Abstract
Background
Persons with multiple sclerosis (pwMS) have higher risk of mortality compared with the general population. Longitudinal studies are important for understanding the evolution of survival in pwMS.
Objective
Examine changes in mortality among pwMS during the past seven decades.
Methods
We followed pwMS from Hordaland and Møre and Romsdal in Western Norway, with disease onset from before 1950, identified from population-based epidemiological surveys and the Norwegian MS Registry and Biobank, until 1 January 2021. Data were linked to the Norwegian Cause of Death Registry to obtain underlying cause of death. We examined all-cause, and cause-specific mortality using standardised mortality ratios (SMR) and excess death rates (EDR). We calculated life expectancies and assessed survival stratified by sex, age and disease phenotype at onset. We compared hazard ratios (HRs) for mortality, in pwMS diagnosed before and after the era of disease-modifying treatment (DMT).
Results
Of 3624 pwMS, 964 (55.5% women) had died, predominantly of multiple sclerosis (49.0%). Median life expectancy for pwMS was 74.3 years (95% CI 73.3 to 75.3), compared with 83.1 years for the general population (p<0.001). From disease onset, pwMS survived 14.6 years shorter than the general population (p<0.001). Overall, SMR was 2.3 (95% CI 2.13 to 2.42) and EDR was 6.8 (95% CI 6.42 to 7.09) for pwMS. Treatment-eligible pwMS diagnosed in the DMT era had the lowest risk of mortality, HR 0.49 (95% CI 0.34 to 0.70, p<0.001).
Conclusion
Excess mortality among pwMS declined during the past seven decades, possibly due to improved diagnostics, better symptomatic treatment and access to DMTs.
Keywords: MULTIPLE SCLEROSIS, NEUROEPIDEMIOLOGY
WHAT IS ALREADY KNOWN ON THIS TOPIC
Studies examining the temporal trends of survival in people with multiple sclerosis (pwMS) have demonstrated conflicting results. To better understand the evolution of survival in pwMS during the past seven decades, we examined population-based longitudinal changes in all-cause and cause-specific mortality.
WHAT THIS STUDY ADDS
Survival in pwMS has improved during the past seven decades, potentially due to access to disease-modifying treatment, better symptomatic treatment and reduced diagnostic delay.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE AND/OR POLICY
In practice, pwMS can be reassured that the prognosis of the disease has improved over time. Survival studies have unambiguous logical validity, and this study demonstrates the importance and feasibility of linking individual data held by national health registries to obtain outcomes otherwise unattainable in randomised clinical trials.
Introduction
Persons with multiple sclerosis (pwMS) have reduced life expectancy compared with the general population,1 and longitudinal studies of temporal trends in multiple sclerosis (MS) survival have demonstrated inconsistent findings.2–6
The era of disease-modifying treatment (DMT) in MS began almost 30 years ago with the pivotal randomised clinical trial comparing injectable interferon beta (IFNβ)−1b to placebo.7 Long-term follow-up of the participants receiving active treatment demonstrated increased longevity more than 20 years later.8 Longer survival of pwMS exposed to IFNβ−1b was confirmed more recently using real-world data,9 emphasising the importance of such studies and their ability to provide data otherwise unattainable in the clinical trial setting due to limited follow-up time.
In addition to their therapeutic benefits, DMTs may cause short-term and long-term side effects. A recent study from Norway indicated an increase in the incidence of cancer among pwMS compared with the general population after DMTs were implemented in routine clinical practice from 1996.10 Thus, longitudinal studies that allow comparison of time periods before and after DMTs became available are important to determine the natural course of MS and to evaluate the benefits and potential side effects of DMTs on mortality in pwMS.
To better understand the evolution of MS survival during the past seven decades, we examined all-cause and cause-specific mortality among pwMS in Hordaland and Møre and Romsdal counties in Western Norway. We hypothesised that cancer and infectious disease-related mortality could change after the introduction of DMTs in Norway.
Methods
Study design
We conducted a retrospective cohort study by linking data from The Norwegian MS Registry and Biobank (NorMSR) and the Norwegian Cause of Death Registry (CoDR). We report our results as suggested in the Strengthening the Reporting of Observational studies in Epidemiology statement guideline.11
Study population
We followed all pwMS registered in the databases of the local hospital trusts held by NorMSR until their time of death, or 1 January 2021, which ever came first. We also followed pwMS, not found in NorMSR, ascertained from previous longitudinal population-based epidemiological studies in the corresponding geographic areas under investigation in the present study.12 13 We avoided duplicate entries in the final data set by filtering the aggregated material using each person’s unique 11-digit national identification number. We included data on the year of disease onset, date of diagnosis and initial disease course, that is, persons with relapsing onset MS (pwROMS) or persons with progressive onset MS (pwPOMS).14 Data on smoking history, time of conversion to secondary progressive MS (SPMS), longitudinal DMT exposure and symptomatic therapy were incomplete.
The NorMSR is synchronised weekly with the National Population Register. This synchronisation automatically updates the vital status, that is, date of death, for pwMS in NorMSR within 1-week postmortem. We crosslinked the final data set with CoDR to identify the underlying cause of death (UCoD) for all participants, including the date of death for those not contained in NorMSR. The WHO defines UCoD as ‘the disease or injury which initiated the train of morbid events leading directly to death, or the circumstances of the accident or violence which produced the fatal injury’.15 The CoDR contains UCoD information from death certificates dating back to 1951. In CoDR, data are coded according to the 6th through 10th revisions of the International Classification of Diseases (ICD) and grouped according to the European Shortlist for Causes of Death (ESLCoD) published by Eurostat.16 We classified the UCoDs using the ESLCoD which comprises 17 main categories, with a total of 65 subspecific groups. For a particular UCoD subcategory to be reported and included in the statistical analyses, there had to be at least 10 deaths and the ESLCoD taxonomy had to be informative. Thus, we excluded main UCoD categories, in which less than 10 deaths occurred from the statistical analyses. Consolidated subcategories, consisting of several ICD codes, labelled using unspecific nomenclature, such as ‘other diseases of the (organ) system’, were reported without further specification under their respective main category. However, and since MS is included in the consolidated ESLCoD subcategory labelled ‘other diseases of the nervous system and sense organs’, we made one exception to this rule to gain access to disease-specific UCoD data.
