Abstract
Objective
To investigate individual and interactive associations of baseline serum leptin levels and physical comorbidity with short- and long-term treatment outcomes in outpatients with depressive disorders who received stepwise antidepressant treatment in a naturalistic prospective study design.
Methods
Baseline serum leptin levels were measured, and the number of concurrent physical disorders ascertained from 1,094 patients. These patients received initial antidepressant monotherapy; then, for patients with an insufficient response or who experienced uncomfortable side effects, treatment was administered using alternative strategies every 3 weeks in the acute treatment phase (at 3, 6, 9, and 12 weeks) and every 3 months in the continuation treatment phase (at 6, 9, and 12 months). Then, 12-week and 12-month remission, defined as a Hamilton Depression Rating Scale score of ≤7, was estimated.
Results
In multivariable logistic regression analyses, individual effects were found only between higher baseline serum leptin levels and 12-week non-remission. Significant interactive effects between higher leptin levels and fewer physical disorders (< 2 physical disorders) on 12-week non-remission were observed. However, neither individual nor interactive effects between leptin levels and physical comorbidity were associated with 12-month remission.
Conclusion
The combination of serum leptin level and number of physical disorders may be a useful predictor of short-term treatment responses in patients with depressive disorders receiving pharmacotherapy.
Keywords: Depression, Treatment outcome, Leptin, Comorbidity, Antidepressant.
INTRODUCTION
Depressive disorders are a major cause of disability, with a prevalence of more than 264 million people globally [1,2]. Antidepressants are the first-line treatment for moderate to severe major depressive disorder [3]. How-ever, currently used antidepressants lead to remission in less than one-third of patients after 8−12 weeks in the first trial [3-5]. Currently, the selection of antidepressants is based on the subjective assessment of symptoms. Peripheral biomarkers could help overcome this limitation by identifying homogenous groups of patients who will benefit most from a particular treatment. Therefore, it is necessary to discover biomarkers that predict antidepressant treatment outcomes to aid selecting personalized treatment strategies for depressive disorders.
Leptin, an adipocyte-derived hormone that can cross the blood-brain barrier (BBB), exerts its biological function by binding to a leptin receptor (LepRb) in the central nervous system (CNS) [6-8]. Previous animal studies reported that leptin levels were decreased in chronic stress mouse models [9,10], and ob/ob mice, which cannot produce leptin due to a mutation in the ob gene, exhibited severe depressive behavior in a forced swimming test [11]. Moreover, several animal studies reported the antidepressant effect of leptin, and LepRb signaling in the CNS, rather than leptin concentration in the periphery, is considered an essential factor determining this effect [9,11-13]. It has been suggested that leptin exerts antidepressant effects by influencing monoamine neurotrans-mission, neurotrophic effect, and hypothalamus-pituitary- adrenal (HPA) axis [9,14-18]. However, clinical studies focused on the effects of peripheral leptin levels have found conflicting results with respect to depression risk and treatment responses. Some studies have reported that leptin levels were lower in patients with depression [19-21], whereas others have found that leptin levels were higher in such patients [22-24]. Moreover, previous studies reported inconsistent results regarding associations between peripheral leptin levels and depression treatment outcomes. Two studies have reported that higher leptin levels were associated with a better treatment response in initial antidepressant trials [25,26]. Among studies conducted on patients with treatment resistance to initial antidepressant treatment, some reported that higher leptin levels were associated with a better treatment response [27], whereas others found a worse treatment response [27] or no significant association [28]. Association between higher leptin levels and lower depression risk or better treatment response was explained by the antidepressant effect of leptin through LepRb signaling in the CNS [9]. On the other hand, association between higher leptin levels and higher depression risk or worse treatment response was explained by leptin resistance, which interprets increased peripheral leptin levels as a surrogate for decreased central LepRb signaling [29].
Physical comorbidities are frequently found in patients with depressive disorders [30]. Depression comorbid with physical disorders is reportedly associated with worse treatment outcomes [4,31-34]. In addition, leptin levels are elevated in many physical disorders [35-40]. Because physical comorbidities are closely related to both leptin levels and depression, it could be postulated that a potentially complex relationship between leptin levels and physical comorbidities may exist in the depression treatment outcomes. However, this has not been studied to date. Despite the close link of physical comorbidities with leptin and depression, previous studies did not consider its influence on the association between leptin levels and future antidepressant treatment outcomes. This may be the cause of inconsistent results regarding the association between peripheral leptin levels and antidepressant treatment outcomes in previous clinical studies.
Using data from a prospective study of Korean patients with depressive disorders receiving stepwise antidepressant treatments, we investigated the individual and interactive effects of baseline leptin levels and physical comorbidities on short- and long-term remission.
METHODS
Study Outline
This study was carried out as a component of the MAKE Biomarker discovery for Enhancing antidepressant Treat-ment Effect and Response (MAKE BETTER) program. Details of the study have been published as a design paper [41] and registered with cris.nih.go.kr (identifier: KCT0001332). Data on socio-demographic and clinical characteristics were obtained using a structured clinical report form (CRF) by clinical research coordinators, who were blind to treatment modalities. They were trained in CRF implementation and data collection methods by the research psychiatrists. Patients’ data were recorded on a CRF, registered via the website of the MAKE BETTER study (http://icreat.nih.go.kr/icreat/webapps/com/hismainweb/jsp/cdc_n2.live ) within 3 days, and monitored by data management center personnel. This study was approved by the Chonnam National University Hospital Institutional Review Board (CNUH 2012-014).
Participants
Patients with depressive disorders were recruited from March 2012 to April 2017 who had attended the outpatient psychiatric department of Chonnam National University Hospital. All inclusion instances represented new treatment episodes, i.e., newly initiated antidepressant treatment, regardless of whether depressive symptoms were first onset or recurrent. Research psychiatrists assessed and diagnosed depressive disorders using the Mini-International Neuropsychiatric Interview [42], a structured diagnostic psychiatric interview based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria. As the aim of the study was to reflect the real-world clinical setting as closely as possible, broad inclusion criteria and minimal exclusion criteria were applied. Inclusion criteria were: i) aged older than 7 years; ii) diagnosed with MDD, dysthymic disorder, or depressive disorder not otherwise specified (NOS); iii) Hamilton Depression Rating Scale (HAMD) score ≥ 14 [43]; iv) able to complete questionnaires, understand the objective of the study, and sign the informed consent form. Exclusion criteria were as follows: i) unstable or uncontrolled medical condition; ii) unable to complete the psychiatric assessment or comply with the medication regimen, due to a severe physical illness; iii) current or lifetime DSM-IV diagnosis of bipolar disorder, schizophrenia, schizoaffective disorder, schizophreniform disorder, psychotic disorder NOS, or other psychotic disorder; iv) history of organic psychosis, epilepsy, or seizure disorder; v) history of anticonvulsant treatment; vi) hospitalization for any psychiatric diagnosis apart from depressive disorder (e.g., alcohol/drug dependence); vii) electroconvulsive therapy received for the current depressive episode; viii) pregnant or breastfeeding. All participants reviewed the consent form and written informed consent was obtained. For participants aged under 16, written consent was obtained from a parent or legal guardian, and written assent was obtained from the participant.
