Abstract
Objective
Present study was designed to investigate behavioral and biochemical role of nimodipine in prenatal valproic acid (Pre-VPA) induced autism in rats.
Methods
Valproic acid was utilized to induce autistic phenotypes in Wistar rats. The rats were assessed for social behavior. Hippocampus and prefrontal cortex (PFC) were utilized for various biochemical assessments, whereas cerebellum was used to assess blood brain barrier (BBB) permeability.
Results
Pre-VPA rats showed reduction social interaction. Pre-VPA administration were decreased PFC levels of interleukin-10 (IL-10), and glutathione along with hippocampus cAMP response element-binding protein (CREB) and brain-derived neurotrophic factor (BDNF). Also, the animals have shown increase in PFC levels of IL-6, tumor necrosis factor-α, thiobarbituric acid reactive substance, Evans blue leakage and water content. Nimodipine countered Pre-VPA administered reduction in social interaction, CREB, BDNF, inflammation, oxidative stress, BBB permeability.
Conclusion
Pre-VPA has induced autistic phenotype, which were attenuated by nimodipine in rats. Nimodipine and other calcium channel blockers should further investigate to check the management of autism.
Keywords: Cyclic AMP response element-binding protein, Nimodipine, IL-6, BDNF, Blood brain barrier, Calcium channel.
INTRODUCTION
A neurodevelopmental disorder, autism spectrum disorder (ASD) is currently being diagnosed with communication problems, repetitive behavior along with social behavior dysfunction. Shockingly, there is no affirmed treatment accessible till presently. This is following an upward example and overall budgetary weight. Males are increasingly inclined to ASD when contrasted with females. More than one percent of the populace overall is experiencing ASD [1]. Several environmental and genetic factors seem to be responsible but the exact cause still unknown. Autism has diverse genetic relations along with different environmental causes including infection, pesticides and drugs [2,3]. It has suggested that maternal gly-phosate exposure can mimic the autistic phenotype in animals [4]. Valproic acid is a typically used antiepileptic drug and it is used to treat depressive disorders, migraine, and neuropathic pain. Prenatal valproic acid (Pre-VPA) treatment is one of the best characterized models of autism rats with solid choice, face and predictive validity [5]. The autistic individuals have some genetic defects in calcium channel genes, which regulates the neurodevel-opment [6].
It has already documented, multiple comorbid conditions including neuroinflammation and oxidative stress in children with autism [7,8]. In autistic patients, glutathione levels were higher, reduced glutathione levels were lower, and glutathione redox ratios were diminished. Additionally, animal models show low levels of glutathione (GSH) and interleukin-10 (IL-10) and high levels of thiobarbituric acid reactive (TBAR), IL-6 and tumor necrosis factor (TNF)-α [9,10]. Valproic acid is used to induce autism in rats by exposure during prenatal life 12.5th day. Valproic acid is widely used in the induction of autism phenotypes because it produces autistic phenotypes like the clinical features of autism. Valproic can produce core symptoms as social behavior impairment along with some other comorbid features in form of oxidative stress and neuro-inflammation [7,11-13].
L-type of calcium channels are commonly spread throughout the brain. L-type of calcium channels control neuronal excitability and combine neuronal activation with gene transcription L-type of calcium channels triggered by De Novo CACNA1D mutations in ASD have been identified [14]. Calcium-channel subunit mutations in CaVβ2 is associated with autism [15]. Nimodipine is an antagonist of calcium dihydropyridine L-type channels with a long record and a high safety score. Nimodipine binds to the pore and transmembrane of voltage receptor alpha-1 subunits and serves as a modulator for the negative allosteric channel role. Nimodipine play significant roles in dendritic and somatic calcium accumulation, gene expression and excitability [16]. Nimodipine is well described for the treatment of subarachnoid hemorrhage documented to encourage improved outcomes and less severe ischemic neurological symptoms. Nimodipine has been found to be neuroprotective in many other disorders in the brain, such as ischemic stroke, traumatic brain injury, migraine [17]. Being an antagonist of calcium channel, Nimodipine is also a strong cerebral vasodilator which act on cell membranes. It is also a molecule that is more lipophilic than nifedipine, which is also a calcium channel antagonist [17]. The neuroprotective effect of nimodipine in inflammation-mediated neurodegenerative disease was attributed to the inhibition of microglial activation, since nimodipine significantly inhibited the production of nitric oxide and further cytokines from lipopolysaccharide-stimulated cells.
There are only two study which stating that nimodipine blocked seizure-induced MeCP2 phosphorylation in developing brain [18,19]. Unfortunately, we don’t have any US Food and Drug Administration (FDA) approved therapeutic to treat core symptoms of autism such as social dysfunction. Our study finds out the novel role of nimodipine in valproic acid induced autism aspect of result such as social behavior, oxidative stress, IL-6, IL-10 and TNF-α, brain-derived neurotrophic factor (BDNF) and cAMP response element-binding protein (CREB) along with blood brain barrier (BBB) permeability. It could enable the scientific community to move forward in autism research.
This research examined the novel role of nimodipine; L-type of calcium channel antagonist in Pre-VPA mediated autism associated phenotypes in rats.
METHODS
Animals
Adult albino Wistar rats were kept in polypropylene cages in Amity University (No. 1327/PO/ReBi/S/10/CPCSEA) at a temperature of 25 ± 2°C with relative humidity of 50 ± 5%. The animals had free access to water and standard laboratory pellet chow diet (Ashirwad Industries, Punjab, India). Animals were exposed to the natural light and dark cycle with 12 hours of light (starting at 07:00 hours and ending at 19:00 hours) followed by 12 hours of dark (starting at 19:00 hours and ending at 07:00 hours). The Institutional Animal Ethics Committee of Amity Univer-sity, Uttar Pradesh, India, has approved all experiments (CPCSEA/IAEC/AIP/2019/01/19).
Drugs Chemicals and Reagents
Analytical and laboratory grade chemicals and reagents were used in the present study. Sodium salt of valproic acid was taken from Sun Pharma Pvt. Ltd. (Gurugram, India). Evans blue, ethylene glycol tetra acetic acid (EGTA) was purchased from SISCO Research Laboratory Pvt. Limited, Mumbai, India. Lowry’s reagent, N-naphthyle-thylenediamine and 5, 5’-dithiobis (2-nitrobenzoic acid) (DTNB) were obtained from Sigma-Aldrich (Bengaluru, India). Hydrogen peroxide and pyridine was obtained from Rankem Laboratories Pvt. Ltd. (Gurugram, India).
Protocol design
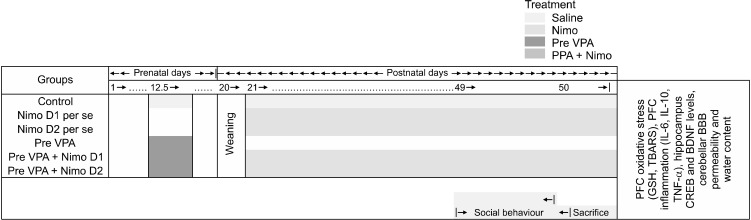
In total five groups of animals, with each group containing eight (n = 8) animals were used for the protocol of the study. Choice of animals was based on research already published, effectively using albino Wistar rats to model experimental ASD like condition [20]. The timeline, groups, and parameters assessed for present study are seen in Figure 1.
