Abstract
Background
One in six infant deaths worldwide are caused by invasive bacterial infections, of which a substantial but unquantified proportion are caused by Gram-negative bacteria.
Methods
We conducted a systematic review of studies published from 31 May 2010 to 1 June 2020 indexed in MEDLINE, Embase and Global Health databases. We performed meta-analyses of the incidence of Gram-negative bacteraemia and of individual Gram-negative species as proportions of all infant bacteraemia, stratified by onset (early vs late) and country income (low/middle vs high).
Results
152 studies from 54 countries were included, 60 in high-income countries (HIC) and 92 in low-income/middle-income countries (LMIC). Gram-negatives represented a higher proportion (53%, 95% CI 49% to 57%) of all infant bacteraemia in LMIC compared with HIC (28%, 95% CI 25% to 32%). Incidence of infant Gram-negative bacteraemia was 2.01 (95% CI 1.15 to 3.51) per 1000 live births; it was five times higher in LMIC (4.35, 95% CI 2.94 to 6.43) compared with HIC (0.73, 95% CI 0.39 to 7.5). In HIC, Escherichia coli was the leading Gram-negative pathogen, representing 19.2% (95% CI 15.6% to 23.4%) of early and 7.3% (95% CI 5.3% to 10.1%) of all late-onset bacteraemia; Klebsiella spp were the next most common cause (5.3%) of late-onset bacteraemia. In LMIC, Klebsiella spp caused 16.4% (95% CI 11.5% to 22.7%) of early and 15.0% (95% CI 10.1% to 21.8%) of late-onset bacteraemia, followed by E. coli (early-onset 7.50%, 95% CI 4.98% to 11.1%; late-onset 6.53%, 95% CI 4.50% to 9.39%) and Pseudomonas spp (early-onset 3.93%, 95% CI 2.04% to 7.44%; late-onset 2.81%, 95% CI 1.99% to 3.95%).
Conclusion
E. coli, Klebsiella and Pseudomonas spp cause 20%–28% of early-onset infant bacteraemia and 14% cases of infant meningitis worldwide. Implementation of preventive measures could reduce the high incidence of Gram-negative bacteraemia in LMIC.
PROSPERO registration number
CRD42020191618.
Keywords: sepsis, neonatology, infectious disease medicine, microbiology
This systematic review confirms the disproportionate burden of Gram negative infections in low and middle income countries, compared to high income countries.
What is already known on this topic
Invasive bacterial infections accounted for approximately 15% of all infant deaths worldwide in 2012, comprising 10% of early and 40% of late neonatal deaths.
Gram-negative neonatal infections constitute a substantial and increasingly difficult to treat proportion of these infections.
The worldwide incidence of Gram-negative invasive bacterial disease in infants and the contributions of different species have not been quantified.
What this study adds
Escherichia coli, Klebsiella spp and Pseudomonas spp cause one-fifth to one-quarter of all early-onset infant bacteraemia worldwide, one-quarter of late-onset infant bacteraemia in low-income/middle-income countries and one in seven cases of meningitis and late-onset bacteraemia in high-income countries.
Incidence of infant Gram-negative bacteraemia was five times higher in low-income/middle-income countries compared with high-income countries (late-onset disease incidence almost ninefold higher) and the proportion of all infant bacteraemia attributable to Gram-negative disease in low-income/middle-income countries was almost double the proportion in high-income countries.
How this study might affect research, practice or policy
Although incidence of Gram-negative neonatal infections is much higher in resource-constrained settings, similarities in aetiology suggest that a better understanding of sources and transmission routes for Gram-negative infections in infants obtained from studies conducted in well-resourced settings could translate into preventive measures that would be effective in any setting, thereby reducing the substantial morbidity and mortality caused by Gram-negative neonatal bacteraemia worldwide.
Introduction
Invasive bacterial infections accounted for approximately 15% of all infant deaths worldwide in 2012, comprising 8% of early (0–6 days) and 37% of late (7–27 days) neonatal deaths.1 The epidemiology of invasive bacterial infections in infants is likely to differ by setting,2 but most systematic reviews have focused on low-income/middle-income countries (LMIC).3–5 In all settings, invasive bacterial infections at birth and during infancy carry a high risk of morbidity and mortality, while increasing prevalence of antimicrobial resistance (AMR) presents a challenge to effective treatment.3–8 This systematic review aimed to quantify worldwide incidence of infant Gram-negative invasive bacterial disease and contributions of different Gram-negative species. It focuses on studies reporting bacteraemia and/or meningitis, defined respectively as growth of a pathogen in a blood or cerebrospinal fluid (CSF) culture.
Methods
Aims, objectives and protocol
The review protocol was registered with PROSPERO (CRD42020191618). The predefined outcomes were incidence of neonatal/infant Gram-negative bacteraemia (GNB) and meningitis per 1000 live births, and the proportions of all infant bacteraemia and CSF infections attributable to specific Gram-negative pathogens.
Searches
MEDLINE, Embase and Global Health databases were searched to identify studies that reported invasive bacterial infections in infants using MeSH terms for bacteremia, sepsis or bacterial meningitis and title words ‘septicemia’, ‘septicaemia’, ‘invasive bacterial infection’ or ‘bloodstream infection’ combined with MeSH terms for newborn or infant and title word ‘neonat*’ (see online supplemental data). Searches were restricted to the 10-year period from 31 May 2010 to 1 June 2020 without language restrictions. Bibliographies of systematic reviews identified during the database searches were scanned to identify additional references. See online supplemental methods for screening and extraction and quality assessment methods.
archdischild-2022-324047supp001.pdf (387.8KB, pdf)
archdischild-2022-324047supp002.pdf (62.7KB, pdf)
Analysis
Studies were split into two groups according to World Bank classifications of high-income countries (HIC) and LMIC. Pooled cumulative incidence of invasive Gram-negative disease (per 1000 live births) and pooled proportions of Escherichia coli and the main Gram-negative genera were estimated using generalised linear mixed model random-effects meta-analysis.9 H-statistics were calculated to compare the SD of the estimated overall effect size from a random-effects meta-analysis with that obtained from a fixed-effect meta-analysis. Between-study variance was estimated as τ2 and the proportion of variation in summary estimates attributable to between-study heterogeneity was quantified using the I2 statistic. Subgroup analyses were planned by country income, timing of disease onset, birth weight and gestational age. Meta-analyses were performed using the meta package in R (V.4.0.4).10
Results
Database searches identified 12 763 unique records (figure 1), of which 416 were retained for full-text review (reviewer agreement for final inclusion 98.5%) plus an additional 20 studies identified from bibliographies of 11 systematic reviews. The quality of 157 eligible studies was assessed and 5 were excluded for poor quality. Of 152 studies included for data extraction, 81% (123/152) were rated good and 19% (29/152) fair quality (see online supplemental data).