Primary Measures
Serum leptin
Participants were instructed to fast (except water) starting the previous night for blood sampling. They were to sit quietly and relax for 25−45 minutes before blood samples were obtained. Serum leptin levels were measured using Human Leptin ELISA (BioVendor Laboratory Medicine, Inc., Modrice, Czech Republic) at Global Clinical Central Lab (Yongin, Korea). Serum samples were incubated in microplate wells pre-coated with polyclonal anti-human leptin antibody. After 60 minutes’ incubation and washing, polyclonal anti-human leptin antibody conjugated with horseradish peroxidase (HRP) was added and incubated for 60 minutes with captured leptin. Following another washing step, the remaining HRP conjugate was allowed to react with the substrate solution. The reaction was stopped by addition of acidic solution, and the absorbance of the resulting yellow product was measured. The absorbance was proportional to the concentration of leptin. A standard curve was constructed by plotting absorbance values against the concentration standards, and the concentrations of unknown samples were determined using this standard curve. Patients were divided according to leptin levels into low- and high-leptin groups based on the median value. In the baseline characteristics analysis, the low- and high-leptin groups were divided according to the median leptin level of all participants in the study (n = 1,086). Because the sex differences in leptin levels were marked, as has been shown in previous studies [44, 45], the low- and high-leptin groups were divided by the median leptin level for each sex in further analyses.
Physical disorders
Information on concurrent physical disorders at baseline was gathered using a questionnaire enquiring about 15 different systems or disorders including allergic or immunologic disorders; cardiac disorders; hypertension; stroke; dermatologic disorders; ear, nose, and throat disorders; endocrine disorders; eye disorders; gastrointestinal disorders; genitourinary disorders; hematologic or oncologic disorders; musculoskeletal disorders; neurologic disorders; respiratory disorders; and others. Concurrent physical disorders were totaled to generate a summary score, and participants were divided into two groups, based on the median value: fewer than two physical disorders (< 2 physical disorders) and two or more physical disorders (≥ 2 physical disorders).
Baseline Covariates
Socio-demographic characteristics obtained comprised age, sex, years of formal education, marital status (currently married or not), cohabiting status (living alone or not), religion (religious observation or not), occupation (current employed status or not), income (above or below 2,000 USD), and body mass index (BMI). BMI was compared as a binary variable (< 23 vs. ≥ 23) in the additional analysis based on the criterion for overweight in Asian populations [46]. Clinical characteristics evaluated comprised diagnoses of depressive disorders as mentioned above with certain specifiers, age at onset and duration of illnesses, history of previous depressive episodes (recurrent or first episode), number of previous depressive episodes, duration of present episode, family history of depression, and history of suicide attempt. Assessment scales for investi-gating symptoms and function were administered. Depressive symptoms were evaluated by the HAMD, anxiety symptoms by the Hospital Anxiety Depression Scale-anxiety subscale [47], quality of life by the EuroQol-5D (EQ-5D) [48], and functioning levels by the Social and Occupational Functioning Assessment Scale.
Pharmacotherapy
Details of the treatment in this study have been previously published [41,49]. Before treatment commencement, a comprehensive review was made of the patients’ clinical manifestations (e.g., psychotic and anxiety symptoms), severity of illness, physical comorbidity and medication profiles, and history of previous treatments. Minimal and maximal dosages of pharmacological agents were determined considering existing treatment guidelines [50,51]. In the first treatment Step 1, patients received antidepressant treatment, taking into consideration these data and treatment guidelines [51-53], for 3 weeks. Antidepressants used were bupropion, desvenlafaxine, duloxetine, escitalopram, fluoxetine, mirtazapine, paroxetine, sertraline, venlafaxine, and vortioxetine. After Step 1 antidepressant monotherapy, next step pharmacotherapy could be administered every 3 weeks during the acute treatment phase (3, 6, 9, and 12 weeks with a 3-day allowable window) and every 3 months during the continuation treatment phase (6, 9, and 12 months with a 7-day allowable window), whenever needed. At the end of each step, overall effectiveness and tolerability were reviewed for proceeding with measurement-based next-step treatments. In cases of insufficient improvement (a HAMD score reduction of < 30% from the baseline) or intolerable side effects, patients were instructed to choose whether they would prefer to remain in the current step or enter the next step strategies with switching (S), augmentation (A), combination (C), S + A, S + C, A + C, and S + A + C treatment. A decline of < 30% in HAMD score from the baseline over 3 weeks was adopted because most previous studies have defined early non-improvement as a decline of < 20% in HAMD score over 2 weeks [54-56]. Patients were also allowed to receive next step treatment if they showed sufficient improvement (a HAMD score reduction of ≥ 30% from the baseline) and absent/tolerable side effects. For determining treatment strategies, each patient’s preference was given priority to maximize medication compliance and treatment outcomes [57]. Antide-pressants switched or combined were bupropion, desvenlafaxine, duloxetine, escitalopram, fluoxetine, mirtazapine, paroxetine, sertraline, venlafaxine, and vortioxetine. Augmented drugs were buspirone, lithium, triiodothyronine, and atypical antipsychotics including aripiprazole, risperidone, olanzapine, quetiapine, and ziprasidone.
Outcome
Remission was defined as a HAMD score ≤ 7. Remission at 12 weeks and at 12 months was used in order to investigate the individual and interactive effects of baseline serum leptin levels and number of physical disorders on the short- and long-term treatment outcomes, respectively.
Statistical Analysis
Baseline data were compared by leptin levels and BMI in the acute treatment phase (up to 12 weeks) using independent ttests or chi-square tests. Correlations of baseline serum leptin levels and baseline BMI with the number of physical disorders were quantified by Spearman’s rank-order correlations. Unadjusted individual associations of baseline serum leptin levels, number of physical disorders, and baseline BMI with 12-week and 12-month remission were investigated using Mann−Whitney Utests for the continuous variables and chi-square tests for the binary variables (leptin low vs. high, number of physical disorders < 2 vs. ≥ 2, and BMI < 23 vs. ≥ 23). Unadjusted individual association of serum leptin levels (low vs. high) with 12-week and 12-month remission according to initially prescribed antidepressant types, including selective serotonin reuptake inhibitors (SSRIs), noradrenergic and specific serotonin antidepressants (NaSSA), serotonin−norepinephrine reuptake inhibitors, and norepinephrine−dopamine reuptake inhibitors, as well as treatment steps and treatment strategies were investigated using chi-square tests. Unadjusted interactive effects of binary variables on leptin and number of physical disorders on remission status were evaluated using the Breslow-Day test. Main individual and two-way interactive effects of leptin and number of physical disorders on remission status were estimated by using multinomial logistic regression tests after adjusted for relevant covariates, chosen considering baseline variables significantly associated with leptin levels (pvalue < 0.05). Additionally, individual and two-way interactive effects of BMI and the number of physical disorders on remission status were estimated using multinominal logistic regression tests after adjustment for relevant covariates, chosen considering baseline variables significantly associated with BMI (pvalues < 0.05). All statistical tests were two-sided with a significance level of 0.05. Statistical analyses were performed using IBM SPSS Statistics (version 25; IBM Co., Armonk, NY, USA).
