Abstract
Multiple sclerosis (MS) is a neuroinflammatory and neurodegenerative disease of unknown etiology. However, several studies suggest that infectious agents, e.g., Human Herpes Viruses (HHV), may be involved in triggering the disease. Molecular mimicry, bystander effect, and epitope spreading are three mechanisms that can initiate immunoreactivity leading to CNS autoimmunity in MS. Theiler’s murine encephalomyelitis virus (TMEV)-induced demyelinating disease (TMEV-IDD) is a pre-clinical model of MS in which intracerebral inoculation of TMEV results in a CNS autoimmune disease that causes demyelination, neuroaxonal damage, and progressive clinical disability. Given the spectra of different murine models used to study MS, this review highlights why TMEV-IDD represents a valuable tool for testing the viral hypotheses of MS. We initially describe how the main mechanisms of CNS autoimmunity have been identified across both MS and TMEV-IDD etiology. Next, we discuss how adaptive, innate, and CNS resident immune cells contribute to TMEV-IDD immunopathology and how this relates to MS. Lastly, we highlight the sexual dimorphism observed in TMEV-IDD and MS and how this may be tied to sexually dimorphic responses to viral infections. In summary, TMEV-IDD is an underutilized murine model that recapitulates many unique aspects of MS; as we learn more about the nature of viral infections in MS, TMEV-IDD will be critical in testing the future therapeutics that aim to intervene with disease onset and progression.
Keywords: TMEV-IDD, Theiler’s, demyelinating disease, viral MS model, multiple sclerosis
Introduction: Evidence for the role of a virus in multiple sclerosis
Multiple Sclerosis (MS) is an inflammatory and neurodegenerative disease that originates in the central nervous system (CNS). Its etiology remains highly enigmatic, with insufficient evidence on the disease’s exact cause. Many studies have highlighted the role of different environmental and genetic factors in its etiopathogenesis, each adding a new wedge to the MS puzzle and making it a multifactorial and polygenic disease.
Viruses have long been suspected of playing a critical role in the development and progression of MS. Data from epidemiological studies analyzing the geographical, socioeconomic, and genetic features of the disease suggest that exposure to an infectious agent may indeed be involved in triggering the disease (Steiner et al., 2007; Alenda et al., 2014; Virtanen et al., 2014; Tarlinton et al., 2020; Jons et al., 2021; Meier et al., 2021; Xu et al., 2021a; Bjornevik et al., 2022). Several viruses have been studied in association with MS, ultimately speculating a potential pathogenic role for Human Herpes Viruses (HHV), such as varicella-zoster virus (VZV) (Steiner et al., 2007; Jons et al., 2021; Perez-Saldivar et al., 2021), herpes simplex virus 1 (HSV-1) (Steiner et al., 2007; Xu et al., 2021a), HHV-6 (Simpson et al., 2012; Alenda et al., 2014; Virtanen et al., 2014; Meier et al., 2021) and Epstein-Barr virus (EBV) (Virtanen et al., 2014; Guan et al., 2019; Jons et al., 2021; Meier et al., 2021; Perez-Saldivar et al., 2021; Bjornevik et al., 2022). Interestingly, all these viruses are persistent and cause life-long infections with “cellular stress-triggered” reactivation cycles that may be associated with the relapsing-remitting nature of MS (Guan et al., 2019; Tarlinton et al., 2020).
Molecular mimicry, bystander activation, and epitope spreading have been proposed to potentially explain the viral pathogenesis of MS mediated by HHV (Figure 1; Smatti et al., 2019; Donati, 2020). HHV-6, for example, may play a role in MS through a mechanism of molecular mimicry. Studies have shown a high sequence identity between the U24 gene in the HHV-6 genome and the myelin basic protein (MBP) (Tejada-Simon et al., 2003; Tait and Straus, 2008) increasing the risk of autoimmunity in the CNS. In line with this observation, researchers found, in MS patients, increased T- and B-cell dependent immune responses against this specific antigenic sequence (Tejada-Simon et al., 2003). Furthermore, the theory of cytomegalovirus (CMV)-induced autoimmunity in organ transplant recipients is that cell surface proteins from infected tissues, e.g., CD13, are incorporated into the viral envelope of the virus, severely affecting the allograft function in these patients (Naucler et al., 1996; Rojas et al., 2018). Similarly, it may be speculated that myelin proteins from infected oligodendrocytes could be incorporated into the HHV-6 envelope, inducing an autoimmune reaction in the CNS.
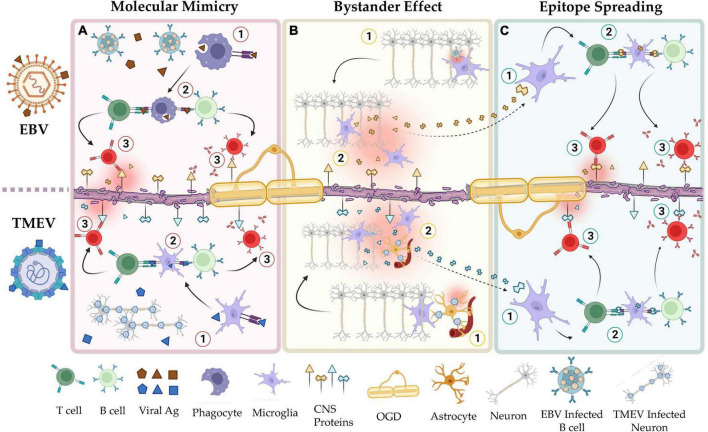
FIGURE 1.
Shared mechanisms of autoimmunity in the viral hypotheses of MS and the TMEV-IDD model of MS. (A) Mechanisms of molecular mimicry in EBV (top) and TMEV infection (bottom). Viral peptides, e.g., EBNA-1, share structural homology with CNS resident self-peptides, e.g., GlialCAM. Natural immunization to these viruses induces autoimmunity toward the CNS resident proteins. (B) Mechanisms of bystander effect in EBV (top) and TMEV infection (bottom). Responses by resident immune cells to latent or reactivated virus in the CNS induces collateral damage to adjacent tissues. (C) Mechanisms of epitope spreading in EBV (top) and TMEV infection (bottom). Peptides released by apoptotic or damaged cells are used to activate self-reactive lymphocytes. In a positive feedback cycle, the autoimmune reaction by these cells releases additional cellular debris. As this cycle continues, the set of self-peptides that are recognized by auto-reactive lymphocytes increases via clonal expansion. This figure was illustrated using Biorender.com.
Bystander activation implies that a viral infection can elicit an over-reactive inflammatory response due to persistent infection, tissue damage, and consequent unveiling and presentation of hidden autoantigens. These events can lead to the presentation of autoantigens to autoreactive T and B cells (Figure 1). α-herpesviruses such as VZV and HSV-1 persist in the CNS by establishing a latent infection (Antinone and Smith, 2010; Gilden et al., 2015). Continuous immune control is essential to keep these viruses in latency (Khanna et al., 2004; Cunningham et al., 2006). However, it may still trigger auto-reactive T and B cells bystander activation in susceptible individuals. Overall, this autoimmune response damages myelin-producing cells, causing a release of myelin fragments in the inflammatory environment and triggering a self-sustained breakdown of myelin with the recognition of new subdominant self−epitopes, in a process known as epitope spreading (Figure 1; Lehmann et al., 1992; Cusick et al., 2013). Epitope spreading usually occurs in the context of chronic inflammation and destruction of the target tissue promoting the spreading of the immune response from one autoantigenic determinant to other epitopes not previously recognized by the immune system (Miller et al., 1997; Vanderlugt and Miller, 2002). Disease progression is thus exacerbated by epitope spreading to self−myelin epitopes.
EBV is a γ-herpesvirus that infects more than 95% of the global population (Balfour et al., 2013; Dowd et al., 2013; Winter et al., 2019). Its latent infection is life-long and is established in quiescent memory B cells, presumably by undergoing the germinal center reaction alongside maturing, uninfected B cells (Thorley-Lawson et al., 2013). As a persistent and frequently reactivating virus with major immunogenic influences and a clear epidemiological association with MS (Thacker et al., 2006; Bjornevik et al., 2022), EBV is currently considered to play a leading role in MS pathogenesis, triggering localized inflammation in the CNS. The persistent presence of the virus in the CNS likely provides the signal for initiating and perpetuating inflammation with a consequent inflammation-induced bystander CNS damage mediated by infiltrating B- and T-cells as well as resident astrocytes and microglial cells. On the other hand, molecular mimicry has been reported between the viral protein Epstein–Barr nuclear antigen 1 (EBNA1) and various CNS proteins, including GlialCAM, an immunoglobulin-like cell adhesion molecule expressed in glial cells of the CNS (Lanz et al., 2022), and Anoctamin 2 (ANO1), a voltage-gated calcium-activated anion channel (Tengvall et al., 2019). Upon their activation, “peptides mimic” specific T and B cells can cross-react with self-epitopes, thus leading to tissue pathology, i.e., autoimmunity. Through epitope spreading, these responses may target additional self-epitopes, eventually leading to the accrual of antibody specificity (James et al., 1995; Jog and James, 2020).
Altogether these findings suggest that MS might develop in susceptible individuals due to an alteration of an HHV–host homeostasis that usually ensures a life-long coexistence under continuous immune surveillance in healthy virus carriers. However, despite many years of research and compelling indirect evidence, the role of infectious and viral agents in MS etiology and pathogenesis is still under debate. Research on well-characterized and controlled viral animal models of MS remains an essential step in studying the viral pathogenesis of MS.
Theiler’s murine encephalomyelitis virus-induced demyelinating diseases as a relevant infectious animal model for multiple sclerosis
Animal models for MS have greatly improved our understanding of the cause and progression of MS. They have proven to be a valuable tool for discovering therapeutic targets and testing their safety and efficacy. However, until recently, pre-clinical studies in animal models of MS took mainly two avenues: one focusing on a purely autoimmune component of the disease and one focusing on the mechanisms of demyelination independent of inflammatory reactions. Neither of these avenues provides clues about the actual triggering of the condition in MS patients.
The experimental autoimmune encephalomyelitis (EAE) series of MS models are mainly used to gain insights into the autoimmune pathogenesis of the disease. In brief, EAE is induced via a peripheral exposure to myelin-specific proteins, e.g., proteolipid protein (PLP), myelin oligodendrocytes glycoprotein (MOG), and MBP, or via adoptive transfer of T cells targeted to these proteins (Lassmann and Bradl, 2017; Glatigny and Bettelli, 2018). Following immunization, autoreactive T cells become active in the secondary lymphoid organs and access the CNS parenchyma through a compromised blood-brain barrier (BBB), subsequently inducing neuroinflammation and demyelination (Lassmann and Bradl, 2017; Glatigny and Bettelli, 2018). Thus, EAE represents a valuable model for studying the development of relapses and acute (neuro)inflammation.
