Abstract
Host protection against Streptococcus pneumoniae is mainly mediated by opsonin-dependent phagocytosis. Several techniques for measuring opsonophagocytic activity (OPA) of antibodies to S. pneumoniae have been standardized and used. These include the viable cell-assay, flow-cytometric assays, and an assay utilizing radiolabeled bacteria. Using these different methods, we measured the OPA of antibodies to S. pneumoniae types 6B and 19F from the sera of infants immunized with a pneumococcal conjugate vaccine, PncCRM. Generally, the results obtained by the various techniques correlated well, although serotype-specific differences were found (6B, r = 0.78 to 0.95, P < 0.001; 19F, r = 0.50 to 0.84, P < 0.001). The same serotype-specific differences were observed for the relationship between the concentrations of specific immunoglobulin G antibodies measured by enzyme immunoassay and the OPA. Since the sensitivities of the OPA assays differed, the most prominent discrepancies between the techniques were found at low antibody concentrations.
Opsonophagocytosis mediated by antibodies and complement is the major mechanism for clearing Streptococcus pneumoniae (Pnc) from the host (19, 22). Therefore, the in vitro opsonophagocytic activity (OPA) of antibodies to pneumococcal capsular polysaccharides (PSs) is believed to be a measure of their functional activity in vivo. Limited data are available on the requirements of protective immune response in humans to conjugate vaccines against pneumococci (3). By contrast, protective levels of human antibodies in animals have been determined in several studies (6, 12, 18). In two different models of passive protection of mice against bacteremia or lung infection, OPA of human immunoglobulin G (IgG) antibody was found to correlate better with the protection than the IgG concentration (6, 17). Thus, to determine the serological correlates or surrogates of protection from the samples of ongoing efficacy trials, both quantitative and qualitative characteristics of antibodies have to be measured reliably. Because the analyses may be done in different laboratories, it is important to use validated methods that give comparable results.
Validation of the enzyme immunoassay (EIA) method for measuring concentrations of serotype-specific antibodies to Pnc has advanced during recent years (10, 14). A multicenter study at 12 laboratories has been completed, and similar results have been published (13). The validation of opsonophagocytic assays is far behind, although several techniques have been reported and standardized (5, 11, 16, 20, 21). Since each laboratory used its own assay for the measurement of OPA of antibodies against Pnc, it is important to determine whether the results obtained are comparable both to each other and to the IgG concentrations measured by EIA. Therefore, using four different opsonophagocytic assays, we analyzed the OPA of antibodies to Pnc serotypes 6B and 19F from the sera of infants immunized with a pneumococcal conjugate vaccine. Thereafter, we compared the results to each other and to the IgG antibody concentrations.
MATERIALS AND METHODS
Vaccines.
PncCRM (Wyeth-Lederle Vaccines and Pediatrics, West Henrietta, N.Y.) is a heptavalent pneumococcal conjugate vaccine containing 2 μg each of types 4, 9V, 14, 19F, and 23F capsular PSs, 2 μg of type-18C oligosaccharide, and 4 μg of type-6B PS conjugated to a nontoxic variant of diphtheria toxin, CRM197. PNU-IMUNE (Wyeth-Lederle Vaccines and Pediatrics) is a commercial 23-valent pneumococcal PS vaccines (PncPS) containing 25 μg of each capsular PS.
Vaccine subjects and sampling.
Infants (n = 16) were immunized at 2, 4, and 6 months of age with PncCRM and given booster injections at 15 months of age with the homologous conjugate vaccine or PncPS (1). Blood samples were obtained from subjects at 7, 15, and 16 months of age. Sera were separated by centrifugation and stored at −20°C until testing. Infants receiving booster injections of either the homologous conjugate vaccine or the PncPS vaccine were retained as one group.
EIA for anti-Pnc PS IgG.
Concentrations of IgG antibodies to pneumococcal PSs were measured by EIA methods as described previously (8). The results are given as micrograms per milliliter calculated on the basis of the officially assigned IgG values of the 89-SF reference serum (15).
Bacteria.
