Abstract
One component of host defense at mucosal surfaces appears to be epithelium-derived antimicrobial peptides. Molecules of the defensin and cathelicidin families have been studied in several species, including human and mouse. We describe in this report the identification and characterization of rhesus monkey homologues of human mucosal antimicrobial peptides. Using reverse transcriptase PCR methodology, we cloned the cDNAs of rhesus monkey β-defensin 1 and 2 (rhBD-1 and rhBD-2) and rhesus monkey LL-37/CAP-18 (rhLL-37/rhCAP-18). The predicted amino acid sequences showed a high degree of homology to the human molecules. The expression of the monkey antimicrobial peptides was analyzed using immunohistochemistry with three polyclonal antibodies to the human molecules. As in humans, rhesus monkey antimicrobial peptides are expressed in epithelia of various organs. The present study demonstrates that β-defensins and cathelicidins of rhesus monkeys are close homologues to the human molecules and indicate that nonhuman primates represent valid model organisms to study innate immune functions.
Antimicrobial peptides have been found in a wide array of animal species ranging from insects to lower vertebrates and mammals (8). These peptides contribute to innate host defense against a number of bacterial and fungal pathogens. Mammalian defensins are small antimicrobial peptides (3.5 to 4.5 kDa) that are characterized by the presence of six cysteines which form three disulfide bonds, whose ordered array defines the classification as α- or β-defensin (12, 13). β-defensins have been described in leukocytes of cattle and fowl, as well as at mucosal surfaces of different organ systems (17). Human β-defensins 1 and 2 (hBD-1 and hBD-2) are expressed in surface organs, such as epithelia of the urinary, intestinal, and respiratory tracts (3, 7, 14, 23). The cathelicidins are a distinct family of antimicrobial peptides and are characterized by a highly conserved signal sequence and proregion (called “cathelin”) but show substantial heterogeneity in their C-terminal domain that encodes the mature peptide (24). The only human cathelicidin, LL-37/hCAP-18, was isolated from human bone marrow. LL-37/hCAP-18 is expressed in myeloid cells and is also found on body surfaces such as skin and respiratory epithelia, where it is secreted into the airway surface fluid (1, 4, 9).
Defects of the function of antimicrobial peptides have been implicated in the development of human diseases such as cystic fibrosis (6, 20). However, lacking appropriate animal models, no final conclusions can be drawn with respect to the role of these molecules in the pathogenesis of human disease. Mice express several antimicrobial peptides homologous to the human molecules, such as mouse β-defensins 1 to 4 or cathelin-related antimicrobial murine peptide (2, 5, 11, 16, 18, 19). For several reasons, murine models seem inappropriate to study human disease. Mice do not express defensins in neutrophils (10), whereas in humans these cells contain large amounts of antimicrobial peptides of the defensin and cathelicidin class. This may indicate that the murine and human innate immune systems differ significantly. Furthermore, murine models often do not reveal the characteristic pathology of human disease.
In the present study we describe the cloning of rhesus monkey (Macaca mulatta) antimicrobial peptides of the β-defensin and cathelicidin families. The newly identified molecules reveal a high degree of homology to the human antimicrobial peptides and are expressed in the same organs and cell types. Nonhuman primates may represent valid animal models to study innate immune functions.
MATERIALS AND METHODS
Cloning of rhBD-1, rhBD-2, and rhLL-37 cDNA.
The cDNA sequences of hBD-1 (GenBank accession number NM_005218), hBD-2 (GenBank accession number NM_004942), and LL-37 (GenBank accession number Z38026) were used to generate primers spanning the entire molecule. Reverse-transcriptase PCR was used to clone the full-length cDNA sequences of rhesus monkey peptides. Total RNA was isolated from lung or bone marrow of a 3-year-old male rhesus monkey (Buckshire Farms, Perkasie, Pa.) by using Trizol (Gibco BRL) and further purified to poly(A)+ RNA using oligo(dT) columns (Qiagen). Poly(A)+ RNA (approximately 100 ng) was reverse transcribed using NotI-(dT)18 as primer (First-Strand cDNA Synthesis kit; Pharmacia Biotech) and 10% of the reaction mixture was used for a PCR. Specific primers to the molecules are listed in Table 1. PCR was carried out using a Hybaid thermocycler. The PCR products were analyzed on a 1.5% agarose gel, and bands of the calculated length were cut out and cloned into pGEM T (Promega). Animal experiments were approved by the Institutional Animal Care and Use Committees of the University of Pennsylvania or the Primatenzentrum Göttingen, Göttingen, Germany.