RESULTS
Recruitment and Treatment Flow
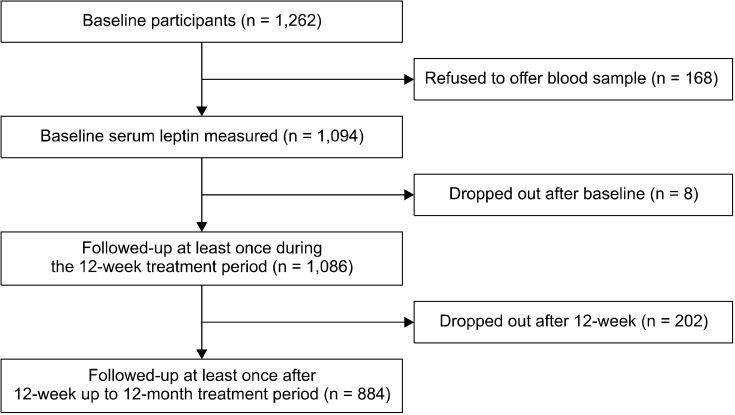
Patient flow over the 12-month period is described in Figure 1. Among 1,262 patients evaluated at baseline, serum leptin levels were measured in 1,094 (86.7%), and 1,086 (86.1%) were followed up at least once during the 12-week treatment period. Reasons for drop-out were a lack of treatment effect (n = 4) and loss to follow-up (n = 4). After the acute treatment phase, 884 (81.4%) patients were followed up at least once up to the 12-month follow-up, and an analysis of remission in the 12-month treatment period was performed. Reasons for drop-out were a lack of treatment effect (n = 129), transfer to another hospital (n = 13), intolerable side effects (n = 12), poor physical condition (n = 9), and loss to follow-up (n = 39). Drop-out at 12 months was significantly associated with unemployed status, melancholic features, and higher EQ-5D scores at baseline. However, drop-out at 12 months was not associated with leptin levels or number of physical disorders.
Fig. 1.
Participant recruitment and flow.
Baseline Characteristics by Leptin Levels
Baseline characteristics by leptin levels in patients who underwent up to 12 weeks of treatment (acute treatment phase) are shown in Table 1. Age, sex, education, religious observance, monthly income, BMI, duration of illness, recurrent depression, and EQ-5D score differed significantly between the low-leptin and high-leptin groups. Considering these findings and potential collinearity among the variables, ten variables (age, sex, education, religious observance, monthly income, BMI, duration of illness, recurrent depression, EuroQol-5D, and type of antidepressant) were included as covariates in the later adjusted analysis.
Table 1.
Baseline characteristics by serum leptin levels in patients with depressive disorders
| Variable | Up to 12-week treatment (n = 1,086) | |||
|---|---|---|---|---|
|
| ||||
| Low leptin (n = 543) | High leptin (n = 543) | Statistical coefficientsa | pvalue | |
| Age (yr) | 55.6 ± 15.2 | 58.3 ± 14.4 | t = −2.947 | 0.003 |
| Sex, female | 267 (49.2) | 478 (88.0) | χ2 = 190.320 | < 0.001 |
| Education (yr) | 9.8 ± 4.7 | 8.4 ± 4.8 | t = 5.060 | < 0.001 |
| Marital status, unmarried | 154 (28.4) | 172 (31.7) | χ2 = 1.420 | 0.233 |
| Living alone | 76 (14.0) | 91 (16.8) | χ2 = 1.592 | 0.207 |
| Religious observance | 272 (50.1) | 335 (61.7) | χ2 = 14.825 | < 0.001 |
| Unemployed status | 149 (27.4) | 167 (30.8) | χ2 = 1.446 | 0.229 |
| Monthly income, < 2,000 USD | 305 (56.2) | 343 (63.2) | χ2 = 5.525 | 0.019 |
| Body mass index (kg/m2) | 22.2 ± 2.8 | 24.3 ± 3.2 | t = −11.678 | < 0.001 |
| Major depressive disorder | 451 (83.1) | 474 (87.3) | χ2 = 3.858 | 0.050 |
| Melancholic feature | 88 (16.2) | 74 (13.6) | χ2 = 1.422 | 0.233 |
| Atypical feature | 33 (6.1) | 36 (6.6) | χ2 = 0.139 | 0.709 |
| Age at onset (yr) | 51.5 ± 16.8 | 52.2 ± 16.5 | t = −0.667 | 0.505 |
| Duration of illness (yr) | 4.1 ± 7.6 | 6.1 ± 10.2 | t = −3.628 | < 0.001 |
| Recurrent depression | 268 (49.4) | 302 (55.6) | χ2 = 4.268 | 0.039 |
| Number of depressive episodes | 1.0 ± 1.5 | 1.1 ± 1.5 | t = −1.320 | 0.187 |
| Duration of present episode (mo) | 7.1 ± 9.6 | 7.7 ± 11.1 | t = −1.092 | 0.275 |
| Family history of depression | 79 (14.5) | 79 (14.5) | χ2 = 0.000 | 1.000 |
| History of suicide attempt | 52 (9.6) | 43 (7.9) | χ2 = 0.934 | 0.334 |
| Hamilton Depression Rating Scale | 20.6 ± 4.1 | 20.9 ± 4.2 | t = −1.441 | 0.150 |
| Hospital Anxiety & Depression Scale-anxiety subscale | 11.7 ± 4.0 | 11.9 ± 4.1 | t = −0.607 | 0.544 |
| EuroQol-5D | 8.8 ± 1.5 | 9.1 ± 1.5 | t = −2.800 | 0.005 |
| Social and Occupational Functional Assessment Scale | 55.9 ± 7.5 | 56.0 ± 7.5 | t = −0.081 | 0.935 |
Values are presented as mean ± standard deviation or number (%).
aIndependent two sample t test or χ2 test, as appropriate.