Statistical methods
Survival
We estimated overall survival using the standard Kaplan-Meier estimator, following pwMS from date of disease onset to date of death, or date of administrative censoring, 1 January 2021. Life expectancy was estimated using the Ederer V.2 estimator using lifetables stratified on sex, age and calendar year (in 1-year intervals). To evaluate the effect of age at onset on survival, we calculated the median age at death and median survival time in years for women and men based on disease course at presentation.
Mortality
To assess all-cause and cause-specific mortality in the study population, we used national aggregated population data on number of deaths, stratified by sex, 10-year age groups and calendar year to calculate the standardised mortality ratio (SMR) and the excess death rate (EDR).17 SMR is the quotient obtained by dividing the number of observed deaths in a sample population, by the number of expected deaths in a standard population matched by age group, sex and calendar-year of death, whereas EDR is the difference between the observed number of deaths per 1000 person-years and the corresponding expected number. Thus, SMR serves as a measure of relative risk, whereas EDR provides an absolute measure of excess mortality. Some authors hold EDR as a better measurement than SMR when performing longitudinal comparisons of mortality.1 4 5 18 19 We calculated overall SMR and EDR by sex, initial disease course and age at onset. To examine the temporal trend in all-cause mortality, we calculated SMR and EDR for seven cohorts defined by year of disease onset. The first onset cohort includes pwMS who experienced their first symptom of MS before 1950, while the second through sixth onset cohorts include pwMS with onset during the five following decades, 1950–2000. The seventh onset cohort includes pwMS with onset of MS between 2000 and 2015. To estimate the contribution of disease duration on excess mortality from all causes, we calculated EDR for different decades after MS onset in women and men based on disease course. Using UCoD data, we assessed cause-specific mortality across the seven onset cohorts stratified by sex, initial course of disease and age at onset.
To estimate the risk of mortality among pwROMS diagnosed before and after DMTs became available, we used multivariate Cox proportional hazards regression to estimate the HR for different cohorts based on time of diagnosis. The first DMTs, that is, injectable interferons, were introduced in Norway from 1996. To account for some delay in the implementation of novel drugs in routine practice, and to allow for inclusion of participants diagnosed recently leading up to DMTs becoming available, we selected the group of pwROMS diagnosed between 1990 and 1999 as the reference group. To adjust for potential confounding, we included sex, age at onset and diagnostic delay as covariates in the model. To account for differences in observation time across the cohorts, we performed a sensitivity analysis, restricting follow-up to 20 years.
The data were analysed in Stata V.17.
Results
We identified 3624 pwMS, of which 3088 were held by NorMSR. 964 (26.6%) were deceased by 1 January 2021, of whom 535 (55.5 %) were women, yielding a total of 79 897 person-years. Initial course of disease was unknown for 12 (1.2 %), and we could not obtain the UCoD for 31 (3.2 %), deceased pwMS. At the time of death, 109 pwMS were residing outside the geographic areas under investigation, but none was lost to follow-up. Among the deceased, 768 (79.7 %) died in a health institution or hospital. Autopsy was performed in 86 (8.9 %) cases. An overview of the study population, including demographics and clinical characteristics, is shown in table 1.
Table 1.
Clinical and demographic data on deceased persons with multiple sclerosis by period of disease onset, in Hordaland and Møre and Romsdal counties, Western Norway
| Period of onset (N) |
≤1950 (n=141) |
1950–1959 (n=122) |
1960–1969 (n=191) |
1970–1979 (n=376) |
1980–1989 (n=538) |
1990–1999 (n=644) |
2000–2015 (n=1312) |
Total |
| Sex | ||||||||
| Female | 81 (8.5%) | 67 (7.0%) | 80 (8.3%) | 139 (14.4%) | 96 (10.0%) | 57 (5.9%) | 15 (1.6%) | 535 (55.5%) |
| Male | 60 (6.2%) | 49 (5.1%) | 67 (7.0%) | 96 (10.0%) | 91 (9.4%) | 42 (4.4%) | 24 (2.5%) | 429 (44.5%) |
| MS-type | ||||||||