On the other hand, mice treated with toxic substances like cuprizone, lysolecithin, or ethidium bromide represent reliable models for inducing and examining demyelinating/remyelinating events (Lassmann and Bradl, 2017). When exposed to the toxicant, these mice exhibit extensive reactive gliosis, activation of microglia, oligodendrocyte apoptosis, and subsequent reversible demyelination. Spontaneous remyelination is immediately observed after withdrawal of the toxic exposure (Lassmann and Bradl, 2017).
The pathogenic complexity of MS is not easily replicated in both EAE and toxic models. This can be somewhat overcome with the use of infectious models of MS to attempt to replicate the potential viral etiology of the disease.
The study of viral models of MS is not novel (Lassmann and Bradl, 2017). However, given the increasing evidence supporting a viral etiology of the disease, these models have become increasingly popular (and accepted) in recent years. The most common animal used in viral infection models for MS is the mouse (Mus musculus), whose CNS can be infected with three different neurotropic viruses, Theiler’s murine encephalomyelitis virus (TMEV), mouse hepatitis virus (MHV), and Semliki Forest virus (SFV), to induce three clinically distinct inflammatory demyelinating diseases all recapitulating different features of MS (Lassmann and Bradl, 2017). Among the three, the TMEV-induced demyelinating disease (TMEV-IDD) is more commonly used to study the viral hypothesis of MS since the other models are much shorter in duration and do not replicate the chronic nature of MS (Mokhtarian et al., 2003; Das Sarma, 2010).
TMEV is a natural single-stranded RNA murine cardiovirus of the Picornaviridae family that infects the gastrointestinal tract; however, intracerebral injection of this virus leads to varying neuropathology (Olitsky, 1940; Liang et al., 2008). TMEV strains are categorized into two neurovirulence groups (Lorch et al., 1981): (1) highly neurovirulent strains, also known as the GDVII subgroup, including GDVII and FA viruses which cause a rapidly fatal encephalomyelitis in mice (Theiler and Gard, 1940), and (2) low neurovirulence strains, also known as TO subgroup, including the BeAn and DA viruses which produce acute poliomyelitis followed by a chronic demyelinating disease (Lipton, 1975). The latter strains are used to induce TMEV-IDD as a model of progressive MS (PMS).
The type and severity of the disease course are variable across mouse strains, mainly affected by variations in the mouse genome, such as those found in the major histocompatibility complex (MHC) regions associated with susceptibility to the virus (Melvold et al., 1987; Perez Gomez et al., 2021). For example, highly susceptible mouse strains such as SJL and FVB succumb to TMEV-IDD, whereas non-susceptible strains such as C57BL/6 or partially susceptible strains such as C3H mice develop alternative pathology like epilepsy or myocarditis (Lipton and Dal Canto, 1979; Gómez et al., 1996; Omura et al., 2018). Distinct strains of TMEV can also induce subtle differences in the pathology and disease course. In SJL mice, for example, the DA strain has a higher propensity to induce demyelination, while the BeAn strain results in more robust antibody responses (Zoecklein et al., 2003).
TMEV-IDD is clinically described as a biphasic disease; the first stage is an acute polioencephalomyelitis which manifests within the first few weeks post-infection, followed by a chronic progressive demyelinating phase, starting about 1 month after infection and progressing throughout the animal’s lifespan (Lipton, 1975). During the first acute phase, TMEV predominantly infects neurons near the site of infection, spreading then down to the spinal cord (Stewart et al., 2010; Kummerfeld et al., 2012). Like EAE, TMEV-IDD pathology is most prevalent in the spinal cord. Here, leukocytes, predominantly virus-specific T cells, are rapidly recruited from the periphery (Pope et al., 1996; Tsunoda et al., 2002; Kang et al., 2020). As the disease becomes chronic, TMEV infection persists mainly in astrocytes, which are resistant to TMEV-induced apoptosis, whereas neurons and oligodendrocytes are not (Tsunoda et al., 1997; Gerhauser et al., 2018). Interestingly, the BBB is intact during the chronic phase, suggesting that any T and B cell activity is maintained locally (DiSano et al., 2019a,b). Like in PMS, meningeal lymphoid structures form in TMEV-IDD and are theorized to be the source of local B cell proliferation and intrathecal antibody production (Magliozzi et al., 2007; Pachner et al., 2007a; DiSano et al., 2019b).
TMEV-IDD etiology parallels well the viral hypothesis of MS (Figure 2). In TMEV-IDD, the early viral infection causes vast neuronal and oligodendroglia apoptosis. These events release myelin and neuronal antigen, which are thought to initiate an autoimmune reaction. The proposed mechanisms by which TMEV may induce autoimmunity are like those that aim to explain the viral pathogenesis of MS: bystander activation, epitope spreading, and even molecular mimicry.
FIGURE 2.
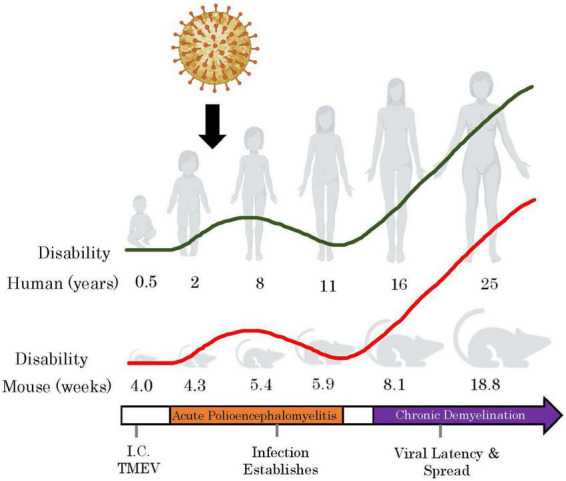
TMEV-IDD models how childhood exposures to common viruses may induce autoimmunity upon adulthood. By mapping the ages of mice in the TMEV-IDD model to human correlates (Wang et al., 2020), we observe that the acute phase of the TMEV infection occurs during childhood, and the chronic phase begins in early adulthood. Red line represents relative clinical disability accumulation in TMEV-IDD over time and the green line shows this with respect to human years. I.C, intracranial. This figure was illustrated using Biorender.com.
The bystander effect is possibly the most apparent mechanism by which TMEV may induce autoimmunity (Figure 1). CNS resident antigen-presenting cells (APCs) present both viral and myelin epitopes during the chronic stage of the disease but only viral epitopes during the acute phase (Katz-Levy et al., 1999, 2000). This suggests that the immune response to the virus results in inflammatory signaling that allows microglia and/or other APCs to present self-antigen to autoreactive lymphocytes. In addition, microglia infected with TMEV can release viral RNA via exosomes, stimulating distal, uninfected cells to become pro-inflammatory (Luong and Olson, 2021).
Researchers have also detected epitope spreading during TMEV-IDD (Figure 1). By mapping the expansion of autoreactive T cell clones throughout the disease course, it was demonstrated that there is a particular order in which the epitopes spread: in wild-type TMEV infections, the earliest T cell clones are PLP139–151 specific, followed by PLP178–191, PLP56–70, and finally MOG92–106 (Miller et al., 1997). This exemplifies how the initial immunity toward a single fragment can result in autoimmunity to other fragments of the same molecule (other PLP fragments) or molecules (MOG).
Lastly, TMEV has been used to model viral molecular mimicry (Figure 1). Although wild-type TMEV does not seem to have direct cross-reactivity with self-peptides, epitopes such as PLP139–151 have been genetically engineered into wild-type TMEV to study this phenomenon (Croxford et al., 2002). These strains induce a much faster disease with earlier CD4 + T cell responses compared to the wild-type virus. In addition, other genetically engineered TMEV strains have been constructed with sequences of Haemophilus influenzae virus (HI574-598), a natural high-homology mimic of PLP (Croxford et al., 2002). This strain also produced a faster disease than wild-type TMEV but slower than the PLP-TMEV.
Interestingly, autoimmunity via the HI574–598 fragment is only possible via a virus (Carrizosa et al., 1998). Although immunization to this fragment alone results in expanded T cell populations that cross-react with PLP, they lack Th1 differentiation. The fact that Th1 differentiated cells are required for the disease induction suggests that a particular cytokine environment is needed to establish autoimmunity, in addition to the presentation of self-antigen.
Although there are many strengths to using TMEV-IDD as a model the viral hypothesis of MS there are some limitations. Firstly, we do not observe relapses and remissions with TMEV-IDD, but rather, it follows a disease course more like that of primary progressive MS. In addition, TMEV-IDD, like EAE, is primarily a spinal cord disease and fails to replicate the level of cerebral damage as we see in MS. Lastly, TMEV is very different in structure and tropism from the HHVs that are linked to MS and may illicit different immunological responses. Given that all mouse models have their strengths and weaknesses, TMEV-IDD is the best available option when studying how viruses can progress to a MS-like disease.
Neuro-immune response in Theiler’s murine encephalomyelitis virus-induced demyelinating diseases
The inflammatory response to viral infection in humans is a dynamic process with complex cell interactions led by the immune system and influenced by both host and viral factors. Due to this complexity, the relative contributions of each factor in shaping the inflammatory response can be studied in animal models. Most viral infections are initially controlled by different innate immune system elements, including phagocytic cells, e.g., microglia, interferons and interferon-stimulated genes (ISGs), and the CS (Chhatbar and Prinz, 2021). If viral replication outpaces innate defenses, a specific adaptive immune response, consisting of B and T lymphocytes, is mobilized.
Following direct CNS infection, TMEV persists in microglia and leads to developing a chronic progressive demyelinating disease associated with innate and adaptive inflammation in the CNS (Tsunoda and Fujinami, 2010). As such, TMEV-IDD is used as a powerful model to examine changes in the innate and adaptive immune responses related to a persistent infection of the CNS leading to demyelination and disability progression.
Role of B cells
MS has historically been viewed as a T cell-mediated autoimmune disease partly because of the T cell-specific pathology observed and studied in the MS murine model EAE (Weiner, 2004). However, the recent and successful use of B-cell depleting therapies (BCDT) reveals a previously unrecognized critical role for B cells in MS. On the other hand, B cells have been implicated with MS since 1942, when increased levels of immunoglobulins (Igs) in the cerebrospinal fluid (CSF) of patients were first described to be suggestive of MS (Kabat et al., 1942). More recent studies also indicate that intrathecal synthesis of Igs correlates with MS disease progression (Joseph et al., 2009; Farina et al., 2017; Ozakbas et al., 2017). Antibody-secreting cells (ASC) in the CNS may be pathogenic either by the direct action of reactive Igs or locally secreting neurotoxic products (Michel et al., 2015; Jain and Yong, 2021). Substantial proof for the involvement of B cells in MS has also come from the finding of ectopic B cell-rich lymphoid-like follicles (ELF) in the meninges of patients with PMS (Magliozzi et al., 2007; Reali et al., 2020). These ELF correlate strongly with cortical pathology and disease severity (Reali et al., 2020).
