Abstract
Fast-acting insulin aspart (faster aspart) is an ultra-rapid-acting formulation of insulin aspart developed to more closely match the prandial endogenous insulin profile, and its accelerated absorption kinetics are expected to provide clinical benefits for patients using insulin pump therapy. A head-to-head trial versus the original insulin aspart formulation in pump therapy did not demonstrate superiority of faster aspart in terms of A1C reduction, but pump settings were not optimized for the pharmacokinetic/pharmacodynamic profile of faster aspart. Nevertheless, meal test and continuous glucose monitoring data suggest that faster aspart is beneficial for postprandial glucose control, and a case study is presented illustrating excellent results using this insulin in pump therapy. Frequent blood glucose monitoring and appropriate patient education are vital for success.
Type 1 Diabetes, Multiple Injection Therapy, and the Need for Fast-Acting Insulin Analogs
Achieving and maintaining glycemic levels close to normoglycemia in people with type 1 diabetes is a recognized goal to prevent or delay the progression of microvascular disease and reduce the risk for macrovascular disease (1) in a condition that requires life-long exogenous insulin therapy. The aim of insulin replacement therapy is to replicate as closely as possible the dynamic circulating insulin levels that, before the onset of diabetes, would have been provided by endogenous pancreatic secretion (2). Insulin therapy in type 1 diabetes has traditionally been provided using a multiple daily injection (MDI) therapy regimen, in which a once- or twice-daily long-acting insulin that provides background basal insulin is used alongside mealtime boluses of a short-acting insulin (2).
Human insulin molecules naturally self-associate into hexamers, and this property means that the absorption kinetics from a subcutaneous depot are suboptimal; formulations of human insulin have been unable to closely replicate either the natural relatively constant basal insulin output or the rapidly elevated secretion made in response to food intake (3). Consequently, research in recent decades has focused on developing analogs of human insulin that have modified self-association properties, resulting in clinically more desirable kinetic profiles when absorbed from a subcutaneous injection depot (3). The three currently available rapid-acting insulin analogs (RAIAs)—insulin lispro, insulin aspart, and insulin glulisine—differ from regular human insulin by having one or two amino acid sequence changes that reduce self-association, allowing these products to better replicate the natural prandial insulin response when given as a subcutaneous bolus injection (3).
With their faster action, these RAIAs limit postprandial hyperglycemic excursions, which are a major contributor to glycemic variability and constrain a patient’s ability to achieve overall glycemic goals. These factors are of clinical concern because increased glycemic variability and decreased time in range (TIR), as assessed by continuous glucose monitoring (CGM), have been independently associated with adverse outcomes in patients with diabetes, including cardiovascular disease and an increased risk of death, as well as an increased risk of microvascular complications (4–13). For example, a retrospective analysis of data from the Diabetes Control and Complications Trial (5) showed that, with each 10% drop in TIR, there was an increase in risk of retinopathy by 64% and of microalbuminuria by 40%. As elaborated upon below, the new CGM-derived glycemic metrics are becoming increasingly important as insulin therapy moves from MDI regimens to insulin pump technology.
The Increasing Role and Scope of Insulin Pump Therapy
The reduced self-association and faster absorption of the RAIAs also make them attractive for use in continuous subcutaneous insulin infusion (CSII) (i.e., insulin pump therapy), which is becoming increasingly used for type 1 diabetes. CSII aims to dynamically change insulin supply to match physiological need, and changes in the pump infusion rate for these RAIAs are more quickly translated into appropriate changes in the circulating concentration than could previously be achieved with regular human insulin. Hence, they are associated clinically with improved glycemic status without an increase in hypoglycemic episodes (14–17).
CSII is gaining popularity as a result of improving technology (18) and the fact that it can be more effective and carry a lower risk of nocturnal and severe hypoglycemia than MDI regimens in patients with type 1 diabetes (19–23). CSII can be especially successful in patients who are invested in their self-care and able to frequently monitor glucose or use CGM, count carbohydrates and/or match food to insulin, and (with training in troubleshooting) deal with issues that may arise with pump use such as infusion-site problems or hypoglycemia (24). Data from the T1D Exchange Registry show that use of an insulin pump increased in the United States from 57% of patients in 2010–2012 to 63% in 2016–2018, with the largest increases seen in children (from 50 to 60% in children <6 years of age and from 58 to 68% in children 6–12 years of age) (25).
Insulin pumps allow for programmable rates of insulin delivery, as well as on-demand changes to adapt for physical activity or illness. They also incorporate several bolus strategies (i.e., standard, square, or extended/dual-wave, depending on specific brands) to account for differences in the nutritional content of meals and bolus calculators that assist users with complex dose calculations. Commonly used devices in the United States include conventional insulin pump models with tubing connecting the subcutaneous cannula to the insulin reservoir, such as the MiniMed pumps (Medtronic Diabetes, Northridge, CA) and the t:slim X2 (Tandem Diabetes, San Diego, CA) brands. Tubeless insulin pumps, such as the Omnipod (Insulet, Acton, MA), for which the cannula is directly attached to the insulin reservoir that sits on the patient’s skin, are also widely used. Both the Medtronic and Tandem pumps have been enhanced by integration with CGM. These sensor-augmented pumps (SAPs) respond in various ways to CGM-derived data (e.g., by suspending insulin delivery when a pre-set glycemic threshold is reached or predicted from trend data).
In 2016, the U.S. Food and Drug Administration (FDA) approved the Medtronic MiniMed 670G system; in December 2019, the Tandem t:slim X2 with Control-IQ Technology; and, in August 2020, the Medtronic MiniMed 770G system, enhancing the CSII field with three hybrid closed-loop (HCL) systems (26–28); in addition, in 2022, the Insulet Omnipod 5 automated insulin delivery system was cleared by the FDA (29). When the automated insulin delivery mode of an HCL insulin pump is turned on, the pump can automatically adjust insulin delivery based on the user’s CGM readings, although users still need to enter carbohydrate data for their meals. The pivotal trial for the t:slim X2 with Control-IQ Technology advanced glucose controller involved 168 patients with type 1 diabetes who were 14–71 years of age and showed that the closed-loop group experienced an increase in percentage of time spent in the target range of 70–180 mg/dL (3.9–10.0 mmol/L) from 61 to 71% (P <0.001), as well as decreased time in hyperglycemia (>180 mg/dL [10.0 mmol/L]) from 36 to 27% (P <0.001) and in hypoglycemia (<70 mg/dL [3.9 mmol/L]) from 3.58 to 1.58% (30). A recent 1-year study following patients starting on the 670G system found a significant correlation between improved A1C and use of the system’s automatic insulin delivery mode (31).