S. pneumoniae serotypes 6B and 19F (reference strains received from Centers for Disease Control, Atlanta, Ga.) (16) were grown in Todd-Hewitt broth supplemented with 0.5% yeast extract and kept frozen (−70°C) in aliquots in Todd-Hewitt broth with 15% glycerol. The growing and labeling (when needed) of the bacteria (Table 1) were performed as described previously (5, 11, 16, 20, 21). The encapsulation of the strains was judged by the quellung test using rabbit antiserum (Statens Seruminstitut, Copenhagen, Denmark) (16).
TABLE 1.
Differences in the protocols of four different opsonophagocytic assays
| Factor | Requirement(s) of opsonophagocytic assay
|
|||
|---|---|---|---|---|
| Viable assay | Radio assay | Flow assay 1 | Flow assay 2 | |
| Bacteria | Live, untreated, grown once to log phase (1 × log) | Live, radiolabeled (tritium, H3), grown 1 × log | Killed, labeled with fluorescein isothiocyanate, grown 3 × log | Killed, labeled with 5,6-carboxyfluorescein, succinimidyl ester, grown 1 × log |
| Phagocytes | Fresh PMNLs | Fresh PMNLs | Fresh PMNLs | Differentiated HL-60 cells |
| Bacterium/phagocyte ratio | 1:400 | 10:1 | 10:1 | 4:1 |
| Complement source | Baby rabbit (aged 3–4 weeks) serum | Pooled serum from hypo- and agammaglobulinemic patients (serotype 6B), IgG-depleted serum from a healthy adult volunteer (serotype 19F) | IgG-depleted human pooled serum | Baby rabbit serum |
| Complement concentration | 12.5% | 5% (6B), 12% (19F) | 2% | 12.5% |
Opsonophagocytic assays.
Functional activity of antibodies from all serum samples was determined by three different techniques: an opsonophagocytic assay using viable bacteria (“viable assay” [16]), an assay using live, radiolabeled bacteria (“radio assay” [20, 21]), and a flow-cytometric assay (“flow assay 1” [5]). In addition, parts of the sera were analyzed by another flow-cytometric technique (“flow assay 2” [11]). The details of the techniques are shown in Table 1. In all assays, serum, from which the internal complement was inactivated, bacteria, external complement source, and phagocytes were mixed, and phagocytosis was allowed to take place. Polymorphonuclear leukocytes (PMNLs) served as phagocytes in viable, radio, and flow 1 assays (Table 1). The PMNLs were isolated from the fresh peripheral blood of healthy adult donors by dextran sedimentation and Ficoll (Paque [Pharmacia Biotech, Uppsala, Sweden] or Histopaque [Sigma, St. Louis, Mo.]) density gradient centrifugation (viable and radio assays) or by Ficoll-Histopaque gradient followed by two hypotonic shocks (flow assay 1). The isolated cells were washed and dissolved in Hanks' balanced salt solution containing 1% bovine serum albumin or 2.5% fetal calf serum. In flow assay 2, differentiated HL-60 cells were used as phagocytes (11).
The viable-cell assay was a modification (2) of the assay described by Romero-Steiner et al. (16). It measured the killing of live pneumococci by PMNLs in the presence of antibody and complement. OPA of antibodies was expressed as a titer, which was the reciprocal of the serum dilution with 50% killing as compared to the bacterial growth in the controls without serum. A titer of 4 was given to sera with undetectable OPA, with a titer of 8 being the lowest positive result.
The radio assay was modified from the assay described previously by Vidarsson et al. (20, 21). Instead of using internal complement of each test serum, an external complement was added. As a complement source, pooled serum of hypo- and agammaglobulinemic patients, provided by J. Plested (Churchill University, Oxford, United Kingdom), or an IgG-depleted serum of a healthy adult volunteer was used (Table 1). Fresh normal serum was depleted of IgG by protein G affinity chromatoraphy (Pharmacia Biotech, Roosendaal, The Netherlands) and stored at −70°C. The success of IgG depletion was assured by radial immunodiffusion (LC Partigen IgG; Behring, Malburg, Germany). Pooled serum of hypo- and agammaglobulinemic patients was used at a concentration of 5% in the experiments with serotype 6B. The experiments with serotype 19F were performed using IgG-depleted serum at a 12% concentration (Table 1). The results were obtained by measuring the radioactivity in a liquid scintillation counter (Packard, Greve, Denmark) and by counting the percent uptake of radiolabeled bacteria in the presence of each serum (21). This was compared to a standard run at various concentrations in every assay. The OPA was then calculated from the standard curve and represented as arbitrary units. Undetectable OPAs were reported as 1 arbitrary unit.
