Abstract
The core planar cell polarity (PCP) protein Vang/Vangl, including Vangl1 and Vangl2 in vertebrates, is indispensable during development. Our previous studies showed that the activity of Vangl is tightly controlled by two important posttranslational modifications, ubiquitination and phosphorylation. Vangl is ubiquitinated through an endoplasmic reticulum-associated degradation (ERAD) pathway and is phosphorylated by casein kinase 1 (CK1) in response to Wnt. Here, we present step-by-step procedures to analyze Vangl ubiquitination and phosphorylation, including cell culture, transfection, sample preparation, and signal detection, as well as the use of newly available phospho-specific antibodies to detect Wnt-induced Vangl2 phosphorylation. The protocol described here can be applicable to the analysis of posttranslational modifications of other membrane proteins.
Keywords: Planar cell polarity (PCP) , Wnt , Wnt/PCP , Vangl1 , Vangl2 , Ubiquitination , Phosphorylation
Background
As a conserved cellular mechanism from invertebrates to vertebrates, planar cell polarity (PCP) refers to the asymmetric pattern of a group of cells within a tissue plane, and it controls the polarized cell behaviors and tissue morphogenesis during embryonic development ( Yang and Mlodzik, 2015 ). PCP function is carried out by a set of core PCP proteins, including Fzd-Dvl/Dgo, Vangl-Pk, and Fmi complexes, which have mutually exclusive localizations within cells. Deletion or mutation of any of the core PCP proteins causes disintegration of the PCP asymmetry, and, thus, developmental defects, such as disorientation of bristles and hairs in Drosophila , convergent extension (CE) impairment in zebrafish, and neural tube defects in mammals ( Butler and Wallingford, 2017 ). Of all the core PCP components, Vangl proteins are more dedicated to PCP signaling, with Vangl2 being developmentally more important than its homolog Vangl1. Disruption and aberrant activation of Vangl lead to a variety of developmental defects and cancer malignancy, respectively ( Butler and Wallingford, 2017 ; Humphries and Mlodzik, 2018 ; Wang et al., 2021). Vangl is a four-pass transmembrane protein with both N-terminal and C-terminal in the cytosol. Our previous studies have shown that phosphorylation and ubiquitination, two posttranslational modifications, are interrelated in the regulation of Vangl protein homeostasis ( Gao et al., 2011 ; Yang et al., 2017 ; Feng et al., 2021 ). Phosphorylation mainly occurs at two clusters in the N-terminus. Casein kinase 1 (CK1), particularly CK1δ and CK1ε, first induces basal phosphorylation of Vangl in the endoplasmic reticulum (ER), leading to its stabilization and trafficking to the plasma membrane, where Vangl is further phosphorylated by CK1 for its normal PCP function. Wnt5a can induce Vangl phosphorylation via its receptor Ror2. On the other hand, Vangl undergoes ubiquitination mainly at two lysine sites (K300/K306) in the C-terminus ( Feng et al., 2021 ; Gao et al., 2011 ). The E3 ubiquitin ligase CUL3-KBTBD7 and the AAA+ ATPase p97/VCP mediate Vangl poly-ubiquitination and subsequent ER-associated degradation (ERAD), leading to its destruction in the proteasome ( Feng et al., 2021 ). We also found that phosphorylation of Vangl prevents its ubiquitination and ERAD ( Feng et al., 2021 ).
The functional significance of Vangl and Vangl phosphorylation in establishing PCP has been validated in various animal models, ranging from Drosophila, Xenopus , and zebrafish to mouse ( Gao et al., 2011 ; Ossipova et al., 2015 ; Kelly et al., 2016 ; Yang et al., 2017 ; Strutt et al., 2019 ; Chuykin et al., 2021 ). However, the role of Vangl ubiquitination is only recently emerging ( Feng et al., 2021 ; Radaszkiewicz et al., 2021 ). Unlike the canonical Wnt/ß-catenin signaling that can be measured by a number of biochemical assays, the noncanonical Wnt/PCP signaling lacks such tools. As Vangl phosphorylation is induced by Wnt5a, but ubiquitination is inhibited by Wnt5a ( Feng et al., 2021 ), the analysis of Vangl ubiquitination and phosphorylation may provide a unique approach to analyzing Wnt/PCP signaling. Hence, here we describe the ubiquitination assay of Vangl proteins by providing two different methods with detailed information regarding reagents, procedure, and analysis. We also introduce the new method for Vangl phosphorylation assay by using the recently available site-specific phospho-Vangl2 monoclonal antibodies, which can sensitively detect the CK1-mediated Vangl2 phosphorylation induced by Wnt. The protocols described below for detection of the ubiquitination and phosphorylation of Vangl may facilitate the future studies of Wnt/PCP signaling.
