/BF02311762 [DOI] [PubMed] [Google Scholar]
- 23.Matzen S, Perko G, Groth S, Friedman DB, Secher NH. Blood volume distribution during head-up tilt induced central hypovolaemia in man. Clin Physiol. (1991) 11:411–22. 10.1111/j.1475-097X.1991.tb00813.x [DOI] [PubMed] [Google Scholar]
- 24.Diedrich A, Biaggioni I. Segmental orthostatic fluid shifts. Clin Auton Res Off J Clin Auton Res Soc. (2004) 14:146–7. 10.1007/s10286-004-0188-9 [DOI] [PubMed] [Google Scholar]
- 25.White CM, Tsikouris JP. A review of pathophysiology and therapy of patients with vasovagal syncope. Pharmacotherapy. (2000) 20:158–65. 10.1592/phco.20.3.158.34786 [DOI] [PubMed] [Google Scholar]
- 26.Ricci F, De Caterina R, Fedorowski A. Orthostatic hypotension: epidemiology, Prognosis, and Treatment. J Am Coll Cardiol. (2015) 66:848–60. 10.1016/j.jacc.2015.06.1084 [DOI] [PubMed] [Google Scholar]
- 27.Boron W, Boulpaep E. Medical Physiology. 2nd ed. Amsterdam: Elsevier; (2008). [Google Scholar]
- 28.El-Bedawi K, Hainsworth R. Combined head-up tilt and lower body suction: a test of orthostatic tolerance. Clin Auton Res. (1994) 4:41–7. 10.1007/BF01828837 [DOI] [PubMed] [Google Scholar]
- 29.Moya A, Sutton R, Ammirati F, Blanc J-J, Brignole M, Dahm JB, et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. (2009) 30:2631–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faghri PD, Yount J. Electrically induced and voluntary activation of physiologic muscle pump: a comparison between spinal cord-injured and able-bodied individuals. Clin Rehabil. (2002) 16:878–85. 10.1191/0269215502cr570oa [DOI] [PubMed] [Google Scholar]
- 31.Raymond J, Davis GM, Clarke J, Bryant G. Cardiovascular responses during arm exercise and orthostatic challenge in individuals with paraplegia. Eur J Appl Physiol. (2001) 85:89–95. 10.1007/s004210100449 [DOI] [PubMed] [Google Scholar]
- 32.Hockin BCD, Claydon VE. Intermittent calf compression delays the onset of presyncope in young healthy individuals. Front Physiol. (2019) 10:1598. 10.3389/fphys.2019.01598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hockin BCD, Ruiz IA, Brar GK, Claydon VE. Intermittent calf compression reverses lower limb pooling and improves cardiovascular control during passive orthostasis. Auton Neurosci. (2020) 217:102–13. 10.1016/j.autneu.2018.12.004 [DOI] [PubMed] [Google Scholar]
- 34.Protheroe CL, Dikareva A, Menon C, Claydon VE. Are compression stockings an effective treatment for orthostatic presyncope? PLoS One. (2011) 6:e28193. 10.1371/journal.pone.0028193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Dijk N, Quartieri F, Blanc J-J, Garcia-Civera R, Brignole M, Moya A, et al. Effectiveness of physical counterpressure maneuvers in preventing vasovagal syncope: the Physical Counterpressure Manoeuvres Trial (PC-Trial). J Am Coll Cardiol. (2006) 48:1652–7. 10.1016/j.jacc.2006.06.059 [DOI] [PubMed] [Google Scholar]
- 36.Krediet CTP, de Bruin IGJM, Ganzeboom KS, Linzer M, van Lieshout JJ, Wieling W. Leg crossing, muscle tensing, squatting, and the crash position are effective against vasovagal reactions solely through increases in cardiac output. J Appl Physiol. (2005) 99:1697–703. 10.1152/japplphysiol.01250.2004 [DOI] [PubMed] [Google Scholar]
- 37.Alboni P, Brignole M, Menozzi C, Raviele A, Del Rosso A, Dinelli M, et al. Clinical spectrum of neurally mediated reflex syncopes. Europace Eur Pacing Arrhythmias Card Electrophysiol J Work Groups Card Pacing Arrhythmias Card Cell Electrophysiol Eur Soc Cardiol. (2004) 6:55–62. 10.1016/j.eupc.2003.09.003 [DOI] [PubMed] [Google Scholar]