Flow assay 1 was performed as described previously by Jansen et al. (5). The OPA of antibodies was expressed as a titer; it was the reciprocal of the serum dilution resulting in 25% fluoroscein isothiocyanate-positive PMNLs. A titer of 1 was given to sera with undetectable OPAs.
Sera taken from 10 infants at 15 and 16 months of age were analyzed by a flow assay 2 described by Martinez et al., using differentiated HL-60 cells as phagocytes (11). The OPA of antibodies was expressed as a titer, which was the reciprocal of the serum dilution with at least a 50% decrease in fluorescence compared with the maximal percent fluorescence of each sample. A titer of 4 was given to sera with an undetectable OPA, 8 being the lowest positive result.
Statistical analysis.
Statistical comparisons were carried out using the paired t-test, Pearson's correlation analysis, and kappa statistics, interpreted as the chance-corrected proportional agreement. When the relationship between two different factors (OPA and concentration) was evaluated, sera taken from infants at different time points were retained separately due to their dependency on each other. In statistical analyses, log-transformed data of concentrations and OPAs were used.
RESULTS
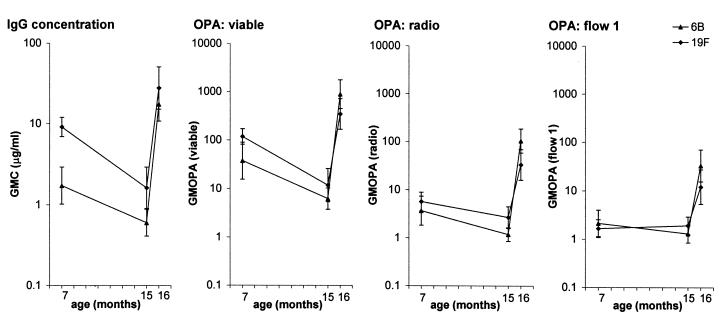
The IgG concentration and the OPA of antibodies measured by different opsonophagocytic techniques showed similar kinetics (Fig. 1). Both the antibody level and the OPA against serotypes 6B and 19F decreased significantly in samples from subjects between the ages of 7 and 15 months (P < 0.01 to 0.001), with the exception of OPAs determined by flow assay 1. A significant increase (P < 0.001) was seen after the booster vaccination by all methods.
FIG. 1.
Geometric mean concentration (GMC) and OPA (GMOPA) of antibodies to Pnc serotypes 6B and 19F PSs in sera of infants (n = 16) taken at 7, 15, and 16 months of age. The OPAs were determined by using three different opsonophagocytic methods: viable assay, radio assay, and flow assay 1.
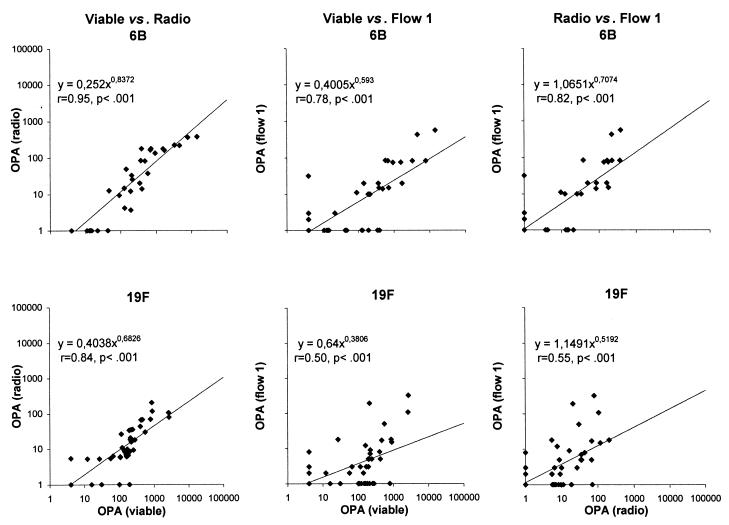
When the data from analyses of serum samples taken from subjects at different ages were combined (n = 48), the OPAs obtained by the three phagocytic assays correlated significantly (Fig. 2). The correlation of the OPAs measured by viable and radio assays was significant at all ages for both serotypes (r = 0.76 to 0.92, P < 0.01 to 0.001). Likewise, after booster vaccination, the correlations between the OPAs of viable and radio assays and of flow assay 1 were mostly significant (r = 0.37 to 0.82, P < 0.2 to 0.001). However, at 7 or 15 months of age, when the activities were low with all assays, no significant correlations were found between flow assay 1 and the other two assays (r = −0.14 to 0.43, P = 0.09 to 0.89).