| PPMS | 21 (2.2%) | 32 (3.3%) | 34 (3.5%) | 51 (5.3%) | 46 (4.8%) | 35 (3.6%) | 18 (1.8%) | 237 (24.6%) |
| RRMS | 118 (12.2%) | 82 (8.5%) | 113 (11.7%) | 180 (18.7%) | 141 (14.6%) | 61 (6.3%) | 20 (2.1%) | 715 (74.2%) |
| Unknown | 2 (0.2%) | 2 (0.2%) | 0 (0.0%) | 4 (0.4%) | 0 (0.0%) | 3 (0.3%) | 1 (0.1%) | 12 (1.2%) |
| Age at onset | ||||||||
| <20 | 26 (2.7%) | 5 (0.5%) | 14 (1.5%) | 13 (1.3%) | 7 (0.7%) | 3 (0.3%) | 0 (0.0%) | 68 (7.1%) |
| 20–29 | 60 (6.2%) | 44 (4.5%) | 55 (5.7%) | 82 (8.5%) | 44 (4.5%) | 19 (2.0%) | 2 (0.2%) | 306 (31.8%) |
| 30–39 | 41 (4.3%) | 40 (4.1%) | 48 (5.0%) | 72 (7.5%) | 70 (7.3%) | 20 (2.1%) | 12 (1.2%) | 303 (31.4%) |
| 40–49 | 12 (1.2%) | 20 (2.1%) | 22 (2.3%) | 42 (4.4%) | 32 (3.3%) | 33 (3.4%) | 8 (0.8%) | 169 (17.5%) |
| ≥50 | 2 (0.2%) | 7 (0.7%) | 8 (0.8%) | 26 (2.7%) | 34 (3.5%) | 24 (2.5%) | 17 (1.8%) | 118 (12.2%) |
| Age at diagnosis | ||||||||
| <20 | 1 (0.1%) | 1 (0.1%) | 1 (0.1%) | 4 (0.4%) | 5 (0.5%) | 2 (0.2%) | 0 (0.0%) | 14 (1.4%) |
| 20–29 | 17 (1.8%) | 9 (0.9%) | 34 (3.5%) | 42 (4.4%) | 28 (2.9%) | 12 (1.2%) | 2 (0.2%) | 144 (14.9%) |
| 30–39 | 42 (4.4%) | 41 (4.3%) | 44 (4.5%) | 68 (7.1%) | 57 (5.9%) | 20 (2.1%) | 8 (0.8%) | 280 (29.1%) |
| 40–49 | 43 (4.5%) | 33 (3.4%) | 36 (3.7%) | 68 (7.1%) | 47 (4.9%) | 29 (3.0%) | 9 (0.9%) | 265 (27.5%) |
| ≥50 | 38 (3.9%) | 32 (3.3%) | 32 (3.3%) | 53 (5.5%) | 50 (5.2%) | 36 (3.7%) | 2 (0.2%) | 261 (27.1%) |
| Median diagnostic delay (years) | 13.0 | 8.0 | 5.0 | 4.0 | 2.0 | 1.9 | 1.0 | 4.3 |
| Autopsy* | ||||||||
| No | 132 (13.7%) | 110 (11.4%) | 130 (13.5%) | 208 (21.6%) | 172 (17.8%) | 92 (9.5%) | 34 (3.5%) | 878 (91.1%) |
| Yes | 9 (0.9%) | 6 (0.6%) | 17 (1.7%) | 27 (2.8%) | 15 (1.6%) | 7 (0.7%) | 5 (0.5%) | 86 (8.9%) |
| Place of death | ||||||||
| Private home | 3 (0.3%) | 6 (0.6%) | 10 (1.0%) | 28 (2.9%) | 35 (3.6%) | 13 (1.3%) | 6 (0.6%) | 101 (10.5%) |
| Hospital and other health institution | 111 (11.5%) | 96 (10.0%) | 126 (13.1%) | 198 (20.5%) | 134 (13.9%) | 77 (8.0%) | 26 (2.7%) | 768 (79.7%) |
| Not specified | 27 (2.8%) | 14 (1.5%) | 11 (1.1%) | 9 (0.9%) | 18 (1.9%) | 9 (0.9%) | 7 (0.7%) | 95 (9.8%) |
*Including forensic, medicolegal and unspecified autopsies.
PPMS, Primary-progressive multiple sclerosis.; RRMS, Relapsing-remitting multiple sclerosis.
Categorical survival
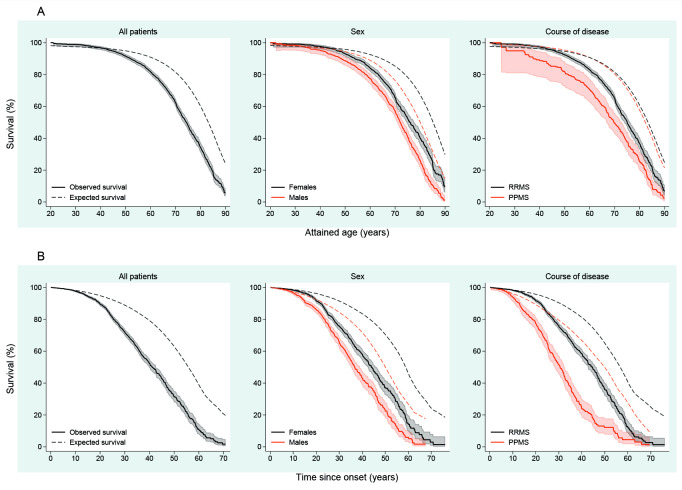
The median life expectancy for pwMS was 74.3 years (95% CI 73.3 to 75.3), compared with 83.1 years for the general population (p<0.001). For women with MS, the median life expectancy was 75.9 years (95% CI 74.6 to 77.6), and for men with MS, it was 72.0 years (95% CI 70.9 to 73.4), compared with 85.2 years for women (p<0.001) and 80.0 years for men (p<0.001) in the general population. The median life expectancy for pwROMS was 75.0 years (95% CI 73.8 to 76.3) and 69.7 years (95% CI 66.7 to 72.9) for pwPOMS (p<0.001). The survival curves are shown in figure 1A.
Figure 1.
Survival for persons with multiple sclerosis, from birth (A) and disease onset (B), stratified by sex and initial course of disease, in Hordaland and Møre and Romsdal.
Calculated from MS onset (figure 1B), the median survival time for pwMS was 14.6 years shorter compared with the general population (p<0.001). Correspondingly, the median survival time for women and men with MS was reduced by 14.9 (p<0.001) and 13.4 (p<0.001) years. Similarly, survival from disease onset of pwROMS and pwPOMS was shortened by 12.0 (p<0.001) and 19.3 (p<0.001) years.
The median age at death, and the median time from MS onset to death, stratified by sex and initial disease course, is found in supplemental material (online supplemental table 1).
jnnp-2022-329169supp001.pdf (99.7KB, pdf)
All-cause mortality
When assessing all-cause mortality, the overall EDR was 6.8 (95% CI 6.42 to 7.09) and SMR was 2.3 (95% CI 2.13 to 2.42, p<0.001) for pwMS compared with the general population. The all-cause EDR and SMR for women were 6.1 (95% CI 5.75 to 6.46) and 2.5 (95% CI 2.25 to 2.67, p<0.001), while the corresponding numbers for men were 7.9 (95% CI 7.22 to 8.60) and 2.1 (95% CI 1.90 to 2.30, p<0.001). For pwROMS, all-cause EDR and SMR were 5.6 (95% CI 5.28 to 5.98) and 2.2 (95% CI 2.03 to 2.35, p<0.001), whereas for pwPOMS, EDR was 14.5 (95% CI 13.24 to 15.60) and the SMR was 2.6 (95% CI 2.25 to 2.92, p<0.001). The all-cause EDRs and SMRs for the different age groups at onset and onset cohorts are shown in table 2.
Table 2.