TABLE 1.
Primers useda
| Molecule | 5′ primer | 3′ primer |
|---|---|---|
| rhBD-1 | CCTGCCAGGCGCCATGAGAACTTC | GCTCACTTGCAGCACTTGGCCTTC |
| rhBD-2 | CATCAGCCATGAGGGTCTTGTATC | CTCATGGCTTTTTGCAGCATTTTG |
| rhLL-37/rhCAP-18 | GAATTCCGGCCATGAAGACCCAAAG | CTCAGAGCCCAGAAGCCTGAGCC |
The primers used to amplify cDNA of rhesus monkey antimicrobial peptides were deduced from the mRNA sequence of hBD-1, hBD-2, and LL-37/hCAP-18.
Expression analysis.
All tissue samples were obtained from the Primatenzentrum Göttingen and were formalin fixed and paraffin embedded using routine methodology. Immunohistochemical methods applying antibodies raised against the human homologous peptides were used to analyze the tissue distribution of the antimicrobial peptides. The antibodies were generated by performing a standard immunization protocol in rabbits using peptides purified from urine (hBD-1), isolated from a recombinant baculovirus system (hBD-2), or synthesized chemically (LL-37) as described previously (3, 4). The antibodies were tested to be specific for the corresponding antimicrobial peptide and showed no cross-reactivity with the other antimicrobial peptides or other cationic substances such as mucins, lactoferrin, or lysozyme. Comparisons of the immune and preimmune sera in blotting experiments showed the specific reactivity of the polyclonal antibodies (data not shown). Based on the high degree of structural similarity between human and rhesus monkey antimicrobial peptides, a cross-reactivity of the antibodies was predicted to be likely. We used a two-step indirect immunohistochemical method. Sections were deparaffinized in xylol (20 min), hydrated in serial dilutions of ethanol, and brought into Tris-buffered saline (TBS, pH 7.4) for 5 min. To eliminate the endogenous peroxidase, the sections were treated with 3% H2O2 for 5 min. After washes in TBS three times, the sections were incubated with a 1:5 dilution of goat serum in TBS for 20 min to reduce unspecific background staining. Sections were incubated with different dilutions of the primary antibody in TBS (1:50 and 1:500) for 18 h at 4°C. After three washes in TBS, the sections were incubated with the biotinylated secondary goat anti-rabbit antibody (1:1000 in TBS) for 30 min. Then the sections were washed in TBS and incubated with peroxidase-conjugated streptavidin lasting 20 min in a 1:150 dilution with TBS. After washes, 6 mg of 3,3-diaminobenzidine-tetrahydrochloride (DAB) in 10 ml of distilled water with 2 drops of 3% H2O2 were applied as chromogen. Alternatively, the detection of the bound primary antibodies was carried out by using the Histostain Plus staining kit (Zymed Laboratories Inc.) using 3-amino-9-ethylcarbazole (AEC) as chromogen according to the manufacturer's instructions. The sections were dehydrated in serial dilutions of ethanol, treated with xylol, and mounted. Negative controls lacked the primary antibody or used preimmune serum instead. Some sections were counterstained using acidic hematoxylin or the periodic acid-schiff reaction using standard methods.
For lectin histochemistry, dehydrated sections were incubated for 1 h with a dilution of the biotinylated lectin peanut agglutinin (5 μg/ml in phosphate-buffered saline). After washes in phosphate-buffered saline the same detection procedure was used, as described above.
The staining intensity of cell types was evaluated using a semiquantitative scoring system, as follows: no staining (0), low staining (1), intermediate staining (2), strong staining (3), and very strong staining (4). The results were evaluated by three independent investigators and averaged. For each organ or tissue, material from three to four animals was used. Negative controls did not show any signal above background.