The Omnipod 5 system consists of a small, tubeless, adhesive-patch pump (Pod) that is worn on the body and uses a novel algorithm with customizable glucose targets (32). The pivotal trial for the Omnipod 5 system was a single-arm, prospective study that included 111 children and 124 adults with type 1 diabetes (32). Change in A1C during a 3-month automated insulin delivery phase compared with a 2-week standard therapy phase (primary effectiveness outcome) was −0.71% (−7.8 mmol/mol) in children (mean ± SD: 7.67 ± 0.95% to 6.99 ± 0.63% [60 ± 10.4 mmol/mol to 53 ± 6.9 mmol/mol], P <0.0001) and −0.38% (−4.2 mmol/mol) in adults (7.16 ± 0.86% to 6.78 ± 0.68% [55 ± 9.4 mmol/mol to 51 ± 7.4 mmol/mol], P <0.0001). The corresponding increase seen in time in range (70–180 mg/dL [3.9–10.0 mmol/L]) was +15.6% in children (52.5 ± 15.6% to 68.0 ± 8.1%, P <0.0001) and +9.3% in adults (64.7 ± 16.6% to 73.9 ± 11.0%, P <0.0001).
Emergence of Important New Metrics From CGM Technology
CGM data are able to provide new insights into glycemic control that were previously invisible with standard end points such as A1C (6,33–35). Although A1C has remained the gold standard for assessment of glycemia for several decades, it has also long been known that A1C is actually a poor indicator of blood glucose control that can give rise to false reassurance or concern because patients with similar A1C values can vary greatly in terms of their glycemic variability and TIR (6). CGM data, however, can be highly revealing about the degree and nature of dysglycemia that patients are experiencing, and the developing closed-loop technologies offer the scope for rapid and/or automated corrections to be made that render longer-term metrics such as A1C almost obsolete for their users. With improving accuracy, affordability, and adoption of pump and CGM technologies in clinical practice, metrics such as TIR, time below range (<70 mg/dL and <54 mg/dL), time above range (>180 mg/dL and >250 mg/dL), coefficient of variation, and others are emerging as important new end points for the assessment of glycemic variability (4,6,33–35). There is ongoing development to make insulin delivery systems even more responsive to glucose trends, with fully closed-loop delivery systems in clinical trials, and these newer CGM metrics are becoming increasingly used as end points in clinical trials of insulins, antihyperglycemic drugs, and pump devices. TIR, in particular, is emerging as an important outcome metric, having been validated as a predictor of microvascular complications in people with diabetes (5–7).
Putting the Two Together: Expectations and Opportunities for Faster Aspart in Pump Therapy
Despite the advances in insulin delivery and CGM technology, many patients with type 1 diabetes still do not achieve glycemic targets (25). The inability to achieve optimal glycemic management is multifactorial, but there are barriers to the uptake of CGM technology, and the majority of patients do not use it (35,36). Issues specific to pump therapy include interruptions to insulin delivery because of absorption problems or malfunctions such as blockage of the infusion set and/or kinking of the cannula, cannula displacement, pump dysfunction, and lack of insulin in the reservoir (37). Insulin absorption can be affected by infusion set type, placement and wear time, pump delivery rate, lipohypertrophy at the infusion site, and the pharmacokinetics of the specific insulin formulation (38,39). Indeed, with the original generation of RAIAs, there is still a lag time after adjusting the insulin infusion rate and the corresponding onset and offset of glucose-lowering action; hence, their absorption kinetics remain a key limitation in the performance of current HCL insulin delivery systems (40,41).
Because the concept of CSII is based on an immediacy of insulin action, some new ultra-rapid-acting insulin formulations are being developed with accelerated absorption kinetics to further reduce the time interval between change in infusion rate and insulin action. Recently, a new formulation of insulin lispro (insulin lispro-aabc) was approved by the FDA (42). Another novel formulation of insulin lispro, BioChaperone lispro, is currently in phase 3 clinical trials (43,44). Faster aspart, a novel formulation of insulin aspart, is the only ultra-rapid-acting insulin currently approved by the FDA for use in insulin pumps (45,46).
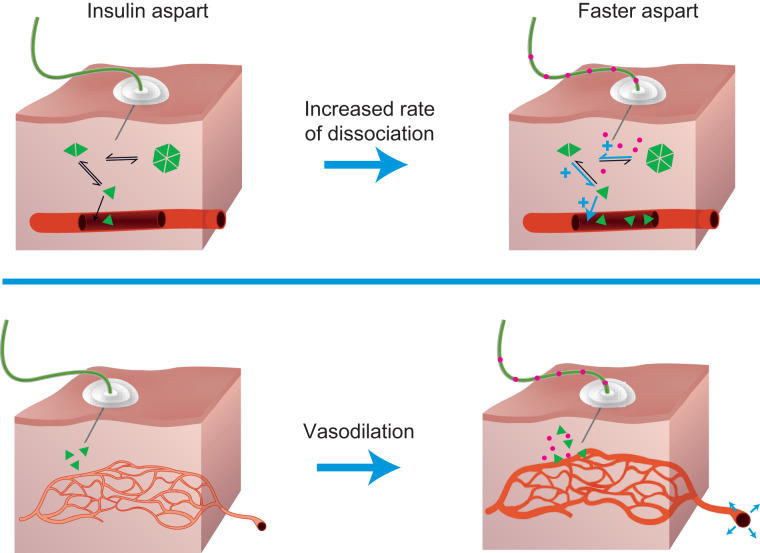
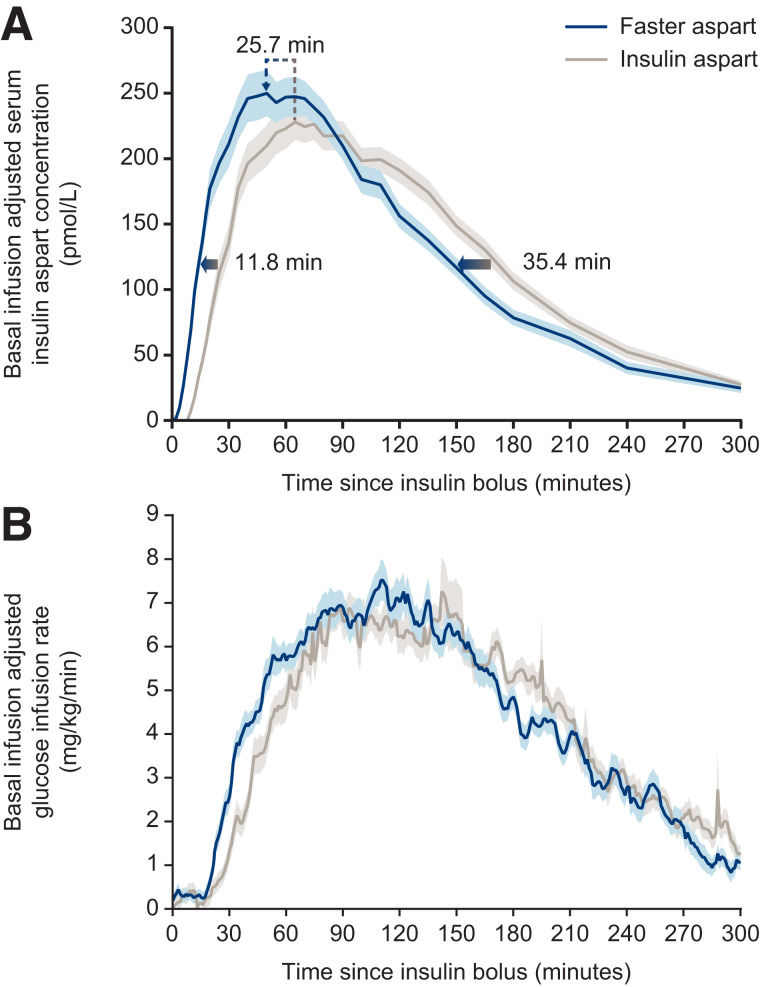
Faster aspart differs from the previous formulation of this insulin analog by the inclusion of the excipients niacinamide and L-arginine (47). Niacinamide (a form of vitamin B3) acts to increase the initial abundance of insulin aspart monomers (which are rapidly absorbed into the circulation) after subcutaneous administration and also mediates a local vasodilatory effect (Figure 1). L-arginine (an amino acid) functions as a stabilizing agent (47). As a consequence of these excipients, the pharmacokinetic/pharmacodynamic (PK/PD) profiles of faster aspart are left-shifted compared with those of insulin aspart (48,49) (Figure 2). The acceleration of the PK/PD profile is particularly marked when faster aspart is administered via CSII, possibly as a result of continuous replenishment of niacinamide and/or a smaller subcutaneous depot when compared with bolus injection (50). Indeed, after a bolus dose delivered in a crossover glucose clamp study of patients receiving faster aspart and insulin aspart via CSII, there was an approximately threefold greater early insulin exposure, and the glucose-lowering effect within the first 30 minutes was approximately twice as great with faster aspart (49). The offset of exposure and of glucose-lowering effect were also accelerated with faster aspart, occurring approximately 35 and 24 minutes earlier, respectively (49).