Figure 1.
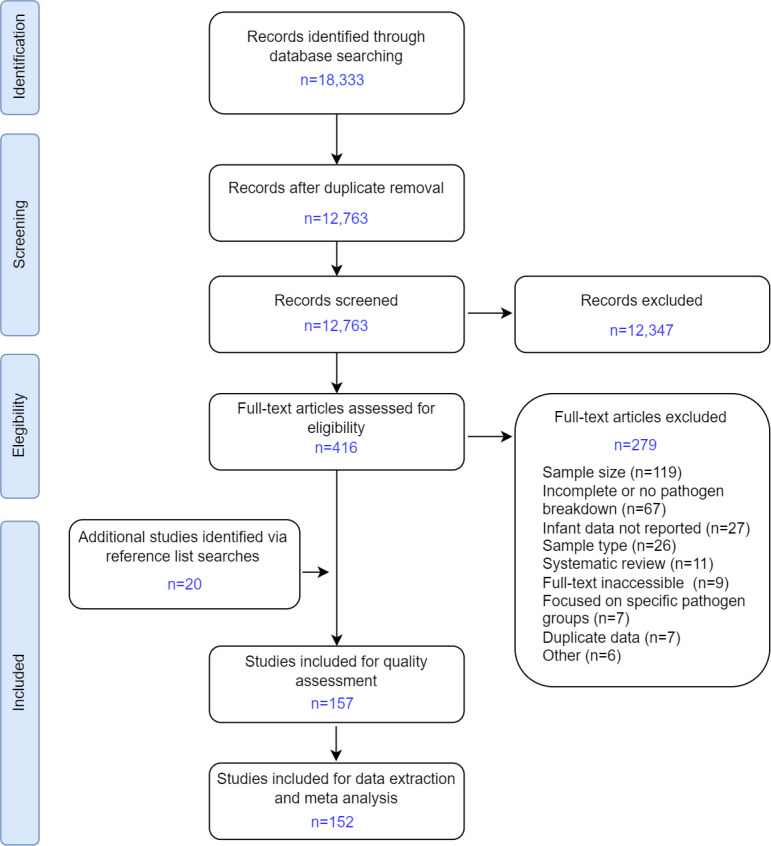
Study selection, Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
Incidence of invasive Gram-negative infection in neonates and infants
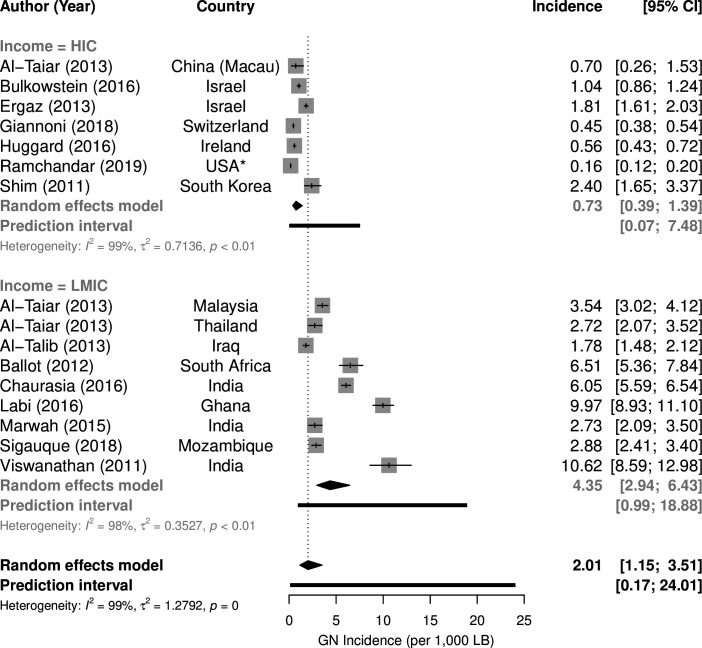
Of the 152 included studies, 135 examined only blood cultures, 10 mainly blood and CSF and 7 CSF cultures only (see online supplemental data). Sixty studies were conducted in HIC and 92 in LMIC, representing 54 countries (figure 2). Most (83%) focused only on neonates (up to 1 month old), the remainder included infants up to 1 year old. Thirteen studies estimated population incidence of GNB (figure 3), 10 for neonatal disease and 3 for neonatal and infant disease.11–24 Overall incidence per 1000 live births in HIC (0.73, 95% CI 0.39 to 1.39) was substantially lower than in LMIC (4.35, 95% CI 2.94 to 6.43, p<0.001). The worldwide incidence of GNB was 2.01 (95% CI 1.15 to 3.51) per 1000 live births.
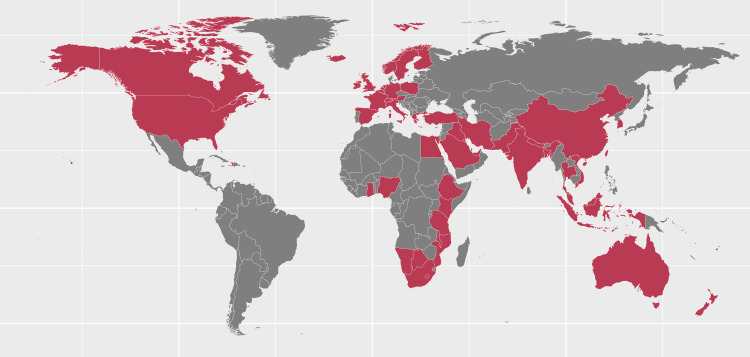
Figure 2.
Countries represented in the included studies that used blood culture as primary diagnostic method (n=152). Ten of the 152 studies provided data on Gram-negative bacteraemia and meningitis (China ×2, India, Kenya, USA ×3, Canada, Israel, Italy); 7 studies (Malawi, Namibia, Canada, France, Korea, Taiwan, UK) provided data on meningitis only.
Figure 3.
Incidence of infant Gram-negative (GN) bacteraemia per 1000 live births by country income. HIC, high-income countries; LMIC, low-income/middle-income countries. *Ramchandar et al 21 include military base study sites outside the USA.