Unadjusted Associations of Serum Leptin Levels and Number of Physical Disorders with Remission Status
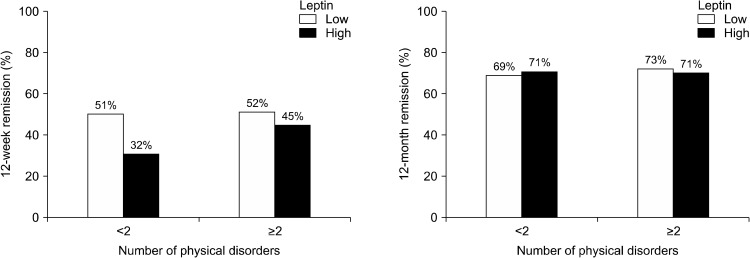
Baseline serum leptin levels and the number of physical disorders were significantly and positively correlated in 1,086 study participants (rs = 0.161, p < 0.001). Baseline serum leptin levels and the number of physical disorders are compared between those who did and did not achieve remission in the acute and continuation treatment phases in Table 2. Of 1086 patients in the acute treatment phase, both baseline serum leptin levels and the proportion of patients with high leptin levels were significantly higher in the non-remission compared to the remission group, whereas the number of physical disorders and proportion of patients with ≥ 2 physical disorders did not differ between the non-remission and remission groups. Of 884 patients in the continuation treatment phase, the baseline serum leptin levels, proportion of patients with high leptin levels, and number of physical disorders or proportion of patients with ≥ 2 physical disorders did not differ between non-remission and remission groups. In summary, the baseline leptin levels and proportion of patients with high leptin levels were higher only in the 12-week non-remission group compared to the remission group. The 12-week and 12-month remission rates by baseline serum leptin levels and number of physical disorders are displayed in Figure 2.The association between higher baseline leptin levels and 12-week non-remission was significant in patients with < 2 physical disorders (χ2 = 20.232, p < 0.001), but not in patients with ≥ 2 physical disorders (χ2 = 2.320, p = 0.128), reflecting a statistically significant interaction (χ2 = 4.569, p = 0.033). However, in neither patients with < 2 physical disorders (χ2 = 0.188, p = 0.664) nor patients with ≥ 2 physical disorders (χ2 = 0.209, p = 0.647) was there an association between higher baseline leptin levels and 12-month non-remission. Baseline serum leptin levels are compared between those with and without remission in the acute and continuation treatment phases according to initial antidepressant types in Supplementary Table 1 (available online). In the acute treatment phase, the non-remission group showed a higher frequency of patients who had high leptin levels among those who received SSRIs and NaSSA. In the continuation treatment phase, the frequency of patients who had high leptin levels did not differ between the non-remission and remission groups for all types of antidepressants. Baseline serum leptin levels are compared between those with and without remission in the acute and continuation treatment phases according to treatment steps in Supplementary Table 2 (available online). In the acute treatment phase, the non-remission group showed a higher frequency of patients who had high leptin levels among those who received Step 2 treat-ment. In the continuation treatment phase, the frequency of patients who had high leptin levels did not differ between the non-remission and remission groups in all steps of treatment. Baseline serum leptin levels are compared between those with and without remission in the acute and continuation treatment phases according to treatment strategies in Supplementary Table 3 (available online). In the acute treatment phase, the non-remission group showed a higher frequency of patients who had high leptin levels among those who received treatment strategy A. In the continuation treatment phase, the frequency of patients who had high leptin levels did not differ between non-remission and remission groups for all treatment strategies.
Table 2.
Baseline serum leptin levels and number of physical disorders by 12-week and 12-month remission status
| Exposure variable | Up to 12-week treatment (n = 1,086) | Up to 12-month treatment (n = 884) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Non-remission (n = 596) | Remission (n = 490) | Statistical coefficientsa | pvalue | Non-remission (n = 259) | Remission (n = 625) | Statistical coefficientsa | pvalue | ||
| Leptin (ng/ml) | |||||||||
| Median (IQR) | 6.1 (6.6) | 5.3 (5.7) | U = 124,592.0 | < 0.001 | 6.0 (6.0) | 5.6 (6.1) | U = 77,199.0 | 0.317 | |
| Low:High, n (%) | 265 (44.5):331 (55.5) | 277 (56.5):213 (43.5) | χ2 = 15.664 | 0.001 | 131 (50.6):128 (49.4) | 315 (50.4):310 (49.6) | χ2 = 0.002 | 0.961 | |
| Number of physical disorders | |||||||||
| Median (IQR) | 1.0 (1.0) | 2.0 (1.0) | U = 138,405.5 | 0.128 | 1.0 (1.0) | 2.0 (1.0) | U = 79,760.0 | 0.726 | |
| < 2: ≥ 2, n (%) | 317 (53.2):279 (46.8) | 233 (47.6):257 (52.4) | χ2 = 3.418 | 0.064 | 133 (51.4):126 (48.6) | 316 (50.6) | χ2 = 0.268 | 0.605 | |
IQR, interquartile range.
aMann−Whitney Utest or χ2 test, as appropriate.
Fig. 2.
Rates of remission according to baseline serum leptin levels and the number of physical disorders.
Adjusted Associations of Serum Leptin Levels and Number of Physical Disorders with Remission Status
The results of multivariable logistic regression analysis for individual and interactive effects of baseline serum leptin levels and number of physical disorders as binary variables (low vs. high leptin and < 2 vs. ≥ 2 physical disorders) on 12-week and 12-month remission are summarized in Table 3. Higher baseline serum leptin levels were associated with 12-week non-remission after adjustment for age, sex, education, religious observance, monthly income, BMI, duration of illness, recurrent depression, and EQ-5D score, but the presence of ≥ 2 physical disorders was not significantly associated. Neither higher baseline serum leptin levels nor the presence of ≥ 2 physical disorders was associated with 12-month non- remission after adjustment. In the interaction model, the leptin × number of physical disorders interaction exhibited a significant effect on 12-week remission status, with non-remission being significantly predicted by the combination of higher leptin levels and the presence of < 2 physical disorders. However, the leptin × number of physical disorders interaction had no effect on 12-month remission status.
Table 3.
Multivariate analysis examining the individual and interactive effects of baseline leptin levels and the number of physical disorders on the 12-week and 12-month remission
| Exposure variable | 12-week remission | 12-month remission | |||
|---|---|---|---|---|---|
|
|
|
||||
| Individual effect | Interactive effect | Individual effect | Interactive effect | ||
| Leptin (high vs. low) | 0.545 (0.415−0.715)‡ | 0.265 (0.121−0.580)† | 1.000 (0.748−1.336) | 1.347 (0.536−3.387) | |
| Number of physical disorders (≥ 2 vs. < 2) | 1.194 (0.917−1.555) | 0.623 (0.289−1.344) | 1.007 (0.730−1.390) | 1.485 (0.588−3.752) | |
| Leptin × number of physical disorders | 0.591 (0.361−0.966)* | 1.230 (0.684−2.210) | |||
Values are presented as odds ratio (95% confidence interval).