FIGURE 1.
The role of niacinamide in the accelerated absorption of faster aspart. Faster aspart is a novel formulation of insulin aspart containing two excipients: niacinamide and L-arginine. The presence of niacinamide increases the early absorption of insulin aspart by reducing the tendency of insulin aspart monomers to remain self-associated as hexamers in the injection depot and by mediating a transient, local vasodilatory effect (47).
FIGURE 2.
Pharmacological properties of faster aspart in insulin pump therapy. (A) Mean serum insulin aspart concentration after bolus dose of 0.15 units/kg faster aspart or insulin aspart administered by CSII. Arrows indicate that the estimated onset and offset of exposure occurred earlier for faster aspart versus insulin aspart and show the left-shift of the time of maximum insulin aspart concentration for faster aspart versus insulin aspart. (B) Mean glucose-lowering effect after bolus dose of 0.15 units/kg faster aspart or insulin aspart. Variability bands show the SEM. Reprinted with permission from ref. 47. Copyright John Wiley and Sons 2016.
It is important to understand, however, that the PK/PD profiles shown in glucose clamp studies represent mean profiles, and there can be large interindividual variability in response; hence, there is a need for careful individualization of therapy. Intraindividual variability in PK response (a major concern with basal insulin injection therapy) from bolus to bolus is less of an issue with RAIAs, but there may still be a 10–20% intraindividual variability in PD response (51). This variability might reflect temporal fluctuations in insulin sensitivity caused by various nutritional, physiological, pathophysiological, and disruptive factors (summarized by Home [52]). This is a major reason why closed-loop insulin pump therapy is appealing, as such factors should be automatically compensated for by the feedback system.
The improved PK/PD properties of faster aspart ought to translate into an ability to further improve glycemic control versus previous RAIAs in insulin pumps, especially with regard to postprandial glucose (PPG) control, but the phase 3 onset clinical trial program for faster aspart mostly compared its performance versus insulin aspart in type 1 diabetes in the setting of MDI regimens (53–55). In summary, these trials demonstrated that faster aspart was noninferior with regard to A1C reduction versus insulin aspart, but was, predictably, superior with regard to early PPG control during a standardized meal test, and the feasibility of postmeal dosing was also demonstrated (53–55). A relative reduction in glucose increments 1 and 2 hours after breakfast and dinner and in the mean of all meals versus insulin aspart was also shown with the use of CGM in pediatric patients (56). Overall hypoglycemia (either severe or blood glucose–confirmed <56 mg/dL [3.1 mmol/L]) and adverse events were similar between treatments (53–55). Although the rates of hypoglycemia reported during the first hour after a meal were very low compared with the overall rate (accounting for 1 in 40 episodes reported for mealtime faster aspart), there was an increase with mealtime faster aspart versus insulin aspart when used in combination with insulin detemir (54); however, there was a reduction in hypoglycemia in favor of mealtime faster aspart 3–4 hours after a meal when used in combination with insulin degludec (53). The onset trial program had some potential limitations, including the artificiality of the meal test to evaluate PPG levels and the lack of individualization of insulin doses during the meal test.
Relatively few clinical trials have assessed the use of faster aspart in a CSII regimen, but the compatibility of the new formulation with pump use was established in a 6-week study in which no microscopically confirmed infusion-set occlusions or particle/crystal formations were observed with either insulin aspart or faster aspart (57). In the subsequent larger-scale onset 5 study, the rates of routine and nonroutine infusion-set changes were similar with faster aspart and insulin aspart (58). The expectation that faster aspart would enable improved glycemic control with CSII was set by a double-blind, randomized, crossover study in 43 individuals with type 1 diabetes using insulin pumps for 14 days (59). Here, faster aspart was associated with a 25% greater plasma glucose-lowering effect during the first 2 hours of a standardized meal test compared with insulin aspart, and the mean postprandial increment in interstitial glucose across all meals, measured by blinded CGM, was ∼50% lower for faster aspart than for insulin aspart.