Gram-negative infections as a proportion of all infant bacteraemia
Gram-negative infection as a percentage of all infant bacteraemia was higher in LMIC (53.1%, 95% CI 48.8% to 57.3%) compared with HIC (28.3%, 95% CI 24.7% to 32.1%, p<0.001) (online supplemental figure S1). Exclusion of possible contaminants was reported by 73% (40/55) of HIC and 34% (31/90) of LMIC studies (online supplemental figure S2). HIC studies that excluded contaminants reported higher prevalence (31.8%, 95% CI 27.8% to 36.0%) of Gram-negative infections as a percentage of all infant bacteraemia compared with studies that did not (20.2%, 95% CI 15.1% to 26.5%, p=0.003), a difference not observed in LMIC studies (p=0.38, online supplemental figure S2). HIC studies were more often multisite (62%, 34/55) than LMIC studies (16%, 14/90) (online supplemental figure S3). While the number of sites made little difference to Gram-negative infections as a percentage of all infant bacteraemia in HIC (p=0.164), multisite studies in LMIC reported a lower percentage of Gram-negatives (44.7%, 95% CI 37.5% to 52.1%) compared with single-site studies (54.7%, 95% CI 49.9% to 59.4%, p=0.03).
archdischild-2022-324047supp003.pdf (144.6KB, pdf)
Aetiology of Gram-negative infant bacteraemia
The 145 studies reporting bacteraemia yielded a total of 26 235 blood cultures positive for a Gram-negative pathogen, with the number of Gram-negative isolates per study ranging from 7 to 2161 (median 111). E. coli was the most reported Gram-negative species in HIC (n=5026 isolates; prevalence 11.7%, 95% CI 9.4% to 14.4%), while Klebsiella spp were most reported in LMIC (n=5915; 19.0%, 95% CI 16.3% to 22.1%) (table 1). In HIC, the next two most common species were Klebsiella spp (n=2401; 3.17%, 95% CI 2.07% to 4.84%) and Enterobacter spp (n=1395; 0.63%, 95% CI 0.31% to 1.29%). In LMIC, the next most reported species were E. coli (n=2758; 7.67%, 95% CI 6.36% to 9.24%) and Acinetobacter spp (n=1996; 3.25%, 95% CI 0.05% to 0.39%) (table 1). Heterogeneity between studies varied by pathogen but was generally very high (online supplemental table S1). A sensitivity analysis restricted to studies that reported neonatal infection where the neonatal period was defined explicitly as up to day 28 or 30 of life showed a similar distribution of species (online supplemental tables S2, S5).
Table 1.
Gram-negative species as percentages of all infant bacteraemia (Gram-negative and Gram-positive) in high-income and low-income/middle-income countries*
| High-income countries (n=55 studies) | Low-income/middle-income countries (n=90 studies) | |||
| Isolates, n | Proportion (95% CI) | Isolates, n | Proportion (95% CI) | |
| Escherichia coli | 5026 | 11.7% (9.40 to 14.4%) | 2758 | 7.67% (6.36 to 9.24%) |
| Klebsiella spp | 2401 | 3.17% (2.07 to 4.84%) | 5915 | 19.0% (16.3 to 22.1%) |
| Pseudomonas spp | 665 | 0.70% (0.04 to 1.09%) | 1127 | 2.53% (1.83 to 3.49%) |
| Enterobacter spp | 1395 | 0.63% (0.31 to 1.29%) | 918 | 1.18% (0.74 to 1.85%) |
| Serratia spp | 441 | 0.15% (0.06 to 0.37%) | 237 | 0.02% (0.00 to 0.09%) |
| Proteus spp | 34 | 0.01% (0.00 to 0.05%) | 134 | 0.04% (0.01 to 0.13%) |
| Salmonella spp | 61 | 0.00% (0.00 to 0.26%) | 215 | 0.00% (0.00 to 0.03%) |
| Citrobacter spp | 74 | 0.01% (0.00 to 0.06%) | 249 | 0.09% (0.04 to 0.23%) |
| Haemophilus spp | 272 | 0.02% (0.01 to 0.10%) | 38 | 0.00% (0.00 to 0.06%) |
| Neisseria spp | 19 | 0.00% (0.00 to 0.07%) | 13 | 0.00% (0.00 to 0.20%) |
| Acinetobacter spp | 394 | 0.14% (0.05 to 0.39%) | 1996 | 3.25% (2.41 to 4.37%) |
| Moraxella spp | 3 | 0.00% (0.00 to 3.05%) | 10 | 0.00% (0.00 to 3.11%) |
| Other species or unspecified Gram-negative | 868 | 0.42% (0.21 to 0.85%) | 978 | 0.43% (0.22 to 0.83%) |
*Estimates obtained by random-effects meta-analysis; see online supplemental file 4 for heterogeneity statistics.