In the context of a viral etiology for MS, it is noteworthy that humoral responses within the CNS are prevalent in neurotropic viral infections, with ASC contributing to local protection. In MS patients, intrathecal synthesis of Igs was detected against measles, rubella, varicella zoster, mumps and EBV, and in most cases, antibodies against more than one virus are present (Arnadottir et al., 1979; Sindic et al., 1994; Kakalacheva et al., 2011). In contrast, antibodies against non-neurotropic viruses, e.g., CMV, are rarely detected in the CSF from MS patients (Sindic et al., 1994).
TMEV-IDD provides an excellent model to interrogate the function of B cells in a viral model of MS (DiSano et al., 2019b). Increased concentrations of intrathecally produced Igs and substantial infiltration of ASC and B memory cells are observed in the spinal cord and the meninges of persistently infected mice, suggesting a potentially critical role for Igs and B cells in the chronic progressive phase of the demyelinating disease (Pachner et al., 2007a,b; DiSano et al., 2019a,b; Jin et al., 2020).
Many of the intrathecal Igs in TMEV-IDD are reactive against the virus (Pachner et al., 2007b; Tsunoda and Fujinami, 2010). However, mice chronically infected with TMEV also produced antibodies to self-antigen within the CNS, primarily in response to damaged tissue and the consequent release of CNS unique antigens (Jin et al., 2020). These autoantibodies target multiple self-antigens (because of epitope spreading) and are independent of the initial trigger once tolerance is broken (Figure 1).
A recent finding in TMEV-IDD also described the development of B cell-rich meningeal aggregates, like those observed in patients with MS (DiSano et al., 2019b,2020). Although these aggregates in TMEV-IDD do not fully develop into EFLs, like in MS, they correlate strongly with pathology and disease severity (DiSano et al., 2019b).
Overall, these findings in TMEV-IDD demonstrate that the model well recapitulates B cell-specific disease phenotypes that are also observed in patients with MS. Therefore, these findings support TMEV-IDD as a valuable model for investigating the role of B cells in a chronic neuroinflammatory condition and test new potential therapeutic strategies.
B cell-depleting therapies in Theiler’s murine encephalomyelitis virus-induced demyelinating diseases
BCDT efficiently suppresses acute inflammatory disease activity in relapsing-remitting MS and may slow progression in a subgroup of primary-progressive MS patients (Bar-Or et al., 2008; Montalban et al., 2017). Considering the increasing interest in these treatments in MS, the susceptibility of B cells to TMEV infection is particularly important. However, studies in our laboratory showed that anti-CD20 BCDT administered to TMEV-DD mice worsened rather than improved the overall disease course (Gilli et al., 2015). In these mice, systemic and CNS antibody responses were suppressed during the treatment. Higher viral loads were detected in treated mice vs. controls, and the viral levels correlated negatively with IgG production in the brain (Gilli et al., 2015).
BCDT causes significant worsening of the early encephalitis and faster progression of disability, as well as exacerbation of the pathology at the end stage of the disease (Gilli et al., 2015). Interestingly, a similar severe acute disease worsening was also seen in the MOG33–35 EAE model when treated with BCDT (Matsushita et al., 2008; Weber et al., 2010). This was thought to be due to heightened inflammation caused by removing IL-10-producing CD20 + regulatory B10 cells in EAE. The similar timing and exacerbation of disease symptoms in TMEV-IDD and EAE studies suggest that a similar B10 mechanism may be operative in both models. The fact that mice had worse neurological function when CNS antibody production was reduced is consistent with a protective role for CNS IgG. Protection could be accomplished by suppression of viral replication (Stohlman et al., 2002; Metcalf and Griffin, 2011), clearance of myelin debris (Vargas et al., 2010), promotion of remyelination (Asakura and Rodriguez, 1998), or other mechanisms. This positive role for some populations of locally produced IgG does not rule out pathological effects for different populations of CNS IgG. These findings have considerable relevance to the use of BCDT in MS. Patients with MS receiving BCDT may develop highly inflammatory syndromes because of the absence of B regulatory cells or worsened CNS or systemic infections because of the inability to mount a solid anti-pathogen humoral immune response.
The innate immune system
According to evidence from the recent decade, the innate immune system plays a critical role in the initiation and progression of MS. This system activates and regulates the effector functions of T and B cells, similar to what happens during infections (Sospedra and Martin, 2005; Weiner, 2008; Gandhi et al., 2010). In the CNS, such modulation is mainly mediated by resident immune cells, i.e., microglia and astrocytes, and locally produced inflammatory proteins, like the interferons and the complement system (CS) (Ingram et al., 2014; Liddelow et al., 2020; Absinta et al., 2021).
TMEV-IDD is an appropriate model to study innate immunity to viral pathogens in the CNS, given the critical role that this arm of the immune system plays in the pathogenesis of the demyelinating disease. As stated above, clinically, TMEV-IDD is considered a biphasic disease (Lipton, 1975). Nevertheless, its immunopathogenesis can be divided into not just two but three discrete stages, two of which mainly involve the innate immune system (Sospedra and Martin, 2005; Gilli et al., 2016). Following TMEV infection, the innate immune response is immediately activated in the CNS (Olson et al., 2001; Olson and Miller, 2009), thereby providing an adjuvant signal for the induction of naive and memory virus-specific adaptive immune responses (stage 1, polioencephalomyelitis). A prolonged adaptive inflammatory reaction, which involves both T and B cells, determines the development of the demyelinating disease (stage 2). Finally, there is a reversion to a chronic innate-like immune response associated with extensive CNS damage and progressive neurodegeneration (stage 3) (Gilli et al., 2016). This last stage is characterized by an upregulation in the CNS of almost all innate immune pathways, including toll-like receptor (TLR)-signaling pathways, e.g., TLR7, TLR8, TLR9, and type I Interferon responses (Ulrich et al., 2010; Gilli et al., 2016).
While triggering these signaling pathways may be beneficial during acute infections, persistent activation of the innate immune system contributes to neurodegeneration (Heneka et al., 2014). Interestingly, a similar multi-stage process is evident in MS, where the relapsing-remitting phase is thought to be mainly driven by the adaptive immune system, while a heightened innate immune response and neurodegeneration primarily characterize the late progressive stage of the disease (Weiner, 2008; De Jager et al., 2009; Kamma et al., 2022).
Another component of the innate immune system critically involved in neurodegenerative processes is the CS. This protein cascade has traditionally been considered a complex innate immune surveillance system, playing a pivotal role in defense against pathogens and host homeostasis (Merle et al., 2015a). The proteins collaborate to opsonize pathogens, enhance inflammatory responses, and induce cytotoxicity, helping the immune cells to fight infections (Merle et al., 2015b). However, in the last few decades, complement activation has been increasingly involved in the progression of neurodegenerative disorders, including MS (Lee et al., 2019; Morgan et al., 2020). Activation of the CS is also involved in the pathogenesis of many antibody-mediated autoimmune diseases (Leffler et al., 2014; Nytrova et al., 2014).
Autoantibodies can directly damage target tissues, and complement activation is a downstream mediator of this injury (Merle et al., 2015a). The classical complement cascade, one of the three activation pathways of the CS, is activated when IgG or IgM are bound to their target antigen on either a pathogen cell membrane or an immune complex (Merle et al., 2015a). Thus, in MS, intrathecally produced IgG and IgM may lead to the activation of the CS, thereby promoting CNS tissue damage. Accordingly, deposition of key complement components and activation products, e.g., C1q, C3, C4b, and the membrane attack complex (MAC), have been detected in MS patient lesions (Ingram et al., 2014; Watkins et al., 2016; Cooze et al., 2022). Complement activation has been demonstrated in acute and chronic MS lesions (Ingram et al., 2014; Watkins et al., 2016).
In TMEV-IDD, activation of the CS is evident at the early and chronic stages of the disease (Libbey et al., 2010; DiSano et al., 2019a; Linzey et al., 2022). In a recent study, the CS, specifically the classical complement pathway, was associated with TMEV-IDD pathogenesis, as the expression of critical complement components like C1q, C3, and C3aR1 were all correlated to a worse disease outcome (Linzey et al., 2022). In line with this finding, C1q and C3 depositions were observed in CNS regions characterized by inflammation, i.e., microglia and astrocytes activation, demyelination, and axonal damage (Linzey et al., 2022). These results reveal an association between TMEV-IDD and activation of the classical complement pathway by antigen-antibody immune complexes that appear to contribute significantly to the disease severity.
Microglia and astrocytes play an essential role in the CNS, contributing to many functions, including homeostasis, immune response, BBB maintenance, and synaptic support (Liddelow et al., 2020). Microglia, the resident phagocytic immune cell of the CNS (Schafer et al., 2012; Favuzzi et al., 2021), are exquisitely sensitive to injury and disease, altering their morphology and phenotype to adopt an “activated” state in response to pathophysiological brain insults. These CNS cells are a culprit of neuroinflammation in several neurodegenerative diseases, including MS (Hickman et al., 2018; Guerrero and Sicotte, 2020; Xu et al., 2021b).
During TMEV-IDD, microglia undergo activation, proliferation, and changes in morphology, with detrimental and beneficial effects (Herder et al., 2015). Activated microglia promote neuronal repair through the secretion of anti-inflammatory growth factors and cytokines. However, activated microglia can also generate secondary neuronal injury via the production of pro-inflammatory cytokines, e.g., type 1 interferons, IL-1, IL-6, IL-23, TNFα, complement components such as C1q (Libbey et al., 2017; Gerhauser et al., 2019), reactive oxygen species (ROS) (Iwahashi et al., 1999), and proteases (Crapser et al., 2021). These MOG drive a CNS compartmentalized inflammation by stimulating local immune cells, a phenotype observed in PMS like in TMEV-IDD (Absinta et al., 2021; Cooze et al., 2022).
Astrocytes are supporting cells within the CNS that have numerous functions, including providing structural support, insulating receptive surfaces, and buffering the extracellular compartment (Santello et al., 2019). During CNS insult or neurodegenerative processes, astrocytes can respond to pathological changes by releasing extracellular MOG, such as neurotrophic factors, e.g., BDNF and VEGF, pro-inflammatory cytokines, e.g., IL-1β, TNF-α, stimulatory MOG like MHC II and CD80/86 (Li et al., 2019; Escartin et al., 2021), inducible nitric oxide synthase (iNOS) and cytotoxins such as Lcn2, through reactive astrogliosis (Li et al., 2019). As a result, like microglia, astrocytes have neuroprotective or neurotoxic effects in the CNS (Linnerbauer et al., 2020).