The largest clinical trial of faster aspart in CSII was the double-blind, treat-to-target onset 5 study, in which 472 people with type 1 diabetes were randomized in equal numbers to a 16-week treatment period with faster aspart or insulin aspart (58). This study showed noninferiority for faster aspart versus insulin aspart for A1C reduction, with an estimated treatment difference (ETD) of 0.09% (95% CI 0.01–0.17%, P <0.001, for clinical noninferiority), but with this small ETD being statistically significantly greater with insulin aspart (58). In contrast, PPG increments at 30 minutes, 1 hour, and 2 hours after a liquid meal test were statistically significantly reduced with faster aspart compared with insulin aspart (ETD at 30 minutes −11.8 mg/dL [95% CI −18.1 to −5.6 mg/dL] or −0.66 mmol/L [−1.00 to −0.31 mmol/L], P <0.001; at 1 hour −16.4 mg/dL [95% CI −25.7 to −7.1 mg/dL] or −0.91 mmol/L [−1.43 to −0.39 mmol/L], P = 0.001; and at 2 hours −16.2 mg/dL [95% CI −28.5 to −4.0 mg/dL] or −0.90 mmol/L [−1.58 to −0.22 mmol/L], P = 0.01) (58). This was corroborated by lower postprandial interstitial glucose increments 1 and 2 hours after a meal with faster aspart (58). CGM data also showed that participants using faster aspart had higher nocturnal and premeal levels of interstitial glucose compared with those using insulin aspart (58). This observation, and the discrepancy between the A1C and PPG results, may have been caused by suboptimal pump settings, as parameters were kept the same as pre-study settings and not adjusted for faster aspart use. There was no difference between treatments in the rate of overall severe or blood glucose– confirmed hypoglycemia (45.07 and 45.29 episodes per patient-year of exposure [PYE] for faster aspart and insulin aspart, respectively), although, again, the rate for the small proportion of episodes that occurred during the first hour after a meal was higher for faster aspart than for insulin aspart (1.25 vs. 0.71 events/PYE) (58).
A few pilot studies of faster aspart in fully closed-loop insulin delivery systems have also now reported results using CGM metrics (60–62). During two 27-hour inpatient periods that included unannounced afternoon moderate-to-vigorous exercise and meals, near-normal glucose concentrations (outside of postprandial periods) with no hypoglycemia were achieved with both faster aspart and insulin aspart in 20 young adults with type 1 diabetes (60). TIR was similar for both insulins at 53.3% (83% overnight) and 57.9% (88% overnight) for faster aspart and insulin aspart, respectively (P = 0.17) (60). In contrast to the previous CSII studies, interstitial prandial glucose increments 1 hour after meals were greater with faster aspart compared with insulin aspart when data for all three meals were combined (30.9 mg/dL [95% CI 25.8–38.9 mg/dL] vs. 21.7 mg/dL [95% CI 7.3–30.6 mg/dL], P = 0.017), while there was no difference between the two groups for each meal separately (60). However, the study authors noted that, in the previous CSII studies of faster aspart, mealtime bolus dosing had been manually optimized based on self-monitored blood glucose measurements before each meal, without dependency on CGM to detect glucose excursions (60). The authors then noted that the closed-loop algorithm (DreaMedGlucoSitter, DreaMed Diabetes, Petah Tikva, Israel) was better adjusted to insulin aspart, with the system not optimized for the faster onset of action and clearance of faster aspart (60). Instead, the system settings were derived from the run-in period based on standard insulin aspart only; because the study was double-blinded, the closed-loop settings were not optimized for each formulation separately (60).
A study in 15 adults with type 2 diabetes using a fully closed-loop system over 22 hours showed similar results for faster aspart and insulin aspart for TIR (101–180 mg/dL [5.6–10.0 mmol/L]; 67.7 vs. 70.9%, P = 0.17) and other CGM metrics such as mean glucose, glucose variability, and times below and above target range (61). There was one episode of hypoglycemia (<63 mg/dL [3.5 mmol/L]) with faster insulin aspart and two with insulin aspart, and higher mean ± SD insulin doses of faster aspart (31.9 ± 22.6 vs. 28.2 ± 20.1 units, mean difference 3.7 units [95% CI 0.7–6.8 units], P = 0.021) were required to achieve these glucometric outcomes (61).
Collectively, these data point to faster aspart having the ability to better limit PPG excursions, but with this benefit being offset in other end points if pump settings are not adjusted relative to those used for standard RAIAs. The clamp study data show a PK/PD profile for faster aspart that is theoretically better suited to CSII than a standard RAIA (49), and the meal-test study shows that this can potentially translate into improved PPG control (59). Indeed, because of its PK/PD profile (with fast onset and offset of action), faster aspart was chosen for a study of fully closed-loop insulin delivery for noncritical care inpatients on enteral and/or parenteral nutrition (62). Compared with standard insulin therapy (multiple regimens), the mean ± SD glucose concentration was lower in the closed-loop group (8.5 ± 1.2 vs. 11.4 ± 3.4 mmol/L, P = 0.001), and TIR was higher (68.4 ± 15.5 vs. 36.4 ± 26.6%, P <0.0001) (62). In the clinical trials of faster aspart versus insulin aspart in similar pump systems, however, few clear advantages were shown for faster aspart, but it is important to note that few adaptations were made in onset 5, and no adaptations to the control algorithms were made in the closed-loop studies to account for the differences in insulin PK profile. The authors of each of these studies noted that optimization of insulin delivery algorithms might be needed to observe any additional benefits from faster aspart. This situation may, therefore, be analogous to that in the late 1990s, when insulin lispro was first introduced and used in CSII. At that time, patients and their physicians also had yet to learn how to use a RAIA optimally in CSII. Again, improvements in PPG control and hypoglycemia were observed in the initial trials, but improved A1C was only shown when basal insulin and/or snack regimens were simultaneously adjusted (63). Now, however, RAIAs have largely replaced soluble human insulin as the insulin of choice in CSII.
Studies using ultra-rapid lispro in CSII have also been published very recently (64–66) but are outside the scope of this article.
Taking Faster Aspart Into Real-World Pump Therapy
The FDA approved faster aspart for use in CSII in 2019 (45), and the authors of this article have used it with success in this indication over the past year. From our experience, patients appreciate the product not only for its ability to reduce PPG peaks, but also for the fact that it allows them to make faster corrections to hyperglycemia. As our case history below illustrates, individual patients can benefit greatly from a switch to faster aspart in their pump therapy, as long as appropriate adjustments are made to account for its PK profile. It should be noted that the use of faster aspart with different mealtime boluses and basal rate adjustments has not been studied in clinical trials; hence, health care providers prescribing faster aspart will need to support patients individually and with perseverance to find their optimal pump settings.
When converting from another insulin to faster aspart in CSII, the initial change can be made on a unit-to-unit basis, but, from our experience, substantial adjustments to both the bolus and basal infusions may be needed, based on real-time CGM or flash glucose monitoring. Patient education is also key to realizing the potential benefits when switching to faster aspart, as patients may otherwise not engage in the behavior changes needed to achieve improvements, including adjusting the timing of boluses, especially with regard to exercise and high-fat meals.