archdischild-2022-324047supp004.pdf (135.6KB, pdf)
Early-onset and late-onset infant Gram-negative bacteraemia
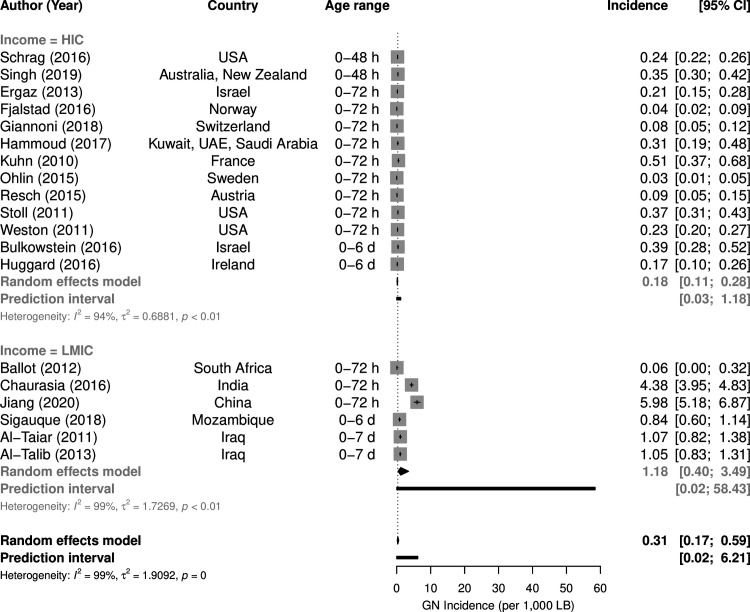
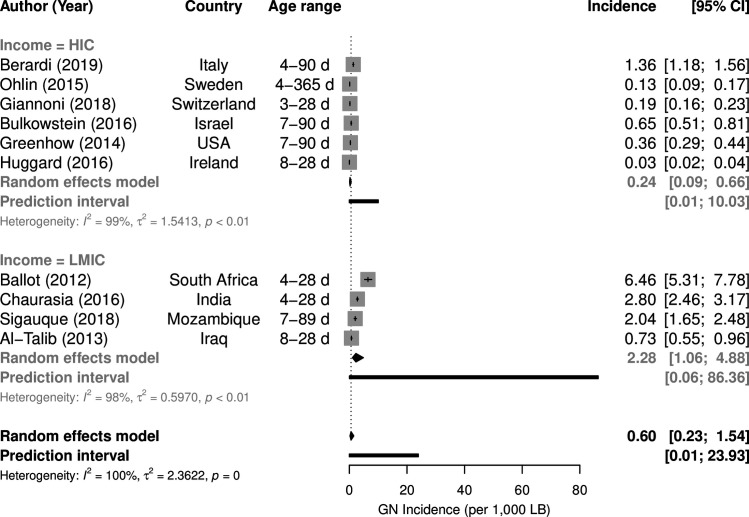
Among the 145 bacteraemia studies, 41 investigated both early-onset diesase (EOD) and late-onset disease (LOD), 15 early-onset only and 15 late-onset only. In these 71 studies, the EOD/LOD boundary was set at 48 hours postbirth in 7 studies, at 72 hours in 47 studies and at 6/7 days of life in 17 studies. The upper age defining ‘neonate’ or ‘newborn’ was ≤28 days in 26 studies, ≤30 days in one study and unspecified in 18 studies, while ‘infant’ was defined as <6 months old in 1 study, <90 days in 3 studies and was unspecified in 5 studies. EOD incidence was estimated by 19 studies (figure 4),7 12–18 23 25–34 LOD incidence by 10 studies (figure 5).12–15 17 18 23 30 35 36 EOD (p=0.0015) and LOD (p=0.0005) incidence was much lower in HIC than in LMIC. Incidence of Gram-negative EOD in HIC was 0.18 (95%CI 0.11 to 0.28) per 1000 live births compared with 1.18 (95%CI 0.40 to 3.49) in LMIC, while LOD incidence was 0.24 (95% CI 0.09 to 0.66) per 1000 live births in HIC compared with 2.28 (95% CI 1.06 to 4.88) in LMIC.
Figure 4.
Incidence per 1000 live births of early-onset Gram-negative (GN) bacteraemia by income group. HIC, high-income countries; LMIC, low-income/middle-income countries.
Figure 5.
Incidence per 1000 live births of late-onset Gram-negative (GN) bacteraemia by country income. HIC, high-income countries; LMIC, low-income/middle-income countries.
Aetiology of Gram-negative infant early-onset and late-onset bacteraemia
In HIC, E. coli was the leading cause of Gram-negative EOD and LOD, representing 19.2% of EOD (n=1454 isolates; 95% CI 15.6% to 23.4%) and 7.34% of LOD (n=1418; 95% CI 5.28% to 10.1%) (table 2). Klebsiella spp, Enterobacter spp and Pseudomonas spp represented 5.28% (95% CI 3.42% to 8.06%), 1.36% (95% CI 0.64% to 2.88%) and 1.21% (95% CI 0.75% to 1.92%) of Gram-negative late-onset bacteraemia. All other identified Gram-negative pathogens represented <1% of early-onset and late-onset GNB (table 2). In LMIC, Klebsiella spp were the leading cause of early-onset and late-onset GNB, representing 16.4% of EOD (n=794 isolates; 95% CI 11.5% to 22.7%) and 15.0% of LOD (n=974, 95% CI 10.1% to 21.8%). E. coli was the second most common cause of EOD (7.50%, 95% CI 4.98% to 11.1%) and LOD (6.53%, 95% CI 4.50% to 9.39%), Pseudomonas spp the third most common cause of EOD (3.93%, 95% CI 2.04% to 7.44%) and LOD (2.81%, 95% CI 1.99% to 3.95%) (table 2). Incidence and aetiology of GNB in very low birthweight (VLBW) infants and by gestational age, and incidence and aetiology of Gram-negative infection detected in CSF are summarised in online supplemental results.
Table 2.
Gram-negative species as percentages of all infant early-onset and late-onset bacteraemia (Gram-negative and Gram-positive) in high-income and low-income/middle-income countries*
| High-income countries | Early-onset disease (n=24 studies) | Late-onset disease (n=25 studies) | ||
| Isolates, n | Proportion (95% CI) | Isolates, n | Proportion (95% CI) | |
| Escherichia coli | 1454 | 19.2% (15.6 to 23.4%) | 1418 | 7.34% (5.28 to 10.1%) |
| Klebsiella spp | 91 | 0.95% (0.36 to 2.49%) | 1401 | 5.28% (3.42 to 8.06%) |
| Pseudomonas spp | 40 | 0.22% (0.01 to 0.79%) | 406 | 1.21% (0.75 to 1.92%) |
| Enterobacter spp | 16 | 0.08% (0.01 to 0.48%) | 708 | 1.36% (0.64 to 2.88%) |
| Serratia spp | 1 | 0.00% (0.00 to 99.5%) | 254 | 0.50% (0.23 to 1.11%) |
| Proteus spp | 4 | 0.03% (0.00 to 0.28%) | 28 | 0.03% (0.01 to 0.19%) |
| Salmonella spp | 0 | N/A | 4 | 0.00% (0.00 to 22.6%) |
| Citrobacter spp | 19 | 0.02% (0.00 to 0.43%) | 36 | 0.01% (0.00 to 0.17%) |
| Haemophilus spp | 184 | 0.54% (0.18 to 1.62%) | 6 | 0.00% (0.00 to 0.25%) |
| Neisseria spp | 0 | N/A | 2 | 0.00% (0.00 to 2.43%) |
| Acinetobacter spp | 31 | 0.08% (0.01 to 0.57%) | 207 | 0.28% (0.09 to 0.90%) |
| Moraxella spp | 0 | N/A | 7 | 0.00% (0.00 to 99.3%) |
| Other species or unspecified Gram-negative | 180 | 1.41% (0.62 to 3.18%) | 275 | 0.39% (0.14 to 1.03%) |
| Low-income/middle-income countries | Early-onset disease (n=32 studies) | Late-onset disease (n=30 studies) | ||
| Escherichia coli | 481 | 7.50% (4.98 to 11.1%) | 489 | 6.53% (4.50 to 9.39%) |
| Klebsiella spp | 794 | 16.4% (11.5 to 22.7%) | 974 | 15.0% (10.1 to 21.8%) |
| Pseudomonas spp | 248 | 3.93% (2.04 to 7.44%) | 232 | 2.81% (1.99 to 3.95%) |
| Enterobacter spp | 203 | 1.76% (0.88 to 3.49%) | 158 | 1.14% (0.55 to 2.34%) |
| Serratia spp | 36 | 0.12% (0.02 to 0.62%) | 67 | 0.12% (0.02 to 0.71%) |
| Proteus spp | 21 | 0.00% (0.00 to 1.61%) | 50 | 0.09% (0.01 to 0.52%) |
| Salmonella spp | 11 | 0.00% (0.00 to 10.9%) | 20 | 0.00% (0.00 to 17.4%) |
| Citrobacter spp | 47 | 0.12% (0.02 to 0.60%) | 75 | 0.10% (0.02 to 0.51%) |
| Haemophilus spp | 0 | N/A | 14 | 0.00% (0.00 to 99.1%) |
| Neisseria spp | 0 | N/A | 6 | 0.00% (0.00 to 97.4%) |
| Acinetobacter spp | 318 | 2.33% (1.17 to 4.61%) | 348 | 2.33% (1.20 to 4.46%) |
| Moraxella spp | 0 | N/A | 0 | N/A |
| Other species or unspecified Gram-negative | 195 | 0.83% (0.29 to 2.37%) | 184 | 1.28% (0.61 to 2.66%) |
*Estimates obtained by random-effects meta-analysis; see online supplemental file 4 for heterogeneity statistics.