Nucleotide sequence accession numbers.
The nucleotide sequences of the identified antimicrobial peptides have been submitted to GenBank and assigned the following accession numbers: rhBD-1, AF288285; rhBD-2, AF288286; and rhLL-37/rhCAP-18, AF288284.
RESULTS
Structure of rhesus monkey antimicrobial peptides.
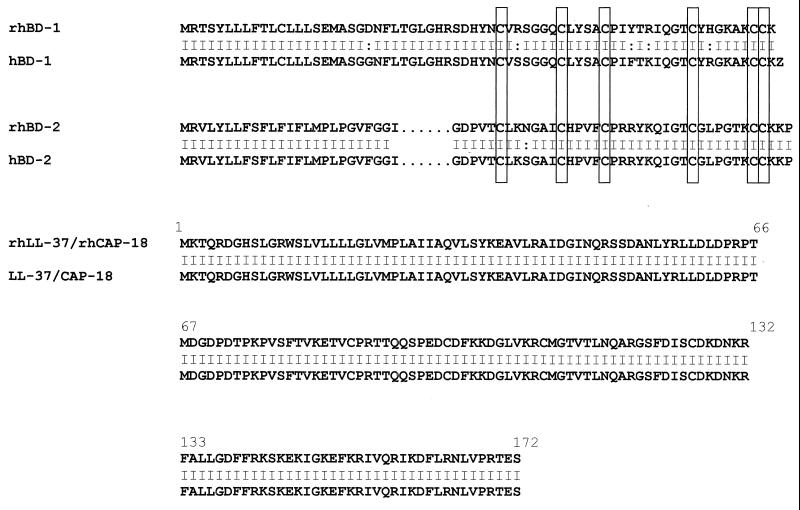
The cDNAs of rhesus monkey antimicrobial peptides were cloned by using a reverse transcriptase PCR methodology based on homology to the human molecules. The degree of similarity on the nucleic level between human and rhesus monkey BD-1 and BD-2 is 93.2 and 99.5%, respectively. Rhesus monkey LL-37 (rhLL-37) revealed 99.6% sequence similarity to human LL-37. The predicted amino acid sequences of rhesus monkey and human peptides are shown in Fig. 1. Like the human molecules, the monkey β-defensin molecules reveal the structural hallmarks of this peptide family. The amino terminal prepro-portions of the peptides contain several hydrophobic residues, which are characteristic for β-defensins and are found in other β-defensin family members expressed on mucosal surfaces. The putative mature peptide contains six cysteine residues spaced in a typical array. Several characteristic charged residues are present in the putative mature peptide. Like its human homologue, rhLL-37 revealed a highly conserved proregion of the molecule, called cathelin (Fig. 1). The cloning procedures were repeated several times with identical results.
FIG. 1.
Comparison of the amino acid sequences of rhBD-1, rhBD-2, and rhLL37/rhCAP-18 with their human homologues. Rhesus monkey β-defensins reveal a high degree of similarity to the human molecules, and rhLL-37 is identical to LL-37, including the cathelin-like proregion (amino acids 31 to 131 of the LL-37 sequence). Conserved cysteines of the defensins are labeled by boxes.
Expression of rhesus monkey antimicrobial peptides.
The tissue distribution of rhesus monkey antimicrobial peptides was analyzed at the tissue level by using immunohistochemistry with three antibodies raised against the homologous human substances. Peptides were detected in epithelial and other cell types identical to the location of the corresponding human antimicrobial molecules and showed an equivalent expression pattern for most organs. Control experiments omitting the primary antibody or using preimmune serum did not reveal a specific signal (Fig. 2L and 3B).
