Abstract
Objective
To detect the clinical characteristics of patients with amyotrophic lateral sclerosis (ALS) carrying an intermediate ATXN2 polyQ number of repeats in a large population-based series of Italian patients with ALS.
Methods
The study population includes 1330 patients with ALS identified through the Piemonte and Valle d’Aosta Register for ALS, diagnosed between 2007 and 2019 and not carrying C9orf72, SOD1, TARDBP and FUS mutations. Controls were 1274 age, sex and geographically matched Italian subjects, identified through patients’ general practitioners.
Results
We found 42 cases and 4 controls with≥31 polyQ repeats, corresponding to an estimated OR of 10.4 (95% CI 3.3 to 29.0). Patients with≥31 polyQ repeats (ATXN2+) compared with those without repeat expansion (ATXN2−) had more frequently a spinal onset (p=0.05), a shorter diagnostic delay (p=0.004), a faster rate of ALSFRS-R progression (p=0.004) and King’s progression (p=0.004), and comorbid frontotemporal dementia (7 (28.0%) vs 121 (13.4%), p=0.037). ATXN2+ patients had a 1-year shorter survival (ATXN2+ patients 1.82 years, 95% CI 1.08 to 2.51; ATXN2− 2.84 years, 95% CI 1.67 to 5.58, p=0.0001). ATXN2 polyQ intermediate repeats was independently related to a worse outcome in Cox multivariable analysis (p=0.006).
Conclusions
In our population-based cohort, ATXN2+ patients with ALS have a distinctive phenotype, characterised by a more rapid disease course and a shorter survival. In addition, ATXN2+ patients have a more severe impairment of cognitive functions. These findings have relevant implications on clinical practice, including the possibility of refining the individual prognostic prediction and improving the design of ALS clinical trials, in particular as regards as those targeted explicitly to ATXN2.
Keywords: ALS, GENETICS
WHAT IS ALREADY KNOWN ON THIS TOPIC.
An intermediate-length CAG number of repeats (encoding≥31 glutamines, polyQ) in the ataxin 2 (ATXN2) gene is recognised to be associated with an increased risk of developing amyotrophic lateral sclerosis (ALS). However, very few is known about the phenotypic characteristics of patients with ATXN2 polyQ intermediate number of repeats.
WHAT THIS STUDY ADDS
Patients with ATXN2 polyQ intermediate number of repeats had more commonly a spinal onset and were characterised by a more rapid clinical course, as shown by a 1.5-fold ALSFRS-R progression and a significantly higher King’s progression at the time of diagnosis compared with patients without the expansion. In addition, patients with ATXN2 PolyQ intermediate number of repeats were more frequently affected by frontotemporal impairment.
WHAT THIS STUDY MAY AFFECT RESEARCH, PRACTICE, OR POLICY
The identification of the specific phenotypic characteristics of patients with ALS with ATXN2 polyQ intermediate number of repeats has many implications, including the possibility of refining the individual prognostic prediction and improving the design of ALS clinical trials, including those targeted explicitly to ATXN2.
Introduction
Amyotrophic lateral sclerosis (ALS) is a multisystem disorder of adult life characterised by progressive degeneration of upper and lower motor neurons and frontotemporal cortex neurons. Several genes have been related to this fatal neurodegenerative disorder, accounting for 10%–20% of ALS cases.1 2 Among these, an intermediate-length CAG number of repeats (encoding ≥31 glutamines, polyQ) in the ataxin 2 (ATXN2) gene, already known as the cause of spinocerebellar ataxia type 2 (characterised by a number of polyQ≥38), is recognised to be associated with an increased risk of developing ALS and has been reported to be a modifier of survival.3 4 More recently, it has been reported that an intermediate number of ATXN2 polyQ repeats can also be a modifier of frontotemporal dementia (FTD) phenotype.5 6 Nevertheless, the phenotypic characteristics of patients with ALS with intermediate-length CAG repeats in the ATXN2 gene are still incompletely understood. This study aimed at detecting the clinical characteristics of patients with ALS carrying an intermediate number of ATXN2 polyQ repeats in a large population-based series of Italian patients with ALS.