All-cause standardised mortality ratios (SMRs) and excess death rates (EDRs) among persons with multiple sclerosis in Hordaland and Møre and Romsdal counties, Western Norway
| Observed | Expected | SMR (95% CI) | EDR (95% CI) | |
| Sex | ||||
| Female | 535 | 218.3 | 2.5* (2.25 to 2.67) | 6.1 (5.75 to 6.46) |
| Male | 429 | 205.4 | 2.1* (1.90 to 2.30) | 7.9 (7.22 to 8.60) |
| Course of disease | ||||
| PPMS† | 237 | 92.3 | 2.6* (2.25 to 2.92) | 14.5 (13.24 to 15.60) |
| RRMS‡ | 715 | 327.3 | 2.2* (2.03 to 2.35) | 5.6 (5.28 to 5.98) |
| Unknown | 12 | N/A | N/A | N/A |
| Age at onset | ||||
| <20 | 68 | 17.8 | 3.8* (2.96 to 4.84) | 6.4 (5.77 to 6.86) |
| 20–29 | 306 | 102.7 | 3.0* (2.66 to 3.33) | 6.9 (6.47 to 7.25) |
| 30–39 | 303 | 134.4 | 2.3* (2.01 to 2.52) | 6.7 (6.04 to 7.24) |
| 40–49 | 169 | 93.9 | 1.8* (1.54 to 2.09) | 6.0 (4.78 to 7.05) |
| ≥50 | 118 | 74.8 | 1.6* (1.31 to 1.89) | 9.2 (6.03 to 11.79) |
| Period of onset | ||||
| <1950 | 141 | 63.7 | 2,2* (1.86 to 2.61) | 13.5 (11.52 to 15.22) |
| 1950–1959 | 116 | 55.4 | 2.1* (1.73 to 2.51) | 13.6 (11.07 to 15.61) |
| 1960–1969 | 147 | 58.6 | 2.5* (2.12 to 2.95) | 12.3 (10.85 to 13.48) |
| 1970–1979 | 235 | 72.8 | 3.2* (2.83 to 3.67) | 12.9 (12.13 to 13.61) |
| 1980–1989 | 187 | 82.5 | 2.3* (1.95 to 2.61) | 6.3 (5.50 to 6.93) |
| 1990–1999 | 99 | 53.3 | 1.9* (1.51 to 2.26) | 2.9 (2.19 to 3.54) |
| 2000–2015 | 39 | 36.0 | 1.1 (0.77 to 1.48) | 0.2 (-0.62 to 0.76) |
| Period of diagnosis | ||||
| <1980 | 475 | 177.5 | 2.7* (2.44 to 2.93) | 15.8 (14.94 to 16.65) |
| 1980–1989 | 249 | 97.2 | 2.6* (2.25 to 2.90) | 10.3 (9.39 to 11.03) |
| 1990–1999 | 152 | 73.3 | 2.1* (1.76 to 2.43) | 4.4 (3.66 to 4.95) |
| 2000–2019 | 88 | 75.7 | 1.2 (0.93 to 1.43) | 0.4 (–0.19 to 0.94) |
| Total | 964 | 423.6 | 2.3* (2.13 to 2.42) | 6.8 (6.42 to 7.09) |
*p < 0.001.
†Primary progressive multiple sclerosis.
‡Relapsing-remitting multiple sclerosis.
EDR, excess death rates; SMR, standardised mortality ratios.
The all-cause EDRs for five decades after MS onset are shown in table 3 stratified by sex and disease course.
Table 3.
Excess death rate (EDR) with 95% CIs by decade after disease onset in deceased persons with multiple sclerosis, in Hordaland and Møre and Romsdal counties, Western Norway
| Decade after disease onset | |||||
| First | Second | Third | Fourth | Fifth | |
| Women | 0.3 (–0.17–0.71) | 3.6 (3.01–4.12) | 13.0 (12.11–13.81) | 16.3 (14.29–17.96) | 23.8 (19.12–27.46) |
| RRMS* | 0.2 (–0.36–0.56) | 3.0 (2.41–3.51) | 11.0 (9.98–11.76) | 12.5 (10.26–14.35) | 21.4 (16.30–25.33) |
| PPMS† | 1.8 (–0.71–3.14) | 8.7 (5.73–10.74) | 30.1 (26.64–32.57) | 48.5 (42.73–52.51) | 45.2 (29.92–53.64) |
| Men | 0.5 (–0.59–1.24) | 5.9 (4.70–6.90) | 16.1 (14.24–17.68) | 24.9 (21.09–28.01) | 23.2 (11.53–31.99) |
| RRMS‡ | 0.1 (–1.01–0.91) | 4.2 (2.87–5.13) | 14.3 (12.37–15.84) | 18.8 (14.58–22.11) | 24.0 (11.90–32.91) |
| PPMS§ | 2.0 (–1.57–4.06) | 13.9 (9.95–16.79) | 24.8 (18.28–29.48) | 62.7 (50.73–70.96) | 17.3 (-39.98–43.02) |
| Total | 0.4 (–0.09–0.77) | 4.4 (3.88–4.93) | 14.1 (13.26–14.88) | 19.2 (17.39–20.74) | 23.6 (18.97–27.46) |
*n=412.
†n=115.
‡n=303.
§n=122.
PPMS, Primary progressive multiple sclerosis.; RRMS, Relapsing-remitting multiple sclerosis.
Cause-specific mortality
Overall, during the study period, the most frequent UCoD was MS (n=473, 49.0 %), followed by diseases of the circulatory system (n=148, 15.4 %) and neoplasms (n=144, 14.9 %).
We found a marked excess in mortality due to epilepsy, SMR 22.4 (95% CI 14.9 to 32.3, p<0.001). Similarly, we found increased cause-specific mortality from diseases of the respiratory system, SMR 1.6 (95% CI 1.17 to 2.09, p<0.01). For this main UCoD category, we found an excess in the number of deaths in men, SMR 1.8 (95% CI 1.12 to 2.61, p<0.05), in pwROMS, SMR 1.5 (95% CI 1.07 to 2.11, p<0.05), in pwMS with onset before 30 years of age, SMR 2.3 (95% CI 1.37 to 3.65, p<0.01) and those with disease onset before 1951, SMR 2.5 (95% CI 1.34 to 4.30, p<0.01). Overall, twenty-one participants died due to pneumonia, SMR 2.3 (95% CI 1.14 to 3.49, p<0.01). For this, UCoD subcategory we found excess mortality both among women with SMR 2.3 (95% CI 1.13 to 4.03, p<0.05) and men with SMR 2.3 (95% CI 1.11 to 4.25, p<0.05), for pwROMS with SMR 2.4 (95% CI 1.34 to 2.82, p<0.01) and among pwMS with disease onset before 1951 with SMR 2.8 (95% CI 1.45 to 6.03, p<0.01) and those experiencing their first MS symptom before 30 years of age with SMR 3.1 (95% CI 1.23 to 6.30, p<0.05).