Astrocytes play multiple roles in the evolution of MS lesions, not only by recruiting lymphocytes and contributing to tissue damage but also by confining inflammation and promoting lesion repair (Zeinstra et al., 2003; Liddelow et al., 2017; Ponath et al., 2018; Li et al., 2019). Likewise, reactive astrocytes have been researched for their role in the disease progression of TMEV-IDD (Allnoch et al., 2019). Astrocytic infection by TMEV stimulates TLR3, which initiates an antiviral response, and increases the production of pro-inflammatory MOG such as TNFα and iNOS (Allnoch et al., 2022). Also, reactive astrocytes have been shown to release, in the CNS, the C3 factor, i.e., the central protein in the CS (Pekna and Pekny, 2021). Accordingly, throughout TMEV-IDD, a significant upregulation of C3 expression and deposition is observed as stimulated by reactive astrocytes (DiSano et al., 2019a; Linzey et al., 2022).
B cells –astrocytes –microglia axis
The inflamed CNS in MS emerges as a B cell fostering environment, as evidenced by the continuous synthesis of intrathecal Igs and the development of B cell-rich ELFs in chronic progressive patients. In this favorable environment, B cells contribute to developing CNS-compartmentalized inflammation and progressive CNS injury. However, it is still unclear how B cells are fostered within the CNS and how they contribute locally to the propagation of neuroinflammation behind a closed BBB. Interestingly, recent studies have shown a potential bi-directional interaction in the CNS between B cells and glial cells, mainly astrocytes and microglia, which may influence the propagation of the neuroinflammatory stimuli associated with MS progression (Touil et al., 2018; Hollen et al., 2020).
Thus far, TMEV-IDD has provided valuable insights into the relationship between B cells and glial cells. For example, in this model, it has been shown that inflammatory microglia produce a range of soluble factors such as cytokines and complement proteins to induce astrogliosis (Liddelow et al., 2017; Linzey et al., 2022). Following their activation, reactive astrocytes further amplify inflammation by releasing pro-inflammatory and neurotoxic mediators, including the complement factor C3 (Allnoch et al., 2019; DiSano et al., 2019a). C3 is the central protein of the CS, and its hydrolysis leads to the production of anaphylatoxins (C3a and C5a), which stimulate the secretion of various pro-inflammatory cytokines (Werneburg et al., 2020). Moreover, recent work has shown that C3 can interact with B cells to aid their maturation, linking the CS and B cell activity (Rossbacher and Shlomchik, 2003).
Another connection linking CNS resident cells to pro-inflammatory B cell activation is CXCL13 and its receptor CXCR5. CXCL13 is a potent B cell chemoattract that neurons can produce during various insults (Trolese et al., 2020). It has also been shown that the receptor for CXCL13, CXCR5, is expressed by astrocytes, and this interaction promotes the development of reactive astrocytes (Jiang et al., 2016). Once activated by CXCL13, astrocytes secrete essential B cells growth factors such as BAFF and APRIL (Touil et al., 2018; DiSano et al., 2019b), ultimately fostering B cells within the CNS. Interestingly, researchers have reported significantly increased CXCL13, BAFF, and APRIL levels in the CNS of TMEV-IDD (DiSano et al., 2019b; Harrer et al., 2021).
Sex differences in Theiler’s murine encephalomyelitis virus-induced demyelinating diseases
Biological sex profoundly affects the susceptibility and course of MS, with a higher disease prevalence and overall better prognosis in women than men [for review, see Gilli et al. (2020)]. The risk of developing MS is 3–4 times higher in women (Orton et al., 2006; Harbo et al., 2013). However, while men are less likely to develop the disease, they are at increased risk for worse clinical outcomes, e.g., more significant disability over time (Ribbons et al., 2015), faster time to progression (Jelinek et al., 2016) and overall, more neurodegenerative lesions compared to women (van Walderveen et al., 2001; Pozzilli et al., 2003).
In the context of the viral etiology of MS, it is worth noting that many viral infections manifest sex differences like those observed in MS: longitudinal studies found, for example, a significant difference in EBV and HSV1 prevalence between men and women, showing an overall infection preponderance and higher antibody titers in females than males (Jacobsen and Klein, 2021). On the other hand, the intensity and severity of disease caused by some neurotropic viruses, including EBV and HSV1, are more significant for males than females (Han, 2001; Jean et al., 2007; Balogun et al., 2009; Murphy et al., 2009).
TMEV-IDD is characterized by a similar sexual dimorphism, with female mice showing an overall infection preponderance and higher antibody titers but male mice being more severely affected (Alley et al., 2003; Fuller et al., 2005). Interestingly, different strains of mice exhibit different susceptibility to the development of TMEV-IDD and variations in their sexual dimorphism. In SJL mice, for example, females seem slightly more susceptible than males (Hill et al., 1998). Still, males exhibit worsened clinical scores over time as well as moderately more demyelination in the CNS compared to their female counterparts (Fuller et al., 2005). Other studies did not confirm the increased TMEV-IDD prevalence in female SJL mice (Alley et al., 2003). However, it is noteworthy that in the SJL strain, the analysis may be confounded by the high susceptibility to TMEV-IDD of these mice. Differently from the SJL strain, in the C57L background, male mice are highly susceptible to TMEV-IDD, whereas females are resistant (Kappel et al., 1990).
Gonadal hormones, especially estrogens and androgens, have long been found to account for some sex differences in MS (Tomassini et al., 2005; Tomassini and Pozzilli, 2009; Ysrraelit and Correale, 2019), although molecular mechanisms mediating these effects remain to be elucidated. Accordingly, in SJL mice, at least partly, susceptibility is sex-hormone specific as castrated male mice exhibit increased susceptibility to TMEV infection (Fuller et al., 2007). At the same time, this effect is rescued by estrogen treatment (Fuller et al., 2007). More recently, the effects of sex chromosome genes have also been implicated as contributors in animal models of MS (Smith-Bouvier et al., 2008; Du et al., 2014; Voskuhl et al., 2018), particularly the potentially harmful impact of the second X chromosome in females and/or the unique Y chromosome in males. It is widely speculated that this genetic difference may account for the different prevalence in the incidence of several diseases, including MS.
Sex chromosomes and sex hormones contribute to differential regulation of immune responses between sexes. Since the immune system plays a critical role in the initiation and progression of MS as well as in viral infections, sex-driven differences in the type and magnitude of the inflammatory immune response represent a vital cue. It is well known the immune system displays widespread sexual dimorphism. Generally, females mount stronger humoral and cell-mediated immunity than males (Klein and Flanagan, 2016; Gal-Oz et al., 2019). The weaker male response to viruses may allow the invader to cause more damage, whereas the intense and persistent female response reduces the tissue injury. In line with this observation, data from our laboratory demonstrate that following TMEV infection, female SJL mice induce a quicker and stronger peripheral anti-TMEV humoral and cellular immune response (Welsh et al., 2020). This finding is in line with the results of a previous study which showed a higher level of the initial antiviral immune (both humoral and cellular) response in female compared to male mice (Fuller et al., 2005).
Despite this clear distinction of similarities to the sexual differences in MS, very little research has utilized the TMEV-IDD model to study these dimorphisms. Additionally, most of the research in TMEV-IDD has been using female mice only due to the aggressive nature of the SJL male mice. The National Institute of Health (NIH) currently expects sex as a biological variable to be factored into research designs, analyses, and reporting in clinical and pre-clinical studies. Given the solid sexual dimorphism reported in TMEV-IDD and the high relevance for MS, appropriate analyses and transparent reporting of data by sex may significantly enhance the rigor and applicability of pre-clinical biomedical research using this model.
Conclusion
TMEV-IDD represents an accurate model system of MS due to several distinct features. In the first place, TMEV-IDD disability slowly progresses, secondary to chronic demyelination, inflammation, and axonal/neuronal damage. Like MS, in TMEV-IDD, the disease progresses through 20–25% of the lifespan and then usually plateaus (Figure 2). Moreover, this murine model parallels typical MS neurodegenerative hallmarks such as atrophy (Umpierre et al., 2014), neuronal death (Mecha et al., 2013), axonal injury (Tsunoda and Fujinami, 2010), and, most importantly, persistent compartmentalized CNS inflammation (Gilli et al., 2016; DiSano et al., 2019a). In this model, inflammation is often sustained behind a relatively closed BBB (DiSano et al., 2019a), supported by diverse CNS-infiltrating immune cells and CNS-resident cells. TMEV-IDD not only represents an excellent model for the study of the pathogenesis of MS, but it also provides a model for studying disease susceptibility factors, sexual dimorphism, CNS viral persistence, and virus-induced autoimmune disease. This model system provides a solid basis for dissecting cell and molecular mechanisms involved in developing and progressing a virus-induced demyelinating disease and characterizing the efficacy of targeted therapeutics.
Author contributions
SP, NW, ML, and FG outlined the subject for the review. SP, NW, and ML reviewed the literature and wrote the manuscript. SP drafted the figures. FG edited and revised the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
We thank Dartmouth College’s Library and Office of the Provost for sponsoring the open-access fees for the submission of this manuscript.