N/A, not available.
archdischild-2022-324047supp005.pdf (221.5KB, pdf)
Discussion
This review has shown that incidence of infant GNB was fivefold higher in LMIC compared with HIC (LOD incidence almost ninefold higher) and that the proportion of all infant bacteraemia attributable to Gram-negative disease in LMIC was almost double the proportion in HIC (53% vs 28%). E. coli and Klebsiella spp caused a substantial proportion of GNB in infants worldwide, although with marked variation between HIC and LMIC and EOD versus LOD. Klebsiella was the predominant Gram-negative species in EOD and LOD in LMIC (15%–16% of early-onset and late-onset bacteraemia) whereas E. coli predominated in EOD (19%) in HIC. The remainder of Gram-negative infections in EOD and LOD were caused by species from many other genera each contributing a small percentage, although with Pseudomonas spp and Acinetobacter spp causing 2%–3% of all bacteraemia in LMIC and 2%–4% of early-onset bacteraemia in LMIC and HIC. Incidence of GNB per 1000 VLBW (28.5) was 40 times higher than overall incidence per 1000 live births in HIC (0.73).
The very large difference in incidence between low-income/middle-income and high-income settings is, presumably, attributable to higher rates of community and healthcare-associated infection in resource-constrained settings where intrapartum and postnatal care is non-existent, minimal or delayed.1 This higher incidence invariably translates into higher mortality among the estimated 2.5 million infants in LMICs affected annually by invasive infections, many of whom will not have access to antibiotics.37 Implementation of preventive measures could reduce the fivefold higher incidence of GNB in lower-income countries.38 A recent scoping review of neonatal healthcare-associated infection prevention and care bundles in LMIC proposed a ‘3+I’ framework comprising (1) primary prevention, (2) detection, (3) case management, plus (I) implementation.39 Primary prevention elements were well represented in studies of care bundle interventions in LMIC, although with bias towards specific devices (catheters and ventilators), whereas detection (secondary prevention) through screening and surveillance and tertiary prevention elements (case management, cohorting, antimicrobial stewardship) were less frequently evaluated. Implementation focused on provision of facilities and equipment, consistent with a lack of these in resource-limited settings.39 Conversely, in well-resourced settings, lapses in preventive measures, including inadequate disinfection of surfaces and shared equipment, combined with resource-related factors such as proximity of cots and understaffing during periods of high demand, have been linked to cross-infection in neonatal units.40 Preventing community-acquired infections presents specific challenges, particularly in resource-poor settings where sanitation and clean water supply are inadequate and rates of AMR in neonatal bacteraemia are high.41
Our aetiological findings for LMIC are consistent with previous systematic reviews based only on studies from LMIC.3–5 Characterisation of neonatal infections is less complete in these settings than in HIC, where surveillance networks such as neonIN (UK),8 Neo-KISS (Germany)42 and NRN (USA)7 have quantified trends, risk factors, aetiologies, antimicrobial susceptibilities and outcomes. Although findings from these studies show some regional variation, consistent aspects of invasive bacterial infection in infants include E. coli being predominant among Gram-negatives in EOD (otherwise dominated by group B Streptococcus (GBS)), a more even distribution of Gram-negative species in LOD (for which prematurity/VLBW was the main risk factor), and higher case fatality associated with GNB.7 8 42 43 Definitions were equivalent across a sufficient number of studies in our review to support a separate meta-analysis for VLBW infants, showing a Gram-negative pathogen distribution similar to that for LOD in HIC, which is consistent with the high relative risk of invasive late-onset bacterial infection in VLBW infants.7 8 42 43
Our review did not address AMR in Gram-negative neonatal infections, a topic that has been covered by recent systematic reviews of studies from LMIC,4 41 44 Middle Eastern countries,45 sub-Saharan African countries3 and China,46 but not HIC. Primary concerns around Gram-negative AMR include neonatal infection by E. coli resistant to ampicillin, gentamicin or both (an estimated 8% of early-onset infections in the USA) and emerging threats posed by extended-spectrum β-lactamase-producing (ESBL) and carbapenemase-producing Gram-negative bacteria (CPGNB).47–49 In LMIC, where challenges to antimicrobial stewardship are far greater, non-susceptibility of neonatal invasive infection isolates to ampicillin (89% in sub-Saharan Africa) and gentamicin (66% of Klebsiella spp, 47% of E. coli) is higher, and ESBL and CPGNB are established.3 4 6 The risk of contributing to AMR presents a challenge to prevention interventions such as intrapartum antibiotics and prophylaxis to prevent catheter-related infection.50 Antimicrobial stewardship programmes within neonatal units, principally reducing the initiation and duration of antibiotics, are supported by a growing body of observational evidence.51 52
Limitations
Between-study heterogeneity and the proportion of variance attributable to it was high for most of the estimates in our meta-analyses, and H-statistics justified the assumption of random rather than fixed effects. The remit of our review was worldwide, but Latin America, Eastern Europe, Central Asia and West and North Africa were not represented. We searched three electronic databases because scoping indicated that studies providing sufficient data for meta-analysis were likely to be indexed in MEDLINE and/or EMBASE. The Global Health database includes book chapters, reports, conference proceedings and theses, serving as our grey literature source. We used the conventional World Bank classification of countries by income in our subgroup analyses, recognising that this encompasses considerable heterogeneity within and between countries grouped into the same classification.
