Abstract
Background
Obesity-associated metabolic complications display sexual dimorphism and can be impacted by cytokines. We previously showed that interleukin-10 (IL-10) was upregulated in white adipose tissue (WAT) of obese women with type 2 diabetes (T2D). Whether this pertains to men is unknown. The aim of this study was to compare the impact of obesity and T2D on WAT IL-10 levels in men versus women.
Methods
Plasma and subcutaneous WAT biopsies were obtained from 108 metabolically well-characterized individuals. WAT IL10 expression/secretion and WAT-resident IL-10-secreting macrophage number were measured. Circulating sex hormone levels were correlated to WAT IL10 expression in 22 individuals and sex hormone effects on macrophage IL10 expression were investigated in vitro.
Results
Obese women with T2D showed increased IL10 expression/secretion and IL-10-secreting WAT macrophage number compared to other female groups. This difference was absent in men. Non-obese women and men with T2D showed similar IL-10 levels compared to healthy controls, indicating that T2D alone does not regulate IL-10. Although WAT IL10 expression correlated with serum estrone (E1) concentrations, recombinant E1 did not affect macrophage IL10 expression in vitro.
Conclusion
WAT IL-10 levels are higher in women with obesity and T2D, but not in men and this effect is primarily attributed to obesity per se. This is less likely to be driven by circulating sex hormones. We propose that the WAT IL-10 might exert protective effects in obesity-associated chronic inflammation in women which could be one of the contributing factors for the decreased morbidity observed in women during obesity than men.
Keywords: IL-10, sex-specific, adipose, macrophages, obesity, women, men
Introduction
Chronic low-grade inflammation in white adipose tissue (WAT) is important for the development of insulin resistance (IR) and type 2 diabetes (T2D) in obesity (1). This low-grade inflammation is characterized by the infiltration and phenotypic change of macrophages as well as other pro-inflammatory immune cell populations like CD8+T, dendritic and B cells (2–5). The infiltration of pro-inflammatory cells is associated with an increased production of chemokines and pro-inflammatory cytokines like CC-motif Chemokine Ligand 2 (CCL2), Tumor Necrosis Factor-α (TNFα), interleukins IL-1β and IL-6 (6–9). Anti-inflammatory cytokines like IL-10 are also regulated by obesity (10, 11).
It is evident that sex influences metabolic disorders like T2D and cardiovascular disease (CVD) (12). Body fat amounts and distribution differ between men and women, where men usually accumulate fat centrally and women peripherally (13). Females have about 10% higher total body fat content than males with the same body mass index (BMI) (14). However, during the reproductive years, women with obesity are better protected from T2D and CVD compared to men, which may at least in part be attributed to the inflammation/immune cell status in WAT (15, 16).
Inflammation has been shown previously to be dependent on age, sex, and ethnicity (17–19). Sex-specific differences are seen in levels of circulating inflammatory cytokines like IL-6, TNFα and also C-reactive protein (CRP) (20) as well as in both arms of immunity (adaptive and non-adaptive) (19, 21). Furthermore, antigen presentation and type I interferon response by dendritic cells are higher in women than in men whereas the number of natural killer cells, pro-inflammatory cytokine production and expression of toll-like receptors are higher in males compared to females (22–25). Thus, sex-specific effects on immune responses may contribute to different susceptibility to cardio-metabolic conditions between men and women.
Variations in inflammatory response between men and women could at least partially depend on sex hormones. Both testosterone (TS) and estrogen have immunomodulatory effects (26–28) where TS has immunosuppressive potency (26–28). Abdominal subcutaneous (sc) and visceral (vis) WAT aromatise androstendione and TS to estrone (E1) and estradiol (E2), respectively (29). Therefore, the local conversion of sex hormones might impact WAT metabolism and inflammation.
Previous studies suggest that IL-10 may have a specific role in obesity/T2D. Both circulating and WAT-expressed IL-10 are upregulated by obesity in women (10, 11, 30) and WAT IL-10 is strongly linked to IR (30). Furthermore, in WAT, pro-inflammatory macrophages (CD14+CD206+CD11c+ cells), that have been shown to correlate with IR in human WAT (31), are the main producers of IL-10 (30). In addition, IL10 expression has been shown to be impacted by sex in different clinical contexts (17, 32, 33). Having in mind anti-inflammatory effect of IL-10 and better protection from obesity-associated metabolic complications in women compared to men, we aimed to compare the impact of obesity and T2D on WAT IL-10 levels in men versus women, intending to define the role of sex in IL-10 production under WAT metabolic challenge.
Materials and methods
Subjects
Cohort 1 consisted of non-obese (BMI<30) healthy, non-obese T2D and obese (BMI ≥30) T2D men and women. Clinical parameters of included individuals are shown in Table 1 . T2D patients were mainly treated by metformin; two men (one non-obese and one obese) and two women (one non-obese and one obese) were also treated with insulin. The diabetes duration was similar for men (10 ± 8 years) and women (11 ± 9 years). Homeostatic model assessment of insulin resistance (HOMA-IR) was used to measure insulin sensitivity and was calculated from the levels of fasting glucose and insulin (34). Fat cell volume (pL) was measured as described previously (35). In brief, the diameter of 100 cells was measured by light microscopy using a scale on the objective. The average fat cell volume was calculated using the formula (π/6) X (3σ2xd + d3) (where d is the mean diameter and σ is the standard deviation of the diameter), which considers the fact that the cube of the diameter (d3) is skewed. Cohort 1 was utilized for the analysis of IL-10 and TS secretion in WAT explants, WAT IL10 mRNA expression, IL-10-secreting cells by flow cytometry, and IL-10 in plasma. Cohort 2 has been described previously in (36). In brief, clinical characteristics of the individuals of cohort 2 (51 obese women and 19 obese men with and without T2D) are shown in Table 2 . IL-10 expression in visWAT and scWAT from these individuals was used for correlation analyses with HOMA-IR. Correlation analyses of IL10 mRNA expression with plasma sex hormone levels were performed in 22 women with obesity from cohort 3 (37). The clinical parameters of included individuals are listed in Table 3 . In addition, stromal vascular fraction obtained from scWAT of four healthy female individuals undergoing plastic surgery were used for the analysis of IL-10 expression upon hormone stimulation in vitro. Only age and BMI from these individuals were available to us (age 48 ± 10 years, BMI 31 ± 5).
Table 1.