FIG. 2.
Relationship between the OPAs of antibodies to Pnc serotypes 6B and 19F PSs determined by the three different phagocytic techniques: viable, radio, and flow 1 assay. The sera taken from infants at 7, 15, and 16 months of age have been retained as a one group (n = 48).
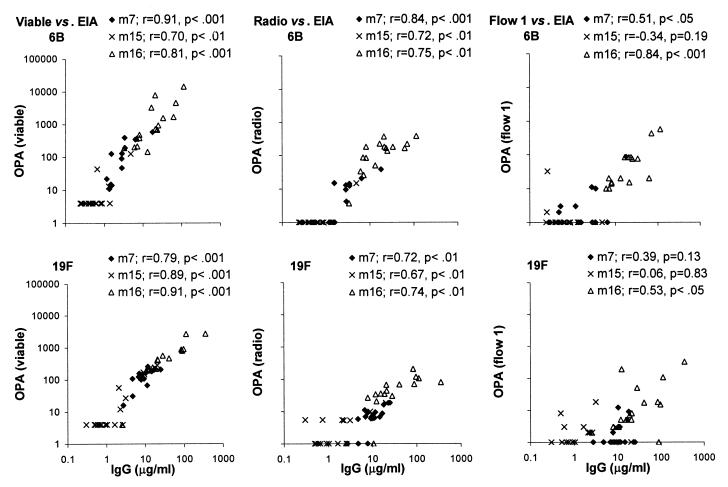
The OPA measured by viable or radio assay correlated strongly with the IgG concentration measured by EIA at all subject ages for both serotypes (Fig. 3). The correlation between the OPAs of flow assay 1 and the IgG concentration was significant after the booster vaccination for both serotypes 6B and 19F and at the subject age of 7 months for serotype 6B. However, when the concentration of antibody was low, e.g., in the sera taken before booster vaccinations, no significant correlation could be found. Furthermore, the sensitivity of the OPA assays differed. For serotype 6B, the detection limits of the viable and radio assays were both about 1 μg/ml, while more antibodies were usually required to get detectable OPAs with flow assay 1 (Fig. 3). For all serotype 19F OPA assays, the detection limit was higher than that for serotype 6B assays. Generally, more anti-19F than anti-6B antibodies were needed to obtain similar OPAs; this was seen by comparing the slopes for the two serotypes (Fig. 3).
FIG. 3.
Relationship between the IgG concentration measured by EIA and OPA of antibodies to serotypes 6B and 19F measured by the three phagocytic techniques: viable, radio, and flow 1 assays. The correlation between the two parameters was analyzed separately for the sera taken from infants at 7, 15, and 16 months of age.
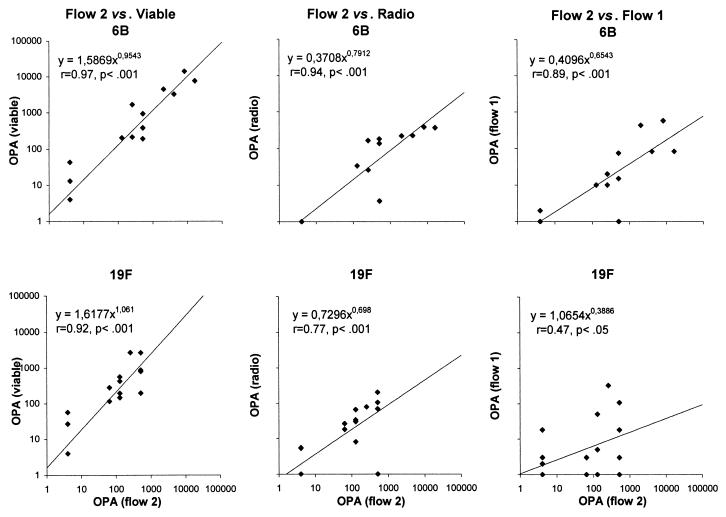
Because two standardized flow-cytometric opsonophagocytosis assays for Pnc have been described in the literature, we wanted to include both of them in the comparison. However, only 20 sera were available for analyses by flow assay 2. Therefore, flow assay 2 and the other three assays were compared using only part of the sera. The OPAs measured by flow assay 2 correlated well with the OPAs of the other assays for serotype 6B (Fig. 4). For serotype 19F, the correlations were not as good between the two flow-cytometric assays but were, however, significant. The correlation between OPAs of flow assay 2 and IgG concentration assays was mostly significant in both age groups for both serotypes (r = 0.49 to 0.80, P < 0.16 to 0.01).