- 38.Brignole M, Alboni P, Benditt D, Bergfeldt L, Blanc JJ, Bloch Thomsen PE, et al. Guidelines on management (diagnosis and treatment) of syncope. Eur Heart J. (2001) 22:1256–306. 10.1053/euhj.2001.2739 [DOI] [PubMed] [Google Scholar]
- 39.Kowalsky JM, France JL, Wissel ME, France CR. Effect of applied muscle tension on cerebral oxygenation in female blood donors. Transfusion. (2011) 51:1802–8. 10.1111/j.1537-2995.2011.03075.x [DOI] [PubMed] [Google Scholar]
- 40.France CR, Ditto B, Wissel ME, France JL, Dickert T, Rader A, et al. Predonation hydration and applied muscle tension combine to reduce presyncopal reactions to blood donation. Transfusion. (2010) 50:1257–64. 10.1111/j.1537-2995.2009.02574.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ditto B, France CR, Lavoie P, Roussos M, Adler PSJ. Reducing reactions to blood donation with applied muscle tension: a randomized controlled trial. Transfusion. (2003) 43:1269–75. 10.1046/j.1537-2995.2003.00488.x [DOI] [PubMed] [Google Scholar]
- 42.Inamura K, Mano T, Iwase S, Amagishi Y, Inamura S. One-minute wave in body fluid volume change enhanced by postural sway during upright standing. J Appl Physiol. (1996) 81:459–69. 10.1152/jappl.1996.81.1.459 [DOI] [PubMed] [Google Scholar]
- 43.Nakamura T, Meguro K, Yamazaki H, Okuzumi H, Tanaka A, Horikawa A, et al. Postural and gait disturbance correlated with decreased frontal cerebral blood flow in Alzheimer disease. Alzheimer Dis Assoc Disord. (1997) 11:132–9. 10.1097/00002093-199709000-00005 [DOI] [PubMed] [Google Scholar]
- 44.Speers RA, Paloski WH, Kuo AD. Multivariate changes in coordination of postural control following spaceflight. J Biomech. (1998) 31:883–9. 10.1016/S0021-9290(98)00065-7 [DOI] [PubMed] [Google Scholar]
- 45.Medow MS, Stewart JM, Sanyal S, Mumtaz A, Sica D, Frishman WH. Pathophysiology, diagnosis, and treatment of orthostatic hypotension and vasovagal syncope. Cardiol Rev. (2008) 16:4–20. 10.1097/CRD.0b013e31815c8032 [DOI] [PubMed] [Google Scholar]
- 46.Anderson JB, Willis M, Lancaster H, Leonard K, Thomas C. The evaluation and management of pediatric syncope. Pediatr Neurol. (2016) 55:6–13. 10.1016/j.pediatrneurol.2015.10.018 [DOI] [PubMed] [Google Scholar]
- 47.Guida P, Iacoviello M, Forleo C, Ferrara A, Sorrentino S, Balducci C, et al. Prevalence, timing, and haemodynamic correlates of prodromes in patients with vasovagal syncope induced by head-up tilt test. Europace Eur Pacing Arrhythmias Card Electrophysiol J Work Groups Card Pacing Arrhythmias Card Cell Electrophysiol Eur Soc Cardiol. (2009) 11:1221–6. 10.1093/europace/eup164 [DOI] [PubMed] [Google Scholar]
- 48.Alboni P, Brignole M, Menozzi C, Raviele A, Del Rosso A, Dinelli M, et al. Diagnostic value of history in patients with syncope with or without heart disease. J Am Coll Cardiol. (2001) 37:1921–8. 10.1016/S0735-1097(01)01241-4 [DOI] [PubMed] [Google Scholar]
- 49.Cubera K, Stryjewski PJ, Kuczaj A, Nessler J, Nowalany-Kozielska E, Pytko-Polończyk J. The impact of gender on the frequency of syncope provoking factors and prodromal signs in patients with vasovagal syncope. Przegl Lek. (2017) 74:147–9. [PubMed] [Google Scholar]
- 50.Dockx K, Avau B, De Buck E, Vranckx P, Vandekerckhove P. Physical manoeuvers as a preventive intervention to manage vasovagal syncope: a systematic review. PLoS One. (2019) 14:e0212012. 10.1371/journal.pone.0212012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jensen JL, Ohshimo S, Cassan P, Meyran D, Greene J, Ng KC, et al. Immediate interventions for presyncope of vasovagal or orthostatic origin: a systematic review. Prehospital Emerg Care. (2020) 24:64–76. 10.1080/10903127.2019.1605431 [DOI] [PubMed] [Google Scholar]