Fig. 1.
Schematic representation of experimental protocol.
E, embryonic day; PFC, prefrontal cortex; P, postnatal day; Pre-VPA, prenatal valproic acid; D1, dose 1; D2, dose 2; Nimo, nimodipine; GSH, glutathione; TBARS, thiobarbituric acid reactive substance; IL, interleukin; TNF, tumor necrosis factor; CREB, cAMP response element-binding protein; BDNF, brain-derived neurotrophic factor.
Fertilization was ascertained using a vaginal examination for the presence of sperm cells, this day was considered as gestational day 1. Pregnant dams were housed individually till the day of parturition and weaning of the pups. The pups were weaned and then distributed randomly into their respective groups on post-natal day 20 [20,21]. Nimodipine is a high lipophilic compound, it crosses the blood-brain barrier and can reach the brain and cerebrospinal fluid. Nimodipine has already tested for various central conditions including subarachnoid hemorrhage, cerebral ischemia-reperfusion injury, multiple sclerosis [22-24]. Valproic acid and nimodipine doses were selected as per our preliminary investigations and previous studies [13,22,25].
Group I–Control group: Pregnant dams were injected with single dose of 0.9% saline (3.3 ml/kg) on gestational 12.5th day. The received male pups were taken as control group.
Group II and III–Nimodipine per se: Rats received Nimodipine D1 (5 mg/kg, i.p.) and Nimodipine D2 (10 mg/kg, i.p.) from post-natal 24th day to till end of the study.
Group III–Prenatal valproic acid (Pre-VPA) group: Pregnant dams were injected with single dose of sodium valproate (500 mg/kg, i.p.) on gestational 12.5th day.
Group IV and V–Pre-VPA + Nimodipine D1 and D2 group: Males exposed to Pre-VPA were randomly selected and received Nimodipine D1 (5 mg/kg, i.p.) and D2 (10 mg/kg, i.p.) from postnatal day 21st to 50th (Fig. 1).
Social Interaction Assessment
The three-chambered task was modified from equipment in behavioral neuroscience to measure rodent social behavior in a simple way. Normal-developing children play with friends, but children with autism tend to play alone, with a toy, in a corner, rather than with other children. On postnatal day 49th−50th day, social interaction was evaluated by Three-Chamber Sociability and Social Novelty Test apparatus (30 cm long and 70 cm wide which divided into three identical chambers) for the rat. We have used this mehod in our previous research adopted from Kim et al. [26], 2011 with slightly modifi-cation. Concisely, the test arena measured 76 × 30 × 35 cm and was divided into three equal chambers with an access point between each chamber. Animals were given free access to all the chambers and Each trial started with the animal placed in the central chamber. To encourage exploration of the side chambers, all animals were accustomed to the apparatus for 5 minutes prior to starting of the test trial. After ending of the habituation period, the rats were tested in the sociability phase lasting for 10 minutes. Animals to be placed under a wire cage were spent 30 minutes at the wire cage, prior to initiation of the sociability phase. In the sociability phase, a stranger animal was placed under the wired cage in either (left or right) side chamber, while in the other chamber an empty cage would be placed. In order to avoid side preferences, the placement of wired cages was randomized and the chamber with the stranger animal and the empty cage were called as stranger chamber and empty chamber, respectively. Upon conclusion of the sociability phase the social preference phase was initiated 2 hours after the last animal trial. In the social preference phase each animal was allotted 10 minutes to look at the complete arena. During this phase, the animal earlier considered as stranger, was now rendered familiar and introducing another novel animal into the paradigm, along with the familiar animal. The two chambers now would be called as familiar and novel chamber. The time spent by test animals in both the side chambers was measured. Sociability index and social preference index were calculated according to the following formula [13,27,28].
Preparation for Biochemical Assessments
On postnatal day 50th, a high dose of thiopental sodium (90 mg/kg, i.p.) was used to isolate the prefrontal cortex (PFC) tissue by anaesthetization. The homogenate supernatant was collected for various biochemical estimates as defined in the procedures below. Absorption was taken with a spectrophotometer (PerkinElmer, Waltham, MA, USA). Prefrontal cortex, hippocampus and cerebellum are the main brain regions implicated in ASD pathogenesis and neurochemical disorders [28,29]. So, for this study, we selected prefrontal cortex and cerebellum brain regions.
Oxidative Stress Assessments
GSH and lipid peroxidation (TBAR) are known as oxidative stress markers, so in this study, we evaluated these markers in prefrontal cortex [13].
Glutathione (GSH)
GSH is an important tripeptide thiol antioxidant and levels its intracellular concentration is an evaluation parameter of oxidative stress. The quantitative estimation of reduced form of GSH was assessed using spectrophotometer (PerkinElmer) at 412 nm [20]. In a test tube, PFC supernatant and trichloroacetic acid (10 percent w/v) were combined at 1:1 ratio and the test tubes were centrifuged (1,000 g at 4°C for 10 minutes). Supernatant (0.5 ml) and 0.3 M disodium hydrogen phosphate (2 ml) were mixed. The spectrophotometric absorbance was observed at 412 nm following the addition of .25 ml of freshly prepared DTNB (DTNB dissolved in 1 percent w/v sodium citrate). For standard curve plotting, 10−100 mM of the reduced form of glutathione was employed. The effects of reduced glutathione consistent with mg of protein were expressed as micromoles.
Lipid peroxidation
Measurement of lipid peroxidation can be accomplished using thiobarbituric acid reactive substance (TBARS) in biological fluids. Lipid peroxidation is the reaction between free radicals attacking double bonds in carbon and carbon in lipids, involving the removal of hydrogen from carbon and the placement of oxygen. Lipid peroxidation was assessed as close to our previous investigations [20]. The PFC supernatant sample (0.2 ml) was taken in test tube. In addition, 8.1 percent sodium dodecyl sulphate (0.2 ml), 30 percent acetic acid pH 3.5 (1.5 ml) and 0.8 percent thiobarbituric acid (1.5 ml) were added, followed by distilled water up to 4 ml. The test tubes were put in the incubator at 95°C for 1 hour. The incubated mixture was then cooled by adding distilled water (1 ml) followed by adding n-butanol-pyridine mixture (15:1 v/v) to 5 ml. The centrifuge tubes were handled at 4,000 g for 10 minutes. The spectrophotometric absorbance was taken on developing pink color. 1, 1, 3, 3-tetra methoxy propane (1−10 nM) was used for plotting standard calibration curve. Nano moles per mg of protein were used for expression of result.