Clinical parameters of cohort 1.
| Non-obese | Non-obese T2D | Obese T2D | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Women | Men | p-value | Women | Men | p-value | Women | Men | p-value | |
| Sample No | 23 | 22 | 12 | 30 | 9 | 12 | |||
| Age | 57 ± 7 (41 - 69) |
59 ± 7 (43 - 69) |
0.475 | 61 ± 4 (53 - 70) |
*57 - 64 (41 - 69) |
0.950 | 60 ± 5 (51 - 68) |
60 ± 4 (52 - 66) |
0.861 |
| BMI (kg/m2) | 25 ± 2 (22 - 29) |
25 ± 2 (22 - 29) |
0.652 | 26 ± 3 (23 - 30) |
26 ± 2 (23 - 30) |
0.999 | 33 ± 3 (30 - 40) |
*31 - 35 (30 - 43) |
0.986 |
| Fat cell volume (pL) | *407 - 542 (210 - 1295) |
542 ± 100 (368 - 770) |
0.104 | 601 ± 123 (419 - 793) |
551 ± 138 (336 - 900) |
0.290 | 715 ± 128 (526 - 919) |
773 ± 169 (531 -1062) |
0.407 |
| WHR | 0.85 ± 0.05 (0.77 - 0.99) | 0.95 ± 0.05 (0.84 - 1.04) | <0.001 | *0.93 - 1 (0.87 - 1.2) | 0.95 ± 0.04 (0.86 - 1.04) | 0.853 | 0.97 ± 0.03 (0.9 - 1.02) |
1.0 ± 0.04 (0.97 - 1.13) | 0.005 |
| TG (mmol/L) | 1.02 ± 0.33 (0.45 - 1.8) | *0.73 - 1.42 (0.55 - 3.4) | 0.438 | *0.83 - 2.6 (0.3 - 6.8) | *0.77 - 1.6 (0.33 - 3.6) | 0.436 | 1.16 ± 0.48 (0.44 - 2) | 1.7 ± 0.73 (0.73 - 3.1) | 0.041 |
| Fasting insulin (μIU/L) | 4.5 ± 1.2 (2.5 – 6.9) | *4.1 - 9.1 (2.4 - 14.2) |
0.524 | 9.18 ± 6 (0.9 - 19.7) |
*4.3 - 10.8 (2.1 - 24) |
0.007 | *5.2 - 18.3 (4.9 - 33.3) | *7 - 18.5 (1.1 - 67.1) |
0.574 |
| Fasting Glucose (mmol/L) | 5.3 ± 0.3 (4.7 - 5.9) | *5.3 - 5.6 (5.1 - 6.7) |
0.140 | *7.1 - 9.3 (4.9 - 20.2) |
7.4 ± 1.3 (5 - 10.8) |
0.108 | 7.3 ± 0.6 (6.2 - 8.3) |
*6.5 - 11.5 (6.2 - 13.6) |
0.875 |
| HbA1C (mmol/mol) | 36 ± 3 (28 - 44) |
34 ± 4 (24 - 44) |
0.119 | 57 ± 15 (42 - 84) |
47.6 ± 7.7 (34 - 65) |
0.006 | 46 ± 5 (39 - 55) |
55 ± 12 (43 - 76) |
0.037 |
| HOMA-IR | 1.1 ± 0.3 (0.5 - 1.6) |
1.6 ± 0.7 (0.5 - 3.2) |
0.007 | *1.1 – 4.5 (0.35 - 17.7) |
*1.7 - 3.3 (0.5 - 7.4) |
0.679 | 4.2. ± 3.2 (1.3 - 11.4) |
*2.1 – 6.2 (0.67 - 40.6) |
0.641 |
For normally distributed values, the mean ± SD with minimal and maximal values is shown. For non–normally distributed values (indicated with *), median–interquartile range with minimal and maximal values are indicated. Unpaired parametric (t-test) and non-parametric (Mann-Whitney)-tests were used to test the significance between men and women for normally and non-normally distributed (indicated with*) values, respectively. Significant values are indicated in bold.
Table 2.
Clinical parameters of cohort 2.
| Obese without T2D | Obese T2D | |||||
|---|---|---|---|---|---|---|
| Women | Men | p-value | Women | Men | p-value | |
| Sample No | 29 | 10 | 22 | 9 | ||
| Age (years) | 45 ± 11 (25 - 63) |
45 ± 12 (22 - 59) |
0.817 | *40 - 62 (31 - 65) |
55 ± 10 (39 - 67) |
0.401 |
| BMI (kg/m2) | 47 ± 5 (39 - 57) |
47 ± 7 (37 - 58) |
0.760 | 51 ± 7 (33 - 64) |
46 ± 8 (38 - 58) |
0.140 |
| TG (mmol/L) | 1.1 ± 0.4 (0.18 - 2.0) |
*0.9 -1.6 (0.8-5) |
0.273 | *1.3 - 2.3 (0.6 - 11.2) |
1.5 ± 0.6 (0.8 - 2.3) |
0.395 |
| Fasting insulin (μIU/L) | *11.1 - 27.6 (3.6 - 88.5) | 15.1 ± 8.2 (3.8 - 30.2) | 0.378 | *15.1 - 46.8 (2.7 - 98.9) | *7.6 - 19.9 (2.2 - 111.3) | 0.103 |
| Fasting glucose(mmol/L) | *4.7 - 5.6 (4.1 - 10.4) | 5.4 ± 0.8 (4.4 - 7.3) | 0.606 | 7.4 ± 1.9 (4.5 - 12.1) | *5.6 - 7.1 (5.2 - 13.4) | 0.287 |
| HbA1C (mmol/mol) | 35.1 ± 4.5 (27.9 - 47.7)) |
*35.5 - 39.4 (32.2 - 54.2) |
0.072 | *43.1 - 52.2 (34.5 - 86) |
47 ± 10 (38 - 64.4) |
0.402 |
| HOMA-IR | *2.10 - 6.1 (0.64 - 18.4) |
2.3 ± 1.4 (0.5 - 4.3) |
0.073 | *3.8 - 14 (0.6 - 30.5) |
*3.7 - 6.8 (2.6 - 58.4) |
0.321 |
For normally distributed values, the mean ± SD with minimal and maximal values is shown. For non–normally distributed values (indicated with *), median–interquartile range with minimal and maximal values is indicated. Unpaired parametric (t-test) and non-parametric (Mann-Whitney)-tests were used to test the significance between men and women for normally and non-normally distributed (indicated with*) values, respectively.
Table 3.
Clinical parameters of cohort 3.
| Factors | Values |
|---|---|
| Sample number | 22 |
| Age (years) | *32 - 41 (23 - 45) |
| BMI (kg/m2) | 37 ± 4 (31 - 49) |
| WHR | 0.94 ± 0.07 (1.1 - 0.78) |
| TG (mmol/L) | 1.5 - 0.7 (3.1 - 0.4) |
For normally distributed values, the mean ± SD with minimal and maximal values is shown. For non–normally distributed values (indicated with *), median–interquartile range with minimal and maximal values are indicated.
Secretion of cytokines and sex hormones
A venous blood sample for each subject was obtained for routine clinical measurements, an abdominal scWAT biopsy was taken, and explant incubations were performed as previously described (35). IL-10 was quantified in adipose tissue explant incubation media and in serum samples using V-plex Plus human IL-10 detection kit (Mesoscale Discovery, K151QU, Research Boulevard, Rockville, US). 50 μl of each sample was analyzed according to manufacturer’s instructions, and data were collected with SECTOR Imager 6000 and analyzed with MSD Discovery Workbench software. Results are expressed in pg/ml. Estrone (E1) and estradiol (E2) concentrations were measured by LC-MS/MS technology as previously described in detail (38). Limits of detection for E1 and E2 were 2.9 pmol/L and 4.0 pmol/L, respectively. Serum concentrations of follicle stimulating hormone (FSH), leutinising hormone (LH) and sex hormone binding globulin (SHBG) were determined using a time-resolved fluoroimmunoassay (Delfia, Wallac, Turku, Finland). Detection limits for LH and FSH were 0.005 IU/L.