Materials and Reagents
-
Materials
Cell culture dish, 100 mm diameter (Corning, catalog number: 9380H79)
Cell culture dish, 60 mm diameter (Corning, catalog number: 9380H77)
12-well plate (Fisher Scientific, catalog number: 08-100-241)
15 mL centrifuge tube (Sigma-Aldrich, catalog number: CLS430791)
10 mL pipet (Corning, catalog number: 4488)
1.5 mL Eppendorf tube (Axygen, catalog number: MCT-150-C)
1,000 µL blue tip (Axygen, catalog number: T-1000-B)
200 µL yellow tip (Axygen, catalog number: T-200-Y)
10 µL clear tip (Axygen, catalog number: T-300)
Eppendorf pipettes (Eppendorf, catalog number: 2231300002)
Electronic pipette (Thermo Scientific, catalog number: 9501)
0.22 µm filter (Fisher Scientific, catalog number: SLGP033RS)
Cell scraper (Fisher Scientific, catalog number: 08-100-241)
-
Cell lines and plasmids
HEK293T (Human Embryonic Kidney 293T) cells (ATCC, catalog number: CRL-3216)
CHO (Chinese Hamster Ovary) cells (ATCC, catalog number: CCL-61)
HA-Vangl2 plasmid (described previously in Gao et al., 2011)
His-Ubiquitin plasmid (described previously in Feng et al., 2021)
FLAG-Wnt5a plasmid (described previously in Gao et al., 2011)
FLAG-Ror2 plasmid (described previously in Gao et al., 2011)
Myc-CK1δ plasmid (described previously in Gao et al., 2011)
-
Antibodies
Anti-HA antibody (Roche, catalog number: 11867431001, 1:5,000 dilution)
Anti-His antibody (Abcam, catalog number: ab18184, 1:5,000 dilution)
Anti-FLAG antibody (Sigma-Aldrich, catalog number: F1804, 1:5,000 dilution)
Anti-Myc antibody (Santa Cruz Biotechnology, catalog number: sc-40, 1:2,000 dilution)
Anti-Ubiquitin antibody (FK2) (Enzo Life Sciences, catalog number: ENZ-ABS840-0100, 1:1,000 dilution)
Anti-Vangl2-Phospho-T78/S79/S82 antibody (ABclonal, catalog number: AP1206, 1:1,000 dilution)
Anti-Vangl2-Phospho-S79/S82/S84 antibody (ABclonal, catalog number: AP1207, 1:1,000 dilution)
Anti-Actin antibody (Sigma-Aldrich, catalog number: A2228, 1:5,000 dilution)
Anti-GAPDH antibody (Santa Cruz Biotechnology, catalog number: sc-47724, 1:5,000 dilution)
Goat anti-Mouse IgG (H+L) Secondary Antibody, HRP (Invitrogen, catalog number: 31430)
Goat anti-Rabbit IgG (H+L) Secondary Antibody, HRP (Invitrogen, catalog number: 31460)
Goat anti-Rat IgG (H+L) Secondary Antibody, HRP (Invitrogen, catalog number: 31470)
-
Reagents
Dulbecco’s Modified Eagle Medium (DMEM) (Gibco, catalog number: 12-800-017)
Fetal Bovine Serum (FBS) (Gibco, catalog number: 10-099-141)
Penicillin-Streptomycin (PS) (Gibco, catalog number: 15140122)
0.25% Trypsin-EDTA (Gibco, catalog number: 25-200-056)
Proteasome inhibitor MG132 (Abcam, catalog number: ab141003)
Lysosome inhibitor chloroquine (CQ) (Sigma-Aldrich, catalog number: C6628)
CK1 inhibitor D4476 (Abcam, catalog number: ab120220)
Deubiquitinase inhibitor N-Ethylmaleimide (NEM) (Thermo Fisher Scientific, catalog number: 23030)
cOmplete, EDTA-free Protease Inhibitor Cocktail (Roche, catalog number: 11836153001)
Phosphatase Inhibitor Cocktail (Thermo Fisher Scientific, catalog number: 78420)
Polyethylenimine (PEI) (Sigma-Aldrich, catalog number: 765090)
Opti-MEM TM I Reduced Serum Medium (Gibco, catalog number: 31985070)
Ni-NTA Agarose (Qiagen, catalog number: 30210)
Protein A/G Plus-agarose (Santa Cruz Biotechnology, catalog number: sc-2003)
Tris (Thermo Scientific, catalog number: J75825)
Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S9888)
Sodium phosphate dibasic (Na 2 HPO 4 ) (Sigma-Aldrich, catalog number: S9763)
Sodium phosphate monobasic (NaH 2 PO 4 ) (Sigma-Aldrich, catalog number: S0751)
Sodium bicarbonate (NaHCO 3 ) (Sigma-Aldrich, catalog number: S6014)
Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P3911)
Sodium phosphate monobasic (KH 2 PO 4 ) (Sigma-Aldrich, catalog number: P5379)
Glycine (Affymetrix, catalog number: 16407)
Sodium dodecyl sulfate (SDS) (Sigma-Aldrich, catalog number: 436143)
Imidazole (Sigma-Aldrich, catalog number: 12399)
Guanidine hydrochloride (Sigma-Aldrich, catalog number: 50950)
4x Laemmli Sample Buffer (Bio-Rad, catalog number: 1610747)
β-Mercaptoethanol (Bio-Rad, catalog number: 1610710)
30% Acrylamide/Bis Solution, 29:1 (Bio-Rad, catalog number: 1610156)
Ammonium persulfate (APS) (Sigma-Aldrich, catalog number: A3678)
Tetramethylethylenediamine (TEMED) (Sigma-Aldrich, catalog number: T9281)
PageRuler Prestained Protein Ladder (Thermo Scientific, catalog number: 26616)
Immobilon-P membrane (PVDF) (Merck, catalog number: IPVH00010)
Methanol (VWR International, catalog number: BDH1135-1LP)
2-Propanol (VWR International, catalog number: BDH1131-1LP)
Bovine Serum Albumin (BSA) (Sigma-Aldrich, catalog number: A3912)