Enzyme-linked Immunosorbent Assay (ELISA) Method for IL-6, IL-10 and TNF-α, BDNF and CREB
ELISA is a technique used to measure the levels of antigens in biological samples. These were assessed by enzyme-linked immune-sorbent assay- sandwich method in PFC and hippocampus area of brain. The IL-6, IL-10 and TNF-α were estimated by using RayBio® Rat ELISA kits were used for the assessments. The procedure mentioned in the product information leaflet was completely followed and samples were run in triplicate for optical density measurement, average optical density was considered for final calculation of concentration. Briefly, IL-6, IL-10, TNF-α, BDNF, and CREB quantitation was made in PFC supernatant at 450 nm on 96-well percolated to specific antibodies plates. Supernatant and standard were taken out in to well. The immobilized antibody was bound with IL-6, IL-10, TNF-α, BDNF, and CREB present in sample, respectively. Washing was given to the tubes, and the biotinylated anti-Rat IL-6, IL-10, TNF-α, BDNF, and CREB antibodies were applied to the plates. Unbound biotiny-lated antibody was removed by washing the well. The horseradish peroxidase (HRP) conjugated streptavidin was added to the wells. After washing a TMB substratum solution was applied to the wells. A blue color formation showed the IL-6, IL-10, TNF, BDNF, and CREB have linked in sample. The stop solution was pipette to the wells which changed the color to yellow. Result were expressed in pg/ml and ng/ml [30].
BBB Permeability and Water Content
BBB permeability and water content performed by the concentration of Evans blue dye in the cerebellum as previously reported methods [27,31]. Concisely, 4% of dye/ Evans blue (intra-peritoneal; 4 ml/kg) was injected to subject and was permitted to circulate for 2 hours. Before collecting the samples, the subjects (anesthetized) were trans-cordially perfused with saline to flush out the remaining dye in the vessels. Weighed cerebellum for quantitative spectroscopic calculations. In short, the cerebel-lum precisely measured. The cerebellum processed at pH 7.4 dye extraction in 3.5 ml of 0.1 mol/L phosphate buffer saline by a homogenizer. Then, for protein precipitation, 6 ml of 60 percent trichloroacetic acid was added. This processed cerebellum was vortexed after 30 minutes of cooling for 2 minutes. The cerebellum was processed for 40 minutes through a centrifuge at 4,000 rpm to obtain pellets. The dye levels were measured at 610 nm via a spectrophotometer. The findings were expressed using a standardized curve of Evans blue as mg of Evans blue/g of cerebellum tissue. For 48 hours wet-weighted cerebellum tissue was put in the oven at 105°C. Weighted out after 48 hours of dry cerebellum. Wet-dry method was used to calculate the volume of water in the cerebellum. The percentage water content was calculated as: (wet weight − dry weight) / wet weight × 100% and results shown in percentage of water content [31].
Statistical Analysis
Sigma Stat (v3.5) was used for statistical analysis. The findings were demonstrated in the form of mean ± standard deviation. Statistics for all variables were analyzed using two-way ANOVA followed by a post-test by Bon-ferroni. This at p < 0.05 was considered statistically im-portant.
RESULTS
Social Behavior
Sociability, sociability index, social preference, and social preference index
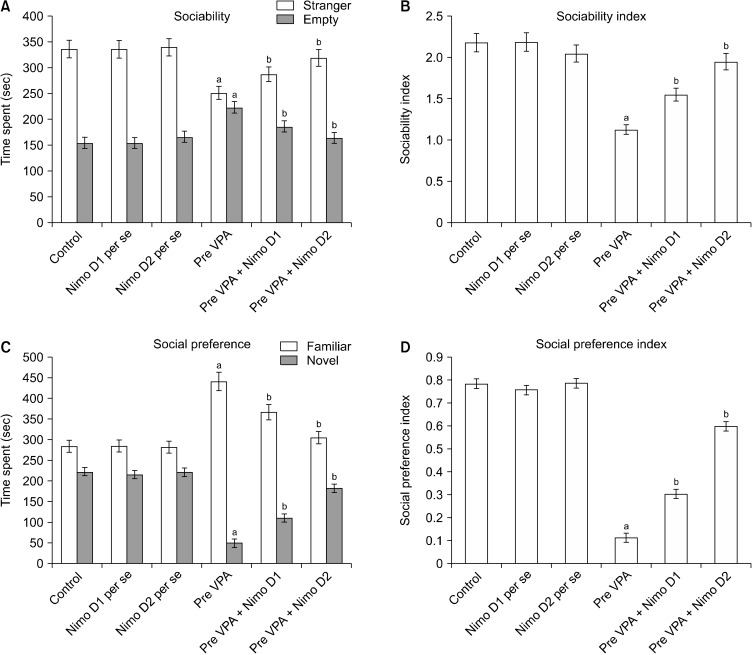
Pre-VPA has decreased time went through in stranger chamber and expanded time went through in empty chamber, with contrast to control rats, which shows lower sociability in Pre-VPA treated rats. Administration of Nimodipine has significantly mitigated Pre-VPA associated reduction of time went through in stranger chamber followed by increased time went through in the empty chamber. Pre-VPA treated rats, has shown lower sociability index when compared to control rats, which was markedly attenuated by Nimodipine (Fig. 2).
Fig. 2.
(A) Sociability, (B) sociability index, (C) social preference, and (D) social preference index on Three-Chamber Sociability and Social Novelty Test apparatus. (A) Stranger: aF1, 42 = 106.277; bF2, 42 = 31.543; Empty: aF1, 42 = 228.979; bF2, 42 = 68.632. (B) Sociability index: aF1, 42 = 502.727; bF2, 42 = 131.026. (C) Familiar: aF1, 42 = 502.086; bF2, 42 = 60.493; Novel: aF1, 42 = 1,477.037; bF2, 42 = 421.444. (D) Social preference index: aF1, 42 = 2,585.419; bF2, 42 = 444.524.
Pre-VPA, prenatal valproic acid; D1, dose 1; D2, dose 2; Nimo, nimodipine.
Results are mean ± standard deviation, two-way ANOVA followed by Bonferroni’s post-test. app < 0.05 vs. control rats, bpp < 0.05 vs. Pre-VPA treated rats.
Pre-VPA administration has decrease time went through in novel chamber and expand time went through in the familiar chamber, with contrast to control rats, which shows lower social preference in Pre-VPA treated rats. Adminis-tration of Nimodipine has significantly mitigated Pre-VPA associated reduction of time went through in novel chamber and increased time went through in the familiar chamber (Fig. 2). Pre-VPA treated rats have shown lower the social preference index, contrast to control rats, which was significantly mitigated by Nimodipine.