Funding
Dartmouth College’s Open-Access Publication Equity Fund was sponsored by the Dartmouth Library and Office of the Provost.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Absinta M., Maric D., Gharagozloo M., Garton T., Smith M. D., Jin J., et al. (2021). A lymphocyte-microglia-astrocyte axis in chronic active multiple sclerosis. Nature 597 709–714. 10.1038/s41586-021-03892-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alenda R., Alvarez-Lafuente R., Costa-Frossard L., Arroyo R., Mirete S., Alvarez-Cermeno J. C., et al. (2014). Identification of the major HHV-6 antigen recognized by cerebrospinal fluid IgG in multiple sclerosis. Eur. J. Neurol. 21 1096–1101. 10.1111/ene.12435 [DOI] [PubMed] [Google Scholar]
- Alley J., Khasabov S., Simone D., Beitz A., Rodriguez M., Njenga M. K. (2003). More severe neurologic deficits in SJL/J male than female mice following Theiler’s virus-induced CNS demyelination. Exp. Neurol. 180 14–24. 10.1016/s0014-4886(02)00054-7 [DOI] [PubMed] [Google Scholar]
- Allnoch L., Baumgärtner W., Hansmann F. (2019). Impact of astrocyte depletion upon inflammation and demyelination in a murine animal model of multiple sclerosis. Int. J. Mol. Sci. 20:3922. 10.3390/ijms20163922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allnoch L., Leitzen E., Zdora I., Baumgärtner W., Hansmann F. (2022). Astrocyte depletion alters extracellular matrix composition in the demyelinating phase of Theiler’s murine encephalomyelitis. PLoS One 17:e0270239. 10.1371/journal.pone.0270239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinone S. E., Smith G. A. (2010). Retrograde axon transport of herpes simplex virus and pseudorabies virus: A live-cell comparative analysis. J. Virol. 84 1504–1512. 10.1128/JVI.02029-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnadottir T., Reunanen M., Meurman O., Salmi A., Panelius M., Halonen P. (1979). Measles and rubella virus antibodies in patients with multiple sclerosis. A longitudinal study of serum and CSF specimens by radioimmunoassay. Arch Neurol. 36 261–265. 10.1001/archneur.1979.00500410039004 [DOI] [PubMed] [Google Scholar]
- Asakura K., Rodriguez M. (1998). A unique population of circulating autoantibodies promotes central nervous system remyelination. Mult. Scler. 4 217–221. 10.1177/135245859800400324 [DOI] [PubMed] [Google Scholar]
- Balfour H. H., Sifakis F., Sliman J. A., Knight J. A., Schmeling D. O., Thomas W. (2013). Age-specific prevalence of Epstein-Barr virus infection among individuals aged 6-19 years in the United States and factors affecting its acquisition. J. Infect. Dis. 208 1286–1293. 10.1093/infdis/jit321 [DOI] [PubMed] [Google Scholar]
- Balogun M. A., Vyse A. J., Hesketh L. M., Kafatos G., Parry J. V., Ramsay M. E. (2009). Estimating hepatitis C infection acquired in England, 1986-2000. Epidemiol. Infect. 137 1249–1254. 10.1017/S0950268809002143 [DOI] [PubMed] [Google Scholar]
- Bar-Or A., Calabresi P. A., Arnold D., Markowitz C., Shafer S., Kasper L. H., et al. (2008). Rituximab in relapsing-remitting multiple sclerosis: A 72-week, open-label, phase I trial. Ann. Neurol. 63 395–400. 10.1002/ana.21363 [DOI] [PubMed] [Google Scholar]
- Bjornevik K., Cortese M., Healy B. C., Kuhle J., Mina M. J., Leng Y., et al. (2022). Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 375 296–301. 10.1126/science.abj8222 [DOI] [PubMed] [Google Scholar]
- Carrizosa A. M., Nicholson L. B., Farzan M., Southwood S., Sette A., Sobel R. A., et al. (1998). Expansion by self antigen is necessary for the induction of experimental autoimmune encephalomyelitis by T cells primed with a cross-reactive environmental antigen. J. Immunol. 161 3307–3314. [PubMed] [Google Scholar]
- Chhatbar C., Prinz M. (2021). The roles of microglia in viral encephalitis: From sensome to therapeutic targeting. Cell Mol. Immunol. 18 250–258. 10.1038/s41423-020-00620-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooze B. J., Dickerson M., Loganathan R., Watkins L. M., Grounds E., Pearson B. R., et al. (2022). The association between neurodegeneration and local complement activation in the thalamus to progressive multiple sclerosis outcome. Brain Pathol. 32:e13054. 10.1111/bpa.13054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapser J. D., Arreola M. A., Tsourmas K. I., Green K. N. (2021). Microglia as hackers of the matrix: Sculpting synapses and the extracellular space. Cell Mol. Immunol. 18 2472–2488. 10.1038/s41423-021-00751-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxford J. L., Olson J. K., Miller S. D. (2002). Epitope spreading and molecular mimicry as triggers of autoimmunity in the Theiler’s virus-induced demyelinating disease model of multiple sclerosis. Autoimmun. Rev. 1 251–260. [DOI] [PubMed] [Google Scholar]
- Cunningham A. L., Diefenbach R. J., Miranda-Saksena M., Bosnjak L., Kim M., Jones C., et al. (2006). The cycle of human herpes simplex virus infection: Virus transport and immune control. J. Infect. Dis. 194:S11–S18. 10.1086/505359 [DOI] [PubMed] [Google Scholar]
- Cusick M. F., Libbey J. E., Fujinami R. S. (2013). Multiple sclerosis: Autoimmunity and viruses. Curr. Opin. Rheumatol. 25 496–501. 10.1097/BOR.0b013e328362004d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das Sarma J. (2010). A mechanism of virus-induced demyelination. Interdiscip. Perspect. Infect. Dis. 2010:109239. 10.1155/2010/109239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jager P. L., Jia X., Wang J., de Bakker P. I., Ottoboni L., Aggarwal N. T., et al. (2009). Meta-analysis of genome scans and replication identify CD6, IRF8 and TNFRSF1A as new multiple sclerosis susceptibility loci. Nat. Genet. 41 776–782. 10.1038/ng.401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiSano K. D., Linzey M. R., Royce D. B., Pachner A. R., Gilli F. (2019a). Differential neuro-immune patterns in two clinically relevant murine models of multiple sclerosis. J. Neuroinflamm. 16:109. 10.1186/s12974-019-1501-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiSano K. D., Royce D. B., Gilli F., Pachner A. R. (2019b). Central nervous system inflammatory aggregates in the theiler’s virus model of progressive multiple sclerosis. Front. Immunol. 10:1821. 10.3389/fimmu.2019.01821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiSano K. D., Linzey M. R., Welsh N. C., Meier J. S., Pachner A. R., Gilli F. (2020). Isolating central nervous system tissues and associated meninges for the downstream analysis of immune cells. J Vis Exp 159. 10.3791/61166 [DOI] [PubMed] [Google Scholar]
- Donati D. (2020). Viral infections and multiple sclerosis. Drug Discov. Today Dis. Models 32 27–33. 10.1016/j.ddmod.2020.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd J. B., Palermo T., Brite J., McDade T. W., Aiello A. (2013). Seroprevalence of epstein-barr virus infection in u.s. children ages 6-19, 2003-2010. PLoS One 8:e64921. 10.1371/journal.pone.0064921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du S., Itoh N., Askarinam S., Hill H., Arnold A. P., Voskuhl R. R. (2014). XY sex chromosome complement, compared with XX, in the CNS confers greater neurodegeneration during experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. U.S.A. 111 2806–2811. 10.1073/pnas.1307091111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escartin C., Galea E., Lakatos A., O’Callaghan J. P., Petzold G. C., Serrano-Pozo A., et al. (2021). Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 24 312–325. 10.1038/s41593-020-00783-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina G., Magliozzi R., Pitteri M., Reynolds R., Rossi S., Gajofatto A., et al. (2017). Increased cortical lesion load and intrathecal inflammation is associated with oligoclonal bands in multiple sclerosis patients: A combined CSF and MRI study. J. Neuroinflamm. 14:40. 10.1186/s12974-017-0812-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favuzzi E., Huang S., Saldi G. A., Binan L., Ibrahim L. A., Fernández-Otero M., et al. (2021). GABA-receptive microglia selectively sculpt developing inhibitory circuits. Cell 184 4048–4063.e32. 10.1016/j.cell.2021.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller A., Yahikozawa H., So E. Y., Dal Canto M., Koh C. S., Welsh C. J., et al. (2007). Castration of male C57L/J mice increases susceptibility and estrogen treatment restores resistance to Theiler’s virus-induced demyelinating disease. J. Neurosci. Res. 85 871–881. 10.1002/jnr.21184 [DOI] [PubMed] [Google Scholar]
- Fuller A. C., Kang B., Kang H. K., Yahikozowa H., Dal Canto M. C., Kim B. S. (2005). Gender bias in Theiler’s virus-induced demyelinating disease correlates with the level of antiviral immune responses. J. Immunol. 175 3955–3963. 10.4049/jimmunol.175.6.3955 [DOI] [PubMed] [Google Scholar]
- Gal-Oz S. T., Maier B., Yoshida H., Seddu K., Elbaz N., Czysz C., et al. (2019). ImmGen report: Sexual dimorphism in the immune system transcriptome. Nat. Commun. 10:4295. 10.1038/s41467-019-12348-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi R., Laroni A., Weiner H. L. (2010). Role of the innate immune system in the pathogenesis of multiple sclerosis. J. Neuroimmunol. 221 7–14. 10.1016/j.jneuroim.2009.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhauser I., Hansmann F., Ciurkiewicz M., Loscher W., Beineke A. (2019). Facets of theiler’s murine encephalomyelitis virus-induced diseases: An update. Int. J. Mol. Sci. 20:448. 10.3390/ijms20020448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhauser I., Li L., Li D., Klein S., Elmarabet S. A., Deschl U., et al. (2018). Dynamic changes and molecular analysis of cell death in the spinal cord of SJL mice infected with the BeAn strain of Theiler’s murine encephalomyelitis virus. Apoptosis 23 170–186. 10.1007/s10495-018-1448-9 [DOI] [PubMed] [Google Scholar]
- Gilden D., Nagel M., Cohrs R., Mahalingam R., Baird N. (2015). Varicella zoster virus in the nervous system. F1000Res 4:F1000FacultyRev–1356. 10.12688/f1000research.7153.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilli F., DiSano K. D., Pachner A. R. (2020). SeXX matters in multiple sclerosis. Front. Neurol. 11:616. 10.3389/fneur.2020.00616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilli F., Li L., Campbell S. J., Anthony D. C., Pachner A. R. (2015). The effect of B-cell depletion in the Theiler’s model of multiple sclerosis. J. Neurol. Sci. 359 40–47. 10.1016/j.jns.2015.10.012 [DOI] [PubMed] [Google Scholar]
- Gilli F., Li L., Pachner A. R. (2016). The immune response in the CNS in Theiler’s virus induced demyelinating disease switches from an early adaptive response to a chronic innate-like response. J. Neurovirol. 22 66–79. 10.1007/s13365-015-0369-4 [DOI] [PubMed] [Google Scholar]