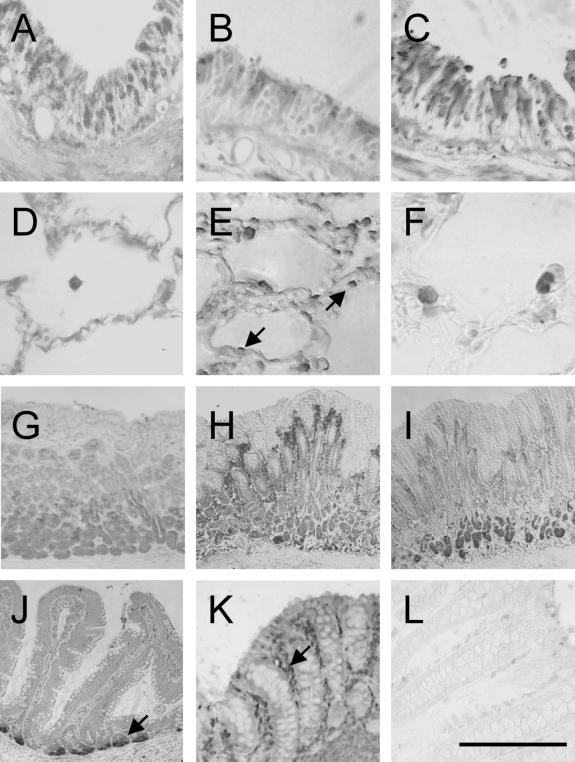
FIG. 2.
Immunohistochemical detection of rhesus monkey antimicrobial peptides in organs of the respiratory and gastrointestinal tract. In large airways (A, rhBD-1; B, rhBD-2; C, rhLL-37), all peptides were detected in ciliated epithelial cells. In distal lung (D, rhBD-1; E, rhBD-2; F, rhLL-37), type II pneumocytes revealed a positive signal for rhBD-2 (arrows), and alveolar macrophages were stained with antibodies to all three antimicrobial peptides. In the stomach, chief cells of the mucosa stained positive for all three peptides (G, rhBD-1; H, rhBD-2; I, rhLL-37); however, the staining pattern is specific for the peptides. Enterocytes of the small (J, rhBD-1) and large bowel (K, rhBD-1) were stained diffusely positive for the presence of the peptides. In the duodenum, cells located at the basal portion of crypts were intensely stained for rhBD-1 (J, arrow). In the subepithelial space of the intestinal mucosa, lymphocytes were stained intensely with the hBD-1 antibodies (K, arrow). A preimmune control section of colon showed no specific signal (L, rhBD-1 preimmune). The bar indicates 30 μm in panels A to F, 15 μm in panel G, and 60 μm in panels H to L. As chromogens, we used DAB (panels A to G, K, and L) or AEC (panels H, I, and J) (see Materials and Methods).
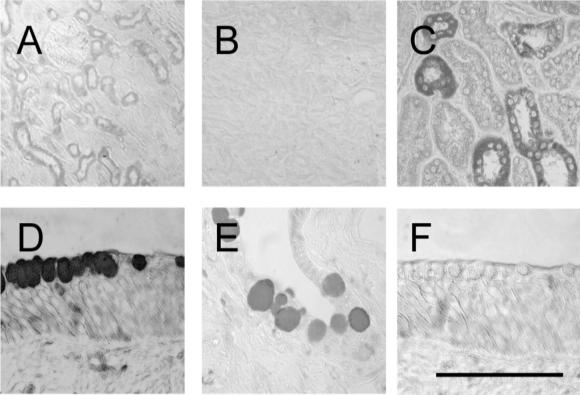
FIG. 3.
Immunohistochemical detection of rhesus monkey antimicrobial peptides in kidney (A, rhBD-1; B, rhBD-1 preimmune; C, rhLL-37) and conjunctival tissue of the eye (D, rhBD-1; E, rhBD-2; F, rhLL-37). The bar indicates 120 μm in panels A to C and 30 μm in panels D to F. As chromogens, we used DAB (panels A, B, D, and F) or AEC to (panels C and E).
In the respiratory tract (Table 2; Fig. 2), antimicrobial peptides are expressed predominantly in the large airways, revealing a proximal-distal gradient. Defensins and the cathelicidin are localized in ciliated cell types of the surface epithelium as well as serous gland cells. A weak or no significant signal was detectable in distal airway epithelia. Pneumocytes revealed a positive signal for rhBD-2. Alveolar macrophages were stained with the antibodies to the defensins and the cathelicidin (Fig. 2).