Methods
The study population includes 1487 patients with ALS identified through the Piemonte and Valle d’Aosta Register for ALS, a prospective population-based register active since 1995. The characteristics of the register have been reported elsewhere.7 For the present paper, we considered ALS cases diagnosed between 2007 and 2019. Patients met the El Escorial revised diagnostic criteria for definite, probable and probable laboratory-supported ALS.8
ALSFRS-R mean monthly decline (∆ALSFRS-R) was calculated using the following formula: (48 – ALSFRS-R score at diagnosis)/(months from onset to diagnosis). Similarly, weight mean monthly decline (∆Weight) as (Weight at diagnosis – healthy body weight)/(months from onset to diagnosis). Finally, to have a proxy of disease spread, we calculated the mean/monthly decline of King’s staging as (King’s staging at diagnosis)/(months from onset to diagnosis).
A total of 928 patients underwent an extensive cognitive battery at the time of diagnosis. These cases were classified into five categories according to the Consensus Criteria for diagnosing frontotemporal cognitive and behavioural syndromes in ALS.9 The battery assessed executive function, memory, visuospatial function, language and social cognition using the following tests Letter Fluency test; Category Fluency Test; Frontal Assessment Battery; Trail Making Test A, B and B-A; Rey-Osterrieth Complex Figure Test, immediate (IR) and delayed recall (DR); Rey Auditory Verbal Learning Test (RAVL), immediate (IR) and DR; BSRT, immediate (IR) and DR; Digit Span Forward and Backward; Raven’s Colored Progressive Matrices (CPM47); Story-Based Empathy Task; and Mini-Mental State Examination. The raw scores of all tests were age, sex and education-corrected using the more recent Italian normative.10
Neurobehavioral dysfunction was determined with the Frontal Systems Behavior Scale (FrSBe), using the Family-form evaluated by a close relative/caregiver (scores: normal≤59, borderline 60–64; pathological≥65). For this study, we considered the change in points for each of the three domains of FrSBe (apathy, disinhibition, executive) from before-disease to disease scores. If a subject had scores reflecting a frontal systems abnormality both in the premorbid and post-illness forms, they were considered pathological only if there was an increase of≥10 points at the T-score between the two states.10 Anxiety and depression were assessed using the Hospital Anxiety and Depression Scale; the item ‘I feel slowed down’ was discussed with patients to have them not refer to physical disability.11
Controls
Controls were 1274 age, sex and geographically matched Italian subjects, identified through patients’ general practitioners.
Genetic analysis
All patients included in the study were tested for SOD1 (all exons), TARDBP (exon 6), FUS (exons 14 and 15) mutations, and C9ORF72 intronic expansion using the methods described elsewhere.12 However, since 1180 cases underwent whole-genome sequencing, no mutation in the other exons of TARDBP and FUS were found.
ATXN2 CAG repeat analysis
In 434 cases and 509 controls, ATXN2 CAG repeat in exon 1 (NM_002973.3) was amplified using a fluorescent primer and sized by capillary electrophoresis on an ABI3130 genetic analyzer (Applied Biosystems, Foster City, California, USA). In 1043 cases and 765 controls, ATXN2 CAG repeats were detected through next-generation sequencing. All subjects were screened for the C9orf72 intronic expansion using a standard repeat-primed PCR13 (Renton et al. 2011 PMID: 21944779). Repeat lengths of≥30 units with the characteristic sawtooth pattern were considered pathogenic. Whole-genome sequencing was performed at The American Genome Center located at the Uniformed Services University, Bethesda, Maryland, USA. Libraries were prepared using TruSeq DNA PCR-Free High Throughput Library Prep Kit (Illumina) per the manufacturer’s instructions. Sequencing was performed on an Illumina HiSeqX10 sequencer using paired-end 150 base pair reads. A significant advantage of next-generation sequencing is its ability to reliably assay repeat expansions, such as those in C9orf72 and ATXN2. ATXN2 CAG repeats were deemed intermediate if they were in the 31–38 range. ExpansionHunter—Targeted software (V.3.0.1) was used to estimate repeat lengths of known, disease-causing expansions in samples that had undergone whole-genome sequencing.14 This algorithm has been validated using experimentally confirmed samples carrying the C9orf72 and ATXN2 repeat expansions. In particular, cases with≥31 polyQ repeats were identified correctly with both PCR and ExpansionHunter.