- 52.Snyder H. Literature review as a research methodology: an overview and guidelines. J Bus Res. (2019) 104:333–9. 10.1016/j.jbusres.2019.07.039 [DOI] [Google Scholar]
- 53.Wong G, Greenhalgh T, Westhorp G, Buckingham J, Pawson R. RAMESES publication standards: meta-narrative reviews. BMC Med. (2013) 11:20. 10.1186/1741-7015-11-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krediet CTP, van Lieshout JJ, Bogert LWJ, Immink RV, Kim Y-S, Wieling W. Leg crossing improves orthostatic tolerance in healthy subjects: a placebo-controlled crossover study. Am J Physiol Heart Circ Physiol. (2006) 291:H1768–72. 10.1152/ajpheart.00287.2006 [DOI] [PubMed] [Google Scholar]
- 55.Krediet CTP, Go-Schön IK, Kim Y-S, Linzer M, Van Lieshout JJ, Wieling W. Management of initial orthostatic hypotension: lower body muscle tensing attenuates the transient arterial blood pressure decrease upon standing from squatting. Clin Sci (Lond). (2007) 113:401–7. 10.1042/CS20070064 [DOI] [PubMed] [Google Scholar]
- 56.Krediet CTP, Go-Schön IK, van Lieshout JJ, Wieling W. Optimizing squatting as a physical maneuver to prevent vasovagal syncope. Clin Auton Res Off J Clin Auton Res Soc. (2008) 18:179–86. 10.1007/s10286-008-0481-0 [DOI] [PubMed] [Google Scholar]
- 57.Privett SE, George KP, Whyte GP, Cable NT. The effectiveness of compression garments and lower limb exercise on post-exercise blood pressure regulation in orthostatically intolerant athletes. Clin J Sport Med Off J Can Acad Sport Med. (2010) 20:362–7. 10.1097/JSM.0b013e3181f20292 [DOI] [PubMed] [Google Scholar]
- 58.Smith ML, Hudson DL, Raven PB. Effect of muscle tension on the cardiovascular responses to lower body negative pressure in man. Med Sci Sports Exerc. (1987) 19:436–42. 10.1249/00005768-198710000-00003 [DOI] [PubMed] [Google Scholar]
- 59.Stewart JM, Medow MS, Montgomery LD, Mcleod K, Julian M, Medow MS, et al. Decreased skeletal muscle pump activity in patients with postural tachycardia syndrome and low peripheral blood flow. Am J Physiol Circ Physiol. (2004) 286:H1216–22. 10.1152/ajpheart.00738.2003 [DOI] [PubMed] [Google Scholar]
- 60.Ten Harkel AD, van Lieshout JJ, Wieling W. Effects of leg muscle pumping and tensing on orthostatic arterial pressure: a study in normal subjects and patients with autonomic failure. Clin Sci (Lond). (1994) 87:553–8. 10.1042/cs0870553 [DOI] [PubMed] [Google Scholar]
- 61.Thijs RD, Wieling W, van den Aardweg JG, van Dijk JG. Respiratory countermaneuvers in autonomic failure. Neurology. (2007) 69:582–5. 10.1212/01.wnl.0000266671.61599.a0 [DOI] [PubMed] [Google Scholar]
- 62.Tutaj M, Marthol H, Berlin D, Brown CM, Axelrod FB, Hilz MJ. Effect of physical countermaneuvers on orthostatic hypotension in familial dysautonomia. J Neurol. (2006) 253:65–72. 10.1007/s00415-005-0928-3 [DOI] [PubMed] [Google Scholar]
- 63.van Dijk N, de Bruin IGJM, Gisolf J, de Bruin-Bon HACMR, Linzer M, van Lieshout JJ, et al. Hemodynamic effects of leg crossing and skeletal muscle tensing during free standing in patients with vasovagal syncope. J Appl Physiol. (2005) 98:584–90. 10.1152/japplphysiol.00738.2004 [DOI] [PubMed] [Google Scholar]
- 64.Bouvette CM, McPhee BR, Opfer-Gehrking TL, Low PA. Role of physical countermaneuvers in the management of orthostatic hypotension: efficacy and biofeedback augmentation. Mayo Clin Proc. (1996) 71:847–53. 10.4065/71.9.847 [DOI] [PubMed] [Google Scholar]