Cell culture
Human monocyte cell line THP-1 (ATCC, Manassas, VA, USA) was cultured as recommended in ATCC protocols (39). 50 ng/ml PMA was added for 48 hours (Sigma-Aldrich, St.Louis, MO, USA P1585-1Mg) to differentiate monocytes to macrophages and then cells were treated with 50 ng/ml E1 (E-075, Sigma-Aldrich), 10 ng/ml TS (A8380, 5-alpha-Androstan-17beta-ol-3-one, Sigma-Aldrich) and 50ng/ml of E2 (E1024, Sigma-Aldrich) for 24 hours. The concentration was selected by titration (data not shown) and was comparable to the previously published in vitro studies (40). No toxic effects, measured as lactate dehydrogenase activity, were observed (data not shown). In parallel, cells were also treated with 20 ng/ml of TNFα (T6674, Sigma-Aldrich). Stromal vascular fraction (SVF) from human WAT was purified and frozen as previously described (35, 41). Frozen SVF was thawed, seeded at a density of 300,000 cells/well in 24-well plates and incubated for 24 hours in RPMI medium supplemented with 10% fetal calf serum (FCS; Gibco, Thermo Fisher Scientific, Waltham, MA, USA) and 2% penicillin-streptomycin (Gibco) at 37 °C followed by hormone stimulation as described for THP-1 cells.
RNA expression analysis
RNeasy Lipid Tissue Mini Kit (Qiagen, Cat.No.74804, Hilden, Germany), NucleoSpin RNA kit (Macherey-Nagel, Duren, Germany) and RNeasy Micro kit (Qiagen) were used for RNA extraction of lipid tissue, THP-1 and SVF, respectively. Concentration and purity of RNA were measured using a Nanodrop ND-1000 Spectrophotometer (Thermo Fisher Scientific). cDNA synthesis for THP-1 and SVF was performed using iScript cDNA synthesis kit (Bio-rad, Hercules, CA) and Super Script III (ThermoFisher Scientific) was used to reverse transcribe RNA from lipid tissue. Quantitative RT-PCR for IL-10 was performed using commercial TaqMan probes (Hs00961622_m1) at 60 °C (Thermo Fisher Scientific) on CFX96 Touch™ qPCR Detection System (Bio-Rad). Microarray profiling of scWAT from cohort 3 was performed using Affymetrix Clariom D platforms and analyzed as described in (37).
Intracellular staining and flow cytometry
Cryopreserved SVF was thawed, immediately washed with PBS/0.5% bovine serum albumin/2 mM EDTA buffer, filtered and seeded overnight in non-tissue culture treated 6-well plates in-order to avoid firm attachment of adherent cells to the plate. Brefeldin A (BD Biosciences, San Diego, CA, USA), a protein transport inhibitor, was used at 1:1000 for the last 3 hours of incubation to inhibit the release of cytokines. After two washes and filtering, cells were incubated with viability aqua dye for 30 minutes. The cells were then incubated with an antibody cocktail (42) for 30 minutes to stain for extracellular markers (BD Biosciences; Biolegend, San Diego, CA, USA; Thermo Fisher scientific). Research resource identifiers (RRIDs) of the antibodies are provided in the Supplementary Table S1 (42). Subsequently, cells were fixed with 4% PFA at 4 °C for 20 minutes and stored at 4°C using freshly prepared PBS/1% FCS buffer. Next day the cells were permeabilized using BD Perm/wash™ buffer (BD Biosciences) and stained with IL-10 antibody for 45 minutes at 4°C ( Supplementary Figure S1 ) (42). BD Fortessa equipped with 405 nm, 488 nm, 561 nm, and 640 nm lasers and with Diva software (BD Biosciences) was used to analyze all the samples. Fluorescence minus one (FMO) control was used for all the antibodies to set marker-positive cell populations. Data analysis was performed with FlowJo software (Tree Star, Ashland, OR, USA) where live/dead cells were first gated based on aqua staining, followed by gating for the single cells, and subsequently for monocytes/macrophages (CD45+CD14+). The M2 adipose tissue macrophages were defined as CD206+CD11c- and M1 as CD206+CD11c+. Relative number of IL-10-secreting macrophages was then determined in the latter population.
Statistical analysis
A statistical power calculation was made on available values for subcutaneous fat IL-10 secretion in 99 subjects including previously published cohort (n=80) (30) and additional cohort of 19 healthy individuals. According to calculations, 86 individuals were needed to detect 30% difference at alpha 0.05 and power 80%. Comparisons between the groups, correlations, linear regressions and graphical illustrations were done using GraphPad prism 7 (GraphPad Software, RRID: SCR_002798, La Jolla, CA). The Shapiro-Wilks test was used to determine the normal distribution of the variables, and logarithm transformation (log10) was performed for variables, such as IL-10, to achieve normal distribution. For normally distributed values, the mean ± SD with minimal and maximal values are indicated whereas median–interquartile range with minimal and maximal values are indicated for non–normally distributed values in the tables. Unpaired parametric (t-test) and non-parametric (Mann-Whitney)-tests were used to test the significance between two groups for normally and non-normally distributed values, respectively. Spearman correlation was used to evaluate association for non-normal distribution and Pearson correlation was used for all normally distributed factors. Data are presented as box plots in the graphs, showing minimum, first quartile, median, third quartile, and maximum values. Two-way ANOVA with Tukey’s and Sidak’s multiple comparisons were used in figures to compare metabolic groups of men and women and to compare the groups between men and women, respectively. A probability level of 0.05 was considered statistically significant.
Results
Participants
Clinical characteristics of cohort 1 are detailed in Table 1 . To determine the effect of BMI and T2D separately, 108 patients from this cohort were classified into the following three groups: non-obese healthy (45 subjects), non-obese with T2D (42 subjects) and obese with T2D (21 subjects). Men and women included in the study were of similar age and matched for BMI in the respective subgroups. Furthermore, fat cell volume and plasma glucose were similar in men and women belonging to the same metabolic group. HOMA-IR and waist-to-hip ratio (WHR) were significantly higher in non-obese healthy men compared to women, whereas, fasting insulin and haemoglobin A1c (HbA1c) was higher in non-obese T2D women compared to men. In addition, WHR, triglycerides (TG) and HbA1c were significantly higher in men with obesity and T2D compared to women.