Statistical methods
Multivariable analysis for survival was performed with the Cox proportional hazards model (stepwise backward) with a retention criterion of p value<0.1. A p value<0.05 was considered significant. Statistical analyses were carried out using the SPSS V.26.0 statistical package (SPSS).
Results
We assessed for intermediate ATXN2 polyQ repeats 1487 patients with ALS diagnosed in Piemonte e Valle d’Aosta between 2007 and 2019. Of these, 157 were subsequently excluded from the present analysis because they carried a genetic mutation of one of the most common ALS-related genes (C9orf72, 97; SOD1, 32; TARDBP, 21; FUS, 6). We decided to exclude C9orf72 patients from this analysis because we found in a previous paper that in patients with this mutation intermediate ATXN2 polyQ repeats do not modify survival.15 As for SOD1, TARDBP and FUS, we chose to also exclude these cases because of the strong influence of these genes on survival. The study was therefore performed on 1330 patients. Controls were 1274 subjects matched to cases by age, gender and geographical origin. Patients and controls did not differ for the main demographic variables (online supplemental table 1).
jnnp-2022-329376supp001.pdf (221.6KB, pdf)
In our cohort, we found 46 cases and 44 controls with 27 to 30 polyQ repeats, and 42 cases and 4 controls with≥31 polyQ repeats. The estimated OR for 27–30 polyQ was 0.99 (95% CI 0.66 to 1.52). The estimated OR for≥31 polyQ was 10.4 (95% CI 3.3 to 29.0). The 4 controls had 31 (3) and 32 (1) polyQ repeats. Their age ranged between 55 and 72 years. They were neurologically normal and they had no family history for neurodegenerative diseases or ataxia. The number of polyQ repeats in patients was 20 (31 repeats), 12 (32 repeats), 4 (33 repeats), 3 (34 repeats), 2 (35 repeats) and 1 (38 repeats).
Phenotype of ATXN2+ patients (table 1). Patients with intermediate ATXN2 polyQ repeats≥31 polyQ repeats (ATXN2+) and those without expansion (<31 polyQ repeats) (ATXN2−) did not differ for the age at onset but ATXN2+ had more frequent spinal onset (p=0.05). In addition, ATXN+ patients had a shorter diagnostic delay (p=0.004) and a faster rate of progression as measured by ΔALSFRS-R (p=0.004) and ΔKing’s (p=0.004).
Table 1.
Demographic and clinical characteristics of patients according to ATXN2 PolyQ intermediate number of repeats (ATXN2+, PolyQ≥31; ATXN2−, PolyQ≤30)
| ATXN2+ (n=42) | ATXN2− (n=1288) | P value | |
| Age at onset (median, IQR) | 69.6 (63.5–75.7) | 68.3 (60.3–74.4) | 0.15 |
| Gender (female) | 15 (35.7%) | 575 (44.6%) | 0.25 |
| Site of onset (bulbar) | 7 (16.7%) | 393 (30.5%) | 0.05 |
| Diagnostic delay (months, IQR) | 6.0 (3.94–10.03) | 9.04 (5.88–13.97) | 0.004 |
| ALSFRS-R score at diagnosis (median, IQR) | 42 (34.75–44) | 42 (37–45) | 0.13 |
| ΔALSFRS-R (median points/month, IQR) | 1.00 (0.50–1.99) | 0.66 (0.31–1.33) | 0.004 |
| FVC% at diagnosis (median, IQR)* | 89 (74–105) | 91 (72–104) | 0.81 |
| ΔWeight (kg/month, median, IQR)† | 0.50 (0–1.26) | 0.28 (0–0.96) | 0.87 |
| MiToS stage at diagnosis (0/1/2/3/4) | 27/12/2/1/0 | 858/374/42/10/2 | 0.80 |
| King’s state at diagnosis (1/2/3/4) | 14/14/11/3 | 530/409/293/50 | 0.59 |
| ΔKing’s (median points/month, IQR) | 0.25 (0.17–0.53) | 0.19 (0.10–0.34) | 0.004 |
| ALS-FTD‡ | 7 (28.0%) | 121 (13.4%) | 0.037 |
*FVC, 1222 (ATXN2+, 38; ATXN2−, 1184).