PFC Neuro-inflammation, Oxidative Stress and Hippocampus CREB and BDNF
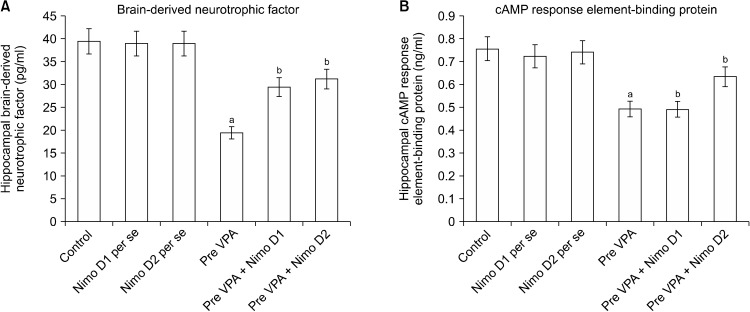
Pre-VPA has increased PFC Ca+ levels, PFC inflam-mation (IL-6 levels, TNF-α levels and decreased IL-10 levels), oxidative stress (decrease GSH levels and increase TBARS levels) along with decreased hippocampus CREB and BDNF. Nimodipine treatment has significantly mitigated Pre-VPA induced increased PFC inflammation (IL-6 levels, TNF-α levels and decreased IL-10 levels), oxidative stress (decrease GSH levels and increase TBARS levels) along with decreased hippocampus CREB and BDNF (Table 1, Figs. 2 and 3).
Table 1.
Effect of various agents on prefrontal cortex oxidative stress and inflammation
| Groups | Oxidative stress | Inflammation | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| GSH | TBARS | IL-10 | IL-6 | TNF-α | ||
| Control | 19.21 ± 0.961 | 4.88 ± 0.342 | 98.00 ± 4.900 | 93.70 ± 4.685 | 43.40 ± 2.170 | |
| Nimo D1 per se | 18.22 ± 0.922 | 4.6 ± 0.337 | 81.50 ± 5.025 | 82.71 ± 4.524 | 44.11 ± 2.130 | |
| Nimo D2 per se | 18.4 ± 0.920 | 4.81 ± 0.321 | 100.50 ± 5.101 | 90.47 ± 4.432 | 42.60 ± 2.121 | |
| Pre-VPA | 13.31 ± 0.656a | 12.75 ± 0.893a | 47.50 ± 2.375a | 194.76 ± 9.738a | 122.99 ± 6.150a | |
| Pre-VPA + Nimo D1 | 14.85 ± 0.743b | 10.23 ± 0.716b | 77.00 ± 3.850b | 169.07 ± 8.454b | 80.30 ± 4.015b | |
| Pre-VPA +Nimo D2 | 17.94 ± 0.897b | 7.33 ± 0.513b | 88.88 ± 4.444b | 125.63 ± 6.282b | 70.56 ± 3.528b | |
Results are mean ± standard deviation.
GSH: aF1, 18 = 163.265 bF2, 18 = 685.007. TBARS: aF1, 18 = 1,257.099 bF2, 18 = 175.039. IL-6 levels: aF1, 18 = 973.189 bF2, 18 = 84.89. IL-10 levels: aF1, 18 = 219.928; bF2, 18 = 138.116. TNF-α levels: aF1, 18 = 1,105.281 bF2, 18 = 364.755.
Pre-VPA, prenatal valproic acid; Nimo, nimodipine; D1, dose 1; D2, dose 2; GSH, glutathione; TBARS, thiobarbituric acid reactive substance; IL, interleukin; TNF, tumor necrosis factor.
Two-way ANOVA followed by Bonferroni’s post-test. ap < 0.05 vs. control rats, bp < 0.05 vs. Pre-VPA treated rats.
Fig. 3.
Effect of various agents on hippocampal (A) brain-derived neurotrophic factor (BDNF) and (B) cAMP response element-binding protein (CREB). (A) aF1, 18 = 476.678; bF2, 18 = 78.896. (B) aF1, 18 = 199.643; bF2, 18 = 29.509.
Pre-VPA, prenatal valproic acid; D1, dose 1; D2, dose 2; Nimo, nimodipine.
Results are mean ± standard deviation, two-way ANOVA followed by Bonferroni’s post-test. app < 0.05 vs. control rats, bpp < 0.05 vs. Pre-VPA treated rats.
Blood Brain Barrier Permeability and Water Content in the Cerebellum
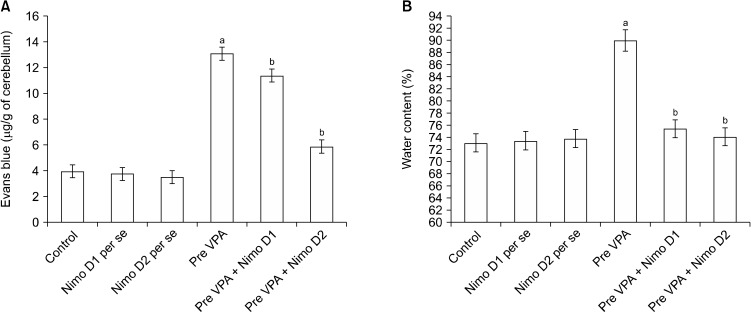
A considerably higher Evans blue concentration and water content were present in the cerebellum of Pre-VPA exposed rats in contrast to control rats. Treatment with Nimodipine expressively mitigates Pre-VPA induced increased concentration of Evans blue and water content in the cerebellum (Fig. 4), which suggest amelioration in Pre-VPA induced BBB dysfunction.
Fig. 4.
Effect of various agents on blood brain barrier permeability measured by (A) Evans blue concentration and (B) water content in cerebellum. (A) aF1, 18 = 971.701; bF2, 18 = 163.993. (B) aF1, 18 = 181.92; bF2, 18 = 94.856. Cerebellum of Pre-VPA treated rat have shown higher staining compare with control rat, while Nimodipine treatment have shown lower staining compare with Pre-VPA rat.
Pre-VPA, prenatal valproic acid; D1, dose 1; D2, dose 2; Nimo, nimodipine.
Results are mean ± standard deviation, two-way ANOVA followed by Bonferroni’s post-test. app < 0.05 vs. control rats, bpp < 0.05 vs. Pre-VPA treated rats.
DISCUSSION
Several neural circuits and brain regions trigger social behaviour in humans and animals, and it is crucial to their survival and neurodevelopment. Social behaviour is rooted in understanding and interpreting social cues; individuals must recognize, process, and interpret social cues, as well as responding appropriately to them. Even though the neurophysiological substrates of abnormal social behavior are unknown, humans and animals suggest that the excitatory/inhibitory imbalance of neuronal activity might contribute to the physiological explanation of abnormal social behavior [32]. There is a strong correlation among social behavior impairment, oxidative stress, neuroinflammation and neuroproteins changes such as BDNF and CREB [7,33]. Hence, we have assessed nimodipine effects on behavior and biochemical parameters.
Calcium stimulated second messenger pathways may activate a CREB transcription factor that could reverse this pathway downstream to BDNF but calcium excito-toxicity. CREB/BDNF signalling plays a significant role in synaptic plasticity, morphology and behaviour. Phospho-CREB regulates the transcription of some neuro-protective proteins such as BDNF. BDNF regulates memory consolidation and synaptic strengthening in brain. Reduction in BDNF level causes abnormal, learning and memory formation, and plasticity [34]. Research has documented that diminishing the levels of p-CREB can result in to the diminishing of spatial memory [35]. CREB activity plays role in double cortin, dendritic development, expression of neuronal microtubule associated protein and BDNF that could result in autistic characteristic features such as altered synaptic plasticity and neuronal survival [36]. In-utero exposer to valproic acid is produced nerve cell death, synaptic function disturbance along with neuronal tube defects in offspring. Pre-VPA has known to produce the change in the CREB and BDNF level in autism. We found significant decrease levels of CREB and BDNF proteins in the hippocampus of the Pre-VPA-exposed rats in parallel to previous finding [37]. Nimodipine activate TrkB phosphorylation associated increased in CREB in hippocampus and induce neuroplasticity and neuropro-tective signalling events [38].