We found excess mortality due to gastrointestinal cancers in the 1980–1989 onset cohort, SMR 2.2 (95% CI 1.02 to 4.24, p<0.05), but, overall, we could not observe excess mortality due to neoplasms, SMR 1.0 (95% CI 0.82 to 1.15). Although we could not find excess mortality due to diseases of the digestive system in total, there was an increase in SMR among pwMS with onset before age 30 in this main category, SMR 2.4 (95% CI 1.11 to 4.62, p<0.05).
Overall, there was no excess mortality due to diseases of the circulatory system, SMR 1.1 (95% CI 0.97 to 1.34). However, we found excess mortality from diseases of the circulatory system, among women with MS for the total study period, SMR 1.4 (95% CI 1.07 to 1.70, p<0.05), and for the 1970–1979 onset cohort, SMR 1.7 (95% CI 1.20 to 2.39, p<0.01). Death due to underlying acute myocardial infarction was increased in the same subgroups, SMR 1.6 (95% CI 1.02 to 2.33, p<0,05) in women and SMR 1.9 (95% CI 1.05 to 3.22, p<0.05) for the 1970–1979 onset cohort. For the latter subgroup mortality from cerebrovascular diseases was increased, SMR 2.5 (95% CI 1.27 to 4.55, p<0.05).
The cause-specific SMRs for all the reported UCoD categories and selected subcategories are shown in table 4.
Table 4.
Overall cause-specific standardised mortality ratios (SMRs) in persons with multiple sclerosis for selected* underlying causes of death (UCoDs)
| Observed | Expected | SMR | 95% CI | |
| Neoplasms (total) | 144 | 147.5 | 1.0 | 0.82 to 1.15 |
| Malignant neoplasm of colon. rectum and anus | 23 | 18.8 | 1.2 | 0.78 to 1.84 |
| Malignant neoplasm of trachea. bronchus. lung | 30 | 28.5 | 1.1 | 0.71 to 1.5 |
| Malignant neoplasm of breast | 10 | 14.3 | 0.7 | 0.34 to 1.29 |
| Diseases of the nervous system and the sense organs (total) | 510 | 14.7 | 34.6† | 31.7 to 37.8 |
| Multiple sclerosis | 473 | 1.9 | 248.9† | 222.4 to 267.0 |
| Epilepsy | 28 | 1.3 | 22.4† | 14.9 to 32.3 |
| Diseases of the circulatory system (total) | 148 | 129.4 | 1.1 | 0.97 to 1.34 |
| Acute myocardial infarction | 55 | 45.4 | 1.2 | 0.91 to 1.58 |
| Other ischaemic heart diseases | 23 | 19.9 | 1.2 | 0.73 to 1.73 |
| Cerebrovascular diseases | 35 | 29.4 | 1.2 | 0.83 to 1.65 |
| Diseases of the respiratory system (total) | 49 | 30.9 | 1.6‡ | 1.17 to 2.09 |
| Pneumonia | 21 | 9.2 | 2.3‡ | 1.14 to 3.49 |
| Other chronic lower respiratory diseases | 18 | 16.2 | 1.1 | 0.66 to 1.75 |
| Diseases of the digestive system (total) | 16 | 13.4 | 1.2 | 0.68 to 1.94 |
| External causes of morbidity and mortality (total) | 29 | 32.2 | 0.9 | 0.60 to 1.30 |
| Accidents | 18 | 20.3 | 0.9 | 0.53 to 1.40 |
| Suicide and intentional self-harm | 11 | 10.5 | 1.0 | 0.52 to 1.87 |
| Unknown underlying cause of death | 32 | N/A | N/A | N/A |
*The following main categories according to the European Shortlist for Causes of Death (ESLCoD) are not reported (see Methods); infectious and parasitic diseases, diseases of the blood and blood-forming organs and certain disorders involving the immune mechanism, endocrine, nutritional and metabolic diseases, mental and behavioural disorders, diseases of the skin and subcutaneous tissue, diseases of the musculoskeletal system/connective tissue, diseases of the genitourinary system, complications of pregnancy, childbirth and puerperium, certain conditions originating in the perinatal period, congenital malformations and chromosomal abnormalities and symptoms, signs, ill-defined causes.
†p<0.001.
‡p<0.01.
All-cause mortality risk before and after the introduction of immunomodulatory therapy
We identified 715 deceased pwROMS. Of these, 559 were diagnosed before 1990, including 190 between 1980 and 1989. Between 1990 and 1999, 112 pwROMS were diagnosed, and 44 in the subsequent years until 2019.
All-cause mortality risk was lower for pwROMS diagnosed between 2000 and 2019, HR 0.49 (95% CI 0.34 to 0.70, p<0.001) compared with the reference group, that is, those diagnosed between 1990 and 1999 (table 5). For pwROMS diagnosed before DMTs became available, those diagnosed between 1980 and 1989 had lower risk of mortality, HR 1.7 (95% CI 1.30 to 2.10, p<0.001), than those diagnosed before 1980, HR 2.4 (95% CI 1.94 to 3.05, p<0.001). These findings remained consistent in the sensitivity analysis in which follow-up time was restricted to 20 years (results not shown).
Table 5.