IGEPAL CA-630 (Sigma-Aldrich, catalog number: 18896)
Triton X-100 (Sigma-Aldrich, catalog number: 11332481001)
Tween-20 (Sigma-Aldrich, catalog number: P1379)
Deoxycholic acid (DCA) (Selleck Chemicals, catalog number: S4689)
SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Scientific, catalog number: PI34096)
PBS (see Recipes)
RIPA lysis buffer (see Recipes)
Buffer A (see Recipes)
Buffer TI (see Recipes)
Buffer B (see Recipes)
8% Resolving Gel (see Recipes)
5% Stacking Gel (see Recipes)
WB Running Buffer (see Recipes)
WB Transfer Buffer (see Recipes)
WB Blocking Buffer (see Recipes)
WB Washing Buffer (see Recipes)
Equipment
37 °C, 5% CO 2 forced-air incubator (Thermo Scientific, catalog number: 4110)
Warm water bath
Ultrasonic cell disruptor (Costar, catalog number: 3513)
Dry bath (Heater) (VWR International, catalog number: SH-1001)
Microcentrifuge (Hitachi, catalog number: CT15RE)
Shaker (VWR, catalog number: 97109-890)
Rotator (VWR, catalog number: 10136-084)
SDS-PAGE gel casting apparatus (Bio-Rad, catalog number: 1658008)
Protein electrophoresis and blotting instruments (Bio-Rad, catalog number: 1656019)
ChemiDoc MP imaging system (Bio-Rad, catalog number: 17001402)
Software
Image Lab 6.1 Software (Bio-Rad)
-
ImageJ (NIH)
Part I: For Vangl2 ubiquitination assay
Procedure
-
Cell culture, transfection, and MG132 treatment
HEK293T cells are maintained in DMEM complete medium (DMEM supplemented with 10% FBS and 1% PS) in a humid incubator at 37 °C and 5% CO 2 .
Culture HEK293T cells in a 100 mm diameter culture dish until 90% confluency.
Pass cells at the ratio of 1:6 into 60 mm diameter cell culture dishes.
Incubate cells at 37 °C, 5% CO 2 for ~20 h until 50%–70% confluency.
Replace with 4 mL of fresh medium 4 h before transfection.
Transfect cells with plasmids expressing HA-Vangl2 and/or His-Ubiquitin, using 1 mg/mL polyethylenimine (PEI) at the ratio of 1:4 (µg DNA: µg PEI). For a 60 mm cell culture dish, transfect 4 µg plasmids in total (2 µg HA-Vangl2 + 2 µg empty vector, 2 µg His-Ubiquitin + 2 µg empty vector, or 2 µg HA-Vangl2 + 2 µg His-Ubiquitin).
Incubate the cells for 12 h.
Replace with 4 mL of fresh medium 12 h post transfection.
Culture the transfected cells for 48 h in total.
Add MG132 to a final concentration of 10 µM, and incubate for 4 h before harvesting the cells.
To ensure the ubiquitination signal is exclusively from Vangl2 rather than its interacting proteins, the ubiquitination assay is performed under denaturing conditions, using either 2% SDS lysis buffer ( Choo and Zhang, 2009 ) or 6 M guanidine lysis buffer ( Wang et al., 2017 ). We provided two methods for analysis of the ubiquitination of a protein of interest because they are both commonly used, and we have verified them in the previous studies. Therefore, here we provide the two methods to detect the ubiquitination of Vangl2. We suggest that readers choose one of the two methods to start their experiments.
-
Ubiquitination assay under 2% SDS denaturing condition
Gently aspirate media, and rinse cells with cold PBS three times immediately before cell harvest.
Lyse cells in a 60 mm dish with 100 µL of RIPA lysis buffer containing 2% SDS, 10 mM deubiquitinase inhibitor NEM, protease and phosphatase inhibitors. Incubate at room temperature (RT) for 5 min before scraping and collecting the cell lysates into 1.5 mL Eppendorf tubes.
Boil at 95 °C for 10 min.
Sonicate with the ultrasonic cell disruptor at 3s ON/7s OFF with 30% amplitude, until the solution is clear.
Dilute the supernatants with nine times (900 µL) of RIPA lysis buffer, to reduce the SDS concentration to 0.2%, and make the final volume of lysates 1 mL.
Incubate on ice for 15 min.
Centrifugate at 16,000 × g , 4 °C for 15 min, and collect the supernatants.
Prepare the input sample by collecting 1/20 of the total supernatants (50 µL). Add 17 µL of 4× Laemmli Sample Buffer containing 10% 2-Mercaptoethanol to the supernatants. Boil at 95 °C for 10 min. Store at -20 °C until use as input sample.
Incubate the remaining 19/20 of total supernatants (950 µL) with anti-HA antibody with rotation, at 4 °C overnight.
Next day, pre-wash the Protein A/G PLUS-Agarose beads twice with cold PBS, and once with cold RIPA lysis buffer.
Add 20 µL of pre-washed Protein A/G PLUS-Agarose beads to the supernatants for further incubation at 4 °C for 2 h.
Wash protein-bound Protein A/G PLUS-Agarose beads four times in RIPA lysis buffer. For each wash, add 1 mL of RIPA lysis buffer to the beads, mix well, and incubate for 2 min. Centrifugate at 2,000 × g , 4 °C for 2 min, and discard the supernatants.