†Weight 1288 (ATXN2+, 40; ATXN2−, 1246).
‡928 cases (ATXN2+, 25, ATXN2−, 903)
A total of 928 patients (25 ATXN2+ and 903 ATXN2−) and 129 controls underwent cognitive examination. The demographic and clinical characteristics of these patients did not differ from that of the overall cohort (online supplemental table 2). ATXN2+ patients were more commonly diagnosed as ALS-FTD (7 (28.0%) vs 121 (13.4%), p=0.037) (table 2).
Table 2.
Frequency of cognitive impairment classified according to the consensus criteria for the diagnosis of frontotemporal cognitive and behavioural syndromes in ALS9
| ATXN2+ (n=25) | ATXN2- (n=903) | |
| Cognitively normal ALS | 10 (40%) | 483 (53.5%) |
| ALSbi | – | 72 (8%) |
| ALSci | 7 (28%) | 164 (18.2%) |
| ALScbi | 1 (4%) | 63 (7%) |
| ALS-FTD | 7 (28%) | 121 (13.4%) |
In patients with ALS with ATXN2 PolyQ intermediate number of repeats ≥31 (ATXN2+) compared with patients with PolyQ ≤30 (ATXN2-). ALS-FTD was significantly more frequent in ATXN2+ patients (p=0.037).
ALS, amyotrophic lateral sclerosis; ALSbi, ALS with behavioural impairment; ALScbi, ALS with cognitive and behavioural impairment; ALSci, ALS with cognitive impairment; ALS-FTD, ALS with comorbid FTD.
When assessing the differences in specific tests, excluding the 128 patients with ALS-FTD, we found a significantly worse performance of ATXN2+ patients in the RAVL − Delayed Recall (p=0.023), the BSRT − Immediate Recall (p=0.044), and in the Executive Functions domain of ECAS (p=0.034) (online supplemental tables 3 and 4). No differences were found in the behavioural function assessed with FrSBe. Finally, no differences were found in anxiety, while depression was significantly more severe in ATXN2+ patients (p=0.04).
Survival analysis
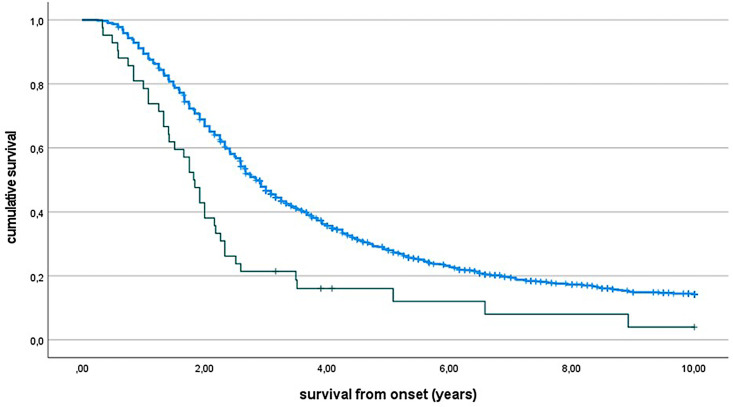
ATXN2+ patients had a 1 year shorter survival (median survival time for ATXN2+ patients = 1.82 years, 95% CI 1.08 to 2.51; ATXN2− =2.84 years, 95% CI 1.67 to 5.58, p=0.0001, figure 1). The Cox multivariable analysis confirmed that the ATXN2 polyQ intermediate number of repeats was independently related to a worse outcome compared with non-expanded patients in our cohort (p=0.006) (table 3). A separate analysis including only the patients not assessed in our previous paper3 gave similar results (data not shown).
Figure 1.
Survival from onset according to ATXN2 polyQ intermediate number of repeats. PolyQ≥31 (green line) versus PolyQ≤30 (blue line). Ticks indicate censored patients. P<0.0001.
Table 3.