All-cause mortality risk in relapsing-remitting multiple sclerosis by period of diagnosis, sex, age at onset and diagnostic delay
| HR | 95% CI | P value | |
| Period of diagnosis | |||
| <1980 | 2.4 | 1.94 to 3.05 | <0.001 |
| 1980–1989 | 1.7 | 1.30 to 2.10 | <0.001 |
| 1990–1999 | Reference | – | – |
| 2000–2019 | 0.49 | 0.34 to 0.70 | <0.001 |
| Sex | |||
| Women | Reference | ||
| Men | 1.4 | 1.18 to 1.59 | <0.001 |
| Age at onset | |||
| <20 | Reference | – | – |
| 20–29 | 1.4 | 1.07 to 1.88 | 0.016 |
| 30–39 | 1.9 | 1.44 to 2.58 | <0.001 |
| 40–49 | 3.2 | 2.33 to 4.52 | <0.001 |
| ≥50 | 8.5 | 5.83 to 12.39 | <0.001 |
| Diagnostic delay | |||
| <2 years | Reference | – | – |
| 2–4 years | 1.4 | 1.13 to 1.74 | 0.002 |
| 5–10 years | 1.7 | 1.34 to 2.05 | <0.001 |
| >10 years | 2.7 | 2.25 to 3.34 | <0.001 |
Discussion
In this longitudinal study, we found shortened lifespans and excess mortality in pwMS, corroborating the findings from a recent meta-analysis.6 Approximately, 8 out of 10 pwMS died while receiving care at a hospital or another health institution, which is similar to a study from Finland published recently.20
The life expectancy for pwMS was, on average, reduced by 8.8 years, which is similar to the findings in a Canadian population-based study.21 The observed 3.9-year difference in median life expectancy between women and men with MS is comparable to the sex difference observed in the general Norwegian population. On average, pwROMS survived, 5.3 years longer than pwPOMS.
When evaluating survivorship from disease onset, the survival of pwMS started to diverge from that of the general population after approximately 10 years of disease duration. Furthermore, this decrement appears to occur earlier in men and is most pronounced for pwPOMS (figure 1B).
When assessing the effect of age at onset on survival, a later onset appears favourable when attained age is considered. However, in terms of absolute survival time in years, those who experience onset of MS at younger ages survive the longest (online supplemental table 1). A similar phenomenon has been observed for clinical, neuropsychological and radiologic markers of disease progression when comparing adult-onset to paediatric-onset MS, where the paediatric group requires longer time to achieve specific disability and MRI milestones but does so at younger age.22
As shown in table 2, the all-cause SMR initially increased for pwMS with disease onset from before 1950 and up to 1979, whereas EDR remained stable in the same period. For the subsequent onset cohorts, we observed a decrease in comparative and absolute excess mortality as measured by all-cause SMR and EDR, corroborating previous findings from Hordaland.3 However, and as pointed out by the authors of a recent meta-analysis, which failed to demonstrate a reduction in all-cause SMR in pwMS during the past 65 years6, shorter follow-up time for participants enrolled towards the end of a study period, among other factors, offers a plausible explanation for the apparent improvement in survivorship observed in previously published reports from Scandinavia.2 4
When assessing all-cause mortality by age of MS onset, SMR was highest for the youngest onset group, and lowest for the oldest onset group, implying a higher relative mortality risk for pwMS that experience early onset compared with the general population (table 2). This observation is expected when acknowledging that competing mortality risks increase with age, and when considering that mortality in the younger background population is low.
In absolute terms, however, we found similar EDRs for all the different age at onset groups, apart from the oldest (≥50 years) group (table 2). Current age was recently shown, in a study modelling EDR trajectories in a large cohort of pwMS from France, to have a stronger effect on mortality in pwROMS compared with duration of disease.23 In the present study, we did not find an excess mortality during the first decade after disease onset, but after more than 10 years of disease duration, we found a time-dependent successive increase in EDR (table 3), which corroborates findings from previous reports.18 19
As expected, the cause-specific mortality due to MS was high (table 4). The SMR for epilepsy was also considerable and comorbid epilepsy has been associated with increased risk of mortality in pwMS.24 However, 26 out of 28 deaths in this subcategory occurred in patients experiencing onset before 1959. Thus, epilepsy appears to be a historical UCoD in this longitudinal study population.
Cancer mortality was not increased among patients with RRMS, nor among the latest onset cohorts, which constitute patients most likely exposed to DMTs. Excess mortality from gastrointestinal neoplasms was only observed for the 1980–1989 onset cohort, possibly due to fluctuations in cancer incidence.
Among pwMS with onset before age 30, we found excess mortality due to diseases of the digestive system, but not in total (table 4).
Overall, we found excess mortality due to disease of the circulatory system in women and for the 1970–1979 onset cohort. Indeed, pwMS, and females more than males, appear to have an increased cardiovascular disease mortality.25 The observed increase in comparative mortality for the 1970–1979 onset cohort is arguably a reflection of known cardiovascular risk factors, for example, smoking, and high dietary intake of saturated fats, which previously were more common.
We observed an excess in mortality from diseases of the respiratory system, particularly pneumonia, and most evident in pwMS with disease onset before 30 years of age. Progressive disability accumulation and immobility are known risk factors for developing respiratory infections, including aspiration pneumonia, which are common causes of death in pwMS.21
When comparing all-cause mortality risk for pwROMS before and after the start of the DMT era, and by using pwROMS diagnosed between 1990 and 1999 as the reference group, we found the risk to be highest among those diagnosed before 1980. Between 1980 and 1989, this risk decreased, and after DMTs were made readily available between 2000 and 2019, this risk was more than halved (table 5).
We suggest that several healthcare improvements, not only access to DMTs, are associated with the observed improvement in all-cause mortality risk for pwROMS, but also the implementation of MRI contributing to reduced diagnostic delay and better symptomatic therapies (table 1).
Strengths and limitations
The use of national health registries, a well-defined study population, and the long duration of follow-up are strengths of the present study.
However, our study has limitations. During the study period, different diagnostic criteria have been applied, including outdated revisions based primarily or exclusively on clinical assessment, and without the use of advanced ancillary examinations. The clinical application of different diagnostic criteria, in combination with early initiation of DMT, is associated with pwMS reaching disability milestones at a slower rate.26 Following a diagnosis of MS, early exposure to DMTs, or treatment of disease related symptoms and signs, could convey beneficial effects on survival and mortality. However, and since we did not have detailed data on symptomatic therapy or DMT use, we are unable to elaborate on these potential effects.