- 65.van Lieshout JJ, ten Harkel AD, Wieling W. Physical manoeuvres for combating orthostatic dizziness in autonomic failure. Lancet (London, England). (1992) 339:897–8. 10.1016/0140-6736(92)90932-S [DOI] [PubMed] [Google Scholar]
- 66.van Lieshout JJ, Pott F, Madsen PL, van Goudoever J, Secher NH. Muscle tensing during standing: effects on cerebral tissue oxygenation and cerebral artery blood velocity. Stroke. (2001) 32:1546–51. 10.1161/01.STR.32.7.1546 [DOI] [PubMed] [Google Scholar]
- 67.Brignole M, Croci F, Menozzi C, Solano A, Donateo P, Oddone D, et al. Isometric arm counter-pressure maneuvers to abort impending vasovagal syncope. J Am Coll Cardiol. (2002) 40:2053–9. 10.1016/S0735-1097(02)02683-9 [DOI] [PubMed] [Google Scholar]
- 68.Cheshire WPJ. Hypotensive akathisia: autonomic failure associated with leg fidgeting while sitting. Neurology. (2000) 55:1923–6. 10.1212/WNL.55.12.1923 [DOI] [PubMed] [Google Scholar]
- 69.France CR, France JL, Patterson SM. Blood pressure and cerebral oxygenation responses to skeletal muscle tension: a comparison of two physical maneuvers to prevent vasovagal reactions. Clin Physiol Funct Imaging. (2006) 26:21–5. 10.1111/j.1475-097X.2005.00642.x [DOI] [PubMed] [Google Scholar]
- 70.Groothuis JT, van Dijk N, Ter Woerds W, Wieling W, Hopman MTE. Leg crossing with muscle tensing, a physical counter-manoeuvre to prevent syncope, enhances leg blood flow. Clin Sci (Lond). (2007) 112:193–201. 10.1042/CS20060241 [DOI] [PubMed] [Google Scholar]
- 71.Harms MPM, Wieling W, Colier WNJM, Lenders JWM, Secher NH, van Lieshout JJ. Central and cerebrovascular effects of leg crossing in humans with sympathetic failure. Clin Sci (Lond). (2010) 118:573–81. 10.1042/CS20090038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Krediet CTP, van Dijk N, Linzer M, van Lieshout JJ, Wieling W. Management of vasovagal syncope: controlling or aborting faints by leg crossing and muscle tensing. Circulation. (2002) 106:1684–9. 10.1161/01.CIR.0000030939.12646.8F [DOI] [PubMed] [Google Scholar]
- 73.Kim KH, Cho JG, Lee KO, Seo TJ, Shon CY, Lim SY, et al. Usefulness of physical maneuvers for prevention of vasovagal syncope. Circ J. (2005) 69:1084–8. 10.1253/circj.69.1084 [DOI] [PubMed] [Google Scholar]
- 74.Killewich LA, Sandager GP, Nguyen AH, Lilly MP, Flinn WR. Venous hemodynamics during impulse foot pumping. J Vasc Surg. (1995) 22:598–605. 10.1016/S0741-5214(95)70046-3 [DOI] [PubMed] [Google Scholar]
- 75.Delis KT, Azizi ZA, Stevens RJG, Wolfe JHN, Nicolaides AN. Optimum intermittent pneumatic compression stimulus for lower-limb venous emptying. Eur J Vasc Endovasc Surg. (2000) 19:261–9. 10.1053/ejvs.1999.1047 [DOI] [PubMed] [Google Scholar]
- 76.Saltin B, Sjøgaard G, Gaffney FA, Rowell LB. Potassium, lactate, and water fluxes in human quadriceps muscle during static contractions. Circ Res. (1981) 48(Pt 2):I18–24. [PubMed] [Google Scholar]
- 77.Sejersted OM, Hargens AR, Kardel KR, Blom P, Jensen O, Hermansen L. Intramuscular fluid pressure during isometric contraction of human skeletal muscle. J Appl Physiol. (1984) 56:287–95. 10.1152/jappl.1984.56.2.287 [DOI] [PubMed] [Google Scholar]
- 78.Raven PB, Potts JT, Shi X. Baroreflex regulation of blood pressure during dynamic exercise. Exerc Sport Sci Rev. (1997) 25:365–89. 10.1249/00003677-199700250-00015 [DOI] [PubMed] [Google Scholar]
- 79.Rowell LB, O’Leary DS. Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J Appl Physiol. (1990) 69:407–18. 10.1152/jappl.1990.69.2.407 [DOI] [PubMed] [Google Scholar]