- Glatigny S., Bettelli E. (2018). Experimental autoimmune encephalomyelitis (eae) as animal models of multiple sclerosis (ms). Cold Spring Harb. Perspect. Med. 8:a028977. 10.1101/cshperspect.a028977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez R. M., Rinehart J. E., Wollmann R., Roos R. P. (1996). Theiler’s murine encephalomyelitis virus-induced cardiac and skeletal muscle disease. J. Virol. 70 8926–8933. 10.1128/JVI.70.12.8926-8933.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y., Jakimovski D., Ramanathan M., Weinstock-Guttman B., Zivadinov R. (2019). The role of Epstein-Barr virus in multiple sclerosis: From molecular pathophysiology to in vivo imaging. Neural. Regen. Res. 14 373–386. 10.4103/1673-5374.245462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero B. L., Sicotte N. L. (2020). Microglia in multiple sclerosis: Friend or foe? Front. Immunol. 11:374. 10.3389/fimmu.2020.00374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B. (2001). The impact of age, gender, and race on the relationship between depression and self-rated health in community-dwelling older adults: A longitudinal study. Home Health Care Serv. Q. 20 27–43. 10.1300/J027v20n03_02 [DOI] [PubMed] [Google Scholar]
- Harbo H. F., Gold R., Tintore M. (2013). Sex and gender issues in multiple sclerosis. Ther. Adv. Neurol. Disord. 6 237–248. 10.1177/1756285613488434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrer C., Otto F., Pilz G., Haschke-Becher E., Trinka E., Hitzl W., et al. (2021). The CXCL13/CXCR5-chemokine axis in neuroinflammation: Evidence of CXCR5+CD4 T cell recruitment to CSF. Fluids Barriers CNS 18:40. 10.1186/s12987-021-00272-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka M. T., Kummer M. P., Latz E. (2014). Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 14 463–477. 10.1038/nri3705 [DOI] [PubMed] [Google Scholar]
- Herder V., Iskandar C. D., Kegler K., Hansmann F., Elmarabet S. A., Khan M. A., et al. (2015). Dynamic changes of microglia/macrophage m1 and m2 polarization in theiler’s murine encephalomyelitis. Brain Pathol. 25 712–723. 10.1111/bpa.12238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman S., Izzy S., Sen P., Morsett L., El Khoury J. (2018). Microglia in neurodegeneration. Nat. Neurosci. 21 1359–1369. 10.1038/s41593-018-0242-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill K. E., Pigmans M., Fujinami R. S., Rose J. W. (1998). Gender variations in early Theiler’s virus induced demyelinating disease: Differential susceptibility and effects of IL-4, IL-10 and combined IL-4 with IL-10. J. Neuroimmunol. 85 44–51. 10.1016/s0165-5728(97)00263-4 [DOI] [PubMed] [Google Scholar]
- Hollen C. W., Paz Soldán M. M., Rinker J. R., Spain R. I. (2020). The future of progressive multiple sclerosis therapies. Fed. Pract. 37:S43–S49. [PMC free article] [PubMed] [Google Scholar]
- Ingram G., Loveless S., Howell O. W., Hakobyan S., Dancey B., Harris C. L., et al. (2014). Complement activation in multiple sclerosis plaques: An immunohistochemical analysis. Acta Neuropathol. Commun. 2:53. 10.1186/2051-5960-2-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwahashi T., Inoue A., Koh C. S., Shin T. K., Kim B. S. (1999). Expression and potential role of inducible nitric oxide synthase in the central nervous system of Theiler’s murine encephalomyelitis virus-induced demyelinating disease. Cell Immunol. 194 186–193. 10.1006/cimm.1999.1482 [DOI] [PubMed] [Google Scholar]
- Jacobsen H., Klein S. L. (2021). Sex Differences in Immunity to Viral Infections. Front. Immunol. 12:720952. 10.3389/fimmu.2021.720952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R. W., Yong V. W. (2021). B cells in central nervous system disease: Diversity, locations and pathophysiology. Nat. Rev. Immunol. 22 513–524. 10.1038/s41577-021-00652-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James J. A., Gross T., Scofield R. H., Harley J. B. (1995). Immunoglobulin epitope spreading and autoimmune disease after peptide immunization: Sm B/B’-derived PPPGMRPP and PPPGIRGP induce spliceosome autoimmunity. J. Exp. Med. 181 453–461. 10.1084/jem.181.2.453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean C. M., Honarmand S., Louie J. K., Glaser C. A. (2007). Risk factors for West Nile virus neuroinvasive disease, California, 2005. Emerg. Infect. Dis. 13 1918–1920. 10.3201/eid1312.061265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek G. A., De Livera A. M., Marck C. H., Brown C. R., Neate S. L., Taylor K. L., et al. (2016). Associations of Lifestyle, Medication, and Socio-Demographic Factors with Disability in People with Multiple Sclerosis: An International Cross-Sectional Study. PLoS One 11:e0161701. 10.1371/journal.pone.0161701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B. C., Cao D. L., Zhang X., Zhang Z. J., He L. N., Li C. H., et al. (2016). CXCL13 drives spinal astrocyte activation and neuropathic pain via CXCR5. J. Clin. Invest. 126 745–761. 10.1172/JCI81950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y. H., Kim C. X., Huang J., Kim B. S. (2020). Infection and Activation of B Cells by Theiler’s Murine Encephalomyelitis Virus (TMEV) Leads to Autoantibody Production in an Infectious Model of Multiple Sclerosis. Cells 9:1787. 10.3390/cells9081787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jog N. R., James J. A. (2020). Epstein Barr Virus and Autoimmune Responses in Systemic Lupus Erythematosus. Front. Immunol. 11:623944. 10.3389/fimmu.2020.623944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jons D., Persson Berg L., Sundstrom P., Haghighi S., Axelsson M., Thulin M., et al. (2021). Follow-up after infectious mononucleosis in search of serological similarities with presymptomatic multiple sclerosis. Mult. Scler. Relat. Disord. 56:103288. 10.1016/j.msard.2021.103288 [DOI] [PubMed] [Google Scholar]
- Joseph F. G., Hirst C. L., Pickersgill T. P., Ben-Shlomo Y., Robertson N. P., Scolding N. J. (2009). CSF oligoclonal band status informs prognosis in multiple sclerosis: A case control study of 100 patients. J. Neurol. Neurosurg Psychiatry 80 292–296. 10.1136/jnnp.2008.150896 [DOI] [PubMed] [Google Scholar]
- Kabat E. A., Moore D. H., Landow H. (1942). An electrophoretic study of the protein components in cerebrospinal fluid and their relationship to the serum proteins. J. Clin. Invest. 21 571–577. 10.1172/JCI101335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakalacheva K., Munz C., Lunemann J. D. (2011). Viral triggers of multiple sclerosis. Biochim. Biophys. Acta 1812 132–140. 10.1016/j.bbadis.2010.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamma E., Lasisi W., Libner C., Ng H. S., Plemel J. R. (2022). Central nervous system macrophages in progressive multiple sclerosis: Relationship to neurodegeneration and therapeutics. J. Neuroinflamm. 19:45. 10.1186/s12974-022-02408-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H. S., Hou W., Kim B. S. (2020). Rapid Expansion of Virus-Specific CD4. Int. J. Mol. Sci. 21:7719. 10.3390/ijms21207719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappel C. A., Melvold R. W., Kim B. S. (1990). Influence of sex on susceptibility in the Theiler’s murine encephalomyelitis virus model for multiple sclerosis. J. Neuroimmunol. 29 15–19. [DOI] [PubMed] [Google Scholar]
- Katz-Levy Y., Neville K. L., Girvin A. M., Vanderlugt C. L., Pope J. G., Tan L. J., et al. (1999). Endogenous presentation of self myelin epitopes by CNS-resident APCs in Theiler’s virus-infected mice. J. Clin. Invest. 104 599–610. 10.1172/jci7292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz-Levy Y., Neville K. L., Padilla J., Rahbe S., Begolka W. S., Girvin A. M., et al. (2000). Temporal development of autoreactive Th1 responses and endogenous presentation of self myelin epitopes by central nervous system-resident APCs in Theiler’s virus-infected mice. J. Immunol. 165 5304–5314. 10.4049/jimmunol.165.9.5304 [DOI] [PubMed] [Google Scholar]
- Khanna K. M., Lepisto A. J., Decman V., Hendricks R. L. (2004). Immune control of herpes simplex virus during latency. Curr. Opin. Immunol. 16 463–469. 10.1016/j.coi.2004.05.003 [DOI] [PubMed] [Google Scholar]
- Klein S. L., Flanagan K. L. (2016). Sex differences in immune responses. Nat. Rev. Immunol. 16 626–638. 10.1038/nri.2016.90 [DOI] [PubMed] [Google Scholar]
- Kummerfeld M., Seehusen F., Klein S., Ulrich R., Kreutzer R., Gerhauser I., et al. (2012). Periventricular demyelination and axonal pathology is associated with subependymal virus spread in a murine model for multiple sclerosis. Intervirology 55 401–416. 10.1159/000336563 [DOI] [PubMed] [Google Scholar]
- Lanz T. V., Brewer R. C., Ho P. P., Moon J. S., Jude K. M., Fernandez D., et al. (2022). Clonally expanded B cells in multiple sclerosis bind EBV EBNA1 and GlialCAM. Nature 603 321–327. 10.1038/s41586-022-04432-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann H., Bradl M. (2017). Multiple sclerosis: Experimental models and reality. Acta Neuropathol. 133 223–244. 10.1007/s00401-016-1631-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. D., Coulthard L. G., Woodruff T. M. (2019). Complement dysregulation in the central nervous system during development and disease. Semin. Immunol. 45:101340. 10.1016/j.smim.2019.101340 [DOI] [PubMed] [Google Scholar]
- Leffler J., Bengtsson A. A., Blom A. M. (2014). The complement system in systemic lupus erythematosus: An update. Ann. Rheum. Dis. 73 1601–1606. 10.1136/annrheumdis-2014-205287 [DOI] [PubMed] [Google Scholar]
- Lehmann P. V., Forsthuber T., Miller A., Sercarz E. E. (1992). Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature 358 155–157. 10.1038/358155a0 [DOI] [PubMed] [Google Scholar]
- Li K., Li J., Zheng J., Qin S. (2019). Reactive astrocytes in neurodegenerative diseases. Aging Dis. 10 664–675. 10.14336/AD.2018.0720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z., Kumar A. S., Jones M. S., Knowles N. J., Lipton H. L. (2008). Phylogenetic analysis of the species Theilovirus: Emerging murine and human pathogens. J. Virol. 82 11545–11554. 10.1128/JVI.01160-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbey J. E., Cusick M. F., Doty D. J., Fujinami R. S. (2017). Complement components are expressed by infiltrating macrophages/activated microglia early following viral infection. Viral. Immunol. 30 304–314. 10.1089/vim.2016.0175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbey J. E., Kirkman N. J., Wilcox K. S., White H. S., Fujinami R. S. (2010). Role for complement in the development of seizures following acute viral infection. J. Virol. 84 6452–6460. 10.1128/JVI.00422-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow S. A., Guttenplan K. A., Clarke L. E., Bennett F. C., Bohlen C. J., Schirmer L., et al. (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541 481–487. 10.1038/nature21029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow S. A., Marsh S. E., Stevens B. (2020). Microglia and astrocytes in disease: Dynamic duo or partners in crime? Trends Immunol. 41 820–835. 10.1016/j.it.2020.07.006 [DOI] [PubMed] [Google Scholar]