Prepare the immunoprecipitation (IP) sample. After the final wash, leave 75 µL of buffer in the Eppendorf tube and add 25 µL of 4× Laemmli Sample Buffer containing 10% 2-Mercaptoethanol to the beads. Boil at 95 °C for 10 min. Store at -20 °C until use as IP sample.
-
Ubiquitination assay under 6 M guanidine denaturing condition
Gently aspirate media, and rinse cells with cold PBS three times immediately before cell harvest.
Collect the cells using 1 mL of cold PBS. Pipette the cells in PBS several times until they detach from the culture dish, and then pipette them into an Eppendorf tube.
Separate the cells into two Eppendorf tubes, with 1/20 of total cells in one tube and the remaining 19/20 in the other tube.
Centrifugate at 1,500 × g , 4 °C for 3 min to collect the two individual parts of cells. Discard the supernatants.
Prepare the input sample. Lyse 1/20 of the cells in 50 µL of RIPA lysis buffer containing protease and phosphatase inhibitors. Incubate on ice for 20 min. Centrifugate at 16,000 × g , 4 °C for 15 min, and collect the supernatants. Add 17 µL of 4× Laemmli Sample Buffer containing 10% 2-Mercaptoethanol to the supernatants. Boil at 95 °C for 10 min. Store at -20 °C until use as input sample.
Lyse the remaining 19/20 of total cells using 1 mL of Buffer A containing 6 M guanidine, 10 mM NEM, protease and phosphatase inhibitors (see Recipes).
Sonicate with the ultrasonic cell disruptor at 3s ON/7s OFF with 30% amplitude, until the solution is clear.
Centrifugate at 16,000 × g for 15 min, and collect the supernatants.
During centrifugation, pre-wash the nickel-nitrilotriacetic acid (Ni-NTA) beads with Buffer A three times.
Add 20 µL of pre-washed Ni-NTA beads to the supernatants and incubate with gentle rotation at RT for 3 h.
Wash protein-bound Ni-NTA beads using Buffer A, Buffer B, and Buffer TI shown below. For each wash, add 1 mL of buffer to the beads, mix well, and incubate for 2 min, centrifugate at 2,000 × g for 2 min, and discard the supernatants.
Wash the beads once in Buffer A (see Recipes).
Wash the beads twice in Buffer B (see Recipes).
Wash the beads twice in Buffer TI (see Recipes).
-
Prepare the pulldown (PD) sample. After the final wash, leave 75 µL of Buffer TI in the Eppendorf tube, and add 25 µL of 4× Laemmli Sample Buffer containing 10% 2-Mercaptoethanol to the beads. Boil at 95 °C for 10 min. Store at -20 °C until use as PD sample.
After sample preparation from either B or C, perform the following detection and analysis procedures.
-
Ubiquitination analysis by western blotting (WB)
Set an 8% SDS-PAGE gel.
Assemble the SDS-PAGE gel into an SDS-PAGE electrophoresis chamber.
Fill the chamber with WB Running Buffer (see Recipes).
Load 1 µg protein ladder and 30 µg input or IP/PD samples in the wells of the stacking gel.
Run for ~20 min using constant 80 V when the samples are in the stacking gel, and for ~120 min in the resolving gel at 120 V for a better separation of proteins.
Rinse PVDF membrane in methanol for 15s for activation. Transfer the gel onto the activated membrane in WB Transfer Buffer (see Recipes) using constant 300 mA at 4 °C for 120 min.
Block the transferred membrane in WB Blocking Buffer (see Recipes) with gentle shaking at RT for 1 h.
Dilute primary antibodies in WB Blocking Buffer (see Recipes) at the indicated dilution. Incubate the membrane with primary antibodies with gentle shaking at 4 °C overnight.
Next day, wash the membrane in WB Washing Buffer (see Recipes, TBST Buffer) at RT 5 × 10 min.
Dilute HRP-conjugated secondary antibodies in WB Blocking Buffer at 1:5,000 dilution. Incubate the membrane with secondary antibodies with gentle shaking at RT for 1 h.
Wash the membrane in WB Washing Buffer (TBST Buffer) at RT 5 × 10 min.
Mix Solutions A and B at 1:1 from SuperSignal West Femto Maximum Sensitivity Substrate kit, and use the mixed solution to cover the membrane. Incubate for an appropriate time for chemiluminescent signal development.
Perform image acquisition using the ChemiDoc MP imaging system, following the manufacturer’s instructions.
Edit gel images using ImageJ, and present the results.