TABLE 2.
Results of the immunohistochemical analysis of expression of rhesus monkey antimicrobial peptidesa
| System | Cell type, tissue, or organ | Staining intensity for:
|
||
|---|---|---|---|---|
| rhBD-1 | rhBD-2 | rhLL-37/rhCAP-18 | ||
| Respiratory tract | Airway surface epithelium (large airways) ciliated cells | 2 | 2 | 2 |
| Airways serous gland cells | 3 | 3 | 3 | |
| Alveolar macrophages | 3 | 3 | 1 | |
| GI tract | Esophagus | 0 | 0 | 0 |
| Stomach chief cells | 2 | 2 | 3 | |
| Small bowel enterocytes | 2 | 2 | 2 | |
| Small bowel crypt cells | 3 | 3 | 3 | |
| Large bowel columnar absorptive cells | 1 | 1 | 3 | |
| Pancreas | 2 | 2 | 1 | |
| Parotid gland goblet cells or secretory cellsb | 4 | 2 | 2 | |
| Urogenital tract | Kidney (distal tubules) | 2 | 2 | 3 |
| Ureter epithelial cells | 1 | 2 | 3 | |
| Bladder epithelial cells | 3 | 2 | 3 | |
| Urethra | 1 | 0 | 3 | |
| Reproductive organs | Mammae (glands) nonlactating, lobular epithelial structures | 3 | 3 | 2 |
| Uterus (glands) | 1 | 2 | 2 | |
| Testis Sertoli cells | 2 | 2 | 0 | |
| Seminal vesicle epithelial cells | 0 | 2 | 3 | |
| Other | Eyelid goblet cells | 3 | 2 | 0 |
| Lymphocytes (in small and large bowel), CD74 positive | 4 | 4 | 0 | |
| Spleen | 0 | 0 | 0 | |
| Epidermis | 1 | 3 | 3 | |
The staining intensity of cell types was evaluated using a semiquantitative scoring system as follows: no staining (0), low staining (1), intermediate staining (2), strong staining (3), and very strong staining (4). The results were evaluated by three independent investigators and averaged. GI, gastrointestinal.
rhBD-1 staining was measured in parotid gland goblet cells in ducts, and rhBD-2 and rhLL-37/rhCAP-18 were measured in parotid gland secretory cells.
In the gastrointestinal tract, we analyzed the esophagus, stomach, and small and large bowel, as well as the parotid gland and pancreas (Table 2; Fig. 2). In the gastric mucosa, the three antibodies stained chief cells in the basal parts of the gastric mucosa. In the small and large bowel, columnar absorptive cells of the surface epithelium stained diffusely positive for the defensins and the cathelicidin. Pancreatic secretory acini were diffusely stained for all three peptides. Interlobular ducts of the parotid gland showed sparse positive cells with the antibody to rhLL-37, whereas acinar secretory cells were weakly positive for the defensins. In the kidney (Table 2; Fig. 3), antibodies to all antimicrobial peptides stained distal tubules and collecting ducts, as indicated by the staining of sections with peanut agglutinin, which selectively labels these structures. In the male reproductive system, cells in the seminiferous tubules, representing most likely Sertoli cells, stained positively for the defensins. In the female reproductive system, epithelia of nonlactating mammae stained positive for all peptides. Glands of the uterus were labeled for rhBD-2 and rhLL-37. In other organs, goblet-like cells of the conjunctiva of the eye were positive for rhBD-1 and rhBD-2 (Fig. 3). Endothelial cells at several locations were positive when probed with antibody to rhLL-37. Lymphocytes in a subepithelial location of the small and large intestine that were identified by positive staining with antibody to CD74 revealed a positive signal for the defensins.
DISCUSSION
In the present study, we described the isolation of cDNA sequences from the rhesus monkey with high homology to the human antimicrobial peptides hBD-1, hBD-2, and LL-37. We have named these antimicrobial peptides rhBD-1, rhBD-2, and rhLL-37.