All data were adjusted for age, sex, education, religious observance, monthly income, body mass index, duration of illness, recurrent depression, EuroQol-5D, and initial antidepressant types.
*p < 0.05; †p < 0.01; ‡p < 0.001.
Associations of Baseline BMI and Number of Physical Disorders with Remission Status
Baseline characteristics by BMI in patients who underwent up to 12 weeks of treatment (acute treatment phase) are shown in Supplementary Table 4 (available online). Age, sex, education, and HAMD score differed signifi-cantly between the < 23 BMI and ≥ 23 BMI groups. Considering these findings, four variables (age, sex, education, and HAMD score) were included as covariates in the later adjusted analysis. Baseline BMI and the number of physical disorders were significantly positively correlated in the 1085 study participants (rs = 0.175, p < 0.001). Baseline BMI is compared between those who did and did not achieve remission in the acute and continuation treatment phases in Supplementary Table 5 (available online). Among the 1085 patients in the acute treatment phase, neither baseline BMI nor the proportion of patients with ≥ 23 BMI differed between the non-remission and remission groups. Among the 883 patients in the continuation treatment phase, baseline BMI was significantly higher in the remission compared to the non-remission group, whereas the proportion of patients with ≥ 23 BMI did not differ between the non-remission and remission groups. The 12-week and 12-month remission rates by baseline BMI and number of physical disorders are displayed in Supplementary Figure 1 (available online). Neither patients with < 2 physical disorders (χ2 = 0.679, p = 0.410 in 12-week remission and χ2 = 1.449, p = 0.229 in 12-month remission) nor patients with ≥ 2 physical disorders (χ2 = 0.202, p = 0.653 in 12-week remission and χ2 = 1.988, p = 0.159 in 12-month remission) showed an association between higher BMI and non-remission at 12-weeks and 12-months. The results of multivariable logistic regression analysis for individual and interactive effects of baseline BMI and the number of physical disorders as binary variables (< 23 vs. ≥ 23 BMI and < 2 vs. ≥ 2 physical disorders) on 12-week and 12-month remission are summarized in Supplementary Table 6 (available online). Neither ≥ 23 BMI nor the presence of ≥ 2 physical disorders were associated with 12-week or 12-month non-remission after adjustment for age, sex, education, and HAMD score. In addition, the BMI × number of physical disorders interaction had no effect on 12-week and 12-month remission status.
DISCUSSION
In the present study, using data from a naturalistic prospective stepwise pharmacotherapeutic study, which reflects real-world clinical practice, we found that higher baseline leptin levels predicted 12-week non-remission in patients with depressive disorders. Moreover, this association was more prominent in patients with fewer physical disorders than in those with more, reflecting a significant interaction.
Associations of peripheral leptin levels with antidepressant treatment outcomes have been inconsistent in previous clinical studies. Some studies conducted on patients who received initial antidepressant treatment or patients who received adjunctive treatment after inadequate response to initial antidepressant trial have reported that higher leptin levels predicted better treatment responses [25-27]. Based on results showing that leptin administration activates LepRb signaling in the CNS, resulting in an antidepressant effect by modulating monoamine neurotransmission, neurotrophic effects, and the HPA axis in animal studies, high baseline leptin levels may predict better antidepressant effects [9,14-18]. In addition, high leptin levels accompanied by high inflammatory activity in patients who received L-methylfolate calcium after showing an inadequate response to a SSRI might have predicted a better treatment response due to the restoration of monoamine synthesis by increasing tetrahydrobiopterin [27]. However, another study reported that higher leptin levels predicted a poorer treatment response in patients with insufficient response to SSRIs [27]. Given that the antidepressant role of leptin is mainly attributable to LepRb signaling in the CNS and, although controversy remains, elevated peripheral leptin levels have been suggested as a surrogate marker of attenuated central LepRb signaling [12,13,29], high baseline leptin levels may predict a poorer antidepressant response. The source of the discrepancy in the results of previous studies does not appear to be differences in study design. The treatment response was assessed at around 4 to 8 weeks in all studies. Moreover, except for one study that included bipolar disorder patients with depressive episodes [25], most studies were conducted on patients with major depressive disorder. Although treatment strategies varied from study to study, inconsistent results were also found in two studies with similar study designs conducted on patients with insufficient treatment response to selective serotonin reuptake inhibitors [27]
Previous studies related to the predictive effect of baseline leptin levels on antidepressant treatment responses did not consider the interactive role of leptin levels with other variables except BMI, which exhibits collinearity with peripheral leptin levels [27]. This may be the cause of the conflicting results regarding the association between baseline peripheral leptin levels and future antidepressant treatment responses. Our finding that higher leptin levels predicted a poorer short-term treatment response only in patients with fewer physical disorders (< 2 physical disorders) may provide clues for addressing the controversy to date. The interactive effects between leptin levels and the number of physical disorders on the 12-week antidepressant treatment outcome in depressive disorders in our cohort are plausible for several reasons. First, elevated peripheral leptin levels represent leptin resistance, which implicates leptin signaling disruption caused by leptin transport dysfunction through the BBB and intracellular LepRb signaling pathway defects [58]. Because the antidepressant role of leptin is determined by the activation of the LepRb signaling pathway in the CNS rather than by peripheral levels of leptin, increased leptin levels, which represent attenuated LepRb signaling, may predict poor antidepressant treatment outcomes. Second, increased leptin levels in patients with the higher number of physical disorders are more likely to be secondary elevations by various types of physical disorders [35-40], meaning that increased leptin levels in this subgroup may be more dependent on physical disorders rather than depression. A higher number of physical disorders might have been a confounding factor in the association between baseline serum leptin levels and short-term antidepressant treatment outcomes in patients with depressive disorders. In patients with < 2 physical disorders, the impact of peripheral leptin levels due to physical disorders per se would have been less; as a result, a clear association between higher leptin levels and 12-week non- remission might have been found.
Unlike in previous studies that analyzed the association between leptin levels and antidepressant treatment outcomes in patients who exhibited insufficient response in an initial antidepressant trial [27,28], we conducted the study without selecting patients. Moreover, we evaluated the treatment response up to not only 12 weeks but also 12 months. However, in contrast to the results for the acute treatment phase, neither higher leptin levels nor the interaction between higher leptin levels and low physical comorbidity was associated with 12-month non-remission. From these results, we speculate that higher baseline leptin levels affect the treatment response up to 12 weeks, but the effect of higher leptin does not last until 12 months if appropriate antidepressant treatment is continued. Because our results were drawn from a naturalistic prospective cohort study using various treatment strategies, the combination of baseline serum leptin levels and concurrent physical comorbidity warrants further evaluation as a useful predictor of short-term treatment outcomes in real-world clinical practice.
