Introduction
The provision of multiple sclerosis (MS) services in England was profoundly impacted by the COVID-19 pandemic in 2020. Specialist staff were re-deployed to frontline services, social distancing measures resulted in reduced access to diagnostics and hospital facilities for infusion treatments, and concerns were raised over the safety of immunosuppressive disease-modifying therapies (DMTs). The Association of British Neurologists issued guidance in March 2020, advising that the risk from COVID-19 may potentially be increased with fingolimod, ocrelizumab, alemtuzumab and cladribine treatment in people with MS, due to the immunosuppressive mechanism of action of these drugs.1
Clinical trials and observational studies suggest that early use of high-efficacy DMTs results in improved long-term outcomes for people with MS.2 If the impacts of the COVID-19 pandemic were to reduce or delay the initiation of DMTs, particularly high-efficacy therapies, this may result in worse long-term outcomes for people with MS.3
Here, we use national data on all DMTs prescribed in NHS England between January 2016 and December 2020 in order to assess whether the COVID-19 pandemic was associated with changes in DMT initiation in 2020 in people with MS.
Methods
As a compulsory requirement of National Health Service (NHS) payment, all licenced DMTs prescribed for MS within NHS England must be registered on the Blueteq high-cost drug database. We obtained fully anonymised metadata on the number of patients with MS initiated on each of the licenced DMTs in England from 1 January 2016 to 31 December 2020.
These data represent any patient in England, across all 77 MS centres, being prescribed any licenced DMT for the first time, including those who are treatment-naïve, or switching from an alternative DMT.
We compared overall rates of DMT initiation, together with trends in prescribing of individual DMTs, between 2016 and 2020 in order to assess whether the COVID-19 pandemic was associated with changes in DMT prescribing patterns in England.
Results
Between 1 January 2016 and 31 December 2020, 41 632 DMT initiations were recorded within NHS England. Between 2016 and 2019, the overall number of DMT initiations consistently increased, from 7241 in 2016 to 9377 in 2019 (+29.5%). This was followed by a 13.3% reduction in the number of DMTs initiated in 2020 compared with 2019.
Changes in the initiation of individual DMTs are shown in figure 1. Similar to the trends in overall DMT initiation, 2016–2019 saw increasing use of high-efficacy, monoclonal antibody therapies. This was reversed in 2020, with a 16.7% reduction in the initiation of monoclonal antibody therapies compared with 2019. The greatest absolute reductions in treatments initiated in 2020, compared with 2019, were seen with alemtuzumab (−597 (−75.0%)), ocrelizumab (−283 (−13.5%)) and dimethyl fumurate (−270 (−13.5%)). While the fall in alemtuzumab initiations continued a trend established in 2019 (2019 vs 2018: −647 (−45.5%)), the reduction in ocrelizumab initiations reversed the previous rapid increase in its use (2019 vs 2018: +1931 (+1192.0%)). Natalizumab, the only high-efficacy monoclonal antibody treatment initially recommended as ‘safe’ during the COVID-19 pandemic, saw a 46.9% increase in the number of initiations in 2020 compared with 2019. Lower efficacy treatments, such as interferon-beta and glatiramer acetate, were relatively unaffected (4.5% increase and 4.6% decrease, 2020 vs 2019, respectively).
Figure 1.
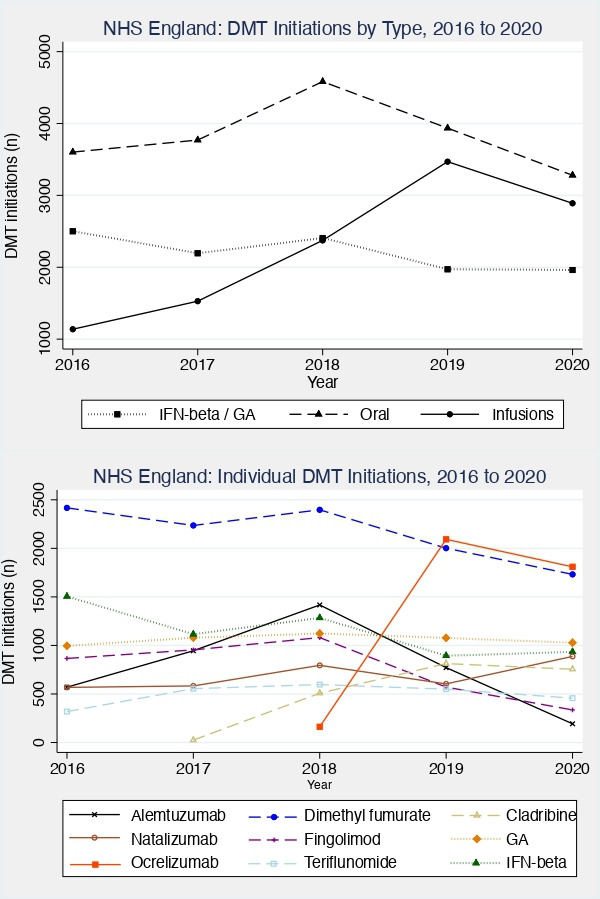
Yearly number of all disease-modifying therapies initiated within NHS England for people with multiple sclerosis from 2016 to 2020. Top: The number of DMTs initiated each year, grouped by DMT category. Injectable treatments (interferon-beta and glatiramer acetate); oral treatments (dimethyl fumarate, fingolimod, teriflunomide, cladribine); monoclonal antibody infusions (alemtuzumab, natalizumab, ocrelizumab). Bottom: The number of each individual DMT initiated within NHS England each year. Solid lines indicate monoclonal antibody infusions, dashed lines oral treatments and dotted lines injectable treatments. DMT, disease-modifying therapy; GA, glatiramer acetate; IFN-beta, interferon-beta; NHS, National Health Service.
