The newer antidiabetic medications have revolutionized management of diabetes. These include the sodium–glucose cotransporter 2 (SGLT2) inhibitors and the long-acting glucagon-like peptide 1 (GLP-1) receptor agonists. These two classes of drugs provide additional cardiovascular and renal benefits beyond glycemic control, making them attractive choices in this new era of diabetes therapy. The benefits of drugs in these two classes include weight loss and improved blood pressure control. They have a limited number of adverse effects, including urinary tract infections in patients on SGLT2 inhibitors and gastrointestinal symptoms in those receiving long-acting GLP-1 receptor agonists. Although the newer GLP-1 receptor agonists have not been noted to have any significant renal adverse effects, there are a few reported instances of renal injury induced by the GLP-1 receptor agonist semaglutide (1). However, there is only one known case report of dulaglutide-induced kidney injury (2). However, this case did not include renal biopsy information to identify the type of renal injury.
We report here a case of biopsy-proven acute interstitial nephritis secondary to dulaglutide use, which has not been reported previously. The incidence of dulaglutide-induced acute kidney injury is extremely rare, although possible. This case also provides an understanding of the possible pathophysiology of this adverse effect.
Case Presentation
A 78-year-old man with type 2 diabetes, hypertension, ischemic heart disease, and obstructive sleep apnea presented to our hospital with a 2-week history of lethargy, loss of appetite, and nausea. Laboratory investigations revealed acute kidney injury (AKI), with creatinine of 8.46 mg/dL (normal <1.25 mg/dL) and blood urea nitrogen of 67 mg/dL (normal <22.4 mg/dL). His renal function had been normal 3 months before this presentation. He was also found to have transaminitis, with ALT of 2,036 units/L and AST of 515 units/L, with normal bilirubin. He had normal urine output.
He had been treated with the GLP-1 receptor agonist exenatide for a period of several years, but this was changed to dulaglutide 4 weeks before presentation because of dulaglutide’s better cardiovascular profile and the ease of its once-weekly administration. There had been no other changes in medications or other recent illnesses to account for the AKI.
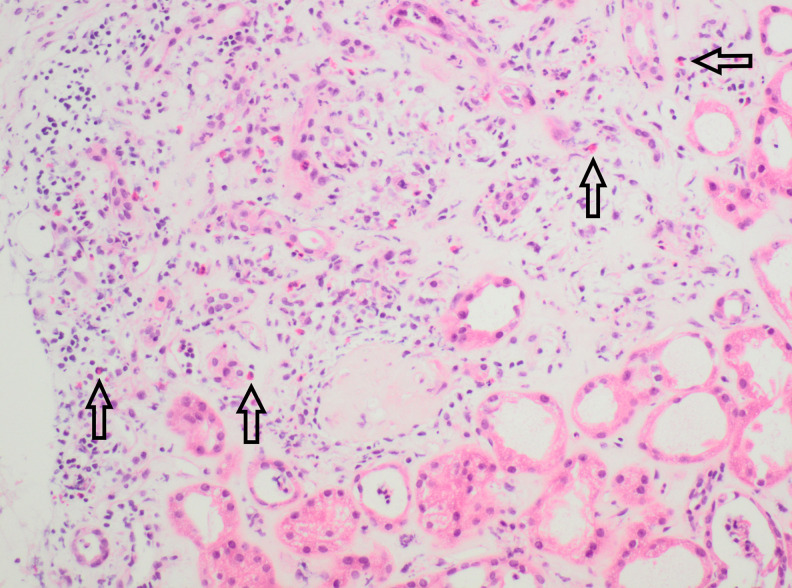
He underwent extensive investigations for an autoimmune process, which were negative, and abdominal imaging, which revealed no evidence of obstructive uropathy, hepatobiliary, or pancreatic abnormalities. His urine always demonstrated microscopic hematuria because of a history of prostate cancer and the presence of a urethral sphincter device. The hematuria varied in severity because of the insertion of an indwelling catheter. Subsequently, he underwent a renal biopsy, which revealed evidence of interstitial nephritis with eosinophilic infiltrates (Figure 1). There was also partial scarring noted on biopsy, with changes secondary to hypertension and diabetes.
FIGURE 1.
High-power view of hematoxylin and eosin stain of renal biopsy, showing eosinophilic infiltrate in the interstitium (marked with arrows).