More problematic was the lack of a consensus definition for the early-onset versus late-onset period and the grouping together in our meta-analyses of neonates (up to 28 days) and older infants (up to 90 or 365 days). The former limitation can only be resolved by global adoption of an agreed definition, which would benefit future studies in the field of neonatal sepsis. We note that two-thirds (of 71 studies) used 72 hours as the early-late boundary.
There were insufficient late-onset studies in each country income subgroup to subdivide these into neonates and older infants. We did not have data to support differentiation in our meta-analyses of community-acquired versus hospital-acquired infection in LOD. The distribution of species arising from these two sources will differ. A recent HIC study identified 63% of late-onset invasive bacterial infections as being hospital-acquired, with causal agents predominantly coagulase-negative staphylococci (CoNS), Staphylococcus aureus, enterococci and Enterobacteriaceae, whereas community-acquired late-onset infections were predominantly GBS and E. coli.35
The total number of isolates in our meta-analyses from HIC (11 653) was less than for LMIC (14 588) but from half the number of studies (55 vs 90) because LMIC studies were more likely to be single-site and retrospective rather than based on multisite surveillance. Of note here are initiatives such as the Burden of Antibiotic Resistance in Neonates from Developing Societies (BARNARDS) network, which uses a standardised methodological framework to characterise Gram-negative species causing neonatal sepsis in sub-Saharan African and South Asian countries, including genotyping to identify sequence types and AMR genes.6 53 54 That multisite studies in LMICs reported a lower percentage of Gram-negatives relative to single site LMIC studies may reflect better standardisation of microbiological techniques in multisite studies that allowed for improved detection of Gram-positive organisms such as GBS.
The differences between LMIC and HIC Gram-negative neonatal disease aetiology in our results will be influenced by different standards in culture/subculturing of bacteria and reporting of disease episodes. Exclusion of skin commensals such as CoNS, Micrococcus spp, Bacillus spp, Corynebacterium spp and Propionibacterium spp as contaminants was more common in HIC than LMIC studies. These exclusions can make a marked difference to estimates of neonatal disease incidence,8 and will increase Gram-negatives as a proportion of all neonatal bacteraemia as we observed when we compared HIC studies which did or did not exclude contaminants. Consensus has yet to emerge in neonatal infection surveillance, with some networks including potential contaminants if two or more blood cultures are positive or if one blood culture is positive but with clinical signs of infection.42 47 Regardless of setting, any tendency for study specimens to be from infections that are not susceptible to antibiotic treatment will bias reported aetiology towards species that are more commonly antimicrobial resistant.
Conclusions
E. coli, Klebsiella spp and Pseudomonas spp cause one-fifth to one-quarter of all early-onset infant bacteraemia worldwide, one-quarter of late-onset infant bacteraemia in LMIC and one in seven cases of meningitis and late-onset bacteraemia in HIC. Although incidence of Gram-negative infections is much higher in resource-constrained settings, similarities in aetiology suggest that a better understanding of sources of infection and transmission routes could translate into preventive measures,55 thereby reducing the substantial burden of morbidity and mortality.
Acknowledgments
We would like to thank Alyson Hyland, Samuel Thomas, Mina Krishnan, Charlotte Prew at UKHSA Knowledge and Library Services for their assistance with this review. We would like to thank Guido Schwarzer for his help and adaptation of the meta package.
Footnotes
Contributors: TL conceived the study. LKH-W performed the database searches. LKH-W, AA and ON screened references, assessed study quality and extracted data. LKH-W performed the meta-analyses. SC provided analytical and methodological input. RJH, AD and TL provided microbiological and clinical input. LKH-W, SC and TL drafted the paper. All authors provided input to the manuscript, contributed to revisions and approved the final version. SC accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: This study was funded by Pfizer Inc. AA is funded by the NIHR Health Protection Research Unit in Healthcare Associated Infections and Antimicrobial Resistance, a partnership between the UK Health Security Agency and the University of Oxford (NIHR200915).
Disclaimer: The funder of the study (Pfizer) had no role in the study design, data collection, data analysis, data interpretation or writing of the report. The corresponding author had full access to the data in the study and final responsibility for the decision to submit this paper.
Map disclaimer: The depiction of boundaries on this map does not imply the expression of any opinion whatsoever on the part of BMJ (or any member of its group) concerning the legal status of any country, territory, jurisdiction or area or of its authorities. This map is provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. Not applicable.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. Lawn JE, Blencowe H, Oza S, et al. Every newborn: progress, priorities, and potential beyond survival. Lancet 2014;384:189–205. 10.1016/S0140-6736(14)60496-7 [DOI] [PubMed] [Google Scholar]
- 2. Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 2012;379:2151–61. 10.1016/S0140-6736(12)60560-1 [DOI] [PubMed] [Google Scholar]
- 3. Okomo U, Akpalu ENK, Le Doare K, et al. Aetiology of invasive bacterial infection and antimicrobial resistance in neonates in sub-Saharan Africa: a systematic review and meta-analysis in line with the STROBE-NI reporting guidelines. Lancet Infect Dis 2019;19:1219–34. 10.1016/S1473-3099(19)30414-1 [DOI] [PubMed] [Google Scholar]