As with any insulin therapy, patients must be educated to recognize and manage hypoglycemia. Patients starting faster aspart should be advised that the accelerated action may cause early postmeal hypoglycemia, especially when eating a protein- or fat-rich meal, although the potential reduction in late postmeal hypoglycemia may help them avoid overtreatment with carbohydrates. With faster aspart having a faster onset and offset of glucose-lowering action, it may be beneficial to suggest that advanced boluses such as dual-wave or extended boluses be implemented if meals are protein- or fat-heavy. Additionally, consideration could be given to reducing the amount of bolus insulin given upfront (e.g., by ∼20%, depending on starting glucose levels) and increasing the duration of the extended bolus to better match food absorption and insulin action. The exact split will be subjectively determined, but the desired effect could be accomplished, for example, by using 40–50% of the bolus upfront and extending the rest (60 or 50%) over 2–3 hours, based on the macronutrient composition of the meals. Alternatively, 50–60% could be given upfront and 40–50% delivered ∼1 hour later. Because the time to late 50% of maximum insulin concentration (tLate 50% Cmax) has been shown to occur 35.4 minutes earlier with faster aspart than with insulin aspart (49), consideration should also be given to shortening active insulin time by 30 minutes. Adjustments to the basal rates may also be needed to compensate for the shorter duration of action of boluses with faster aspart. However, it may be prudent initially to only adjust bolus settings and keep the basal rate the same, so as not to increase the risk of dysglycemia (potentially including nocturnal hypoglycemia) arising from multiple simultaneous changes. Use of faster aspart could potentially also increase the risk of nocturnal hyperglycemia in patients who eat protein-rich evening meals unless compensatory adjustments are made to the basal infusion rate in a standard (not HCL) insulin pump system.
Although administration of faster aspart is recommended 0–2 minutes before a meal, postprandial administration (up to 20 minutes after a meal) via subcutaneous injection is included in the FDA-approved label (45). Although not tested in a CSII setting, health care providers could consider switching patients who frequently dose after meals to CSII with faster aspart. Postprandial bolus administration could also be useful in younger patients, when the meal content is unknown, when eating restaurant meals, and for patients in nursing facilities, who may have unpredictable food intake and/or delivery of meals.
Some patients have reported pain or tissue irritation around the infusion site when using faster aspart (50), and a numerically higher number of infusion-site changes resulting from infusion-site reactions were reported with faster aspart than with insulin aspart (21 vs. 13 events, respectively) in the onset 5 trial (58). The reasons for these reactions are not fully understood but may be associated with the excipients. Changing the bolus speed to a slower rate of delivery and having a longer cannula length can help with pain or tissue irritation around the infusion site. Empirically, from the authors’ experience, patients on faster aspart have also reported an increased need to change infusion site after 2 days, especially during the summer, because of hyperglycemia, which is perceived to be the result of reduced insulin potency. As with insulin aspart, patients should change their infusion set every 3 days and rotate infusion sites. Longer periods of time between infusion-set changes with faster aspart have not been trialed.
A Case History
To conclude this article, we outline and show data from a case history that we hope will illustrate how faster aspart can be used to great clinical benefit in selected patients if pump settings are carefully adjusted to individual need.
Presentation
A 27-year-old woman with type 1 diabetes, diagnosed at the age of 13 years, presented on 19 May 2018 to the endocrinology practice on an MDI insulin regimen and CGM. On that date, her initial A1C was 8.3%, and she requested to transition to insulin pump therapy. In July 2018, she started using Tandem t:slim X2 with Basal IQ insulin pump with insulin aspart, and Basal IQ technology was continued for the duration of the case study period. Despite being on insulin pump therapy and CGM, her A1C did not improve, and, in October 2018, her repeat A1C was elevated at 9.1%. She also reported significant challenges with postmeal hyperglycemia and timing of insulin doses at mealtimes. Her initial basal rate was 0.85 units/hour, her insulin-to-carbohydrate ratio was 1:11, her insulin sensitivity factor was 45, her blood glucose target was 130 mg/dL [7.2 mmol/L], and her insulin active time was 4 hours.
The patient’s insulin was therefore changed to faster aspart in an effort to reduce her postmeal hyperglycemia, and her pump settings were adjusted. She was also instructed to modify the frequency of insulin infusion-set changes to every 2 days instead of every 3 days. In June 2019, her A1C had improved to 7.8% with her insulin pump settings as follows: basal rates, midnight 0.9 units/hour; 6:00 a.m. 1 unit/hour; insulin-to-carbohydrate ratio 1:9; insulin sensitivity factor 45; blood glucose target 130 mg/dL [7.2 mmol/L]; and insulin active time 3 hours. She had no adverse events, no hospitalizations for diabetic ketoacidosis, and no severe hypoglycemic episodes requiring assistance from others during that period of time.
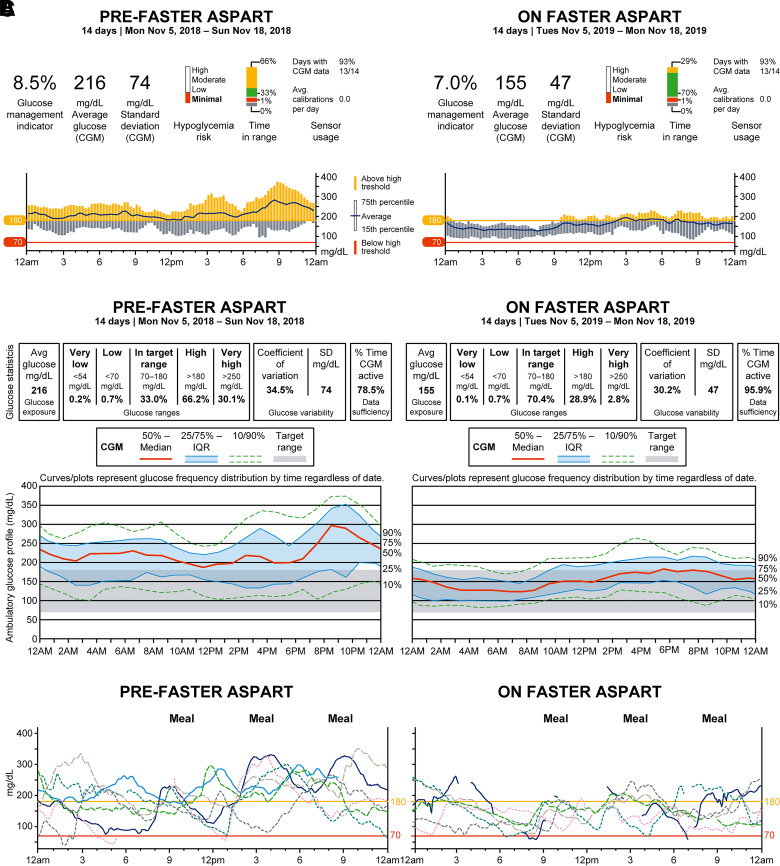
At her follow-up appointment in November 2019, her A1C had decreased further to 7.0% without further changes to her insulin pump settings. She has been administering boluses with meals and uses extended boluses for dinner. Since changing to faster aspart, her overall glucose management indicator decreased from 8.5 to 7.0%, when comparing two 2-week periods on the same dates but 1 year apart (November 2018, before starting faster aspart, and November 2019, 1 year after starting faster aspart) (Figure 3A); her TIR increased from 33 to 70% (Figure 3B); and her PPG excursion became much smaller without any increase in hypoglycemic events (Figure 3C). The 2-week periods reported in the figures were chosen based on recommendations from the International Consensus on Time in Range (33) regarding optimal duration of CGM review and based on the fact that 14 days of CGM data correlate strongly with 3 months of mean glucose; time in, above, and below the target range; and hyperglycemia metrics as published previously (67,68).