Analysis of the cloned sequences revealed a high degree of similarity to the human molecules on the nucleic and amino acid level. Based on the structural similarity, we used antibodies against the human molecules for an expression analysis. Our data indicate that rhesus monkey antimicrobial peptides are expressed in a similar pattern as their human homologues (3, 7, 15). Immunohistochemical analysis of human material revealed an identical tissue distribution of β-defensins (unpublished data). The most abundant peptide levels are found in organs lining outer or inner body surfaces, such as organs of the respiratory or gastrointestinal tract. Peptides are expressed in secretory cells, indicating that their secreted forms contribute to the host defense on body surfaces. The immunohistochemical analysis revealed new insight into the biology of antimicrobial peptides, expanding expression studies of human molecules on the transcript level. In contrast to RNA studies, antimicrobial peptides were also found in phagocytes (i.e., alveolar macrophages) and lymphocytes. Also, goblet cells of the conjunctiva of the eye showed expression of defensin molecules. These results highlight the functions of antimicrobial peptides as direct endogenous antibiotics as well as mediator substances in innate and adaptive immunity. The peptides described in this study revealed a high degree of homology to human molecules. The sequences of rhesus macaque α-defensins are also closely related to the human molecules (21). Another class of defensins, the so called θ-defensins, has been described in monkeys but has not yet been identified in humans (22).
The results presented in this study indicate that rhesus monkey mucosal antimicrobial peptides resemble those of humans. Therefore, rhesus monkeys may allow the development of valid animal models to study the function of the innate immune system in health and disease, overcoming limitations that are part of many murine model systems used to study human disease.
ACKNOWLEDGMENTS
We thank F. J. Kaup, E. Fuchs, and K. Mätz-Rensing, Deutsches Primatenzentrum, Göttingen, Germany, for supplying formalin-fixed rhesus monkey tissue samples. The expert immunohistochemical contribution of K. Verch from the Institute of Anatomy of the University of Munich was greatly appreciated.
This study was supported by grants of the Cystic Fibrosis Foundation, NIDDK, and NHLBI of the NIH, as well as Genovo, Inc., a biotechnology company that J. Wilson founded and holds equity in. Robert Bals was supported by the Deutsche Forschungsgemeinschaft (Ba 1641/1, Ba 1641/3-1).
REFERENCES
- 1.Agerberth B, Gunne H, Odeberg J, Kogner P, Boman H G, Gudmundsson G H. FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc Natl Acad Sci USA. 1995;92:195–199. doi: 10.1073/pnas.92.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bals R, Goldman M J, Wilson J M. Mouse β-defensin 1 is a salt-sensitive antimicrobial peptide present in epithelia of the lung and urogenital tract. Infect Immun. 1998;66:1225–1232. doi: 10.1128/iai.66.3.1225-1232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bals R, Wang X, Wu Z, Freeman T, Banfa V, Zasloff M, Wilson J. Human beta-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. J Clin Investig. 1998;102:874–880. doi: 10.1172/JCI2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bals R, Wang X, Zasloff M, Wilson J M. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci USA. 1998;95:9541–9546. doi: 10.1073/pnas.95.16.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bals R, Wattler S, Nehls M, Meegalla R, Wilson J. Mouse β-defensin 3 is an inducible antimicrobial peptide expressed in the epithelia of multiple organs. Infect Immun. 1999;67:3542–3547. doi: 10.1128/iai.67.7.3542-3547.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bals R, Weiner D, Wilson J. The innate immune system in cystic fibrosis lung disease. J Clin Investig. 1999;103:303–307. doi: 10.1172/JCI6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bensch K, Raida M, Magert H-J, Schulz-Knappe P, Forssmann W-G. hBD-1: a novel β-defensin from human plasma. FEBS Lett. 1995;368:331–335. doi: 10.1016/0014-5793(95)00687-5. [DOI] [PubMed] [Google Scholar]