Data analysis
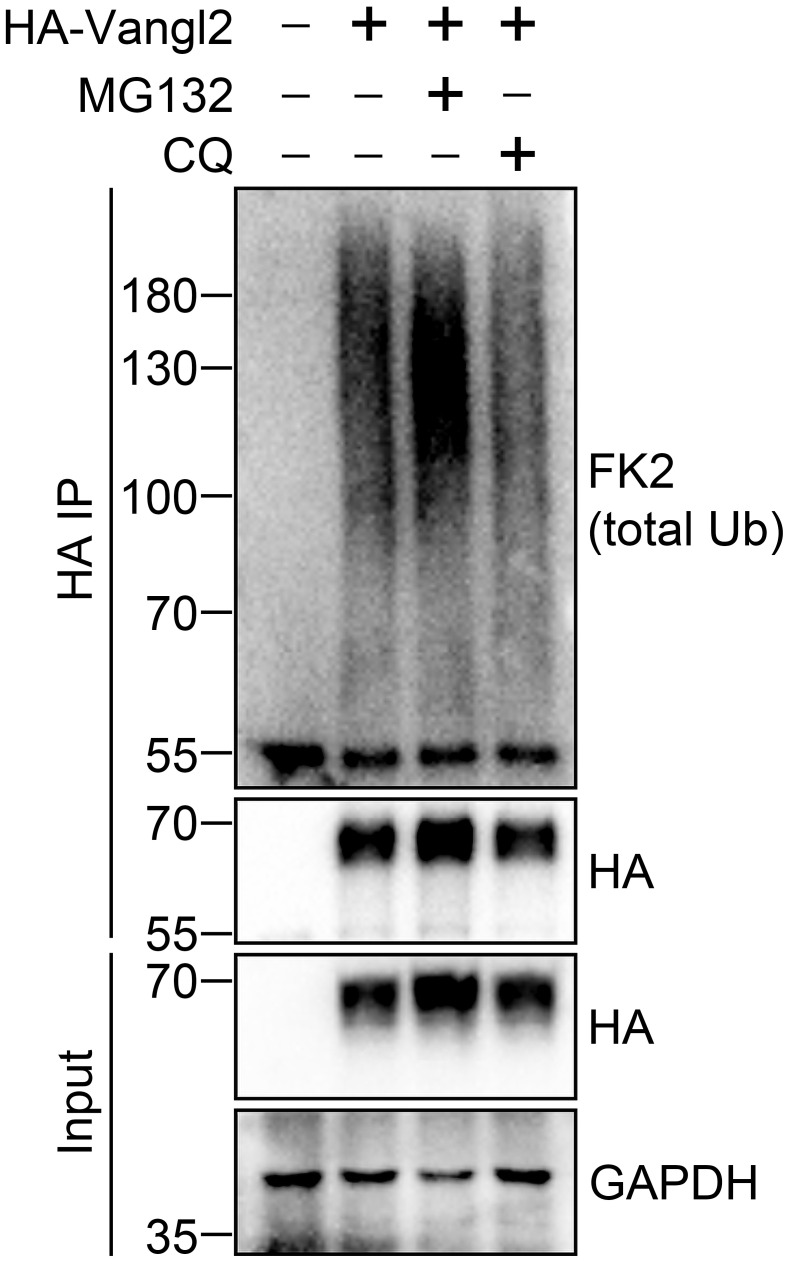
Here is a representative result showing the ubiquitination of Vangl2 under 2% SDS denaturing condition ( Figure 1 ).
-
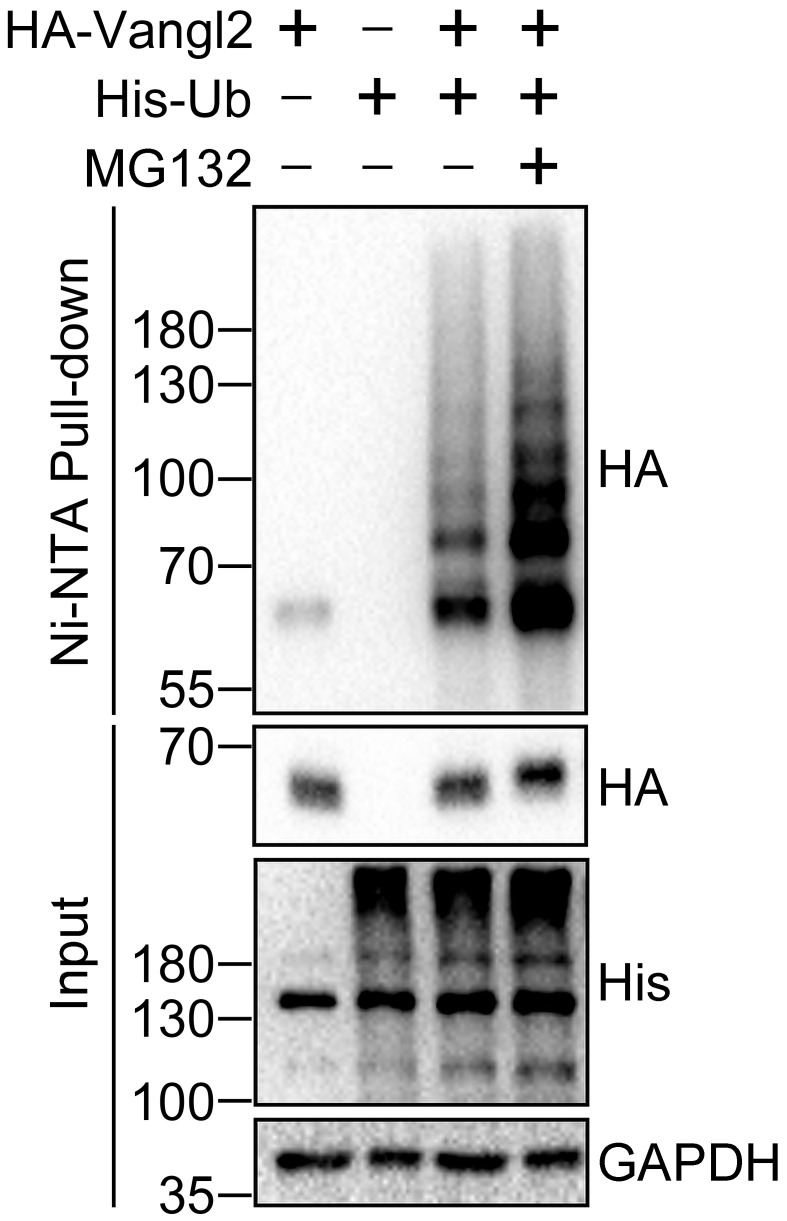
Here is a representative result showing the ubiquitination of Vangl2 under 6 M guanidine denaturing condition ( Figure 2 ).