- 4. Wen SCH, Ezure Y, Rolley L, et al. Gram-Negative neonatal sepsis in low- and lower-middle-income countries and who empirical antibiotic recommendations: a systematic review and meta-analysis. PLoS Med 2021;18:e1003787. 10.1371/journal.pmed.1003787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zelellw DA, Dessie G, Worku Mengesha E, et al. A systemic review and meta-analysis of the leading pathogens causing neonatal sepsis in developing countries. Biomed Res Int 2021;2021:6626983. 10.1155/2021/6626983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sands K, Carvalho MJ, Portal E, et al. Characterization of antimicrobial-resistant gram-negative bacteria that cause neonatal sepsis in seven low- and middle-income countries. Nat Microbiol 2021;6:512–23. 10.1038/s41564-021-00870-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stoll BJ, Hansen NI, Sánchez PJ, et al. Early onset neonatal sepsis: the burden of group B streptococcal and E. coli disease continues. Pediatrics 2011;127:817–26. 10.1542/peds.2010-2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cailes B, Kortsalioudaki C, Buttery J, et al. Epidemiology of UK neonatal infections: the neonIN infection surveillance network. Arch Dis Child Fetal Neonatal Ed 2018;103:F547–53. 10.1136/archdischild-2017-313203 [DOI] [PubMed] [Google Scholar]
- 9. Lin L, Chu H. Meta-Analysis of proportions using generalized linear mixed models. Epidemiology 2020;31:713–7. 10.1097/EDE.0000000000001232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schwarzer G. Schwarzer MGJTRffsc. Package ‘meta’ 2012;9. [Google Scholar]
- 11. Al-Taiar A, Hammoud MS, Cuiqing L, et al. Neonatal infections in China, Malaysia, Hong Kong and Thailand. Arch Dis Child Fetal Neonatal Ed 2013;98:F249–55. 10.1136/archdischild-2012-301767 [DOI] [PubMed] [Google Scholar]
- 12. Al-Talib HA-K. Neonatal septicemia in neonatal intensive care units: epidemiological and microbiological analysis of causative organisms and antimicrobial susceptibility. Int Medical J 2013;20:36–40. [Google Scholar]
- 13. Ballot DE, Nana T, Sriruttan C, et al. Bacterial bloodstream infections in neonates in a developing country. ISRN Pediatr 2012;2012:508512. 10.5402/2012/508512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bulkowstein S, Ben-Shimol S, Givon-Lavi N, et al. Comparison of early onset sepsis and community-acquired late onset sepsis in infants less than 3 months of age. BMC Pediatr 2016;16:82. 10.1186/s12887-016-0618-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Investigators of the Delhi Neonatal Infection Study (DeNIS) collaboration . Characterisation and antimicrobial resistance of sepsis pathogens in neonates born in tertiary care centres in Delhi, India: a cohort study. Lancet Glob Health 2016;4:e752–60. 10.1016/S2214-109X(16)30148-6 [DOI] [PubMed] [Google Scholar]
- 16. Ergaz Z, Benenson S, Cohen MJ, et al. No change in antibiotic susceptibility patterns in the neonatal ICU over two decades. Pediatr Crit Care Med 2013;14:164–70. 10.1097/PCC.0b013e31824fbc19 [DOI] [PubMed] [Google Scholar]
- 17. Giannoni E, Agyeman PKA, Stocker M, et al. Neonatal sepsis of early onset, and hospital-acquired and community-acquired late onset: a prospective population-based cohort study. J Pediatr 2018;201:106–14. 10.1016/j.jpeds.2018.05.048 [DOI] [PubMed] [Google Scholar]
- 18. Huggard D, Drew R, McCallion N. Neonatal bacteraemia among 112,360 live births. Ir Med J 2016;109:467. [PubMed] [Google Scholar]
- 19. Labi A-K, Obeng-Nkrumah N, Bjerrum S, et al. Neonatal bloodstream infections in a Ghanaian tertiary Hospital: are the current antibiotic recommendations adequate? BMC Infect Dis 2016;16:598. 10.1186/s12879-016-1913-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marwah P, Chawla D, Chander J, et al. Bacteriological profile of neonatal sepsis in a tertiary-care hospital of northern India. Indian Pediatr 2015;52:158–9. [PubMed] [Google Scholar]
- 21. Ramchandar N, Gierhart S, Creppage KE, et al. Epidemiology of serious bacterial infections in infants less than 90 days in a military health system cohort. Pediatr Infect Dis J 2019;38:849–53. 10.1097/INF.0000000000002346 [DOI] [PubMed] [Google Scholar]
- 22. Shim GH, Kim SD, Kim HS, et al. Trends in epidemiology of neonatal sepsis in a tertiary center in Korea: a 26-year longitudinal analysis, 1980-2005. J Korean Med Sci 2011;26:284–9. 10.3346/jkms.2011.26.2.284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sigaúque B, Kobayashi M, Vubil D, et al. Invasive bacterial disease trends and characterization of group B streptococcal isolates among young infants in southern Mozambique, 2001-2015. PLoS One 2018;13:e0191193. 10.1371/journal.pone.0191193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Viswanathan R, Singh AK, Mukherjee S, et al. Aetiology and antimicrobial resistance of neonatal sepsis at a tertiary care centre in eastern India: a 3 year study. Indian J Pediatr 2011;78:409–12. 10.1007/s12098-010-0272-1 [DOI] [PubMed] [Google Scholar]
- 25. Al-Taiar A, Hammoud MS, Thalib L, et al. Pattern and etiology of culture-proven early-onset neonatal sepsis: a five-year prospective study. Int J Infect Dis 2011;15:e631–4. 10.1016/j.ijid.2011.05.004 [DOI] [PubMed] [Google Scholar]
- 26. Fjalstad JW, Stensvold HJ, Bergseng H, et al. Early-Onset sepsis and antibiotic exposure in term infants: a nationwide population-based study in Norway. Pediatr Infect Dis J 2016;35:1–6. 10.1097/INF.0000000000000906 [DOI] [PubMed] [Google Scholar]
- 27. Hammoud MS, Al-Taiar A, Al-Abdi SY, et al. Culture-Proven early-onset neonatal sepsis in Arab states in the Gulf region: two-year prospective study. Int J Infect Dis 2017;55:11–15. 10.1016/j.ijid.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 28. Jiang SH. Early-Onset sepsis among preterm neonates in China, 2015 to 2018. Pediatr Infect Dis J 2020:1236–41. [DOI] [PubMed] [Google Scholar]
- 29. Kuhn P, Dheu C, Bolender C, et al. Incidence and distribution of pathogens in early-onset neonatal sepsis in the era of antenatal antibiotics. Paediatr Perinat Epidemiol 2010;24:479–87. 10.1111/j.1365-3016.2010.01132.x [DOI] [PubMed] [Google Scholar]
- 30. Ohlin A, Björkman L, Serenius F, et al. Sepsis as a risk factor for neonatal morbidity in extremely preterm infants. Acta Paediatr 2015;104:1070–6. 10.1111/apa.13104 [DOI] [PubMed] [Google Scholar]