IL-10 secretion and gene expression are regulated by obesity/T2D in women but not in men
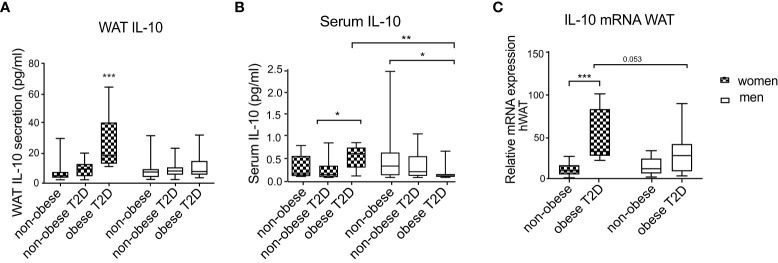
To determine whether IL-10 levels are influenced by BMI, T2D and sex, we examined WAT IL-10 secretion in cohort 1. While IL-10 secretion was similar in healthy non-obese men and women, it was significantly increased in women with obesity and T2D compared to controls, but this increase was absent in men ( Figure 1A ). IL-10 release was also significantly higher in women with obesity and T2D compared to men from the same metabolic subgroup ( Figure 1A ). Non-obese women and men with T2D showed similar levels of WAT IL-10 secretion and no upregulation in comparison to healthy controls ( Figure 1A ), indicating that this cytokine is not regulated by T2D alone. Furthermore, in men, all three groups had similar levels of IL-10 secretion. To evaluate if this local sex-specific IL-10 regulation is also reflected in circulating levels, we determined IL-10 levels in serum of the same patient cohort. In serum of healthy non-obese men and women, IL-10 levels were similar ( Figure 1B ). Circulating IL-10 levels of healthy women did not differ from the other female groups. However, women with obesity and T2D had significantly higher circulating IL-10 compared to non-obese women with T2D (p=0.022) ( Figure 1B ). In contrast, IL-10 was lower in serum of men with obesity and T2D compared to non-obese healthy men ( Figure 1B ). Women with obesity and T2D had significantly higher circulating IL-10 compared to men belonging to the same metabolic subgroup ( Figure 1B ). Correlations of IL-10 to the inflammatory cytokines didn’t show sex differences where IL-10 was correlating positively with inflammatory factors in WAT and negatively in serum ( Supplementary Tables S2 , S3 ).
Figure 1.
IL-10 secretion and mRNA expression in scWAT and serum of men and women. Three metabolic groups (non-obese healthy, non-obese T2D and obese T2D) of both women and men are represented by box plots. IL-10 secretion in WAT explants (A), circulating serum IL-10 (B) and IL10 mRNA expression in scWAT (C) are shown. Two-way ANOVA with Tukey’s and Sidak’s multiple comparisons were used in (A–C) to compare between the groups in men and women and to compare the groups between men and women, respectively. Patients from cohort 1 have been used for this analysis and number of individuals per group corresponds to Table 1 , *** <0.001, ** <0.01, * <0.05.
We further investigated if IL10 levels are regulated transcriptionally (i.e., mRNA) level in WAT. Since non-obese T2D individuals showed similar IL-10 secretion compared to the control group, in this analysis we included only men and women from the healthy non-obese and obese T2D groups. The mRNA expression measurements revealed that IL10 mRNA levels did not differ between healthy non-obese men and women. Similar to the secretion results, IL10 expression was upregulated in women with obesity and T2D compared to healthy controls, and this regulation was not observed in men ( Figure 1C ). The difference between IL10 expression in men and women with obesity and T2D had a borderline significance (p=0.053) ( Figure 1C ). Sex differences were further confirmed by positive correlations of WAT IL-10 production to the measures of glucose metabolism (plasma glucose, HOMA-IR and HbA1C) found in women, but not in men ( Supplementary Table S4 ).
It is widely accepted that in obesity, inflammation is even more pronounced in visceral fat. To further investigate whether this sex-specific regulation of WAT IL-10 is also seen in visWAT, we used another cohort of obese individuals (cohort 2) where visWAT samples were available. Because all individuals in this cohort were obese, we could not evaluate effect of obesity and T2D due to the lack of the control group. Therefore, we evaluated if visWAT IL-10 levels were associated with metabolic status of these obese individuals measured by HOMA-IR. In visWAT, IL10 did not correlate with HOMA-IR neither in men nor in women ( Supplementary Figure S2 ), whereas scWAT IL-10 had a trend towards a positive correlation (p=0.096) in women and but not in men ( Supplementary Figure S2 ) indicating that the sex specific regulation of IL-10 in obesity/T2D is only attributed to scWAT.
Women with obesity and T2D have higher numbers of IL-10-positive macrophages than men with obesity and T2D
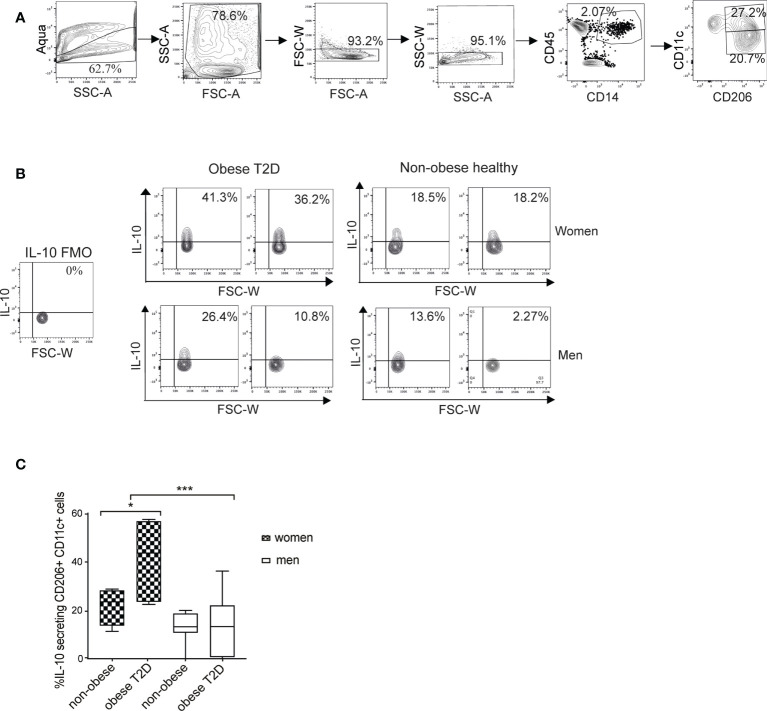
The M1 macrophages (CD11c+CD206+) are the major source of IL-10 in WAT (30). Therefore, we examined the possible difference in the amounts of IL-10-secreting cells between men and women. For this purpose, we established an intracellular staining protocol to detect cytokines in ex vivo WAT macrophages ( Supplementary Figure S1 ). To quantify IL-10-expressing cells, we used a gating strategy detailed in Figure 2A and in the material and methods. Data analysis visualized by representative images of the four groups ( Figure 2B ) and the compiled results ( Figure 2C ) demonstrated that healthy women and men showed no difference in the IL-10-secreting cell fraction while women with obesity and T2D had significantly higher proportion of IL-10-secreting macrophages compared to all other examined groups ( Figure 2C ). In men, the amounts of IL-10-secreting cells were not different in the healthy non-obese compared to individuals with obesity and T2D.