Because peripheral leptin levels are highly correlated with BMI [59] and BMI measurements are a more readily available method in real-world clinical settings compared to leptin measurements, we evaluated whether BMI could be used as a predictive marker of antidepressant response instead of leptin. Interestingly, BMI did not show individual effects or interactive effects with the number of physical disorders on 12-week non-remission. Considering that leptin levels increase in proportion to fat body mass [60] whereas BMI does not differentiate between fat and lean body mass, high fat mass, not high body mass, may be an important factor predicting the 12-week antidepressant treatment response.
In this study, we performed subgroup analysis according to initially prescribed antidepressant types, treatment steps, and treatment strategies to find clues as to which treatment method would be effective in patients with high leptin levels. The results revealed that among patients who received SSRIs, Step 2 treatment, and the A strategy, there was a significant association between the frequency of high leptin levels and 12-week non-remission. These findings suggest that, in cases with high leptin levels, using non-SSRI antidepressants initially, using the S or C rather than the A strategy as an adjunctive treatment, and proceeding to Step 3 treatment as soon as possible if an insufficient response occurred in Step 2 treatment might maximize the pharmacotherapeutic response in patients with depressive disorders.
This study has several limitations. First, we were not able to assess the associations between treatment-related changes in serum leptin and treatment outcomes, as longitudinal data on serum leptin levels were lacking. Given that the leptin/LepRb signaling pathway exerts antidepressant effects, treatment-related changes in serum leptin may be associated with treatment outcomes. Second, because of the naturalistic design of the study, treatment was decided by patient preference under physician’s guidance rather than by a determined protocol; thus, inter-physician variability might have influenced the outcomes. However, as physicians guided treatment decisions without awareness of the baseline leptin levels, it is unlikely that inter-physician variability affected the outcomes. Third, follow-up rates for the continuation treatment phase were relatively low compared to those for the acute treatment phase. Due to poor prognosis-associated characteristics of the participants who were lost to follow-up, such as unemployed status, melancholic features, and higher EQ-5D scores, their absence might have affected the results. However, this possibility is unlikely, as baseline leptin levels and the number of physical disorders did not vary with continuation treatment phase follow-up status. Fourth, because various antidepressants were initially used and different treatment strategies (switching, augmentation, and combination or mixtures of these approaches) were applied from 3 weeks after the start of antidepressant monotherapy, too many variables were in play to evaluate the effect of leptin on the antidepressant response depending on the individual psychopharmacological treatments. However, since our results were drawn after adjusting for initial antidepressant type, the predictive value of leptin levels for 12-week non-remission is more likely to be a generalized conclusion irrespective of the type of treatment regimen. Fifth, physical comorbidities were assessed by number of physical disorders arbitrarily rather than well validated scales such as Charlson comorbidity index [61].
Despite the clear antidepressant role of leptin/LepRb signaling pathways in animal studies, the association between baseline peripheral leptin levels and antidepressant treatment outcomes in clinical studies has been contro-versial. This study verified that higher baseline serum leptin levels and the interaction between higher leptin levels and fewer physical comorbidities predicted 12-week non-remission in patients with depressive disorders who received stepwise antidepressant therapy. Based on these results, we suggest that the combination of baseline serum leptin level and number of concurrent physical comorbidities could be a useful predictor of future 12-week antidepressant treatment outcome. As this was a non- randomized trial, the effectiveness of baseline serum leptin levels and the interaction between serum leptin levels and physical comorbidities as a predictor of treatment outcomes in patients who receive antidepressants should be evaluated in randomized trials in the future.
Supplemental Materials
Footnotes
Funding
The study was funded by a grant of National Research Foundation of Korea Grant (NRF-2019M3C7A1031345, NRF-2020R1A2C2003472) to Jae-Min Kim. Robert Stewart is part-funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London. Robert Stewart is also a National Institute for Health Research (NIHR) Senior Investigator.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: Wonsuk Choi, Robert Stewart, Jae-Min Kim. Data curation: Wonsuk Choi, Hee-Ju Kang, Hee Kyung Kim, Ho-Cheol Kang, Ju-Yeon Lee, Sung-Wan Kim, Jae-Min Kim. Validation: Hee Kyung Kim, Ho-Cheol Kang, Ju-Yeon Lee, Sung-Wan Kim. Project administration: Hee Kyung Kim, Ho-Cheol Kang, Ju-Yeon Lee, Sung-Wan Kim. Methodology: Hee-Ju Kang, Ju-Wan Kim. Formal analysis: Wonsuk Choi, Ju-Wan Kim, Robert Stewart, Jae-Min Kim. Writing: Wonsuk Choi, Hee-Ju Kang, Ju-Wan Kim, Robert Stewart, Jae-Min Kim.