FIG. 4.
Relationship between the OPAs determined for serotypes 6B and 19F by flow cytometric assay 2 and by the other three methods: viable, radio, and flow 1 assays. The sera from 10 infants obtained at 15 and 16 months of age were retained as one group (n = 20).
Comparison of the proportions of sera with detectable (+) or undetectable (−) OPAs determined by the different assays further emphasized the better agreement between OPA assays for serotype 6B than those for serotype 19F (Table 2). The agreement between viable and radio assays was generally good for both serotypes. Moderate or poor agreement was found between flow assay 1 and viable or radio assays. Exactly the same samples were detectable for serotype 6B OPAs with the flow assay 2 and the radio assay (κ = 1.00). Since the other two assays detected mostly the same sera (κ = 0.80), very good agreement was found between flow assay 2 and the other assays for serotype 6B. By contrast, conflicting OPAs were obtained with the two flow-cytometric assays for serotype 19F.
TABLE 2.
Number of sera with detectable and undetectable OPA of anti-serotype 6B and anti-serotype 19F antibodies measured by the viable, radio, and flow 1 assays (n = 48), as well as with the flow 2 assay (n = 20)a
| Assay resultb | No. of sera with indicated combination of results for sample
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serotype 6B by assay
|
Serotype 19F by assay
|
|||||||||||||||||
| Radio
|
Flow 1
|
Flow 2
|
Radio
|
Flow 1
|
Flow 2
|
|||||||||||||
| + | − | κ | + | − | κ | + | − | κ | + | − | κ | + | − | κ | + | − | κ | |
| Viable | 0.75 | 0.34 | 0.80 | 0.67 | 0.13 | 0.79 | ||||||||||||
| + | 25 | 6 | 19 | 12 | 10 | 2 | 33 | 4 | 23 | 14 | 11 | 2 | ||||||
| − | 0 | 17 | 4 | 13 | 0 | 8 | 2 | 9 | 5 | 6 | 0 | 7 | ||||||
| Radio | 0.50 | 1.00 | 0.18 | 0.59 | ||||||||||||||
| + | 18 | 7 | 10 | 0 | 23 | 13 | 10 | 3 | ||||||||||
| − | 5 | 18 | 0 | 10 | 5 | 7 | 1 | 6 | ||||||||||
| Flow 1 | 0.80 | 0.17 | ||||||||||||||||
| + | 9 | 1 | 8 | 5 | ||||||||||||||
| − | 1 | 9 | 3 | 4 | ||||||||||||||
The measure of agreement between the different assays was analyzed by kappa statistics (see text). κ, kappa value; measure of agreement. κ < 0.4 indicates poor agreement, κ = 0.4 to 0.6 indicates moderate agreement, κ > 0.6 indicates good agreement
+, detectable; −, undetectable.
DISCUSSION
In this study, comparable results were obtained by the opsonophagocytic assay techniques, which, themselves, differed in many respects. However, the levels of OPAs were different, which may be due to the dissimilarities in the details of the assays (e.g., bacterium/PMNL ratio, complement source, and concentration) and in the ways results are calculated. In addition, there were serotype-specific differences. For serotype 6B, the OPAs obtained by all assays correlated well, but for serotype 19F, the correlations were poorer. High concentrations of anti-19F antibodies were often required to get detectable opsonic activities, and OPAs measured by different assays from the sera with low anti-19F concentrations varied. Sera with high antibody concentrations had generally high OPAs with all methods.