- 80.Hainsworth R. Pathophysiology of syncope. Clin Auton Res. (2004) 14(Suppl. 1):18–24. 10.1007/s10286-004-1004-2 [DOI] [PubMed] [Google Scholar]
- 81.Thijs RD, Wieling W, Kaufmann H, van Dijk G. Defining and classifying syncope. Clin Auton Res Off J Clin Auton Res Soc. (2004) 14(Suppl. 1):4–8. 10.1007/s10286-004-1002-4 [DOI] [PubMed] [Google Scholar]
- 82.Kontos HA. Validity of cerebral arterial blood flow calculations from velocity measurements. Stroke. (1989) 20:1–3. 10.1161/01.STR.20.1.1 [DOI] [PubMed] [Google Scholar]
- 83.Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke. (2000) 31:1672–8. 10.1161/01.STR.31.7.1672 [DOI] [PubMed] [Google Scholar]
- 84.Poole DC, Ward SA, Whipp BJ. Control of blood-gas and acid-base status during isometric exercise in humans. J Physiol. (1988) 396:365–77. 10.1113/jphysiol.1988.sp016966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wieling W, van Lieshout JJ. Investigation and treatment of autonomic circulatory failure. Curr Opin Neurol Neurosurg. (1993) 6:537–43. [PubMed] [Google Scholar]
- 86.Claydon VE, Hainsworth R. Salt supplementation improves orthostatic cerebral and peripheral vascular control in patients with syncope. Hypertens (Dallas, Tex 1979). (2004) 43:809–13. 10.1161/01.HYP.0000122269.05049.e7 [DOI] [PubMed] [Google Scholar]
- 87.Williams EL, Raj SR, Schondorf R, Shen WK, Wieling W, Claydon VE. Salt supplementation in the management of orthostatic intolerance: vasovagal syncope and postural orthostatic tachycardia syndrome. Auton Neurosci. (2021) 237:102906. 10.1016/j.autneu.2021.102906 [DOI] [PubMed] [Google Scholar]
- 88.Alizadeh A, Peighambari M, Keikhavani A, Emkanjoo Z, Rad MA, Ghadrdoost B, et al. The role of acute physical maneuver in preventing vasovagal syncope: a randomized clinical trial. Age. (2016) 42:e5348. [Google Scholar]
- 89.Croci F, Brignole M, Menozzi C, Solano A, Donateo P, Oddone D, et al. Efficacy and feasibility of isometric arm counter-pressure manoeuvres to abort impending vasovagal syncope during real life. Europace Eur Pacing Arrhythmias Card Electrophysiol J Work Groups Card Pacing Arrhythmias Card Cell Electrophysiol Eur Soc Cardiol. (2004) 6:287–91. 10.1016/j.eupc.2004.03.008 [DOI] [PubMed] [Google Scholar]
- 90.Tomaino M, Romeo C, Vitale E, Kus T, Moya A, van Dijk N, et al. Physical counter-pressure manoeuvres in preventing syncopal recurrence in patients older than 40 years with recurrent neurally mediated syncope: a controlled study from the Third International Study on Syncope of Uncertain Etiology (ISSUE-3)†. Europace Eur Pacing arrhythmias Card Electrophysiol J Work Groups Card Pacing Arrhythmias Card Cell Electrophysiol Eur Soc Cardiol. (2014) 16:1515–20. 10.1093/europace/euu125 [DOI] [PubMed] [Google Scholar]
- 91.Fenton AM, Hammill SC, Rea RF, Low PA, Shen WK. Vasovagal syncope. Ann Intern Med. (2000) 133:714–25. 10.7326/0003-4819-133-9-200011070-00014 [DOI] [PubMed] [Google Scholar]
- 92.Sheldon R, Rose S, Flanagan P, Koshman ML, Killam S. Risk factors for syncope recurrence after a positive tilt-table test in patients with syncope. Circulation. (1996) 93:973–81. 10.1161/01.CIR.93.5.973 [DOI] [PubMed] [Google Scholar]
- 93.Sahota I, Sheldon R, Pournazari P. Clinical improvement of vasovagal syncope in the absence of specific therapies: the Seinfeld effect. Cardiol J. (2014) 21:637–42. 10.5603/CJ.2014.0096 [DOI] [PubMed] [Google Scholar]
- 94.Hilz MJ, Ehmann EC, Pauli E, Baltadzhieva R, Koehn J, Moeller S, et al. Combined counter-maneuvers accelerate recovery from orthostatic hypotension in familial dysautonomia. Acta Neurol Scand. (2012) 126:162–70. 10.1111/j.1600-0404.2012.01670.x [DOI] [PubMed] [Google Scholar]