- 31. Resch BR. Comparison of pathogen associated laboratory and clinical parameters in early-onset sepsis of the newborn. J Perinat Med 2015;43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schrag SJ, Farley MM, Petit S, et al. Epidemiology of invasive early-onset neonatal sepsis, 2005 to 2014. Pediatrics 2016;138:12. 10.1542/peds.2016-2013 [DOI] [PubMed] [Google Scholar]
- 33. Singh T, Barnes EH, Isaacs D, et al. Early-Onset neonatal infections in Australia and New Zealand, 2002-2012. Arch Dis Child Fetal Neonatal Ed 2019;104:F248–52. 10.1136/archdischild-2017-314671 [DOI] [PubMed] [Google Scholar]
- 34. Weston EJ, Pondo T, Lewis MM, et al. The burden of invasive early-onset neonatal sepsis in the United States, 2005-2008. Pediatr Infect Dis J 2011;30:937–41. 10.1097/INF.0b013e318223bad2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Berardi A, Sforza F, Baroni L, et al. Epidemiology and complications of late-onset sepsis: an Italian area-based study. PLoS One 2019;14:e0225407. 10.1371/journal.pone.0225407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Greenhow TL, Hung Y-Y, Herz AM, et al. The changing epidemiology of serious bacterial infections in young infants. Pediatr Infect Dis J 2014;33:595–9. 10.1097/INF.0000000000000225 [DOI] [PubMed] [Google Scholar]
- 37. Seale AC, Blencowe H, Zaidi A, et al. Neonatal severe bacterial infection impairment estimates in South Asia, sub-Saharan Africa, and Latin America for 2010. Pediatr Res 2013;74 Suppl 1:73–85. 10.1038/pr.2013.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Allegranzi B, Bagheri Nejad S, Combescure C, et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet 2011;377:228–41. 10.1016/S0140-6736(10)61458-4 [DOI] [PubMed] [Google Scholar]
- 39. Molina García A, Cross JH, Fitchett EJA, et al. Infection prevention and care bundles addressing health care-associated infections in neonatal care in low-middle income countries: a scoping review. EClinicalMedicine 2022;44:101259. 10.1016/j.eclinm.2021.101259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Collin SM, Lamb P, Jauneikaite E, et al. Hospital clusters of invasive group B streptococcal disease: a systematic review. J Infect 2019;79:521–7. 10.1016/j.jinf.2019.11.008 [DOI] [PubMed] [Google Scholar]
- 41. Downie L, Armiento R, Subhi R, et al. Community-acquired neonatal and infant sepsis in developing countries: efficacy of WHO's currently recommended antibiotics--systematic review and meta-analysis. Arch Dis Child 2013;98:146–54. 10.1136/archdischild-2012-302033 [DOI] [PubMed] [Google Scholar]
- 42. Piening BC, Geffers C, Gastmeier P, et al. Pathogen-Specific mortality in very low birth weight infants with primary bloodstream infection. PLoS One 2017;12:e0180134. 10.1371/journal.pone.0180134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Simonsen KA, Anderson-Berry AL, Delair SF, et al. Early-Onset neonatal sepsis. Clin Microbiol Rev 2014;27:21–47. 10.1128/CMR.00031-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Le Doare K, Bielicki J, Heath PT, et al. Systematic review of antibiotic resistance rates among gram-negative bacteria in children with sepsis in resource-limited countries. J Pediatric Infect Dis Soc 2015;4:11–20. 10.1093/jpids/piu014 [DOI] [PubMed] [Google Scholar]
- 45. Khalil N, Blunt HB, Li Z, et al. Neonatal early onset sepsis in middle Eastern countries: a systematic review. Arch Dis Child 2020;105:639–47. 10.1136/archdischild-2019-317110 [DOI] [PubMed] [Google Scholar]
- 46. Yu Y-Q, He X-R, Wan L-J, et al. Etiology, antimicrobial resistance, and risk factors of neonatal sepsis in China: a systematic review and meta-analysis from data of 30 years. J Matern Fetal Neonatal Med 2021:1–10. 10.1080/14767058.2021.1951217 [DOI] [PubMed] [Google Scholar]
- 47. Flannery DD, Puopolo KM, Hansen NI, et al. Antimicrobial susceptibility profiles among neonatal early-onset sepsis pathogens. Pediatr Infect Dis J 2022;41:263–71. 10.1097/INF.0000000000003380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. UKHSA . English surveillance programme for antimicrobial utilisation and resistance (ESPAUR) report 2020-2021. London, UK: UKHSA, 2021. [Google Scholar]
- 49. Akselsen AB, Sheth CC, Veses V. Efficacy of empiric antibiotic treatment of late-onset neonatal sepsis caused by Enterobacteriaceae: a systematic review. Lett Appl Microbiol 2021. 10.1111/lam.13640. [Epub ahead of print: 24 Dec 2021]. [DOI] [PubMed] [Google Scholar]
- 50. Lodha A, Furlan AD, Whyte H, et al. Prophylactic antibiotics in the prevention of catheter-associated bloodstream bacterial infection in preterm neonates: a systematic review. J Perinatol 2008;28:526–33. 10.1038/jp.2008.31 [DOI] [PubMed] [Google Scholar]
- 51. Araujo da Silva AR, Marques A, Di Biase C, et al. Effectiveness of antimicrobial stewardship programmes in neonatology: a systematic review. Arch Dis Child 2020;105:563–8. 10.1136/archdischild-2019-318026 [DOI] [PubMed] [Google Scholar]
- 52. Rajar P, Saugstad OD, Berild D, et al. Antibiotic stewardship in premature infants: a systematic review. Neonatology 2020;117:673–86. 10.1159/000511710 [DOI] [PubMed] [Google Scholar]
- 53. Milton R, Gillespie D, Dyer C, et al. Neonatal sepsis and mortality in low-income and middle-income countries from a facility-based birth cohort: an international multisite prospective observational study. Lancet Glob Health 2022;10:e661–72. 10.1016/S2214-109X(22)00043-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Thomson KM, Dyer C, Liu F, et al. Effects of antibiotic resistance, drug target attainment, bacterial pathogenicity and virulence, and antibiotic access and affordability on outcomes in neonatal sepsis: an international microbiology and drug evaluation prospective substudy (BARNARDS). Lancet Infect Dis 2021;21:1677–88. 10.1016/S1473-3099(21)00050-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schlapbach LJ, Hagmann C, Giannoni E. Time to tackle early-onset sepsis in low-income and middle-income countries. Lancet Glob Health 2022;10:e592–3. 10.1016/S2214-109X(22)00086-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
archdischild-2022-324047supp001.pdf (387.8KB, pdf)
archdischild-2022-324047supp002.pdf (62.7KB, pdf)
archdischild-2022-324047supp003.pdf (144.6KB, pdf)
archdischild-2022-324047supp004.pdf (135.6KB, pdf)
archdischild-2022-324047supp005.pdf (221.5KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information. Not applicable.