Patients’ survival
| Variable | Value | HR (95% CI) | P value |
| Age at onset (years) | Per each year of age at onset | 1.030 (1.024 to 1.037) | 0.0001 |
| Diagnostic delay (months) | Per each month of delay | 0.953 (0.945 to 0.962) | 0.0001 |
| Site of onset | Spinal Bulbar |
1 (reference) 1.45 (1.27–1.67) |
0.0001 |
| ΔALSFRS-R | Per each point loss/month | 1.25 (1.20 to 1.29) | 0.0001 |
| ΔKing’s | Per each point loss/month | 1.56 (1.16 to 2.09) | 0.003 |
| ATXN2 polyQ | <31 ≥31 |
1 (reference) 1.58 (1.14 to 2.19) |
0.006 |
Cox multivariable analysis.
Analysis of oligogenicity
All but two patients with≥31 polyQ repeats underwent whole-genome sequencing. We extracted variant information for 47 genes previously implicated in ALS pathogenesis (see online supplemental table 5 for the list of extracted genes). The following six mutations of ALS-related genes, all in heterozygosis, of ALS-related genes were detected (one for each patient): OPTN: p.L111R; SETX: p.H476R; CCNF: p.R123H; EWSR1: p.A132S; SETX: p.V919I; DTCN1: p.A354V. Allele frequencies and predicted functional effects of identified genetic variants are reported in online supplemental table 6. Patients carrying one of these mutations (n=6) did not differ from those not carrying other genetic mutations (n=34) for any clinical characteristic but the median weight decline (Δweight) (see online supplemental table 7).
Discussion
We have assessed a large population-based cohort of Italian patients with ALS without mutations for C9orf72, SOD1, TARDBP and FUS genes to identify the clinical signature of ATXN2 polyQ intermediate number of repeats. We have found that ATXN2+ patients, despite having more commonly spinal onset, were characterised by a more rapid clinical course, as shown by (1) a 1.5-fold ΔALSFRS-R at the time of diagnosis compared with ATXN2- patients; (2) a shorter diagnostic delay, a factor related to a faster disease progression; and (3) a significantly higher ΔKing’s, indicating a more rapid spreading of symptoms from one to three body regions. The greater aggressiveness of ALS in subjects with ATXN2 polyQ intermediate number of repeats is reflected in the 1 year shorter survival (median survival time, 1.82 vs 2.84), confirming our previous findings.3 This result was independent of relevant prognostic factors in Cox multivariable analysis. Finally, patients with ATXN2 PolyQ intermediate number of repeats were more frequently affected by frontotemporal impairment.
ATXN2 polyQ intermediate number of repeats were first recognised as a risk factor for ALS in 2010 in a cohort of US patients (using a cut-off≥27)16 and subsequently confirmed in populations of different ethnic origin,3 with the only exception of South Africans.17 Although a length of 27–33 polyQ was initially considered significantly associated with ALS, later studies have shown that the cut-off is≥31 polyQ repeats. In our population, we found that the best risk cut-off for ALS is≥31 polyQ since the distribution of alleles in the 27–30 polyQ repeats range was substantially similar among patients and controls.
ATXN2 is an RNA binding protein with an essential function in the nucleocytoplasmic shuttling of RNA and regulation of transcription.18 However, the pathogenic mechanism of ATXN2 in ALS is unknown. It has been reported that ATXN2 induces an increase of phosphorylated TDP-43 in the spinal anterior horn but not in motor cortex neurons of patients with ALS.19 Interestingly, our data show that the spinal phenotype is more common in patients with ATXN2 polyQ intermediate number of repeats than those without expansion.