FIGURE 3.
Patient case study before and after use of faster aspart in insulin pump therapy. (A) Dexcom Clarity CGM report showing glucose management indicator (GMI), average glucose, and SD. GMI improved from 8.5 to 7.0%, matching the improvement in A1C. The sensor glucose average decreased from 216 to 155 mg/dL, and the SD decreased from 74 to 47 mg/dL. (B) Dexcom Clarity CGM ambulatory glucose report and glucometrics comparison between pre- and post-adoption of faster aspart. Sensor glucose average, SD, and coefficient of variation improved with faster aspart use. TIR improved from 33 to 70.4% on faster aspart. Time in hypoglycemia did not change overall; time in hyperglycemia >180 mg/dL [10.0 mmol/L] and >250 mg/dL [13.9 mmol/L] decreased from 66.2 and 30.1% to 28.9 and 2.8%, respectively. (C) Comparison of postmeal glycemic fluctuation between pre- and post-use of faster aspart. Postprandial glucose fluctuations were reduced with faster aspart use.
It should, of course, be noted that the improved PPG excursion and A1C would have resulted from the increases in basal rate and insulin-to-carbohydrate ratio, but we consider it likely that the use of faster aspart made these changes possible; increasing the basal rate and mealtime insulin-to-carbohydrate ratio with regular insulin aspart might have risked an increase in late postprandial hypoglycemia.
This patient is still on faster aspart in 2021 and continues to change infusion sets every 2.2 days. Her most recent A1C was 6.7% in June 2021.
Conclusion
Insulin formulations that can translate a change in pump infusion rate into a rapid increase or decrease of glucose-lowering action are theoretically ideally suited to CSII, particularly in automated or semiautomated CGM-guided systems. Faster aspart comes close to providing an ideal PK/PD profile, and meal-test studies show that it is better able to limit PPG excursions than conventional RAIAs. Available clinical trial data, however, may have underestimated the potential benefits of faster aspart as a result of the confounding influence of pump settings, which were optimized for the trial comparator rather than for faster aspart.
Empirically, faster aspart can be used to bring about marked improvements in the glycemic control of CSII-treated patients, but this requires attention to both the prandial and basal pump settings, and to meal content. To illustrate the full therapeutic potential of ultra-fast-acting insulins in CSII, future trials should allow pump settings to be fully optimized independently for each comparator, and, in addition to conventional end points such as A1C, CGM metrics should be used to capture information on glycemic stability.
Article Information
Acknowledgments
Medical writing and editorial support for the development of this manuscript, under the direction of the authors, was provided by Murray Edmunds and Helen Marshall, of Ashfield MedComms, an Ashfield Health company.
Funding
Medical writing and editorial support services for this article were funded by Novo Nordisk, Inc.
Duality of Interest
G.A. has received research support from AstraZeneca, Dexcom, Eli Lilly, Insulet, and Novo Nordisk and is a consultant for Dexcom and Insulet. B.B. has served on advisory panels for Medtronic and Novo Nordisk; has been a consultant for Lilly, Medtronic, and Novo Nordisk; has received research support from Abbott, Advance, DexCom, Diasome, Insulet, Janssen, Lilly/Boehringer Ingelheim, Mannkind, Medtronic, the National Institutes of Health, Nova Biomedical, Novo Nordisk, Provention Bio, Sanofi, and Senseonics; has participated in speakers’ bureaus for AstraZeneca, Janssen, Lilly/Boehringer Ingelheim, MannKInd, Medtronic, Novo Nordisk, Sanofi, and Senseonics; and is a stock shareholder in Aseko. A.L.C. has received speaker honoraria from Minimed Medtronic; has served on advisory panels for Eli Lilly, Medtronic, and Sanofi; and has received research support from Abbott, Dexcom, Eli Lilly, Insulet, Medtronic, Novo Nordisk, Sanofi, and UnitedHealth. A.L.C.’s employer, the nonprofit HealthPartners Institute, contracts for his services, and no personal income goes to A.L.C.
Author Contributions
All authors confirm that they meet the International Committee of Medical Journal Editors requirements for authorship. All authors contributed to the conception of the work and to researching references, drafting, and critically revising the article, and they share the final responsibility for the content of the manuscript and the decision to submit it for publication. G.A. provided the case history. G.A. is the guarantor of this work and, as such, had full access to all the data presented and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1. Diabetes Control and Complications Trial Research Group; Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 2. Lefever E, Vliebergh J, Mathieu C. Improving the treatment of patients with diabetes using insulin analogues: current findings and future directions. Expert Opin Drug Saf 2021;20:155–169 [DOI] [PubMed] [Google Scholar]
- 3. Mathieu C, Gillard P, Benhalima K. Insulin analogues in type 1 diabetes mellitus: getting better all the time. Nat Rev Endocrinol 2017;13:385–399 [DOI] [PubMed] [Google Scholar]
- 4. Ceriello A, Monnier L, Owens D. Glycaemic variability in diabetes: clinical and therapeutic implications. Lancet Diabetes Endocrinol 2019;7:221–230 [DOI] [PubMed] [Google Scholar]
- 5. Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care 2019;42:400–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hirsch IB, Sherr JL, Hood KK. Connecting the dots: validation of time in range metrics with microvascular outcomes. Diabetes Care 2019;42:345–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lu J, Ma X, Zhou J, et al. Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care 2018;41:2370–2376 [DOI] [PubMed] [Google Scholar]
- 8. Mayeda L, Katz R, Ahmad I, et al. Glucose time in range and peripheral neuropathy in type 2 diabetes mellitus and chronic kidney disease. BMJ Open Diabetes Res Care 2020; 8:e000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lu J, Ma X, Shen Y, et al. Time in range is associated with carotid intima-media thickness in type 2 diabetes. Diabetes Technol Ther 2020;22:72–78 [DOI] [PubMed] [Google Scholar]
- 10. Guo Q, Zang P, Xu S, et al. Time in range, as a novel metric of glycemic control, is reversely associated with presence of diabetic cardiovascular autonomic neuropathy independent of HbA1c in Chinese type 2 diabetes. J Diabetes Res 2020;2020:5817074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lu J, Wang C, Shen Y, et al. Time in range in relation to all-cause and cardiovascular mortality in patients with type 2 diabetes: a prospective cohort study. Diabetes Care 2021;44:549–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ranjan AG, Rosenlund SV, Hansen TW, Rossing P, Andersen S, Nørgaard K. Improved time in range over 1 year is associated with reduced albuminuria in individuals with sensor-augmented insulin pump-treated type 1 diabetes. Diabetes Care 2020;43:2882–2885 [DOI] [PubMed] [Google Scholar]