An inflammation of the brain, even at low levels, can adversely affect synaptic function and result in behavioral abnormalities. Pre-VPA causes neuro-inflammation via accelerate inflammatory cytokines like IL-6, TNF-α along with decreased IL-10 [39]. Pre-VPA causes neuro-inflammation followed by neuronal disorganization and reactive gliosis in frontal cortex that may be capable for social behavior impairment and cognitive impairment [40]. Studies are evident for existence of the relation between increased inflammations linked social behavior impairment, repetitive behavior in autism. Increased neuro-inflammatory markers like TNF-α and IL-6 may modulate social and repetitive behavior via deviations in the brain microvascular system [41], and equilibrium of excitatory and inhibitory state responsible for glutamate signaling [40,42,43]. The exposure to valproic acid during pregnancy resulted in the development of long-term microglial changes in brain structures. Neuroinflammation makes for pro-inflammatory cytokines that can change the neuronal homeostasis of ca+2 by l-type calcium channel. Previous research has shown that nimodipine counter the effect of IL-6, IL-10, TNF-α [44]. Nimodipine may be involved through inhibition of proinflammatory cytokines [45] and reduced calcium influx [46]. A reduction in inflammation is known to help to keep the BBB more robust. By preserving calcium homeostasis, nimodipine reduces the permeability of the blood brain barrier in rats [47,48]. This regulation of nimodipine in the neuroinflammatory signals would be responsible for correcting behaviors for propionic acid induced ASD in rats [49]. It could be proposed that the correction of proinflammatory and anti-inflammatory cytokines in the current research may be one of the pathways for corrective behavior.
Oxidative stress is one of the sponsors for autistic characteristic in clinical as well pre-clinical findings [50-52]. Previous findings also indicating, Pre-VPA leads to increase in oxidative markers in rats [20]. Impaired glutathione associated antioxidant pathways such as methionine cycle, redox system, and transculturation reported in autistic candidates [53]. Increased level of oxidative stress can leads to activation of the Wnt Signaling Pathway, which has important role in autism pathogenesis [54-56]. Valproic acid, as well as other induction models, have been used to describe oxidative stress and inflammation [7,30,57,58]. The cerebellar damage along with irregular glutathione redox grade is associated with communication problems along with social deficits in rodents and autistic subjects [59,60]. Oxidative homeostasis dysregulation via catenin path activation was found responsible for the impairment of social, repetitive behavior and cognition in rats [61]. There are multiple antioxidant has shown neuroprotective effects in Pre-VPA exposed animals In present study, Nimodipine was found to reduce the TBAR levels followed by increase in GSH in Pre-VPA exposed rats. Several previous finding also suggest for antioxidant activity of nimodipine by working on antioxidant and related pathways such as activation of CREB and AKT signaling pathways [62,63]. The above line of discussion that may conclude, nimodipine possibly modulates the endogenous antioxidant pathways and results in decrease oxidative stress in Pre-VPA exposed rats.
BBB dysfunction has been found to be involved in autism which supports the gut barrier hypothesis of autism. Postmortem of the cerebellum of ASD was found to altered gene expression responsible for BBB integrity [64]. Interestingly, in our previous research, Pre-VPA exposed rats was found with increased BBB permeability measured by Evans blue dye staining [20]. Altered cytokines levels, endogenous redox mechanism, mitochondrial function, elevated neuro dermatitis levels may contributes to BBB dysfunction [65,66]. Increased in oxidative stress may be responsible for increase BBB permeability [67]. BBB permeability is mainly ruled by the tight junctions between adjacent cells and together low and high levels of Ca+ have been shown to have an adverse effect on these endothelial junctions. Collective intracellular Ca+ levels has been shown to affect in tight junction structure through apoptosis and protein kinase C-alpha [68]. Nimodipine modulates the water homeostasis and blood-brain barrier permeability possibly via maintain Ca+ homeostasis within the brain [69,70].
These experiments were limited by the fact that they were conducted only on male young-adult offspring. Males were believed to suffer from ASD more often than females for a long period of time. Recent works have raised more doubts about sex differences in autism, though, and strengthened discussions in this area. In spite of this, studies on the VPA model have shown both neurochemical and behavioral abnormalities in male offspring, while only marginal differences were seen in female offspring [71]. Females are presumably underreported as having autism-related disorders due to different modes of masking social deficiency. As a result, we only used male rat in this study, which focused mainly on the behavioral and biochemical changes within the brain. Though autism's complex pathophysiology remains a mystery, significant research has been made in deciphering it and developing effective therapies. Genetic modification and environmental exposure have made it easier to develop these models like Pre-VPA but have not masked the complexity of their mechanisms. Animal models cannot fully approximate the symptoms of autism, which is a particular disorder that affects humans [72]. Nimodipine can be tested in some other induction model of autism for supporting and pushing this research towards translation study. Still, there is a huge amount of molecular level research required to elucidate protective effect of nimodipine in autism.
On the basic of the above line of discussion, L-type calcium channel blocker; Nimodipine could ameliorate Pre-VPA induced remarkable decrease social behavior followed by NADH dehydrogenase activity, SDH activity, cytochrome oxidase activity, GSH, CREB and BDNF. Furthermore, nimodipine was found to counter Pre-VPA associated increase in anxiety, PFC IL-6, IL-10, and TNF-α and TBARS. Pre-VPA has been increase in BBB permeability and water content, which was ameliorated by nimodipine treatment in rats. Further, future study is required to explore the role of nimodipine in autism.
ACKNOWLEDGMENTS
Authors are thankful to Dr. A. K. Chauhan, Hon’ble Founder President, Ritnand Balve Education Foundation, India, and Dr. Atul Chauhan, Hon’ble Chancellor, Amity University Uttar Pradesh, India for providing all the necessary experimental facilities and motivation to conduct this research work. We are also thankful to Prof (Dr.). Nirmal Singh, Pharmacology Division, Department of Pharma-ceutical Sciences and Drug Research, Faculty of Medi-cine, Punjab University, Patiala (Punjab), India, for his va-luable suggestions.
Footnotes
Funding
None.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: Hariom Kumar, Bhupesh Sharma. Data acquisition: Hariom Kumar. Formal analysis: Hariom Kumar, Bhupesh Sharma. Supervision: Vishal Diwan, Bhupesh Sharma. Writing—original draft: Hariom Kumar. Writing—review & editing: Vishal Diwan, Bhupesh Sharma.