- 95.Convertino VA, Tripp LD, Ludwig DA, Duff J, Chelette TL. Female exposure to high G: chronic adaptations of cardiovascular functions. Aviat Space Environ Med. (1998) 69:875–82. [PubMed] [Google Scholar]
- 96.Romme JJCM, Reitsma JB, Go-Schön IK, Harms MPM, Ruiter JH, Luitse JSK, et al. Prospective evaluation of non-pharmacological treatment in vasovagal syncope. Europace Eur Pacing Arrhythmias Card Electrophysiol J Work Groups Card Pacing Arrhythmias Card Cell Electrophysiol Eur Soc Cardiol. (2010) 12:567–73. 10.1093/europace/eup414 [DOI] [PubMed] [Google Scholar]
- 97.Sheikh NA, Phillips AA, Ranada S, Lloyd M, Kogut K, Bourne KM, et al. Mitigating initial orthostatic hypotension: mechanistic roles of muscle contraction versus sympathetic activation. Hypertens (Dallas, Tex 1979). (2022) 79:638–47. 10.1161/HYPERTENSIONAHA.121.18580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Grossman SA, Chiu D, Lipsitz L, Mottley JL, Shapiro NI. Can elderly patients without risk factors be discharged home when presenting to the emergency department with syncope? Arch Gerontol Geriatr. (2014) 58:110–4. 10.1016/j.archger.2013.07.010 [DOI] [PubMed] [Google Scholar]
- 99.Brignole M, Menozzi C, Moya A, Andresen D, Blanc JJ, Krahn AD, et al. Pacemaker therapy in patients with neurally mediated syncope and documented asystole: third International Study on Syncope of Uncertain Etiology (ISSUE-3): a randomized trial. Circulation. (2012) 125:2566–71. 10.1161/CIRCULATIONAHA.111.082313 [DOI] [PubMed] [Google Scholar]
- 100.Clarke DA, Medow MS, Taneja I, Ocon AJ, Stewart JM. Initial orthostatic hypotension in the young is attenuated by static handgrip. J Pediatr. (2010) 156:1019–22.e1. 10.1016/j.jpeds.2010.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thieben MJ, Sandroni P, Sletten DM, Benrud-Larson LM, Fealey RD, Vernino S, et al. Postural orthostatic tachycardia syndrome: the mayo clinic experience. Mayo Clin Proc. (2007) 82:308–13. 10.4065/82.3.308 [DOI] [PubMed] [Google Scholar]
- 102.Low PA, Sandroni P, Joyner M, Shen W-K. Postural tachycardia syndrome (POTS). J Cardiovasc Electrophysiol. (2009) 20:352–8. 10.1111/j.1540-8167.2008.01407.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stewart JM, McLeod KJ, Sanyal S, Herzberg G, Montgomery LD. Relation of postural vasovagal syncope to splanchnic hypervolemia in adolescents. Circulation. (2004) 110:2575–81. 10.1161/01.CIR.0000145543.88293.21 [DOI] [PubMed] [Google Scholar]
- 104.Kowalsky JM, Conatser R, Ritz T, France CR. Effects of respiratory and applied muscle tensing interventions on responses to a simulated blood draw among individuals with high needle fear. J Behav Med. (2018) 41:771–83. 10.1007/s10865-018-9925-8 [DOI] [PubMed] [Google Scholar]
- 105.Meuret AE, Simon E, Bhaskara L, Ritz T. Ultra-brief behavioral skills trainings for blood injection injury phobia. Depress Anxiety. (2017) 34:1096–105. 10.1002/da.22616 [DOI] [PubMed] [Google Scholar]
- 106.Thijsen A, Gemelli CN, Davison TE, O’Donovan J, Bell B, Masser B. Does using applied muscle tension at strategic time points during donation reduce phlebotomist- and donor-reported vasovagal reaction rates? A three-armed randomized controlled trial. Transfusion. (2018) 58:2352–9. 10.1111/trf.14940 [DOI] [PubMed] [Google Scholar]
- 107.Thijsen A, Masser B, Davison TE. Reduced risk of vasovagal reactions in Australian whole blood donors after national implementation of applied muscle tension and water loading. Transfusion. (2020) 60:918–21. 10.1111/trf.15701 [DOI] [PubMed] [Google Scholar]