- 13. Yoo JH, Choi MS, Ahn J, et al. Association between continuous glucose monitoring-derived time in range, other core metrics, and albuminuria in type 2 diabetes. Diabetes Technol Ther 2020;22:768–776 [DOI] [PubMed] [Google Scholar]
- 14. Zinman B, Tildesley H, Chiasson JL, Tsui E, Strack T. Insulin lispro in CSII: results of a double-blind crossover study. Diabetes 1997;46:440–443 [DOI] [PubMed] [Google Scholar]
- 15. Garg SK, Anderson JH, Gerard LA, et al. Impact of insulin lispro on HbA1c values in insulin pump users. Diabetes Obes Metab 2000;2:307–311 [DOI] [PubMed] [Google Scholar]
- 16. Home PD, Lindholm A; European Insulin Aspart Study Group . Insulin aspart vs. human insulin in the management of long-term blood glucose control in type 1 diabetes mellitus: a randomized controlled trial. Diabet Med 2000;17:762–770 [DOI] [PubMed] [Google Scholar]
- 17. Becker RH, Frick AD. Clinical pharmacokinetics and pharmacodynamics of insulin glulisine. Clin Pharmacokinet 2008;47:7–20 [DOI] [PubMed] [Google Scholar]
- 18. Kravarusic J, Aleppo G. Diabetes technology use in adults with type 1 and type 2 diabetes. Endocrinol Metab Clin North Am 2020;49:37–55 [DOI] [PubMed] [Google Scholar]
- 19. Karges B, Schwandt A, Heidtmann B, et al. Association of insulin pump therapy vs insulin injection therapy with severe hypoglycemia, ketoacidosis, and glycemic control among children, adolescents, and young adults with type 1 diabetes. JAMA 2017;318:1358–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Misso ML, Egberts KJ, Page M, O’Connor D, Shaw J. Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. Cochrane Database Syst Rev 2010(1):CD005103. [DOI] [PubMed] [Google Scholar]
- 21. Senn JD, Fischli S, Slahor L, Schelbert S, Henzen C. Long-term effects of initiating continuous subcutaneous insulin infusion (CSII) and continuous glucose monitoring (CGM) in people with type 1 diabetes and unsatisfactory diabetes control. J Clin Med 2019;8:394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Benkhadra K, Alahdab F, Tamhane SU, McCoy RG, Prokop LJ, Murad MH. Continuous subcutaneous insulin infusion versus multiple daily injections in individuals with type 1 diabetes: a systematic review and meta-analysis. Endocrine 2017;55:77–84 [DOI] [PubMed] [Google Scholar]
- 23. Sherr JL, Hermann JM, Campbell F, et al.; T1D Exchange Clinic Network; the DPV Initiative; and the National Paediatric Diabetes Audit and the Royal College of Paediatrics and Child Health Registries . Use of insulin pump therapy in children and adolescents with type 1 diabetes and its impact on metabolic control: comparison of results from three large, transatlantic paediatric registries. Diabetologia 2016;59:87–91 [DOI] [PubMed] [Google Scholar]
- 24. Scheiner G, Sobel RJ, Smith DE, et al. Insulin pump therapy: guidelines for successful outcomes. Diabetes Educ 2009;35(Suppl. 2):29S–41S; quiz 28S, 42S–43S [DOI] [PubMed] [Google Scholar]
- 25. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016–2018. Diabetes Technol Ther 2019;21:66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. U.S. Food and Drug Administration . Minimed 670G system - P160017/S031. Available from https://www.accessdata.fda.gov/cdrh_docs/pdf16/P160017A.pdf. Accessed March 2021
- 27. U.S. Food and Drug Administration . t:Slim X2 insulin pump with interoperable technology. Available from https://www.accessdata.fda.gov/cdrh_docs/pdf18/DEN180058.pdf. Accessed March 2021
- 28. U.S. Food and Drug Administration . MiniMed 770G system - P160017/S076. Available from https://www.fda.gov/medical-devices/recently-approved-devices/minimed-770g-system-p160017s076#:∼:text=The%20Medtronic%20MiniMed%20770G%20System%20is%20the%20first,glucose%20reading%20in%20users%20two%20years%20and%20up. Accessed April 2021
- 29. U.S. Food and Drug Administration . Omnipod 5 ACE Pump (Pod) clearance letter. Available from https://www.accessdata.fda.gov/cdrh_docs/pdf20/K203768.pdf. Accessed 28 February 2022
- 30. Brown SA, Kovatchev BP, Raghinaru D, et al.; iDCL Trial Research Group . Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med 2019;381:1707–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lal RA, Basina M, Maahs DM, Hood K, Buckingham B, Wilson DM. One year clinical experience of the first commercial hybrid closed-loop system. Diabetes Care 2019;42:2190–2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brown SA, Forlenza GP, Bode BW, et al.; Omnipod 5 Research Group . Multicenter trial of a tubeless, on-body automated insulin delivery system with customizable glycemic targets in pediatric and adult participants with type 1 diabetes. Diabetes Care 2021;44:1630–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care 2019;42:1593–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chehregosha H, Khamseh ME, Malek M, Hosseinpanah F, Ismail-Beigi F. A view beyond HbA1c: role of continuous glucose monitoring. Diabetes Ther 2019;10:853–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Advani A. Positioning time in range in diabetes management. Diabetologia 2020;63:242–252 [DOI] [PubMed] [Google Scholar]
- 36. Tanenbaum ML, Hanes SJ, Miller KM, Naranjo D, Bensen R, Hood KK. Diabetes device use in adults with type 1 diabetes: barriers to uptake and potential intervention targets. Diabetes Care 2017;40:181–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Paldus B, Lee MH, O’Neal DN. Insulin pumps in general practice. Aust Prescr 2018;41:186–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Heinemann L, Steiner S. Subcutaneous injection versus subcutaneous infusion of insulin: are the rates of absorption truly the same? J Diabetes Sci Technol 2011;5:1027–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Heinemann L, Krinelke L. Insulin infusion set: the Achilles heel of continuous subcutaneous insulin infusion. J Diabetes Sci Technol 2012;6:954–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gingras V, Taleb N, Roy-Fleming A, Legault L, Rabasa-Lhoret R. The challenges of achieving postprandial glucose control using closed-loop systems in patients with type 1 diabetes. Diabetes Obes Metab 2018;20:245–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bekiari E, Kitsios K, Thabit H, et al. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. BMJ 2018;361:k1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Eli Lilly and Co . Lyumjev (insulin lispro-aabc) prescribing information. Available from https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761109Orig1s000lbl.pdf. Accessed March 2021