The highest correlation was found for both serotypes between the OPAs obtained by the viable and radio assays. Likewise, functional activities measured by these assays correlated well with IgG concentrations measured by EIA. For data deduced from sera with detected OPAs by viable assay but undetectable OPAs by radio assay, the viable assay seemed to be somewhat more sensitive. This was further confirmed for results of the correlation between IgG concentration and OPA; for serotype 19F, more antibodies were usually required to get detectable activity by radio assay than by viable assay.
The OPAs of flow assay 1 correlated well with the OPAs of the other types of assays for serotype 6B. For serotype 19F, the correlations were not as high, and there were discrepancies with the sera of low functional activities. The same was seen when the OPAs were correlated to IgG concentration. In fact, the flow assay 1 seemed to be somewhat less sensitive than either the viable or radio assay for both serotypes. This may partly explain the lack of correlation between the concentration and OPA of serotype 19F in the sera having low antibody levels. Considerably better correlations were found for serotype 6B in the postbooster sera that had higher concentrations and OPAs.
Unfortunately, all sera were not available for flow assay 2. Based on the data received by using 20 sera, the OPAs obtained by flow assay 2 correlated well with the OPAs of the other assays for serotype 6B. For serotype 19F, however, there were discrepancies in the OPAs measured by the two flow-cytometric assays. These differences may have resulted from differences between the assays in source and concentration of complement, bacterium/phagocyte ratio, methods growing and labeling the bacteria, etc. The difference was most probably not due to the use of HL-60 cells instead of fresh PMNLs in flow assay 2.
Each method had its own advantages and drawbacks. The main advantage of the viable-cell assay was its sensitivity and the fact that it was the only assay that measured the killing of the bacteria. The other assays measured binding between the phagocytes and bacteria, but the good correlation between these later assays and the viable assay suggests that binding most likely indicates killing. This was demonstrated using 10 adult postvaccination sera and removing aliquots from each tube at the end of the radio assay for plating onto agar. A significant correlation was found between percent uptake and percent killing (E. Saeland, unpublished data). The viable assay was more laborious than the other assays and consumed more PMNLs. Therefore, when fresh PMNLs were used, a large volume of blood was needed for their isolation. The number of sera that could be analyzed per day was also lower when the viable assay was used when the radio assay or both flow assays were used. Radio and flow assays were fast, convenient, and easy to perform. In addition, an advantage of the flow assays is the possibility they afford to semiautomate the technique and measure OPAs for multiple serotypes (using different dyes) in one tube (4). A drawback of the radio assay was the need of large volumes (up to 200 μl per serotype) of the test sera; large volumes were required to be able to count β emissions reliably. Furthermore, the radio assay is very dependent on human complement, and the best sensitivity is gained when intact test sera (21) or sera from the agammaglobulinemic patients, both retaining the full complement activity, are used as sources of complement. Radioactive waste may also be considered as a drawback of the radio assay. Flow assay 1, though very easy to perform if a laboratory has good equipment, is at the moment too insensitive for analyzing samples from infants whose sera contain low concentrations of specific antibodies. However, there is a conflict between sensitivity and specificity; flow assay 1 has been made completely specific for anticapsular PS antibodies by using highly encapsulated bacteria grown three times to log phase. Bacteria encapsulated this heavily may be difficult to phagocytize (7), which would impair the sensitivity of an assay.
We ended up using the mentioned sources and concentrations of complement, ways of growing and labeling the bacteria, bacterium/phagocyte ratios, etc., to perform the assays as described previously in the literature. Because none of the assays was optimal, the influence of different factors on each technique should be evaluated in the future. Nevertheless, taking into consideration the large differences in the performance of the assays, the OPAs obtained were fairly comparable; every method correctly detected the sera with high activity and identified the activity as high, although there were variations near the detection limit of the assays. As pointed out earlier, different methods had different sensitivities, and those having the highest sensitivity gave OPAs that correlated best with each other and with IgG concentrations. The issue of sensitivity is especially important when serological correlates of protection induced by immunizing infants with pnuemococcal conjugate vaccines are analyzed. The reports of animal studies and efficacy trials suggest that the minimal protective antibody level might vary between 0.05 and 1.15 μg of specific antibodies per ml, depending on the serotype and the disease in question (different for invasive and mucosal infections) (6, 9, 12, 18). This range is mostly below the detection limit of any tested opsonophagocytic assay. Therefore, effort is needed, in the future on increasing the sensitivity of the assays.