A recent paper has reported that ATXN+patients (cut-off limits≥31) did not show any survival difference compared with ATXN-.20 The discrepancy with the present study is likely related to the different nature of the two cohorts, that is, the prevalent, referral centers, population in the US study (as indicated by the young age at onset (~60 years), the median survival (~3.5 years) and finally the very low percentage of patients with ALS-FTD (3.8%)) and the incident population in our study. It is therefore possible that at least a part of fast progressor patients, including those who are ATXN+, have not been caught in the US study. Several papers have demonstrated that prevalent and incident populations strongly differ from the clinical point of view, including survival, supporting the notion that studies derived from clinical cohorts should be cautiously interpreted.21 22
A novel finding in this paper is the identification of a correlation between ATXN2 polyQ intermediate number of repeats in ALS and cognitive impairment. ALS-FTD was two times more frequent among the ATXN2+ patients (28% vs 13.4%). Furthermore, some cognitive tests related to frontal function (RAVL – delayed recall, BSRT – immediate recall, and Executive Functions domain of ECAS) were significantly more compromised in ATXN2+ patients. Interestingly, ATXN2 polyQ intermediate number of repeats (using a cut-off of≥27) have been recently proposed to be a modifier of the behavioural variant FTD phenotype, with earlier age at onset and more frequent parkinsonian and psychotic symptoms.5 6 23 However, no increased risk of developing behavioural variant FTD was reported by other studies.24 25 An antagonistic pleiotropic role in cognition of ATXN2 has been identified, with a positive influence on verbal–numerical reasoning, reaction time, educational attainment and cognitive resilience,26 27 while in spinocerebellar ataxia 2 polyQ expansions are related to cognitive impairment in executive functions, memory and visuoconstructive skills.28 Finally, a postmortem study in patients with non-fluent primary progressive aphasia with 39 polyQ repeat expansion showed neuronal loss and gliosis, associated with a superficial laminar spongiosis, were severe in the superficial layers of the middle frontal gyrus, motor cortex, supramarginal gyrus, CA1 and the subiculum, but not in the cerebellum.29
One limitation of this study is that not all patients were tested for cognitive function. However, the clinical characteristics of tested and non-tested patients were similar, excluding a selection bias. A remarkable feature of our study is its population-based nature, including some 80% of incident patients.
In conclusion, in our population-based cohort of patients of Italian ancestry, we found that patients with ALS carrying an intermediate ATXN2 polyQ number of repeats≥31 have a distinctive phenotype, characterised by a more rapid disease course, with a 1.5-fold increase of ΔALSFRS-R rate and a higher ΔKing’s. Compared with ATXN2− patients, this greater aggressiveness resulted in a 1 year reduction in survival. In addition, ATXN2+ patients have a more severe impairment of cognitive functions, with relative preservation of the behavioural domain. Identifying the specific phenotypic characteristics of patients with ALS with ATXN2 polyQ intermediate number of repeats has many implications. These include the possibility of refining the individual prognostic prediction and improving the design of ALS clinical trials, including those targeted explicitly to ATXN2.
Footnotes
Contributors: AC had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: AC, CM, AC, UM, MG, RV, FP, SG, MB, MB, FDM, CD, RC, GM, BT, LC, SD’A, LM, AC. Acquisition, analysis, or interpretation of data: AC, CM, AC, UM, MG, RV, FP, SG, MB, MB, FDM, CD, RC, GM, BT, LC, SD’A, LM, AC. Drafting of the manuscript: AC, CM, AC, BT, SD’A, LM, AC. Critical revision of the manuscript for important intellectual content: AC, CM, AC, UM, MG, RV, FP, SG, MB, MB, FDM, CD, RC, GM, BT, LC, SD’A, LM, AC. Obtained funding: BT, AC. Administrative, technical, or material support: MG, FP, SG, MB. Study supervision: AC, CM, AC, FD’A, LM, AC. AC is responsible for the overall content as guarantor.
Funding: This work was supported by the Italian Ministry of Health (Ministero della Salute, Ricerca Sanitaria Finalizzata, grant RF-2016-02362405); the Progetti di Rilevante Interesse Nazionale program of the Ministry of Education, University and Research (grant 2017SNW5MB); the European Commission’s Health Seventh Framework Programme (FP7/2007–2013 under grant agreement 259867); the Horizon 2020 Programme (project Brainteaser under grant agreement 101017598); and the Joint Programme–Neurodegenerative Disease Research (Strength, ALS-Care and Brain-Mend projects), granted by Italian Ministry of Education, University and Research. This work was supported in part by the Intramural Research Programs of the National Institute on Aging (grant Z01-AG000949-02). This study was performed under the Department of Excellence grant of the Italian Ministry of University and Research to the “Rita Levi Montalcini” Department of Neuroscience, University of Torino, Italy, and to the Department of Health Sciences, University of Eastern Piedmont, Novara, Italy.