Figure 2.
Amount of IL-10 secreting macrophages is increased in scWAT of women with obesity and T2D but not in men. (A) A flow cytometry gating strategy. Selection of alive single cells (four panels on the left), macrophage/monocyte (CD45+/CD14+) and M1 macrophage (CD45+/CD14+/CD206+/CD11c+) selection (two right-side panels). (B) The M1 macrophage population was then analyzed for IL-10 secretion. Representative contour plots from two individuals/group are shown for obese T2D and non-obese healthy women and men. FMO control samples are shown on the left side. (C) Compiled results of IL-10-secreting macrophage populations for non-obese healthy (n=8 for both men and women) and obese T2D (n=7 for women, n=8 for men) men and women from cohort 1. Two-way ANOVA with Tukey’s and Sidak’s multiple comparisons were used in (C) to compare between the groups in men and women and to compare the groups between men and women respectively. *** <0.001, * <0.05.
IL10 expression correlates with estrone secretion
We next examined if the sex-specific differences in IL-10 regulation by obesity and T2D associated with the levels of circulating hormones linked to the gonadotropin axis (FSH, LH, SHBG, E1 and E2). For this, we used available samples from cohort 3 (37). We found weak but significant correlations of E1 (r=0.221 and p=0.027) and free E1 (r=0.216 and p=0.029) with WAT IL10 expression ( Table 4 ). All the patients in this cohort were obese and there was no correlation between IL-10 and BMI (p=0.139). Therefore, the IL-10/E1 correlation was not driven by BMI in this cohort.
Table 4.
Correlations of serum hormones with human WAT IL10 mRNA expression in cohort 3 (n=22).
| Linear regression | ||
|---|---|---|
| Hormones | r-value | p-value |
| E1 | 0.221 | 0.027 |
| E2 | 0.053 | 0.303 |
| FSH | < 0.001 | 0.923 |
| LH | 0.142 | 0.084 |
| SHBG | 0.034 | 0.413 |
| Free E1 | 0.216 | 0.029 |
| Free E2 | 0.059 | 0.275 |
p- and r- values of Pearson correlations are indicated. Significant values are indicated in bold.
Recombinant E1 does not upregulate IL10 expression in vitro
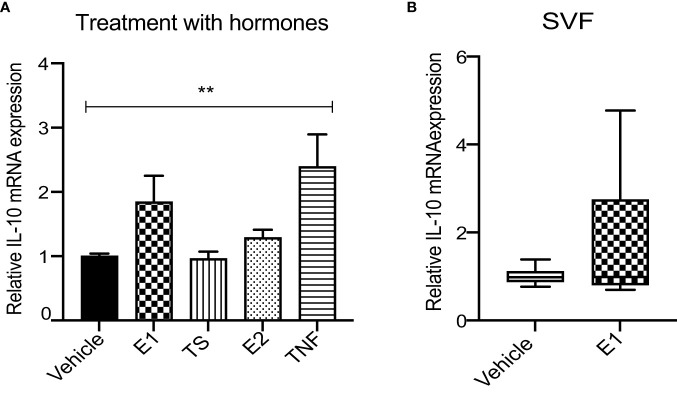
Finally, to investigate whether the observed correlation between circulating E1 and WAT IL10 expression could reflect causality, we examined the effect of recombinant human E1 (rhE1) on IL10 expression in vitro. Differentiated THP-1 cells were stimulated by rhE1, rhE2, TS or TNFα (as a positive control) and IL10 expression was measured. The results demonstrated no significant effect of rhE1 or any other tested hormone on IL10 mRNA levels ( Figure 3A ). In addition, no effect of rhE1 was observed on primary WAT SVF cells ( Figure 3B ).
Figure 3.
E1 has no effect on IL-10 expression neither in in vitro stimulated THP-1 macrophages nor in primary WAT SVF. (A) Relative IL10 mRNA expression in E1, TS, E2 and TNFα-treated THP-1 macrophages. Two-way ANOVA with Sidak’s multiple comparison was used to test the significance. ** <0.01 (n=4). (B) Relative IL10 mRNA in E1-stimulated WAT SVF; (Mann-whitney test, n=4 subjects in triplicates).
Discussion
In this study, we demonstrate that although WAT IL-10 levels are similar in non-obese men and women, T2D in combination with obesity is linked to a marked increase in IL-10 in scWAT of women, but not men. Furthermore, numbers of IL-10-secreting macrophages are increased in WAT of women with obesity and T2D, but not in men. Together, the findings suggest a sex-specific regulation of this cytokine upon WAT mass expansion. In contrast, circulating IL-10 levels were mostly affected (reduced) in men with obesity and T2D. This indicates that obesity/T2D has different impacts on circulating and WAT IL-10. Although WAT IL10 mRNA significantly correlates with circulating E1, we could not establish a direct effect of this hormone on IL10 expression using in vitro cell models.
As discussed earlier, sexual dimorphism in regulation of pro- and anti-inflammatory cytokines is observed in humans and animal models (20, 43–45). For example, female mice show higher periodontal bone loss than male mice, which is associated with higher inflammatory cytokines (IL-6, IL-1b and IL-17a) production (44). On the other hand, young female mice are protected against osteoarthritis and bone degeneration, which associates with higher levels of TGFb1 and IL-4 (45). It is also widely accepted that the pathogen (human immune deficiency virus, coxsachievirus, respiratory virus etc)-induced inflammation is sex dependent in both mouse and humans where males are more susceptible than females (46–48).
IL-10 has been shown to have a gender-specific regulation by several earlier studies not related to adipose tissue. IL-10 is significantly higher upregulated in men compared to women when exposed to endotoxin (17). Depression-related symptoms were shown to be associated with low circulating IL-10 levels in women but not in men (32). In addition, female IL-10 knockout mice are more prone to Pseudomonas aerigonas infection than male (33). Therefore, it is likely that IL-10 production is associated with sex-specific factors that are at present unknown.
Although sex hormones are mostly produced by ovaries and testis, they can also be aromatized in peripheral tissues like adipose, breast and brain tissues (49–51). Furthermore, their influence on cytokine and chemokine secretion is established (52, 53). For example, in vivo treatment of mice with estradiol has been shown to increase insulin sensitivity and decrease basal lipolysis in WAT, which was connected to lower levels of TNFα and IL-6 in serum (43). In this study, correlation analysis of sex hormones and WAT IL10 expression showed that only E1 was associated with increased IL-10 levels. Adipose tissue is a major site of aromatization of E1, the main circulating estrogen form in post-menopausal women (29). Furthermore, the production of circulating E1 is increased in obesity due to increased aromatization in adipose tissue (54). E1 treatment significantly increases IL-10 secretion in T cells (40) and increased circulating IL-10 levels are observed in post-menopausal compared to pre-menopausal women (55). Although we did not observe a direct effect of E1 or any other tested sex hormone on IL10 expression in WAT SVF or THP-1 macrophages in vitro, we cannot exclude that sex hormones (and E1 in particular) are playing a role in obesity-related IL-10 upregulation in women in vivo.