- Linnerbauer M., Wheeler M. A., Quintana F. J. (2020). Astrocyte crosstalk in cns inflammation. Neuron 108 608–622. 10.1016/j.neuron.2020.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzey M., DiSano K., Welsh N., Pachner A., Gilli F. (2022). Divergent complement system activation in two clinically distinct murine models of multiple sclerosis. Front. Immunol. 13:924734. 10.3389/fimmu.2022.924734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton H. L. (1975). Theiler’s virus infection in mice: An unusual biphasic disease process leading to demyelination. Infect. Immun. 11 1147–1155. 10.1128/iai.11.5.1147-1155.1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton H. L., Dal Canto M. C. (1979). Susceptibility of inbred mice to chronic central nervous system infection by Theiler’s murine encephalomyelitis virus. Infect. Immun. 26 369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorch Y., Friedmann A., Lipton H. L., Kotler M. (1981). Theiler’s murine encephalomyelitis virus group includes two distinct genetic subgroups that differ pathologically and biologically. J. Virol. 40 560–567. 10.1128/JVI.40.2.560-567.1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luong N., Olson J. K. (2021). Exosomes secreted by microglia during virus infection in the central nervous system activate an inflammatory response in bystander cells. Front. Cell Dev. Biol. 9:661935. 10.3389/fcell.2021.661935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magliozzi R., Howell O., Vora A., Serafini B., Nicholas R., Puopolo M., et al. (2007). Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 130 1089–1104. 10.1093/brain/awm038 [DOI] [PubMed] [Google Scholar]
- Matsushita T., Yanaba K., Bouaziz J. D., Fujimoto M., Tedder T. F. (2008). Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J. Clin. Invest. 118 3420–3430. 10.1172/jci36030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecha M., Carrillo-Salinas F. J., Mestre L., Feliu A., Guaza C. (2013). Viral models of multiple sclerosis: Neurodegeneration and demyelination in mice infected with Theiler’s virus. Prog. Neurobiol. 10 46–64. 10.1016/j.pneurobio.2012.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier U. C., Cipian R. C., Karimi A., Ramasamy R., Middeldorp J. M. (2021). Cumulative roles for epstein-barr virus, human endogenous retroviruses, and human herpes virus-6 in driving an inflammatory cascade underlying ms pathogenesis. Front. Immunol. 12:757302. 10.3389/fimmu.2021.757302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melvold R. W., Jokinen D. M., Knobler R. L., Lipton H. L. (1987). Variations in genetic control of susceptibility to Theiler’s murine encephalomyelitis virus (TMEV)-induced demyelinating disease. I. Differences between susceptible SJL/J and resistant BALB/c strains map near the T cell beta-chain constant gene on chromosome 6. J. Immunol. 138 1429–1433. [PubMed] [Google Scholar]
- Merle N. S., Church S. E., Fremeaux-Bacchi V., Roumenina L. T. (2015a). Complement system part i - molecular mechanisms of activation and regulation. Front. Immunol. 6:262. 10.3389/fimmu.2015.00262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merle N. S., Noe R., Halbwachs-Mecarelli L., Fremeaux-Bacchi V., Roumenina L. T. (2015b). Complement system part ii: Role in immunity. Front. Immunol. 6:257. 10.3389/fimmu.2015.00257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf T. U., Griffin D. E. (2011). Alphavirus-induced encephalomyelitis: Antibody-secreting cells and viral clearance from the nervous system. J. Virol. 85 11490–11501. 10.1128/jvi.05379-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel L., Touil H., Pikor N. B., Gommerman J. L., Prat A., Bar-Or A. (2015). B Cells in the multiple sclerosis central nervous system: Trafficking and contribution to cns-compartmentalized inflammation. Front. Immunol. 6:636. 10.3389/fimmu.2015.00636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. D., Vanderlugt C. L., Begolka W. S., Pao W., Yauch R. L., Neville K. L., et al. (1997). Persistent infection with Theiler’s virus leads to CNS autoimmunity via epitope spreading. Nat. Med. 3 1133–1136. [DOI] [PubMed] [Google Scholar]
- Mokhtarian F., Huan C. M., Roman C., Raine C. S. (2003). Semliki Forest virus-induced demyelination and remyelination–involvement of B cells and anti-myelin antibodies. J. Neuroimmunol. 137 19–31. 10.1016/s0165-5728(03)00039-0 [DOI] [PubMed] [Google Scholar]
- Montalban X., Hauser S. L., Kappos L., Arnold D. L., Bar-Or A., Comi G., et al. (2017). Ocrelizumab versus placebo in primary progressive multiple sclerosis. N. Engl. J. Med. 376 209–220. 10.1056/NEJMoa1606468 [DOI] [PubMed] [Google Scholar]
- Morgan B. P., Gommerman J. L., Ramaglia V. (2020). An “outside-in” and “inside-out” consideration of complement in the multiple sclerosis brain: Lessons from development and neurodegenerative diseases. Front. Cell Neurosci. 14:600656. 10.3389/fncel.2020.600656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Pfeiffer R., Camargo M. C., Rabkin C. S. (2009). Meta-analysis shows that prevalence of Epstein-Barr virus-positive gastric cancer differs based on sex and anatomic location. Gastroenterology 137 824–833. 10.1053/j.gastro.2009.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naucler C. S., Larsson S., Moller E. (1996). A novel mechanism for virus-induced autoimmunity in humans. Immunol. Rev. 152 175–192. 10.1111/j.1600-065x.1996.tb00916.x [DOI] [PubMed] [Google Scholar]
- Nytrova P., Potlukova E., Kemlink D., Woodhall M., Horakova D., Waters P., et al. (2014). Complement activation in patients with neuromyelitis optica. J. Neuroimmunol. 274 185–191. 10.1016/j.jneuroim.2014.07.001 [DOI] [PubMed] [Google Scholar]
- Olitsky P. K. (1940). A transmissible agent (theiler’s virus) in the intestines of normal mice. J. Exp. Med. 72 113–127. 10.1084/jem.72.2.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson J. K., Girvin A. M., Miller S. D. (2001). Direct activation of innate and antigen-presenting functions of microglia following infection with Theiler’s virus. J. Virol. 75 9780–9789. 10.1128/jvi.75.20.9780-9789.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson J. K., Miller S. D. (2009). The innate immune response affects the development of the autoimmune response in Theiler’s virus-induced demyelinating disease. J. Immunol. 182 5712–5722. 10.4049/jimmunol.0801940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura S., Kawai E., Sato F., Martinez N. E., Minagar A., Al-Kofahi M., et al. (2018). Theiler’s virus-mediated immunopathology in the cns and heart: Roles of organ-specific cytokine and lymphatic responses. Front. Immunol. 9:2870. 10.3389/fimmu.2018.02870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orton S. M., Herrera B. M., Yee I. M., Valdar W., Ramagopalan S. V., Sadovnick A. D., et al. (2006). Sex ratio of multiple sclerosis in Canada: A longitudinal study. Lancet Neurol. 5 932–936. 10.1016/s1474-4422(06)70581-6 [DOI] [PubMed] [Google Scholar]
- Ozakbas S., Cinar B. P., Ozcelik P., Baser H., Kosehasanogullari G. (2017). Intrathecal IgM index correlates with a severe disease course in multiple sclerosis: Clinical and MRI results. Clin. Neurol. Neurosurg 160 27–29. 10.1016/j.clineuro.2017.05.026 [DOI] [PubMed] [Google Scholar]
- Pachner A. R., Brady J., Narayan K. (2007a). Antibody-secreting cells in the central nervous system in an animal model of MS: Phenotype, association with disability, and in vitro production of antibody. J. Neuroimmunol. 190 112–120. 10.1016/j.jneuroim.2007.09.001 [DOI] [PubMed] [Google Scholar]
- Pachner A. R., Li L., Narayan K. (2007b). Intrathecal antibody production in an animal model of multiple sclerosis. J. Neuroimmunol. 185 57–63. 10.1016/j.jneuroim.2007.01.017 [DOI] [PubMed] [Google Scholar]
- Pekna M., Pekny M. (2021). The complement system: A powerful modulator and effector of astrocyte function in the healthy and diseased central nervous system. Cells 10:1812. 10.3390/cells10071812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez Gomez A. A., Karmakar M., Carroll R. J., Lawley K. S., Amstalden K., Young C. R., et al. (2021). Genetic and immunological contributors to virus-induced paralysis. Brain Behav. Immun. Health 18:100395. 10.1016/j.bbih.2021.100395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Saldivar M., Ordonez G., Pineda B., Sotelo J., Martinez-Palomo A., Flores-Rivera J., et al. (2021). T-cell response against varicella zoster virus in patients with multiple sclerosis during relapse and remission. Int. J. Mol. Sci. 23 298. 10.3390/ijms23010298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponath G., Park C., Pitt D. (2018). The role of astrocytes in multiple sclerosis. Front. Immunol. 9:217. 10.3389/fimmu.2018.00217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope J. G., Karpus W. J., VanderLugt C., Miller S. D. (1996). Flow cytometric and functional analyses of central nervous system-infiltrating cells in SJL/J mice with Theiler’s virus-induced demyelinating disease. Evidence for a CD4+ T cell-mediated pathology. J. Immunol. 156 4050–4058. [PubMed] [Google Scholar]
- Pozzilli C., Tomassini V., Marinelli F., Paolillo A., Gasperini C., Bastianello S. (2003). ‘Gender gap’ in multiple sclerosis: Magnetic resonance imaging evidence. Eur. J. Neurol. 10 95–97. 10.1046/j.1468-1331.2003.00519.x [DOI] [PubMed] [Google Scholar]
- Reali C., Magliozzi R., Roncaroli F., Nicholas R., Howell O. W., Reynolds R. (2020). B cell rich meningeal inflammation associates with increased spinal cord pathology in multiple sclerosis. Brain Pathol. 30 779–793. 10.1111/bpa.12841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribbons K. A., McElduff P., Boz C., Trojano M., Izquierdo G., Duquette P., et al. (2015). Male sex is independently associated with faster disability accumulation in relapse-onset ms but not in primary progressive ms. PLoS One 10:e0122686. 10.1371/journal.pone.0122686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas M., Restrepo-Jimenez P., Monsalve D. M., Pacheco Y., Acosta-Ampudia Y., Ramirez-Santana C., et al. (2018). Molecular mimicry and autoimmunity. J. Autoimmun. 95 100–123. 10.1016/j.jaut.2018.10.012 [DOI] [PubMed] [Google Scholar]
- Rossbacher J., Shlomchik M. J. (2003). The B cell receptor itself can activate complement to provide the complement receptor 1/2 ligand required to enhance B cell immune responses in vivo. J. Exp. Med. 198 591–602. 10.1084/jem.20022042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santello M., Toni N., Volterra A. (2019). Astrocyte function from information processing to cognition and cognitive impairment. Nat. Neurosci. 22 154–166. 10.1038/s41593-018-0325-8 [DOI] [PubMed] [Google Scholar]