ACKNOWLEDGMENTS
This study was supported by World Health Organization (GVP/VRD contract V23/181/76) and by the Academy of Finland, the Nederlandse Organisatie voor Wetenschappelijk Onderzoek, and the Federation of European Microbiological Societies (FEMS). The clinical part of the study was supported by Wyeth-Lederle Vaccines and Pediatrics.
We are grateful to Joseph Martinez and Sandra Romero-Steiner for teaching us one of the flow-cytometric techniques, as well as a method for the treatment and differentiation of the HL-60 cells. We also thank George M. Carlone for giving us an opportunity to do part of the analyses in his laboratory. Furthermore, we thank Maijastiina Voutilainen, Arja Vuorela, Hannele Lehtonen, and Sirkka-Liisa Wahlman for excellent technical assistance; Heidi Åhman for the IgG concentration data; Joyce Plested for providing us with the sera of hypo- and agammaglobulinemic patients; and Virva Jäntti for statistical help. Personnel of the study centers are acknowledged for their help in the clinical part of the study.
REFERENCES
- 1.Anttila M, Eskola J, Åhman H, Käyhty H. Differences in the avidity of antibodies evoked by four different pneumococcal conjugate vaccines in early childhood. Vaccine. 1999;17:1970–1977. doi: 10.1016/s0264-410x(98)00458-7. [DOI] [PubMed] [Google Scholar]
- 2.Anttila M, Voutilainen M, Jäntti V, Eskola J, Käyhty H. Contribution of serotype specific IgG concentration, IgG subclasses, and relative antibody avidity to opsonophagocytic activity against Streptococcus pneumoniae. Clin Exp Immunol. 1999;118:402–407. doi: 10.1046/j.1365-2249.1999.01077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr Infect Dis J. 2000;19:187–195. doi: 10.1097/00006454-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Carlone G M. Workshop on standardization of serologic assays for the evaluation of immune responses. Washington, D.C.: World Health Organization, Pan American Health Organization, and National Institutes of Health; 1999. Comparison of FACS and viable cell assay, including simultaneous measurement of opsonic activity for multiple serotypes. [Google Scholar]
- 5.Jansen W T M, Gootjes J, Zelle M, Madore D V, Verhoef J, Snippe H, Verheul A F M. Use of highly encapsulated Streptococcus pneumoniae strains in a flow-cytometric assay for assessment of the phagocytic capacity of serotype-specific antibodies. Clin Diagn Lab Immunol. 1998;5:703–710. doi: 10.1128/cdli.5.5.703-710.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson S E, Rubin L, Romero-Steiner S, Dykes J K, Pais L B, Rizvi A, Ades E, Carlone G M. Correlation of opsonophagocytosis and passive protection assays using human anticapsular antibodies in infant mouse model of bacteremia for Streptococcus pneumoniae. J Infect Dis. 1999;180:133–140. doi: 10.1086/314845. [DOI] [PubMed] [Google Scholar]
- 7.Kim J O, Romero-Steiner S, Sorensen U B S, Blom J, Carvalho M, Barnard S, Carlone G, Weiser J. Relationship between cell surface carbohydrates and intrastrain variation on opsonophagocytosis of Streptococcus pneumoniae. Infect Immun. 1999;67:2327–2333. doi: 10.1128/iai.67.5.2327-2333.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Käyhty H, Åhman H, Rönnberg P-R, Tillikainen R, Eskola J. Pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine is immunogenic in infants and children. J Infect Dis. 1995;172:1273–1278. doi: 10.1093/infdis/172.5.1273. [DOI] [PubMed] [Google Scholar]
- 9.Madore D. Workshop on standardization of serologic assays for the evaluation of immune responses. Washington, D.C.: World Health Organization, Pan American Health Organization, and National Institutes of Health; 1999. Efficacy and immunogenicity of heptavalent PncCRM: proposal for serological correlate of protection. [Google Scholar]