Part II: For Vangl2 phosphorylation assay
Figure 1. Detection of Vangl2 ubiquitination under 2% SDS denaturing condition.
HEK293T cells were transfected with HA-Vangl2 plasmid for 48 h, and treated with proteasome inhibitor MG132 (10 µM) or lysosome inhibitor chloroquine (CQ, 25 μM) for 4 h before cell harvest. HA antibody conjugated Protein A/G agarose beads were used for immunoprecipitation (IP) of HA-Vangl2 and its covalently bound endogenous ubiquitin, under 2% SDS denaturing condition. HA-Vangl2 and endogenous ubiquitin from input and IP samples were subjected to immunoblotting with the indicated antibodies. Endogenous ubiquitin was examined by total ubiquitin FK2 antibody. GAPDH serves as a loading control. The 55kDa bands in the HA IP portion indicate the IgG heavy chains. This result is from Feng et al. (2021).
Figure 2. Detection of Vangl2 ubiquitination under 6 M guanidine denaturing condition.
HEK293T cells were transfected with HA-Vangl2 and/or His-Ubiquitin (His-Ub) plasmids for 48 h and treated with proteasome inhibitor MG132 (10 µM) for 4 h before cell harvest. Ni-NTA beads were used to pull down His-Ubiquitin and its covalently bound proteins under 6 M guanidine denaturing condition. HA-Vangl2 and His-Ubiquitin from Input and Pulldown samples were subjected to immunoblotting by the indicated antibodies. GAPDH serves as a loading control. The band in the leftmost pulldown sample should be the background. This result is from Feng et al. (2021).
Procedure
HEK293T cells are commonly used cell line for protein ubiquitination studies, and Vangl2 ubiquitination is easily detected under our investigation. Based on our experimental experience, Wnt5a/CK1 could induce more robust phosphorylation of Vangl proteins in CHO cells than in HEK293T cells (the band shift is more remarkable in CHO cells), so we switch from HEK293T cells to CHO cells for better demonstration of Vangl phosphorylation assay. To analyze the phosphorylation of Vangl2, we transfect CHO cells with plasmids expressing Wnt5a/Ror2 or CK1δ, to induce Vangl2 phosphorylation ( Feng et al., 2021 ; Gao et al., 2011 ; Yang et al., 2017 ). The phosphorylation is detected by western blotting analysis with HA and Vangl2 phospho-specific antibodies. Procedures of cell culture, transfection, harvest, sample preparation, and western blotting analysis are similar to those in the ubiquitination assay.
CHO cells are maintained in DMEM complete medium (DMEM supplemented with 10% FBS and 1% PS) in a humid incubator at 37 °C and 5% CO 2 .
Culture CHO cells in a 100 mm diameter culture dish until 90% confluency.
Pass cells at the ratio of 1:30 into a 12-well cell culture plate.
Incubate the cells at 37 °C, 5% CO 2 for ~20 h until 50%–70% confluency.
Replace with 1 mL of fresh medium 4 h before transfection.
Transfect cells with plasmids expressing HA-Vangl2, and/or FLAG-Wnt5a/Ror2 and Myc-CK1δ, using 1 mg/mL PEI at the ratio of 1:4 (µg DNA: µg PEI). For each well of a 12-well culture plate, transfect 1 µg plasmids in total (0.5 µg HA-Vangl2 + 0.5 µg empty vector, 0.5 µg HA-Vangl2 + 0.5 µg FLAG-Wnt5a/Ror2, or 0.5 µg HA-Vangl2 + 0.5 µg Myc-CK1δ).
Incubate the cells for 12 h.
Replace with 1 mL of fresh medium 12 h post transfection.
Culture the transfected cells for 48 h in total.
Gently aspirate media, and rinse cells with cold PBS three times immediately before cell harvest.
Collect cells as follows: detach cells using trypsin, and inactivate trypsin using DMEM complete medium. Pipette cells into an Eppendorf tube for centrifugation, and resuspend the cell pellet using 200 µL of cold PBS.
Centrifugate at 1,500 × g , 4 °C for 3 min, to collect the cells. Discard the supernatants.
Prepare the sample for western blotting. Lyse the cells in 75 µL of RIPA lysis buffer containing protease and phosphatase inhibitors. Incubate on ice for 20 min. Centrifugate at 16,000 × g , 4 °C for 15 min, and collect the supernatants. Add 25 µL of 4× Laemmli Sample Buffer containing 10% 2-Mercaptoethanol to the supernatants. Boil at 95 °C for 10 min. Store at -20 °C until use.
Detect and analyze the phosphorylation of Vangl2 by western blotting. Run an 8% Tris/Bis SDS-PAGE gel at 120 V for ~2 h, until the 55 kDa marker band runs to the bottom of the gel.
Use HA and Vangl2 phospho-specific antibodies for detection.