Our results suggest that sex-specific IL-10 regulation by obesity/T2D is mediated at the transcriptional level because mRNA expression was found to be increased in parallel to secretion. This induction occurs in the M1-like macrophage population in scWAT, but only in women with obesity and T2D. However, at present we do not know the molecular mechanism of this upregulation and which factor increasing IL-10 production is absent in men with obesity and T2D. Therefore, additional studies in larger cohorts and WAT-derived macrophages obtained from men are required. On the other hand, such cohorts with large adipose tissue samples obtained from men are difficult to access. For the same reason, it is not feasible to study possible sex differences for WAT IL-10 in lean (BMI<25) patients with T2D. For ethical reasons, visceral depot cannot be investigated in a clinical setting like cohort 1. We have previously shown that IL-10 production is positively correlated to HOMA-IR in a cohort of women with broad range of BMI. Even in a cohort of obese only women (cohort 2 in this study), IL-10 had a clear tendency for correlation to HOMA-IR. However, such association was completely absent in visceral fat of the same women cohort and in men (both scWAT and visWAT). Therefore, we suggest that IL-10 regulation in obesity is specific to female scWAT.
Several measures of glucose metabolism were found to be different in men versus women in specific metabolic groups. For example, heathy men had significantly higher HOMA-IR than women and obese T2D men had higher HbA1C than women in the same metabolic group. On the other hand, non-obese T2D women had higher insulin and HbA1C compared to men. Therefore, we cannot fully exclude those differences in glucose metabolism in itself could have affected IL-10 regulation presented in Figure 1 . The fact that measures of glucose metabolism were not matched between sexes, can be considered as a limitation of this study.
Although the associations of IL-10 with obesity and metabolic complications have been studied previously (10, 30, 56–58), the regulation of IL-10 in different sexes has, to the best of our knowledge, not been addressed. Few IL-10 studies that included both genders (adolescent and young men and women) (57, 58) did not specifically examine the gender effect on IL-10 associations with metabolic abnormalities. We show that WAT and serum IL-10 levels of healthy men and women are the same, but a gender-specific effect is observed in connection to obesity. In murine models, Gotoh et al., demonstrated that high fat diet-induced obesity decreased serum IL-10 levels in male mice (59), which is similar to our current finding in men with obesity and T2D. The same study also suggested that the majority of IL-10 produced in the body is coming from spleen and this may be one of the reasons that the WAT-specific upregulation of IL-10 observed in women with obesity and T2D was not observed in circulation (59–61).
In summary, there is a sex-specific obesity/T2D regulation of the anti-inflammatory cytokine IL-10 in scWAT and in serum. We propose that the scWAT IL-10 might exert protective effects in obesity-associated chronic inflammation but that this mechanism is absent in men. However, the molecular mechanisms for such sex-specific regulation remain to be revealed.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Regional ethics board of the Stockholm County Council. The patients/participants provided their written informed consent to participate in this study.
Author contributions
NS, BT, PA and JL designed a study, NS, BT, KH, BG, LM, JA, AJ, MR performed data collection and analysis, as well as participated in discussions of the results, NS and JL drafted the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Swedish research council (2016-00694), Novo Nordisk Foundation including NNF210C0069989, the Tripartite Immuno-metabolism Consortium (TrIC) Grant Number NNF15CC0018486 and the MSAM consortium NNF15SA0018346 and the Strategic Research Programme in Diabetes at Karolinska Institutet, KID grant at Karolinska Institutet.
Acknowledgments
The technical assistance of Gaby Åström, Elisabeth Dungner, Kerstin Wåhlén, Yvonne Widlund and Katarina Hertel (Dept. of Medicine Huddinge, Karolinska Institutet, Sweden) is greatly appreciated. Cell sorting was performed at MedH Flow Cytometry Core Facility.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.996954/full#supplementary-material
References
- 1. Zatterale F, Longo M, Naderi J, Raciti GA, Desiderio A, Miele C, et al. Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Front Physiol (2019) 10:1607. doi: 10.3389/fphys.2019.01607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med (2009) 15(8):914–20. doi: 10.1038/nm.1964 [DOI] [PubMed] [Google Scholar]
- 3. Bertola A, Ciucci T, Rousseau D, Bourlier V, Duffaut C, Bonnafous S, et al. Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients. Diabetes (2012) 61(9):2238–47. doi: 10.2337/db11-1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab (2008) 8(4):301–9. doi: 10.1016/j.cmet.2008.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. DeFuria J, Belkina AC, Jagannathan-Bogdan M, Snyder-Cappione J, Carr JD, Nersesova YR, et al. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc Natl Acad Sci U S A (2013) 110(13):5133–8. doi: 10.1073/pnas.1215840110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arner E, Mejhert N, Kulyte A, Balwierz PJ, Pachkov M, Cormont M, et al. Adipose tissue microRNAs as regulators of CCL2 production in human obesity. Diabetes (2012) 61(8):1986–93. doi: 10.2337/db11-1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Caer C, Rouault C, Le Roy T, Poitou C, Aron-Wisnewsky J, Torcivia A, et al. Immune cell-derived cytokines contribute to obesity-related inflammation, fibrogenesis and metabolic deregulation in human adipose tissue. Sci Rep (2017) 7(1):3000. doi: 10.1038/s41598-017-02660-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest (1995) 95(5):2409–15. doi: 10.1172/JCI117936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bastard JP, Jardel C, Bruckert E, Blondy P, Capeau J, Laville M, et al. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J Clin Endocrinol Metab (2000) 85(9):3338–42. doi: 10.1210/jcem.85.9.6839 [DOI] [PubMed] [Google Scholar]
- 10. Esposito K, Pontillo A, Giugliano F, Giugliano G, Marfella R, Nicoletti G, et al. Association of low interleukin-10 levels with the metabolic syndrome in obese women. J Clin Endocrinol Metab (2003) 88(3):1055–8. doi: 10.1210/jc.2002-021437 [DOI] [PubMed] [Google Scholar]
- 11. Juge-Aubry CE, Somm E, Pernin A, Alizadeh N, Giusti V, Dayer JM, et al. Adipose tissue is a regulated source of interleukin-10. Cytokine (2005) 29(6):270–4. doi: 10.1016/j.cyto.2004.10.017 [DOI] [PubMed] [Google Scholar]
- 12. Hu G, Jousilahti P, Qiao Q, Katoh S, Tuomilehto J. Sex differences in cardiovascular and total mortality among diabetic and non-diabetic individuals with or without history of myocardial infarction. Diabetologia (2005) 48(5):856–61. doi: 10.1007/s00125-005-1730-6 [DOI] [PubMed] [Google Scholar]