References
- 1.Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, et al. Prevalence of autism spectrum disorder among children aged 8 years- Autism and Developmental Dis-abilities Monitoring Network, 11 Sites, United States. MMWR Surveill Summ. 2014;67:1–23. doi: 10.15585/mmwr.mm6719a8. Erratum in: MMWR Morb Mortal Wkly Rep 2018;67:564. Erratum in: MMWR Morb Mortal Wkly Rep 2018;67:1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Markram K, Rinaldi T, La Mendola D, Sandi C, Markram H. Abnormal fear conditioning and amygdala processing in an animal model of autism. Neuropsychopharmacology. 2008;33:901–912. doi: 10.1038/sj.npp.1301453. [DOI] [PubMed] [Google Scholar]
- 3.Barrett CE, Hennessey TM, Gordon KM, Ryan SJ, McNair ML, Ressler KJ, et al. Developmental disruption of amygdala transcriptome and socioemotional behavior in rats exposed to valproic acid prenatally. Mol Autism. 2017;8:42. doi: 10.1186/s13229-017-0160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pu Y, Ma L, Shan J, Wan X, Hammock BD, Hashimoto K. Autism-like behaviors in male juvenile offspring after maternal glyphosate exposure. Clin Psychopharmacol Neurosci. 2021;19:554–558. doi: 10.9758/cpn.2021.19.3.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirsch MM, Deckmann I, Santos-Terra J, Staevie GZ, Fontes- Dutra M, Carello-Collar G, et al. Effects of single-dose antipurinergic therapy on behavioral and molecular alterations in the valproic acid-induced animal model of autism. Neuro-pharmacology. 2020;167:107930. doi: 10.1016/j.neuropharm.2019.107930. [DOI] [PubMed] [Google Scholar]
- 6.Li J, You Y, Yue W, Jia M, Yu H, Lu T, et al. Genetic evidence for possible involvement of the calcium channel gene CACNA1A in autism pathogenesis in Chinese Han popu-lation. PLoS One. 2015;10:e0142887. doi: 10.1371/journal.pone.0142887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luhach K, Kulkarni GT, Singh VP, Sharma B. Cilostazol attenuated prenatal valproic acid-induced behavioural and biochemical deficits in a rat model of autism spectrum disorder. J Pharm Pharmacol. 2021;73:1460–1469. doi: 10.1093/jpp/rgab115. [DOI] [PubMed] [Google Scholar]
- 8.Santocchi E, Guiducci L, Fulceri F, Billeci L, Buzzigoli E, Apicella F, et al. Gut to brain interaction in Autism Spectrum Disorders: a randomized controlled trial on the role of probiotics on clinical, biochemical and neurophysiological para-meters. BMC Psychiatry. 2016;16:183. doi: 10.1186/s12888-016-0887-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang JP, Su KP. Nutritional neuroscience as mainstream of psychiatry: the evidence- based treatment guidelines for using omega-3 fatty acids as a new treatment for psychiatric disorders in children and adolescents. Clin Psychopharmacol Neurosci. 2020;18:469–483. doi: 10.9758/cpn.2020.18.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sünnetçi E, Durankuş F, Albayrak Y, Erdoğan MA, Atasoy Ö, Erbaş O. Effects of the prenatal administration of tetanus toxoid on the sociability and explorative behaviors of rat offspring: a preliminary study. Clin Psychopharmacol Neurosci. 2021;19:84–92. doi: 10.9758/cpn.2021.19.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kazlauskas N, Seiffe A, Campolongo M, Zappala C, Depino AM. Sex-specific effects of prenatal valproic acid exposure on sociability and neuroinflammation: relevance for susceptibility and resilience in autism. Psychoneuroendocrinology. 2019;110:104441. doi: 10.1016/j.psyneuen.2019.104441. [DOI] [PubMed] [Google Scholar]
- 12.Luhach K, Kulkarni GT, Singh VP, Sharma B. Attenuation of neurobehavioural abnormalities by papaverine in prenatal valproic acid rat model of ASD. Eur J Pharmacol. 2021;890:173663. doi: 10.1016/j.ejphar.2020.173663. [DOI] [PubMed] [Google Scholar]
- 13.Kumar H, Sharma B. Minocycline ameliorates prenatal valproic acid induced autistic behaviour, biochemistry and blood brain barrier impairments in rats. Brain Res. 2016;1630:83–97. doi: 10.1016/j.brainres.2015.10.052. [DOI] [PubMed] [Google Scholar]
- 14.Pinggera A, Lieb A, Benedetti B, Lampert M, Monteleone S, Liedl KR, et al. CACNA1D de novo mutations in autism spectrum disorders activate Cav1.3 L-type calcium channels. Biol Psychiatry. 2015;77:816–822. doi: 10.1016/j.biopsych.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Despang P, Salamon S, Breitenkamp AF, Kuzmenkina E, Herzig S, Matthes J. Autism-associated mutations in the CaVβ2 calcium-channel subunit increase Ba2+-currents and lead to differential modulation by the RGK-protein Gem. Neurobiol Dis. 2020;136:104721. doi: 10.1016/j.nbd.2019.104721. [DOI] [PubMed] [Google Scholar]
- 16.Rosenberg EC, Lippman-Bell JJ, Handy M, Soldan SS, Rakhade S, Hilario-Gomez C, et al. Regulation of seizure-induced MeCP2 Ser421 phosphorylation in the developing brain. Neurobiol Dis. 2018;116:120–130. doi: 10.1016/j.nbd.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlson AP, Hänggi D, Macdonald RL, Shuttleworth CW. Nimodipine reappraised: an old drug with a future. Curr Neuropharmacol. 2020;18:65–82. doi: 10.2174/1570159X17666190927113021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li B, Tadross MR, Tsien RW. Sequential ionic and conformational signaling by calcium channels drives neuronal gene expression. Science. 2016;351:863–867. doi: 10.1126/science.aad3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tao J, Hu K, Chang Q, Wu H, Sherman NE, Martinowich K, et al. Phosphorylation of MeCP2 at serine 80 regulates its chromatin association and neurological function. Proc Natl Acad Sci U S A. 2009;106:4882–4887. doi: 10.1073/pnas.0811648106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar H, Sharma BM, Sharma B. Benefits of agomelatine in behavioral, neurochemical and blood brain barrier alterations in prenatal valproic acid induced autism spectrum disorder. Neurochem Int. 2015;91:34–45. doi: 10.1016/j.neuint.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Hajisoltani R, Karimi SA, Rahdar M, Davoudi S, Borjkhani M, Hosseinmardi N, et al. Hyperexcitability of hippocampal CA1 pyramidal neurons in male offspring of a rat model of autism spectrum disorder (ASD) induced by prenatal exposure to valproic acid: a possible involvement of Ih channel current. Brain Res. 2019;1708:188–199. doi: 10.1016/j.brainres.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 22.Ansari MA, Iqubal A, Ekbbal R, Haque SE. Effects of nimodipine, vinpocetine and their combination on isoproterenol- induced myocardial infarction in rats. Biomed Pharmacother. 2019;109:1372–1380. doi: 10.1016/j.biopha.2018.10.199. [DOI] [PubMed] [Google Scholar]