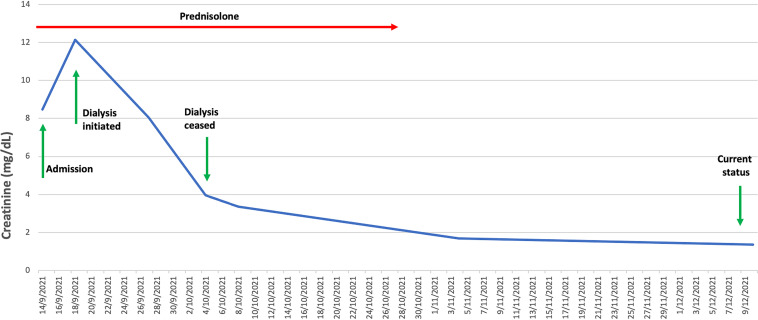
He was initiated on prednisolone 50 mg, which was continued for 1 week, then tapered to a dose of 30 mg and maintained at this level for 2 weeks, and then gradually tapered over 3 more weeks. He underwent dialysis for 2 weeks. Two weeks after initiating therapy, he was noted to have stabilization and improvement in kidney function, with his most recent creatinine level at 1.36 mg/dL (Figure 2). The transaminitis had normalized as well.
FIGURE 2.
Renal function (creatinine mg/dL) from admission until recovery from AKI (reference range <1.25 mg/dL).
Questions
How likely is the development of AKI secondary to interstitial nephritis with dulaglutide or other long-acting GLP-1 receptor agonists?
Is there a unique molecular structure creating increased immunogenicity for some GLP-1 receptor agonists despite tolerance to other drugs within this class?
Are long-acting GLP-1 receptor agonists safe in the context of these rare adverse events?
Commentary
SGLT2 inhibitors and long-acting GLP-1 receptor agonists have been shown to provide cardiovascular and renal benefits in addition to glycemic control. The common side effects of long-acting GLP-1 receptor agonists are gastrointestinal in nature and managed symptomatically, with most patients able to overcome these issues and continue therapy. However, with wider usage of these agents over a longer period of time, rare adverse effects may become evident and may be worth monitoring for in the initial phase of treatment.
Here, we report a case of AKI caused by acute interstitial nephritis requiring dialysis after introduction of dulaglutide. There is emerging literature regarding the incidence of AKI with semaglutide and one report of dulaglutide-induced AKI. However, there is no information from large clinical trials on the overall incidence of AKI and other serious renal adverse events.
The pathophysiology of AKI in our patient was allergic interstitial nephritis related to the only new medication, namely dulaglutide, which had been initiated 4 weeks earlier. It is interesting to note that he had no problems with exenatide during several years of use. Hence, dulaglutide probably elicited a unique immunogenic response not seen with exenatide.
The outcome of allergic interstitial nephritis associated with newer long-acting GLP-1 receptor agonists is mixed, with a poorer response noted with semaglutide (1) and a better outcome noted with dulaglutide (2). However, the data are too sparse to establish an actual incidence rate or risk factors for AKI associated with long-acting GLP-1 receptor agonists. Moreover, the actual incidence of AKI as a direct effect of these agents is difficult to establish, as AKI may be a consequence of other adverse effects—especially gastrointestinal side effects resulting in pre-renal insult.
A registry of adverse events in the post-marketing phase would greatly help in documenting the actual incidence of AKI as a direct adverse event. Importantly, despite rare adverse events, long-acting GLP-1 receptor agonists have revolutionized the treatment of diabetes and should always be considered in patients with diabetes for better long-term cardiovascular and renal outcomes in addition to glycemic control.
Clinical Pearls
Acute interstitial nephritis is a rare but significant complication associated with the use of newer long-acting GLP-1 receptor agonists.
Available evidence suggests that early diagnosis and withdrawal of these agents, along with treatment with prednisolone, may be helpful in limiting and reversing the kidney injury in some cases.
Tolerance to one GLP-1 receptor agonist agent may not necessarily mean a different drug in the same class will be as well tolerated.
Article Information
Acknowledgments
The authors thank the patient depicted in this case for providing permission to publish this information and thereby contributing to our evolving knowledge about this condition.
Duality of Interest
No potential conflicts of interest relevant to this article were reported.
Author Contributions
Both authors were actively involved in the discussion and preparation of this manuscript. M.G.K. drafted the initial manuscript. J.R. critically revised and edited the manuscript. M.G.K. is the guarantor of this work and, as such, takes responsibility for the accuracy of the information provided.
References
- 1. Leehey DJ, Rahman MA, Borys E, Picken MM, Clise CE. Acute kidney injury associated with semaglutide. Kidney Med 2021;3:282–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Taylor SR, Moody MT. Acute kidney injury occurring in a patient initiated on dulaglutide. J Pharm Technol 2018;34:231–232 [DOI] [PMC free article] [PubMed] [Google Scholar]