- 10.Madore D V, Strong N M, Quataert S A. Validation and standardization of serologic methods for evaluation of clinical immune response to vaccines. In: Paoletti L C, McInnes P M, editors. Vaccines, from concept to clinic: a guide to the development and clinic testing of vaccines for human use. Boca Raton, Fla: CRC Press LLC; 1999. pp. 43–75. [Google Scholar]
- 11.Martinez J E, Romero-Steiner S, Pilishvili T, Barnard S, Schinsky J, Goldblatt D, Carlone G. A flow cytometric opsonophagocytic assay for measurement of functional antibodies elicited after vaccination with the 23-valent pneumococcal polysaccharide vaccine. Clin Diagn Lab Immunol. 1999;6:581–586. doi: 10.1128/cdli.6.4.581-586.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Musher D M, Johnson B, Jr, Watson D A. Quantitative relationship between anticapsular antibody measured by enzyme-linked immunosorbent assay or radioimmunoassay and protection of mice against challenge with Streptococcus pneumoniae serotype 4. Infect Immun. 1990;58:3871–3876. doi: 10.1128/iai.58.12.3871-3876.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plikaytis B D, Goldblatt D, Frasch C E, Blondeau C, Bybel M J, Giebink G S, Jonsdottir I, Käyhty H, Konradsen H B, Madore D V, Nahm M H, Schulman C A, Holder P F, Lezhava T, Elie C, Carlone G M. An analytical model applied to a multi-center pneumococcal ELISA study. J Clin Microbiol. 2000;38:2043–2050. doi: 10.1128/jcm.38.6.2043-2050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quataert S. Workshop on standardization of serologic assays for the evaluation of immune responses. Washington, D.C.: World Health Organization, Pan American Health Organization, and National Institutes of Health; 1999. Strategies for validation of a pneumococcal polysaccharide ELISA. [Google Scholar]
- 15.Quataert S A, Kirch C S, Wiedl L J, Phipps D C, Strohmeyer S, Cimino C O, Skuse J, Madore D V. Assignment of weight-based antibody units to a human antipneumococcal standard reference serum, Lot 89-S. Clin Diagn Lab Immunol. 1995;2:590–597. doi: 10.1128/cdli.2.5.590-597.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romero-Steiner S, Libutti D, Pais L B, Dykes J, Anderson P, Whitin J C, Keyserling H L, Carlone G M. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus penumoniae using differentiated HL-60 cells. Clin Diagn Lab Immunol. 1997;4:415–422. doi: 10.1128/cdli.4.4.415-422.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saeland E, Jakobsen H, Ingolfsdottir G, Sigurdardottir S, Jonsdottir I. Sera from infants vaccinated with a pneumococcal conjugate vaccine, PncT, protect mice against invasive infection caused by serotypes 6A and 6B. J Infect Dis. 2001;183:253–260. doi: 10.1086/317934. [DOI] [PubMed] [Google Scholar]
- 18.Stack A M, Malley R, Thompson C M, Les K, Siber G R, Saladino R A. Minimum protective serum concentrations of pneumococcal anti-capsular antibodies in infant rats. J Infect Dis. 1998;177:986–990. doi: 10.1086/515259. [DOI] [PubMed] [Google Scholar]
- 19.Tuomanen E I, Austrian R, Masure H R. Pathogenesis of pneumococcal infection. N Engl J Med. 1995;332:1280–1284. doi: 10.1056/NEJM199505113321907. [DOI] [PubMed] [Google Scholar]
- 20.Vidarsson G, Jonsdottir I, Jonsson S, Valdimarsson H. Opsonization and antibodies to capsular and cell wall polysaccharides of Streptococcus pneumoniae. J Infect Dis. 1994;170:592–599. doi: 10.1093/infdis/170.3.592. [DOI] [PubMed] [Google Scholar]
- 21.Vidarsson G, Sigurdardottir S T, Gudnason T, Kjartansson S, Kristinsson K G, Ingolfsdottir G, Jonsson S, Valdimarsson H, Schiffman G, Schneerson R, Jonsdottir I. Isotypes and opsonophagocytosis of pneumococcus type 6B antibodies elicited in infants and adults by an experimental pneumococcus type 6B-tetanus toxoid vaccine. Infect Immun. 1998;66:2866–2870. doi: 10.1128/iai.66.6.2866-2870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watson D A, Musher D M, Verhoef J. Pneumococcal virulence factors and host immune responses to them. Eur J Clin Microbiol Infect Dis. 1995;14:479–490. doi: 10.1007/BF02113425. [DOI] [PubMed] [Google Scholar]