- 23.Desai RA, Davies AL, Del Rossi N, Tachrount M, Dyson A, Gustavson B, et al. Nimodipine reduces dysfunction and demyelination in models of multiple sclerosis. Ann Neurol. 2020;88:123–136. doi: 10.1002/ana.25749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang HY, Zhou HF, He Y, Yu L, Li C, Yang JH, et al. Protective effect of Naoxintong Capsule (NXTC, 脑心通胶囊) combined with Guhong Injection (GHI, 谷红注射液) on rat brain microvascular endothelial cells during cerebral ischemia-reperfusion injury. Chin J Integr Med. 2021;27:744–751. doi: 10.1007/s11655-020-3215-3. [DOI] [PubMed] [Google Scholar]
- 25.Mishra PR, Barik M, Ray SB. Effect of nimodipine on morphine-related withdrawal syndrome in rat model: an observat-ional study. J Pediatr Neurosci. 2017;12:7–14. doi: 10.4103/1817-1745.205652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim KC, Kim P, Go HS, Choi CS, Yang SI, Cheong JH, et al. The critical period of valproate exposure to induce autistic symptoms in Sprague-Dawley rats. Toxicol Lett. 2011;201:137–142. doi: 10.1016/j.toxlet.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 27.Kumar H, Ranjan RK, Yadav S, Kumar A, Ramanathan AL. Hydrogeochemistry and arsenic distribution in the Gorakhpur district in the middle Gangetic Plain, India. In: Ramanathan AL, Johnston S, Mukherjee A, Nath B, editors. Safe and sustainable use of arsenic-contaminated aquifers in the Gangetic Plain. Springer; Cham: 2015. pp. 97–107. [DOI] [Google Scholar]
- 28.Kumar H, Sharma B. Memantine ameliorates autistic behavior, biochemistry & blood brain barrier impairments in rats. Brain Res Bull. 2016;124:27–39. doi: 10.1016/j.brainresbull.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 29.Chauhan A, Gu F, Essa MM, Wegiel J, Kaur K, Brown WT, et al. Brain region-specific deficit in mitochondrial electron transport chain complexes in children with autism. J Neuro-chem. 2011;117:209–220. doi: 10.1111/j.1471-4159.2011.07189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mirza R, Sharma B. Selective modulator of peroxisome proliferator-activated receptor-α protects propionic acid induced autism-like phenotypes in rats. Life Sci. 2018;214:106–117. doi: 10.1016/j.lfs.2018.10.045. [DOI] [PubMed] [Google Scholar]
- 31.Manaenko A, Chen H, Kammer J, Zhang JH, Tang J. Comparison Evans Blue injection routes: Intravenous versus intraperitoneal, for measurement of blood-brain barrier in a mice hemorrhage model. J Neurosci Methods. 2011;195:206–210. doi: 10.1016/j.jneumeth.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barak B, Feng G. Neurobiology of social behavior abnormalities in autism and Williams syndrome. Nat Neurosci. 2016;19:647–655. doi: 10.1038/nn.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luhach K, Kulkarni GT, Singh VP, Sharma B. Vinpocetine amended prenatal valproic acid induced features of ASD possibly by altering markers of neuronal function, inflammation, and oxidative stress. Autism Res. 2021;14:2270–2286. doi: 10.1002/aur.2597. [DOI] [PubMed] [Google Scholar]
- 34.Bathina S, Das UN. Brain-derived neurotrophic factor and its clinical implications. Arch Med Sci. 2015;11:1164–1178. doi: 10.5114/aoms.2015.56342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 36.de la Torre-Ubieta L, Bonni A. Transcriptional regulation of neuronal polarity and morphogenesis in the mammalian brain. Neuron. 2011;72:22–40. doi: 10.1016/j.neuron.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu H, Wang X, Gao J, Liang S, Hao Y, Sun C, et al. Fingolimod (FTY720) attenuates social deficits, learning and memory impairments, neuronal loss and neuroinflammation in the rat model of autism. Life Sci. 2017;173:43–54. doi: 10.1016/j.lfs.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 38.Koskimäki J, Matsui N, Umemori J, Rantamäki T, Castrén E. Nimodipine activates TrkB neurotrophin receptors and induces neuroplastic and neuroprotective signaling events in the mouse hippocampus and prefrontal cortex. Cell Mol Neurobiol. 2015;35:189–196. doi: 10.1007/s10571-014-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kany S, Vollrath JT, Relja B. Cytokines in inflammatory dis-ease. Int J Mol Sci. 2019;20:6008. doi: 10.3390/ijms20236008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Codagnone MG, Podestá MF, Uccelli NA, Reinés A. Differen-tial local connectivity and neuroinflammation profiles in the medial prefrontal cortex and hippocampus in the valproic acid rat model of autism. Dev Neurosci. 2015;37:215–231. doi: 10.1159/000375489. [DOI] [PubMed] [Google Scholar]
- 41.Pober JS, Sessa WC. Inflammation and the blood microvascular system. Cold Spring Harb Perspect Biol. 2014;7:a016345. doi: 10.1101/cshperspect.a016345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei H, Zou H, Sheikh AM, Malik M, Dobkin C, Brown WT, et al. IL-6 is increased in the cerebellum of autistic brain and alters neural cell adhesion, migration and synaptic formation. J Neuroinflammation. 2011;8:52. doi: 10.1186/1742-2094-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Theije CG, Koelink PJ, Korte-Bouws GA, Lopes da Silva S, Korte SM, Olivier B, et al. Intestinal inflammation in a murine model of autism spectrum disorders. Brain Behav Immun. 2014;37:240–247. doi: 10.1016/j.bbi.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 44.Abdel-Fattah MM, Messiha BAS, Mansour AM. Modulation of brain ACE and ACE2 may be a promising protective strategy against cerebral ischemia/reperfusion injury: an experimental trial in rats. Naunyn Schmiedebergs Arch Pharmacol. 2018;391:1003–1020. doi: 10.1007/s00210-018-1523-3. [DOI] [PubMed] [Google Scholar]
- 45.Sanz JM, Chiozzi P, Colaianna M, Zotti M, Ferrari D, Trabace L, et al. Nimodipine inhibits IL-1b release stimulated by amyloid b from microglia. Br J Pharmacol. 2012;167:1702–1711. doi: 10.1111/j.1476-5381.2012.02112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zamora NN, Cheli VT, Santiago González DA, Wan R, Paez PM. Deletion of voltage-gated calcium channels in astrocytes during demyelination reduces brain inflammation and promotes myelin regeneration in mice. J Neurosci. 2020;40:3332–3347. doi: 10.1523/JNEUROSCI.1644-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li S, Bian L, Fu X, Ai Q, Sui Y, Zhang A, et al. Gastrodin pretreatment alleviates rat brain injury caused by cerebral ischemic-reperfusion. Brain Res. 2019;1712:207–216. doi: 10.1016/j.brainres.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 48.Chen LH, Liu LX, Yang YJ, Liu YS, Cao MH. Neuroprotective effects of nimodipine and MK-801 on acute infectious brain edema induced by injection of pertussis bacilli to neocortex of rats. Chin J Traumatol. 2003;6:118–123. [PubMed] [Google Scholar]