Data analysis
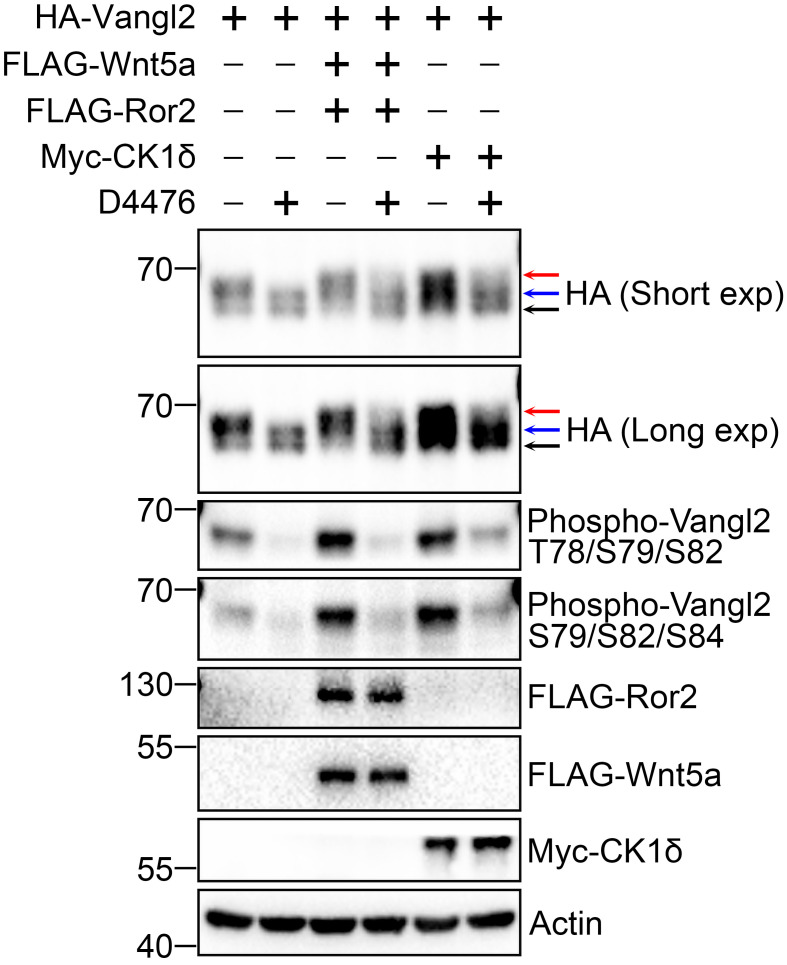
Here is a representative result showing that Wnt5a/Ror2 and CK1δ markedly promote phosphorylation, especially the hyperphosphorylation of Vangl2 ( Figure 3 ).
Figure 3. Detection of Vangl2 phosphorylation.
Wnt5a/Ror2 and CK1δ largely promote the phosphorylation of HA-Vangl2. CHO cells were transfected with HA-Vangl2 and/or co-transfected with FLAG-Wnt5a/Ror2 and Myc-CK1δ plasmids for 48 h before cell harvest, in the presence or absence of CK1 inhibitor D4476 (100 μM) for 6 h. The cell lysates were subjected to immunoblotting by the indicated antibodies. For detection of the phosphorylation of Vangl2, HA and two site-specific phospho-Vangl2 antibodies (p-T78/S79/S82 and p-S79/S82/S84) were used. Actin serves as a loading control. The lower-black, middle-blue, and upper-red arrows denote unphosphorylated, basally phosphorylated, and hyperphosphorylated Vangl2, respectively.
Recipes
-
Phosphate Buffered Saline (PBS)
Reagent Concentration NaCl 137 mM KCl 2.7 mM Na 2 HPO 4 10 mM KH 2 PO 4 1.8 mM Adjust the pH to 7.4.
-
RIPA Lysis Buffer
Reagent Concentration Tris [pH 7.4] 50 mM NaCl 150 mM IGEPAL CA-630 1% (v/v) Deoxycholic acid 0.25% (v/v) SDS 0.1% (m/v) For cell lysis, add protease and phosphatase inhibitors (final concentration 1×) immediately before use.
-
Buffer A
Reagent Concentration Guanidine 6 M Na 2 HPO 4 /NaH 2 PO 4 0.1 M Imidazole 10 mM Adjust the pH to 8.0, by preparing 1 L of 0.1 M Na 2 HPO 4 /NaH 2 PO 4 buffer (mix 93.2 mL of 1 M Na 2 HPO 4 and 6.8 mL of 1 M NaH 2 PO 4 ).
For cell lysis before the ubiquitination assay, add deubiquitinase inhibitor NEM (10 mM), and protease and phosphatase inhibitors (final concentration 1×) immediately before use.
-
Buffer TI
Reagent Concentration Tris 25 mM Imidazole 20 mM Adjust the pH to 6.8.
-
Buffer B
A mixture of Buffer A: Buffer TI with a volume ratio of 1:3.
-
8% Tris/Bis SDS-PAGE Gel
An 8% Tris/Bis SDS-PAGE gel contains the lower resolving gel (8%) and the upper stacking gel (5%).