- 13. Camhi SM, Bray GA, Bouchard C, Greenway FL, Johnson WD, Newton RL, et al. The relationship of waist circumference and BMI to visceral, subcutaneous, and total body fat: Sex and race differences. Obes (Silver Spring) (2011) 19(2):402–8. doi: 10.1038/oby.2010.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jackson AS, Stanforth PR, Gagnon J, Rankinen T, Leon AS, Rao DC, et al. The effect of sex, age and race on estimating percentage body fat from body mass index: The heritage family study. Int J Obes Relat Metab Disord (2002) 26(6):789–96. doi: 10.1038/sj.ijo.0802006 [DOI] [PubMed] [Google Scholar]
- 15. Pettersson US, Walden TB, Carlsson PO, Jansson L, Phillipson M. Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PloS One (2012) 7(9):e46057. doi: 10.1371/journal.pone.0046057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nordstrom A, Hadrevi J, Olsson T, Franks PW, Nordstrom P. Higher prevalence of type 2 diabetes in men than in women is associated with differences in visceral fat mass. J Clin Endocrinol Metab (2016) 101(10):3740–6. doi: 10.1210/jc.2016-1915 [DOI] [PubMed] [Google Scholar]
- 17. Engler H, Benson S, Wegner A, Spreitzer I, Schedlowski M, Elsenbruch S. Men and women differ in inflammatory and neuroendocrine responses to endotoxin but not in the severity of sickness symptoms. Brain Behav Immun (2016) 52:18–26. doi: 10.1016/j.bbi.2015.08.013 [DOI] [PubMed] [Google Scholar]
- 18. Paalani M, Lee JW, Haddad E, Tonstad S. Determinants of inflammatory markers in a bi-ethnic population. Ethn Dis (2011) 21(2):142–9. [PMC free article] [PubMed] [Google Scholar]
- 19. Hirokawa K, Utsuyama M, Hayashi Y, Kitagawa M, Makinodan T, Fulop T. Slower immune system aging in women versus men in the Japanese population. Immun Ageing (2013) 10(1):19. doi: 10.1186/1742-4933-10-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cartier A, Cote M, Lemieux I, Perusse L, Tremblay A, Bouchard C, et al. Sex differences in inflammatory markers: What is the contribution of visceral adiposity? Am J Clin Nutr (2009) 89(5):1307–14. doi: 10.3945/ajcn.2008.27030 [DOI] [PubMed] [Google Scholar]
- 21. Gal-Oz ST, Maier B, Yoshida H, Seddu K, Elbaz N, Czysz C, et al. ImmGen report: Sexual dimorphism in the immune system transcriptome. Nat Commun (2019) 10(1):4295. doi: 10.1038/s41467-019-12348-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marriott I, Bost KL, Huet-Hudson YM. Sexual dimorphism in expression of receptors for bacterial lipopolysaccharides in murine macrophages: A possible mechanism for gender-based differences in endotoxic shock susceptibility. J Reprod Immunol (2006) 71(1):12–27. doi: 10.1016/j.jri.2006.01.004 [DOI] [PubMed] [Google Scholar]
- 23. Marriott I, Huet-Hudson YM. Sexual dimorphism in innate immune responses to infectious organisms. Immunol Res (2006) 34(3):177–92. doi: 10.1385/IR:34:3:177 [DOI] [PubMed] [Google Scholar]
- 24. Weinstein Y, Ran S, Segal S. Sex-associated differences in the regulation of immune responses controlled by the MHC of the mouse. J Immunol (1984) 132(2):656–61. [PubMed] [Google Scholar]
- 25. Phan MT, Chun S, Kim SH, Ali AK, Lee SH, Kim S, et al. Natural killer cell subsets and receptor expression in peripheral blood mononuclear cells of a healthy Korean population: Reference range, influence of age and sex, and correlation between NK cell receptors and cytotoxicity. Hum Immunol (2017) 78(2):103–12. doi: 10.1016/j.humimm.2016.11.006 [DOI] [PubMed] [Google Scholar]
- 26. Furman D, Hejblum BP, Simon N, Jojic V, Dekker CL, Thiebaut R, et al. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci U S A (2014) 111(2):869–74. doi: 10.1073/pnas.1321060111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miyagi M, Aoyama H, Morishita M, Iwamoto Y. Effects of sex hormones on chemotaxis of human peripheral polymorphonuclear leukocytes and monocytes. J Periodontol (1992) 63(1):28–32. doi: 10.1902/jop.1992.63.1.28 [DOI] [PubMed] [Google Scholar]
- 28. Cassidy RA. Influence of steroids on oxidant generation in activated human granulocytes and mononuclear leukocytes. Shock (2003) 20(1):85–90. doi: 10.1097/01.shk.0000070740.34700.cd [DOI] [PubMed] [Google Scholar]
- 29. Hetemaki N, Savolainen-Peltonen H, Tikkanen MJ, Wang F, Paatela H, Hamalainen E, et al. Estrogen metabolism in abdominal subcutaneous and visceral adipose tissue in postmenopausal women. J Clin Endocrinol Metab (2017) 102(12):4588–95. doi: 10.1210/jc.2017-01474 [DOI] [PubMed] [Google Scholar]
- 30. Acosta JR, Tavira B, Douagi I, Kulyte A, Arner P, Ryden M, et al. Human-specific function of IL-10 in adipose tissue linked to insulin resistance. J Clin Endocrinol Metab (2019) 104(10):4552–62. doi: 10.1210/jc.2019-00341 [DOI] [PubMed] [Google Scholar]
- 31. Wentworth JM, Naselli G, Brown WA, Doyle L, Phipson B, Smyth GK, et al. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes (2010) 59(7):1648–56. doi: 10.2337/db09-0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Majd M, Graham-Engeland JE, Smyth JM, Sliwinski MJ, Lipton RB, Katz MJ, et al. Distinct inflammatory response patterns are evident among men and women with higher depressive symptoms. Physiol Behav (2018) 184:108–15. doi: 10.1016/j.physbeh.2017.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guilbault C, Stotland P, Lachance C, Tam M, Keller A, Thompson-Snipes L, et al. Influence of gender and interleukin-10 deficiency on the inflammatory response during lung infection with pseudomonas aeruginosa in mice. Immunology (2002) 107(3):297–305. doi: 10.1046/j.1365-2567.2002.01508.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia (1985) 28(7):412–9. doi: 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 35. Acosta JR, Douagi I, Andersson DP, Backdahl J, Ryden M, Arner P, et al. Increased fat cell size: A major phenotype of subcutaneous white adipose tissue in non-obese individuals with type 2 diabetes. Diabetologia (2016) 59(3):560–70. doi: 10.1007/s00125-015-3810-6 [DOI] [PubMed] [Google Scholar]
- 36. Krieg L, Didt K, Karkossa I, Bernhart SH, Kehr S, Subramanian N, et al. Multiomics reveal unique signatures of human epiploic adipose tissue related to systemic insulin resistance. Gut (2021) 0:1–15. doi: 10.1136/gutjnl-2021-324603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kwok KHM, Ryden M, Andersson DP, Beauchef G, Guere C, Vie K, et al. Prospective analyses of white adipose tissue gene expression in relation to long-term body weight changes. Int J Obes (Lond) (2020) 44(2):377–87. doi: 10.1038/s41366-019-0385-1 [DOI] [PubMed] [Google Scholar]
- 38. Frederiksen H, Johannsen TH, Andersen SE, Albrethsen J, Landersoe SK, Petersen JH, et al. Sex-specific estrogen levels and reference intervals from infancy to late adulthood determined by LC-MS/MS. J Clin Endocrinol Metab (2020) 105(3):754–68. doi: 10.1210/clinem/dgz196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pettersson AM, Acosta JR, Bjork C, Kratzel J, Stenson B, Blomqvist L, et al. MAFB as a novel regulator of human adipose tissue inflammation. Diabetologia (2015) 58(9):2115–23. doi: 10.1007/s00125-015-3673-x [DOI] [PubMed] [Google Scholar]
- 40. Correale J, Arias M, Gilmore W. Steroid hormone regulation of cytokine secretion by proteolipid protein-specific CD4+ T cell clones isolated from multiple sclerosis patients and normal control subjects. J Immunol (1998) 161(7):3365–74. [PubMed] [Google Scholar]
- 41. van Harmelen V, Skurk T, Hauner H. Primary culture and differentiation of human adipocyte precursor cells. Methods Mol Med (2005) 107:125–35. doi: 10.1385/1-59259-861-7 [DOI] [PubMed] [Google Scholar]
- 42. Subramanian N, Hofwimmer K, Abildgaard J, Juul A, Rydén M, Arner P, et al. (2022). Supplementary table and figure. Figshare.