Due to the longitudinal study design, pwMS diagnosed during the earliest periods may have inherent differences in disease severity and interval from onset to diagnosis. The median diagnostic delay from MS onset varied noticeably for the different onset cohorts (table 1). Using survival measures based on time of disease onset negates this effect partially, but a person’s recollection of their first suggestive symptom of MS is subject to recall bias, particularly for pwMS brought to late diagnostic attention or who initially developed mild or unspecific transient symptoms. Furthermore, retrospective determination of disease onset based on suggestive symptomatology, as provided by pwMS themselves or through scrutinising medical records, is unlikely to be accurate, especially when considering the increasing amount of evidence of a protracted prodromal, preclinical and presymptomatic phase in MS.27–29
For the survival and mortality analyses, we were unable to evaluate objective measures of individual disease severity, for example, frequency of relapses and clinical signs of disability progression, and paraclinical evidence of disease activity, that is, formation of new lesions on MRI and accelerated brain atrophy. We did not have comprehensive data on smoking history for estimating the amount of tobacco exposure in the study population. Smoking in pwMS has been associated with disability progression, conversion to SPMS and premature mortality.30 31 Similarly, we did not have access to data on comorbid conditions to assess individual disease burden beyond MS. Comorbidities are common in pwMS and affect disability progression and survival negatively.32 33 This also pertains to certain types of malignancies, which appear to be more prevalent among pwMS,34 and the incidence of cancer was higher among pwMS in Norway after DMTs became available.10 However, in the present study, we did not find excess cancer mortality among persons considered eligible for treatment with DMTs, that is, pwROMS.
Conclusion
In summary, our study provides longitudinal real-world data in a well-defined cohort of pwMS demonstrating shortened lifespans compared with the general population. We observed a decrease in excess mortality parallel to the reduced delay from MS onset to diagnosis, which could imply a prognostic role of early diagnostic verification. For pwROMS, those diagnosed after the start of the DMT era had a more favourable outcome. We did not observe an increase in cancer or infectious disease-related mortality after DMTs were introduced.
To identify disease features associated with reduced longevity in pwMS, future studies should employ detailed clinical data, particularly exposure to specific DMTs and treatment of disease manifestations and comorbidities, as well as paraclinical measures of individual disease severity, including promising biomarkers.
Acknowledgments
The authors thank Stephanie Jebsen Fagerås, Gunhild Forland Slungård and Yngve Pedersen at the Norwegian Institute of Public Health for providing national background data.
Footnotes
Contributors: JSW: designed and conceptualised the study, collected the data, analysed the data, drafted the manuscript for intellectual content and is responsible for the overall content as guarantor. NG: collected the data and revised the manuscript for intellectual content. JHA: analysed the data and revised the manuscript for intellectual content. TÅM: performed the statistical analyses, drafted the figure and revised the manuscript for intellectual content. K-MM: designed and conceptualised the study, and revised the manuscript for intellectual content. RM: designed and conceptualised the study, collected the data, and revised the manuscript for intellectual content.
Funding: The study was funded by grants from Møre og Romsdal Hospital Trust.
Competing interests: JSW reports personal fees from Biogen Idec, NG, JHA, RM and TÅM report no disclosures. K-MM reports grants and personal fees from Biogen Idec and Novartis; personal fees from Genzyme, Roche, Almirall, and Merck; personal fees and nonfinancial support from Teva, outside the submitted work.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Data may be obtained from a third party and are not publicly available.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Scalfari A, Knappertz V, Cutter G, et al. Mortality in patients with multiple sclerosis. Neurology 2013;81:184–92. 10.1212/WNL.0b013e31829a3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burkill S, Montgomery S, Hajiebrahimi M, et al. Mortality trends for multiple sclerosis patients in Sweden from 1968 to 2012. Neurology 2017;89:555–62. 10.1212/WNL.0000000000004216 [DOI] [PubMed] [Google Scholar]
- 3. Lunde HMB, Assmus J, Myhr K-M, et al. Survival and cause of death in multiple sclerosis: a 60-year longitudinal population study. J Neurol Neurosurg Psychiatry 2017;88:621–5. 10.1136/jnnp-2016-315238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Koch-Henriksen N, Laursen B, Stenager E, et al. Excess mortality among patients with multiple sclerosis in Denmark has dropped significantly over the past six decades: a population based study. J Neurol Neurosurg Psychiatry 2017;88:626–31. 10.1136/jnnp-2017-315907 [DOI] [PubMed] [Google Scholar]
- 5. Manouchehrinia A, Tanasescu R, Tench CR, et al. Mortality in multiple sclerosis: meta-analysis of standardised mortality ratios. J Neurol Neurosurg Psychiatry 2016;87:324–31. 10.1136/jnnp-2015-310361 [DOI] [PubMed] [Google Scholar]
- 6. Smyrke N, Dunn N, Murley C, et al. Standardized mortality ratios in multiple sclerosis: systematic review with meta-analysis. Acta Neurol Scand 2022;145:360–70. 10.1111/ane.13559 [DOI] [PubMed] [Google Scholar]
- 7. Group TIMSS . Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. The IFNB multiple sclerosis Study Group. Neurology 1993;43:655–61. 10.1212/WNL.43.4.655 [DOI] [PubMed] [Google Scholar]
- 8. Goodin DS, Ebers GC, Cutter G, et al. Cause of death in MS: long-term follow-up of a randomised cohort, 21 years after the start of the pivotal IFNβ-1b study. BMJ Open 2012;2:e001972. 10.1136/bmjopen-2012-001972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kingwell E, Leray E, Zhu F, et al. Multiple sclerosis: effect of beta interferon treatment on survival. Brain 2019;142:1324–33. 10.1093/brain/awz055 [DOI] [PubMed] [Google Scholar]
- 10. Grytten N, Myhr K-M, Celius EG, et al. Incidence of cancer in multiple sclerosis before and after the treatment era- a registry- based cohort study. Mult Scler Relat Disord 2021;55:103209. 10.1016/j.msard.2021.103209 [DOI] [PubMed] [Google Scholar]
- 11. von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495–9. 10.1016/j.ijsu.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 12. Grytten N, Aarseth JH, Lunde HMB, et al. A 60-year follow-up of the incidence and prevalence of multiple sclerosis in Hordaland County, Western Norway. J Neurol Neurosurg Psychiatry 2016;87:100–5. 10.1136/jnnp-2014-309906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Willumsen JS, Aarseth JH, Myhr K-M, et al. High incidence and prevalence of MS in Møre and Romsdal County, Norway, 1950-2018. Neurol Neuroimmunol Neuroinflamm 2020;7:e713. 10.1212/NXI.0000000000000713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Myhr K-M, Grytten N, Torkildsen Ø, et al. The Norwegian multiple sclerosis registry and Biobank. Acta Neurol Scand 2015;132:24–8. 10.1111/ane.12427 [DOI] [PubMed] [Google Scholar]
- 15. WHO . International classification of diseases for mortality and morbidity statistics (ICD-11). World Health Organization; [Eleventh Revision]. Available: https://icd.who.int/icd11refguide/en/index.html#2.17.0Mortalitystatistics|mortality-statistics|c2-17-1 [Accessed 12 Nov 2021].