-
8% Resolving Gel
Reagent Volume (mL) per 10 mL resolving gel H 2 O 4.6 30% Acrylamide/Bis solution 29:1 2.7 1.5 M Tris (pH 8.8) 2.5 10% SDS 0.1 10% APS 0.1 TEMED 0.006 -
5% Stacking Gel
Reagent Volume (mL) per 2 mL stacking gel H 2 O 1.4 30% Acrylamide/Bis solution 29:1 0.33 1.0 M Tris (pH 6.8) 0.25 10% SDS 0.02 10% APS 0.02 TEMED 0.002 -
WB Running Buffer
Reagent Concentration Tris-HCl [pH 7.6] 25 mM Glycine 192 mM SDS 0.1% (m/v) -
WB Transfer Buffer
Reagent Concentration Tris-HCl [pH 7.6] 25 mM Glycine 192 mM Methanol 10% (v/v) -
WB Blocking Buffer (5% BSA in TBST Buffer)
Reagent Concentration Tris 19 mM NaCl 137 mM KCl 2.7 mM Tween-20 0.1% (v/v) BSA 5% (m/v) -
WB Washing Buffer (TBST Buffer)
Reagent Concentration Tris 19 mM NaCl 137 mM KCl 2.7 mM Tween-20 0.1% (v/v)
Acknowledgments
Funding: This research was supported by grants from the Hong Kong Research Grants Council (ECS_27115317), (GRF_17122119), and (GRF_17118120) to B.G., and grants from the National Natural Science Foundation (general program_31771561 and 32170711) to B.G. We appreciate our original research papers where this protocol was derived from: Feng et al. (2021), Gao et al. (2011), and Yang et al. (2017).
Competing interests
The authors declare that they have no competing interests.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
Q&A
Post your question about this protocol in Q&A and get help from the authors of the protocol and some of its users.
References
- 1. Butler M. T. and Wallingford J. B. ( 2017 . ). Planar cell polarity in development and disease . Nat Rev Mol Cell Biol 18 ( 6 ): 375 - 388 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Choo Y. S. and Zhang Z. ( 2009 . ). Detection of protein ubiquitination . J Vis Exp ( 30 ): 1293 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chuykin I. , Itoh K. , Kim K. and Sokol S. Y. ( 2021 . ). Frizzled3 inhibits Vangl2-Prickle3 association to establish planar cell polarity in the vertebrate neural plate . J Cell Sci 134 ( 24 ): jcs258864 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Feng D. , Wang J. , Yang W. , Li J. , Lin X. , Zha F. , Wang X. , Ma L. , Choi N. T. , Mii Y. , et al. .( 2021 . ). Regulation of Wnt/PCP signaling through p97/VCP-KBTBD7-mediated Vangl ubiquitination and endoplasmic reticulum-associated degradation . Sci Adv 7 ( 20 ): eabg2099 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gao B. , Song H. , Bishop K. , Elliot G. , Garrett L. , English M. A. , Andre P. , Robinson J. , Sood R. , Minami Y. , et al. .( 2011 . ). Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror 2. Dev Cell 20 ( 2 ): 163 - 176 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Humphries A. C. and Mlodzik M. ( 2018 . ). From instruction to output: Wnt/PCP signaling in development and cancer . Curr Opin Cell Biol 51 : 110 - 116 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kelly L. K. , Wu J. , Yanfeng W. A. and Mlodzik M. ( 2016 . ). Frizzled-Induced Van Gogh Phosphorylation by CK1epsilon Promotes Asymmetric Localization of Core PCP Factors in Drosophila . Cell Rep 16 ( 2 ): 344 - 356 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ossipova O. , Kim K. and Sokol S. Y. ( 2015 . ). Planar polarization of Vangl2 in the vertebrate neural plate is controlled by Wnt and Myosin II signaling . Biol Open 4 ( 6 ): 722 - 730 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Radaszkiewicz T. , Noskova M. , Gomoryova K. , Vondalova Blanarova O. , Radaszkiewicz K. A. , Pickova M. , Vichova R. , Gybel T. , Kaiser K. , Demkova L. , et al. .( 2021 . ). RNF43 inhibits WNT5A-driven signaling and suppresses melanoma invasion and resistance to the targeted therapy . Elife 10 : e65759 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Strutt H. , Gamage J. and Strutt D. ( 2019 . ). Reciprocal action of Casein Kinase Iepsilon on core planar polarity proteins regulates clustering and asymmetric localisation . Elife 8 : e45107 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang B. , Jie Z. , Joo D. , Ordureau A. , Liu P. , Gan W. , Guo J. , Zhang J. , North B. J. , Dai X. , et al. .( 2017 . ). TRAF2 and OTUD7B govern a ubiquitin-dependent switch that regulates mTORC2 signalling . Nature 545 ( 7654 ): 365 - 369 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang J. , Feng D. and Gao B. ( 2021 . ). An Overview of Potential Therapeutic Agents Targeting WNT/PCP Signaling . Handb Exp Pharmacol 269 : 175 - 213 . [DOI] [PubMed] [Google Scholar]
- 13. Yang W. , Garrett L. , Feng D. , Elliott G. , Liu X. , Wang N. , Wong Y. M. , Choi N. T. , Yang Y. and Gao B. ( 2017 . ). Wnt-induced Vangl2 phosphorylation is dose-dependently required for planar cell polarity in mammalian development . Cell Res 27 ( 12 ): 1466 - 1484 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang Y. and Mlodzik M. ( 2015 . ). Wnt-Frizzled/planar cell polarity signaling: cellular orientation by facing the wind(Wnt) . Annu Rev Cell Dev Biol 31 : 623 - 646 . [DOI] [PMC free article] [PubMed] [Google Scholar]