- 108.Goldman M, Uzicanin S, Marquis-Boyle L, O’Brien SF. Implementation of measures to reduce vasovagal reactions: donor participation and results. Transfusion. (2021) 61:1764–71. 10.1111/trf.16375 [DOI] [PubMed] [Google Scholar]
- 109.Tomasulo P, Kamel H, Bravo M, James RC, Custer B. Interventions to reduce the vasovagal reaction rate in young whole blood donors. Transfusion. (2011) 51:1511–21. 10.1111/j.1537-2995.2011.03074.x [DOI] [PubMed] [Google Scholar]
- 110.McIntyre-Patton L, Wanderski S, Graef D, Woessner L, Baker R. Randomized trial evaluating the effectiveness of a leg crossing and muscle tensing technique on decreasing vasovagal symptoms among pediatric and young adult patients undergoing peripheral IV catheter insertion. J Pediatr Nurs. (2018) 38:53–6. 10.1016/j.pedn.2017.09.012 [DOI] [PubMed] [Google Scholar]
- 111.Foulds J, Wiedmann K, Patterson J, Brooks N. The effects of muscle tension on cerebral circulation in blood-phobic and non-phobic subjects. Behav Res Ther. (1990) 28:481–6. 10.1016/0005-7967(90)90134-5 [DOI] [PubMed] [Google Scholar]
- 112.Ditto B, France CR, Albert M, Byrne N, Smyth-Laporte J. Effects of applied muscle tension on the likelihood of blood donor return. Transfusion. (2009) 49:858–62. 10.1111/j.1537-2995.2008.02067.x [DOI] [PubMed] [Google Scholar]
- 113.Ditto B, France CR, Holly C. Applied tension may help retain donors who are ambivalent about needles. Vox Sang. (2010) 98(Pt 1):e225–30. 10.1111/j.1423-0410.2009.01273.x [DOI] [PubMed] [Google Scholar]
- 114.Claydon VE, Hainsworth R. Postural sway in patients with syncope and poor orthostatic tolerance. Heart (British Cardiac Society). (2006) 92:1688–9. 10.1136/hrt.2005.083907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Claydon VE, Hainsworth R. Increased postural sway in control subjects with poor orthostatic tolerance. J Am Coll Cardiol. (2005) 46:1309–13. 10.1016/j.jacc.2005.07.011 [DOI] [PubMed] [Google Scholar]
- 116.Fitzgibbon-Collins LK, Noguchi M, Heckman GA, Hughson RL, Robertson AD. Acute reduction in cerebral blood velocity on supine-to-stand transition increases postural instability in young adults. Am J Physiol Heart Circ Physiol. (2019) 317:H1342–53. 10.1152/ajpheart.00360.2019 [DOI] [PubMed] [Google Scholar]
- 117.Fitzgibbon-Collins LK, Heckman GA, Bains I, Noguchi M, McIlroy WE, Hughson RL. Older adults’ drop in cerebral oxygenation on standing correlates with postural instability and may improve with sitting prior to standing. J Gerontol A Biol Sci Med Sci. (2021) 76:1124–33. 10.1093/gerona/glaa194 [DOI] [PubMed] [Google Scholar]
- 118.Garg A, Xu D, Laurin A, Blaber AP. Physiological interdependence of the cardiovascular and postural control systems under orthostatic stress. Am J Physiol Heart Circ Physiol. (2014) 307:H259–64. 10.1152/ajpheart.00171.2014 [DOI] [PubMed] [Google Scholar]
- 119.Xu D, Verma AK, Garg A, Bruner M, Fazel-Rezai R, Blaber AP, et al. Significant role of the cardiopostural interaction in blood pressure regulation during standing. Am J Physiol Heart Circ Physiol. (2017) 313:H568–77. 10.1152/ajpheart.00836.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Peterka RJ. Sensorimotor integration in human postural control. J Neurophysiol. (2002) 88:1097–118. 10.1152/jn.2002.88.3.1097 [DOI] [PubMed] [Google Scholar]