- 16. Eurostat . European Shortlist for causes of death, 2012. Available: https://ec.europa.eu/eurostat/ramon/nomenclatures/index.cfm?TargetUrl=LST_NOM_DTL&StrNom=COD_2012 [Accessed 01 Nov 2021].
- 17. Norwegian Institute of Public Health . Norwegian, 2021. Available: http://statistikkbank.fhi.no/dar/ [Accessed 01 Nov 2021].
- 18. Smestad C, Sandvik L, Celius EG. Excess mortality and cause of death in a cohort of Norwegian multiple sclerosis patients. Mult Scler 2009;15:1263–70. 10.1177/1352458509107010 [DOI] [PubMed] [Google Scholar]
- 19. Brønnum-Hansen H, Koch-Henriksen N, Stenager E. Trends in survival and cause of death in Danish patients with multiple sclerosis. Brain 2004;127:844–50. 10.1093/brain/awh104 [DOI] [PubMed] [Google Scholar]
- 20. Murtonen A, Lehto JT, Sumelahti M-L. End of life in multiple sclerosis: disability, causes and place of death among cases diagnosed from 1981 to 2010 in Pirkanmaa Hospital district in Western Finland. Mult Scler Relat Disord 2021;54:103139. 10.1016/j.msard.2021.103139 [DOI] [PubMed] [Google Scholar]
- 21. Harding K, Zhu F, Alotaibi M, et al. Multiple cause of death analysis in multiple sclerosis: a population-based study. Neurology 2020;94:e820–9. 10.1212/WNL.0000000000008907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bonacchi R, Meani A, Pagani E, et al. Association of age at onset with gray matter volume and white matter microstructural abnormalities in people with multiple sclerosis. Neurology 2021;97:e2007–19. 10.1212/WNL.0000000000012869 [DOI] [PubMed] [Google Scholar]
- 23. Rollot F, Fauvernier M, Uhry Z, et al. Effects of age and disease duration on excess mortality in patients with multiple sclerosis from a French nationwide cohort. Neurology 2021;97:e403–13. 10.1212/WNL.0000000000012224 [DOI] [PubMed] [Google Scholar]
- 24. Mahamud Z, Burman J, Zelano J. Prognostic impact of epilepsy in multiple sclerosis. Mult Scler Relat Disord 2020;38:101497. 10.1016/j.msard.2019.101497 [DOI] [PubMed] [Google Scholar]
- 25. Palladino R, Marrie RA, Majeed A, et al. Evaluating the risk of macrovascular events and mortality among people with multiple sclerosis in England. JAMA Neurol 2020;77:820. 10.1001/jamaneurol.2020.0664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tintore M, Cobo-Calvo A, Carbonell P, et al. Effect of changes in MS diagnostic criteria over 25 years on time to treatment and prognosis in patients with clinically isolated syndrome. Neurology 2021;97:e1641–52. 10.1212/WNL.0000000000012726 [DOI] [PubMed] [Google Scholar]
- 27. Gout O, Lebrun-Frenay C, Labauge P, et al. Prior suggestive symptoms in one-third of patients consulting for a "first" demyelinating event. J Neurol Neurosurg Psychiatry 2011;82:323–5. 10.1136/jnnp.2008.166421 [DOI] [PubMed] [Google Scholar]
- 28. Cortese M, Riise T, Bjørnevik K, et al. Preclinical disease activity in multiple sclerosis: a prospective study of cognitive performance prior to first symptom. Ann Neurol 2016;80:616–24. 10.1002/ana.24769 [DOI] [PubMed] [Google Scholar]
- 29. Bjornevik K, Munger KL, Cortese M, et al. Serum neurofilament light chain levels in patients with presymptomatic multiple sclerosis. JAMA Neurol 2020;77:58. 10.1001/jamaneurol.2019.3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Healy BC, Ali EN, Guttmann CRG, et al. Smoking and disease progression in multiple sclerosis. Arch Neurol 2009;66. 10.1001/archneurol.2009.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Manouchehrinia A, Weston M, Tench CR, et al. Tobacco smoking and excess mortality in multiple sclerosis: a cohort study. J Neurol Neurosurg Psychiatry 2014;85:1091–5. 10.1136/jnnp-2013-307187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chou IJ, Kuo CF, Tanasescu R, et al. Comorbidity in multiple sclerosis: its temporal relationships with disease onset and dose effect on mortality. Eur J Neurol 2020;27:105-112. 10.1111/ene.14040 [DOI] [PubMed] [Google Scholar]
- 33. Marrie RA, Elliott L, Marriott J, et al. Effect of comorbidity on mortality in multiple sclerosis. Neurology 2015;85:240–7. 10.1212/WNL.0000000000001718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marrie RA, Maxwell C, Mahar A, et al. Cancer incidence and mortality rates in multiple sclerosis: a matched cohort study. Neurology 2021;96:e501–12. 10.1212/WNL.0000000000011219 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnnp-2022-329169supp001.pdf (99.7KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data may be obtained from a third party and are not publicly available.