- Schafer D. P., Lehrman E. K., Kautzman A. G., Koyama R., Mardinly A. R., Yamasaki R., et al. (2012). Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74 691–705. 10.1016/j.neuron.2012.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson S., Jr., Taylor B., Dwyer D. E., Taylor J., Blizzard L., Ponsonby A. L., et al. (2012). Anti-HHV-6 IgG titer significantly predicts subsequent relapse risk in multiple sclerosis. Mult. Scler. 18 799–806. 10.1177/1352458511428081 [DOI] [PubMed] [Google Scholar]
- Sindic C. J., Monteyne P., Laterre E. C. (1994). The intrathecal synthesis of virus-specific oligoclonal IgG in multiple sclerosis. J. Neuroimmunol. 54 75–80. 10.1016/0165-5728(94)90233-x [DOI] [PubMed] [Google Scholar]
- Smatti M. K., Cyprian F. S., Nasrallah G. K., Al Thani A. A., Almishal R. O., Yassine H. M. (2019). Viruses and autoimmunity: A review on the potential interaction and molecular mechanisms. Viruses 11:762. 10.3390/v11080762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Bouvier D. L., Divekar A. A., Sasidhar M., Du S., Tiwari-Woodruff S. K., King J. K., et al. (2008). A role for sex chromosome complement in the female bias in autoimmune disease. J. Exp. Med. 205 1099–1108. 10.1084/jem.20070850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sospedra M., Martin R. (2005). Immunology of multiple sclerosis. Annu. Rev. Immunol. 23 683–747. 10.1146/annurev.immunol.23.021704.115707 [DOI] [PubMed] [Google Scholar]
- Steiner I., Kennedy P. G., Pachner A. R. (2007). The neurotropic herpes viruses: Herpes simplex and varicella-zoster. Lancet Neurol. 6 1015–1028. 10.1016/S1474-4422(07)70267-3 [DOI] [PubMed] [Google Scholar]
- Stewart K. A., Wilcox K. S., Fujinami R. S., White H. S. (2010). Theiler’s virus infection chronically alters seizure susceptibility. Epilepsia 51 1418–1428. 10.1111/j.1528-1167.2009.02405.x [DOI] [PubMed] [Google Scholar]
- Stohlman S. A., Ramakrishna C., Tschen S. I., Hinton D. R., Bergmann C. C. (2002). The art of survival during viral persistence. J. Neurovirol. 8 53–58. 10.1080/13550280290167884 [DOI] [PubMed] [Google Scholar]
- Tait A. R., Straus S. K. (2008). Phosphorylation of U24 from Human Herpes Virus type 6 (HHV-6) and its potential role in mimicking myelin basic protein (MBP) in multiple sclerosis. FEBS Lett. 582 2685–2688. 10.1016/j.febslet.2008.06.050 [DOI] [PubMed] [Google Scholar]
- Tarlinton R. E., Martynova E., Rizvanov A. A., Khaiboullina S., Verma S. (2020). Role of viruses in the pathogenesis of multiple sclerosis. Viruses 12:643. 10.3390/v12060643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejada-Simon M. V., Zang Y. C., Hong J., Rivera V. M., Zhang J. Z. (2003). Cross-reactivity with myelin basic protein and human herpesvirus-6 in multiple sclerosis. Ann. Neurol. 53 189–197. 10.1002/ana.10425 [DOI] [PubMed] [Google Scholar]
- Tengvall K., Huang J., Hellström C., Kammer P., Biström M., Ayoglu B., et al. (2019). Molecular mimicry between Anoctamin 2 and Epstein-Barr virus nuclear antigen 1 associates with multiple sclerosis risk. Proc. Natl. Acad. Sci. U.S.A. 116 16955–16960. 10.1073/pnas.1902623116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacker E. L., Mirzaei F., Ascherio A. (2006). Infectious mononucleosis and risk for multiple sclerosis: A meta-analysis. Ann. Neurol. 59 499–503. 10.1002/ana.20820 [DOI] [PubMed] [Google Scholar]
- Theiler M., Gard S. (1940). Encephalomyelitis of mice : I. characteristics and pathogenesis of the virus. J. Exp. Med. 72 49–67. 10.1084/jem.72.1.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Hawkins J. B., Tracy S. I., Shapiro M. (2013). The pathogenesis of Epstein-Barr virus persistent infection. Curr. Opin. Virol. 3 227–232. 10.1016/j.coviro.2013.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassini V., Onesti E., Mainero C., Giugni E., Paolillo A., Salvetti M., et al. (2005). Sex hormones modulate brain damage in multiple sclerosis: MRI evidence. J. Neurol. Neurosurg Psychiatry 76 272–275. 10.1136/jnnp.2003.033324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassini V., Pozzilli C. (2009). Sex hormones, brain damage and clinical course of Multiple Sclerosis. J. Neurol. Sci. 286 35–39. 10.1016/j.jns.2009.04.014 [DOI] [PubMed] [Google Scholar]
- Touil H., Kobert A., Lebeurrier N., Rieger A., Saikali P., Lambert C., et al. (2018). Human central nervous system astrocytes support survival and activation of B cells: Implications for MS pathogenesis. J. Neuroinflamm. 15:114. 10.1186/s12974-018-1136-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trolese M. C., Mariani A., Terao M., de Paola M., Fabbrizio P., Sironi F., et al. (2020). CXCL13/CXCR5 signalling is pivotal to preserve motor neurons in amyotrophic lateral sclerosis. eBioMedicine 62:103097. 10.1016/j.ebiom.2020.103097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda I., Fujinami R. S. (2010). Neuropathogenesis of Theiler’s murine encephalomyelitis virus infection, an animal model for multiple sclerosis. J. Neuroimmune Pharmacol. 5 355–369. 10.1007/s11481-009-9179-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda I., Kuang L. Q., Fujinami R. S. (2002). Induction of autoreactive CD8+ cytotoxic T cells during Theiler’s murine encephalomyelitis virus infection: Implications for autoimmunity. J. Virol. 76 12834–12844. 10.1128/jvi.76.24.12834-12844.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda I., Kurtz C. I., Fujinami R. S. (1997). Apoptosis in acute and chronic central nervous system disease induced by Theiler’s murine encephalomyelitis virus. Virology 228 388–393. 10.1006/viro.1996.8382 [DOI] [PubMed] [Google Scholar]
- Ulrich R., Kalkuhl A., Deschl U., Baumgartner W. (2010). Machine learning approach identifies new pathways associated with demyelination in a viral model of multiple sclerosis. J. Cell Mol. Med. 14 434–448. 10.1111/j.1582-4934.2008.00646.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umpierre A. D., Remigio G. J., Dahle E. J., Bradford K., Alex A. B., Smith M. D., et al. (2014). Impaired cognitive ability and anxiety-like behavior following acute seizures in the Theiler’s virus model of temporal lobe epilepsy. Neurobiol. Dis. 64 98–106. 10.1016/j.nbd.2013.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Walderveen M. A., Lycklama A. N. G. J., Ader H. J., Jongen P. J., Polman C. H., Castelijns J. A., et al. (2001). Hypointense lesions on T1-weighted spin-echo magnetic resonance imaging: Relation to clinical characteristics in subgroups of patients with multiple sclerosis. Arch Neurol. 58 76–81. 10.1001/archneur.58.1.76 [DOI] [PubMed] [Google Scholar]
- Vanderlugt C. L., Miller S. D. (2002). Epitope spreading in immune-mediated diseases: Implications for immunotherapy. Nat. Rev. Immunol. 2 85–95. 10.1038/nri724 [DOI] [PubMed] [Google Scholar]
- Vargas M. E., Watanabe J., Singh S. J., Robinson W. H., Barres B. A. (2010). Endogenous antibodies promote rapid myelin clearance and effective axon regeneration after nerve injury. Proc. Natl. Acad. Sci. U.S.A. 107 11993–11998. 10.1073/pnas.1001948107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen J. O., Wohler J., Fenton K., Reich D. S., Jacobson S. (2014). Oligoclonal bands in multiple sclerosis reactive against two herpesviruses and association with magnetic resonance imaging findings. Mult. Scler. 20 27–34. 10.1177/1352458513490545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskuhl R. R., Sawalha A. H., Itoh Y. (2018). Sex chromosome contributions to sex differences in multiple sclerosis susceptibility and progression. Mult. Scler. 24 22–31. 10.1177/1352458517737394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Lai X., Deng Y., Song Y. (2020). Correlation between mouse age and human age in anti-tumor research: Significance and method establishment. Life Sci. 242 117242. 10.1016/j.lfs.2019.117242 [DOI] [PubMed] [Google Scholar]
- Watkins L. M., Neal J. W., Loveless S., Michailidou I., Ramaglia V., Rees M. I., et al. (2016). Complement is activated in progressive multiple sclerosis cortical grey matter lesions. J. Neuroinflamm. 13:161. 10.1186/s12974-016-0611-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M. S., Prod’homme T., Patarroyo J. C., Molnarfi N., Karnezis T., Lehmann-Horn K., et al. (2010). B-cell activation influences T-cell polarization and outcome of anti-CD20 B-cell depletion in central nervous system autoimmunity. Ann. Neurol. 68 369–383. 10.1002/ana.22081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H. L. (2004). Multiple sclerosis is an inflammatory T-cell-mediated autoimmune disease. Arch Neurol. 61 1613–1615. 10.1001/archneur.61.10.1613 [DOI] [PubMed] [Google Scholar]
- Weiner H. L. (2008). A shift from adaptive to innate immunity: A potential mechanism of disease progression in multiple sclerosis. J. Neurol. 255 3–11. 10.1007/s00415-008-1002-8 [DOI] [PubMed] [Google Scholar]
- Welsh N. C., DiSano K., Linzey M., Pachner A. R., Gilli F. (2020). “Sexually dimorphic intrathecal inflammation in a murine model of progressive MS,” in Poster at the ACTRIMS forum, ed. Journals S. (West Palm Beach, FL: Multiple Sclerosis Journal; ). [Google Scholar]
- Werneburg S., Jung J., Kunjamma R. B., Ha S. K., Luciano N. J., Willis C. M., et al. (2020). Targeted complement inhibition at synapses prevents microglial synaptic engulfment and synapse loss in demyelinating disease. Immunity 52 167.e–182.e. 10.1016/j.immuni.2019.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter J. R., Taylor G. S., Thomas O. G., Jackson C., Lewis J. E. A., Stagg H. R. (2019). Predictors of epstein-barr virus serostatus in young people in england. BMC Infect. Dis. 19:1007. 10.1186/s12879-019-4578-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Zhang L. J., Yang L., Yang C. S., Yi M., Zhang S. N., et al. (2021a). Positive association of herpes simplex virus-IgG with multiple sclerosis: A systematic review and meta-analysis. Mult. Scler. Relat. Disord. 47:102633. 10.1016/j.msard.2020.102633 [DOI] [PubMed] [Google Scholar]
- Xu Y., Jin M. Z., Yang Z. Y., Jin W. L. (2021b). Microglia in neurodegenerative diseases. Neural. Regen. Res. 16 270–280. 10.4103/1673-5374.290881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ysrraelit M. C., Correale J. (2019). Impact of sex hormones on immune function and multiple sclerosis development. Immunology 156 9–22. 10.1111/imm.13004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeinstra E., Wilczak N., De Keyser J. (2003). Reactive astrocytes in chronic active lesions of multiple sclerosis express co-stimulatory molecules B7-1 and B7-2. J. Neuroimmunol. 135 166–171. 10.1016/s0165-5728(02)00462-9 [DOI] [PubMed] [Google Scholar]
- Zoecklein L. J., Pavelko K. D., Gamez J., Papke L., McGavern D. B., Ure D. R., et al. (2003). Direct comparison of demyelinating disease induced by the daniel’s strain and bean strain of theiler’s murine encephalomyelitis virus. Brain Pathol. 13 291–308. 10.1111/j.1750-3639.2003.tb00029.x [DOI] [PMC free article] [PubMed] [Google Scholar]