- 49.Weber MD, Godbout JP, Sheridan JF. Repeated social defeat, neuroinflammation, and behavior: monocytes carry the signal. Neuropsychopharmacology. 2017;42:46–61. doi: 10.1038/npp.2016.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nadeem A, Ahmad SF, Attia SM, Bakheet SA, Al-Harbi NO, Al-Ayadhi LY. Activation of IL-17 receptor leads to increased oxidative inflammation in peripheral monocytes of autistic children. Brain Behav Immun. 2018;67:335–344. doi: 10.1016/j.bbi.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 51.Frye RE, Rossignol DA. Mitochondrial dysfunction can connect the diverse medical symptoms associated with autism spectrum disorders. Pediatr Res. 2011;69(5 Pt 2):41R–7R. doi: 10.1203/PDR.0b013e318212f16b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rossignol DA, Frye RE. Evidence linking oxidative stress, mitochondrial dysfunction, and inflammation in the brain of individuals with autism. Front Physiol. 2014;5:150. doi: 10.3389/fphys.2014.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.James SJ, Cutler P, Melnyk S, Jernigan S, Janak L, Gaylor DW, et al. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am J Clin Nutr. 2004;80:1611–1617. doi: 10.1093/ajcn/80.6.1611. [DOI] [PubMed] [Google Scholar]
- 54.Bae SM, Hong JY. The Wnt signaling pathway and related therapeutic drugs in autism spectrum disorder. Clin Psycho-pharmacol Neurosci. 2018;16:129–135. doi: 10.9758/cpn.2018.16.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y, Yang C, Yuan G, Wang Z, Cui W, Li R. Sulindac attenuates valproic acid-induced oxidative stress levels in primary cultured cortical neurons and ameliorates repetitive/stereotypic-like movement disorders in Wistar rats prenatally exposed to valproic acid. Int J Mol Med. 2015;35:263–270. doi: 10.3892/ijmm.2014.1996. [DOI] [PubMed] [Google Scholar]
- 56.Kwan V, Unda BK, Singh KK. Wnt signaling networks in autism spectrum disorder and intellectual disability. J Neurodev Disord. 2016;8:45. doi: 10.1186/s11689-016-9176-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kumar H, Diwan V, Sharma B. Remediate Effect of ryanodine receptor antagonist in valproic-acid induced autism. Biomed Pharmacol J. 2022;15:59–71. doi: 10.13005/bpj/2343. [DOI] [Google Scholar]
- 58.Kumar H, Kulkarni GT, Diwan V, Sharma B. Shielding effect of ryanodine receptor modulator in rat model of autism. Basic Clin Neurosci J. 2021:10. doi: 10.32598/bcn.2021.2966.1. doi: 10.32598/bcn.2021.2966.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frye RE, Melnyk S, Macfabe DF. Unique acyl-carnitine profiles are potential biomarkers for acquired mitochondrial disease in autism spectrum disorder. Transl Psychiatry. 2013;3:e220. doi: 10.1038/tp.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morakotsriwan N, Wattanathorn J, Kirisattayakul W, Chaisiwamongkol K. Autistic-like behaviors, oxidative stress status, and histopathological changes in cerebellum of valproic acid rat model of autism are improved by the combined extract of purple rice and silkworm pupae. Oxid Med Cell Longev. 2016;2016:3206561. doi: 10.1155/2016/3206561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Y, Sun Y, Wang F, Wang Z, Peng Y, Li R. Downregulating the canonical Wnt/b-catenin signaling pathway attenuates the susceptibility to autism-like phenotypes by decreasing oxidative stress. Neurochem Res. 2012;37:1409–1419. doi: 10.1007/s11064-012-0724-2. [DOI] [PubMed] [Google Scholar]
- 62.Kamasak K, Basarslan K, Dagli AT, Ogden M, Alabalik U, Ekinci A, et al. Effects of Nimodipine and Nigella sativa on oxidative stress and apoptosis in serum and brain tissue of rats with experimental head trauma. Turk Neurosurg. 2021;31:8–17. doi: 10.5137/1019-5149.JTN.25523-19.3. [DOI] [PubMed] [Google Scholar]
- 63.Leisz S, Simmermacher S, Prell J, Strauss C, Scheller C. Nimo-dipine-dependent protection of Schwann cells, astrocytes and neuronal cells from osmotic, oxidative and heat stress is associated with the activation of AKT and CREB. Int J Mol Sci. 2019;20:4578. doi: 10.3390/ijms20184578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fiorentino M, Sapone A, Senger S, Camhi SS, Kadzielski SM, Buie TM, et al. Blood-brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Mol Autism. 2016;7:49. doi: 10.1186/s13229-016-0110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sorby-Adams AJ, Marcoionni AM, Dempsey ER, Woenig JA, Turner RJ. The role of neurogenic inflammation in blood-brain barrier disruption and development of cerebral oedema following acute central nervous system (CNS) Injury. Int J Mol Sci. 2017;18:1788. doi: 10.3390/ijms18081788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dong B, Yang Y, Zhang Z, Xie K, Su L, Yu Y. Hemopexin alleviates cognitive dysfunction after focal cerebral ischemia-reperfusion injury in rats. BMC Anesthesiol. 2019;19:13. doi: 10.1186/s12871-019-0681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lochhead JJ, McCaffrey G, Quigley CE, Finch J, DeMarco KM, Nametz N, et al. Oxidative stress increases blood-brain barrier permeability and induces alterations in occludin during hypoxia-reoxygenation. J Cereb Blood Flow Metab. 2010;30:1625–1636. doi: 10.1038/jcbfm.2010.29. Erratum in: J Cereb Blood Flow Metab 2011;31: 790-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rakkar K, Bayraktutan U. Increases in intracellular calcium perturb blood-brain barrier via protein kinase C-alpha and apoptosis. Biochim Biophys Acta. 2016;1862:56–71. doi: 10.1016/j.bbadis.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 69.Brown RC, Davis TP. Calcium modulation of adherens and tight junction function: a potential mechanism for blood-brain barrier disruption after stroke. Stroke. 2002;33:1706–1711. doi: 10.1161/01.STR.0000016405.06729.83. [DOI] [PubMed] [Google Scholar]
- 70.Shutov L, Kruglikov I, Gryshchenko O, Khomula E, Viatchenko- Karpinski V, Belan P, et al. The effect of nimodipine on calcium homeostasis and pain sensitivity in diabetic rats. Cell Mol Neurobiol. 2006;26:1541–1557. doi: 10.1007/s10571-006-9107-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jeon SJ, Gonzales EL, Mabunga DFN, Valencia ST, Kim DG, Kim Y, et al. Sex-specific behavioral features of rodent models of autism spectrum disorder. Exp Neurobiol. 2018;27:321–343. doi: 10.5607/en.2018.27.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mabunga DF, Gonzales EL, Kim JW, Kim KC, Shin CY. Exploring the validity of valproic acid animal model of autism. Exp Neurobiol. 2015;24:285–300. doi: 10.5607/en.2015.24.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]