Disclaimer: The funders had no role in data collection or analysis and did not participate in writing or approving the manuscript.
Competing interests: Adriano Chiò serves on scientific advisory boards for Mitsubishi Tanabe, Biogen, Roche, Denali Pharma, Cytokinetics, Lilly, and Amylyx Pharmaceuticals and has received a research grant from Biogen. Cristina Moglia, Antonio Canosa, Umberto Manera, Maurizio Grassano, Rosario Vasta, Francesca Palumbo, Salvatore Gallone, Maura Brunetti, Fabiola De Marchi, Clifton L. Dalgard, Ruth Chia, Gabriele Mora, Lucia Corrado, Sandra D’Alfonso, Letizia Mazzini no disclosures. Dr Traynor holds the US, Canadian, and European patents on the clinical testing and therapeutic intervention for the hexanucleotide repeat expansion in C9orf72. Andrea Calvo has received a research grant from Cytokinetics.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Data will be available upon reasonable request by interested researchers.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Comitato Etico Azienda Ospedaliero-Universitaria Città della Salute e della Scienza, Torino, and Comitato Etico Azienda Ospedaliero-Universitaria Maggiore della Carità, Novara (protocol number #0038876). Patients and controls provided written informed consent before enrolment. The databases were anonymised according to the Italian law for protecting privacy.
References
- 1. Goutman SA, Hardiman O, Al-Chalabi A, et al. Emerging insights into the complex genetics and pathophysiology of amyotrophic lateral sclerosis. Lancet Neurol 2022;21:465–79. 10.1016/S1474-4422(21)00414-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grassano M, Calvo A, Moglia C, et al. Mutational analysis of known ALS genes in an Italian population-based cohort. Neurology 2021;96:e600–9. 10.1212/WNL.0000000000011209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chiò A, Calvo A, Moglia C, et al. ATXN2 polyQ intermediate repeats are a modifier of ALS survival. Neurology 2015;84:251–8. 10.1212/WNL.0000000000001159 [DOI] [PubMed] [Google Scholar]
- 4. Sproviero W, Shatunov A, Stahl D, et al. ATXN2 trinucleotide repeat length correlates with risk of ALS. Neurobiol Aging 2017;51:178.e1–178.e9. 10.1016/j.neurobiolaging.2016.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fournier C, Anquetil V, Camuzat A, et al. Interrupted CAG expansions in ATXN2 gene expand the genetic spectrum of frontotemporal dementias. Acta Neuropathol Commun 2018;6:41. 10.1186/s40478-018-0547-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rubino E, Mancini C, Boschi S, et al. ATXN2 intermediate repeat expansions influence the clinical phenotype in frontotemporal dementia. Neurobiol Aging 2019;73:231.e7–231.e9. 10.1016/j.neurobiolaging.2018.09.009 [DOI] [PubMed] [Google Scholar]
- 7. Chiò A, Mora G, Moglia C, et al. Secular trends of amyotrophic lateral sclerosis: the Piemonte and Valle d'Aosta register. JAMA Neurol 2017;74:1097–104. 10.1001/jamaneurol.2017.1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brooks BR, Miller RG, Swash M. World Federation of Neurology Research group on motor neuron diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 2000;1:293–9. 10.1080/146608200300079536 [DOI] [PubMed] [Google Scholar]
- 9. Strong MJ, Abrahams S, Goldstein LH. Revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener 2017;18:153–74. 10.1080/21678421.2016.1267768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iazzolino B, Peotta L, Zucchetti JP, et al. Differential neuropsychological profile of patients with amyotrophic lateral sclerosis with and without C9orf72 mutation. Neurology 2021;96:e141–52. 10.1212/WNL.0000000000011093 [DOI] [PubMed] [Google Scholar]
- 11. Montuschi A, Iazzolino B, Calvo A, et al. Cognitive correlates in amyotrophic lateral sclerosis: a population-based study in Italy. J Neurol Neurosurg Psychiatry 2015;86:168–73. 10.1136/jnnp-2013-307223 [DOI] [PubMed] [Google Scholar]