- 8.Boman H. Antibacterial peptides: key components needed in immunity. Cell. 1991;65:205–207. doi: 10.1016/0092-8674(91)90154-q. [DOI] [PubMed] [Google Scholar]
- 9.Cowland J, Johnsen A, Borregaard N. hCAP-18, a cathelin/pro-bactenecin-like protein of human neutrophil specific granules. FEBS Lett. 1995;368:173–176. doi: 10.1016/0014-5793(95)00634-l. [DOI] [PubMed] [Google Scholar]
- 10.Eisenhauer P B, Lehrer R I. Mouse neutrophils lack defensins. Infect Immun. 1992;60:3446–3447. doi: 10.1128/iai.60.8.3446-3447.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallo R, Kim K, Bernfield M, Kozak C, Zanetti M, Merluzzi L, Gennaro R. Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse. J Biol Chem. 1997;272:13088–13093. doi: 10.1074/jbc.272.20.13088. [DOI] [PubMed] [Google Scholar]
- 12.Ganz T, Lehrer R I. Defensins. Pharmacol Ther. 1995;66:191–205. doi: 10.1016/0163-7258(94)00076-f. [DOI] [PubMed] [Google Scholar]
- 13.Ganz T, Weiss J. Antimicrobial peptides of phagocytes and epithelia. Semin Hematol. 1997;34:343–354. [PubMed] [Google Scholar]
- 14.Harder J, Bartels J, Christophers E, Schroeder J-M. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 15.Harder J, Siebert R, Zhang Y, Matthiesen P, Christophers E, Schlegelberger B, Schroder J M. Mapping of the gene encoding human beta-defensin-2 (DEFB2) to chromosome region 8p22-p23.1. Genomics. 1997;46:472–475. doi: 10.1006/geno.1997.5074. [DOI] [PubMed] [Google Scholar]
- 16.Huttner K M, Kozak C A, Bevins C L. The mouse genome encodes a single homolog of the antimicrobial peptide human beta-defensin 1. FEBS Lett. 1997;413:45–49. doi: 10.1016/s0014-5793(97)00875-2. [DOI] [PubMed] [Google Scholar]
- 17.Lehrer R, Ganz T. Antimicrobial peptides in mammalian and insect host defense. Curr Opin Immunol. 1999;11:23–27. doi: 10.1016/s0952-7915(99)80005-3. [DOI] [PubMed] [Google Scholar]
- 18.Morrison G, Davidson D, Dorin J. A novel mouse beta defensin, Defb2, which is upregulated in the airways by lipopolysaccharide. FEBS Lett. 1999;442:112–116. doi: 10.1016/s0014-5793(98)01630-5. [DOI] [PubMed] [Google Scholar]
- 19.Morrison G M, Davidson D J, Kilanowski F M, Borthwick D W, Crook K, Maxwell A I, Govan J R, Dorin J R. Mouse beta defensin-1 is a functional homolog of human beta defensin-1. Mamm Genome. 1998;9:453–457. doi: 10.1007/s003359900795. [DOI] [PubMed] [Google Scholar]
- 20.Smith J, Travis S, Greenberg E, Welsh M. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell. 1996;85:229–236. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 21.Tang Y Q, Yuan J, Miller C J, Selsted M E. Isolation, characterization, cDNA cloning, and antimicrobial properties of two distinct subfamilies of alpha-defensins from rhesus macaque leukocytes. Infect Immun. 1999;67:6139–6144. doi: 10.1128/iai.67.11.6139-6144.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang Y-Q, Yaun J, Osapay G, Osapay C, Tran D, Miller C, Quellette A, Selsted M. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated alpha-defensins. Science. 1999;286:498–502. doi: 10.1126/science.286.5439.498. [DOI] [PubMed] [Google Scholar]
- 23.Valore E V, Park C H, Quayle A J, Wiles K R, McCray P B, Jr, Ganz T. Human beta-defensin-1: an antimicrobial peptide of urogenital tissues. J Clin Investig. 1998;101:1633–1642. doi: 10.1172/JCI1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zanetti M, Gennaro R, Romeo D. Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 1995;374:1–5. doi: 10.1016/0014-5793(95)01050-o. [DOI] [PubMed] [Google Scholar]