Discussion
Through a longitudinal national survey of all licenced DMTs initiated in people with MS in England from 2016 to 2020, we found that the start of the COVID-19 pandemic in 2020 coincided with changes in national prescribing patterns. Recent trends towards a greater number of DMT initiations were reversed, with the greatest reductions seen for the high-efficacy monoclonal antibody therapies. Prescribing of lower efficacy injectable treatments (glatiramer acetate and interferon-beta) was relatively similar.
Factors independent of the COVID-19 pandemic may have also influenced prescribing patterns in England in 2020, particularly licencing changes for alemtuzumab in Europe in 2019. However, reductions in alemtuzumab use prior to the COVID-19 pandemic were more than offset by increases in ocrelizumab prescribing, such that initiations of high-efficacy monoclonal antibody treatments actually increased by 46.1% in 2019 compared with 2018. In 2020, however, alemtuzumab use continued to fall, now with similar reductions in ocrelizumab initiation.
The likely causal role of the COVID-19 pandemic in the changes we observe is supported by the observation that initiations of DMTs perceived to be of lower risk during the pandemic either remained stable (interferon-beta and glatiramer acetate) or increased (natalizumab). For natalizumab, the only high-efficacy monoclonal antibody treatment recommended to be safe during the COVID-19 pandemic, the 46.9% increase in 2020 was insufficient to counter the reductions seen with alemtuzumab and ocrelizumab.
Despite initial concerns over immunosuppressive DMTs during the COVID-19 pandemic, registry studies have found no increase in the risk of severe COVID-19 for most of the available MS therapies. The exception is the anti-CD20 agents, which have consistently been found to increase the risk of severe COVID-19.4 5 Changes in the use of anti-CD20 agents may therefore have been appropriate, particularly in older patients and those with comorbidities known to increase the risk of severe COVID-19.
A limitation of this nationwide study is that we are unable to determine the extent to which changes in MS treatment decisions in 2020 were due to drug safety concerns or other factors that have impacted on patient care, including reduced access to specialist services and diagnostics, tele-medicine and delays in treatment initiation. All of these factors could potentially contribute to the fall in DMT prescribing in 2020 and the fall in high-efficacy DMT use. The Scottish Multiple Sclerosis Register has reported fewer new MS diagnoses in 2020 compared with previous years (450 new diagnoses in 2020, compared with 540 diagnosed in 2019 (16.7% reduction)), suggesting the impact on MS care extends beyond DMT initiation.6 The increased use of natalizumab in 2020, however, suggests that drug safety concerns are likely to have played an important role in the changes in DMT prescribing we observe. Independent of the underlying cause, our findings of suboptimal DMT provision during 2020 indicate that the long-term impact of the COVID-19 pandemic may extend beyond the short-term disruptions in the care for people with MS.
Footnotes
Twitter: @DrWBRO
Contributors: TW: Study conception, data analysis, drafting the manuscript, reviewing and amending the manuscript; study guarantor. RM: Data provision, reviewing and amending the manuscript. BB: Reviewing and amending the manuscript. RD: Reviewing and amending the manuscript. AS: Reviewing and amending the manuscript. JC: Reviewing and amending the manuscript. WB: Study conception, reviewing and amending the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: TW has participated in educational events funded by Novartis and Merck. RM: Nil relevant disclosures. BB: nil relevant disclosures. RD has participated in educational events, advisory boards and conference attendance funded by Roche, Sanofi, Merck, Biogen and Novartis. AS: Nil relevant disclosures. JC has received support from the Efficacy and Mechanism Evaluation Programme and Health Technology Assessment Programme (NIHR); UK Multiple Sclerosis Society and National Multiple Sclerosis Society. In the last three years, he has been a local principal investigator for trials in multiple sclerosis funded by Receptos, Novartis and Biogen Idec, and has received an investigator grant from Novartis outside this work. He has taken part in Advisory Boards/consultancy for Roche, Merck, MedDay, Biogen and Celgene. WB has received speaker honoraria for educational activities and/or participation in advisory boards for Biogen, Celgene, Merck, Mylan, Novartis, Roche and Sanofi Genzyme.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Coles AJ, MS Advisory Group . ABN guidance on the use of disease-modifying therapies in multiple sclerosis in response to the threat of a coronavirus epidemic. Assoc Br Neurol 2020. [Google Scholar]
- 2. Hartung H-P, Meuth SG, Thompson AJ. Paradigm shifts: Early initiation of high-efficacy disease-modifying treatment in multiple sclerosis. Mult Scler 2021;27:1473–6. 10.1177/13524585211033190 [DOI] [PubMed] [Google Scholar]
- 3. He A, Merkel B, Brown JWL, et al. Timing of high-efficacy therapy for multiple sclerosis: a retrospective observational cohort study. Lancet Neurol 2020;19:307–16. 10.1016/S1474-4422(20)30067-3 [DOI] [PubMed] [Google Scholar]
- 4. Sormani MP, De Rossi N, Schiavetti I, et al. Disease‐Modifying Therapies and Coronavirus Disease 2019 Severity in Multiple Sclerosis. Ann Neurol 2021;89:780–9. 10.1002/ana.26028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Salter A, Fox RJ, Newsome SD, et al. Outcomes and Risk Factors Associated with SARS-CoV-2 Infection in a North American Registry of Patients With Multiple Sclerosis. JAMA Neurol 2021;78:699–708. 10.1001/jamaneurol.2021.0688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Public Health Scotland . Scottish multiple sclerosis register national report 2020, 2020. [Google Scholar]