- 121.Tanabe H, Fujii K, Suzuki Y, Kouzaki M. Effect of intermittent feedback control on robustness of human-like postural control system. Sci Rep. (2016) 6:22446. 10.1038/srep22446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Binder MD, Hirokawa N, Windhorst U. editors. Center of Mass (CoM) BT - Encyclopedia of Neuroscience. Berlin: Springer; (2009). p. 604. 10.1007/978-3-540-29678-2 [DOI] [Google Scholar]
- 123.Binder MD, Hirokawa N, Windhorst U. editors. Base of Support (BOS) BT - Encyclopedia of Neuroscience. Berlin: Springer; (2009). p. 354. [Google Scholar]
- 124.Hsu W-L, Scholz JP, Schöner G, Jeka JJ, Kiemel T. Control and estimation of posture during quiet stance depends on multijoint coordination. J Neurophysiol. (2007) 97:3024–35. 10.1152/jn.01142.2006 [DOI] [PubMed] [Google Scholar]
- 125.Soames RW, Atha J. The role of the antigravity musculature during quiet standing in man. Eur J Appl Physiol Occup Physiol. (1981) 47:159–67. 10.1007/BF00421668 [DOI] [PubMed] [Google Scholar]
- 126.Naruse R, Taki C, Yaegashi M, Sakaue Y, Shiozawa N, Kimura T. Attenuated spontaneous postural sway enhances diastolic blood pressure during quiet standing. Eur J Appl Physiol. (2021) 121:251–64. 10.1007/s00421-020-04519-x [DOI] [PubMed] [Google Scholar]
- 127.Protheroe CL, Ravensbergen HRJC, Inskip JA, Claydon VE. Tilt testing with combined lower body negative pressure: a “gold standard” for measuring orthostatic tolerance. J Vis Exp. (2013) 73:e4315. 10.3791/4315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Guyton AC, Richardson TQ, Langston JB. Regulation of cardiac output and venous return. Clin Anesth. (1964) 3:1–34. [PubMed] [Google Scholar]
- 129.Finucane C, O’Connell MDL, Donoghue O, Richardson K, Savva GM, Kenny RA. Impaired orthostatic blood pressure recovery is associated with unexplained and injurious falls. J Am Geriatr Soc. (2017) 65:474–82. 10.1111/jgs.14563 [DOI] [PubMed] [Google Scholar]
- 130.Murata J, Murata S, Horie J, Ohtao H, Miyazaki J. Relationship between orthostatic blood pressure changes and postural sway when standing up from a chair in older adult females. Int J Gerontol. (2012) 6:182–6. 10.1016/j.ijge.2012.01.011 [DOI] [Google Scholar]
- 131.Nordahl SH, Aasen T, Owe JO, Molvaer OI. Effects of hypobaric hypoxia on postural control. Aviat Space Environ Med. (1998) 69:590–5. [PubMed] [Google Scholar]
- 132.Garg A, Xu D, Blaber AP. Statistical validation of wavelet transform coherence method to assess the transfer of calf muscle activation to blood pressure during quiet standing. Biomed Eng Online. (2013) 12:132. 10.1186/1475-925X-12-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Grinsted A, Moore JC, Jevrejeva S. Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlinear Process Geophys. (2004) 11:561–6. 10.5194/npg-11-561-2004 [DOI] [Google Scholar]
- 134.Sugihara G, May R, Ye H, Hsieh C, Deyle E, Fogarty M, et al. Detecting causality in complex ecosystems. Science. (2012) 338:496–500. 10.1126/science.1227079 [DOI] [PubMed] [Google Scholar]
- 135.Bernardi L, Bissa M, DeBarbieri G, Bharadwaj A, Nicotra A. Arterial baroreflex modulation influences postural sway. Clin Auton Res Off J Clin Auton Res Soc. (2011) 21:151–60. 10.1007/s10286-010-0099-x [DOI] [PubMed] [Google Scholar]
- 136.Yates BJ, Bolton PS, Macefield VG. Vestibulo-sympathetic responses. Compr Physiol. (2014) 4:851–87. 10.1002/cphy.c130041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Verma AK, Garg A, Xu D, Bruner M, Fazel-Rezai R, Blaber AP, et al. Skeletal muscle pump drives control of cardiovascular and postural systems. Sci Rep. (2017) 7:45301. [DOI] [PMC free article] [PubMed] [Google Scholar]