- 43. Camporez JP, Lyu K, Goldberg EL, Zhang D, Cline GW, Jurczak MJ, et al. Anti-inflammatory effects of oestrogen mediate the sexual dimorphic response to lipid-induced insulin resistance. J Physiol (2019) 597(15):3885–903. doi: 10.1113/JP277270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Duan X, Gleason RC, Li F, Hosur KB, Duan X, Huang D, et al. Sex dimorphism in periodontitis in animal models. J Periodontal Res (2016) 51(2):196–202. doi: 10.1111/jre.12298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mahr S, Menard J, Krenn V, Muller B. Sexual dimorphism in the osteoarthritis of STR/ort mice may be linked to articular cytokines. Ann Rheum Dis (2003) 62(12):1234–7. doi: 10.1136/ard.2002.005041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chamekh M, Deny M, Romano M, Lefevre N, Corazza F, Duchateau J, et al. Differential susceptibility to infectious respiratory diseases between males and females linked to sex-specific innate immune inflammatory response. Front Immunol (2017) 8:1806. doi: 10.3389/fimmu.2017.01806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Koenig A, Buskiewicz I, Huber SA. Age-associated changes in estrogen receptor ratios correlate with increased female susceptibility to coxsackievirus B3-induced myocarditis. Front Immunol (2017) 8:1585. doi: 10.3389/fimmu.2017.01585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ziegler SM, Altfeld M. Human immunodeficiency virus 1 and type I interferons-where sex makes a difference. Front Immunol (2017) 8:1224. doi: 10.3389/fimmu.2017.01224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nelson LR, Bulun SE. Estrogen production and action. J Am Acad Dermatol (2001) 45(3 Suppl):S116–24. doi: 10.1067/mjd.2001.117432 [DOI] [PubMed] [Google Scholar]
- 50. Simpson ER. Sources of estrogen and their importance. J Steroid Biochem Mol Biol (2003) 86(3-5):225–30. doi: 10.1016/S0960-0760(03)00360-1 [DOI] [PubMed] [Google Scholar]
- 51. Kato J, Yamada-Mouri N, Hirata S. Structure of aromatase mRNA in the rat brain. J Steroid Biochem Mol Biol (1997) 61(3-6):381–5. doi: 10.1016/S0960-0760(97)80036-2 [DOI] [PubMed] [Google Scholar]
- 52. Verthelyi D, Klinman DM. Sex hormone levels correlate with the activity of cytokine-secreting cells in vivo. Immunology (2000) 100(3):384–90. doi: 10.1046/j.1365-2567.2000.00047.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev (2013) 34(3):309–38. doi: 10.1210/er.2012-1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kley HK, Deselaers T, Peerenboom H, Kruskemper HL. Enhanced conversion of androstenedione to estrogens in obese males. J Clin Endocrinol Metab (1980) 51(5):1128–32. doi: 10.1210/jcem-51-5-1128 [DOI] [PubMed] [Google Scholar]
- 55. Jgarkava M, Pantsulaia I, Rukhadze R, Karanadze N, Chikovani T. Association of il-10 and resistin in apparently healthy elderly population. Georgian Med News (2021) 318:119–24. [PubMed] [Google Scholar]
- 56. van Exel E, Gussekloo J, de Craen AJ, Frolich M, Bootsma-Van Der Wiel A, Westendorp RG, et al. Low production capacity of interleukin-10 associates with the metabolic syndrome and type 2 diabetes: The Leiden 85-plus study. Diabetes (2002) 51(4):1088–92. doi: 10.2337/diabetes.51.4.1088 [DOI] [PubMed] [Google Scholar]
- 57. Straczkowski M, Kowalska I, Nikolajuk A, Krukowska A, Gorska M. Plasma interleukin-10 concentration is positively related to insulin sensitivity in young healthy individuals. Diabetes Care (2005) 28(8):2036–7. doi: 10.2337/diacare.28.8.2036 [DOI] [PubMed] [Google Scholar]
- 58. Chang JS, Chang CC, Chien E, Lin SS, Cheng-Shiuan T, Bai CH, et al. Association between interleukin 1beta and interleukin 10 concentrations: A cross-sectional study in young adolescents in Taiwan. BMC Pediatr (2013) 13:123. doi: 10.1186/1471-2431-13-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gotoh K, Inoue M, Masaki T, Chiba S, Shimasaki T, Ando H, et al. A novel anti-inflammatory role for spleen-derived interleukin-10 in obesity-induced hypothalamic inflammation. J Neurochem (2012) 120(5):752–64. doi: 10.1111/j.1471-4159.2011.07617.x [DOI] [PubMed] [Google Scholar]
- 60. Gotoh K, Inoue M, Masaki T, Chiba S, Shiraishi K, Shimasaki T, et al. Obesity-related chronic kidney disease is associated with spleen-derived IL-10. Nephrol Dial Transplant (2013) 28(5):1120–30. doi: 10.1093/ndt/gfs440 [DOI] [PubMed] [Google Scholar]
- 61. Gotoh K, Inoue M, Shiraishi K, Masaki T, Chiba S, Mitsutomi K, et al. Spleen-derived interleukin-10 downregulates the severity of high-fat diet-induced non-alcoholic fatty pancreas disease. PloS One (2012) 7(12):e53154. doi: 10.1371/journal.pone.0053154 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.