- 12. Chiò A, Calvo A, Mazzini L, et al. Extensive genetics of ALS: a population-based study in Italy. Neurology 2012;79:1983–9. 10.1212/WNL.0b013e3182735d36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Renton AE, Majounie E, Waite A, et al. A hexanucleotide repeat expansion in C9orf72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 2011;72:257–68. 10.1016/j.neuron.2011.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dolzhenko E, Deshpande V, Schlesinger F, et al. ExpansionHunter: a sequence-graph-based tool to analyze variation in short tandem repeat regions. Bioinformatics 2019;35:4754–6. 10.1093/bioinformatics/btz431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chiò A, Mora G, Sabatelli M, et al. ATNX2 is not a regulatory gene in Italian amyotrophic lateral sclerosis patients with C9orf72 GGGGCC expansion. Neurobiol Aging 2016;39:218.e5–218.e8. 10.1016/j.neurobiolaging.2015.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Elden AC, Kim H-J, Hart MP, et al. Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature 2010;466:1069–75. 10.1038/nature09320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nel M, Mavundla T, Gultig K, et al. Repeats expansions in ATXN2, NOP56, NIPA1 and ATXN1 are not associated with ALS in Africans. IBRO Neurosci Rep 2021;10:130–5. 10.1016/j.ibneur.2021.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao M, Kim JR, van Bruggen R, et al. RNA-binding proteins in amyotrophic lateral sclerosis. Mol Cells 2018;41:818–29. 10.14348/molcells.2018.0243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang Y, Halliday GM, Kiernan MC, et al. TDP-43 levels in the brain tissue of ALS cases with and without C9ORF72 or ATXN2 gene expansions. Neurology 2019;93:e1748–55. 10.1212/WNL.0000000000008439 [DOI] [PubMed] [Google Scholar]
- 20. Glass JD, Dewan R, Ding J, et al. ATXN2 intermediate expansions in amyotrophic lateral sclerosis. Brain 2022:awac167. 10.1093/brain/awac167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sorenson EJ, Mandrekar J, Crum B, et al. Effect of referral bias on assessing survival in ALS. Neurology 2007;68:600–2. 10.1212/01.wnl.0000254501.58158.e7 [DOI] [PubMed] [Google Scholar]
- 22. Logroscino G, Marin B, Piccininni M, et al. Referral bias in ALS epidemiological studies. PLoS One 2018;13:e0195821. 10.1371/journal.pone.0195821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rosas I, Martínez C, Clarimón J, et al. Role for ATXN1, ATXN2, and HTT intermediate repeats in frontotemporal dementia and Alzheimer’s disease. Neurobiol Aging 2020;87:139.e1–139.e7. 10.1016/j.neurobiolaging.2019.10.017 [DOI] [PubMed] [Google Scholar]
- 24. Ross OA, Rutherford NJ, Baker M, et al. Ataxin-2 repeat-length variation and neurodegeneration. Hum Mol Genet 2011;20:3207–12. 10.1093/hmg/ddr227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lattante S, Millecamps S, Stevanin G, et al. Contribution of ATXN2 intermediary polyQ expansions in a spectrum of neurodegenerative disorders. Neurology 2014;83:990–5. 10.1212/WNL.0000000000000778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fitzgerald J, Fahey L, Holleran L, et al. Thirteen independent genetic loci associated with preserved processing speed in a study of cognitive resilience in 330,097 individuals in the UK Biobank. Genes 2022;13:122. 10.3390/genes13010122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Davies G, Marioni RE, Liewald DC, et al. Genome-wide association study of cognitive functions and educational attainment in UK Biobank (N=112 151). Mol Psychiatry 2016;21:758–67. 10.1038/mp.2016.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gigante AF, Lelli G, Romano R, et al. The relationships between ataxia and cognition in spinocerebellar ataxia type 2. Cerebellum 2020;19:40–7. 10.1007/s12311-019-01079-5 [DOI] [PubMed] [Google Scholar]
- 29. Fournier C, Anquetil V, Camuzat A, et al. Interrupted CAG expansions in ATXN2 gene expand the genetic spectrum of frontotemporal dementias. Acta Neuropathol Commun 2018;6:41. 10.1186/s40478-018-0547-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnnp-2022-329376supp001.pdf (221.6KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data will be available upon reasonable request by interested researchers.