- 43. Andersen G, Meiffren G, Lamers D, et al. Ultra-rapid BioChaperone lispro improves postprandial blood glucose excursions vs insulin lispro in a 14-day crossover treatment study in people with type 1 diabetes. Diabetes Obes Metab 2018;20:2627–2632 [DOI] [PubMed] [Google Scholar]
- 44. Heise T, Meiffren G, Alluis B, et al. BioChaperone lispro versus faster aspart and insulin aspart in patients with type 1 diabetes using continuous subcutaneous insulin infusion: a randomized euglycemic clamp study. Diabetes Obes Metab 2019;21:1066–1070 [DOI] [PubMed] [Google Scholar]
- 45. Novo Nordisk A/S . Fiasp prescribing information. Available from https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/208751s010s011lbl.pdf. Accessed March 2021
- 46. American Diabetes Association . 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021;44(Suppl. 1):S111–S124 [DOI] [PubMed] [Google Scholar]
- 47. Kildegaard J, Buckley ST, Nielsen RH, et al. Elucidating the mechanism of absorption of fast-acting insulin aspart: the role of niacinamide. Pharm Res 2019;36:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Heise T, Pieber TR, Danne T, Erichsen L, Haahr H. A pooled analysis of clinical pharmacology trials investigating the pharmacokinetic and pharmacodynamic characteristics of fast-acting insulin aspart in adults with type 1 diabetes. Clin Pharmacokinet 2017;56:551–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Heise T, Zijlstra E, Nosek L, Rikte T, Haahr H. Pharmacological properties of faster-acting insulin aspart vs insulin aspart in patients with type 1 diabetes receiving continuous subcutaneous insulin infusion: a randomized, double-blind, crossover trial. Diabetes Obes Metab 2017;19:208–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Evans M, Ceriello A, Danne T, et al. Use of fast-acting insulin aspart in insulin pump therapy in clinical practice. Diabetes Obes Metab 2019;21:2039–2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Heinemann L, Weyer C, Rauhaus M, Heinrichs S, Heise T. Variability of the metabolic effect of soluble insulin and the rapid-acting insulin analog insulin aspart. Diabetes Care 1998;21:1910–1914 [DOI] [PubMed] [Google Scholar]
- 52. Home PD. Plasma insulin profiles after subcutaneous injection: how close can we get to physiology in people with diabetes? Diabetes Obes Metab 2015;17:1011–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Buse JB, Carlson AL, Komatsu M, et al. Fast-acting insulin aspart versus insulin aspart in the setting of insulin degludec-treated type 1 diabetes: efficacy and safety from a randomized double-blind trial. Diabetes Obes Metab 2018;20:2885–2893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Russell-Jones D, Bode BW, De Block C, et al. Fast-acting insulin aspart improves glycemic control in basal-bolus treatment for type 1 diabetes: results of a 26-week multicenter, active-controlled, treat-to-target, randomized, parallel-group trial (onset 1). Diabetes Care 2017;40:943–950 [DOI] [PubMed] [Google Scholar]
- 55. Mathieu C, Bode BW, Franek E, et al. Efficacy and safety of fast-acting insulin aspart in comparison with insulin aspart in type 1 diabetes (onset 1): a 52-week, randomized, treat-to-target, phase III trial. Diabetes Obes Metab 2018;20:1148–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bode BW, Iotova V, Kovarenko M, et al. Efficacy and safety of fast-acting insulin aspart compared with insulin aspart, both in combination with insulin degludec, in children and adolescents with type 1 diabetes: the onset 7 trial. Diabetes Care 2019;42:1255–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zijlstra E, Demissie M, Graungaard T, Heise T, Nosek L, Bode B. Investigation of pump compatibility of fast-acting insulin aspart in subjects with type 1 diabetes. J Diabetes Sci Technol 2018;12:145–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Klonoff DC, Evans ML, Lane W, et al. A randomized, multicentre trial evaluating the efficacy and safety of fast-acting insulin aspart in continuous subcutaneous insulin infusion in adults with type 1 diabetes (onset 5). Diabetes Obes Metab 2019;21:961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bode BW, Johnson JA, Hyveled L, Tamer SC, Demissie M. Improved postprandial glycemic control with faster-acting insulin aspart in patients with type 1 diabetes using continuous subcutaneous insulin infusion. Diabetes Technol Ther 2017;19:25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dovc K, Piona C, Yeşiltepe Mutlu G, et al. Faster compared with standard insulin aspart during day-and-night fully closed-loop insulin therapy in type 1 diabetes: a double-blind randomized crossover trial. Diabetes Care 2020;43:29–36 [DOI] [PubMed] [Google Scholar]
- 61. Bally L, Herzig D, Ruan Y, et al. Short-term fully closed-loop insulin delivery using faster insulin aspart compared with standard insulin aspart in type 2 diabetes. Diabetes Obes Metab 2019;21:2718–2722 [DOI] [PubMed] [Google Scholar]
- 62. Boughton CK, Bally L, Martignoni F, et al. Fully closed-loop insulin delivery in inpatients receiving nutritional support: a two-centre, open-label, randomised controlled trial. Lancet Diabetes Endocrinol 2019;7:368–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Radermecker RP, Scheen AJ. Continuous subcutaneous insulin infusion with short-acting insulin analogues or human regular insulin: efficacy, safety, quality of life, and cost-effectiveness. Diabetes Metab Res Rev 2004;20:178–188 [DOI] [PubMed] [Google Scholar]
- 64. Bode BW, Garg SK, Norwood P, et al. Compatibility and safety of ultra rapid lispro with continuous subcutaneous insulin infusion in patients with type 1 diabetes: PRONTO-Pump study. Diabetes Technol Ther 2021;23:41–50 [DOI] [PubMed] [Google Scholar]
- 65. Bode B, Carlson A, Liu R, et al. Ultrarapid lispro demonstrates similar time in target range to lispro with a hybrid closed-loop system. Diabetes Technol Ther 2021;23:828–836 [DOI] [PubMed] [Google Scholar]
- 66. Malecki MT, Cao D, Liu R, et al. Ultra-rapid lispro improves postprandial glucose control and time in range in type 1 diabetes compared to lispro: PRONTO-T1D continuous glucose monitoring substudy. Diabetes Technol Ther 2020;22:853–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Xing D, Kollman C, Beck RW, et al.; Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group . Optimal sampling intervals to assess long-term glycemic control using continuous glucose monitoring. Diabetes Technol Ther 2011;13:351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Riddlesworth TD, Beck RW, Gal RL, et al. Optimal sampling duration for continuous glucose monitoring to determine long-term glycemic control. Diabetes Technol Ther 2018;20:314–316 [DOI] [PubMed] [Google Scholar]