References
- 1.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators, author. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fabbri C, Serretti A. How to utilize clinical and genetic information for personalized treatment of major depressive disorder: step by step strategic approach. Clin Psychopharmacol Neurosci. 2020;18:484–492. doi: 10.9758/cpn.2020.18.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391:1357–1366. doi: 10.1016/S0140-6736(17)32802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 5.Sanches M, Quevedo J, Soares JC. New agents and perspectives in the pharmacological treatment of major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2021;106:110157. doi: 10.1016/j.pnpbp.2020.110157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Otero M, Lago R, Gómez R, Lago F, Gómez-Reino JJ, Gualillo O. Leptin: a metabolic hormone that functions like a proinflammatory adipokine. Drug News Perspect. 2006;19:21–26. doi: 10.1358/dnp.2006.19.1.966243. [DOI] [PubMed] [Google Scholar]
- 7.Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 8.Feinkohl I, Janke J, Slooter AJC, Winterer G, Spies C, Pischon T. Plasma leptin, but not adiponectin, is associated with cognitive impairment in older adults. Psychoneuroendocrinology. 2020;120:104783. doi: 10.1016/j.psyneuen.2020.104783. [DOI] [PubMed] [Google Scholar]
- 9.Lu XY, Kim CS, Frazer A, Zhang W. Leptin: a potential novel antidepressant. Proc Natl Acad Sci U S A. 2006;103:1593–1598. doi: 10.1073/pnas.0508901103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chuang JC, Krishnan V, Yu HG, Mason B, Cui H, Wilkinson MB, et al. A beta3-adrenergic-leptin-melanocortin circuit regulates behavioral and metabolic changes induced by chronic stress. Biol Psychiatry. 2010;67:1075–1082. doi: 10.1016/j.biopsych.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamada N, Katsuura G, Ochi Y, Ebihara K, Kusakabe T, Hosoda K, et al. Impaired CNS leptin action is implicated in depression associated with obesity. Endocrinology. 2011;152:2634–2643. doi: 10.1210/en.2011-0004. [DOI] [PubMed] [Google Scholar]
- 12.Guo M, Huang TY, Garza JC, Chua SC, Lu XY. Selective deletion of leptin receptors in adult hippocampus induces depression-related behaviours. Int J Neuropsychopharmacol. 2013;16:857–867. doi: 10.1017/S1461145712000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo M, Lu XY. Leptin receptor deficiency confers resistance to behavioral effects of fluoxetine and desipramine via separable substrates. Transl Psychiatry. 2014;4:e486. doi: 10.1038/tp.2014.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garza JC, Guo M, Zhang W, Lu XY. Leptin restores adult hippocampal neurogenesis in a chronic unpredictable stress model of depression and reverses glucocorticoid-induced inhibition of GSK-3b/b-catenin signaling. Mol Psychiatry. 2012;17:790–808. doi: 10.1038/mp.2011.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arvaniti K, Huang Q, Richard D. Effects of leptin and corticosterone on the expression of corticotropin-releasing hormone, agouti-related protein, and proopiomelanocortin in the brain of ob/ob mouse. Neuroendocrinology. 2001;73:227–236. doi: 10.1159/000054639. [DOI] [PubMed] [Google Scholar]
- 16.Komori T, Morikawa Y, Nanjo K, Senba E. Induction of brain- derived neurotrophic factor by leptin in the ventromedial hypothalamus. Neuroscience. 2006;139:1107–1115. doi: 10.1016/j.neuroscience.2005.12.066. [DOI] [PubMed] [Google Scholar]
- 17.Charnay Y, Cusin I, Vallet PG, Muzzin P, Rohner-Jeanrenaud F, Bouras C. Intracerebroventricular infusion of leptin decreases serotonin transporter binding sites in the frontal cortex of the rat. Neurosci Lett. 2000;283:89–92. doi: 10.1016/S0304-3940(00)00951-4. [DOI] [PubMed] [Google Scholar]
- 18.Zou X, Zhong L, Zhu C, Zhao H, Zhao F, Cui R, et al. Role of leptin in mood disorder and neurodegenerative disease. Front Neurosci. 2019;13:378. doi: 10.3389/fnins.2019.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kraus T, Haack M, Schuld A, Hinze-Selch D, Pollmächer T. Low leptin levels but normal body mass indices in patients with depression or schizophrenia. Neuroendocrinology. 2001;73:243–247. doi: 10.1159/000054641. [DOI] [PubMed] [Google Scholar]
- 20.Yang K, Xie G, Zhang Z, Wang C, Li W, Zhou W, et al. Levels of serum interleukin (IL)-6, IL-1beta, tumour necrosis factor- alpha and leptin and their correlation in depression. Aust N Z J Psychiatry. 2007;41:266–273. doi: 10.1080/00048670601057759. [DOI] [PubMed] [Google Scholar]
- 21.Jow GM, Yang TT, Chen CL. Leptin and cholesterol levels are low in major depressive disorder, but high in schizophrenia. J Affect Disord. 2006;90:21–27. doi: 10.1016/j.jad.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 22.Gecici O, Kuloglu M, Atmaca M, Tezcan AE, Tunckol H, Emül HM, et al. High serum leptin levels in depressive disorders with atypical features. Psychiatry Clin Neurosci. 2005;59:736–738. doi: 10.1111/j.1440-1819.2005.01445.x. [DOI] [PubMed] [Google Scholar]
- 23.Milaneschi Y, Lamers F, Bot M, Drent ML, Penninx BW. Leptin dysregulation is specifically associated with major depression with atypical features: evidence for a mechanism connecting obesity and depression. Biol Psychiatry. 2017;81:807–814. doi: 10.1016/j.biopsych.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 24.Morris AA, Ahmed Y, Stoyanova N, Hooper WC, De Staerke C, Gibbons G, et al. The association between depression and leptin is mediated by adiposity. Psychosom Med. 2012;74:483–488. doi: 10.1097/PSY.0b013e31824f5de0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kloiber S, Ripke S, Kohli MA, Reppermund S, Salyakina D, Uher R, et al. Resistance to antidepressant treatment is associated with polymorphisms in the leptin gene, decreased leptin mRNA expression, and decreased leptin serum levels. Eur Neuropsychopharmacol. 2013;23:653–662. doi: 10.1016/j.euroneuro.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopresti AL, Maes M, Meddens MJ, Maker GL, Arnoldussen E, Drummond PD. Curcumin and major depression: a randomised, double-blind, placebo-controlled trial investigating the potential of peripheral biomarkers to predict treatment response and antidepressant mechanisms of change. Eur Neuropsychopharmacol. 2015;25:38–50. doi: 10.1016/j.euroneuro.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Shelton RC, Pencina MJ, Barrentine LW, Ruiz JA, Fava M, Zajecka JM, et al. Association of obesity and inflammatory marker levels on treatment outcome: results from a double- blind, randomized study of adjunctive L-methylfolate calcium in patients with MDD who are inadequate responders to SSRIs. J Clin Psychiatry. 2015;76:1635–1641. doi: 10.4088/JCP.14m09587. [DOI] [PubMed] [Google Scholar]
- 28.Machado-Vieira R, Gold PW, Luckenbaugh DA, Ballard ED, Richards EM, Henter ID, et al. The role of adipokines in the rapid antidepressant effects of ketamine. Mol Psychiatry. 2017;22:127–133. doi: 10.1038/mp.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Münzberg H, Myers MG., Jr Molecular and anatomical determinants of central leptin resistance. Nat Neurosci. 2005;8:566–570. doi: 10.1038/nn1454. [DOI] [PubMed] [Google Scholar]
- 30.Wells KB, Golding JM, Burnam MA. Psychiatric disorder in a sample of the general population with and without chronic medical conditions. Am J Psychiatry. 1988;145:976–981. doi: 10.1176/ajp.145.8.976. [DOI] [PubMed] [Google Scholar]
- 31.Keitner GI, Ryan CE, Miller IW, Kohn R, Epstein NB. 12-month outcome of patients with major depression and comorbid psychiatric or medical illness (compound depression) Am J Psychiatry. 1991;148:345–350. doi: 10.1176/ajp.148.3.345. [DOI] [PubMed] [Google Scholar]
- 32.Koike AK, Unützer J, Wells KB. Improving the care for depression in patients with comorbid medical illness. Am J Psychiatry. 2002;159:1738–1745. doi: 10.1176/appi.ajp.159.10.1738. [DOI] [PubMed] [Google Scholar]
- 33.Oslin DW, Datto CJ, Kallan MJ, Katz IR, Edell WS, TenHave T. Association between medical comorbidity and treatment outcomes in late-life depression. J Am Geriatr Soc. 2002;50:823–828. doi: 10.1046/j.1532-5415.2002.50206.x. [DOI] [PubMed] [Google Scholar]
- 34.Kim JM, Stewart R, Bae KY, Yang SJ, Yoon JS, Jung SW, et al. Physical comorbidity and 12-week treatment outcomes in Korean patients with depressive disorders: the CRESCEND study. J Psychosom Res. 2011;71:311–318. doi: 10.1016/j.jpsychores.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Ciprandi G, De Amici M, Tosca MA, Marseglia G. Serum leptin levels depend on allergen exposure in patients with seasonal allergic rhinitis. Immunol Invest. 2009;38:681–689. doi: 10.3109/08820130903107965. [DOI] [PubMed] [Google Scholar]
- 36.Seven A, Güzel S, Aslan M, Hamuryudan V. Serum and synovial fluid leptin levels and markers of inflammation in rheumatoid arthritis. Rheumatol Int. 2009;29:743–747. doi: 10.1007/s00296-008-0764-8. [DOI] [PubMed] [Google Scholar]
- 37.Esteghamati A, Khalilzadeh O, Anvari M, Rashidi A, Mokhtari M, Nakhjavani M. Association of serum leptin levels with homeostasis model assessment-estimated insulin resistance and metabolic syndrome: the key role of central obesity. Metab Syndr Relat Disord. 2009;7:447–452. doi: 10.1089/met.2008.0100. [DOI] [PubMed] [Google Scholar]
- 38.de Boer TN, van Spil WE, Huisman AM, Polak AA, Bijlsma JW, Lafeber FP, et al. Serum adipokines in osteoarthritis; comparison with controls and relationship with local parameters of synovial inflammation and cartilage damage. Osteoarthritis Cartilage. 2012;20:846–853. doi: 10.1016/j.joca.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Trejo-Vazquez F, Garza-Veloz I, Villela-Ramirez GA, Ortiz- Castro Y, Mauricio-Saucedo P, Cardenas-Vargas E, et al. Positive association between leptin serum levels and disease activity on endoscopy in inflammatory bowel disease: a case-control study. Exp Ther Med. 2018;15:3336–3344. doi: 10.3892/etm.2018.5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gu L, Wang CD, Cao C, Cai LR, Li DH, Zheng YZ. Association of serum leptin with breast cancer: a meta-analysis. Medicine (Baltimore) 2019;98:e14094. doi: 10.1097/MD.0000000000014094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang HJ, Kim JW, Kim SY, Kim SW, Shin HY, Shin MG, et al. The MAKE Biomarker discovery for Enhancing anTidepressant Treatment Effect and Response (MAKE BETTER) study: design and methodology. Psychiatry Investig. 2018;15:538–545. doi: 10.30773/pi.2017.10.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59 Suppl 20:22–33. quiz 34–57. [PubMed] [Google Scholar]
- 43.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hickey MS, Israel RG, Gardiner SN, Considine RV, McCammon MR, Tyndall GL, et al. Gender differences in serum leptin levels in humans. Biochem Mol Med. 1996;59:1–6. doi: 10.1006/bmme.1996.0056. [DOI] [PubMed] [Google Scholar]
- 45.Hellström L, Wahrenberg H, Hruska K, Reynisdottir S, Arner P. Mechanisms behind gender differences in circulating leptin levels. J Intern Med. 2000;247:457–462. doi: 10.1046/j.1365-2796.2000.00678.x. [DOI] [PubMed] [Google Scholar]
- 46.WHO Expert Consultation, author. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 47.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 48.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33:337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 49.Kim JM, Stewart R, Kang HJ, Kim JW, Lee HJ, Jhon M, et al. Short and long-term treatment outcomes of stepwise psychopharmacotherapy based on early clinical decision in patients with depressive disorders. J Affect Disord. 2020;274:315–325. doi: 10.1016/j.jad.2020.05.002. [DOI] [PubMed] [Google Scholar]
- 50.Anderson IM, Ferrier IN, Baldwin RC, Cowen PJ, Howard L, Lewis G, et al. Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2000 British Association for Psychopharmacology guidelines. J Psychopharmacol. 2008;22:343–396. doi: 10.1177/0269881107088441. [DOI] [PubMed] [Google Scholar]
- 51.Bauer M, Pfennig A, Severus E, Whybrow PC, Angst J, Möller HJ. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J Biol Psychiatry. 2013;14:334–385. doi: 10.3109/15622975.2013.804195. [DOI] [PubMed] [Google Scholar]
- 52.Malhi GS, Bassett D, Boyce P, Bryant R, Fitzgerald PB, Fritz K, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders. Aust N Z J Psychiatry. 2015;49:1087–1206. doi: 10.1177/0004867415617657. [DOI] [PubMed] [Google Scholar]
- 53.Kennedy SH, Lam RW, McIntyre RS, Tourjman SV, Bhat V, Blier P, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. Pharmacological treatments. Can J Psychiatry. 2016;61:540–560. doi: 10.1177/0706743716659417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim JM, Kim SY, Stewart R, Yoo JA, Bae KY, Jung SW, et al. Improvement within 2 weeks and later treatment outcomes in patients with depressive disorders: the CRESCEND study. J Affect Disord. 2011;129:183–190. doi: 10.1016/j.jad.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 55.Stassen HH, Angst J, Hell D, Scharfetter C, Szegedi A. Is there a common resilience mechanism underlying antidepressant drug response? Evidence from 2848 patients. J Clin Psychiatry. 2007;68:1195–1205. doi: 10.4088/JCP.v68n0805. [DOI] [PubMed] [Google Scholar]
- 56.Szegedi A, Müller MJ, Anghelescu I, Klawe C, Kohnen R, Benkert O. Early improvement under mirtazapine and paroxetine predicts later stable response and remission with high sensitivity in patients with major depression. J Clin Psychiatry. 2003;64:413–420. doi: 10.4088/JCP.v64n0410. [DOI] [PubMed] [Google Scholar]
- 57.Swift JK, Callahan JL. The impact of client treatment preferences on outcome: a meta-analysis. J Clin Psychol. 2009;65:368–381. doi: 10.1002/jclp.20553. [DOI] [PubMed] [Google Scholar]
- 58.Gruzdeva O, Borodkina D, Uchasova E, Dyleva Y, Barbarash O. Leptin resistance: underlying mechanisms and diagnosis. Diabetes Metab Syndr Obes. 2019;12:191–198. doi: 10.2147/DMSO.S182406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 60.Havel PJ, Kasim-Karakas S, Mueller W, Johnson PR, Gingerich RL, Stern JS. Relationship of plasma leptin to plasma insulin and adiposity in normal weight and overweight women: effects of dietary fat content and sustained weight loss. J Clin Endocrinol Metab. 1996;81:4406–4413. doi: 10.1210/jcem.81.12.8954050. [DOI] [PubMed] [Google Scholar]
- 61.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.